Stilbenes from Vine Extracts: Therapeutic Potential and Mechanisms
Abstract
1. Introduction
2. Stilbenes from Grapevine: Biosynthesis, Structures, and Extraction
2.1. Polyphenols in Grapevine: General Classification and Biological Roles
2.2. Focus on Stilbenes: Structure, Diversity, Biosynthesis, and Natural Functions
2.3. Extraction Methods of Polyphenols and Stilbenes
3. Biological Activities of Stilbenes and Health Implications
3.1. Bioavailability and Metabolism of Stilbenes
3.2. Antioxidant and Anti-Inflammatory Properties
3.3. Antimicrobial and Antifungal Activities
3.4. Anticancer Effects: Preclinical and Clinical Evidence
| Stilbenes | Experimental In Vitro Model | Methodology | Experimental Conditions | Significant Results | Ref |
|---|---|---|---|---|---|
| Resveratrol | D407 cells | H2O2-induced cytotoxicity or MTT assay | 0, 25, 50, and 100 µM for 24 h | Resveratrol offered protection to D407 retinal pigment epithelial cells against H2O2-induced cytotoxicity, resulting in reduced cytotoxicity. | [261] |
| N2a cells | Fluorescein diacetate assay | 1.5, 3.125, 6.25, 12.5, 25, 50, and 100 µM for 48 h | Plasma membrane integrity was compromised in resveratrol-treated N2a cells, reducing cell viability. | [262] | |
| T24 cells | Cell Proliferation Kit II | 50, 100, 150, 200, and 250 µM for 24 h | Resveratrol exposure led to a decrease in cell viability, which was greater for s higher concentration of the stilbene. The determined IC50 was 178.73 µM. | [263] | |
| MCF-7 cells | MTS assay | 20, 40, 60, 80, and 100 µM for 48 h | Resveratrol was cytotoxic in MCF-7 cells, aligning with the compound’s anticancer properties. Changes in membrane fluidity and, consequently, in cellular signaling pathways were detected. | [264] | |
| HRT cells | MTT assay | 25, 50, 100, 200, and 300 µM for 72 h | Resveratrol inhibits HRT cell growth in a concentration-dependent manner. | [265] | |
| MCF-7 cells | Sulforhodamine B (SRB) assay | 15 μg/mL for 24 h | Co-treatment with resveratrol and doxorubicin drastically lowered doxorubicin’s IC50 from 0.417 μg/mL to 0.035 μg/mL, showing it to be a good adjuvant in antitumor therapy. | [266] | |
| Trans-resveratrol | PC-3 cells | MTS assay | 0, 3, 10, 30, and 100 μM for 72 h | Trans-resveratrol exhibited an inhibitory effect on cell viability in PC3 cell line at concentrations ranging from 10 to 100 μM. | [267] |
| MCF-7 cells JURKAT E.6, and THP-1 | MTT or XTT assay | 10, 30, 50, 70, 90, and 100 μM for 24, 48, 72, and 96 h | As trans-resveratrol concentrations increased, cell viability showed a greater or lesser pronounced decrease, depending on the cell line. Therefore, the effect of trans-resveratrol depends not only on dose and treatment duration but also on the cell type considered. | [268] | |
| MCF-7, Du145, and PC-3 cells | MTT assay | 1 × 10−15–1×10−3 M for 48 h | Trans-resveratrol exhibited a cytotoxic effect on cancer cells at concentrations ranging from 1 × 10−7 to 1 × 10−4 M. | [269] | |
| MCF-7 cells | MTT or neutral red uptake (NRU) assay | 1, 5, 10, 25, and 50 µM for 24 h | Solely an administration of trans-resveratrol did not lead to a decrease in cell viability in MCF-7 cell line. However, a pre-treatment with trans-resveratrol conferred protection against rotenone-induced toxicity. | [270] | |
| HepG2, Vero, and MCF-7 cells | MTT assay | 0, 0.2, 0.4, 2, 4, 6.25, 12.5, 25, 50, and 100 µM for 24, 48, 72 h | Trans-resveratrol demonstrated cytotoxicity in all mentioned cell types at concentrations equal to or greater than 50 µM after 48 h. | [271] | |
| PC12 cells | MTT or NRU assay | 0, 5, 10, and 25 µM for 24 h | Trans-resveratrol decreases the viability of P12 cells, as shown by both performed assays. | [272] | |
| HCT-116, HCT-116/p53(−/−), HepG2, and Hep3B cells | CellTiter-Blue® and SRB assay | 0, 1, 10, and 100 µM for 72 h | Trans-resveratrol demonstrated a significant ability to decrease cell viability at a concentration of 100 μM after 72 h. | [273] | |
| HepG2 cells | WST-1 assay | 0.5–100 g/mL for 24, 36, and 48 h | Trans-resveratrol decreased the viability of HepG2 cells, evidencing the compound’s anticancer activity. The calculated IC50 was shown to decreased over time. | [273] | |
| MCF-10A, MCF-7, MDA-MB-231, and ZR-75-1 cells | CellTiter-Glo® Luminescent Cell Viability assay | 1–350 µM for 48 h | Trans-resveratrol was proven to be an efficient inhibitor of the cancer cell lines MCF-7, ZR-75-1, and MDA-MB-231, with IC50 values of 68.3 ± 2.6, 82.2 ± 4.8, and 67.6 ± 4.1 µM, respectively. Moreover, it was three times more potent in the MCF-10A cell line, with an IC50 of 20.0 ± 2.9 µM. | [274] | |
| SW480 cells | MTT assay | 30 µM for 48, 72, and 96 h | There was a time- and dose-dependent decline in cell survival. | [275] | |
| MCF-7 cells | Annexin V-FITC and propidium iodide assay | 6.25–50 µg/mL for 24 h | The viability of MCF-7 cells was suppressed following trans-resveratrol treatment. | [276] | |
| ARPE-19 cells, transmitochondrial normal RPE cybrid, and transmitochondrial AMD RPE cybrid cells | MTT assay | 1000 µM for 48 h | Viability assays demonstrated that trans-resveratrol has beneficial properties for cybrid cells, increasing their viability compared to untreated cells. | [277] | |
| HepG2 cells | MTT assay | 2.5, 10, 30, 50, 70, 100, 140, and 200 µM for 24, and 48 h | Trans-resveratrol has inhibitory effects on the cell viability of HepG2 cells, having greater impact in higher concentrations and prolonged exposure times. | [278] | |
| LTC-14 cells | MTT assay | 0, 0.1, 1, 10, 100, and 1000 µM for 24, and 48 h | LTC-14 cells experienced a decrease in cell viability to below 50% in the presence of trans-resveratrol at a concentration of 200 µM. | [279] | |
| NCTC clone 929 cells | NRU assay | 15.63, 31.25, 62.5, 125, and 250 μM for 24 h | Trans-resveratrol caused significant cell injury and death with an associated IC50 of 50 μM. | [280] | |
| A549 cells | MTT assay | 0, 5.5, 11, 21.9, 32.9, 43.8, 87.6, 131.4, and 175.2 μM for 24 h | Trans-resveratrol treatment led to cell viability inhibition in a dose-dependent manner. The IC50 was determined to be 85.5 μM. | [281] | |
| Pterostilbene | MCF-7, MDA-MD-231, and ZR-75-1 | MTT assay | 10 µmol/L, 20 µmol/L, 30 µmol/L (pterostilbene) + 5 µmol/L (Tamoxifen) for 24, 48, and 72 h | Combined therapy with pterostilbene and tamoxifen reduced cell viability in all cell lines. A greater decrease in viability was observed for the 24 h treatment. | [282] |
| MDA-MB-231 and T-47D cells | MTT assay | 10–100 µM for 48 h | A decrease in cell viability and significant morphological changes were observed in both cell lines following the treatment with pterostilbene. The IC50 concentrations for MDA-MB-231 and T-47D cells were 45.7 ± 0.01 and 63.1 ± 0.11 µM, respectively. | [283] | |
| MCF-7, SK-BR-3, and MDA-MB-468 cells | American Type Culture Collection | 0–100 µM for 72 h | Treatment with pterostilbene arrested cells growth in a dose-dependent manner for all three cell lines, exhibiting a greater impact in MDA-MB-468 cells. The calculated IC50 values were 87.6 ± 9.0 µM for MCF-7 cells, 64.4 ± 4.6 µM for SK-BR-3 cells, and 45.7 ± 5.2 µM for MDA-MB-468 cells. | [284] | |
| HeLa, CaSki, and SiHa cells | MTT assay | 0–200 µM for 72 h | All cell lines’ proliferation was inhibited by pterostilbene in a manner that varied in a directly proportional way to concentration. The IC50 for each cell line was calculated as follows: IC50 = 32.67 µM for HeLa, IC50 = 14.83 µM for CaSki, and IC50 = 34.17 µM for SiHa, indicating growth-inhibitory effects. | [285] | |
| TC-1 mouse cells after co-transformation with HPV16-E6, HPV16-E7, and c-Ha-Ras oncogenes | WST-1 assay | 0–100 µM for 72 h | Pterostilbene exhibits significant cytotoxicity, evidenced by the formation of cytoplasmic blebs observed after 48 h. The number of apoptotic cells increased in a dose-dependent manner and the obtained IC50 of pterostilbene was 15.61 µM. | [286] | |
| CL187, C COLO 205, HCT-8, SW480, Lovo, and HCT-116 cells | Cell Counting Kit-8 (CCK-8) assay | 1–100 µM for 24, 48, and 72 h | Pterostilbene inhibited cellular activity of all cell lines in a dose-dependent manner. After 72 h of treatment, the IC50 of pterostilbene for most of the cell lines used (except SW480 cells) was determined to be below 30 µM. | [287] | |
| HT-29 cells | SRB assay | 5–100 µM for 48 h | A significant decrease in cell growth was only observed at concentrations equal to or greater than 10 µM. The extent of inhibitory effects was shown to be dependent on pterostilbene dosage. | [288] | |
| HT-29 cells | MTT assay | 5 and 20 µM for 24 and 48 h | Treatment with pterostilbene at 20 μM inhibited the metabolic activity of HT-29 cells up to 80.2 ± 5.9%. | [289] | |
| HEC-1A and ECC-1 cells | MTS assay | 0, 18.75, 37.5, 75, 150, and 300 µM for 48 h | Pterostilbene treatment significantly reduced cell viability in a dose-dependent manner, with associated IC50 values ranging between 72 and 78 µM for both cell lines. | [290] | |
| Kuramochi, Caov-3, OVCAR-4, OVCAR-8, and SKOV3 cells | MTT assay | 0, 37.5, 75, 150, and 300 µM for 48 h | Cell viability was markedly reduced by pterostilbene in a dose-related way, with the IC50 for each cell line as follows: 161.2 μM for Kuramochi, 100.6 μM for Caov-3, 143.8 μM for OVCAR-4, 74.8 μM for OVCAR-8, and 95.2 μM for SKOV3 cells. | [291] | |
| LNCaP and PC3 cells | MTT assay | 0, 20, 40, 60, 80, and 100 µM for 48 h | Pterostilbene reduced cell viability for both cell lines and in a dose-dependent manner. The IC50 values ranged between 70–80 μM for LNCaP cells, and 80–100 μM for PC3 cells. | [292] | |
| MIA PaCa-2 and PANC-1 cells | MTT assay | 10–100 μM for 24, 48, and 72 h | Pterostilbene inhibited cell viability in a dose- and time-dependent manner in both cell lines. The IC50 concentration values varied depending on cell type and selected time points, with MIA PaCa-2 showing 72 μM at 24 h, 51 μM at 48 h, and 32 μM at 72 h, while PANC-1 showed 84 μM at 24 h, 33 μM at 48 h, and 29 μM at 72 h. | [293] | |
| A375, A549, HT29, and MCF-7 cells | Countess Automated Cell Counter and SRB Toxicology Assay | 0–200 μM for 24, 48, and 72 h | Pterostilbene inhibited cell viability in a dose- and time-dependent manner in both cell lines. The IC50 values determined were cell type-dependent, being much lower for HT29 (IC50 = 60.3 mmol/L) and MCF7 (IC50 = 44.0 mmol/L) cells than for A375 (IC50 = 14.7 mmol/L) and A549 (IC50 = 28.6 mmol/L) cells. | [217] | |
| 11–18, HCC827, HCC4006, H1975, and PC9 cells | MTT assay | 0–150 μM for 72 h | Pterostilbene inhibited cell viability in all cell lines, with IC50 values ranging between 23.8 and 40.7 μM. | [294] | |
| HepG2 cells | MTT assay and CCK-8 assay | 12.5–100 µM for 24 h | Cell viability and proliferation were reduced for all concentrations considered in a dose-dependent manner. | [295] | |
| HT29, MKN74, and CT26 cells | MTS assay | 10, 50, and 100 µM for 48 h | Pterostilbene reduces cell viability in all three cell lines. The determined IC50 values were 21 µM for CT26, 63 µM for HT29, and 65 µM for MKN74 cells. | [296] | |
| CAR cells | MTT assay | 5, 10, 25, 50, 75, and 100 µM for 24, 48, and 72 h | Pterostilbene induces cytotoxicity in a time- and dose-dependent manner. The IC50 values after 24, 48, and 72 h of incubation were 78.26 ± 4.33, 48.04 ± 3.68, and 20.65 ± 4.88 µM, respectively. | [297] | |
| MDA-MB-231 cells | MTT assay | 1, 5, 20, 30, and 50 µg/mL for 48 h | Pterostilbene exhibits an inhibitory associated with an IC50 value of 79.5 ± 6.36 µg/mL. | [298] | |
| AsPC-1, BxPC-3, MIA PaCa-2, and PANC-1 cells | MTT assay | 0, 50, 75, 100, 125, and 150 µM for 48 h | Increasing concentrations of pterostilbene reduced viability in all cell lines tested, pointing towards a dose-dependent sensitivity to the mentioned compound. The IC50 values ranged from 110 to 130 µM. | [299] | |
| MDA-MB-231 cells | MTT assay | 2.5, 5, 10, 20, 40, and 80 µM for 24 h | A 24 h treatment with pterostilbene at 5 μM resulted in a 12% reduction in survival of MDA-MB-231 cells. The IC50, IC80, and IC85 doses against MDA-MB-231 cells were 30.4, 12.1, and 9.7 µM, respectively, confirming selective anticancer toxicity. | [300] | |
| GBC-SD, NOZ, and SGC-996 cells | CCK-8 assay | 0–80 µmol/L for 48 h | Pterostilbene exhibits cytotoxic effects on all three cell lines. The estimated IC50 for GBC-SD cells was above 80 µmol/L, between 40–60 µmol/L for NOZ cells, and approximately 80 µmol/L for SGC-996 cells. | [301] | |
| C6, LN18, LN229, T98G, U87, and HUVECs cells | MTT assay | 0, 20, 40, 80, and 100 µM for 24, 48, and 72 h | Pterostilbene inhibited cell viability on C6, LN18, LN229, T98G, and U87 cells. The IC50 values of pterostilbene treatment for 48 h were 30.10 μM for C6 cells, 22.30 μM for LN18 cells, 37.56 μM for LN229 cells, 32.93 μM for T98G cells, and 46.18 μM for U87 cells. Pterostilbene had a minimal impact on HUVEC cells compared to the previously mentioned cell lines. | [302] | |
| Piceatannol | AGS, SK-MES-1, and J82 cells | MTT assay | 0–100 µg/mL for 72 h | Besides enhancing gemcitabine’s cytotoxic and apoptotic effects, piceatannol actively inhibited SK-MES-1 cell viability. The synergistic combination increased the expression of the Bcl-2 pro-apoptotic protein family. IC50 concentrations for AGS, SK-MES-1, and J82 cells were 10.8 ± 0.7, 7.64 ± 0.5, and 6.7 ± 0.3 µg/mL, respectively. | [303] |
| T24 and HT1376 cells | XTT assay | 0.5, 2.5, 5, and 10 µM for 48 h | Piceatannol showed a dose-dependent inhibitory effect on the proliferation of both T24 and HT1376 cell lines. The IC50 values were 3.9 and 4.6 µM, respectively. | [304] | |
| HL-60 cells | MTT assay | 10–200 µM for 24, 48, and 72 h | Piceatannol significantly inhibited HL-60 cell growth in a time- and dose-dependent manner. A moderate inhibition of HL-60 cells viability was observed after a 72 h treatment with piceatannol at 10, 20, and 50 µM. The highest inhibition was observed after 24, 48, and 72 h treatment with 100–200 µM concentration range. | [305] | |
| WM266-4 and A2058 cells | MTT assay | 0, 1, 10, 20, 40, 100, and 200 µM for 36 h | Both cell lines exhibited decreased viability following piceatannol treatment. The calculated IC50 was 29.4 μM for WM266-4 cells, and 15.6 μM for A2058 cells. | [306] | |
| LNCaP, Du145, and PC3M cells | MTS assay | 1, 5, 10, 25, 50, and 100 µM for 6 days. | All cell lines were susceptible to piceatannol treatment, exhibiting declining cellular activity. The IC50 values obtained were 31.7 μM for LNCaP, 23.2 µM for Du145, and 34.6 μM for PC3M cells. | [307] | |
| B16 cells | MTT assay | 5–400 µM for 24 h | Piceatannol exhibited cytotoxicity effects, resulting in decreased cell viability. The obtained IC50 was 1.53 μM. | [308] | |
| U937 cells | MTT assay | 0–100 µM for 24 h | Exposure to piceatannol inhibited cell viability, with an associated IC50 of 5 µM. | [309] | |
| NCI-H522 cells | WST-8 assay | 10, 30, 50, 80, and 100 µM for 24, 48, and 72 h | Piceatannol treatment notably decreased NCI-H522 cell viability. The IC50 values at each timepoint were 53, 23, and 17 µM, respectively. | [310] | |
| Caco-2 and HCT-116 cells | Crystal violet assay | 12.5, 25, 50, 100, and 200 µM for 24, 48, and 72 h | Piceatannol cytotoxic effects led to a decrease in cell viability fin both cell lines after a 72 h treatment. The obtained IC50 of piceatannol in Caco-2 and HCT-116 cells was 50 µM. | [311] | |
| L1210, K562, and HL-60 cells | Trypan blue dye exclusion | 0–500 µM for 24 h | All cell lines were sensible to piceatannol’s cytotoxic effects. The calculated IC50 values of piceatannol were 50 µmol/L, <10 µmol/L, and <20 µmol/L for K562, HL-60, and L1210 cells, respectively. | [312] | |
| RAW 264.7 cells | MTT assay | 0–50 µg/mL for 48 h | Piceatannol exhibits inhibitory activity, with an associated IC50 value of 5.7 µg/mL. | [313] | |
| HSG, HL-60 HSC-2, and HSC-3, (tumor cell lines) HPC, HGF, and HPLF (normal cells line), | MTT assay (HGF, HPC, HPLF, HSC-2, HSC-3, AND HSG) Trypan blue dye exclusion (HL-60) | 10–1000 μM for 24 h | Piceatannol exhibits greater inhibitory effects on cancer cells compared to normal cells. The IC50 values for cancer cell lines were 63 µM for HSC-2, 232 µM for HSC-3, 373 µM for HSG, and 11 µM for HL-60 cells. In contrast, the IC50 values for normal cells were 367 µM for HGF, 414 µM for HPC, and >1000 µM for HPLF cells. | [314] | |
| SW1990 and PANC-1 cells | CCK-8 assay | 1, 10, 20, 40, 100, and 200 μM for 72 h | Piceatannol inhibited up to 50% cell proliferation for both cell lines. The IC50 value for SW1990 cells was 30.69 µM, while for PANC-1 cells it was 21.82 µM. | [315] | |
| MOLT-4 cells | NRU assay | 0.05, 15, 25, 50, and 100 μM for 48 h | Piceatannol reduced cellular viability with a calculated IC50 of 45.5 µM. | [316] | |
| HeLa cells | MTT assay | 0–250 µM for 48 h | Piceatannol decreased cell viability and the associated IC50 value was 375.20 µM. | [317] | |
| Mouse embryonic stem cells (ESCs) | MTT assay | 1–20 µM for 72 h | High concentrations of piceatannol exhibited cytotoxicity. The obtained IC50 value was 13.5 µM. | [318] | |
| C6 cells (proliferating and growth arrested) | Lowry method | 1–100 µM for 72 h in proliferating cells and 24 h in growth-arrested cells | Piceatannol exhibits cytotoxic effects on both growth-arrested and proliferating cells. The IC50 concentration for growth-arrested cells was 20 ± 2 µM, while for proliferating cells it was 28 ± 4 µM. | [319] | |
| 10ScNCr/23, A-431, RAW 264.7, and CCR-CEM cells | Trypan blue dye exclusion | 0–50 µM for 24 h | Piceatannol exhibits inhibitory effects on all cell lines. The IC50 concentration in RAW 264.7 cells were 1.30 ± 0.12 µM. | [320] | |
| THP-1 cells | Light microscopy | 10, 20, 30, 40, and 50 µM for 48 h | Significant cytotoxic effects with noticeable cell shrinkage were observed at concentrations above 30 µM. | [321] | |
| (-)-ε-viniferin | HSC-2, HSC-3, HCF, HPC, HPLF, HSG, and HL-60 cells | MTT assay in adherent cells Trypan blue dye exclusion in non-adherent cells | 0–1000 µM for 24 h | The four tumor cell lines (HSC-2, HSC-3, HSG, and HL-60) were more sensitive to (-)-ε-viniferin than the remaining normal cell lines. The IC50 values were 42 µM for HSC-2 cells, 84 µM for HSC-3 cells, 111 µM for HCF cells, 146 µM for HPC cells, 94 µM for HPLF cells, 110 µM for HSG cells, and 31 µM for HL-60 cells. | [314] |
| P-388 cells | MTT assay | 0–100 µM for 48 h | ε-viniferin moderately inhibited cell viability in comparison to hopeaphenol, which exhibited a greater effect. The IC50 measured at 18.1 ± 0.7 µM. | [255] | |
| HepG2 and Chang cells | MTT assay | 1.56–200 µg/mL for 72 h | No cytotoxic effect was detected in either cell lines. | [322] | |
| (+)-ε-viniferin | RAW 264.7 cells | MTT assay | 1, 5, and 10 µM for 12 h | Cell viability was significantly reduced to 60% after exposure of 10 µM. IC50 was not determined. | [323] |
| trans-ε-viniferin | K562, L1210, and HCT116 cells | MTT assay | 0–50 µM for 48 h | No cytotoxicity was detected. The IC50 was assumed to be above 50 µM. | [324] |
| AGS, MRC-5, SK-MES-1, and J82 cells | MTT assay | 0–100 µg/mL for 72 h | Cytotoxicity was observed for all cell lines tested. The IC50 values were 42.6 ± 1.7 µM for AGS cells, 49.9 ± 3 µM for MRC-5 cells, 78.8 ± 3.3 µM in SK-MES-1 cells, for 56.7 ± 1.2 µM in J82 cells. | [303] | |
| Mouse primary astrocytes and neurons co-culture | CellTitel 96 ® Aqueous assay | 1, 5, 10, 20, 50, and 100 µM for 24 h | Cell viability was significantly reduced when cells were exposed to concentrations of 50 and 100 µM. | [325] | |
| AGS, COLO 205, HepG2, HL-60, and HT-29 cells | MTT assay | 0–100 µg/mL for 48 h | Dose-dependent cytotoxicity was reported, with a greater effect observed in HL-60 cells. The determined IC50 values were: 9.3 ± 0.3 µM in AGS cells, 85.5 ± 8.1 µM in COLO 205 cells, 7.7 ± 0.2 µM in HepG2 cells, 5.6 ± 1.4 µM in HL-60 cells, and 13.9 ± 0.1 µM in HT-29 cells. | [326] | |
| Hep3B, HepG2, and HH4 cells | Crystal violet assay | 0–200 µM for 24, 48, and 72 h | It was more cytotoxic to Hep3B cells and reduced cell quantity in a dose- and time-dependent manner. Higher amounts were required to cause toxicity in HH4 cells. The IC50 values obtained were the following: - Hep3B cells: 108.1 ± 31.8 µM (24 h), 73.9 ± 17.3 µM (48 h), 63.1 ± 10.8 µM (72 h). - HepG2 cells: 140 ± 39.7 µM (24 h), 103.8 ± 19.2 µM (48 h), 94.8 ± 28.3 µM (72 h). - HH4 cells: >200 µM (24 h), 192.7 ± 21.1 µM (48 h), 177.9 ± 20.5 µM (72 h). | [326] | |
| HepG2 and Caco-2 cells | MTS assay, NRU, and protein content | 0–100 µg/mL for 24 and 48 h | For every endpohint examined, both cell lines showed a time-dependent decline in cell viability. The IC50 values were: - HepG2: 28.28 ± 2.15 µg/mL 24 h and 17.85 ± 3.03 µg/mL for 48 h. - Caco-2 cells: 36.72 ± 3.01 µg/mL for 24 h and 20.63 ± 1.25 µg/mL 48 h. | [327] | |
| trans-ε-viniferin and cis-ε-viniferin | HeLa, MCF-7, C6, HepG2, and HT-29 cells | MTT assay | 0–100 μM for 70 h | Cis- and trans-ε-viniferin to all cell lines, although greater significance was registered for C6 and HeLa cells. The IC50 values for trans-ε-viniferin were: - 20.4 µM in HeLa cells, 44.8 µM in MCF-7 cells, 18.4 µM in C6 cells, 74.3 µM in HepG2 cells, and 88.4 µM in HT-29 cells. The IC50 values for cis-ε-viniferin were: - 21.5 µM in HeLa cells, and 47.2 µM in MCF-7 cells, 20.1 µM in C6 cells, 76.2 µM in HepG2 cells, and 90.2 µM in HT-29 cells. | [328] |
| ε-viniferin | WSU-CLL cells | Trypan blue dye exclusion | 0–100 µM for 24, 48, and 72 h | A concentration- and time-dependent decrease in cell viability was observed, with resveratrol overperforming ε-viniferin. Inhibited cell proliferation was accompanied by a reduction in DNA synthesis. The IC50 value determined at 72 h was 60 µM. | [248] |
| HL-60 cells | MTT assay | 10–200 µM for 24 h | Cell viability decreased in a concentration-dependent manner. The IC50 was 33 µM. | [329] | |
| HepG2 cells | Trypan blue dye exclusion | 30 µM for 24, 48, and 72 h. 1, 5, 10, 30, 60, and 100 µM for 48 h | At 60 µM, ε-viniferin completely blocks cell proliferation. After 48 h, the toxicity potential of ε-viniferin was lower than resveratrol. The IC50 for 48 h was 58.4 µM. | [258] | |
| SW480 cells | Trypan blue dye exclusion and MTT assay | 30 µM for 24, 48, 72, and 96 h in trypan blue dye exclusion. 3, 30, 60, and 100 µM for 48 h in coulter counter | Cells exposed to ε-viniferin grew similarly to the control group, with a reduced growth rate and increasing percentage of cell inhibition. In the MTT assay, no significant inhibition of cell proliferation was recorded. | [275] | |
| VSMCs cells | MTS assay | 10, 20, and 30 µM for 48 h | The potential for arresting cell proliferation rate of ε-viniferin at 20 µM was significantly higher than resveratrol’s at 20 and 30 µM. | [330] | |
| SK-MEL-25 and HT-144 cells | MTT assay Trypan blue dye exclusion | 25–200 µM for 24, 48, and 72 h | Both melanoma lines showed time- and dose-dependent reduction in survival. The IC50 for 48 h was 60 µM. | [249] | |
| C6 cells | WST-1 assay | 95 and 130 µM 12, 24, and 48 h | Proliferation decreased at all doses and times tested in C6 cells. | [253] | |
| Caco-2 cells | MTT and NRU assays | 1.56, 3.12, 6.25, 12.5, 25, 50, and 100 µM for 24 h | At and above 25 µM, cell viability in Caco-2 cells decreased. ε-viniferin was slightly more effective than resveratrol. | [331] | |
| Vascular endothelial cells (VECs) | H2O2-induced cytotoxicity | 10, 20, and 30 µM for 24 h | ε-viniferin effectively protected cells from cytotoxic effects of H2O2. A 24 h pre-treatment with ε-viniferin reduced intracellular ROS. | [332] | |
| VECs | H2O2-induced cytotoxicity | 5 and 10 µM for 24 h | At 10 μM, a pre-treatment with ε-viniferin conferred VEC with resistance against H2O2-induced oxidative stress. | [333] | |
| A2058, A549, HOS, U2OS, and MCF-10A cells | MTT assay | 1–15 μM for 24, 48, and 72 h | ε-viniferin exhibited a time- and dose-dependent decrease in the viability of HOS, U2OS, and A549 cells, but not in A2058 cells. | [334] | |
| ε-viniferin glucoside | PC12 cells | MTT assay | 0–10 µM for 24 h | Cell viability was not significantly altered following the exposure to the stilbene. | [335] |
3.5. Cardiovascular and Metabolic Benefits
3.6. Neuroprotective Effects and Cognitive Function
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| UV | Ultraviolet |
| STS | Stilbene synthase |
| DW | Dry weight |
| MAE | Microwave-assisted extraction |
| UAE | Ultrasound-assisted extraction |
| PSE | Pressurized solvent extraction |
| COX | Cyclooxygenase |
| Aβ | Beta-amyloid |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| SOD | Superoxide dismutase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| GPx | Glutathione peroxidase |
| GST | Glutathione-S-transferase |
| NF-κB | Nuclear factor κB |
| eNOS | Endothelial nitric oxide synthase |
| LDL | Low-density lipoprotein |
| SIRT1 | Sirtuin 1 |
| H2O2 | Hydrogen peroxide |
| TNF-α | Tumor necrosis factor-α |
| AMPK | Adenosine monophosphate-activated protein kinase |
| LPS | Lipopolysaccharide |
| HO-1 | Heme oxygenase-1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| NRU | Neutral red uptake |
| BH4 | Tetrahydrobiopterin |
| cAMP | Cyclic adenosine monophosphate |
| cGMP | Cyclic guanosine monophosphate |
| NDs | Neurodegenerative diseases |
| CCR1 | Chemokine receptor |
| PGE2 | Prostaglandin E2 |
| GSH | Glutathione |
| PTEN | Phosphatase and TENsin homolog |
| PKB | Protein kinase B |
| GSK-3β | Glycogen synthase kinase-3β |
| VEGF | Vascular endothelial growth factor |
| CSC | Cancer stem cell |
| VECs | Vascular endothelial cells |
| PI3K | Phosphoinositide 3-kinase |
| Akt | Protein kinase B |
| HDL | High-density lipoprotein |
References
- International Organisation of Vine and Wine. 2023 World Wine Production—Oiv First Estimates. Available online: https://www.fao.org/3/i7042e/i7042e.pdf (accessed on 11 August 2025).
- OIV. State of the World Vine and Wine Sector in 2023; International Organisation of Vine and Wine: Paris, France, 2023. [Google Scholar]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M.D. Agrochemical characterisation of the solid by-products and residues from the winery and distillery industry. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M.; Arlorio, M.; Coïsson, J.D. Spent grape pomace as a still potential by-product. Int. J. Food Sci. Technol. 2015, 50, 2022–2031. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Broome, J.C.; Warner, K.D. Agro-environmental partnerships facilitate sustainable wine-grape production and assessment. Calif. Agric. 2008, 62, 133–141. [Google Scholar] [CrossRef]
- Zacharof, M.-P. Grape Winery Waste as Feedstock for Bioconversions: Applying the Biorefinery Concept. Waste Biomass Valorization 2017, 8, 1011–1025. [Google Scholar] [CrossRef]
- Prozil, S.O.; Evtuguin, D.V.; Lopes, L.P.C. Chemical composition of grape stalks of Vitis vinifera L. from red grape pomaces. Ind. Crops Prod. 2012, 35, 178–184. [Google Scholar] [CrossRef]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel application and industrial exploitation of winery by-products. Bioresour. Bioprocess. 2018, 5, 46. [Google Scholar] [CrossRef]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current trends and possibilities for exploitation of Grape pomace as a potential source for value addition. Environ. Pollut. 2021, 278, 116796. [Google Scholar] [CrossRef] [PubMed]
- Toma, D.-I.; Baroi, A.M.; Din, A.; Vizitiu, D.E.; Fierascu, I.; Fierascu, R.C. Grapevine Plant Waste Utilization in Nanotechnology. AgroLife Sci. J. 2024, 13, 203–216. [Google Scholar] [CrossRef]
- Wei, M.; Ma, T.; Ge, Q.; Li, C.; Zhang, K.; Fang, Y.; Sun, X. Challenges and opportunities of winter vine pruning for global grape and wine industries. J. Clean. Prod. 2022, 380, 135086. [Google Scholar] [CrossRef]
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable green processing of grape pomace for the production of value-added products: An overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar] [CrossRef]
- Khan, Z.A.; Iqbal, A.; Shahzad, S.A. Synthetic approaches toward stilbenes and their related structures. Mol. Divers. 2017, 21, 483–509. [Google Scholar] [CrossRef] [PubMed]
- Baroi, A.M.; Popitiu, M.; Fierascu, I.; Sărdărescu, I.-D.; Fierascu, R.C. Grapevine Wastes: A Rich Source of Antioxidants and Other Biologically Active Compounds. Antioxidants 2022, 11, 393. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Cuccia, P. Ethics+ economy+ environment= sustainability: Gambero Rosso on the front lines with a new concept of sustainability. Wine Econ. Policy 2015, 4, 69–70. [Google Scholar] [CrossRef]
- Iuga, M.; Mironeasa, S. Potential of grape byproducts as functional ingredients in baked goods and pasta. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2473–2505. [Google Scholar] [CrossRef]
- Contreras, M.d.M.; Romero-García, J.M.; López-Linares, J.C.; Romero, I.; Castro, E. Residues from grapevine and wine production as feedstock for a biorefinery. Food Bioprod. Process. 2022, 134, 56–79. [Google Scholar] [CrossRef]
- Bensid, A.; El Abed, N.; Houicher, A.; Regenstein, J.M.; Özogul, F. Antioxidant and antimicrobial preservatives: Properties, mechanism of action and applications in food—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2985–3001. [Google Scholar] [CrossRef]
- Vilela, A.; Pinto, T. Grape Infusions: Between Nutraceutical and Green Chemistry. Sustain. Chem. 2021, 2, 441–466. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Gallego, R.; Silva, P. The Wine Industry By-Products: Applications for Food Industry and Health Benefits. Antioxidants 2022, 11, 2025. [Google Scholar] [CrossRef]
- Nunes, M.A.; Rodrigues, F.; Oliveira, M.B.P.P. 11—Grape Processing By-Products as Active Ingredients for Cosmetic Proposes. In Handbook of Grape Processing By-Products; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 267–292. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Igrejas, G.; Aires, A.; Falco, V.; Valentão, P.; Poeta, P. Phenolic compounds classification and their distribution in winemaking by-products. Eur. Food Res. Technol. 2023, 249, 207–239. [Google Scholar] [CrossRef]
- Constantin, O.E.; Stoica, F.; Rațu, R.N.; Stănciuc, N.; Bahrim, G.E.; Râpeanu, G. Bioactive Components, Applications, Extractions, and Health Benefits of Winery By-Products from a Circular Bioeconomy Perspective: A Review. Antioxidants 2024, 13, 100. [Google Scholar] [CrossRef]
- Ianni, A.; Di Maio, G.; Pittia, P.; Grotta, L.; Perpetuini, G.; Tofalo, R.; Cichelli, A.; Martino, G. Chemical–nutritional quality and oxidative stability of milk and dairy products obtained from Friesian cows fed with a dietary supplementation of dried grape pomace. J. Sci. Food Agric. 2019, 99, 3635–3643. [Google Scholar] [CrossRef] [PubMed]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C.; Caponio, F. Bioactive Compounds from Vine Shoots, Grape Stalks, and Wine Lees: Their Potential Use in Agro-Food Chains. Foods 2021, 10, 342. [Google Scholar] [CrossRef]
- Ahmad, B.; Yadav, V.; Yadav, A.; Rahman, M.U.; Yuan, W.Z.; Li, Z.; Wang, X. Integrated biorefinery approach to valorize winery waste: A review from waste to energy perspectives. Sci. Total Environ. 2020, 719, 137315. [Google Scholar] [CrossRef]
- Bordiga, M.; Montella, R.; Travaglia, F.; Arlorio, M.; Coïsson, J.D. Characterization of polyphenolic and oligosaccharidic fractions extracted from grape seeds followed by the evaluation of prebiotic activity related to oligosaccharides. Int. J. Food Sci. Technol. 2019, 54, 1283–1291. [Google Scholar] [CrossRef]
- Gabur, G.-D.; Teodosiu, C.; Fighir, D.; Cotea, V.V.; Gabur, I. From Waste to Value in Circular Economy: Valorizing Grape Pomace Waste through Vermicomposting. Agriculture 2024, 14, 1529. [Google Scholar] [CrossRef]
- Vitalini, S.; Gardana, C.; Zanzotto, A.; Simonetti, P.; Faoro, F.; Fico, G.; Iriti, M. The presence of melatonin in grapevine (Vitis vinifera L.) berry tissues. J. Pineal Res. 2011, 51, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Barroso, M.F.; Porto, J.V.; Ramalhosa, M.J.; Švarc-Gajić, J.; Estevinho, L.; Morais, S.; Delerue-Matos, C. Potential of Portuguese vine shoot wastes as natural resources of bioactive compounds. Sci. Total Environ. 2018, 634, 831–842. [Google Scholar] [CrossRef]
- Peña-Portillo, G.-C.; Acuña-Nelson, S.-M.; Bastías-Montes, J.-M. From Waste to Wealth: Exploring the Bioactive Potential of Wine By-Products—A Review. Antioxidants 2024, 13, 992. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. A Reference List of Phenolic Compounds (Including Stilbenes) in Grapevine (Vitis vinifera L.) Roots, Woods, Canes, Stems, and Leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Zwingelstein, M.; Draye, M.; Besombes, J.-L.; Piot, C.; Chatel, G. trans-Resveratrol and trans-ε-Viniferin in Grape Canes and Stocks Originating from Savoie Mont Blanc Vineyard Region: Pre-extraction Parameters for Improved Recovery. ACS Sustain. Chem. Eng. 2019, 7, 8310–8316. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Cheng, L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Barry, T.N.; Forss, D.A. The condensed tannin content of vegetative Lotus pedunculatus, its regulation by fertiliser application, and effect upon protein solubility. J. Sci. Food Agric. 1983, 34, 1047–1056. [Google Scholar] [CrossRef]
- Sies, H. Polyphenols and health: Update and perspectives. Arch. Biochem. Biophys. 2010, 501, 2–5. [Google Scholar] [CrossRef]
- Kampa, M.; Nifli, A.-P.; Notas, G.; Castanas, E. Polyphenols and cancer cell growth. In Reviews of Physiology, Biochemistry and Pharmacology; Amara, S.G., Bamberg, E., Fleischmann, B., Gudermann, T., Hebert, S.C., Jahn, R., Lederer, W.J., Lill, R., Miyajima, A., Offermanns, S., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 79–113. [Google Scholar]
- Guo, W.; Kong, E.; Meydani, M. Dietary Polyphenols, Inflammation, and Cancer. Nutr. Cancer 2009, 61, 807–810. [Google Scholar] [CrossRef]
- Korkina, L.G.; De Luca, C.; Kostyuk, V.A.; Pastore, S. Plant Polyphenols and Tumors: From Mechanisms to Therapies, Prevention, and Protection Against Toxicity of Anti-Cancer Treatments. Curr. Med. Chem. 2009, 16, 3943–3965. [Google Scholar] [CrossRef]
- Zhao, B. Natural Antioxidants Protect Neurons in Alzheimer’s Disease and Parkinson’s Disease. Neurochem. Res. 2009, 34, 630–638. [Google Scholar] [CrossRef]
- Joseph, J.; Cole, G.; Head, E.; Ingram, D. Nutrition, Brain Aging, and Neurodegeneration. J. Neurosci. 2009, 29, 12795–12801. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Merino, C.; Lopez-Sanchez, C.; Lagoa, R.; Samhan-Arias, A.K.; Bueno, C.; Garcia-Martinez, V. Neuroprotective Actions of Flavonoids. Curr. Med. Chem. 2011, 18, 1195–1212. [Google Scholar] [CrossRef]
- Michalska, M.; Gluba, A.; Mikhailidis, D.P.; Nowak, P.; Bielecka-Dabrowa, A.; Rysz, J.; Banach, M. The role of polyphenols in cardiovascular disease. Med. Sci. Monit. 2010, 16, RA110–RA119. [Google Scholar]
- Grassi, D.; Desideri, G.; Croce, G.; Tiberti, S.; Aggio, A.; Ferri, C. Flavonoids, Vascular Function and Cardiovascular Protection. Curr. Pharm. Des. 2009, 15, 1072–1084. [Google Scholar] [CrossRef]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B.; et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Zunino, S.J. Type 2 Diabetes and Glycemic Response to Grapes or Grape Products1,2. J. Nutr. 2009, 139, 1794S–1800S. [Google Scholar] [CrossRef] [PubMed]
- Borriello, A.; Cucciolla, V.; Della Ragione, F.; Galletti, P. Dietary polyphenols: Focus on resveratrol, a promising agent in the prevention of cardiovascular diseases and control of glucose homeostasis. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 618–625. [Google Scholar] [CrossRef]
- Ferrari, C.K.B. Functional foods, herbs and nutraceuticals: Towards biochemical mechanisms of healthy aging. Biogerontology 2004, 5, 275–289. [Google Scholar] [CrossRef]
- Pearson, K.J.; Baur, J.A.; Lewis, K.N.; Peshkin, L.; Price, N.L.; Labinskyy, N.; Swindell, W.R.; Kamara, D.; Minor, R.K.; Perez, E.; et al. Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span. Cell Metab. 2008, 8, 157–168. [Google Scholar] [CrossRef]
- Queen, B.L.; Tollefsbol, T.O. Polyphenols and Aging. Curr. Aging Sci. 2010, 3, 34–42. [Google Scholar] [CrossRef]
- Agouni, A.; Lagrue-Lak-Hal, A.-H.; Mostefai, H.A.; Tesse, A.; Mulder, P.; Rouet, P.; Desmoulin, F.; Heymes, C.; Martínez, M.C.; Andriantsitohaina, R. Red Wine Polyphenols Prevent Metabolic and Cardiovascular Alterations Associated with Obesity in Zucker Fatty Rats (Fa/Fa). PLoS ONE 2009, 4, e5557. [Google Scholar] [CrossRef] [PubMed]
- Cherniack, E.P. Polyphenols: Planting the seeds of treatment for the metabolic syndrome. Nutrition 2011, 27, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Chung, S. Dietary polyphenols, deacetylases and chromatin remodeling in inflammation. Pers. Nutr. 2010, 101, 84–94. [Google Scholar]
- Kostyuk, V.; Potapovich, A.; De Luca, C. The Promise of Plant Polyphenols as the Golden Standard Skin Anti-Inflammatory Agents. Curr. Drug Metab. 2010, 11, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, R.; Iauk, L. Natural polyphenols as anti-inflammatory agents. J. Front. Biosci. 2010, 2, 318–331. [Google Scholar]
- Sáez, V.; Gayoso, C.; Riquelme, S.; Pérez, J.; Vergara, C.; Mardones, C.; von Baer, D. C18 core-shell column with in-series absorbance and fluorescence detection for simultaneous monitoring of changes in stilbenoid and proanthocyanidin concentrations during grape cane storage. J. Chromatogr. B 2018, 1074–1075, 70–78. [Google Scholar] [CrossRef]
- Waffo-Teguo, P.; Krisa, S.; Richard, T.; Mérillon, J.-M. Grapevine Stilbenes and Their Biological Effects. In Bioactive Molecules and Medicinal Plants; Ramawat, K.G., Merillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 25–54. [Google Scholar]
- Mattivi, F.; Vrhovsek, U.; Malacarne, G.; Masuero, D.; Zulini, L.; Stefanini, M.; Moser, C.; Velasco, R.; Guella, G. Profiling of Resveratrol Oligomers, Important Stress Metabolites, Accumulating in the Leaves of Hybrid Vitis vinifera (Merzling × Teroldego) Genotypes Infected with Plasmopara viticola. J. Agric. Food Chem. 2011, 59, 5364–5375. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Liu, Z.P. Natural Products as Anti-Invasive and Anti-Metastatic Agents. Curr. Med. Chem. 2011, 18, 808–829. [Google Scholar] [CrossRef]
- Park, E.-J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2015, 1852, 1071–1113. [Google Scholar] [CrossRef]
- Seyed, M.A.; Jantan, I.; Bukhari, S.N.A.; Vijayaraghavan, K. A Comprehensive Review on the Chemotherapeutic Potential of Piceatannol for Cancer Treatment, with Mechanistic Insights. J. Agric. Food Chem. 2016, 64, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Billet, K.; Houillé, B.; Besseau, S.; Mélin, C.; Oudin, A.; Papon, N.; Courdavault, V.; Clastre, M.; Giglioli-Guivarc’h, N.; Lanoue, A. Mechanical stress rapidly induces E-resveratrol and E-piceatannol biosynthesis in grape canes stored as a freshly-pruned byproduct. Food Chem. 2018, 240, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.F.; Biais, B.; Richard, T.; Puertas, B.; Waffo-Teguo, P.; Merillon, J.-M.; Cantos-Villar, E. Grapevine cane’s waste is a source of bioactive stilbenes. Ind. Crops Prod. 2016, 94, 884–892. [Google Scholar] [CrossRef]
- Navarro-Orcajada, S.; Conesa, I.; Vidal-Sánchez, F.J.; Matencio, A.; Albaladejo-Maricó, L.; García-Carmona, F.; López-Nicolás, J.M. Stilbenes: Characterization, bioactivity, encapsulation and structural modifications. A review of their current limitations and promising approaches. Crit. Rev. Food Sci. Nutr. 2023, 63, 7269–7287. [Google Scholar] [CrossRef]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Bábíková, P.; Vrchotová, N.; Tříska, J.; Kyseláková, M. Content of trans-resveratrol in leaves and berries of interspecific grapevine (Vitis sp.) varieties. Czech J. Food Sci. 2008, 26, S13–S17. [Google Scholar] [CrossRef]
- Rusjan, D.; Halbwirth, H.; Stich, K.; Mikulič-Petkovšek, M.; Veberič, R. Biochemical response of grapevine variety ‘Chardonnay’ (Vitis vinifera L.) to infection with grapevine yellows (Bois noir). Eur. J. Plant Pathol. 2012, 134, 231–237. [Google Scholar] [CrossRef]
- Parage, C.; Tavares, R.; Réty, S.; Baltenweck-Guyot, R.; Poutaraud, A.; Renault, L.; Heintz, D.; Lugan, R.; Marais, G.A.B.; Aubourg, S.; et al. Structural, Functional, and Evolutionary Analysis of the Unusually Large Stilbene Synthase Gene Family in Grapevine. Plant Physiol. 2012, 160, 1407–1419. [Google Scholar] [CrossRef]
- Çetin, E.S.; Altinöz, D.; Tarçan, E.; Göktürk Baydar, N. Chemical composition of grape canes. Ind. Crops Prod. 2011, 34, 994–998. [Google Scholar] [CrossRef]
- Ewald, P.; Delker, U.; Winterhalter, P. Quantification of stilbenoids in grapevine canes and grape cluster stems with a focus on long-term storage effects on stilbenoid concentration in grapevine canes. Food Res. Int. 2017, 100, 326–331. [Google Scholar] [CrossRef]
- Cebrián, C.; Sánchez-Gómez, R.; Salinas, M.R.; Alonso, G.L.; Zalacain, A. Effect of post-pruning vine-shoots storage on the evolution of high-value compounds. Ind. Crops Prod. 2017, 109, 730–736. [Google Scholar] [CrossRef]
- Ben Mohamed, H.; Vadel, A.M.; Geuns, J.M.C.; Khemira, H. Biochemical changes in dormant grapevine shoot tissues in response to chilling: Possible role in dormancy release. Sci. Hortic. 2010, 124, 440–447. [Google Scholar] [CrossRef]
- Aaviksaar, A.; Haga, M.; Pussa, T.; Roasto, M.; Tsoupras, G. Purification of resveratrol from vine stems. Proc.-Est. Acad. Sci. Chem. 2003, 52, 155–164. [Google Scholar] [CrossRef]
- Püssa, T.; Floren, J.; Kuldkepp, P.; Raal, A. Survey of Grapevine Vitis vinifera Stem Polyphenols by Liquid Chromatography−Diode Array Detection−Tandem Mass Spectrometry. J. Agric. Food Chem. 2006, 54, 7488–7494. [Google Scholar] [CrossRef]
- Cruz, S.; Raposo, R.; Ruiz-Moreno, M.J.; Garde-Cerdán, T.; Puertas, B.; Gonzalo-Diago, A.; Moreno-Rojas, J.M.; Cantos-Villar, E. Grapevine-shoot stilbene extract as a preservative in white wine. Food Packag. Shelf Life 2018, 18, 164–172. [Google Scholar] [CrossRef]
- Cebrián-Tarancón, C.; Sánchez-Gómez, R.; Salinas, M.R.; Alonso, G.L.; Oliva, J.; Zalacain, A. Toasted vine-shoot chips as enological additive. Food Chem. 2018, 263, 96–103. [Google Scholar] [CrossRef]
- Rätsep, R.; Karp, K.; Maante-Kuljus, M.; Aluvee, A.; Kaldmäe, H.; Bhat, R. Recovery of Polyphenols from Vineyard Pruning Wastes—Shoots and Cane of Hybrid Grapevine (Vitis sp.) Cultivars. Antioxidants 2021, 10, 1059. [Google Scholar] [CrossRef]
- Pietarinen, S.P.; Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Holmbom, B.R. Knotwood and bark extracts: Strong antioxidants from waste materials. J. Wood Sci. 2006, 52, 436–444. [Google Scholar] [CrossRef]
- García-Pérez, M.-E.; Royer, M.; Herbette, G.; Desjardins, Y.; Pouliot, R.; Stevanovic, T. Picea mariana bark: A new source of trans-resveratrol and other bioactive polyphenols. Food Chem. 2012, 135, 1173–1182. [Google Scholar] [CrossRef]
- Rajbhar, K.; Dawda, H.; Mukundan, U. Polyphenols: Methods of extraction. Sci. Revs. Chem. Commun. 2015, 5, 1–6. [Google Scholar]
- Zwingelstein, M.; Draye, M.; Besombes, J.-L.; Piot, C.; Chatel, G. Viticultural wood waste as a source of polyphenols of interest: Opportunities and perspectives through conventional and emerging extraction methods. Waste Manag. 2020, 102, 782–794. [Google Scholar] [CrossRef]
- Angelov, G.; Boyadzhieva, S.; Georgieva, S. Rosehip extraction: Process optimization and antioxidant capacity of extracts. Open Chem. 2014, 12, 502–508. [Google Scholar] [CrossRef]
- Angelov, G.; Georgieva, S.; Boyadzhieva, S.; Boyadzhiev, L. Optimizing the extraction of globe artichoke wastes. Comptes Rendus De. L’Academie Bulg. Des. Sci. 2015, 68, 1235–1240. [Google Scholar]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Carvalho, T.M.J. Extraction of Raw Plant Material Using Supercritical Carbon Dioxide. Master’s Thesis, Warsaw University of Technology, Warsaw, Poland, 2016. [Google Scholar]
- Mahindrakar, K.V.; Rathod, V.K. Chapter 5—Ultrasound-assisted extraction of lipids, carotenoids, and other compounds from marine resources. In Innovative and Emerging Technologies in the Bio-Marine Food Sector; Garcia-Vaquero, M., Rajauria, G., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 81–128. [Google Scholar]
- Šimat, V.; Frleta, R.; Čagalj, M.; Skroza, D. Technological and Analytical Aspects of Bioactive Compounds and Nutraceuticals from Marine Algae. In Bioactive Compounds and Nutraceuticals from Dairy, Marine, and Nonconventional Sources; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 159–196. [Google Scholar]
- Maddaloni, M.; Vassalini, I.; Alessandri, I. Green Routes for the Development of Chitin/Chitosan Sustainable Hydrogels. Sustain. Chem. 2020, 1, 325–344. [Google Scholar] [CrossRef]
- Schnee, S.; Queiroz, E.F.; Voinesco, F.; Marcourt, L.; Dubuis, P.-H.; Wolfender, J.-L.; Gindro, K. Vitis vinifera Canes, a New Source of Antifungal Compounds against Plasmopara viticola, Erysiphe necator, and Botrytis cinerea. J. Agric. Food Chem. 2013, 61, 5459–5467. [Google Scholar] [CrossRef]
- Gabaston, J.; Leborgne, C.; Waffo-Teguo, P.; Valls, J.; Palos Pinto, A.; Richard, T.; Cluzet, S.; Mérillon, J.-M. Wood and roots of major grapevine cultivars and rootstocks: A comparative analysis of stilbenes by UHPLC-DAD-MS/MS and NMR. Phytochem. Anal. 2019, 30, 320–331. [Google Scholar] [CrossRef]
- Bavaresco, L.; Fregoni, C. Physiological Role and Molecular Aspects of Grapevine Stilbenic Compounds. In Molecular Biology & Biotechnology of the Grapevine; Roubelakis-Angelakis, K.A., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 153–182. [Google Scholar]
- El Khawand, T.; Courtois, A.; Valls, J.; Richard, T.; Krisa, S. A review of dietary stilbenes: Sources and bioavailability. Phytochem. Rev. 2018, 17, 1007–1029. [Google Scholar] [CrossRef]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef]
- Marier, J.-F.; Vachon, P.; Gritsas, A.; Zhang, J.; Moreau, J.-P.; Ducharme, M.P. Metabolism and Disposition of Resveratrol in Rats: Extent of Absorption, Glucuronidation, and Enterohepatic Recirculation Evidenced by a Linked-Rat Model. J. Pharmacol. Exp. Ther. 2002, 302, 369–373. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Lin, H.-S.; Ho, P.C.; Ng, K.-Y. The Impact of Aqueous Solubility and Dose on the Pharmacokinetic Profiles of Resveratrol. Pharm. Res. 2008, 25, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Teplova, V.V.; Isakova, E.P.; Klein, O.I.; Dergachova, D.I.; Gessler, N.N.; Deryabina, Y.I. Natural Polyphenols: Biological Activity, Pharmacological Potential, Means of Metabolic Engineering (Review). Appl. Biochem. Microbiol. 2018, 54, 221–237. [Google Scholar] [CrossRef]
- Schouten, A.; Wagemakers, L.; Stefanato, F.L.; Kaaij, R.M.v.d.; Kan, J.A.L.v. Resveratrol acts as a natural profungicide and induces self-intoxication by a specific laccase. Mol. Microbiol. 2002, 43, 883–894. [Google Scholar] [CrossRef]
- Seppänen, S.K.; Syrjälä, L.; von Weissenberg, K.; Teeri, T.H.; Paajanen, L.; Pappinen, A. Antifungal activity of stilbenes in in vitro bioassays and in transgenic Populus expressing a gene encoding pinosylvin synthase. Plant Cell Rep. 2004, 22, 584–593. [Google Scholar] [CrossRef]
- Liu, Q.; Yeo, W.S.; Bae, T. The SaeRS Two-Component System of Staphylococcus aureus. Genes. 2016, 7, 81. [Google Scholar] [CrossRef]
- Duan, J.; Li, M.; Hao, Z.; Shen, X.; Liu, L.; Jin, Y.; Wang, S.; Guo, Y.; Yang, L.; Wang, L.; et al. Subinhibitory concentrations of resveratrol reduce alpha-hemolysin production in Staphylococcus aureus isolates by downregulating saeRS. Emerg. Microbes Infect. 2018, 7, 1–10. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Kattke, M.D.; Gosschalk, J.E.; Martinez, O.E.; Kumar, G.; Gale, R.T.; Cascio, D.; Sawaya, M.R.; Philips, M.; Brown, E.D.; Clubb, R.T. Structure and mechanism of TagA, a novel membrane-associated glycosyltransferase that produces wall teichoic acids in pathogenic bacteria. PLoS Pathog. 2019, 15, e1007723. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm Formation Mechanisms and Targets for Developing Antibiofilm Agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Ramadan, H.H. Chronic rhinosinusitis and bacterial biofilms. Curr. Opin. Otolaryngol. Head. Neck Surg. 2006, 14, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-B.; Lai, H.-C.; Hsueh, P.-R.; Chiou, R.Y.-Y.; Lin, S.-B.; Liaw, S.-J. Inhibition of swarming and virulence factor expression in Proteus mirabilis by resveratrol. J. Med. Microbiol. 2006, 55, 1313–1321. [Google Scholar] [CrossRef]
- Fernández-Alvarez, A.; Llorente-Izquierdo, C.; Mayoral, R.; Agra, N.; Boscá, L.; Casado, M.; Martín-Sanz, P. Evaluation of epigenetic modulation of cyclooxygenase-2 as a prognostic marker for hepatocellular carcinoma. Oncogenesis 2012, 1, e23. [Google Scholar] [CrossRef]
- Akinwumi, B.C.; Bordun, K.-A.M.; Anderson, H.D. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [PubMed]
- Remsberg, C.M.; Yáñez, J.A.; Ohgami, Y.; Vega-Villa, K.R.; Rimando, A.M.; Davies, N.M. Pharmacometrics of pterostilbene: Preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytother. Res. 2008, 22, 169–179. [Google Scholar] [CrossRef]
- Kim, J.; Min, J.S.; Kim, D.; Zheng, Y.F.; Mailar, K.; Choi, W.J.; Lee, C.; Bae, S.K. A simple and sensitive liquid chromatography–tandem mass spectrometry method for trans-ε-viniferin quantification in mouse plasma and its application to a pharmacokinetic study in mice. J. Pharm. Biomed. Anal. 2017, 134, 116–121. [Google Scholar] [CrossRef]
- Santucci, C.; Mignozzi, S.; Levi, F.; Malvezzi, M.; Boffetta, P.; Negri, E.; La Vecchia, C. European cancer mortality predictions for the year 2025 with focus on breast cancer. Ann. Oncol. 2025, 36, 460–468. [Google Scholar] [CrossRef]
- Pereira, M.; Peleteiro, B.; Capewell, S.; Bennett, K.; Azevedo, A.; Lunet, N. Changing patterns of cardiovascular diseases and cancer mortality in Portugal, 1980–2010. BMC Public Health 2012, 12, 1126. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J. Clin. 2012, 62, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.J.; Shin, S.H.; Lee, H.J.; Lee, K.W. Polyphenols as small molecular inhibitors of signaling cascades in carcinogenesis. Pharmacol. Ther. 2011, 130, 310–324. [Google Scholar] [CrossRef]
- Mena, S.; Ortega, A.; Estrela, J.M. Oxidative stress in environmental-induced carcinogenesis. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2009, 674, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ronghe, A.; Singh, B.; Bhat, N.K.; Chen, J.; Bhat, H.K. Natural Antioxidants Exhibit Chemopreventive Characteristics through the Regulation of CNC b-Zip Transcription Factors in Estrogen-Induced Breast Carcinogenesis. J. Biochem. Mol. Toxicol. 2014, 28, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Sirerol, J.A.; Feddi, F.; Mena, S.; Rodriguez, M.L.; Sirera, P.; Aupí, M.; Pérez, S.; Asensi, M.; Ortega, A.; Estrela, J.M. Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis. Free Radic. Biol. Med. 2015, 85, 1–11. [Google Scholar] [CrossRef]
- Alfaras, I.; Juan, M.E.; Planas, J.M. trans-Resveratrol Reduces Precancerous Colonic Lesions in Dimethylhydrazine-Treated Rats. J. Agric. Food Chem. 2010, 58, 8104–8110. [Google Scholar] [CrossRef]
- Azorín-Ortuño, M.; Yáñez-Gascón, M.J.; Vallejo, F.; Pallarés, F.J.; Larrosa, M.; Lucas, R.; Morales, J.C.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Metabolites and tissue distribution of resveratrol in the pig. Mol. Nutr. Food Res. 2011, 55, 1154–1168. [Google Scholar] [CrossRef]
- Roubille, C.; Martel-Pelletier, J.; Davy, J.-M.; Haraoui, B.; Pelletier, J.-P. Cardiovascular adverse effects of anti-inflammatory drugs. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2013, 12, 55–67. [Google Scholar] [CrossRef]
- Levy, G.N. Prostaglandin H synthases, nonsteroidal antiinflammatory drugs, and colon cancer. FASEB J. 1997, 11, 234–247. [Google Scholar] [CrossRef]
- Kitasato, A.; Kuroki, T.; Adachi, T.; Ono, S.; Tanaka, T.; Tsuneoka, N.; Hirabaru, M.; Takatsuki, M.; Eguchi, S. A Selective Cyclooxygenase-2 Inhibitor (Etodolac) Prevents Spontaneous Biliary Tumorigenesis in a Hamster Bilioenterostomy Model. Eur. Surg. Res. 2014, 52, 73–82. [Google Scholar] [CrossRef]
- Cianciulli, A.; Calvello, R.; Cavallo, P.; Dragone, T.; Carofiglio, V.; Panaro, M.A. Modulation of NF-κB activation by resveratrol in LPS treated human intestinal cells results in downregulation of PGE2 production and COX-2 expression. Toxicol. Vitr. 2012, 26, 1122–1128. [Google Scholar] [CrossRef]
- Schwartz, S.A.; Hernandez, A.; Mark Evers, B. The role of NF-κB/IκB proteins in cancer: Implications for novel treatment strategies. Surg. Oncol. 1999, 8, 143–153. [Google Scholar] [CrossRef]
- Potter, G.A.; Patterson, L.H.; Wanogho, E.; Perry, P.J.; Butler, P.C.; Ijaz, T.; Ruparelia, K.C.; Lamb, J.H.; Farmer, P.B.; Stanley, L.A.; et al. The cancer preventative agent resveratrol is converted to the anticancer agent piceatannol by the cytochrome P450 enzyme CYP1B1. Br. J. Cancer 2002, 86, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.H. Role of Phytostilbenes in Decay and Disease Resistance. Annu. Rev. Phytopathol. 1981, 19, 437–458. [Google Scholar] [CrossRef]
- Adrian, M.; Jeandet, P. Effects of resveratrol on the ultrastructure of Botrytis cinerea conidia and biological significance in plant/pathogen interactions. Fitoterapia 2012, 83, 1345–1350. [Google Scholar] [CrossRef]
- Collado-González, M.; Guirao-Abad, J.P.; Sánchez-Fresneda, R.; Belchí-Navarro, S.; Argüelles, J.-C. Resveratrol lacks antifungal activity against Candida albicans. World J. Microbiol. Biotechnol. 2012, 28, 2441–2446. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Douillet-Breuil, A.-C.; Bessis, R.; Debord, S.; Sbaghi, M.; Adrian, M. Phytoalexins from the Vitaceae: Biosynthesis, Phytoalexin Gene Expression in Transgenic Plants, Antifungal Activity, and Metabolism. J. Agric. Food Chem. 2002, 50, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, J.-H.; Ryu, S.Y.; Cho, M.H.; Lee, J. Stilbenes Reduce Staphylococcus aureus Hemolysis, Biofilm Formation, and Virulence. Foodborne Pathog. Dis. 2014, 11, 710–717. [Google Scholar] [CrossRef]
- Beaumont, P.; Courtois, A.; Atgié, C.; Richard, T.; Krisa, S. In the shadow of resveratrol: Biological activities of epsilon-viniferin. J. Physiol. Biochem. 2022, 78, 465–484. [Google Scholar] [CrossRef]
- Cho, H.S.; Lee, J.-H.; Ryu, S.Y.; Joo, S.W.; Cho, M.H.; Lee, J. Inhibition of Pseudomonas aeruginosa and Escherichia coli O157:H7 Biofilm Formation by Plant Metabolite ε-Viniferin. J. Agric. Food Chem. 2013, 61, 7120–7126. [Google Scholar] [CrossRef]
- Frazzi, R.; Valli, R.; Tamagnini, I.; Casali, B.; Latruffe, N.; Merli, F. Resveratrol-mediated apoptosis of hodgkin lymphoma cells involves SIRT1 inhibition and FOXO3a hyperacetylation. Int. J. Cancer 2013, 132, 1013–1021. [Google Scholar] [CrossRef]
- Shankar, S.; Chen, Q.; Siddiqui, I.; Sarva, K.; Srivastava, R.K. Sensitization of TRAIL-resistant LNCaP cells by resveratrol (3, 4′, 5 tri-hydroxystilbene): Molecular mechanisms and therapeutic potential. J. Mol. Signal. 2007, 2, 7. [Google Scholar] [CrossRef]
- van Ginkel, P.R.; Yan, M.B.; Bhattacharya, S.; Polans, A.S.; Kenealey, J.D. Natural products induce a G protein-mediated calcium pathway activating p53 in cancer cells. Toxicol. Appl. Pharmacol. 2015, 288, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-S.; Yue, B.-D.; Ho, P.C. Determination of pterostilbene in rat plasma by a simple HPLC-UV method and its application in pre-clinical pharmacokinetic study. Biomed. Chromatogr. 2009, 23, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Nigam, M.; Ranjan, V.; Sharma, R.; Balapure, A.K.; Rath, S.K. Caspase Mediated Enhanced Apoptotic Action of Cyclophosphamide- and Resveratrol-Treated MCF-7 Cells. J. Pharmacol. Sci. 2009, 109, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Kai, L.; Samuel, S.K.; Levenson, A.S. Resveratrol enhances p53 acetylation and apoptosis in prostate cancer by inhibiting MTA1/NuRD complex. Int. J. Cancer 2010, 126, 1538–1548. [Google Scholar] [CrossRef]
- Rimando, A.M.; Cuendet, M.; Desmarchelier, C.; Mehta, R.G.; Pezzuto, J.M.; Duke, S.O. Cancer Chemopreventive and Antioxidant Activities of Pterostilbene, a Naturally Occurring Analogue of Resveratrol. J. Agric. Food Chem. 2002, 50, 3453–3457. [Google Scholar] [CrossRef]
- Stivala, L.A.; Savio, M.; Carafoli, F.; Perucca, P.; Bianchi, L.; Maga, G.; Forti, L.; Pagnoni, U.M.; Albini, A.; Prosperi, E.; et al. Specific Structural Determinants Are Responsible for the Antioxidant Activity and the Cell Cycle Effects of Resveratrol. J. Biol. Chem. 2001, 276, 22586–22594. [Google Scholar] [CrossRef]
- McCormack, D.; McFadden, D. Pterostilbene and Cancer: Current Review. J. Surg. Res. 2012, 173, e53–e61. [Google Scholar] [CrossRef]
- McCormack, D.E.; Mannal, P.; McDonald, D.; Tighe, S.; Hanson, J.; McFadden, D. Genomic Analysis of Pterostilbene Predicts Its Antiproliferative Effects Against Pancreatic Cancer In Vitro and In Vivo. J. Gastrointest. Surg. 2012, 16, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, P.; Asensi, M.; Segarra, R.; Ortega, A.; Benlloch, M.; Obrador, E.; Varea, M.T.; Asensio, G.; Jordá, L.; Estrela, J.M. Association between Pterostilbene and Quercetin Inhibits Metastatic Activity of B16 Melanoma. Neoplasia 2005, 7, 37–47. [Google Scholar] [CrossRef]
- Tolomeo, M.; Grimaudo, S.; Cristina, A.D.; Roberti, M.; Pizzirani, D.; Meli, M.; Dusonchet, L.; Gebbia, N.; Abbadessa, V.; Crosta, L.; et al. Pterostilbene and 3′-hydroxypterostilbene are effective apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia cells. Int. J. Biochem. Cell Biol. 2005, 37, 1709–1726. [Google Scholar] [CrossRef]
- Alosi, J.A.; McDonald, D.E.; Schneider, J.S.; Privette, A.R.; McFadden, D.W. Pterostilbene Inhibits Breast Cancer In Vitro Through Mitochondrial Depolarization and Induction of Caspase-Dependent Apoptosis1. J. Surg. Res. 2010, 161, 195–201. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, L.; Wang, X.; Zhang, J.; Han, W.; Feng, L.; Sun, J.; Jin, H.; Wang, X.J. Pterostilbene simultaneously induces apoptosis, cell cycle arrest and cyto-protective autophagy in breast cancer cells. Am. J. Transl. Res. 2012, 4, 44. [Google Scholar]
- Moon, D.; McCormack, D.; McDonald, D.; McFadden, D. Pterostilbene induces mitochondrially derived apoptosis in breast cancer cells in vitro. J. Surg. Res. 2013, 180, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-J.; Tsai, S.-J.; Ho, C.-T.; Pan, M.-H.; Ho, Y.-S.; Wu, C.-H.; Wang, Y.-J. Chemopreventive Effects of Pterostilbene on Urethane-Induced Lung Carcinogenesis in Mice via the Inhibition of EGFR-Mediated Pathways and the Induction of Apoptosis and Autophagy. J. Agric. Food Chem. 2012, 60, 11533–11541. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Gupta, N.; Ghosh, K.; Roy, P. In vitro evaluation of the cytotoxic, anti-proliferative and anti-oxidant properties of pterostilbene isolated from Pterocarpus marsupium. Toxicol. Vitr. 2010, 24, 1215–1228. [Google Scholar] [CrossRef]
- Chiou, Y.-S.; Tsai, M.-L.; Wang, Y.-J.; Cheng, A.-C.; Lai, W.-M.; Badmaev, V.; Ho, C.-T.; Pan, M.-H. Pterostilbene Inhibits Colorectal Aberrant Crypt Foci (ACF) and Colon Carcinogenesis via Suppression of Multiple Signal Transduction Pathways in Azoxymethane-Treated Mice. J. Agric. Food Chem. 2010, 58, 8833–8841. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Rimando, A.M.; Lee, H.J.; Ji, Y.; Reddy, B.S.; Suh, N. Anti-inflammatory Action of Pterostilbene Is Mediated through the p38 Mitogen-Activated Protein Kinase Pathway in Colon Cancer Cells. Cancer Prev. Res. 2009, 2, 650–657. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Reinisalo, M.; Kårlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol Stilbenes: Molecular Mechanisms of Defence against Oxidative Stress and Aging-Related Diseases. Oxidative Med. Cell. Longev. 2015, 2015, 340520. [Google Scholar] [CrossRef]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Wilson, B.J.; Tremblay, A.M.; Deblois, G.v.; Sylvain-Drolet, G.; Giguère, V. An Acetylation Switch Modulates the Transcriptional Activity of Estrogen-Related Receptor α. Mol. Endocrinol. 2010, 24, 1349–1358. [Google Scholar] [CrossRef]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Resveratrol as a Novel Anti-Herpes Simplex Virus Nutraceutical Agent: An Overview. Viruses 2018, 10, 473. [Google Scholar] [CrossRef]
- Kolouchová, I.; Maťátková, O.; Paldrychová, M.; Kodeš, Z.; Kvasničková, E.; Sigler, K.; Čejková, A.; Šmidrkal, J.; Demnerová, K.; Masák, J. Resveratrol, pterostilbene, and baicalein: Plant-derived anti-biofilm agents. Folia Microbiol. 2018, 63, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Martelli, G.; Giacomini, D. Antibacterial and antioxidant activities for natural and synthetic dual-active compounds. Eur. J. Med. Chem. 2018, 158, 91–105. [Google Scholar] [CrossRef]
- Kumar, S.N.; Nambisan, B. Antifungal Activity of Diketopiperazines and Stilbenes Against Plant Pathogenic Fungi In Vitro. Appl. Biochem. Biotechnol. 2014, 172, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Xiong, B.; Qiu, S. Progress of Antimicrobial Mechanisms of Stilbenoids. Pharmaceutics 2024, 16, 663. [Google Scholar] [CrossRef]
- Mattio, L.M.; Dallavalle, S.; Musso, L.; Filardi, R.; Franzetti, L.; Pellegrino, L.; D’Incecco, P.; Mora, D.; Pinto, A.; Arioli, S. Antimicrobial activity of resveratrol-derived monomers and dimers against foodborne pathogens. Sci. Rep. 2019, 9, 19525. [Google Scholar] [CrossRef] [PubMed]
- Gerits, E.; Defraine, V.; Vandamme, K.; Cremer, K.D.; Brucker, K.D.; Thevissen, K.; Cammue, B.P.A.; Beullens, S.; Fauvart, M.; Verstraeten, N.; et al. Repurposing Toremifene for Treatment of Oral Bacterial Infections. Antimicrob. Agents Chemother. 2017, 61, 10.1128/aac.01846-01816. [Google Scholar] [CrossRef]
- Lim, Y.R.I.; Preshaw, P.M.; Lim, L.P.; Ong, M.M.A.; Lin, H.-S.; Tan, K.S. Pterostilbene complexed with cyclodextrin exerts antimicrobial and anti-inflammatory effects. Sci. Rep. 2020, 10, 9072. [Google Scholar] [CrossRef]
- Yang, B.; Yao, H.; Li, D.; Liu, Z. The phosphatidylglycerol phosphate synthase PgsA utilizes a trifurcated amphipathic cavity for catalysis at the membrane-cytosol interface. Curr. Res. Struct. Biol. 2021, 3, 312–323. [Google Scholar] [CrossRef]
- Hrast, M.; Rožman, K.; Ogris, I.; Škedelj, V.; Patin, D.; Sova, M.; Barreteau, H.; Gobec, S.; Grdadolnik, S.G.; Zega, A. Evaluation of the published kinase inhibitor set to identify multiple inhibitors of bacterial ATP-dependent mur ligases. J. Enzym. Inhib. Med. Chem. 2019, 34, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-Z.; Cheng, A.-X.; Sun, L.-M.; Lou, H.-X. Effect of plagiochin E, an antifungal macrocyclic bis(bibenzyl), on cell wall chitin synthesis in Candida albicans. Acta Pharmacol. Sin. 2008, 29, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guo, X.; Jiang, G.; Wu, G.; Miao, H.; Liu, K.; Chen, S.; Sakamoto, N.; Kuno, T.; Yao, F.; et al. NADPH-Cytochrome P450 Reductase Ccr1 Is a Target of Tamoxifen and Participates in Its Antifungal Activity via Regulating Cell Wall Integrity in Fission Yeast. Antimicrob. Agents Chemother. 2020, 64, 10.1128/aac.00079-00020. [Google Scholar] [CrossRef]
- Park, H.B.; Crawford, J.M. Lumiquinone A, an α-Aminomalonate-Derived Aminobenzoquinone from Photorhabdus luminescens. J. Nat. Prod. 2015, 78, 1437–1441. [Google Scholar] [CrossRef]
- Houillé, B.; Papon, N.; Boudesocque, L.; Bourdeaud, E.; Besseau, S.; Courdavault, V.; Enguehard-Gueiffier, C.; Delanoue, G.; Guérin, L.; Bouchara, J.-P.; et al. Antifungal Activity of Resveratrol Derivatives against Candida Species. J. Nat. Prod. 2014, 77, 1658–1662. [Google Scholar] [CrossRef]
- Li, D.-D.; Zhao, L.-X.; Mylonakis, E.; Hu, G.-H.; Zou, Y.; Huang, T.-K.; Yan, L.; Wang, Y.; Jiang, Y.-Y. In Vitro and In Vivo Activities of Pterostilbene against Candida albicans Biofilms. Antimicrob. Agents Chemother. 2014, 58, 2344–2355. [Google Scholar] [CrossRef]
- De Filippis, B.; Ammazzalorso, A.; Amoroso, R.; Giampietro, L. Stilbene derivatives as new perspective in antifungal medicinal chemistry. Drug Dev. Res. 2019, 80, 285–293. [Google Scholar] [CrossRef]
- Mora-Pale, M.; Bhan, N.; Masuko, S.; James, P.; Wood, J.; McCallum, S.; Linhardt, R.J.; Dordick, J.S.; Koffas, M.A.G. Antimicrobial mechanism of resveratrol-trans-dihydrodimer produced from peroxidase-catalyzed oxidation of resveratrol. Biotechnol. Bioeng. 2015, 112, 2417–2428. [Google Scholar] [CrossRef]
- Kim, S.; Lee, D.G. Oxyresveratrol-induced DNA cleavage triggers apoptotic response in Candida albicans. Microbiology 2018, 164, 1112–1121. [Google Scholar] [CrossRef]
- Lee, W.; Lee, D.G. Resveratrol induces membrane and DNA disruption via pro-oxidant activity against Salmonella typhimurium. Biochem. Biophys. Res. Commun. 2017, 489, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Lee, J.; Lee, D.G. Novel Antifungal Mechanism of Resveratrol: Apoptosis Inducer in Candida albicans. Curr. Microbiol. 2015, 70, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Dadi, P.K.; Ahmad, M.; Ahmad, Z. Inhibition of ATPase activity of Escherichia coli ATP synthase by polyphenols. Int. J. Biol. Macromol. 2009, 45, 72–79. [Google Scholar] [CrossRef]
- Hwang, D.; Lim, Y.-H. Resveratrol antibacterial activity against Escherichia coli is mediated by Z-ring formation inhibition via suppression of FtsZ expression. Sci. Rep. 2015, 5, 10029. [Google Scholar] [CrossRef] [PubMed]
- Barrows, J.M.; Goley, E.D. FtsZ dynamics in bacterial division: What, how, and why? Curr. Opin. Cell Biol. 2021, 68, 163–172. [Google Scholar] [CrossRef]
- Lou, F.; Wang, K.; Hou, Y.; Shang, X.; Tang, F. Inhibitory effect of resveratrol on swimming motility and adhesion ability against Salmonella enterica serovar Typhimurium infection. Microb. Pathog. 2023, 184, 106323. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Wallis, C.M.; Rogers, E.E.; Burbank, L.P. Grapevine phenolic compounds influence cell surface adhesion of Xylella fastidiosa and bind to lipopolysaccharide. PLoS ONE 2020, 15, e0240101. [Google Scholar] [CrossRef]
- Kugaji, M.S.; Kumbar, V.M.; Peram, M.R.; Patil, S.; Bhat, K.G.; Diwan, P.V. Effect of Resveratrol on biofilm formation and virulence factor gene expression of Porphyromonas gingivalis in periodontal disease. APMIS 2019, 127, 187–195. [Google Scholar] [CrossRef]
- Uppuluri, P.; Chaturvedi, A.K.; Srinivasan, A.; Banerjee, M.; Ramasubramaniam, A.K.; Köhler, J.R.; Kadosh, D.; Lopez-Ribot, J.L. Dispersion as an Important Step in the Candida albicans Biofilm Developmental Cycle. PLoS Pathog. 2010, 6, e1000828. [Google Scholar] [CrossRef]
- Rosman, C.W.K.; van der Mei, H.C.; Sjollema, J. Influence of sub-inhibitory concentrations of antimicrobials on micrococcal nuclease and biofilm formation in Staphylococcus aureus. Sci. Rep. 2021, 11, 13241. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Jones, S.B. Cancer in the developing world: A call to action. BMJ 1999, 319, 505–508. [Google Scholar] [CrossRef]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef]
- Malhotra, A.; Nair, P.; Dhawan, D.K. Study to Evaluate Molecular Mechanics behind Synergistic Chemo-Preventive Effects of Curcumin and Resveratrol during Lung Carcinogenesis. PLoS ONE 2014, 9, e93820. [Google Scholar] [CrossRef] [PubMed]
- Mazué, F.; Delmas, D.; Murillo, G.; Saleiro, D.; Limagne, E.; Latruffe, N. Differential protective effects of red wine polyphenol extracts (RWEs) on colon carcinogenesis. Food Funct. 2014, 5, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Back, J.H.; Zhu, Y.; Calabro, A.; Queenan, C.; Kim, A.S.; Arbesman, J.; Kim, A.L. Resveratrol-Mediated Downregulation of Rictor Attenuates Autophagic Process and Suppresses UV-Induced Skin Carcinogenesis. Photochem. Photobiol. 2012, 88, 1165–1172. [Google Scholar] [CrossRef]
- Hsieh, T.-c.; Yang, C.-J.; Lin, C.-Y.; Lee, Y.-S.; Wu, J.M. Control of stability of cyclin D1 by quinone reductase 2 in CWR22Rv1 prostate cancer cells. Carcinogenesis 2012, 33, 670–677. [Google Scholar] [CrossRef]
- Lin, C.; Crawford, D.R.; Lin, S.; Hwang, J.; Sebuyira, A.; Meng, R.; Westfall, J.E.; Tang, H.-Y.; Lin, S.; Yu, P.-Y.; et al. Inducible COX-2-dependent apoptosis in human ovarian cancer cells. Carcinogenesis 2011, 32, 19–26. [Google Scholar] [CrossRef]
- Rajasekaran, D.; Elavarasan, J.; Sivalingam, M.; Ganapathy, E.; Kumar, A.; Kalpana, K.; Sakthisekaran, D. Resveratrol interferes with N-nitrosodiethylamine-induced hepatocellular carcinoma at early and advanced stages in male Wistar rats. Mol. Med. Rep. 2011, 4, 1211–1217. [Google Scholar]
- Shrotriya, S.; Tyagi, A.; Deep, G.; Orlicky, D.J.; Wisell, J.; Wang, X.-J.; Sclafani, R.A.; Agarwal, R.; Agarwal, C. Grape seed extract and resveratrol prevent 4-nitroquinoline 1-oxide induced oral tumorigenesis in mice by modulating AMPK activation and associated biological responses. Mol. Carcinog. 2015, 54, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Youn, Y.-K.; Hong, M.-K.; Kim, L.S. Antiproliferation and Redifferentiation in Thyroid Cancer Cell Lines by Polyphenol Phytochemicals. JKMS 2011, 26, 893–899. [Google Scholar] [CrossRef]
- Tsan, M.-f.; White, J.E.; Maheshwari, J.G.; Chikkappa, G. Anti-leukemia Effect of Resveratrol. Leuk. Lymphoma 2002, 43, 983–987. [Google Scholar] [CrossRef]
- Temraz, S.; Mukherji, D.; Shamseddine, A. Potential Targets for Colorectal Cancer Prevention. Int. J. Mol. Sci. 2013, 14, 17279–17303. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; DuBois, R.N. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 2010, 29, 781–788. [Google Scholar] [CrossRef]
- Mosalpuria, K.; Hall, C.; Krishnamurthy, S.; Lodhi, A.; Hallman, D.M.; Baraniuk, M.S.; Bhattacharyya, A.; Lucci, A. Cyclooxygenase-2 expression in non-metastatic triple-negative breast cancer patients. Mol. Clin. Oncol. 2014, 2, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Guo, X.-Z.; Li, H.-Y.; Zhao, J.-J.; Shao, X.-D.; Wu, C.-Y. Prognostic significance of cyclooxygenase-2 protein in pancreatic cancer: A meta-analysis. Tumor Biol. 2014, 35, 10301–10307. [Google Scholar] [CrossRef]
- Jiao, J.; Ishikawa, T.-O.; Dumlao, D.S.; Norris, P.C.; Magyar, C.E.; Mikulec, C.; Catapang, A.; Dennis, E.A.; Fischer, S.M.; Herschman, H.R. Targeted Deletion and Lipidomic Analysis Identify Epithelial Cell COX-2 as a Major Driver of Chemically Induced Skin Cancer. Mol. Cancer Res. 2014, 12, 1677–1688. [Google Scholar] [CrossRef]
- Marnett, L.J. Aspirin and the potential role of prostaglandins in colon cancer. Cancer Res. 1992, 52, 5575. [Google Scholar]
- Yi, C.-O.; Jeon, B.T.; Shin, H.J.; Jeong, E.A.; Chang, K.C.; Lee, J.E.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; et al. Resveratrol activates AMPK and suppresses LPS-induced NF-κB-dependent COX-2 activation in RAW 264.7 macrophage cells. ACB 2011, 44, 194–203. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, C.; Tang, J.; Wang, C.; Wu, P.; Zhang, G.; Liu, W.; Jamangulova, N.; Wu, X.; Song, X. Resveratrol reduces morphine tolerance by inhibiting microglial activation via AMPK signalling. Eur. J. Pain 2014, 18, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- GÜROCAK, Ş.; Karabulut, E.; KARADAĞ SOYLU, N.; ÖZGÖR, D.; Ozkeles, N.; Karabulut, A. Preventive effects of resveratrol against azoxymethane induced damage in rat liver. Asian Pac. J. Cancer Prev. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Akca, H.; Demiray, A.; Aslan, M.; Acikbas, I.; Tokgun, O. Tumour suppressor PTEN enhanced enzyme activity of GPx, SOD and catalase by suppression of PI3K/AKT pathway in non-small cell lung cancer cell lines. J. Enzym. Inhib. Med. Chem. 2013, 28, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Mena, S.; Rodríguez, M.L.; Ponsoda, X.; Estrela, J.M.; Jäättela, M.; Ortega, A.L. Pterostilbene-Induced Tumor Cytotoxicity: A Lysosomal Membrane Permeabilization-Dependent Mechanism. PLoS ONE 2012, 7, e44524. [Google Scholar] [CrossRef]
- Casanova, F.; Quarti, J.; da Costa, D.C.F.; Ramos, C.A.; da Silva, J.L.; Fialho, E. Resveratrol chemosensitizes breast cancer cells to melphalan by cell cycle arrest. J. Cell. Biochem. 2012, 113, 2586–2596. [Google Scholar] [CrossRef]
- Yu, X.-D.; Yang, J.-l.; Zhang, W.-L.; Liu, D.-X. Resveratrol inhibits oral squamous cell carcinoma through induction of apoptosis and G2/M phase cell cycle arrest. Tumor Biol. 2016, 37, 2871–2877. [Google Scholar] [CrossRef]
- Gokbulut, A.A.; Apohan, E.; Baran, Y. Resveratrol and quercetin-induced apoptosis of human 232B4 chronic lymphocytic leukemia cells by activation of caspase-3 and cell cycle arrest. Hematology 2013, 18, 144–150. [Google Scholar] [CrossRef] [PubMed]
- McCormack, D.; McFadden, D. A Review of Pterostilbene Antioxidant Activity and Disease Modification. Oxidative Med. Cell. Longev. 2013, 2013, 575482. [Google Scholar] [CrossRef]
- Schneider, J.G.; Alosi, J.A.; McDonald, D.E.; McFadden, D.W. Pterostilbene Inhibits Lung Cancer Through Induction of Apoptosis1. J. Surg. Res. 2010, 161, 18–22. [Google Scholar] [CrossRef]
- Suh, N.; Paul, S.; Hao, X.; Simi, B.; Xiao, H.; Rimando, A.M.; Reddy, B.S. Pterostilbene, an Active Constituent of Blueberries, Suppresses Aberrant Crypt Foci Formation in the Azoxymethane-Induced Colon Carcinogenesis Model in Rats. Clin. Cancer Res. 2007, 13, 350–355. [Google Scholar] [CrossRef]
- Cichocki, M.; Paluszczak, J.; Szaefer, H.; Piechowiak, A.; Rimando, A.M.; Baer-Dubowska, W. Pterostilbene is equally potent as resveratrol in inhibiting 12-O-tetradecanoylphorbol-13-acetate activated NFκB, AP-1, COX-2, and iNOS in mouse epidermis. Mol. Nutr. Food Res. 2008, 52, S62–S70. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Kumar, A.; Rimando, A.M.; Zhang, X.; Levenson, A.S. Resveratrol and pterostilbene epigenetically restore PTEN expression by targeting oncomiRs of the miR-17 family in prostate cancer. Oncotarget 2015, 6, 27214. [Google Scholar] [CrossRef]
- Paul, S.; DeCastro, A.J.; Lee, H.J.; Smolarek, A.K.; So, J.Y.; Simi, B.; Wang, C.X.; Zhou, R.; Rimando, A.M.; Suh, N. Dietary intake of pterostilbene, a constituent of blueberries, inhibits the β-catenin/p65 downstream signaling pathway and colon carcinogenesis in rats. Carcinogenesis 2010, 31, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- McCormack, D.; Schneider, J.; McDonald, D.; McFadden, D. The antiproliferative effects of pterostilbene on breast cancer in vitro are via inhibition of constitutive and leptin-induced Janus kinase/signal transducer and activator of transcription activation. Am. J. Surg. 2011, 202, 541–544. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Wu, Y.; Lv, C.; Li, X.; Cao, X.; Yang, M.; Feng, D.; Luo, Z. Pterostilbene exerts antitumor activity against human osteosarcoma cells by inhibiting the JAK2/STAT3 signaling pathway. Toxicology 2013, 304, 120–131. [Google Scholar] [CrossRef]
- Chen, R.-J.; Ho, C.-T.; Wang, Y.-J. Pterostilbene induces autophagy and apoptosis in sensitive and chemoresistant human bladder cancer cells. Mol. Nutr. Food Res. 2010, 54, 1819–1832. [Google Scholar] [CrossRef]
- Chakraborty, A.; Bodipati, N.; Demonacos, M.K.; Peddinti, R.; Ghosh, K.; Roy, P. Long term induction by pterostilbene results in autophagy and cellular differentiation in MCF-7 cells via ROS dependent pathway. Mol. Cell. Endocrinol. 2012, 355, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Magee, J.A.; Piskounova, E.; Morrison, S.J. Cancer Stem Cells: Impact, Heterogeneity, and Uncertainty. Cancer Cell 2012, 21, 283–296. [Google Scholar] [CrossRef]
- Nguyen, L.V.; Vanner, R.; Dirks, P.; Eaves, C.J. Cancer stem cells: An evolving concept. Nat. Rev. Cancer 2012, 12, 133–143. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer Stem Cells: Current Status and Evolving Complexities. Cell Stem Cell 2012, 10, 717–728. [Google Scholar] [CrossRef]
- Hagiwara, K.; Kosaka, N.; Yoshioka, Y.; Takahashi, R.-u.; Takeshita, F.; Ochiya, T. Stilbene derivatives promote Ago2-dependent tumour-suppressive microRNA activity. Sci. Rep. 2012, 2, 314. [Google Scholar] [CrossRef]
- Mak, K.-K.; Wu, A.T.H.; Lee, W.-H.; Chang, T.-C.; Chiou, J.-F.; Wang, L.-S.; Wu, C.-H.; Huang, C.-Y.F.; Shieh, Y.-S.; Chao, T.-Y.; et al. Pterostilbene, a bioactive component of blueberries, suppresses the generation of breast cancer stem cells within tumor microenvironment and metastasis via modulating NF-κB/microRNA 448 circuit. Mol. Nutr. Food Res. 2013, 57, 1123–1134. [Google Scholar] [CrossRef]
- Lee, C.-M.; Su, Y.-H.; Huynh, T.-T.; Lee, W.-H.; Chiou, J.-F.; Lin, Y.-K.; Hsiao, M.; Wu, C.-H.; Lin, Y.-F.; Wu, A.T.H.; et al. BlueBerry Isolate, Pterostilbene, Functions as a Potential Anticancer Stem Cell Agent in Suppressing Irradiation-Mediated Enrichment of Hepatoma Stem Cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 258425. [Google Scholar] [CrossRef]
- Hsieh, T.-C.; Bennett, D.J.; Lee, Y.-S.; Wu, E.; Wu, J.M. In Silico and Biochemical Analyses Identify Quinone Reductase 2 as a Target of Piceatannol. Curr. Med. Chem. 2013, 20, 4195–4202. [Google Scholar] [CrossRef] [PubMed]
- Murias, M.; Jäger, W.; Handler, N.; Erker, T.; Horvath, Z.; Szekeres, T.; Nohl, H.; Gille, L. Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: Structure–activity relationship. Biochem. Pharmacol. 2005, 69, 903–912. [Google Scholar] [CrossRef]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res./Rev. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.S.; Bhat, S.H.; Hadi, S.M. Resveratrol–Cu(II) induced DNA breakage in human peripheral lymphocytes: Implications for anticancer properties. FEBS Lett. 2005, 579, 3131–3135. [Google Scholar] [CrossRef] [PubMed]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxidative Med. Cell. Longev. 2016, 2016, 3128951. [Google Scholar] [CrossRef] [PubMed]
- Ashikawa, K.; Majumdar, S.; Banerjee, S.; Bharti, A.C.; Shishodia, S.; Aggarwal, B.B. Piceatannol Inhibits TNF-Induced NF-κB Activation and NF-κB-Mediated Gene Expression Through Suppression of IκBα Kinase and p65 Phosphorylation1. J. Immunol. 2002, 169, 6490–6497. [Google Scholar] [CrossRef]
- Islam, S.; Hassan, F.; Mu, M.M.; Ito, H.; Koide, N.; Mori, I.; Yoshida, T.; Yokochi, T. Piceatannol Prevents Lipopolysaccharide (LPS)-Induced Nitric Oxide (NO) Production and Nuclear Factor (NF)-κB Activation by Inhibiting IκB Kinase (IKK). Microbiol. Immunol. 2004, 48, 729–736. [Google Scholar] [CrossRef]
- Jin, C.-Y.; Moon, D.-O.; Lee, K.-J.; Kim, M.-O.; Lee, J.-D.; Choi, Y.H.; Park, Y.-M.; Kim, G.-Y. Piceatannol attenuates lipopolysaccharide-induced NF-κB activation and NF-κB-related proinflammatory mediators in BV2 microglia. Pharmacol. Res. 2006, 54, 461–467. [Google Scholar] [CrossRef]
- Xueyan, R.; Jia, Y.; Xuefeng, Y.; Lidan, T.; Qingjun, K. Isolation and purification of five phenolic compounds from the Xinjiang wine grape (Vitis Vinifera) and determination of their antioxidant mechanism at cellular level. Eur. Food Res. Technol. 2018, 244, 1569–1579. [Google Scholar] [CrossRef]
- Sharma, A.; Boise, L.H.; Shanmugam, M. Cancer Metabolism and the Evasion of Apoptotic Cell Death. Cancers 2019, 11, 1144. [Google Scholar] [CrossRef]
- Barjot, C.; Tournaire, M.; Castagnino, C.; Vigor, C.; Vercauteren, J.; Rossi, J.-F. Evaluation of antitumor effects of two vine stalk oligomers of resveratrol on a panel of lymphoid and myeloid cell lines: Comparison with resveratrol. Life Sci. 2007, 81, 1565–1574. [Google Scholar] [CrossRef]
- Billard, C.; Izard, J.-C.; Roman, V.; Kern, C.; Mathiot, C.; Mentz, F.; Kolb, J.-P. Comparative Antiproliferative and Apoptotic Effects of Resveratrol, ϵ-viniferin and Vine-shots Derived Polyphenols (Vineatrols) on Chronic B Lymphocytic Leukemia Cells and Normal Human Lymphocytes. Leuk. Lymphoma 2002, 43, 1991–2002. [Google Scholar] [CrossRef]
- Nivelle, L.; Aires, V.; Rioult, D.; Martiny, L.; Tarpin, M.; Delmas, D. Molecular analysis of differential antiproliferative activity of resveratrol, epsilon viniferin and labruscol on melanoma cells and normal dermal cells. Food Chem. Toxicol. 2018, 116, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, F.; Akalın, G.; Şen, M.; Önder, N.I.; Işcan, A.; Kutlu, H.M.; Incesu, Z. Towards Novel Anti-tumor Strategies for Hepatic Cancer: ɛ-Viniferin in Combination with Vincristine Displays Pharmacodynamic Synergy at Lower Doses in HepG2 Cells. OMICS J. Integr. Biol. 2014, 18, 324–334. [Google Scholar] [CrossRef]
- Özdemir, F.; İncesu, Z.; Şena, M.; Öndera, N.İ.; Dikme, M. Implications of Enhanced Effectiveness of Vincristine Sulfate/ε-Viniferin Combination Compared to Vincristine Sulfate Only on HepG2 Cells. Dicle Tıp Derg. 2016, 43, 534–541. [Google Scholar]
- Tarhan, S.; Özdemir, F.; İncesu, Z.; Demirkan, E.S. Direct and protective effects of single or combined addition of vincristine and ε-viniferin on human HepG2 cellular oxidative stress markers in vitro. Cytotechnology 2016, 68, 1081–1094. [Google Scholar] [CrossRef]
- Özdemir, F.; Apaydın, E.; Önder, N.İ.; Şen, M.; Ayrım, A.; Öğünç, Y.; İncesu, Z. Apoptotic effects of ε-viniferin in combination with cis-platin in C6 cells. Cytotechnology 2018, 70, 1061–1073. [Google Scholar] [CrossRef]
- Ito, T.; Akao, Y.; Yi, H.; Ohguchi, K.; Matsumoto, K.; Tanaka, T.; Iinuma, M.; Nozawa, Y. Antitumor effect of resveratrol oligomers against human cancer cell lines and the molecular mechanism of apoptosis induced by vaticanol C. Carcinogenesis 2003, 24, 1489–1497. [Google Scholar] [CrossRef]
- Muhtadi; Hakim, E.H.; Juliawaty, L.D.; Syah, Y.M.; Achmad, S.A.; Latip, J.; Ghisalberti, E.L. Cytotoxic resveratrol oligomers from the tree bark of Dipterocarpus hasseltii. Fitoterapia 2006, 77, 550–555. [Google Scholar] [CrossRef]
- Nur Ainaa, A.; Norizan Ahmat, N.A.; Mashita Abdullah, M.A.; Norrizah Jaafar Sidik, N.J.S.; Syed Ahmad, T. Antioxidant, antimicrobial and cytotoxic activities of resveratrol oligomers of Shorea macroptera dyer. Aust. J. Basic. Appl. Sci. 2012, 6, 431–436. [Google Scholar]
- Wibowo, A.; Ahmat, N.; Hamzah, A.S.; Latif, F.A.; Norrizah, J.S.; Khong, H.Y.; Takayama, H. Identification and biological activity of secondary metabolites from Dryobalanops beccarii. Phytochem. Lett. 2014, 9, 117–122. [Google Scholar] [CrossRef]
- Colin, D.; Lancon, A.; Delmas, D.; Lizard, G.; Abrossinow, J.; Kahn, E.; Jannin, B.; Latruffe, N. Antiproliferative activities of resveratrol and related compounds in human hepatocyte derived HepG2 cells are associated with biochemical cell disturbance revealed by fluorescence analyses. Biochimie 2008, 90, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Aja, I.; Ruiz-Larrea, M.B.; Courtois, A.; Krisa, S.; Richard, T.; Ruiz-Sanz, J.-I. Screening of Natural Stilbene Oligomers from Vitis vinifera for Anticancer Activity on Human Hepatocellular Carcinoma Cells. Antioxidants 2020, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef]
- Pintea, A.; Rugină, D.; Pop, R.; Bunea, A.; Socaciu, C.; Diehl, H.A. Antioxidant Effect of Trans-Resveratrol in Cultured Human Retinal Pigment Epithelial Cells. J. Ocul. Pharmacol. Ther. 2011, 27, 315–321. [Google Scholar] [CrossRef]
- Ksila, M.; Vejux, A.; Prost-Camus, E.; Durand, P.; Ghzaiel, I.; Nury, T.; Duprey, D.; Meziane, S.; Masmoudi-Kouki, O.; Latruffe, N.; et al. Cytotoxic and Antioxidant Activities of Imine Analogs of Trans-Resveratrol towards Murine Neuronal N2a Cells. Molecules 2022, 27, 4713. [Google Scholar] [CrossRef]
- Almeida, T.C.; Seibert, J.B.; Almeida, S.H.d.S.; Amparo, T.R.; Teixeira, L.F.d.M.; Barichello, J.M.; Postacchini, B.B.; Santos, O.D.H.d.; Silva, G.N.d. Polymeric micelles containing resveratrol: Development, characterization, cytotoxicity on tumor cells and antimicrobial activity. Braz. J. Pharm. Sci. 2020, 56, e18411. [Google Scholar] [CrossRef]
- Selvaraj, S.; Mohan, A.; Narayanan, S.; Sethuraman, S.; Krishnan, U.M. Dose-Dependent Interaction of trans-Resveratrol with Biomembranes: Effects on Antioxidant Property. J. Med. Chem. 2013, 56, 970–981. [Google Scholar] [CrossRef]
- Khayoon, H.A.; Al-Rekabi, F.M. Cytotoxic effect of resveratrol on colorectal cancer cell line. Iraqi J. Vet. Med. 2020, 44, 68–74. [Google Scholar] [CrossRef]
- Osman, A.-M.M.; Bayoumi, H.M.; Al-Harthi, S.E.; Damanhouri, Z.A.; ElShal, M.F. Modulation of doxorubicin cytotoxicity by resveratrol in a human breast cancer cell line. Cancer Cell Int. 2012, 12, 47. [Google Scholar] [CrossRef]
- Jeon, D.; Jo, M.; Lee, Y.; Park, S.-H.; Phan, H.T.L.; Nam, J.H.; Namkung, W. Inhibition of ANO1 by Cis- and Trans-Resveratrol and Their Anticancer Activity in Human Prostate Cancer PC-3 Cells. Int. J. Mol. Sci. 2023, 24, 1186. [Google Scholar] [CrossRef]
- Fernández-Pérez, F.; Belchí-Navarro, S.; Almagro, L.; Bru, R.; Pedreño, M.A.; Gómez-Ros, L.V. Cytotoxic Effect of Natural trans-Resveratrol Obtained from Elicited Vitis vinifera Cell Cultures on Three Cancer Cell Lines. Plant Foods Hum. Nutr. 2012, 67, 422–429. [Google Scholar] [CrossRef]
- Anisimova, N.Y.U.; Kiselevsky, M.V.; Sosnov, A.V.; Sadovnikov, S.V.; Stankov, I.N.; Gakh, A.A. Trans-, cis-, and dihydro-resveratrol: A comparative study. Chem. Cent. J. 2011, 5, 88. [Google Scholar] [CrossRef]
- Siddiqui, M.; Saquib, Q.; Ahamed, M.; Ahmad, J.; Al-Khedhairy, A.; Abou-Tarboush, F.; Musarrat, J. Effect of trans-resveratrol on rotenone-induced cytotoxicity in human breast adenocarcinoma cells. Toxicol. Int. 2011, 18, 105. [Google Scholar]
- Kocsis, Z.; Marcsek, Z.L.; Jakab, M.G.; Szende, B.; Tompa, A. Chemopreventive properties of trans—Resveratrol against the cytotoxicity of chloroacetanilide herbicides in vitro. Int. J. Hyg. Environ. Health 2005, 208, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Kashyap, M.P.; Kumar, V.; Al-Khedhairy, A.A.; Musarrat, J.; Pant, A.B. Protective potential of trans-resveratrol against 4-hydroxynonenal induced damage in PC12 cells. Toxicol. Vitr. 2010, 24, 1592–1598. [Google Scholar] [CrossRef]
- Leischner, C.; Burkard, M.; Michel, A.; Berchtold, S.; Niessner, H.; Marongiu, L.; Busch, C.; Frank, J.; Lauer, U.M.; Venturelli, S. Comparative Analysis of the Antitumor Activity of Cis- and Trans-Resveratrol in Human Cancer Cells with Different p53 Status. Molecules 2021, 26, 5586. [Google Scholar] [CrossRef] [PubMed]
- Miksits, M.; Wlcek, K.; Svoboda, M.; Kunert, O.; Haslinger, E.; Thalhammer, T.; Szekeres, T.; Jäger, W. Antitumor activity of resveratrol and its sulfated metabolites against human breast cancer cells. Planta Medica 2009, 75, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Marel, A.-K.; Lizard, G.; Izard, J.-C.; Latruffe, N.; Delmas, D. Inhibitory effects of trans-resveratrol analogs molecules on the proliferation and the cell cycle progression of human colon tumoral cells. Mol. Nutr. Food Res. 2008, 52, 538–548. [Google Scholar] [CrossRef]
- Filomeni, G.; Graziani, I.; Rotilio, G.; Ciriolo, M.R. trans-Resveratrol induces apoptosis in human breast cancer cells MCF-7 by the activation of MAP kinases pathways. Genes. Nutr. 2007, 2, 295–305. [Google Scholar] [CrossRef]
- Nashine, S.; Nesburn, A.B.; Kuppermann, B.D.; Kenney, M.C. Role of Resveratrol in Transmitochondrial AMD RPE Cells. Nutrients 2020, 12, 159. [Google Scholar] [CrossRef]
- El-Melegy, M.G.; Eltaher, H.M.; Gaballah, A.; El-Kamel, A.H. Enhanced oral permeability of Trans-Resveratrol using nanocochleates for boosting anticancer efficacy; in-vitro and ex-vivo appraisal. Eur. J. Pharm. Biopharm. 2021, 168, 166–183. [Google Scholar] [CrossRef]
- Tsang, S.W.; Zhang, H.; Lin, Z.; Mu, H.; Bian, Z.-X. Anti-fibrotic effect of trans-resveratrol on pancreatic stellate cells. Biomed. Pharmacother. 2015, 71, 91–97. [Google Scholar] [CrossRef]
- dos Santos Moreno, C.; Rogero, S.O.; Ikeda, T.I.; Cruz, Á.S.; Rogero, J.R. Resveratrol and radiation biological effects. Int. J. Nutrology 2012, 5, 28–33. [Google Scholar] [CrossRef]
- Lucas, I.K.; Kolodziej, H. Trans-resveratrol induces apoptosis through ROS-triggered mitochondria-dependent pathways in A549 human lung adenocarcinoma epithelial cells. Planta Medica 2015, 81, 1038–1044. [Google Scholar] [CrossRef]
- Mannal, P.; McDonald, D.; McFadden, D. Pterostilbene and tamoxifen show an additive effect against breast cancer in vitro. Am. J. Surg. 2010, 200, 577–580. [Google Scholar] [CrossRef]
- Elsherbini, A.M.; Sheweita, S.A.; Sultan, A.S. Pterostilbene as a Phytochemical Compound Induces Signaling Pathways Involved in the Apoptosis and Death of Mutant P53-Breast Cancer Cell Lines. Nutr. Cancer 2021, 73, 1976–1984. [Google Scholar] [CrossRef]
- Wakimoto, R.; Ono, M.; Takeshima, M.; Higuchi, T.; Nakano, S. Differential Anticancer Activity of Pterostilbene Against Three Subtypes of Human Breast Cancer Cells. Anticancer Res. 2017, 37, 6153–6159. [Google Scholar]
- Shin, H.J.; Han, J.M.; Choi, Y.S.; Jung, H.J. Pterostilbene Suppresses both Cancer Cells and Cancer Stem-Like Cells in Cervical Cancer with Superior Bioavailability to Resveratrol. Molecules 2020, 25, 228. [Google Scholar] [CrossRef]
- Chatterjee, K.; Mukherjee, S.; Vanmanen, J.; Banerjee, P.; Fata, J.E. Dietary Polyphenols, Resveratrol and Pterostilbene Exhibit Antitumor Activity on an HPV E6-Positive Cervical Cancer Model: An in vitro and in vivo Analysis. Front. Oncol. 2019, 9, 352. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Sun, C.; Chen, X.; Han, L.; Wang, T.; Liu, J.; Chen, X.; Zhao, D. Effect of Pterostilbene, a Natural Derivative of Resveratrol, in the Treatment of Colorectal Cancer through Top1/Tdp1-Mediated DNA Repair Pathway. Cancers 2021, 13, 4002. [Google Scholar] [CrossRef] [PubMed]
- Wawszczyk, J.; Jesse, K.; Smolik, S.; Kapral, M. Mechanism of Pterostilbene-Induced Cell Death in HT-29 Colon Cancer Cells. Molecules 2022, 27, 369. [Google Scholar] [CrossRef]
- Hsiao, Y.-H.; Chen, N.-C.; Koh, Y.-C.; Nagabhushanam, K.; Ho, C.-T.; Pan, M.-H. Pterostilbene Inhibits Adipocyte Conditioned-Medium-Induced Colorectal Cancer Cell Migration through Targeting FABP5-Related Signaling Pathway. J. Agric. Food Chem. 2019, 67, 10321–10329. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Lowe, G.; Roberts, C.M.; Finlay, J.; Han, E.S.; Glackin, C.A.; Dellinger, T.H. Pterostilbene, a natural phenolic compound, synergizes the antineoplastic effects of megestrol acetate in endometrial cancer. Sci. Rep. 2017, 7, 12754. [Google Scholar] [CrossRef]
- Wen, W.; Lowe, G.; Roberts, C.M.; Finlay, J.; Han, E.S.; Glackin, C.A.; Dellinger, T.H. Pterostilbene Suppresses Ovarian Cancer Growth via Induction of Apoptosis and Blockade of Cell Cycle Progression Involving Inhibition of the STAT3 Pathway. Int. J. Mol. Sci. 2018, 19, 1983. [Google Scholar] [CrossRef]
- Lin, V.C.-H.; Tsai, Y.-C.; Lin, J.-N.; Fan, L.-L.; Pan, M.-H.; Ho, C.-T.; Wu, J.-Y.; Way, T.-D. Activation of AMPK by Pterostilbene Suppresses Lipogenesis and Cell-Cycle Progression in p53 Positive and Negative Human Prostate Cancer Cells. J. Agric. Food Chem. 2012, 60, 6399–6407. [Google Scholar] [CrossRef]
- Mannal, P.W.; Alosi, J.A.; Schneider, J.G.; McDonald, D.E.; McFadden, D.W. Pterostilbene Inhibits Pancreatic Cancer In Vitro. J. Gastrointest. Surg. 2010, 14, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Bracht, J.W.P.; Karachaliou, N.; Berenguer, J.; Pedraz-Valdunciel, C.; Filipska, M.; Codony-Servat, C.; Codony-Servat, J.; Rosell, R. Osimertinib and pterostilbene in EGFR-mutation-positive non-small cell lung cancer (NSCLC). Int. J. Biol. Sci. 2019, 15, 2607–2614. [Google Scholar] [CrossRef]
- Guo, L.; Tan, K.; Wang, H.; Zhang, X. Pterostilbene inhibits hepatocellular carcinoma through p53/SOD2/ROS-mediated mitochondrial apoptosis. Oncol. Rep. 2016, 36, 3233–3240. [Google Scholar] [CrossRef]
- Mori, S.; Kishi, S.; Honoki, K.; Fujiwara-Tani, R.; Moriguchi, T.; Sasaki, T.; Fujii, K.; Tsukamoto, S.; Fujii, H.; Kido, A.; et al. Anti-Stem Cell Property of Pterostilbene in Gastrointestinal Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9347. [Google Scholar] [CrossRef]
- Chang, H.-P.; Lu, C.-C.; Chiang, J.-H.; Tsai, F.-J.; Juan, Y.-N.; Tsao, J.-W.; Chiu, H.-Y.; Yang, J.-S. Pterostilbene modulates the suppression of multidrug resistance protein 1 and triggers autophagic and apoptotic mechanisms in cisplatin-resistant human oral cancer CAR cells via AKT signaling. Int. J. Oncol. 2018, 52, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.; El-Kamel, A.H.; Sheta, E.; El-Habashy, S.E. Chondroitin/Lactoferrin-dual functionalized pterostilbene-solid lipid nanoparticles as targeted breast cancer therapy. Int. J. Pharm. 2023, 642, 123163. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-J.; Lyu, Y.-J.; Chen, Y.-Y.; Lee, Y.-C.; Pan, M.-H.; Ho, Y.-S.; Wang, Y.-J. Chloroquine Potentiates the Anticancer Effect of Pterostilbene on Pancreatic Cancer by Inhibiting Autophagy and Downregulating the RAGE/STAT3 Pathway. Molecules 2021, 26, 6741. [Google Scholar] [CrossRef]
- Kumar, V.; Haldar, S.; Das, N.S.; Ghosh, S.; Dhankhar, P.; Sircar, D.; Roy, P. Pterostilbene-isothiocyanate inhibits breast cancer metastasis by selectively blocking IKK-β/NEMO interaction in cancer cells. Biochem. Pharmacol. 2021, 192, 114717. [Google Scholar] [CrossRef]
- Tong, C.; Wang, Y.; Li, J.; Cen, W.; Zhang, W.; Zhu, Z.; Yu, J.; Lu, B. Pterostilbene inhibits gallbladder cancer progression by suppressing the PI3K/Akt pathway. Sci. Rep. 2021, 11, 4391. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Liu, Z.; Xu, W.; Wang, Q.; Zhang, C.; Ding, Y.; Nie, W.; Lai, J.; Chen, Y.; Huang, H. Pterostilbene promotes mitochondrial apoptosis and inhibits proliferation in glioma cells. Sci. Rep. 2021, 11, 6381. [Google Scholar] [CrossRef]
- Sáez, V.; Pastene, E.; Vergara, C.; Mardones, C.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; Gómez, M.V.; Theoduloz, C.; Riquelme, S.; von Baer, D. Oligostilbenoids in Vitis vinifera L. Pinot Noir grape cane extract: Isolation, characterization, in vitro antioxidant capacity and anti-proliferative effect on cancer cells. Food Chem. 2018, 265, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.-L.; Hsu, Y.-L. The grape and wine constituent piceatannol inhibits proliferation of human bladder cancer cells via blocking cell cycle progression and inducing Fas/membrane bound Fas ligand-mediated apoptotic pathway. Mol. Nutr. Food Res. 2008, 52, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Haza, A.I. Selective apoptotic effects of piceatannol and myricetin in human cancer cells. J. Appl. Toxicol. 2012, 32, 986–993. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Z.; Gao, T. Piceatannol induced apoptosis through up-regulation of microRNA-181a in melanoma cells. Biol. Res. 2017, 50, 36. [Google Scholar] [CrossRef]
- Dias, S.J.; Li, K.; Rimando, A.M.; Dhar, S.; Mizuno, C.S.; Penman, A.D.; Levenson, A.S. Trimethoxy-Resveratrol and Piceatannol Administered Orally Suppress and Inhibit Tumor Formation and Growth in Prostate Cancer Xenografts. Prostate 2013, 73, 1135–1146. [Google Scholar] [CrossRef]
- Yokozawa, T.; Kim, Y.J. Piceatannol Inhibits Melanogenesis by Its Antioxidative Actions. Biol. Pharm. Bull. 2007, 30, 2007–2011. [Google Scholar] [CrossRef]
- Liu, W.-H.; Chang, L.-S. Piceatannol induces Fas and FasL up-regulation in human leukemia U937 cells via Ca2+/p38α MAPK-mediated activation of c-Jun and ATF-2 pathways. Int. J. Biochem. Cell Biol. 2010, 42, 1498–1506. [Google Scholar] [CrossRef]
- Takasawa, R.; Akahane, H.; Tanaka, H.; Shimada, N.; Yamamoto, T.; Uchida-Maruki, H.; Sai, M.; Yoshimori, A.; Tanuma, S.-i. Piceatannol, a natural trans-stilbene compound, inhibits human glyoxalase I. Bioorganic Med. Chem. Lett. 2017, 27, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Wolter, F.; Clausnitzer, A.; Akoglu, B.; Stein, J. Piceatannol, a Natural Analog of Resveratrol, Inhibits Progression through the S Phase of the Cell Cycle in Colorectal Cancer Cell Lines. J. Nutr. 2002, 132, 298–302. [Google Scholar] [CrossRef]
- Ovesná, Z.; Kozics, K.; Bader, Y.; Saiko, P.; Handler, N.; Erker, T.; Szekeres, T. Antioxidant activity of resveratrol, piceatannol and 3,3′,4,4′,5,5′-hexahydroxy-trans-stilbene in three leukemia cell lines. Oncol. Rep. 2006, 16, 617–624. [Google Scholar] [CrossRef]
- Duarte, N.; Kayser, O.; Abreu, P.; Ferreira, M.-J.U. Antileishmanial activity of piceatannol isolated from Euphorbia lagascae seeds. Phytother. Res. 2008, 22, 455–457. [Google Scholar] [CrossRef]
- Chowdhury, S.A.; Kishino, K.; Satoh, R.; Hashimoto, K.; Kikuchi, H.; Nishikawa, H.; Shirataki, Y.; Sakagami, H. Tumor-specificity and Apoptosis-inducing Activity of Stilbenes and Flavonoids. Anticancer Res. 2005, 25, 2055–2063. [Google Scholar]
- Zhang, Y.; Gu, Y.; Xie, J.; Hu, Y. Anti-tumor effect of piceatannol through induction of cell apoptosis via up-regulation of microRNA-125b expression on pancreatic cancer. Int. J. Clin. Exp. Med. 2017, 10, 14495–14502. [Google Scholar]
- Siedlecka-Kroplewska, K.; Ślebioda, T.; Kmieć, Z. Induction of autophagy, apoptosis and aquisition of resistance in response to piceatannol toxicity in MOLT-4 human leukemia cells. Toxicol. Vitr. 2019, 59, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, M.; Guo, Q.; Liu, Y.; Zhao, Y.; Wu, Y.; Sun, B.; Wang, Q.; Liu, J.; Han, J. Investigation of binary and ternary systems of human serum albumin with oxyresveratrol/piceatannol and/or mitoxantrone by multipectroscopy, molecular docking and cytotoxicity evaluation. J. Mol. Liq. 2020, 311, 113364. [Google Scholar] [CrossRef]
- Arai, D.; Kataoka, R.; Otsuka, S.; Kawamura, M.; Maruki-Uchida, H.; Sai, M.; Ito, T.; Nakao, Y. Piceatannol is superior to resveratrol in promoting neural stem cell differentiation into astrocytes. Food Funct. 2016, 7, 4432–4441. [Google Scholar] [CrossRef]
- Rüweler, M.; Gülden, M.; Maser, E.; Murias, M.; Seibert, H. Cytotoxic, cytoprotective and antioxidant activities of resveratrol and analogues in C6 astroglioma cells in vitro. Chem.-Biol. Interact. 2009, 182, 128–135. [Google Scholar] [CrossRef]
- Billack, B.; Radkar, V.; Adiabouah, C. In Vitro evaluation of the cytotoxic and anti-proliferative properties of resveratrol and several of its analogs. Cell. Mol. Biol. Lett. 2008, 13, 553–569. [Google Scholar] [CrossRef]
- Kang, C.-H.; Moon, D.-O.; Choi, Y.H.; Choi, I.-W.; Moon, S.-K.; Kim, W.-J.; Kim, G.-Y. Piceatannol enhances TRAIL-induced apoptosis in human leukemia THP-1 cells through Sp1- and ERK-dependent DR5 up-regulation. Toxicol. Vitr. 2011, 25, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Rohaiza, S.; Yaacob, W.; Din, L.; Nazlina, I. Cytotoxic oligostilbenes from Shorea hopeifolia. Afr. J. Pharm. Pharmacol. 2011, 5, 1272–1277. [Google Scholar] [CrossRef]
- Chang, C.-I.; Chien, W.-C.; Huang, K.-X.; Hsu, J.-L. Anti-Inflammatory Effects of Vitisinol A and Four Other Oligostilbenes from Ampelopsis brevipedunculata var. Hancei. Molecules 2017, 22, 1195. [Google Scholar] [CrossRef]
- Ha, D.T.; Chen, Q.C.; Hung, T.M.; Youn, U.J.; Ngoc, T.M.; Thuong, P.T.; Kim, H.J.; Seong, Y.H.; Min, B.S.; Bae, K. Stilbenes and oligostilbenes from leaf and stem of Vitis amurensis and their cytotoxic activity. Arch. Pharmacal Res. 2009, 32, 177–183. [Google Scholar] [CrossRef]
- Vion, E.; Page, G.; Bourdeaud, E.; Paccalin, M.; Guillard, J.; Rioux Bilan, A. Trans ε-viniferin is an amyloid-β disaggregating and anti-inflammatory drug in a mouse primary cellular model of Alzheimer’s disease. Mol. Cell. Neurosci. 2018, 88, 1–6. [Google Scholar] [CrossRef]
- Tian, X.; Guo, S.; Zhang, S.; Li, P.; Wang, T.; Ho, C.-T.; Pan, M.-H.; Bai, N. Chemical characterization of main bioactive constituents in Paeonia ostii seed meal and GC-MS analysis of seed oil. J. Food Biochem. 2020, 44, e13088. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Padial, C.; Puerto, M.; Merchán-Gragero, M.d.M.; Moreno, F.J.; Richard, T.; Cantos-Villar, E.; Pichardo, S. Cytotoxicity studies of a stilbene extract and its main components intended to be used as preservative in the wine industry. Food Res. Int. 2020, 137, 109738. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Chang, E.J.; Bae, S.J.; Shim, S.M.; Park, H.D.; Rhee, C.H.; Park, J.H.; Choi, S.W. Cytotoxic and antimutagenic stilbenes from seeds ofPaeonia lactiflora. Arch. Pharmacal Res. 2002, 25, 293–299. [Google Scholar] [CrossRef]
- Kang, J.H.; Park, Y.H.; Choi, S.W.; Yang, E.K.; Lee, W.J. Resveratrol derivatives potently induce apoptosis in human promyelocytic leukemia cells. Exp. Mol. Med. 2003, 35, 467–474. [Google Scholar] [CrossRef]
- Zghonda, N.; Yoshida, S.; Araki, M.; Kusunoki, M.; Mliki, A.; Ghorbel, A.; Miyazaki, H. Greater Effectiveness of ε-Viniferin in Red Wine Than Its Monomer Resveratrol for Inhibiting Vascular Smooth Muscle Cell Proliferation and Migration. Biosci. Biotechnol. Biochem. 2011, 75, 1259–1267. [Google Scholar] [CrossRef]
- Courtois, A.; Garcia, M.; Krisa, S.; Atgié, C.; Sauvant, P.; Richard, T.; Faure, C. Encapsulation of ε-viniferin in onion-type multi-lamellar liposomes increases its solubility and its photo-stability and decreases its cytotoxicity on Caco-2 intestinal cells. Food Funct. 2019, 10, 2573–2582. [Google Scholar] [CrossRef]
- Zghonda, N.; Yoshida, S.; Ezaki, S.; Otake, Y.; Murakami, C.; Mliki, A.; Ghorbel, A.; Miyazaki, H. ε-Viniferin Is More Effective Than Its Monomer Resveratrol in Improving the Functions of Vascular Endothelial Cells and the Heart. Biosci. Biotechnol. Biochem. 2012, 76, 954–960. [Google Scholar] [CrossRef]
- Wu, C.W.; Nakamoto, Y.; Hisatome, T.; Yoshida, S.; Miyazaki, H. Resveratrol and its dimers ε-viniferin and δ-viniferin in red wine protect vascular endothelial cells by a similar mechanism with different potency and efficacy. Kaohsiung J. Med. Sci. 2020, 36, 535–542. [Google Scholar] [CrossRef]
- Huang, C.; Lin, Z.-J.; Lee, C.-J.; Lai, W.-H.; Chen, J.-C.; Huang, H.-C. ε-Viniferin and α-viniferin alone or in combination induced apoptosis and necrosis in osteosarcoma and non-small cell lung cancer cells. Food Chem. Toxicol. 2021, 158, 112617. [Google Scholar] [CrossRef]
- Richard, T.; Poupard, P.; Nassra, M.; Papastamoulis, Y.; Iglésias, M.-L.; Krisa, S.; Waffo-Teguo, P.; Mérillon, J.-M.; Monti, J.-P. Protective effect of ε-viniferin on β-amyloid peptide aggregation investigated by electrospray ionization mass spectrometry. Bioorganic Med. Chem. 2011, 19, 3152–3155. [Google Scholar] [CrossRef]
- Frombaum, M.; Therond, P.; Djelidi, R.; Beaudeux, J.-L.; Bonnefont-Rousselot, D.; Borderie, D. Piceatannol is more effective than resveratrol in restoring endothelial cell dimethylarginine dimethylaminohydrolase expression and activity after high-glucose oxidative stress. Free Radic. Res. 2011, 45, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.; Min, B.; Ryoo, S. Piceatannol-3′-O-β-D-glucopyranoside as an active component of rhubarb activates endothelial nitric oxide synthase through inhibition of arginase activity. Exp. Mol. Med. 2010, 42, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Peng, J.; Liu, S.-Y.; Wang, C.-J.; Xiang, D.-X.; Xiong, X.-M.; Hu, C.-P.; Li, Y.-J. Inhibitory effect of resveratrol derivative BTM-0512 on high glucose-induced cell senescence involves dimethylaminohydrolase/asymmetric dimethylarginine pathway. Clin. Exp. Pharmacol. Physiol. 2010, 37, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Zorita, S.; Fernández-Quintela, A.; Lasa, A.; Aguirre, L.; Rimando, A.M.; Portillo, M.P. Pterostilbene, a Dimethyl Ether Derivative of Resveratrol, Reduces Fat Accumulation in Rats Fed an Obesogenic Diet. J. Agric. Food Chem. 2014, 62, 8371–8378. [Google Scholar] [CrossRef]
- Spanier, G.; Xu, H.; Xia, N.; Tobias, S.; Deng, S.; Wojnowski, L.; Forstermann, U.; Li, H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J. Physiol. Pharmacol. 2009, 60, 111–116. [Google Scholar]
- Wallerath, T.; Deckert, G.; Ternes, T.; Anderson, H.; Li, H.; Witte, K.; Förstermann, U. Resveratrol, a Polyphenolic Phytoalexin Present in Red Wine, Enhances Expression and Activity of Endothelial Nitric Oxide Synthase. Circulation 2002, 106, 1652–1658. [Google Scholar] [CrossRef]
- Frombaum, M.; Le Clanche, S.; Bonnefont-Rousselot, D.; Borderie, D. Antioxidant effects of resveratrol and other stilbene derivatives on oxidative stress and NO bioavailability: Potential benefits to cardiovascular diseases. Biochimie 2012, 94, 269–276. [Google Scholar] [CrossRef]
- Yang, J.; Wang, N.; Li, J.; Zhang, J.; Feng, P. Effects of resveratrol on NO secretion stimulated by insulin and its dependence on SIRT1 in high glucose cultured endothelial cells. Endocrine 2010, 37, 365–372. [Google Scholar] [CrossRef]
- Arunachalam, G.; Yao, H.; Sundar, I.K.; Caito, S.; Rahman, I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol. Biochem. Biophys. Res. Commun. 2010, 393, 66–72. [Google Scholar] [CrossRef]
- Gresele, P.; Pignatelli, P.; Guglielmini, G.; Carnevale, R.; Mezzasoma, A.M.; Ghiselli, A.; Momi, S.; Violi, F. Resveratrol, at Concentrations Attainable with Moderate Wine Consumption, Stimulates Human Platelet Nitric Oxide Production123. J. Nutr. 2008, 138, 1602–1608. [Google Scholar] [CrossRef]
- Nicholson, S.K.; Tucker, G.A.; Brameld, J.M. Physiological concentrations of dietary polyphenols regulate vascular endothelial cell expression of genes important in cardiovascular health. Br. J. Nutr. 2009, 103, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Klinger, J.R. The Nitric Oxide/cGMP Signaling Pathway in Pulmonary Hypertension. Clin. Chest Med. 2007, 28, 143–167. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.; Morón, R.; Zarzuelo, A.; Galisteo, M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem. Pharmacol. 2009, 77, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.-G.; Wang, Z.-R.; Huang, Y.-Z.; Cao, K.-J.; Wu, J.M. Effect of red wine and wine polyphenol resveratrol on endothelial function in hypercholesterolemic rabbits. Int. J. Mol. Med. 2003, 11, 317–320. [Google Scholar] [CrossRef]
- Yoshino, J.; Conte, C.; Fontana, L.; Mittendorfer, B.; Imai, S.I.; Schechtman, K.B.; Gu, C.; Kunz, I.; Fanelli, F.R.; Patterson, B.W.; et al. Resveratrol Supplementation Does Not Improve Metabolic Function in Nonobese Women with Normal Glucose Tolerance. Cell Metab. 2012, 16, 658–664. [Google Scholar] [CrossRef]
- Juan, M.E.; Vinardell, M.P.; Planas, J.M. The Daily Oral Administration of High Doses of trans-Resveratrol to Rats for 28 Days Is Not Harmful. J. Nutr. 2002, 132, 257–260. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; García-Conesa, M.T.; Tomás-Barberán, F.A.; Espín, J.C. One-Year Consumption of a Grape Nutraceutical Containing Resveratrol Improves the Inflammatory and Fibrinolytic Status of Patients in Primary Prevention of Cardiovascular Disease. Am. J. Cardiol. 2012, 110, 356–363. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; García-Almagro, F.J.; Avilés-Plaza, F.; Parra, S.; Yáñez-Gascón, M.J.; Ruiz-Ros, J.A.; García-Conesa, M.T.; Tomás-Barberán, F.A.; et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol. Nutr. Food Res. 2012, 56, 810–821. [Google Scholar] [CrossRef]
- Sahebkar, A. Effects of resveratrol supplementation on plasma lipids: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2013, 71, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Riche, D.M.; Riche, K.D.; Blackshear, C.T.; McEwen, C.L.; Sherman, J.J.; Wofford, M.R.; Griswold, M.E. Pterostilbene on Metabolic Parameters: A Randomized, Double-Blind, and Placebo-Controlled Trial. Evid.-Based Complement. Altern. Med. 2014, 2014, 459165. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Grape Resveratrol Increases Serum Adiponectin and Downregulates Inflammatory Genes in Peripheral Blood Mononuclear Cells: A Triple-Blind, Placebo-Controlled, One-Year Clinical Trial in Patients with Stable Coronary Artery Disease. Cardiovasc. Drugs Ther. 2013, 27, 37–48. [Google Scholar] [CrossRef]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Penumathsa, S.V.; Thirunavukkarasu, M.; Zhan, L.; Maulik, G.; Menon, V.P.; Bagchi, D.; Maulik, N. Resveratrol enhances GLUT-4 translocation to the caveolar lipid raft fractions through AMPK/Akt/eNOS signalling pathway in diabetic myocardium. J. Cell. Mol. Med. 2008, 12, 2350–2361. [Google Scholar] [CrossRef]
- Moridi, H.; Karimi, J.; Sheikh, N.; Goodarzi, M.T.; Saidijam, M.; Yadegarazari, R.; Khazaei, M.; Khodadadi, I.; Tavilani, H.; Piri, H.; et al. Resveratrol-Dependent Down-regulation of Receptor for Advanced Glycation End-products and Oxidative Stress in Kidney of Rats With Diabetes. Int. J. Endocrinol. Metab. 2015, 13, e23542. [Google Scholar] [CrossRef]
- Soufi, F.G.; Mohammad-nejad, D.; Ahmadieh, H. Resveratrol improves diabetic retinopathy possibly through oxidative stress—Nuclear factor κB—Apoptosis pathway. Pharmacol. Rep. 2012, 64, 1505–1514. [Google Scholar] [CrossRef]
- Um, J.-H.; Park, S.-J.; Kang, H.; Yang, S.; Foretz, M.; McBurney, M.W.; Kim, M.K.; Viollet, B.; Chung, J.H. AMP-Activated Protein Kinase–Deficient Mice Are Resistant to the Metabolic Effects of Resveratrol. Diabetes 2009, 59, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Movahed, A.; Nabipour, I.; Lieben Louis, X.; Thandapilly, S.J.; Yu, L.; Kalantarhormozi, M.; Rekabpour, S.J.; Netticadan, T. Antihyperglycemic Effects of Short Term Resveratrol Supplementation in Type 2 Diabetic Patients. Evid.-Based Complement. Altern. Med. 2013, 2013, 851267. [Google Scholar] [CrossRef] [PubMed]
- Thazhath, S.S.; Wu, T.; Bound, M.J.; Checklin, H.L.; Standfield, S.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Administration of resveratrol for 5 wk has no effect on glucagon-like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: A randomized controlled trial12. Am. J. Clin. Nutr. 2016, 103, 66–70. [Google Scholar] [CrossRef]
- Bo, S.; Ponzo, V.; Ciccone, G.; Evangelista, A.; Saba, F.; Goitre, I.; Procopio, M.; Pagano, G.F.; Cassader, M.; Gambino, R. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol. Res. 2016, 111, 896–905. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Fernández-Quintela, A.; Aguirre, L.; Macarulla, M.T.; Rimando, A.M.; Portillo, M.P. Pterostilbene improves glycaemic control in rats fed an obesogenic diet: Involvement of skeletal muscle and liver. Food Funct. 2015, 6, 1968–1976. [Google Scholar] [CrossRef]
- Bhakkiyalakshmi, E.; Shalini, D.; Sekar, T.V.; Rajaguru, P.; Paulmurugan, R.; Ramkumar, K.M. Therapeutic potential of pterostilbene against pancreatic beta-cell apoptosis mediated through Nrf2. Br. J. Pharmacol. 2014, 171, 1747–1757. [Google Scholar] [CrossRef]
- Manickam, M.; Ramanathan, M.; Farboodniay Jahromi, M.A.; Chansouria, J.P.N.; Ray, A.B. Antihyperglycemic Activity of Phenolics from Pterocarpus marsupium. J. Nat. Prod. 1997, 60, 609–610. [Google Scholar] [CrossRef]
- Grover, J.K.; Vats, V.; Yadav, S.S. Pterocarpus marsupium extract (Vijayasar) prevented the alteration in metabolic patterns induced in the normal rat by feeding an adequate diet containing fructose as sole carbohydrate. Diabetes Obes. Metab. 2005, 7, 414–420. [Google Scholar] [CrossRef]
- Nemes-Nagy, E.; Szőcs-Molnár, T.; Dunca, I.; Balogh-Sămărghiţan, V.; Hobai, Ş.; Morar, R.; Pusta, D.L.; Crăciun, E.C. Effect of a dietary supplement containing blueberry and sea buckthorn concentrate on antioxidant capacity in type 1 diabetic children. Acta Physiol. Hung. 2008, 95, 383–393. [Google Scholar] [CrossRef]
- Minakawa, M.; Miura, Y.; Yagasaki, K. Piceatannol, a resveratrol derivative, promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and suppresses blood glucose levels in type 2 diabetic model db/db mice. Biochem. Biophys. Res. Commun. 2012, 422, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Towler, M.C.; Hardie, D.G. AMP-Activated Protein Kinase in Metabolic Control and Insulin Signaling. Circ. Res. 2007, 100, 328–341. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Hijona, E.; Aguirre, L.; Pérez-Matute, P.; Villanueva-Millán, M.J.; Mosqueda-Solis, A.; Hasnaoui, M.; Nepveu, F.; Senard, J.M.; Bujanda, L.; Aldámiz-Echevarría, L.; et al. Limited beneficial effects of piceatannol supplementation on obesity complications in the obese Zucker rat: Gut microbiota, metabolic, endocrine, and cardiac aspects. J. Physiol. Biochem. 2016, 72, 567–582. [Google Scholar] [CrossRef]
- Oritani, Y.; Okitsu, T.; Nishimura, E.; Sai, M.; Ito, T.; Takeuchi, S. Enhanced glucose tolerance by intravascularly administered piceatannol in freely moving healthy rats. Biochem. Biophys. Res. Commun. 2016, 470, 753–758. [Google Scholar] [CrossRef]
- Kershaw, J.; Kim, K.-H. The Therapeutic Potential of Piceatannol, a Natural Stilbene, in Metabolic Diseases: A Review. J. Med. Food 2017, 20, 427–438. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Jeong, S.O.; Son, Y.; Lee, J.H.; Cheong, Y.K.; Park, S.H.; Chung, H.T.; Pae, H.O. Resveratrol analog piceatannol restores the palmitic acid-induced impairment of insulin signaling and production of endothelial nitric oxide via activation of anti-inflammatory and antioxidative heme oxygenase-1 in human endothelial cells. Mol. Med. Rep. 2015, 12, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. General Aspects of Neurodegeneration; Spring: Vienna, Austria, 2003; pp. 101–144. [Google Scholar]
- de A Boleti, A.P.; Almeida, J.A.; Migliolo, L. Impact of the metabolic syndrome on the evolution of neurodegenerative diseases. Neural Regen. Res. 2021, 16, 688–689. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants-an overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef] [PubMed]
- Olajide, O.A.; Sarker, S.D. Alzheimer’s disease: Natural products as inhibitors of neuroinflammation. Inflammopharmacology 2020, 28, 1439–1455. [Google Scholar] [CrossRef] [PubMed]
- Carradori, S.; D’Ascenzio, M.; Chimenti, P.; Secci, D.; Bolasco, A. Selective MAO-B inhibitors: A lesson from natural products. Mol. Divers. 2014, 18, 219–243. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef]
- Fantacuzzi, M.; Amoroso, R.; Carradori, S.; De Filippis, B. Resveratrol-based compounds and neurodegeneration: Recent insight in multitarget therapy. Eur. J. Med. Chem. 2022, 233, 114242. [Google Scholar] [CrossRef] [PubMed]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.S. Natural Products and Their Bioactive Compounds: Neuroprotective Potentials against Neurodegenerative Diseases. Evid.-Based Complement. Altern. Med. 2020, 2020, 6565396. [Google Scholar] [CrossRef]
- Lu, K.-T.; Ko, M.-C.; Chen, B.-Y.; Huang, J.-C.; Hsieh, C.-W.; Lee, M.-C.; Chiou, R.Y.Y.; Wung, B.-S.; Peng, C.-H.; Yang, Y.-L. Neuroprotective Effects of Resveratrol on MPTP-Induced Neuron Loss Mediated by Free Radical Scavenging. J. Agric. Food Chem. 2008, 56, 6910–6913. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, C.; Wang, B.; Ma, Z.; Wang, D.; Gong, B.; Di, S.; Jiang, S.; Li, Y.; Li, T.; et al. Pterostilbene attenuates high glucose-induced oxidative injury in hippocampal neuronal cells by activating nuclear factor erythroid 2-related factor 2. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017, 1863, 827–837. [Google Scholar] [CrossRef]
- Wang, B.; Liu, H.; Yue, L.; Li, X.; Zhao, L.; Yang, X.; Wang, X.; Yang, Y.; Qu, Y. Neuroprotective effects of pterostilbene against oxidative stress injury: Involvement of nuclear factor erythroid 2-related factor 2 pathway. Brain Res. 2016, 1643, 70–79. [Google Scholar] [CrossRef]
- Su, Q.; Pu, H.; Hu, C. Neuroprotection by combination of resveratrol and enriched environment against ischemic brain injury in rats. Neurol. Res. 2016, 38, 60–68. [Google Scholar] [CrossRef]
- Fang, L.; Gao, H.; Zhang, W.; Zhang, W.; Wang, Y. Resveratrol alleviates nerve injury after cerebral ischemia and reperfusion in mice by inhibiting inflammation and apoptosis. Int. J. Clin. Exp. Med. 2015, 8, 3219. [Google Scholar]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical Application-A comprehensive review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, J.-S.; Zhou, H.; Wilson, B.; Hong, J.-S.; Gao, H.-M. Resveratrol Protects Dopamine Neurons Against Lipopolysaccharide-Induced Neurotoxicity through Its Anti-Inflammatory Actions. Mol. Pharmacol. 2010, 78, 466–477. [Google Scholar] [CrossRef]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef] [PubMed]
- Biais, B.; Krisa, S.; Cluzet, S.; Da Costa, G.; Waffo-Teguo, P.; Mérillon, J.-M.; Richard, T. Antioxidant and Cytoprotective Activities of Grapevine Stilbenes. J. Agric. Food Chem. 2017, 65, 4952–4960. [Google Scholar] [CrossRef] [PubMed]
- Chaher, N.; Arraki, K.; Dillinseger, E.; Temsamani, H.; Bernillon, S.; Pedrot, E.; Delaunay, J.-C.; Mérillon, J.-M.; Monti, J.-P.; Izard, J.-C.; et al. Bioactive stilbenes from Vitis vinifera grapevine shoots extracts. J. Sci. Food Agric. 2014, 94, 951–954. [Google Scholar] [CrossRef]
- Ren, J.; Fan, C.; Chen, N.; Huang, J.; Yang, Q. Resveratrol Pretreatment Attenuates Cerebral Ischemic Injury by Upregulating Expression of Transcription Factor Nrf2 and HO-1 in Rats. Neurochem. Res. 2011, 36, 2352–2362. [Google Scholar] [CrossRef]
- Simão, F.; Matté, A.; Pagnussat, A.S.; Netto, C.A.; Salbego, C.G. Resveratrol prevents CA1 neurons against ischemic injury by parallel modulation of both GSK-3β and CREB through PI3-K/Akt pathways. Eur. J. Neurosci. 2012, 36, 2899–2905. [Google Scholar] [CrossRef]
- Ma, X.; Sun, Z.; Liu, Y.; Jia, Y.; Zhang, B.; Zhang, J. Resveratrol improves cognition and reduces oxidative stress in rats with vascular dementia. Neural Regen. Res. 2013, 8, 2050–2059. [Google Scholar]
- Ban, J.Y.; Jeon, S.-Y.; Nguyen, T.T.H.; Bae, K.; Song, K.-S.; Seonga, Y.H. Neuroprotective Effect of Oxyresveratrol from Smilacis Chinae Rhizome on Amyloid Beta Protein (25–35)-Induced Neurotoxicity in Cultured Rat Cortical Neurons. Biol. Pharm. Bull. 2006, 29, 2419–2424. [Google Scholar] [CrossRef]
- Andrabi, S.A.; Spina, M.G.; Lorenz, P.; Ebmeyer, U.; Wolf, G.; Horn, T.F.W. Oxyresveratrol (trans-2,3′,4,5′-tetrahydroxystilbene) is neuroprotective and inhibits the apoptotic cell death in transient cerebral ischemia. Brain Res. 2004, 1017, 98–107. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S.; et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Kerti, L.; Margulies, D.S.; Flöel, A. Effects of Resveratrol on Memory Performance, Hippocampal Functional Connectivity, and Glucose Metabolism in Healthy Older Adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.X.; Raederstorff, D.; Howe, P.R.C. Acute Resveratrol Consumption Improves Neurovascular Coupling Capacity in Adults with Type 2 Diabetes Mellitus. Nutrients 2016, 8, 425. [Google Scholar] [CrossRef]
- Wong, R.H.X.; Nealon, R.S.; Scholey, A.; Howe, P.R.C. Low dose resveratrol improves cerebrovascular function in type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 393–399. [Google Scholar] [CrossRef] [PubMed]
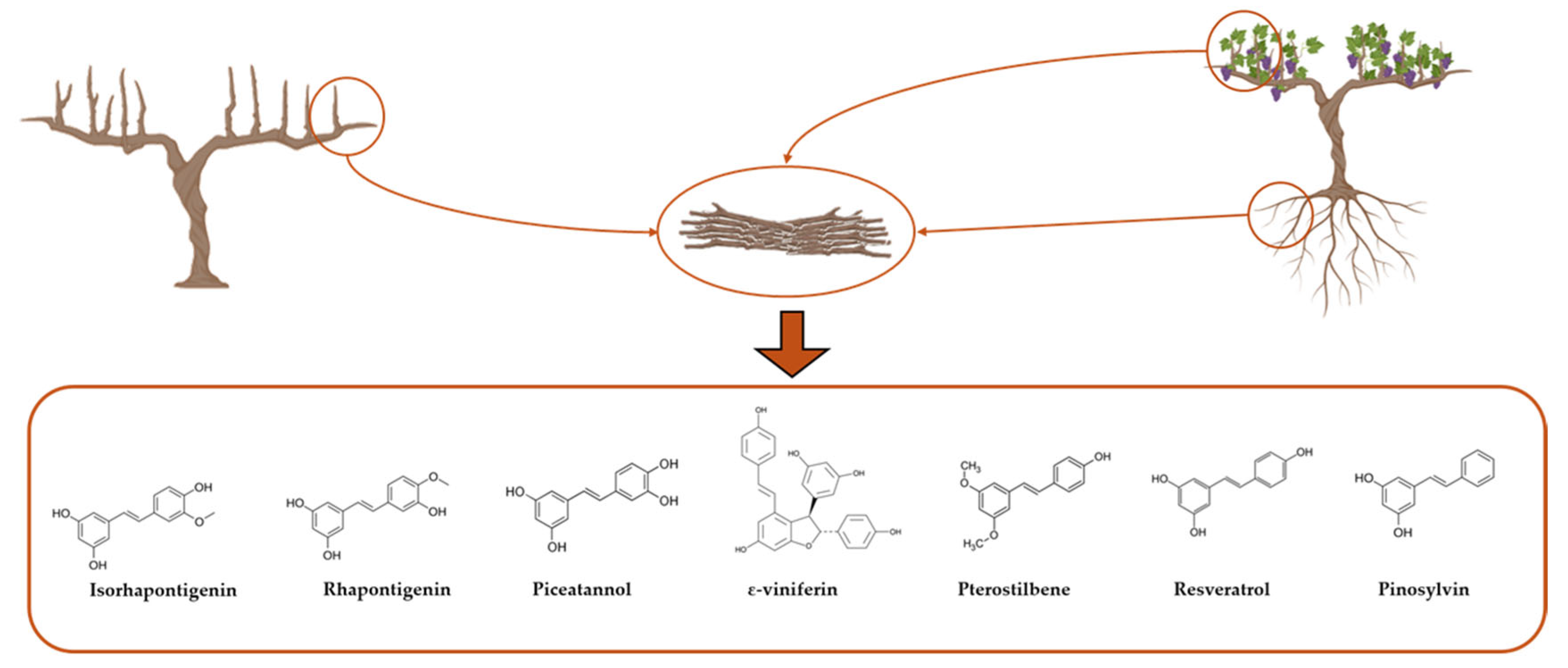

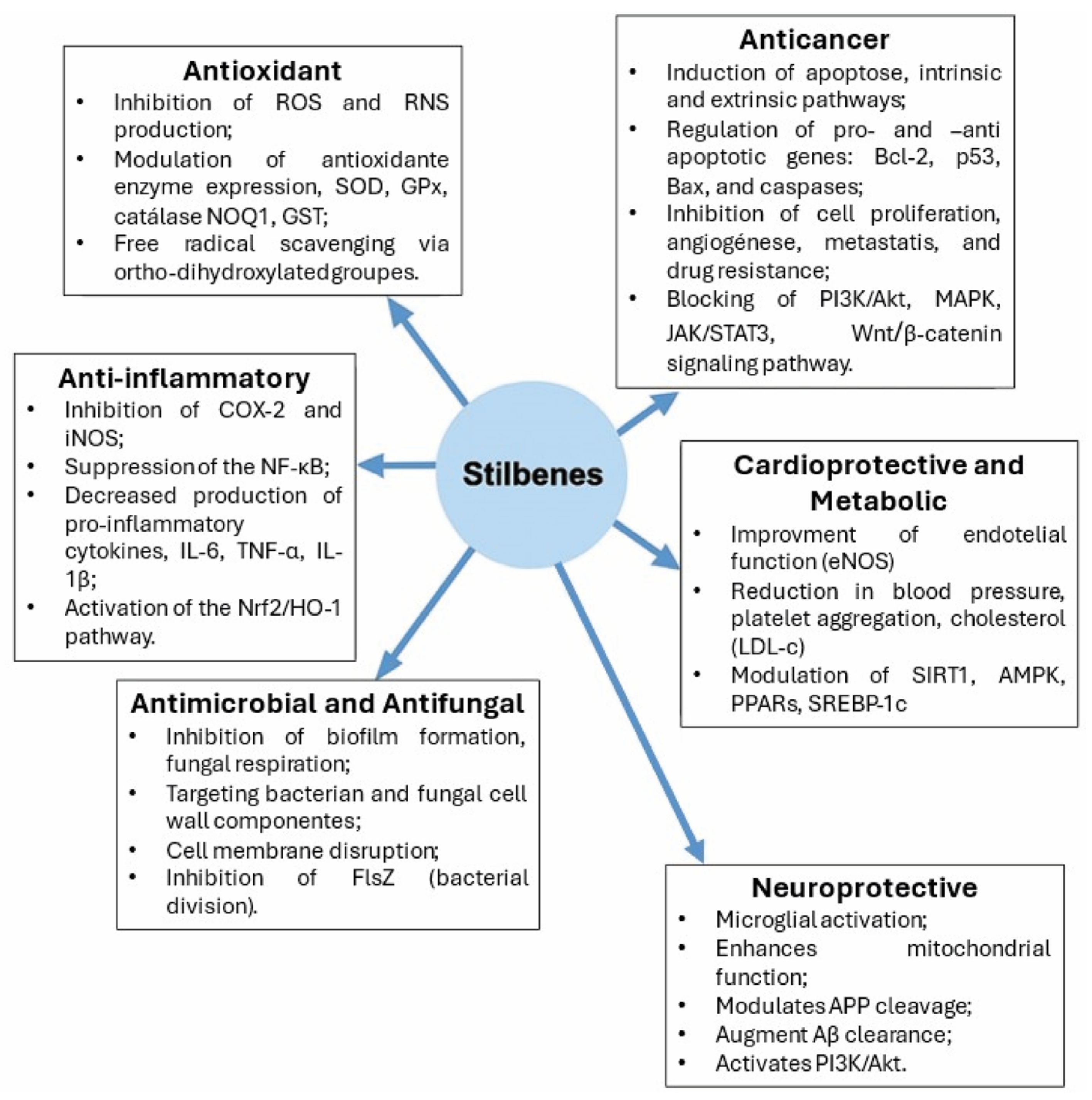
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brás, L.P.; Luís, Â.; Chatel, G.; Socorro, S.; Duarte, A.P. Stilbenes from Vine Extracts: Therapeutic Potential and Mechanisms. Int. J. Mol. Sci. 2025, 26, 8269. https://doi.org/10.3390/ijms26178269
Brás LP, Luís Â, Chatel G, Socorro S, Duarte AP. Stilbenes from Vine Extracts: Therapeutic Potential and Mechanisms. International Journal of Molecular Sciences. 2025; 26(17):8269. https://doi.org/10.3390/ijms26178269
Chicago/Turabian StyleBrás, Luís P., Ângelo Luís, Gregory Chatel, Sílvia Socorro, and Ana Paula Duarte. 2025. "Stilbenes from Vine Extracts: Therapeutic Potential and Mechanisms" International Journal of Molecular Sciences 26, no. 17: 8269. https://doi.org/10.3390/ijms26178269
APA StyleBrás, L. P., Luís, Â., Chatel, G., Socorro, S., & Duarte, A. P. (2025). Stilbenes from Vine Extracts: Therapeutic Potential and Mechanisms. International Journal of Molecular Sciences, 26(17), 8269. https://doi.org/10.3390/ijms26178269









