The Role of the Wnt/β-Catenin Pathway in the Modulation of Doxorubicin-Induced Cytotoxicity in Cardiac H9c2 Cells by Sulforaphane and Quercetin
Abstract
1. Introduction
2. Results
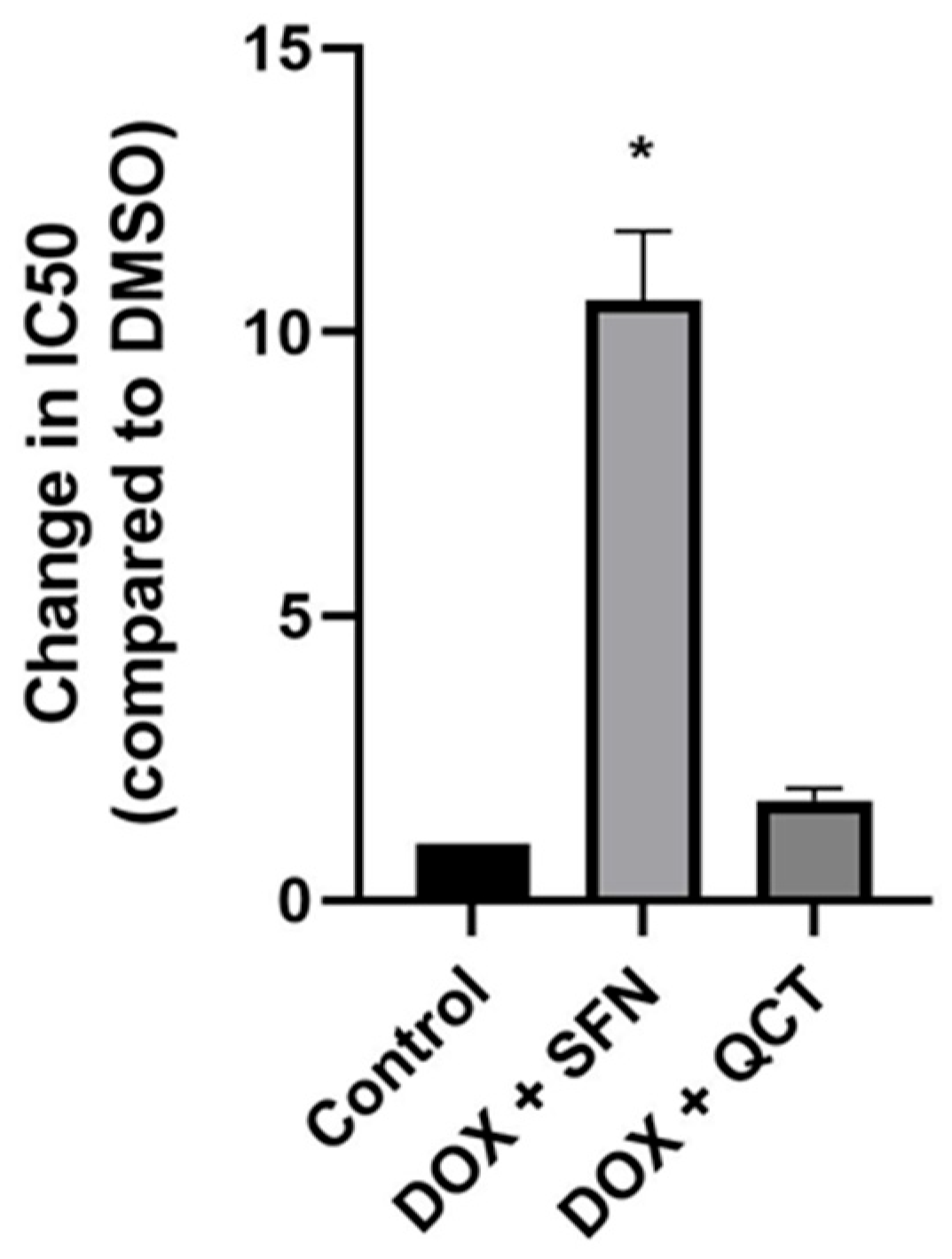
2.1. Effects of Sulforaphane and Quercetin on Modulation of Doxorubicin-Induced Cytotoxicity in H9c2 Cells
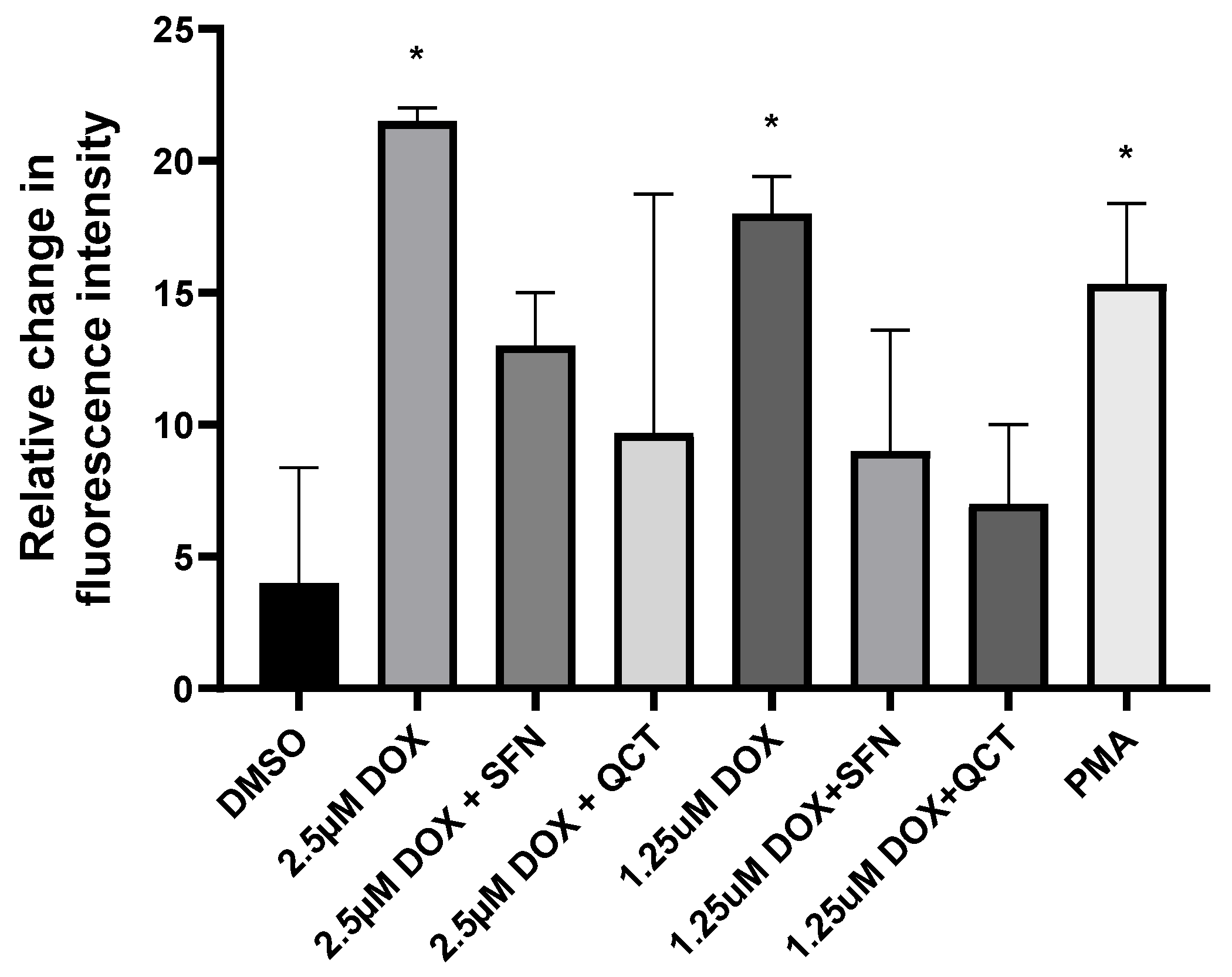
2.2. Effects of Sulforaphane and Quercetin on Production of Cellular Reactive Oxygen Species
2.3. Effects of Sulforaphane and Quercetin on Superoxide Dismutase and Catalase Activities
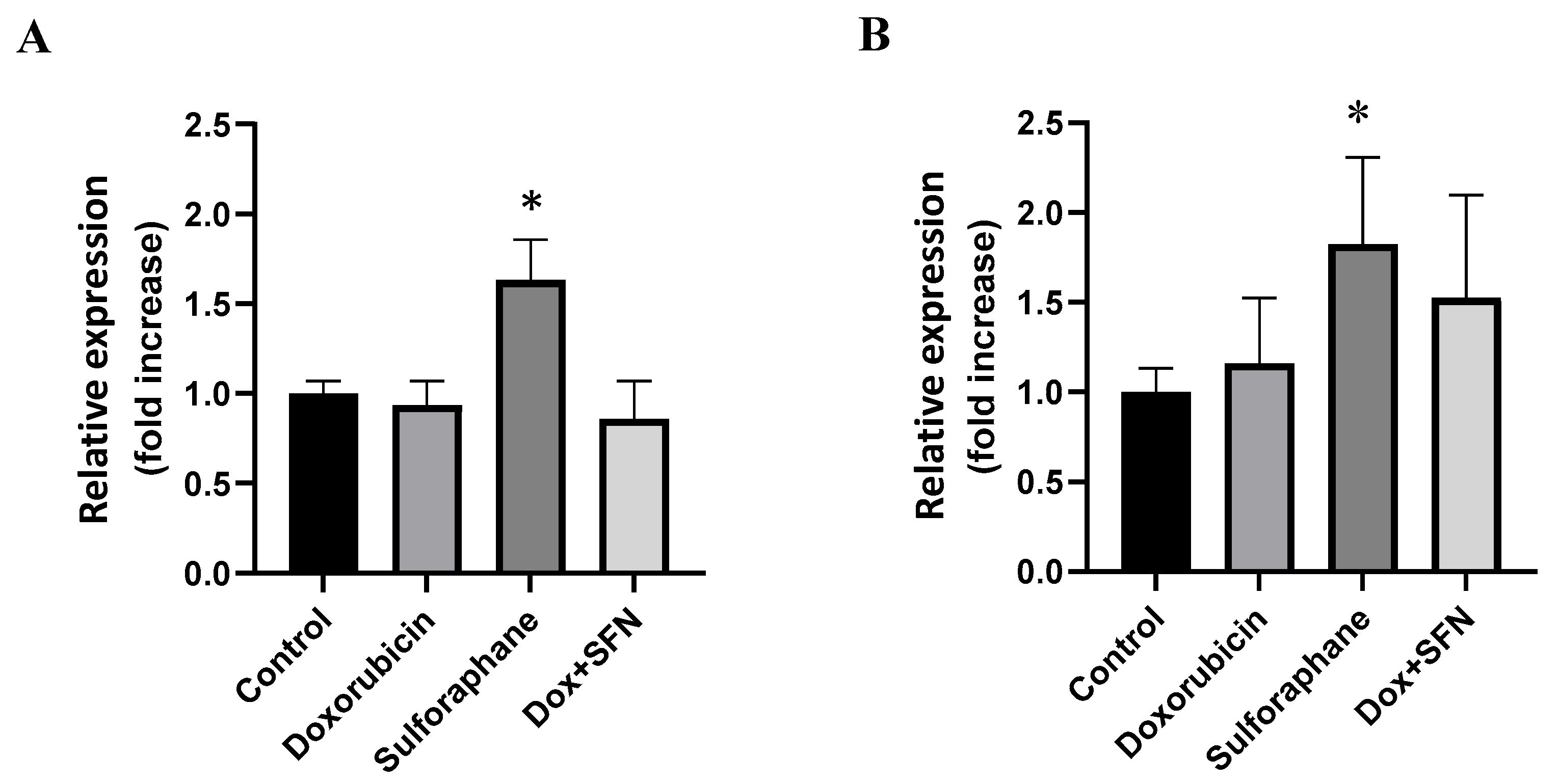
2.4. Effects of Sulforaphane on the Modulation of Superoxide Dismutase Isoforms Protein Levels
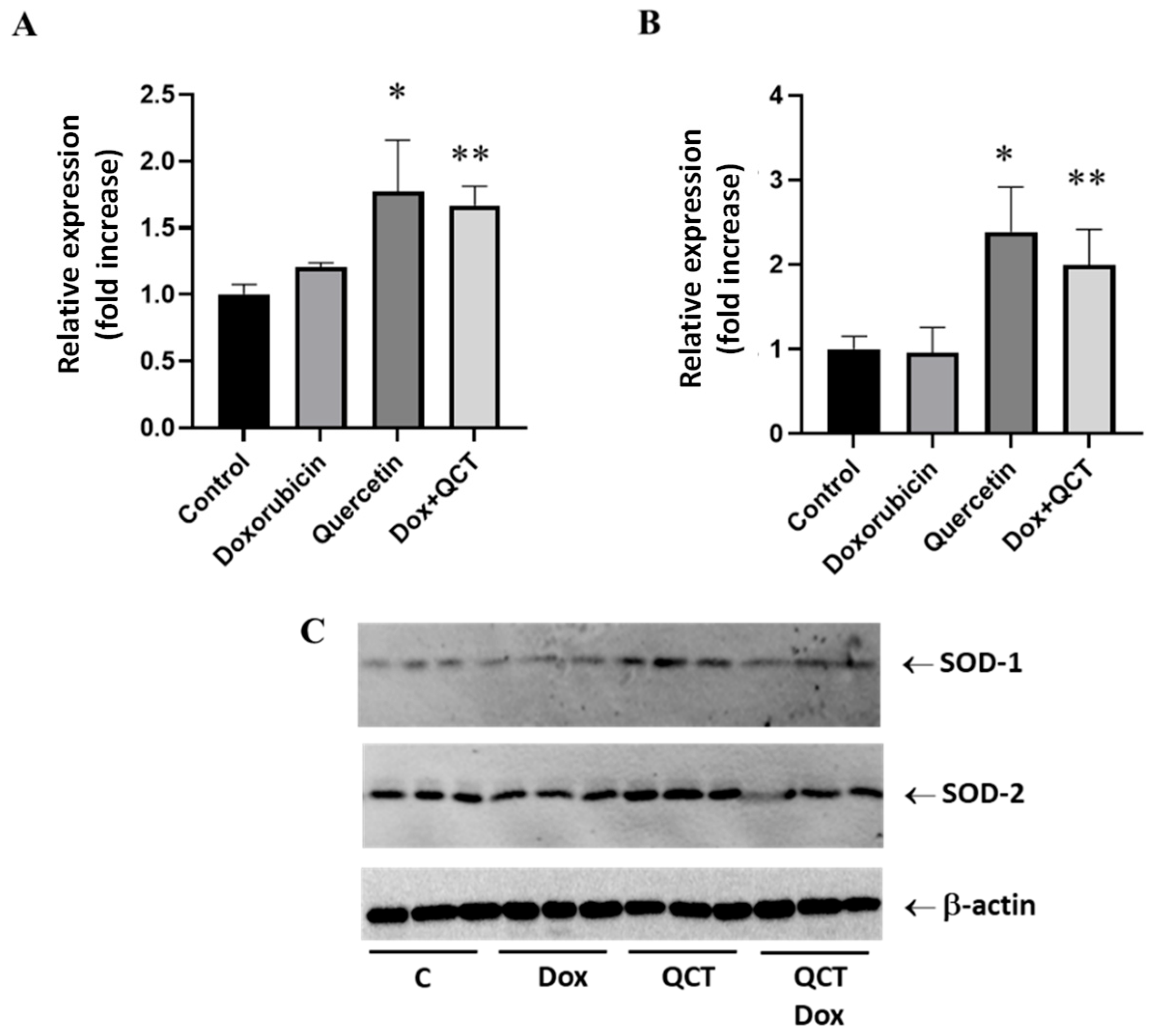
2.5. Effects of Quercetin on the Modulation of Superoxide Dismutase Isoforms Protein Levels
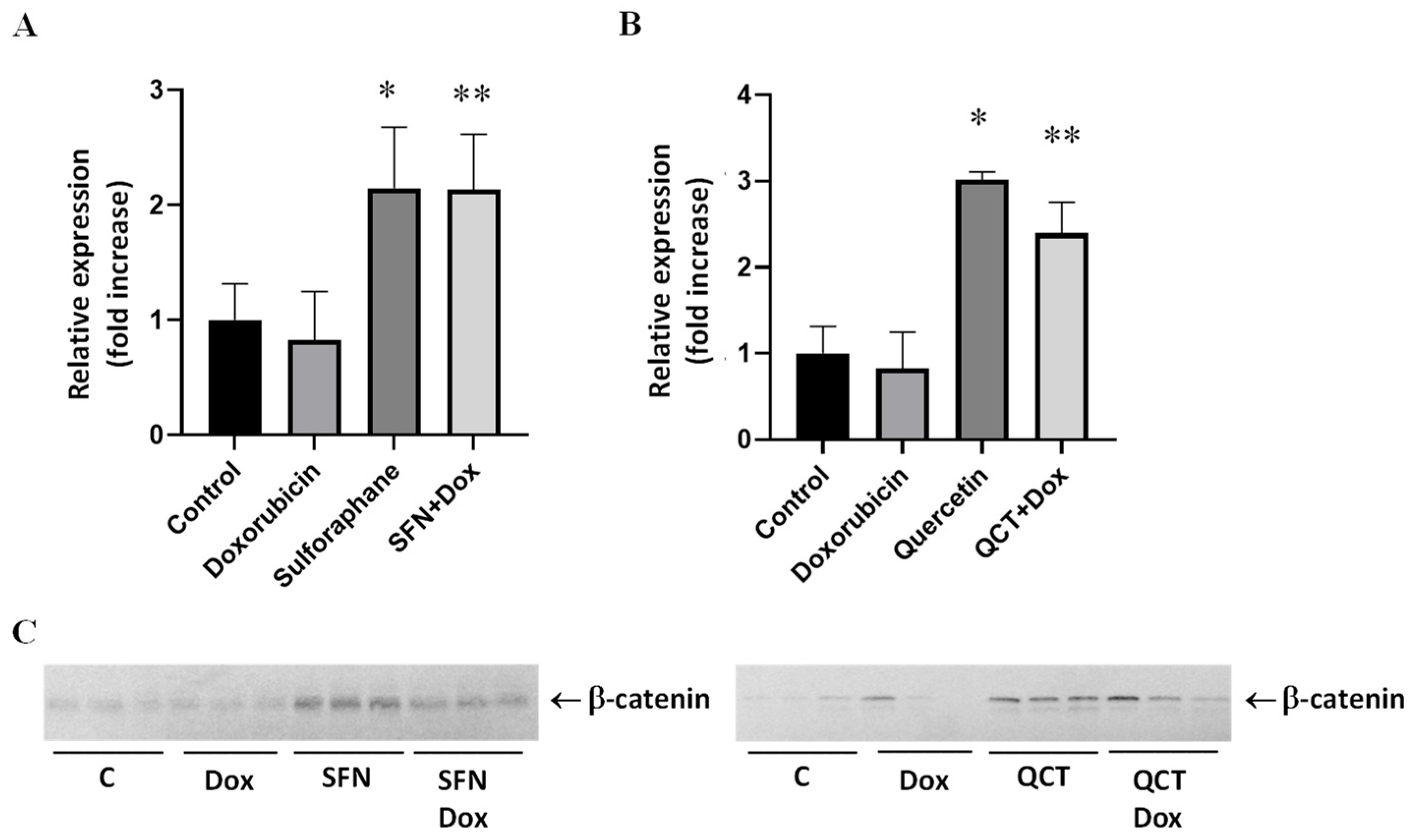
2.6. Effects of Sulforaphane and Quercetin on Doxorubicin-Induced Modulation of Beta-Catenin Protein Levels
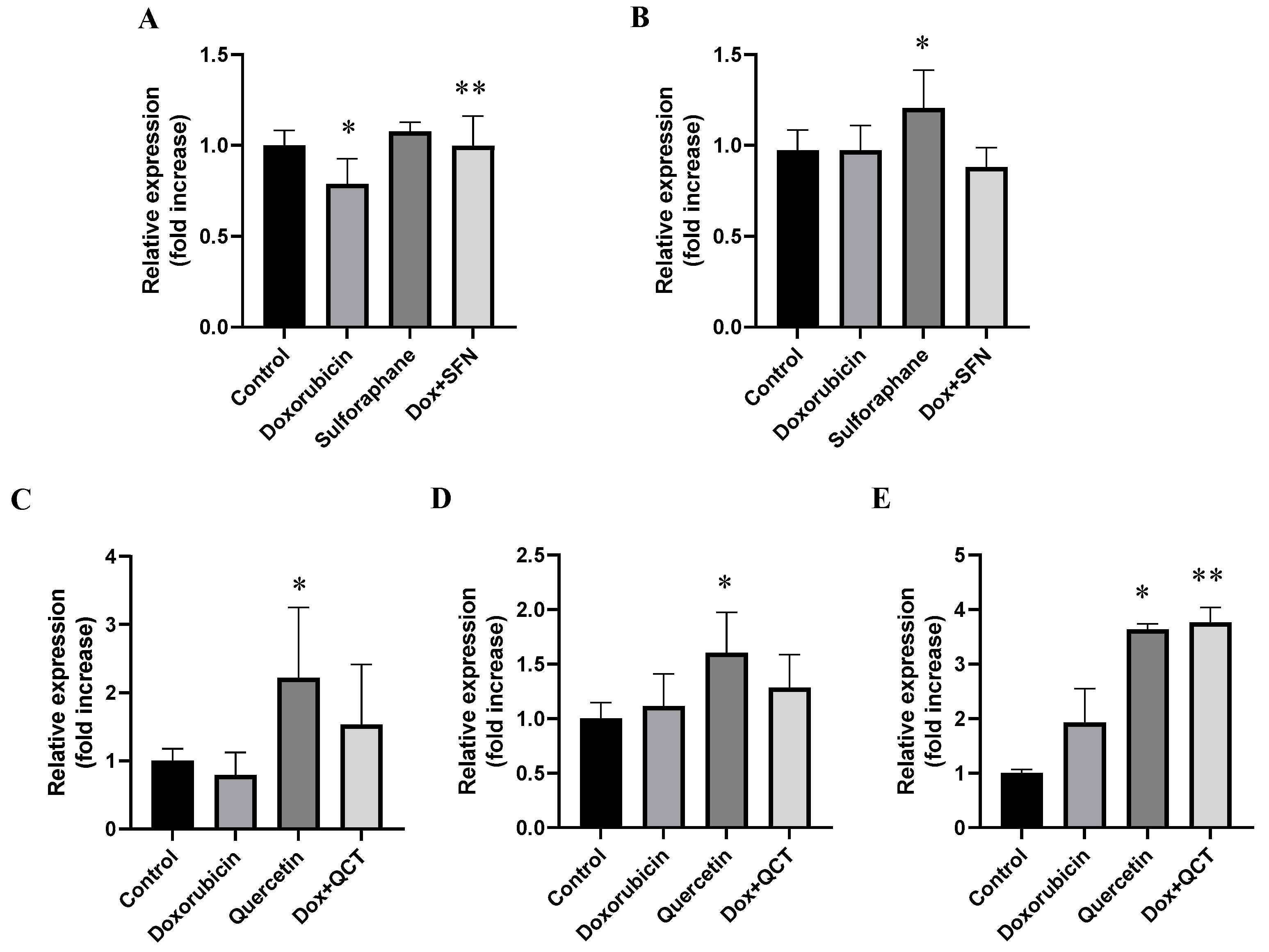
2.7. Effects of Sulforaphane and Quercetin on Doxorubicin-Induced Modulation of GSK-3α/β Protein Levels and Specific Phosphorylation
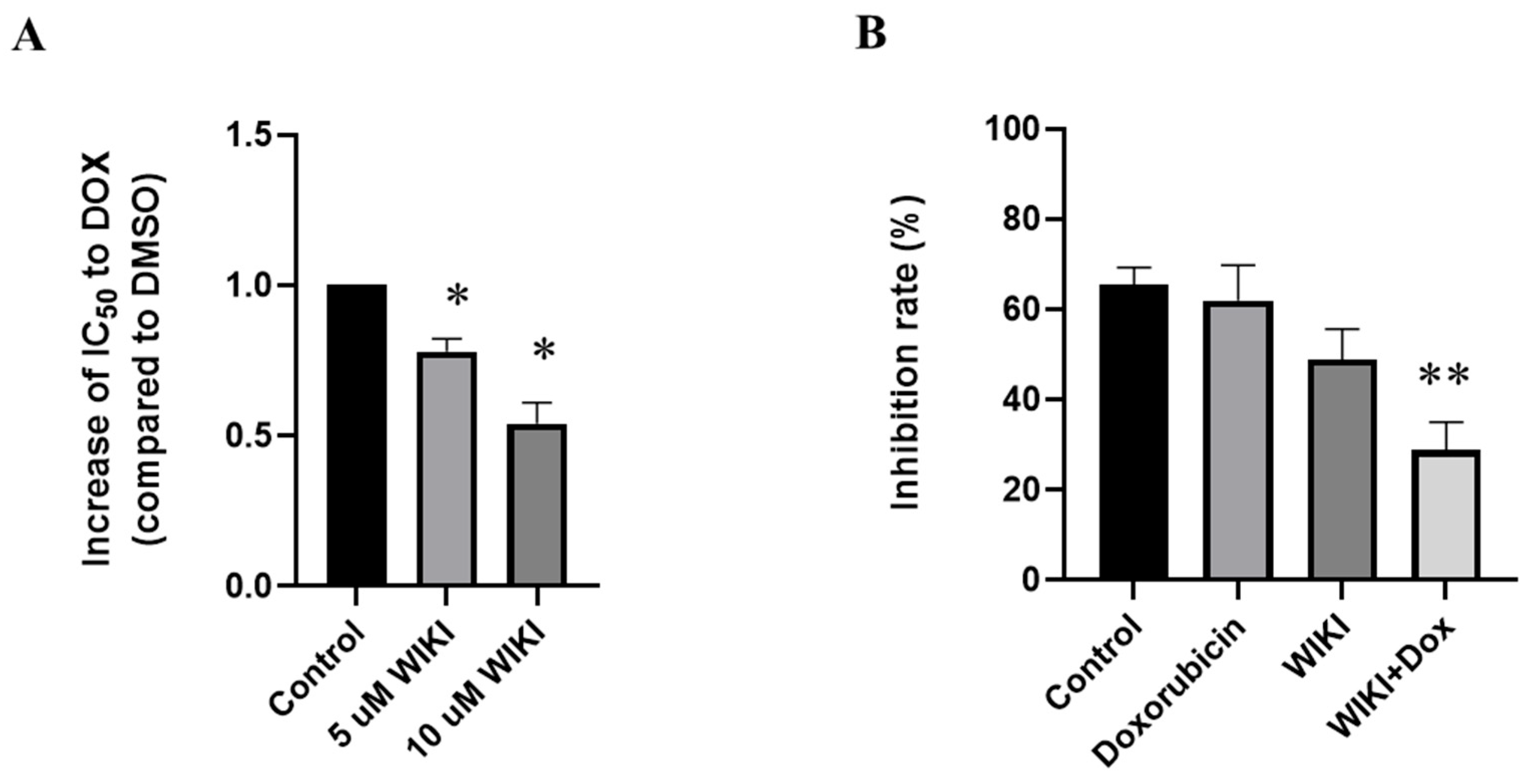
2.8. Influence of WIKI-4, a Wnt Pathway Inhibitor, on Doxorubicin-Induced Changes in H9c2 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Treatment
4.3. Determination of Effects of Doxorubicin on Viability of H9c2 Cells
4.4. Determination of the Influence of Sulforaphane, Quercetin, and WIKI-4 on Doxorubicin-Induced Cytotoxicity
4.5. MTT Assay to Analyze Cell Viability
4.6. Samples Preparation
4.7. Western Blot Analysis
4.8. Determination of Superoxide Dismutase Activity
4.9. Determination of Catalase Activity
4.10. Determination of Cellular ROS by DCFDA Assay
4.11. Statistical Evaluation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | Adenosine monophosphate-activated protein kinase |
| APC | Adenomatous polyposis coli |
| CAT | Catalase |
| DCFDA | 2′,7′-dichlorofluorescin diacetate |
| DKK | Dickkopf |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acid |
| DOX | Doxorubicin |
| Dvl | Dishevelled |
| FBS | Fetal bovine serum |
| GSK-3β | Glycogen synthase kinase-3 beta |
| HO-1 | Heme oxygenase 1 |
| mTOR | Mammalian target of rapamycin |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| Nrf-2 | Nuclear factor erythroid 2-related factor 2 |
| PBS | Phosphate-buffered saline |
| PI3K/AKT | Phosphoinositide-3-kinase/protein kinase B |
| PMA | Phorbol 12-myristate 13-acetate |
| QCT | Quercetin |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| SFN | Sulforaphane |
| Sfrps | Secreted frizzled-related proteins |
| SOD | Superoxide dismutase |
| TCF/LEF | T cell factor/lymphoid enhancer factor |
| TSC2 | Tuberous sclerosis complex 2 |
| Wif1 | Wnt inhibitory factor 1 |
| Wnt | Wingless/Int-1 |
| β-TrCP | E3 ligase beta-transducin repeat-containing protein |
References
- Yang, F.; Kemp, C.J.; Henikoff, S. Anthracyclines induce double-strand DNA breaks at active gene promoters. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2015, 773, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.C.; Magarkar, A.; Horta, M.; Lima, J.L.F.C.; Bunker, A.; Nunes, C.; Reis, S. Influence of doxorubicin on model cell membrane properties: Insights from in vitro and in silico studies. Sci. Rep. 2017, 7, 6343. [Google Scholar] [CrossRef] [PubMed]
- Takemura, G.; Fujiwara, H. Doxorubicin-Induced Cardiomyopathy. Prog. Cardiovasc. Dis. 2007, 49, 330–352. [Google Scholar] [CrossRef]
- Elgendy, I.Y.; Mahtta, D.; Pepine, C.J. Medical Therapy for Heart Failure Caused by Ischemic Heart Disease. Circ. Res. 2019, 124, 1520–1535. [Google Scholar] [CrossRef]
- Algoet, M.; Janssens, S.; Himmelreich, U.; Gsell, W.; Pusovnik, M.; Van den Eynde, J.; Oosterlinck, W. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc. Med. 2023, 33, 357–366. [Google Scholar] [CrossRef]
- Zhai, M.; Li, B.; Duan, W.; Jing, L.; Zhang, B.; Zhang, M.; Yu, L.; Liu, Z.; Yu, B.; Ren, K.; et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J. Pineal Res. 2017, 63, e12419. [Google Scholar] [CrossRef]
- Enos, M.D.; Gavagan, M.; Jameson, N.; Zalatan, J.G.; Weis, W.I. Structural and functional effects of phosphopriming and scaffolding in the kinase GSK-3β. Sci. Signal. 2024, 17, eado0881. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Rada, P.; Rojo, A.I.; Offergeld, A.; Feng, G.J.; Velasco-Martín, J.P.; González-Sancho, J.M.; Valverde, Á.M.; Dale, T.; Regadera, J.; Cuadrado, A. WNT-3A Regulates an Axin1/NRF2 Complex That Regulates Antioxidant Metabolism in Hepatocytes. Antioxid. Redox Signal. 2015, 22, 555–571. [Google Scholar] [CrossRef]
- Inoki, K.; Ouyang, H.; Zhu, T.; Lindvall, C.; Wang, Y.; Zhang, X.; Yang, Q.; Bennett, C.; Harada, Y.; Stankunas, K.; et al. TSC2 Integrates Wnt and Energy Signals via a Coordinated Phosphorylation by AMPK and GSK3 to Regulate Cell Growth. Cell 2006, 126, 955–968. [Google Scholar] [CrossRef]
- Dong, J.; Xu, X.; Zhang, Q.; Yuan, Z.; Tan, B. The PI3K/AKT pathway promotes fracture healing through its crosstalk with Wnt/β-catenin. Exp. Cell Res. 2020, 394, 112137. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Pan, X.; Fu, H.; Zheng, Y.; Dai, Y.; Yin, Y.; Chen, Q.; Hao, Q.; Bao, D.; Hou, D. Effect of curcumin on glycerol-induced acute kidney injury in rats. Sci. Rep. 2017, 7, 10114. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Bai, Y.; Jiang, X.; Zhou, S.; Wang, Y.; Wintergerst, K.A.; Cui, T.; Ji, H.; Tan, Y.; Cai, L. Sulforaphane prevents angiotensin II-induced cardiomyopathy by activation of Nrf2 via stimulating the Akt/GSK-3ß/Fyn pathway. Redox Biol. 2018, 15, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimpour, S.; Shahidi, S.B.; Abbasi, M.; Tavakoli, Z.; Esmaeili, A. Quercetin-conjugated superparamagnetic iron oxide nanoparticles (QCSPIONs) increases Nrf2 expression via miR-27a mediation to prevent memory dysfunction in diabetic rats. Sci. Rep. 2020, 10, 15957. [Google Scholar] [CrossRef]
- Tanito, M.; Masutani, H.; Kim, Y.-C.; Nishikawa, M.; Ohira, A.; Yodoi, J. Sulforaphane Induces Thioredoxin through the Antioxidant-Responsive Element and Attenuates Retinal Light Damage in Mice. Investig. Opthalmol. Vis. Sci. 2005, 46, 979. [Google Scholar] [CrossRef]
- Qazi, A.; Pal, J.; Maitah, M.; Fulciniti, M.; Pelluru, D.; Nanjappa, P.; Lee, S.; Batchu, R.B.; Prasad, M.; Bryant, C.S.; et al. Anticancer Activity of a Broccoli Derivative, Sulforaphane, in Barrett Adenocarcinoma: Potential Use in Chemoprevention and as Adjuvant in Chemotherapy. Transl. Oncol. 2010, 3, 389–399. [Google Scholar] [CrossRef]
- Silva-Palacios, A.; Ostolga-Chavarría, M.; Sánchez-Garibay, C.; Rojas-Morales, P.; Galván-Arzate, S.; Buelna-Chontal, M.; Pavón, N.; Pedraza-Chaverrí, J.; Königsberg, M.; Zazueta, C. Sulforaphane protects from myocardial ischemia-reperfusion damage through the balanced activation of Nrf2/AhR. Free Radic. Biol. Med. 2019, 143, 331–340. [Google Scholar] [CrossRef]
- Yoon, H.-Y.; Kang, N.-I.; Lee, H.-K.; Jang, K.Y.; Park, J.-W.; Park, B.-H. Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the Nrf2-dependent phase 2 enzyme. Biochem. Pharmacol. 2008, 75, 2214–2223. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, R.; McElhanon, K.; Allen, C.D.; Megyesi, J.K.; Beneš, H.; Singh, S.P. Sulforaphane protects the heart from doxorubicin-induced toxicity. Free Radic. Biol. Med. 2015, 86, 90–101. [Google Scholar] [CrossRef]
- Bose, C.; Awasthi, S.; Sharma, R.; Beneš, H.; Hauer-Jensen, M.; Boerma, M.; Singh, S.P. Sulforaphane potentiates anticancer effects of doxorubicin and attenuates its cardiotoxicity in a breast cancer model. PLoS ONE 2018, 13, e0193918. [Google Scholar] [CrossRef]
- Chowdhury, F.I.; Yasmin, T.; Akter, R.; Islam, M.N.; Hossain, M.M.; Khan, F.; Aldhahrani, A.; Soliman, M.M.; Subhan, N.; Haque, M.A.; et al. Resveratrol treatment modulates several antioxidant and anti-inflammatory genes expression and ameliorated oxidative stress mediated fibrosis in the kidneys of high-fat diet-fed rats. Saudi Pharm. J. 2022, 30, 1454–1463. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Shaheen, S.; El Haouari, M.; Azzini, E.; Butnariu, M.; Sarac, I.; Pentea, M.; Ramírez-Alarcón, K.; Martorell, M.; et al. Flavonoids as potential anti-platelet aggregation agents: From biochemistry to health promoting abilities. Crit. Rev. Food Sci. Nutr. 2022, 62, 8045–8058. [Google Scholar] [CrossRef] [PubMed]
- Hanganu, D.; Niculae, M.; Ielciu, I.; Olah, N.-K.; Munteanu, M.; Burtescu, R.; Ștefan, R.; Olar, L.; Pall, E.; Andrei, S.; et al. Chemical Profile, Cytotoxic Activity and Oxidative Stress Reduction of Different Syringa vulgaris L. Extracts. Molecules 2021, 26, 3104. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Rubab, M.; Daliri, E.B.-M.; Chelliah, R.; Javed, A.; Oh, D.-H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Predes, D.; Maia, L.A.; Matias, I.; Araujo, H.P.M.; Soares, C.; Barros-Aragão, F.G.Q.; Oliveira, L.F.S.; Reis, R.R.; Amado, N.G.; Simas, A.B.C.; et al. The Flavonol Quercitrin Hinders GSK3 Activity and Potentiates the Wnt/β-Catenin Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 12078. [Google Scholar] [CrossRef]
- Dorostkar, H.; Haghiralsadat, B.F.; Hemati, M.; Safari, F.; Hassanpour, A.; Naghib, S.M.; Roozbahani, M.H.; Mozafari, M.R.; Moradi, A. Reduction of Doxorubicin-Induced Cardiotoxicity by Co-Administration of Smart Liposomal Doxorubicin and Free Quercetin: In Vitro and In Vivo Studies. Pharmaceutics 2023, 15, 1920. [Google Scholar] [CrossRef]
- Haybar, H.; Khodadi, E.; Shahrabi, S. Wnt/β-catenin in ischemic myocardium: Interactions and signaling pathways as a therapeutic target. Heart Fail. Rev. 2019, 24, 411–419. [Google Scholar] [CrossRef]
- Svetláková, B.B.; Líšková, V.P.; Barančík, M. Wnt Signaling Inhibitors as Therapeutic Approach in Ischemic Heart Disease. Molecules 2024, 29, 5958. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Wang, C.; Hong, X.; Miao, J.; Liao, Y.; Zhou, L.; Liu, Y. An essential role for Wnt/β-catenin signaling in mediating hypertensive heart disease. Sci. Rep. 2018, 8, 8996. [Google Scholar] [CrossRef]
- Vejpongsa, P.; Yeh, E.T.H. Prevention of Anthracycline-Induced Cardiotoxicity. J. Am. Coll. Cardiol. 2014, 64, 938–945. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.-S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T.H. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Amador-Martínez, I.; Aparicio-Trejo, O.E.; León-Contreras, J.C.; Hernández-Pando, R.; Saavedra, E.; García-Arroyo, F.E.; Pedraza-Chaverri, J.; Sánchez-Lozada, L.G.; Tapia, E. Sulforaphane Restores Mitochondrial β-Oxidation and Reduces Renal Lipid Accumulation in a Model of Releasing Unilateral Ureteral Obstruction. Antioxidants 2025, 14, 288. [Google Scholar] [CrossRef]
- Song, J.; Li, S.; Zhang, B.; Wu, J.; Zhong, A. Quercetin protects human coronary artery endothelial cells against hypoxia/reoxygenation-induced mitochondrial apoptosis via the Nrf2/HO-1 axis. Biomed. Res. 2024, 45, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.L.; Westman, N.G.; Lundgren, E.; Roos, G. Copper- and zinc-containing superoxide dismutase, manganese-containing superoxide dismutase, catalase, and glutathione peroxidase in normal and neoplastic human cell lines and normal human tissues. Cancer Res. 1982, 42, 1955–1961. [Google Scholar] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Zhou, N.; Wei, S.; Sun, T.; Xie, S.; Liu, J.; Li, W.; Zhang, B. Recent progress in the role of endogenous metal ions in doxorubicin-induced cardiotoxicity. Front. Pharmacol. 2023, 14, 1292088. [Google Scholar] [CrossRef]
- Li, B.; Kim, D.S.; Yadav, R.K.; Kim, H.R.; Chae, H.J. Sulforaphane prevents doxorubicin-induced oxidative stress and cell death in rat H9c2 cells. Int. J. Mol. Med. 2015, 36, 53–64. [Google Scholar] [CrossRef]
- Wang, H.; Yang, G.; Tian, Y.; Li, J.; Meng, L.; Jiang, X.; Xin, Y. Sulforaphane inhibits angiotensin II-induced cardiomyocyte apoptosis by acetylation modification of Nrf2. Aging 2022, 14, 6740–6755. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Liu, B.; Tang, L.; Kuang, R.; Wang, X.; Zhao, C.; Song, X.; Cao, X.; Wu, X.; et al. Sulforaphane prevents rat cardiomyocytes from hypoxia/reoxygenation injury in vitro via activating SIRT1 and subsequently inhibiting ER stress. Acta Pharmacol. Sin. 2016, 37, 344–353. [Google Scholar] [CrossRef]
- Sharma, A.; Parikh, M.; Shah, H.; Gandhi, T. Modulation of Nrf2 by quercetin in doxorubicin-treated rats. Heliyon 2020, 6, e03803. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Valko, R.; Liska, J.; Nepovimova, E.; Kuca, K.; Valko, M. Flavonoids and their role in oxidative stress, inflammation, and human diseases. Chem. Biol. Interact. 2025, 413, 111489. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Jung, Y.; Shin, S.Y.; Lee, Y.H.; Lim, Y. Flavones with inhibitory effects on glycogen synthase kinase 3β. Appl. Biol. Chem. 2017, 60, 227–232. [Google Scholar] [CrossRef]
- Qian, M.; Zhu, Y.; Lin, W.; Lian, H.; Xia, Y.; Papadimos, T.; Wang, J. PICK1 overexpression ameliorates endotoxin-induced acute lung injury by regulating mitochondrial quality control via maintaining Nrf-2 stabilization through activating the PI3K/Akt/GSK-3β pathway and disrupting the E3 ubiquitin ligase adapter β-TrCP. Int. Immunopharmacol. 2025, 156, 114685. [Google Scholar] [CrossRef]
- Li, L.; Xiang, Y.; Zeng, Y.; Xiao, B.; Yu, W.; Duan, C.; Xia, X.; Zhang, T.; Zeng, Y.; Liu, Y.; et al. GSK-3β inhibition promotes doxorubicin-induced apoptosis in human cholangiocarcinoma cells via FAK/AKT inhibition. Mol. Med. Rep. 2020, 22, 4432–4441. [Google Scholar] [CrossRef]
- Aisagbonhi, O.; Rai, M.; Ryzhov, S.; Atria, N.; Feoktistov, I.; Hatzopoulos, A.K. Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis. Model. Mech. 2011, 4, 469–483. [Google Scholar] [CrossRef]
- Malekar, P.; Hagenmueller, M.; Anyanwu, A.; Buss, S.; Streit, M.R.; Weiss, C.S.; Wolf, D.; Riffel, J.; Bauer, A.; Katus, H.A.; et al. Wnt Signaling Is Critical for Maladaptive Cardiac Hypertrophy and Accelerates Myocardial Remodeling. Hypertension 2010, 55, 939–945. [Google Scholar] [CrossRef]
- Grimes, C.A.; Jope, R.S. The multifaceted roles of glycogen synthase kinase 3β in cellular signaling. Prog. Neurobiol. 2001, 65, 391–426. [Google Scholar] [CrossRef]
- Jope, R.S.; Johnson, G.V. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 2004, 29, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.; Gao, S.; Holle, E.; Yu, X.; Yatani, A.; Wagner, T.; Sadoshima, J. Glycogen Synthase Kinase-3α Reduces Cardiac Growth and Pressure Overload-induced Cardiac Hypertrophy by Inhibition of Extracellular Signal-regulated Kinases. J. Biol. Chem. 2007, 282, 33181–33191. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Tu, Y.; Lu, J.; Wang, P.; Guo, Z.; Wang, Q.; Guo, K.; Lan, R.; Li, H.; Liu, P. Dkk1 exacerbates doxorubicin-induced cardiotoxicity by inhibiting the Wnt/β-catenin signaling pathway. J. Cell Sci. 2019, 132, jcs228478. [Google Scholar] [CrossRef] [PubMed]
- Bastakoty, D. Temporary, Systemic Inhibition of the WNT/β-Catenin Pathway promotes Regenerative Cardiac Repair following Myocardial Infarct. Cell Stem Cells Regen. Med. 2016, 2. [Google Scholar] [CrossRef]
- Lin, J.C.; Kuo, W.-W.; Baskaran, R.; Chen, M.-C.; Ho, T.-J.; Chen, R.-J.; Chen, Y.-F.; Vijaya Padma, V.; Lay, I.-S.; Huang, C.-Y. Enhancement of beta-catenin in cardiomyocytes suppresses survival protein expression but promotes apoptosis and fibrosis. Cardiol. J. 2017, 24, 195–205. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Líšková, V.; Svetláková, B.; Barančík, M. The Role of the Wnt/β-Catenin Pathway in the Modulation of Doxorubicin-Induced Cytotoxicity in Cardiac H9c2 Cells by Sulforaphane and Quercetin. Int. J. Mol. Sci. 2025, 26, 7858. https://doi.org/10.3390/ijms26167858
Líšková V, Svetláková B, Barančík M. The Role of the Wnt/β-Catenin Pathway in the Modulation of Doxorubicin-Induced Cytotoxicity in Cardiac H9c2 Cells by Sulforaphane and Quercetin. International Journal of Molecular Sciences. 2025; 26(16):7858. https://doi.org/10.3390/ijms26167858
Chicago/Turabian StyleLíšková, Viktória, Barbora Svetláková, and Miroslav Barančík. 2025. "The Role of the Wnt/β-Catenin Pathway in the Modulation of Doxorubicin-Induced Cytotoxicity in Cardiac H9c2 Cells by Sulforaphane and Quercetin" International Journal of Molecular Sciences 26, no. 16: 7858. https://doi.org/10.3390/ijms26167858
APA StyleLíšková, V., Svetláková, B., & Barančík, M. (2025). The Role of the Wnt/β-Catenin Pathway in the Modulation of Doxorubicin-Induced Cytotoxicity in Cardiac H9c2 Cells by Sulforaphane and Quercetin. International Journal of Molecular Sciences, 26(16), 7858. https://doi.org/10.3390/ijms26167858



