The Dual Role of Perivascular Adipose Tissue in Vascular Homeostasis and Atherogenesis: From Physiology to Pathological Implications
Abstract
1. Introduction
2. Anatomy and Physiological Role of PVAT
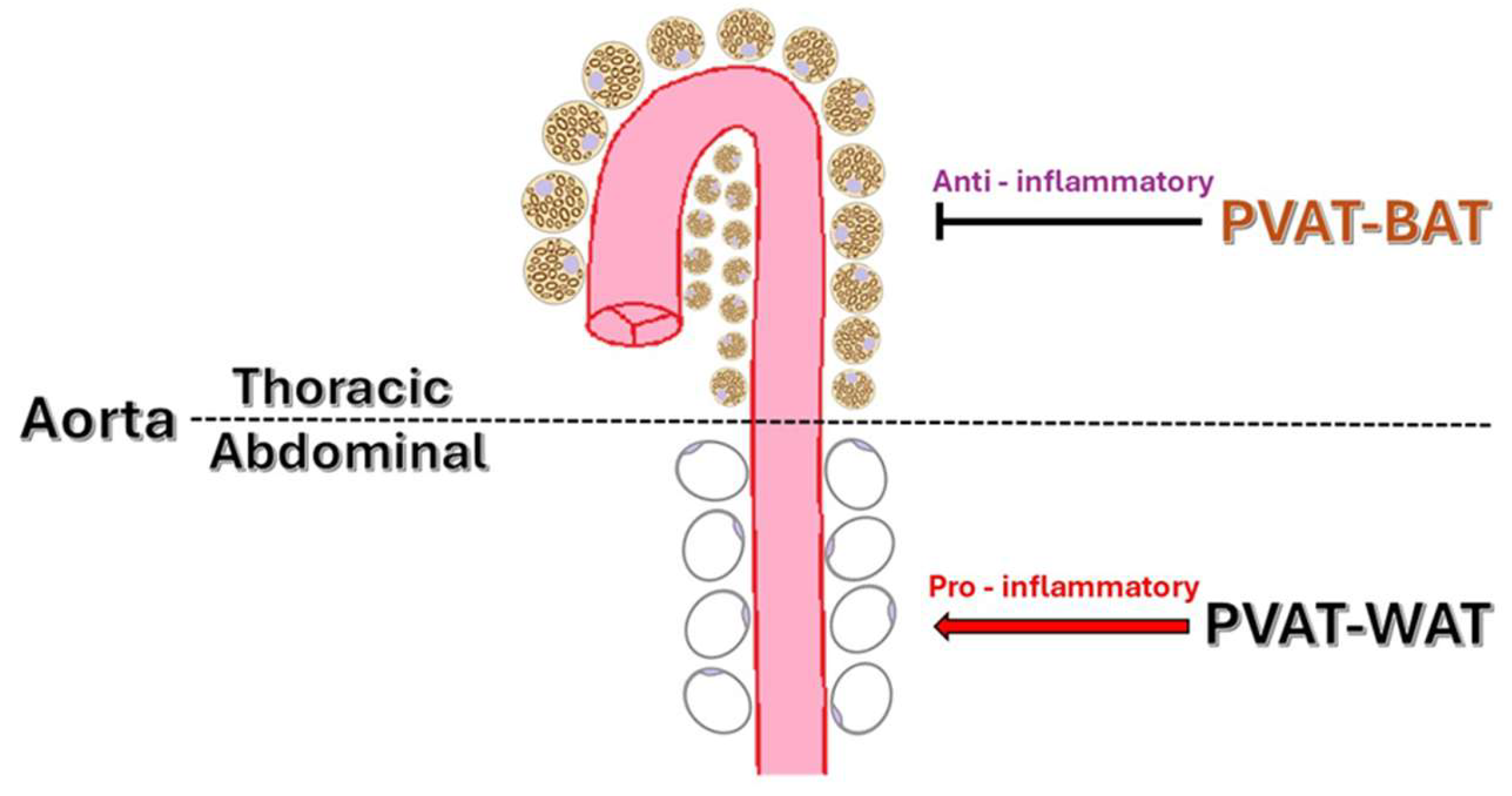
2.1. Anatomical Distribution and Structural Differences of PVAT
2.2. Normal Paracrine Functions and Physiological Roles of PVAT
2.3. Physiological Vascular Tone Modulation by PVAT
3. SAT, VAT, and PVAT Characteristics
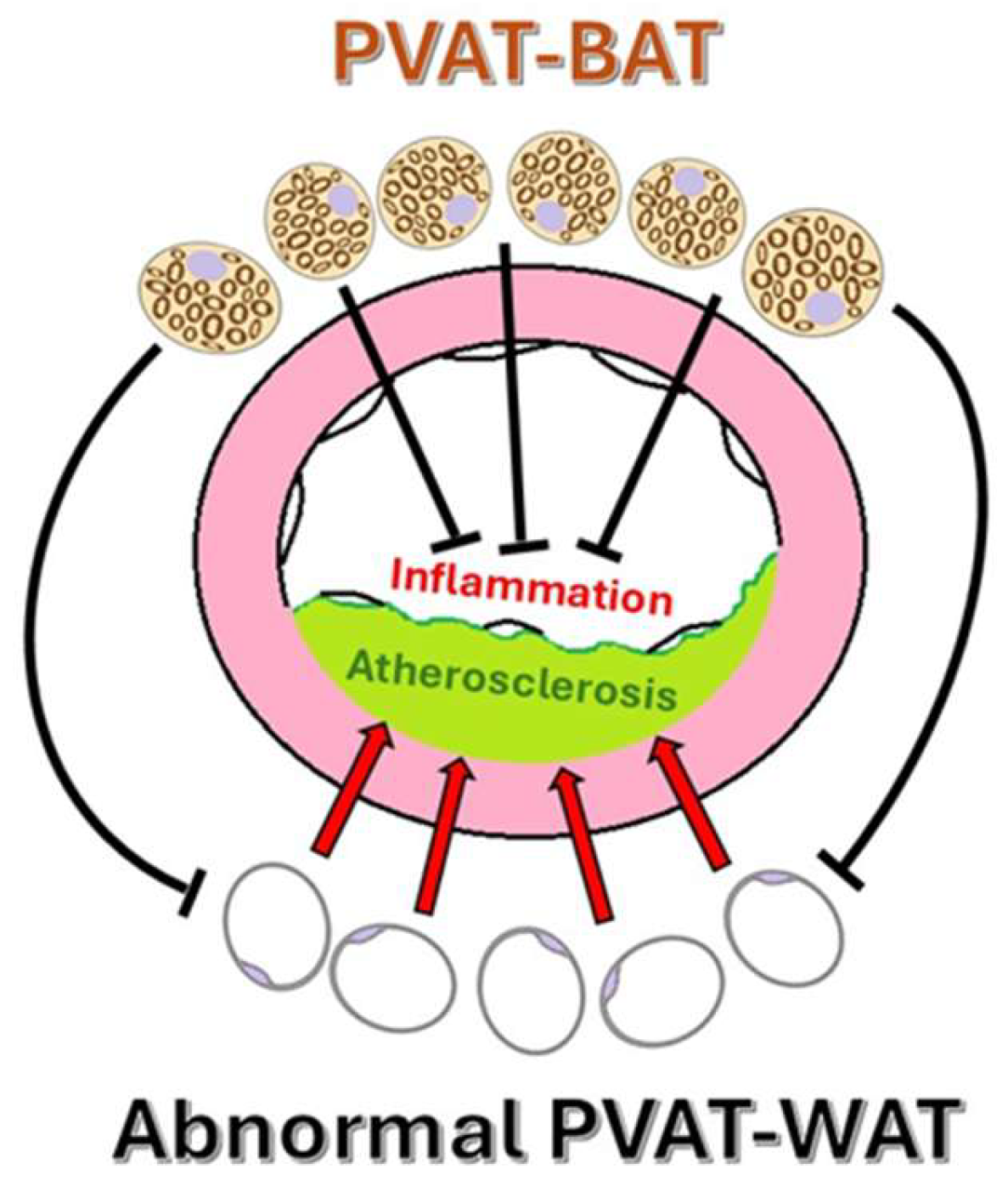
4. PVAT in Pathological States
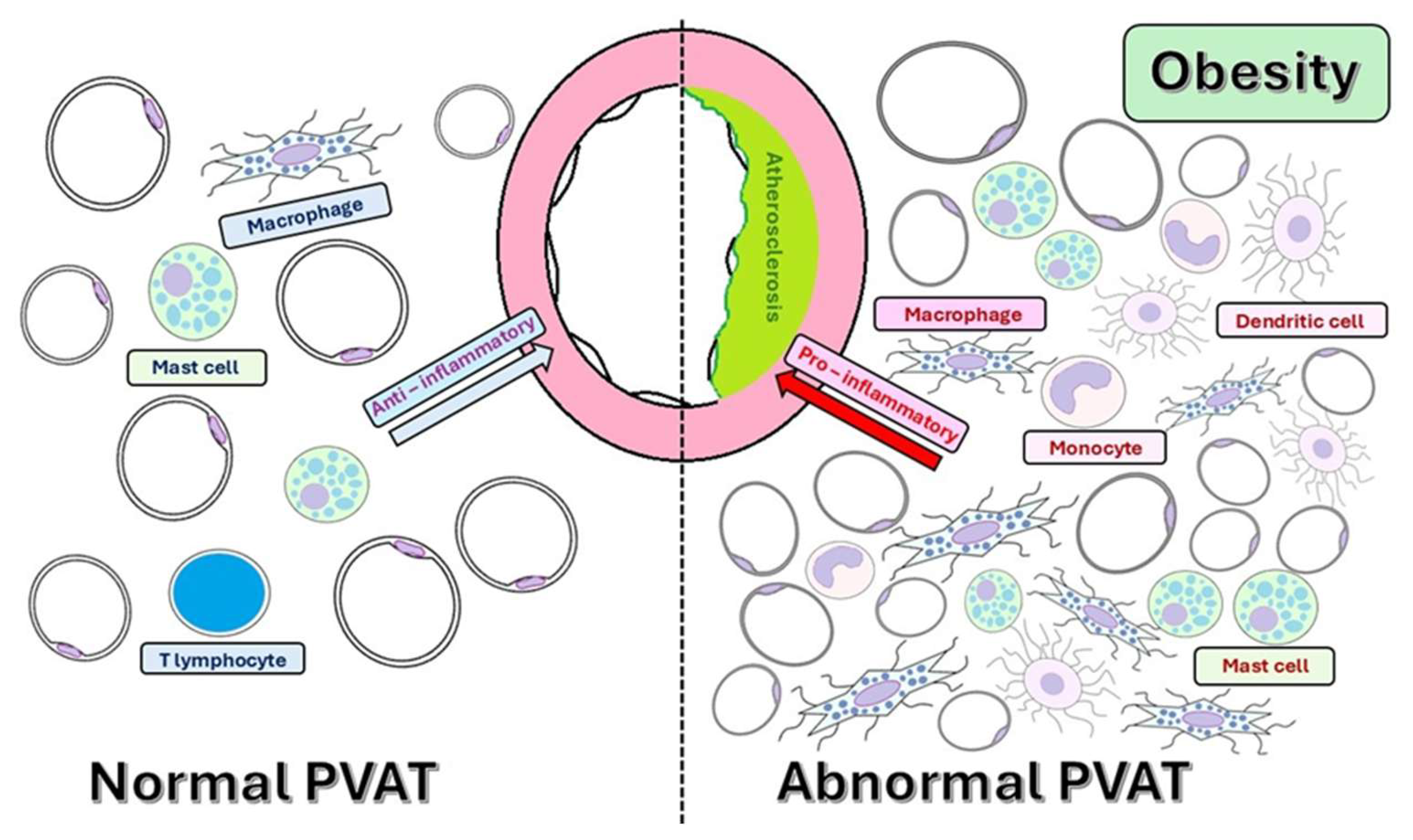
4.1. Cellular Composition of PVAT and Crosstalk with the Systemic Metabolism
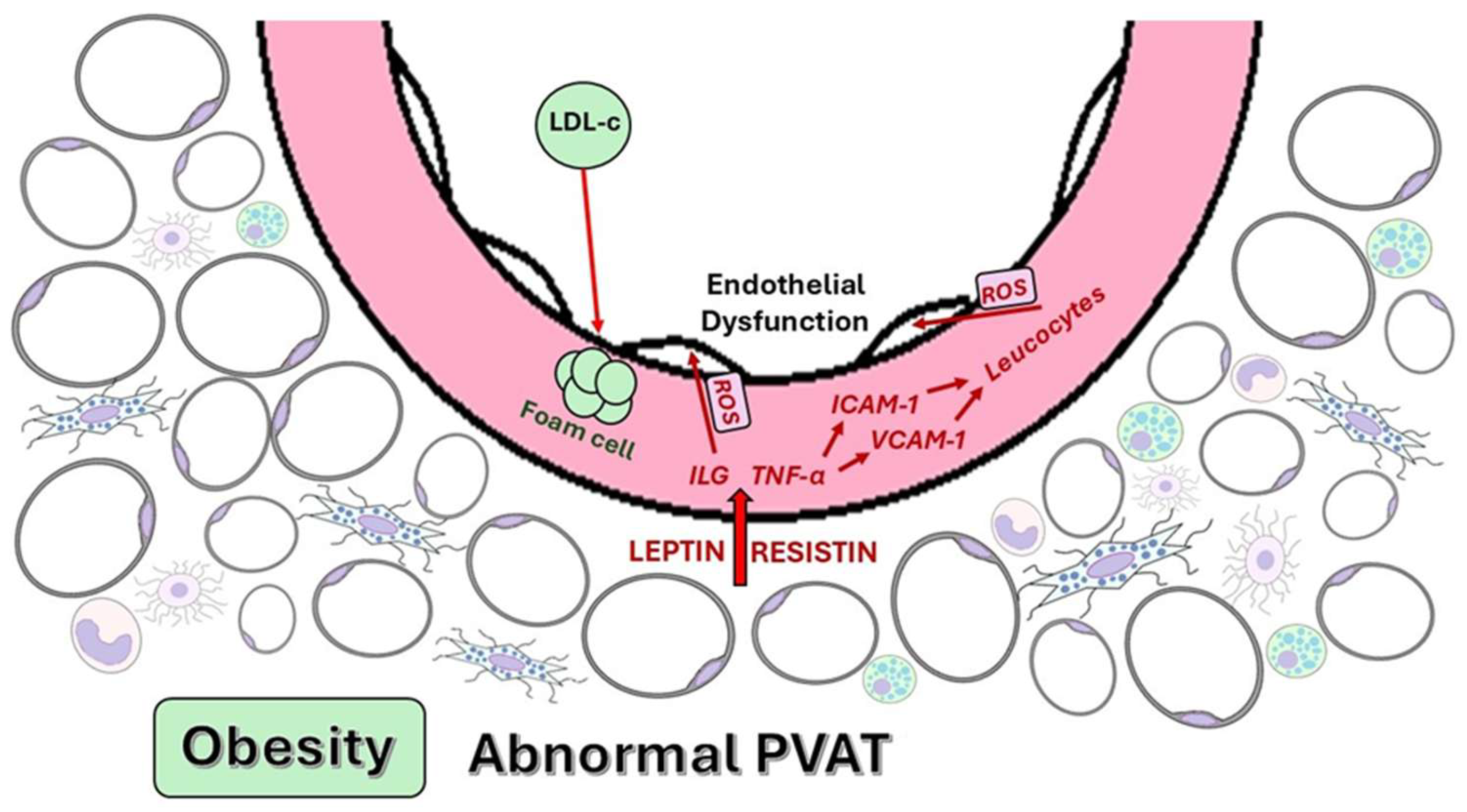
4.2. Adipokines and Cytokines Secreted by Dysfunctional PVAT
4.3. Atherosclerosis and Pathophysiological Mechanisms Linking PVAT to Atherosclerosis
4.3.1. Endothelial Dysfunction
4.3.2. VSMC Modulation
4.3.3. Immune Cell Recruitment and Local Inflammation
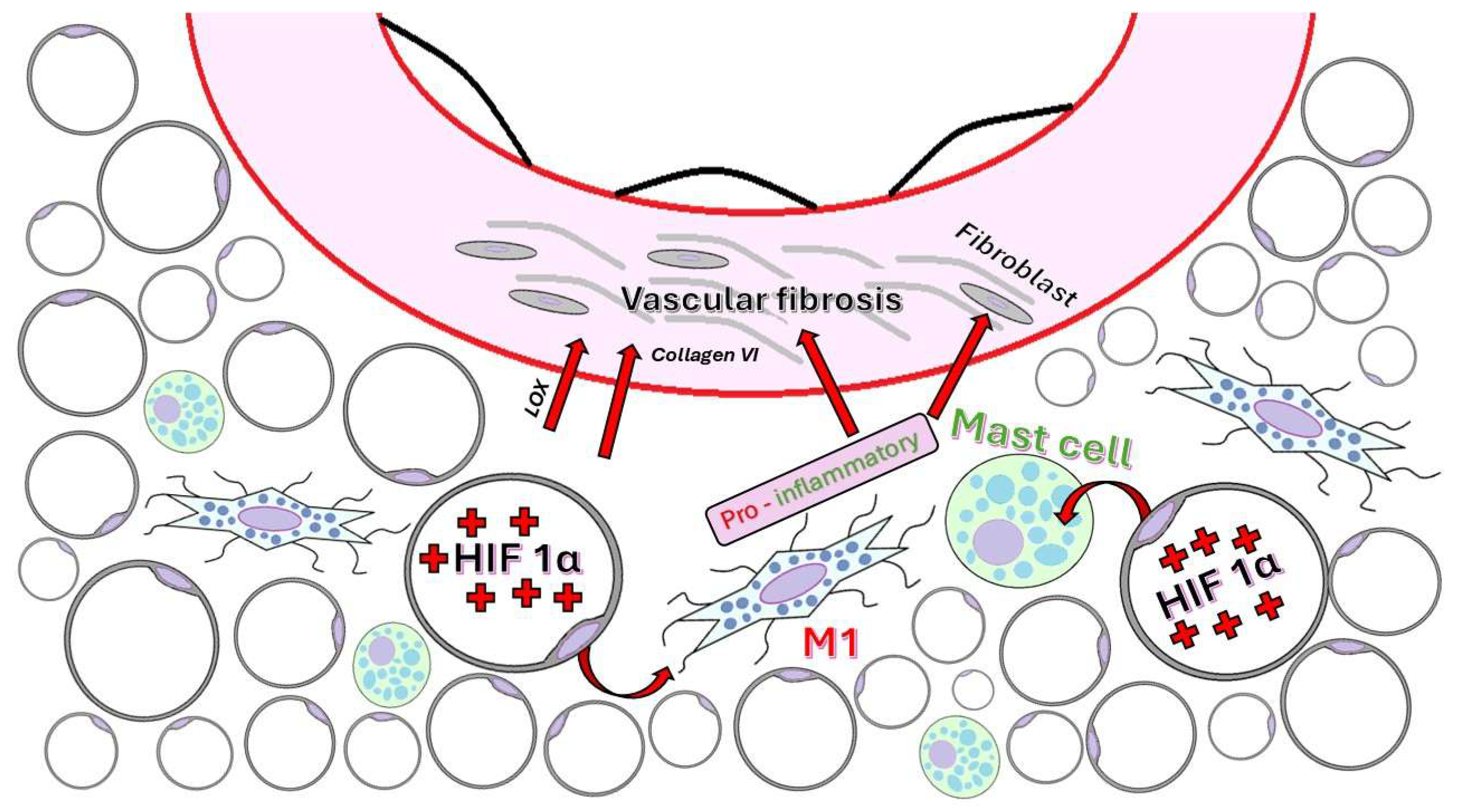
4.3.4. ECM Remodeling and Fibrosis
- (1)
- (2)
- (3)
- (4)
- (5)
5. Pathological Features of PVAT in Human Atherosclerosis
6. Human vs. Animal Model Findings
7. Imaging Modalities for the Evaluation of PVAT
8. Emerging Biomarkers of PVAT Dysfunction
9. Challenges, Limitations, and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | Advanced Glycation End Products |
| ALEs | Advanced Lipoxidation End Products |
| AI | Artificial Intelligence |
| BAT | Brown Adipose Tissue |
| BMI | Body Mass Index |
| CCTA | Coronary Computed Tomography Angiography |
| CCL5 | C-C Motif Chemokine Ligand 5 |
| CCL2 | C-C Motif Chemokine Ligand 2 |
| CXCL10 | C-X-C Motif Chemokine Ligand 10 |
| CXCR3 | C-X-C Chemokine Receptor 3 |
| CRD | Carbohydrate Recognition Domain |
| CRP | C-Reactive Protein |
| CT | Computed Tomography |
| EAT | Epicardial Adipose Tissue |
| ECM | Extracellular Matrix |
| EPC | Endothelial Progenitor Cells |
| ERK | Extracellular Signal-Regulated Kinase |
| FABP | Fatty Acid-Binding Protein |
| FAI | Fat Attenuation Index |
| FDG | Fludeoxyglucose |
| FRP | Fat Radiomic Profile |
| HDL | High-Density Lipoprotein |
| HOMA-IR | Homeostasis Model Assessment For Insulin Resistance |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| IFN-γ | Interferon Gamma |
| IL-17A | Interleukin-17a |
| IL-18 | Interleukin-18 |
| IL-1β | Interleukin-1 Beta |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IP-10 | Interferon Gamma-Induced Protein 10 |
| LAD | Left Anterior Descending Artery |
| LCN-2 | Lipocalin-2 |
| LDL | Low-Density Lipoprotein |
| LDLR | Low-Density Lipoprotein Receptor |
| LOX | Lysyl Oxidase |
| MAPK | Mitogen-Activated Protein Kinase |
| MCP | Modified Citrus Pectin |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MMPs | Matrix Metalloproteinases |
| MMP-2 | Matrix Metalloproteinase-2 |
| MMP-9 | Matrix Metalloproteinase-9 |
| MRI | Magnetic Resonance Imaging |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NF-kB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NLRP3 | Nod-, Lrr-, And Pyrin Domain-Containing Protein 3 |
| NO | Nitric Oxide |
| PET | Positron Emission Tomography |
| PET-CT | Positron Emission Tomography–Computed Tomography |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha |
| PKC-β | Protein Kinase C Beta |
| PVAT | Perivascular Adipose Tissue |
| RAGE | Receptor for Advanced Glycation End Products |
| RANTES | Regulated Upon Activation, Normal T-Cell Expressed and Secreted |
| ROS | Reactive Oxygen Species |
| SAT | Subcutaneous Adipose Tissue |
| α-SMA | Alpha-Smooth Muscle Actin |
| SM22α | Smooth Muscle 22 A |
| SPARC | Secreted Protein, Acidic and Rich in Cysteine |
| TGF-β | Transforming Growth Factor Beta |
| TNF-α | Tumor Necrosis Factor-Alpha |
| TSP-1 | Thrombospondin-1 |
| UCP-1 | Uncoupling Protein-1 |
| VAT | Visceral Adipose Tissue |
| VCAM-1 | Vascular Cell Adhesion Molecule-1 |
| VEGF-A | Vascular Endothelial Growth Factor |
| VSMC | Vascular Smooth Muscle Cell |
| WAT | White Adipose Tissue |
References
- Andraws, R.; Berger, J.S.; Brown, D.L. Effects of Antibiotic Therapy on Outcomes of Patients with Coronary Artery DiseaseA Meta-Analysis of Randomized Controlled Trials. JAMA 2005, 293, 2641–2647. [Google Scholar] [CrossRef] [PubMed]
- Liuzzo, G.; Biasucci, L.M.; Gallimore, J.R.; Grillo, R.L.; Rebuzzi, A.G.; Pepys, M.B.; Maseri, A. The Prognostic Value of C-Reactive Protein and Serum Amyloid A Protein in Severe Unstable Angina. N. Engl. J. Med. 1994, 331, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.K.; Stoll, L.L.; Denning, G.M.; Harrelson, A.; Blomkalns, A.L.; Idelman, G.; Rothenberg, F.G.; Neltner, B.; Romig-Martin, S.A.; Dickson, E.W.; et al. Proinflammatory Phenotype of Perivascular Adipocytes: Influence of High-Fat Feeding. Circ. Res. 2009, 104, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Ketonen, J.; Shi, J.; Martonen, E.; Mervaala, E. Periadventitial Adipose Tissue Promotes Endothelial Dysfunction via Oxidative Stress in Diet-Induced Obese C57Bl/6 Mice. Circ. J. 2010, 74, 1479–1487. [Google Scholar] [CrossRef]
- Lin, A.; Dey, D.; Wong, D.T.L.; Nerlekar, N. Perivascular Adipose Tissue and Coronary Atherosclerosis: From Biology to Imaging Phenotyping. Curr. Atheroscler. Rep. 2019, 21, 47. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Q.; Feng, J.; Xiao, Z.; Zhang, X.; Zhao, L. GLP-1 Alleviates NLRP3 Inflammasome-Dependent Inflammation in Perivascular Adipose Tissue by Inhibiting the NF-κB Signalling Pathway. J. Int. Med. Res. 2021, 49, 300060521992981. [Google Scholar] [CrossRef]
- Siegel-Axel, D.I.; Häring, H.U. Perivascular Adipose Tissue: An Unique Fat Compartment Relevant for the Cardiometabolic Syndrome. Rev. Endocr. Metab. Disord. 2016, 17, 51–60. [Google Scholar] [CrossRef]
- Contreras, G.A.; Thelen, K.; Ayala-Lopez, N.; Watts, S.W. The Distribution and Adipogenic Potential of Perivascular Adipose Tissue Adipocyte Progenitors Is Dependent on Sexual Dimorphism and Vessel Location. Physiol. Rep. 2016, 4, e12993. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Qi, X.-Y.; Qu, S.-L.; Xiong, W.-H.; Rom, O.; Chang, L.; Jiang, Z.-S. Perivascular Adipose Tissue (PVAT) in Atherosclerosis: A Double-Edged Sword. Cardiovasc. Diabetol. 2018, 17, 134. [Google Scholar] [CrossRef]
- Hildebrand, S.; Stümer, J.; Pfeifer, A. PVAT and Its Relation to Brown, Beige, and White Adipose Tissue in Development and Function. Front. Physiol. 2018, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Rendon, C.J.; Watts, S.W.; Contreras, G.A. PVAT Adipocyte: Energizing, Modulating, and Structuring Vascular Function during Normotensive and Hypertensive States. Am. J. Physiol. Heart Circ. Physiol. 2025, 328, H1204–H1217. [Google Scholar] [CrossRef]
- Ivatt, L.; Paul, M.; Miguelez-Crespo, A.; Hadoke, P.W.F.; Bailey, M.A.; Morgan, R.A.; Nixon, M. Obesity-Induced Mesenteric PVAT Remodelling Is Sexually Dimorphic, but Not Driven by Ovarian Hormones. Short Title: Obesity Induces Sex-Specific Responses in Mesenteric PVAT. Cardiovasc. Diabetol. 2025, 24, 39. [Google Scholar] [CrossRef]
- Wang, Z.; Mu, W.; Xu, R.; Zhong, J.; Xiong, W.; Zhao, X.; Liang, X.; Guo, Y.; Zhang, J.; Jiang, Z.-S.; et al. Perivascular Adipose Tissue Dysfunction Contributes to Thoracic Aortic Aneurysm Development. Cardiovasc. Diabetol. 2025, 24, 223. [Google Scholar] [CrossRef]
- Watts, S.W.; Krieger-Burke, T.; Nault, R.; Contreras, G.A. Mechanotransduction in the Perivascular Adipose Tissue. Arterioscler. Thromb. Vasc. Biol. 2025, 45, 461–467. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in Inflammation and Metabolic Disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Vasamsetti, S.B.; Natarajan, N.; Sadaf, S.; Florentin, J.; Dutta, P. Regulation of Cardiovascular Health and Disease by Visceral Adipose Tissue-Derived Metabolic Hormones. J. Physiol. 2023, 601, 2099–2120. [Google Scholar] [CrossRef]
- Hara, T.; Sata, M. Roles of Perivascular Adipose Tissue in the Pathogenesis of Atherosclerosis-an Update on Recent Findings. Front. Physiol. 2024, 15, 1522471. [Google Scholar] [CrossRef]
- Chang, L.; Villacorta, L.; Li, R.; Hamblin, M.; Xu, W.; Dou, C.; Zhang, J.; Wu, J.; Zeng, R.; Chen, Y.E. Loss of Perivascular Adipose Tissue on Peroxisome Proliferator–Activated Receptor-γ Deletion in Smooth Muscle Cells Impairs Intravascular Thermoregulation and Enhances Atherosclerosis. Circulation 2012, 126, 1067–1078. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Hutt Centeno, E.; Thomas, S.; Herdman, L.; Kotanidis, C.P.; Thomas, K.E.; et al. Non-Invasive Detection of Coronary Inflammation Using Computed Tomography and Prediction of Residual Cardiovascular Risk (the CRISP CT Study): A Post-Hoc Analysis of Prospective Outcome Data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef]
- Sidossis, L.; Kajimura, S. Brown and Beige Fat in Humans: Thermogenic Adipocytes That Control Energy and Glucose Homeostasis. J. Clin. Investig. 2015, 125, 478–486. [Google Scholar] [CrossRef]
- Cypess, A.M.; Weiner, L.S.; Roberts-Toler, C.; Elía, E.F.; Kessler, S.H.; Kahn, P.A.; English, J.; Chatman, K.; Trauger, S.A.; Doria, A.; et al. Activation of Human Brown Adipose Tissue by a Β3-Adrenergic Receptor Agonist. Cell Metab. 2015, 21, 33–38. [Google Scholar] [CrossRef]
- Hiraike, Y.; Waki, H.; Yu, J.; Nakamura, M.; Miyake, K.; Nagano, G.; Nakaki, R.; Suzuki, K.; Kobayashi, H.; Yamamoto, S.; et al. NFIA Co-Localizes with PPARγ and Transcriptionally Controls the Brown Fat Gene Program. Nat. Cell Biol. 2017, 19, 1081–1092. [Google Scholar] [CrossRef]
- Angueira, A.R.; Sakers, A.P.; Holman, C.D.; Cheng, L.; Arbocco, M.N.; Shamsi, F.; Lynes, M.D.; Shrestha, R.; Okada, C.; Batmanov, K.; et al. Defining the Lineage of Thermogenic Perivascular Adipose Tissue. Nat. Metab. 2021, 3, 469–484. [Google Scholar] [CrossRef]
- Adachi, Y.; Ueda, K.; Nomura, S.; Ito, K.; Katoh, M.; Katagiri, M.; Yamada, S.; Hashimoto, M.; Zhai, B.; Numata, G.; et al. Beiging of Perivascular Adipose Tissue Regulates Its Inflammation and Vascular Remodeling. Nat. Commun. 2022, 13, 5117. [Google Scholar] [CrossRef]
- Okamoto, E.; Couse, T.; De Leon, H.; Vinten-Johansen, J.; Goodman, R.B.; Scott, N.A.; Wilcox, J.N. Perivascular Inflammation After Balloon Angioplasty of Porcine Coronary Arteries. Circulation 2001, 104, 2228–2235. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, A.S.; Khavandi, K.; Withers, S.B.; Sonoyama, K.; Clancy, O.; Jeziorska, M.; Laing, I.; Yates, A.P.; Pemberton, P.W.; Malik, R.A.; et al. Local Inflammation and Hypoxia Abolish the Protective Anticontractile Properties of Perivascular Fat in Obese Patients. Circulation 2009, 119, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Diaba-Nuhoho, P.; Mittag, J.; Brunssen, C.; Morawietz, H.; Brendel, H. The Vascular Function of Resistance Arteries Depends on NADPH Oxidase 4 and Is Exacerbated by Perivascular Adipose Tissue. Antioxidants 2024, 13, 503. [Google Scholar] [CrossRef] [PubMed]
- Friederich-Persson, M.; Nguyen Dinh Cat, A.; Persson, P.; Montezano, A.C.; Touyz, R.M. Brown Adipose Tissue Regulates Small Artery Function Through NADPH Oxidase 4–Derived Hydrogen Peroxide and Redox-Sensitive Protein Kinase G-1α. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 455–465. [Google Scholar] [CrossRef]
- Dashwood, M.R.; Dooley, A.; Shi-Wen, X.; Abraham, D.J.; Dreifaldt, M.; Souza, D.S.R. Perivascular Fat-Derived Leptin: A Potential Role in Improved Vein Graft Performance in Coronary Artery Bypass Surgery. Interact. Cardiovasc. Thorac. Surg. 2011, 12, 170–173. [Google Scholar] [CrossRef]
- Sun, X.; Hou, N.; Han, F.; Guo, Y.; Hui, Z.; Du, G.; Zhang, Y. Effect of High Free Fatty Acids on the Anti-Contractile Response of Perivascular Adipose Tissue in Rat Aorta. J. Mol. Cell. Cardiol. 2013, 63, 169–174. [Google Scholar] [CrossRef]
- Fernández-Alfonso, M.S.; Gil-Ortega, M.; Aranguez, I.; Souza, D.; Dreifaldt, M.; Somoza, B.; Dashwood, M.R. Role of PVAT in Coronary Atherosclerosis and Vein Graft Patency: Friend or Foe? Br. J. Pharmacol. 2017, 174, 3561–3572. [Google Scholar] [CrossRef] [PubMed]
- Bełtowski, J. Leptin and the Regulation of Endothelial Function in Physiological and Pathological Conditions. Clin. Exp. Pharmacol. Physiol. 2012, 39, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, H.; Kuramoto, J.; Kameshima, S.; Usui, T.; Okada, M.; Hara, Y. Omentin, a Novel Adipocytokine Inhibits TNF-Induced Vascular Inflammation in Human Endothelial Cells. Biochem. Biophys. Res. Commun. 2011, 408, 339–343. [Google Scholar] [CrossRef]
- Maeda, K.; Okubo, K.; Shimomura, I.; Funahashi, T.; Matsuzawa, Y.; Matsubara, K. cDNA Cloning and Expression of a Novel Adipose Specific Collagen-like Factor, apM1 (Adipose Most Abundant Gene Transcript 1). Biochem. Biophys. Res. Commun. 1996, 221, 286–289. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical Decrease of an Adipose-Specific Protein, Adiponectin, in Obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Fesus, G.; Dubrovska, G.; Gorzelniak, K.; Kluge, R.; Huang, Y.; Luft, F.; Gollasch, M. Adiponectin Is a Novel Humoral Vasodilator. Cardiovasc. Res. 2007, 75, 719–727. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional Cloning of the Mouse Obese Gene and Its Human Homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Tartaglia, L.A.; Dembski, M.; Weng, X.; Deng, N.; Culpepper, J.; Devos, R.; Richards, G.J.; Campfield, L.A.; Clark, F.T.; Deeds, J.; et al. Identification and Expression Cloning of a Leptin Receptor, OB-R. Cell 1995, 83, 1263–1271. [Google Scholar] [CrossRef]
- Kang, S.M.; Kwon, H.M.; Hong, B.K.; Kim, D.; Kim, I.J.; Choi, E.Y.; Jang, Y.; Kim, H.S.; Kim, M.S.; Kwon, H.C. Expression of Leptin Receptor (Ob-R) in Human Atherosclerotic Lesions: Potential Role in Intimal Neovascularization. Yonsei Med. J. 2000, 41, 68–75. [Google Scholar] [CrossRef]
- Schäffler, A.; Neumeier, M.; Herfarth, H.; Fürst, A.; Schölmerich, J.; Büchler, C. Genomic Structure of Human Omentin, a New Adipocytokine Expressed in Omental Adipose Tissue. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 2005, 1732, 96–102. [Google Scholar] [CrossRef]
- Boydens, C.; Maenhaut, N.; Pauwels, B.; Decaluwé, K.; Van de Voorde, J. Adipose Tissue as Regulator of Vascular Tone. Curr. Hypertens. Rep. 2012, 14, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.; Sena, C.M. Perivascular Adipose Tissue: A Central Player in the Triad of Diabetes, Obesity, and Cardiovascular Health. Cardiovasc. Diabetol. 2024, 23, 455. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Tousoulis, D.; Vavlukis, M.; Fleming, I.; Duncker, D.J.; Eringa, E.; Manfrini, O.; Antonopoulos, A.S.; Oikonomou, E.; Padró, T.; et al. Perivascular Adipose Tissue as a Source of Therapeutic Targets and Clinical Biomarkers. Eur. Heart J. 2023, 44, 3827–3844. [Google Scholar] [CrossRef]
- Guo, E.; Liu, D.; Zhu, Z. Phenotypic and Functional Disparities in Perivascular Adipose Tissue. Front. Physiol. 2024, 15, 1499340. [Google Scholar] [CrossRef]
- Mittal, B. Subcutaneous Adipose Tissue & Visceral Adipose Tissue. Indian. J. Med. Res. 2019, 149, 571–573. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and Visceral Adipose Tissue: Structural and Functional Differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Kong, L.-R.; Zhou, Y.-P.; Chen, D.-R.; Ruan, C.-C.; Gao, P.-J. Decrease of Perivascular Adipose Tissue Browning Is Associated with Vascular Dysfunction in Spontaneous Hypertensive Rats During Aging. Front. Physiol. 2018, 9, 400. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of Adipose Tissue: An Endocrine Organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Kahn, D.; Macias, E.; Zarini, S.; Garfield, A.; Zemski Berry, K.; MacLean, P.; Gerszten, R.E.; Libby, A.; Solt, C.; Schoen, J.; et al. Exploring Visceral and Subcutaneous Adipose Tissue Secretomes in Human Obesity: Implications for Metabolic Disease. Endocrinology 2022, 163, bqac140. [Google Scholar] [CrossRef]
- Spoto, B.; Di Betta, E.; Mattace-Raso, F.; Sijbrands, E.; Vilardi, A.; Parlongo, R.M.; Pizzini, P.; Pisano, A.; Vermi, W.; Testa, A.; et al. Pro- and Anti-Inflammatory Cytokine Gene Expression in Subcutaneous and Visceral Fat in Severe Obesity. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1137–1143. [Google Scholar] [CrossRef]
- Berry, D.C.; Jiang, Y.; Graff, J.M. Emerging Roles of Adipose Progenitor Cells in Tissue Development, Homeostasis, Expansion and Thermogenesis. Trends Endocrinol. Metab. 2016, 27, 574–585. [Google Scholar] [CrossRef]
- Pellegrinelli, V.; Carobbio, S.; Vidal-Puig, A. Adipose Tissue Plasticity: How Fat Depots Respond Differently to Pathophysiological Cues. Diabetologia 2016, 59, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Ladeiras-Lopes, R.; Sampaio, F.; Bettencourt, N.; Fontes-Carvalho, R.; Ferreira, N.; Leite-Moreira, A.; Gama, V. The Ratio Between Visceral and Subcutaneous Abdominal Fat Assessed by Computed Tomography Is an Independent Predictor of Mortality and Cardiac Events. Rev. Española De Cardiol. (Engl. Ed.) 2017, 70, 331–337. [Google Scholar] [CrossRef]
- Hwang, I.; Kim, J.B. Two Faces of White Adipose Tissue with Heterogeneous Adipogenic Progenitors. Diabetes Metab. J. 2019, 43, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Szasz, T.; Webb, R.C. Perivascular Adipose Tissue: More than Just Structural Support. Clin Sci 2012, 122, 1–12. [Google Scholar] [CrossRef]
- Frayn, K.N. Visceral Fat and Insulin Resistance--Causative or Correlative? Br. J. Nutr. 2000, 83 (Suppl. S1), S71–S77. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ayers, C.R.; Rohatgi, A.K.; Turer, A.T.; Berry, J.D.; Das, S.R.; Vega, G.L.; Khera, A.; McGuire, D.K.; Grundy, S.M.; et al. Associations of Visceral and Abdominal Subcutaneous Adipose Tissue with Markers of Cardiac and Metabolic Risk in Obese Adults. Obesity 2013, 21, E439–E447. [Google Scholar] [CrossRef]
- Szasz, T.; Bomfim, G.F.; Webb, R.C. The Influence of Perivascular Adipose Tissue on Vascular Homeostasis. Vasc. Health Risk Manag. 2013, 9, 105–116. [Google Scholar] [CrossRef]
- Hu, H.; Garcia-Barrio, M.; Jiang, Z.; Chen, Y.E.; Chang, L. Roles of Perivascular Adipose Tissue in Hypertension and Atherosclerosis. Antioxid. Redox Signal. 2021, 34, 736–749. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Margaritis, M.; Coutinho, P.; Shirodaria, C.; Psarros, C.; Herdman, L.; Sanna, F.; De Silva, R.; Petrou, M.; Sayeed, R.; et al. Adiponectin as a Link Between Type 2 Diabetes and Vascular NADPH Oxidase Activity in the Human Arterial Wall: The Regulatory Role of Perivascular Adipose Tissue. Diabetes 2015, 64, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149. [Google Scholar] [CrossRef]
- Cai, M.; Zhao, D.; Han, X.; Han, S.; Zhang, W.; Zang, Z.; Gai, C.; Rong, R.; Gao, T. The Role of Perivascular Adipose Tissue-Secreted Adipocytokines in Cardiovascular Disease. Front. Immunol. 2023, 14, 1271051. [Google Scholar] [CrossRef]
- Kumar, R.K.; Yang, Y.; Contreras, A.G.; Garver, H.; Bhattacharya, S.; Fink, G.D.; Rockwell, C.E.; Watts, S.W. Phenotypic Changes in T Cell and Macrophage Subtypes in Perivascular Adipose Tissues Precede High-Fat Diet-Induced Hypertension. Front. Physiol. 2021, 12, 616055. [Google Scholar] [CrossRef] [PubMed]
- Azul, L.; Leandro, A.; Boroumand, P.; Klip, A.; Seiça, R.; Sena, C.M. Increased Inflammation, Oxidative Stress and a Reduction in Antioxidant Defense Enzymes in Perivascular Adipose Tissue Contribute to Vascular Dysfunction in Type 2 Diabetes. Free. Radic. Biol. Med. 2020, 146, 264–274. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and Metabolic Health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Saxton, S.N.; Clark, B.J.; Withers, S.B.; Eringa, E.C.; Heagerty, A.M. Mechanistic Links Between Obesity, Diabetes, and Blood Pressure: Role of Perivascular Adipose Tissue. Physiol. Rev. 2019, 99, 1701–1763. [Google Scholar] [CrossRef]
- Police, S.B.; Thatcher, S.E.; Charnigo, R.; Daugherty, A.; Cassis, L.A. Obesity Promotes Inflammation in Periaortic Adipose Tissue and Angiotensin II-Induced Abdominal Aortic Aneurysm Formation. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1458–1464. [Google Scholar] [CrossRef]
- Cybularz, M.; Langbein, H.; Zatschler, B.; Brunssen, C.; Deussen, A.; Matschke, K.; Morawietz, H. Endothelial Function and Gene Expression in Perivascular Adipose Tissue from Internal Mammary Arteries of Obese Patients with Coronary Artery Disease. Atheroscler. Suppl. 2017, 30, 149–158. [Google Scholar] [CrossRef]
- Bartuskova, H.; Kauerova, S.; Petras, M.; Poledne, R.; Fronek, J.; Janousek, L.; Kralova Lesna, I. Links between Macrophages in Perivascular Adipose Tissue and Arterial Wall: A Role in Atherosclerosis Initiation? Int. Angiol. 2022, 41, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Shore, A.; Karamitri, A.; Kemp, P.; Speakman, J.R.; Lomax, M.A. Role of Ucp1 Enhancer Methylation and Chromatin Remodelling in the Control of Ucp1 Expression in Murine Adipose Tissue. Diabetologia 2010, 53, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, C.; Basu, S.; Gower, A.C.; Karki, S.; Farb, M.G.; Sroczynski, E.; Zizza, E.; Sarhan, A.; Pande, A.N.; Walsh, K.; et al. Perivascular Adipose Tissue Inflammation in Ischemic Heart Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1239–1250. [Google Scholar] [CrossRef]
- Stoian, A.; Muntean, C.; Babă, D.-F.; Manea, A.; Dénes, L.; Simon-Szabó, Z.; Kosovski, I.B.; Nemes-Nagy, E.; Gliga, F.I.; Stoian, M. Update on Biomarkers of Chronic Inflammatory Processes Underlying Diabetic Neuropathy. Int. J. Mol. Sci. 2024, 25, 10395. [Google Scholar] [CrossRef]
- Victorio, J.A.; da Costa, R.M.; Tostes, R.C.; Davel, A.P. Modulation of Vascular Function by Perivascular Adipose Tissue: Sex Differences. Curr. Pharm. Des. 2020, 26, 3768–3777. [Google Scholar] [CrossRef]
- Gruzdeva, O.V.; Dyleva, Y.A.; Belik, E.V.; Sinitsky, M.Y.; Stasev, A.N.; Kokov, A.N.; Brel, N.K.; Krivkina, E.O.; Bychkova, E.E.; Tarasov, R.S.; et al. Relationship between Epicardial and Coronary Adipose Tissue and the Expression of Adiponectin, Leptin, and Interleukin 6 in Patients with Coronary Artery Disease. J. Pers. Med. 2022, 12, 129. [Google Scholar] [CrossRef]
- Karampetsou, N.; Tzani, A.; Doulamis, I.P.; Bletsa, E.; Minia, A.; Pliaka, V.; Tsolakos, N.; Oikonomou, E.; Tousoulis, D.; Kontzoglou, K.; et al. Epicardial Adipocyte-Derived TNF-α Modulates Local Inflammation in Patients with Advanced Coronary Artery Disease. Curr. Vasc. Pharmacol. 2022, 20, 87–93. [Google Scholar] [CrossRef]
- Du, B.; Ouyang, A.; Eng, J.S.; Fleenor, B.S. Aortic Perivascular Adipose-Derived Interleukin-6 Contributes to Arterial Stiffness in Low-Density Lipoprotein Receptor Deficient Mice. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1382–H1390. [Google Scholar] [CrossRef]
- Small, H.Y.; McNeilly, S.; Mary, S.; Sheikh, A.M.; Delles, C. Resistin Mediates Sex-Dependent Effects of Perivascular Adipose Tissue on Vascular Function in the Shrsp. Sci. Rep. 2019, 9, 6897. [Google Scholar] [CrossRef]
- Romacho, T.; Valencia, I.; Ramos-González, M.; Vallejo, S.; López-Esteban, M.; Lorenzo, O.; Cannata, P.; Romero, A.; San Hipólito-Luengo, A.; Gómez-Cerezo, J.F.; et al. Visfatin/eNampt Induces Endothelial Dysfunction in Vivo: A Role for Toll-Like Receptor 4 and NLRP3 Inflammasome. Sci. Rep. 2020, 10, 5386. [Google Scholar] [CrossRef]
- Lima, A.F.R.; Rodrigues, D.; Machado, M.R.; Oliveira-Neto, J.T.; Bressan, A.F.M.; Pedersoli, C.A.; Alves, J.V.; Silva-Neto, J.A.; Barros, P.R.; Dias, T.B.; et al. Endothelin-1 down-Regulates Nuclear Factor Erythroid 2-Related Factor-2 and Contributes to Perivascular Adipose Tissue Dysfunction in Obesity. Clin. Sci. 2024, 138, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yan, Y.; Wu, Y.; Lu, M.; Xing, Y.; Bai, Y.; Zhao, H.; Ding, L.; Wu, Y.; Xu, J.; et al. Lactoferrin Ameliorated Obesity-Induced Endothelial Dysfunction by Inhibiting the Tak1/IL-18/eNOS Pathway between PVAT and Vascular Endothelium. Free. Radic. Biol. Med. 2024, 212, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Oh, S.; Lee, H.S.; Chung, D.-M.; Jang, J.T.; Jeon, Y.-J.; Choi, C.H.; Park, K.Y.; Son, K.H.; Byun, K. Ecklonia Cava Extract Attenuates Endothelial Cell Dysfunction by Modulation of Inflammation and Brown Adipocyte Function in Perivascular Fat Tissue. Nutrients 2019, 11, 2795. [Google Scholar] [CrossRef] [PubMed]
- Schüler, R.; Efentakis, P.; Wild, J.; Lagrange, J.; Garlapati, V.; Molitor, M.; Kossmann, S.; Oelze, M.; Stamm, P.; Li, H.; et al. T Cell-Derived IL-17A Induces Vascular Dysfunction via Perivascular Fibrosis Formation and Dysregulation of ·NO/cGMP Signaling. Oxid. Med. Cell Longev. 2019, 2019, 6721531. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Y.; Zhang, X.; Xu, W.; Jin, K.; Pang, Y.; Wu, Y.; Luo, J.; Xu, R.; Jiao, L.; et al. Mechanism of Oxidized Phospholipid-Related Inflammatory Response in Vascular Ageing. Ageing Res. Rev. 2023, 86, 101888. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, Atherosclerosis, and Coronary Artery Disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Cybulsky, M.I.; Gimbrone, M.A. Endothelial Expression of a Mononuclear Leukocyte Adhesion Molecule During Atherogenesis. Science 1991, 251, 788–791. [Google Scholar] [CrossRef]
- Gisterå, A.; Hansson, G.K. The Immunology of Atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef]
- Kadl, A.; Meher, A.K.; Sharma, P.R.; Lee, M.Y.; Doran, A.C.; Johnstone, S.R.; Elliott, M.R.; Gruber, F.; Han, J.; Chen, W.; et al. Identification of a Novel Macrophage Phenotype That Develops in Response to Atherogenic Phospholipids via Nrf2. Circ. Res. 2010, 107, 737–746. [Google Scholar] [CrossRef]
- van der Wal, A.C.; Becker, A.E.; van der Loos, C.M.; Das, P.K. Site of Intimal Rupture or Erosion of Thrombosed Coronary Atherosclerotic Plaques Is Characterized by an Inflammatory Process Irrespective of the Dominant Plaque Morphology. Circulation 1994, 89, 36–44. [Google Scholar] [CrossRef]
- Niculescu, R.; Mureșan, A.; Radu, C.C.; Hogea, T.R.; Cocuz, I.G.; Sabău, A.H.; Russu, E.; Arbănași, E.M.; Arbănași, E.M.; Mureșan, A.V.; et al. Predictive Value of Epicardial Adipose Tissue Thickness for Plaque Vulnerability in Left Coronary Arteries: Histological Evidence from 245 Sudden Cardiac Death Cases. Diagnostics 2025, 15, 1491. [Google Scholar] [CrossRef]
- Carvalho, K.F.S.; de Lima, J.F.; Silva, J.L.M.; de Almeida, C.R.; Cunha, R.G.A.; Alves, J.V.; Tostes, R.C.; Lobato, N.S.; Costa, R.M. Toll-like Receptor 9 Contributes to Perivascular Adipose Tissue Dysfunction in Spontaneously Hypertensive Rats. Eur. J. Pharmacol. 2025, 998, 177524. [Google Scholar] [CrossRef]
- Wang, J.; Polaki, V.; Chen, S.; Bihl, J.C. Exercise Improves Endothelial Function Associated with Alleviated Inflammation and Oxidative Stress of Perivascular Adipose Tissue in Type 2 Diabetic Mice. Oxid. Med. Cell. Longev. 2020, 2020, 8830537. [Google Scholar] [CrossRef] [PubMed]
- Pandzic Jaksic, V.; Grizelj, D.; Livun, A.; Ajduk, M.; Boscic, D.; Vlasic, A.; Marusic, M.; Gizdic, B.; Kusec, R.; Jaksic, O. Inflammatory Gene Expression in Neck Perivascular and Subcutaneous Adipose Tissue in Men with Carotid Stenosis. Angiology 2022, 73, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Li, H. The Role of Perivascular Adipose Tissue in Obesity-Induced Vascular Dysfunction. Br. J. Pharmacol. 2017, 174, 3425–3442. [Google Scholar] [CrossRef]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Perivascular Adipose Tissue Oxidative Stress in Obesity. Antioxidants 2023, 12, 1595. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xu, L.; Yu, X.; Li, W.; Sun, X.; Xiao, S.; Guo, M.; Wang, H. Protective Effect of KLF15 on Vascular Endothelial Dysfunction Induced by TNF-α. Mol. Med. Rep. 2018, 18, 1987–1994. [Google Scholar] [CrossRef]
- Naryzhnaya, N.V.; Koshelskaya, O.A.; Kologrivova, I.V.; Suslova, T.E.; Kharitonova, O.A.; Andreev, S.L.; Gorbunov, A.S.; Kurbatov, B.K.; Boshchenko, A.A. Production of Reactive Oxygen Species by Epicardial Adipocytes Is Associated with an Increase in Postprandial Glycemia, Postprandial Insulin, and a Decrease in Serum Adiponectin in Patients with Severe Coronary Atherosclerosis. Biomedicines 2022, 10, 2054. [Google Scholar] [CrossRef]
- Sena, C.M.; Pereira, A.; Fernandes, R.; Letra, L.; Seiça, R.M. Adiponectin Improves Endothelial Function in Mesenteric Arteries of Rats Fed a High-fat Diet: Role of Perivascular Adipose Tissue. Br. J. Pharmacol. 2017, 174, 3514–3526. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, J.; Liu, B.; Huang, F.; Li, Y. Exosomes Derived from Mangiferin-stimulated Perivascular Adipose Tissue Ameliorate Endothelial Dysfunction. Mol. Med. Rep. 2019, 19, 4797–4805. [Google Scholar] [CrossRef]
- Miao, C.-Y.; Li, Z.-Y. The Role of Perivascular Adipose Tissue in Vascular Smooth Muscle Cell Growth. Br. J. Pharmacol. 2012, 165, 643–658. [Google Scholar] [CrossRef]
- Chang, L.; Garcia-Barrio, M.T.; Chen, Y.E. Perivascular Adipose Tissue Regulates Vascular Function by Targeting Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1094–1109. [Google Scholar] [CrossRef]
- Ding, Q.; Chai, H.; Mahmood, N.; Tsao, J.; Mochly-Rosen, D.; Zhou, W. Matrix Metalloproteinases Modulated by PKCε Mediate Resistin-Induced Migration of Human Coronary Artery Smooth Muscle Cells. J. Vasc. Surg. 2011, 53, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Lachaud, C.C.; López-Beas, J.; Soria, B.; Hmadcha, A. EGF-Induced Adipose Tissue Mesothelial Cells Undergo Functional Vascular Smooth Muscle Differentiation. Cell Death Dis. 2014, 5, e1304. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Shi, H.; Winkler, M.A.; Lee, R.; Weintraub, N.L. Perivascular Adipose Tissue and Vascular Perturbation/Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Márquez, A.B.; van der Vorst, E.P.C.; Maas, S.L. Key Chemokine Pathways in Atherosclerosis and Their Therapeutic Potential. J. Clin. Med. 2021, 10, 3825. [Google Scholar] [CrossRef]
- Rami, A.Z.A.; Hamid, A.A.; Anuar, N.N.M.; Aminuddin, A.; Ugusman, A. Exploring the Relationship of Perivascular Adipose Tissue Inflammation and the Development of Vascular Pathologies. Mediat. Inflamm. 2022, 2022, 2734321. [Google Scholar] [CrossRef]
- Whytock, K.L.; Divoux, A.; Sun, Y.; Pino, M.F.; Yu, G.; Jin, C.A.; Robino, J.J.; Plekhanov, A.; Varlamov, O.; Smith, S.R.; et al. Aging Human Abdominal Subcutaneous White Adipose Tissue at Single Cell Resolution. Aging Cell 2024, 23, e14287. [Google Scholar] [CrossRef]
- Zhu, Y.; Crewe, C.; Scherer, P.E. Hyaluronan in Adipose Tissue: Beyond Dermal Filler and Therapeutic Carrier. Sci. Transl. Med. 2016, 8, 323ps4. [Google Scholar] [CrossRef]
- Sun, K.; Li, X.; Scherer, P.E. Extracellular Matrix (ECM) and Fibrosis in Adipose Tissue: Overview and Perspectives. In Comprehensive Physiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 4387–4407. ISBN 978-0-470-65071-4. [Google Scholar]
- Sun, K.; Park, J.; Gupta, O.T.; Holland, W.L.; Auerbach, P.; Zhang, N.; Goncalves Marangoni, R.; Nicoloro, S.M.; Czech, M.P.; Varga, J.; et al. Endotrophin Triggers Adipose Tissue Fibrosis and Metabolic Dysfunction. Nat. Commun. 2014, 5, 3485. [Google Scholar] [CrossRef]
- Khan, T.; Muise, E.S.; Iyengar, P.; Wang, Z.V.; Chandalia, M.; Abate, N.; Zhang, B.B.; Bonaldo, P.; Chua, S.; Scherer, P.E. Metabolic Dysregulation and Adipose Tissue Fibrosis: Role of Collagen VI. Mol. Cell. Biol. 2009, 29, 1575–1591. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Chen, C.; Yang, L.; Lee, H.-H.; Wang, Z.; Zhang, N.; Kolonin, M.G.; An, Z.; Ge, X.; et al. Critical Role of Matrix Metalloproteinase 14 in Adipose Tissue Remodeling during Obesity. Mol. Cell. Biol. 2020, 40, e00564-19. [Google Scholar] [CrossRef]
- Sun, K.; Halberg, N.; Khan, M.; Magalang, U.J.; Scherer, P.E. Selective Inhibition of Hypoxia-Inducible Factor 1α Ameliorates Adipose Tissue Dysfunction. Mol. Cell. Biol. 2013, 33, 904–917. [Google Scholar] [CrossRef]
- Nakayama, Y.; Komuro, R.; Yamamoto, A.; Miyata, Y.; Tanaka, M.; Matsuda, M.; Fukuhara, A.; Shimomura, I. RhoA Induces Expression of Inflammatory Cytokine in Adipocytes. Biochem. Biophys. Res. Commun. 2009, 379, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, Inflammation and Metabolic Disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic Inflammation in Fat Plays a Crucial Role in the Development of Obesity-Related Insulin Resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Memetimin, H.; Li, D.; Tan, K.; Zhou, C.; Liang, Y.; Wu, Y.; Wang, S. Myeloid-Specific Deletion of Thrombospondin 1 Protects against Inflammation and Insulin Resistance in Long-Term Diet-Induced Obese Male Mice. Am. J. Physiol.-Endocrinol. Metab. 2018, 315, E1194–E1203. [Google Scholar] [CrossRef]
- Kong, P.; Gonzalez-Quesada, C.; Li, N.; Cavalera, M.; Lee, D.-W.; Frangogiannis, N.G. Thrombospondin-1 Regulates Adiposity and Metabolic Dysfunction in Diet-Induced Obesity Enhancing Adipose Inflammation and Stimulating Adipocyte Proliferation. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E439–E450. [Google Scholar] [CrossRef]
- Halberg, N.; Khan, T.; Trujillo, M.E.; Wernstedt-Asterholm, I.; Attie, A.D.; Sherwani, S.; Wang, Z.V.; Landskroner-Eiger, S.; Dineen, S.; Magalang, U.J.; et al. Hypoxia-Inducible Factor 1α Induces Fibrosis and Insulin Resistance in White Adipose Tissue. Mol. Cell. Biol. 2009, 29, 4467–4483. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Huang, X.; Liu, K.; Wang, Q.-D.; Chen, A.F.; Sun, K.; Lui, K.O.; Zhou, B. Seamless Genetic Recording of Transiently Activated Mesenchymal Gene Expression in Endothelial Cells During Cardiac Fibrosis. Circulation 2021, 144, 2004–2020. [Google Scholar] [CrossRef]
- Hashimoto, N.; Phan, S.H.; Imaizumi, K.; Matsuo, M.; Nakashima, H.; Kawabe, T.; Shimokata, K.; Hasegawa, Y. Endothelial–Mesenchymal Transition in Bleomycin-Induced Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2010, 43, 161–172. [Google Scholar] [CrossRef]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach, M.A.R.; Wunderlich, F.T. Macrophage Function in Obesity-Induced Inflammation and Insulin Resistance. Pflugers Arch. 2017, 469, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Poblete, J.M.S.; Ballinger, M.N.; Bao, S.; Alghothani, M.; Nevado, J.B.; Eubank, T.D.; Christman, J.W.; Magalang, U.J. Macrophage HIF-1α Mediates Obesity-Related Adipose Tissue Dysfunction via Interleukin-1 Receptor-Associated Kinase M. Am. J. Physiol.-Endocrinol. Metab. 2020, 318, E689–E700. [Google Scholar] [CrossRef] [PubMed]
- Iwayama, T.; Steele, C.; Yao, L.; Dozmorov, M.G.; Karamichos, D.; Wren, J.D.; Olson, L.E. PDGFRα Signaling Drives Adipose Tissue Fibrosis by Targeting Progenitor Cell Plasticity. Genes. Dev. 2015, 29, 1106–1119. [Google Scholar] [CrossRef]
- Marcelin, G.; Ferreira, A.; Liu, Y.; Atlan, M.; Aron-Wisnewsky, J.; Pelloux, V.; Botbol, Y.; Ambrosini, M.; Fradet, M.; Rouault, C.; et al. A PDGFRα-Mediated Switch toward CD9high Adipocyte Progenitors Controls Obesity-Induced Adipose Tissue Fibrosis. Cell Metab. 2017, 25, 673–685. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef]
- Hirai, S.; Ohyane, C.; Kim, Y.-I.; Lin, S.; Goto, T.; Takahashi, N.; Kim, C.-S.; Kang, J.; Yu, R.; Kawada, T. Involvement of Mast Cells in Adipose Tissue Fibrosis. Am. J. Physiol.-Endocrinol. Metab. 2014, 306, E247–E255. [Google Scholar] [CrossRef]
- Wolosowicz, M.; Prokopiuk, S.; Kaminski, T.W. The Complex Role of Matrix Metalloproteinase-2 (MMP-2) in Health and Disease. Int. J. Mol. Sci. 2024, 25, 13691. [Google Scholar] [CrossRef]
- Moe, K.T.; Naylynn, T.M.; Yin, N.O.; Khairunnisa, K.; Allen, J.C.; Wong, M.C.; Chin-Dusting, J.; Wong, P. Tumor Necrosis Factor-α Induces Aortic Intima-Media Thickening via Perivascular Adipose Tissue Inflammation. J. Vasc. Res. 2013, 50, 228–237. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, Z.; Wang, Y.; Li, X.; Zheng, Y.; Liu, Y. Role of Inflammation in Vascular Disease-Related Perivascular Adipose Tissue Dysfunction. Front. Endocrinol. 2021, 12, 710842. [Google Scholar] [CrossRef]
- Farias-Itao, D.S.; Pasqualucci, C.A.; de Andrade, R.A.; da Silva, L.F.F.; Yahagi-Estevam, M.; Lage, S.H.G.; Leite, R.E.P.; Campo, A.B.; Suemoto, C.K. Macrophage Polarization in the Perivascular Fat Was Associated with Coronary Atherosclerosis. J. Am. Heart Assoc. 2022, 11, e023274. [Google Scholar] [CrossRef]
- Farias-Itao, D.S.; Pasqualucci, C.A.; Nishizawa, A.; da Silva, L.F.F.; Campos, F.M.; Bittencourt, M.S.; da Silva, K.C.S.; Leite, R.E.P.; Grinberg, L.T.; de Lucena Ferretti-Rebustini, R.E. B Lymphocytes and Macrophages in the Perivascular Adipose Tissue Are Associated with Coronary Atherosclerosis: An Autopsy Study. J. Am. Heart Assoc. 2019, 8, e013793. [Google Scholar] [CrossRef]
- Siegel-Axel, D.I.; Ullrich, S.; Stefan, N.; Rittig, K.; Gerst, F.; Klingler, C.; Schmidt, U.; Schreiner, B.; Randrianarisoa, E.; Schaller, H.-E.; et al. Fetuin-A Influences Vascular Cell Growth and Production of Proinflammatory and Angiogenic Proteins by Human Perivascular Fat Cells. Diabetologia 2014, 57, 1057–1066. [Google Scholar] [CrossRef]
- Contreras, G.A.; Rendon, C.J.; Shadowens, A.; Chirivi, M.; Salcedo-Tacuma, D.; Lauver, D.A.; Watts, S.W. Perivascular Adipocytes’ Adipogenesis Is Defined by Their Anatomical Location in the Descending Thoracic Aorta. Cells 2025, 14, 579. [Google Scholar] [CrossRef]
- Grodecki, K.; Geers, J.; Kwiecinski, J.; Lin, A.; Slipczuk, L.; Slomka, P.J.; Dweck, M.R.; Nerlekar, N.; Williams, M.C.; Berman, D.; et al. Phenotyping Atherosclerotic Plaque and Perivascular Adipose Tissue: Signalling Pathways and Clinical Biomarkers in Atherosclerosis. Nat. Rev. Cardiol. 2025, 22, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Hu, C.; Liu, J.; Gao, A.; Han, H.; Chai, M.; Zhang, J.; Zhou, Y.; Zhao, Y. Perivascular Adipose Tissue as an Indication, Contributor to, and Therapeutic Target for Atherosclerosis. Front. Physiol. 2020, 11, 615503. [Google Scholar] [CrossRef] [PubMed]
- Getz, G. T Cells in Atherosclerosis in Ldlr-/- and Apoe-/- Mice. J. Immunol. Sci. 2018, 2, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.-M.; Akoumianakis, I.; et al. Detecting Human Coronary Inflammation by Imaging Perivascular Fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef]
- Asada, K.; Saito, Y.; Takaoka, H.; Kitahara, H.; Kobayashi, Y. Relation of Perivascular Adipose Tissues on Computed Tomography to Coronary Vasospasm. Rev. Cardiovasc. Med. 2025, 26, 26327. [Google Scholar] [CrossRef]
- Tu, Y.-B.; Gu, M.; Zhou, S.-Q.; Xie, G.; Liu, L.-L.; Deng, F.-B.; Li, K. Pericoronary Adipose Tissue Attenuation in Patients with Acute Aortic Dissection Based on Coronary Computed Tomography Angiography. Quant. Imaging Med. Surg. 2024, 14, 31–42. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, S.; Huo, R.; Chen, X.; Song, X.; Qiao, H.; Ning, Z.; Xu, H.; Yang, D.; Meng, D.; et al. Amount of Carotid Perivascular Adipose Tissue Measured by MR Imaging: Potential Indicators for the Risk of Ischemic Stroke. Arterioscler. Thromb. Vasc. Biol. 2025, 45, 1440–1447. [Google Scholar] [CrossRef]
- Nakladal, D.; Sijbesma, J.W.A.; Visser, L.M.; Tietge, U.J.F.; Slart, R.H.J.A.; Deelman, L.E.; Henning, R.H.; Hillebrands, J.L.; Buikema, H. Perivascular Adipose Tissue-Derived Nitric Oxide Compensates Endothelial Dysfunction in Aged Pre-Atherosclerotic Apolipoprotein E-Deficient Rats. Vasc. Pharmacol. 2022, 142, 106945. [Google Scholar] [CrossRef]
- Antoniades, C.; Kotanidis, C.P.; Berman, D.S. State-of-the-Art Review Article. Atherosclerosis Affecting Fat: What Can We Learn by Imaging Perivascular Adipose Tissue? J. Cardiovasc. Comput. Tomogr. 2019, 13, 288–296. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Williams, M.C.; Kotanidis, C.P.; Desai, M.Y.; Marwan, M.; Antonopoulos, A.S.; Thomas, K.E.; Thomas, S.; Akoumianakis, I.; Fan, L.M.; et al. A Novel Machine Learning-Derived Radiotranscriptomic Signature of Perivascular Fat Improves Cardiac Risk Prediction Using Coronary CT Angiography. Eur. Heart J. 2019, 40, 3529–3543. [Google Scholar] [CrossRef] [PubMed]
- Pouraliakbar, H.; Abouie, A.; Ziaeifar, E.; Rakhshankhah, N.; Shabestari, A.A.; Rabiei, P.; Mohebbi, B.; Alemzade-Ansari, M.J.; Mahdieh, N. Determinants of Perivascular Adipose Tissue Stranding as a Novel Imaging Marker and Its Relation to Inflammatory Biomarker High-Sensitivity C-Reactive Protein. Pol. J. Radiol. 2023, 88, e141–e148. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Li, Z.; Zhou, H.; Liu, Y.; Wu, H.; Wang, Z. The Predictive Value of the Perivascular Adipose Tissue CT Fat Attenuation Index for Coronary In-Stent Restenosis. Front. Cardiovasc. Med. 2022, 9, 822308. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef]
- Zeytinli Aksit, M.; Demet Arslan, F.; Karakoyun, I.; Aydin, C.; Turgut, E.; Parildar, H.; Gokbalci, U.; Isbilen Basok, B.; Duman, C.; Emiroglu, M. Galectin-3 Levels and Inflammatory Response in Patients Undergoing Bariatric Surgery. Cytokine 2022, 151, 155793. [Google Scholar] [CrossRef]
- Menini, S.; Iacobini, C.; Blasetti Fantauzzi, C.; Pesce, C.M.; Pugliese, G. Role of Galectin-3 in Obesity and Impaired Glucose Homeostasis. Oxidative Med. Cell. Longev. 2016, 2016, 9618092. [Google Scholar] [CrossRef]
- Pugliese, G. Do Advanced Glycation End Products Contribute to the Development of Long-Term Diabetic Complications? Nutr. Metab. Cardiovasc. Dis. 2008, 18, 457–460. [Google Scholar] [CrossRef]
- Rhodes, D.H.; Pini, M.; Castellanos, K.J.; Montero-Melendez, T.; Cooper, D.; Perretti, M.; Fantuzzi, G. Adipose Tissue-Specific Modulation of Galectin Expression in Lean and Obese Mice: Evidence for Regulatory Function. Obesity 2013, 21, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Kiwaki, K.; Novak, C.M.; Hsu, D.K.; Liu, F.-T.; Levine, J.A. Galectin-3 Stimulates Preadipocyte Proliferation and Is Up-Regulated in Growing Adipose Tissue. Obesity 2007, 15, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, E.; Calvier, L.; Rossignol, P.; Rousseau, E.; Fernández-Celis, A.; Jurado-López, R.; Laville, M.; Cachofeiro, V.; López-Andrés, N. Galectin-3 Inhibition Prevents Adipose Tissue Remodelling in Obesity. Int. J. Obes. 2016, 40, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Sime, K.; Choy, E.H.; Williams, A.S. Alterations to Adipose Tissue Morphology during Inflammatory Arthritis Is Indicative of Vasculopathology in DBA/1 Mice. Adipocyte 2017, 6, 87–101. [Google Scholar] [CrossRef]
- Khan, A.W.; Paneni, F.; Jandeleit-Dahm, K.A.M. Cell-Specific Epigenetic Changes in Atherosclerosis. Clin. Sci. 2021, 135, 1165–1187. [Google Scholar] [CrossRef]
- Chen, Y.; Siegel, F.; Kipschull, S.; Haas, B.; Fröhlich, H.; Meister, G.; Pfeifer, A. miR-155 Regulates Differentiation of Brown and Beige Adipocytes via a Bistable Circuit. Nat. Commun. 2013, 4, 1769. [Google Scholar] [CrossRef]
- Karkeni, E.; Astier, J.; Tourniaire, F.; El Abed, M.; Romier, B.; Gouranton, E.; Wan, L.; Borel, P.; Salles, J.; Walrand, S.; et al. Obesity-Associated Inflammation Induces microRNA-155 Expression in Adipocytes and Adipose Tissue: Outcome on Adipocyte Function. J. Clin. Endocrinol. Metab. 2016, 101, 1615–1626. [Google Scholar] [CrossRef]
- Sun, X.; Kang, Y.; Xue, S.; Zou, J.; Xu, J.; Tang, D.; Qin, H. In Vivo Therapeutic Success of MicroRNA-155 Antagomir in a Mouse Model of Pulmonary Fibrosis Induced by Bleomycin. Korean J. Intern. Med. 2021, 36, S160–S169. [Google Scholar] [CrossRef]
- Schober, A.; Nazari-Jahantigh, M.; Weber, C. MicroRNA-Mediated Mechanisms of the Cellular Stress Response in Atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 361–374. [Google Scholar] [CrossRef]
- Kong, Z.; Wang, Y.; Zhang, Y.; Shan, W.; Wu, J.; Wang, Q. MicroRNA-126 Promotes Endothelial Progenitor Cell Proliferation and Migration Ability via the Notch Pathway. Cardiovasc. Diagn. Ther. 2020, 10, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Giral, H.; Kratzer, A.; Landmesser, U. MicroRNAs in Lipid Metabolism and Atherosclerosis. Best. Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Perivascular Adipose Tissue as a Target for Antioxidant Therapy for Cardiovascular Complications. Antioxidants 2020, 9, 574. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in Nanomaterial-Based Targeted Drug Delivery Systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Kumarasamy, R.V.; Natarajan, P.M.; Umapathy, V.R.; Roy, J.R.; Mironescu, M.; Palanisamy, C.P. Clinical Applications and Therapeutic Potentials of Advanced Nanoparticles: A Comprehensive Review on Completed Human Clinical Trials. Front. Nanotechnol. 2024, 6, 1479993. [Google Scholar] [CrossRef]
- Mazurek, T.; Opolski, G. Pericoronary Adipose Tissue: A Novel Therapeutic Target in Obesity-Related Coronary Atherosclerosis. J. Am. Coll. Nutr. 2015, 34, 244–254. [Google Scholar] [CrossRef]
- Sousa, A.S.; Sponton, A.C.S.; Delbin, M.A. Perivascular Adipose Tissue and Microvascular Endothelial Dysfunction in Obese Mice: Beneficial Effects of Aerobic Exercise in Adiponectin Receptor (AdipoR1) and peNOSSer1177. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1430–1440. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, X.; Hu, J.; Ding, M.; Lee, S.K.; Korivi, M.; Qian, Y.; Li, T.; Wang, L.; Li, W. Exercise Attenuates High-Fat Diet-Induced PVAT Dysfunction through Improved Inflammatory Response and BMP4-Regulated Adipose Tissue Browning. Front. Nutr. 2024, 11, 1393343. [Google Scholar] [CrossRef]
- Atakan, M.M.; Koşar, Ş.N.; Güzel, Y.; Tin, H.T.; Yan, X. The Role of Exercise, Diet, and Cytokines in Preventing Obesity and Improving Adipose Tissue. Nutrients 2021, 13, 1459. [Google Scholar] [CrossRef]

| Adipokine | First Mentioned | First Described in Connection with PVAT | Functional Role in PVAT Under Physiological Conditions |
|---|---|---|---|
| Adiponectin | 1996—was mentioned as apM1 [35] 1999—mentioned as adiponectin [36] | 2007 [37] | Enhances endothelial function and increases NO bioavailability |
| Leptin | 1994—was mentioned as ob gene [38] 1995—was mentioned as leptin [39] | 2000 [40] | Promotes neovascularization and enhances endothelial function |
| Omentin | 2005 [41]—was mentioned in an article published in PubMed | 2012 [42] | Exerts anti-inflammatory and anti-atherosclerotic effects, enhances vascular tone |
| Feature | SAT | VAT | PVAT |
|---|---|---|---|
| Adipocyte size [45,50] | Small | Large | Heterogeneous (varies by the surrounding vessel) |
| Adipokines [45,51] | ↑ Resistin, ↑ leptin, and no difference in adiponectin | ↑ IL-12 IL-13, TNF-α, IL-6, IL-8, MCP-1, PAI-1, TBX2, PGE2; ↓ resistin, leptin ↓, adiponectin | Variable Secretion: Includes adiponectin, leptin, IL-6, TNF-α, context- and location-dependent |
| Immune cell infiltration [45,52] | Lower | Higher (especially macrophages and T-cells) | Mixed (either anti-inflammatory or pro-inflammatory) |
| Types of adipose tissue [45,53] | White adipose tissue | White adipose tissue | BAT-like (thoracic), WAT-like (abdominal) |
| Embryological origin [43,45,53,54] | Mesoderm: Derived from Wt1-negative precursor cells | Mesoderm: Partially derived from Wt1-positive precursor cells | Mixed (mesoderm + neural crest in some regions). Heterogeneous embryonic origin is shown by different precursors depending on the region, such as SM22α+, Myf5+, Wt1+, or Pdgfra+ cells. |
| Clinical relevance [45,55] | Indicative of increased cardiovascular and metabolic risk | May have a relatively protective role | Depends on positioning whether it has a cardiovascular protective or proatherogenic role |
| Vascularization [45,54,56] | Moderate capillary supply | Rich vascular supply | Directly connected to the vessel vasculature |
| Innervation [45,56] | Low | Moderate | High (dense sympathetic nerve supply) |
| UCP1 expression/browning [53,57] | Absent | Absent | High in the thoracic region (UCP1+) and absent in other areas |
| Insulin sensitivity [45,58] | High | Low | Variable (dependent on localization) |
| CVD association/atherosclerosis risk [10,59] | Low association | High risk predictor | Independent predictor for CVD/atherosclerosis |
| Phenotypic plasticity [14,54] | Low | Limited | High |
| Inflammatory Marker | Main Source in PVAT | Effects on Blood Vessels | Study Type | Number of Subjects |
|---|---|---|---|---|
| Leptin | Adipocytes | Stimulates production of TNF-α and IL-6; promotes vascular inflammation and atherosclerosis | Cross-sectional observational study [76] | 125 patients with coronary artery disease (CAD) who needed direct myocardial revascularization through coronary artery bypass graft (CABG) surgery |
| TNF-α | Macrophages, adipocytes | Induces expression of ICAM-1 and VCAM-1; promotes leukocyte infiltration and endothelial dysfunction | Cross-sectional observational study [77] | 32 patients with known CAD who underwent CABG surgery |
| IL-6 | Macrophages, adipocytes | Promotes arterial stiffness and endothelial dysfunction via inflammation and extracellular matrix remodeling mechanisms | Experimental study [78] | 8 low-density lipoprotein receptor (LDLr)-deficient mice (LDLr−/−) |
| Resistin | Macrophages | PVAT-derived resistin disrupts endothelial potassium channel–mediated vasorelaxation in hypertensive rats | Experimental study [79] (SHRSP rats) | 5 rats per sex/group |
| Visfatin/eNampt | Adipocytes | Impairment of endothelium-dependent vasodilation and increased oxidative stress through pro-inflammatory signaling | Original experimental studies [80] | 6 C57BL/6 mice per group |
| Endothelin-1 (ET-1) | Endothelial cells, adipocytes | Plays a crucial role in obesity-induced PVAT dysfunction by inhibiting Nrf2 and elevating oxidative stress | Experimental study performed on animal models (mice) [81] | 55 male C57BL/6J mice |
| IL-18 | Adipocytes, macrophages | Inhibits eNOS through the Tak1 pathway, impairing endothelium-dependent relaxation | Experimental study [82] | 8 high-fat diet-fed mice and adipocyte cultures |
| Chemokine (C-C motif) ligand 2 (CCL2) (MCP-1) | Adipocytes, macrophages | Promotes monocyte infiltration and endothelial dysfunction | Diet-induced obese mice, interventional study [83] | 10–12 mice/group |
| IL-17 | T helper 17 lymphocytes | Causes oxidative stress, vascular fibrosis, and impairs vasorelaxation | In vivo/ex vivo transgenic mouse study [84] | 8 mice/group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niculescu, R.; Stoian, A.; Arbănași, E.M.; Russu, E.; Babă, D.-F.; Manea, A.; Stoian, M.; Gliga, F.I.; Cocuz, I.G.; Sabău, A.H.; et al. The Dual Role of Perivascular Adipose Tissue in Vascular Homeostasis and Atherogenesis: From Physiology to Pathological Implications. Int. J. Mol. Sci. 2025, 26, 8320. https://doi.org/10.3390/ijms26178320
Niculescu R, Stoian A, Arbănași EM, Russu E, Babă D-F, Manea A, Stoian M, Gliga FI, Cocuz IG, Sabău AH, et al. The Dual Role of Perivascular Adipose Tissue in Vascular Homeostasis and Atherogenesis: From Physiology to Pathological Implications. International Journal of Molecular Sciences. 2025; 26(17):8320. https://doi.org/10.3390/ijms26178320
Chicago/Turabian StyleNiculescu, Raluca, Adina Stoian, Emil Marian Arbănași, Eliza Russu, Dragoș-Florin Babă, Andrei Manea, Mircea Stoian, Florina Ioana Gliga, Iuliu Gabriel Cocuz, Adrian Horațiu Sabău, and et al. 2025. "The Dual Role of Perivascular Adipose Tissue in Vascular Homeostasis and Atherogenesis: From Physiology to Pathological Implications" International Journal of Molecular Sciences 26, no. 17: 8320. https://doi.org/10.3390/ijms26178320
APA StyleNiculescu, R., Stoian, A., Arbănași, E. M., Russu, E., Babă, D.-F., Manea, A., Stoian, M., Gliga, F. I., Cocuz, I. G., Sabău, A. H., Szabo, D.-A., & Cotoi, O. S. (2025). The Dual Role of Perivascular Adipose Tissue in Vascular Homeostasis and Atherogenesis: From Physiology to Pathological Implications. International Journal of Molecular Sciences, 26(17), 8320. https://doi.org/10.3390/ijms26178320










