Fine Particulate Matter (PM2.5) Disrupts Intestinal Barrier Function by Inducing Oxidative Stress and PI3K/AKT-Mediated Inflammation in Caco-2 Cells
Abstract
1. Introduction
2. Results
2.1. Cell Viability Assay
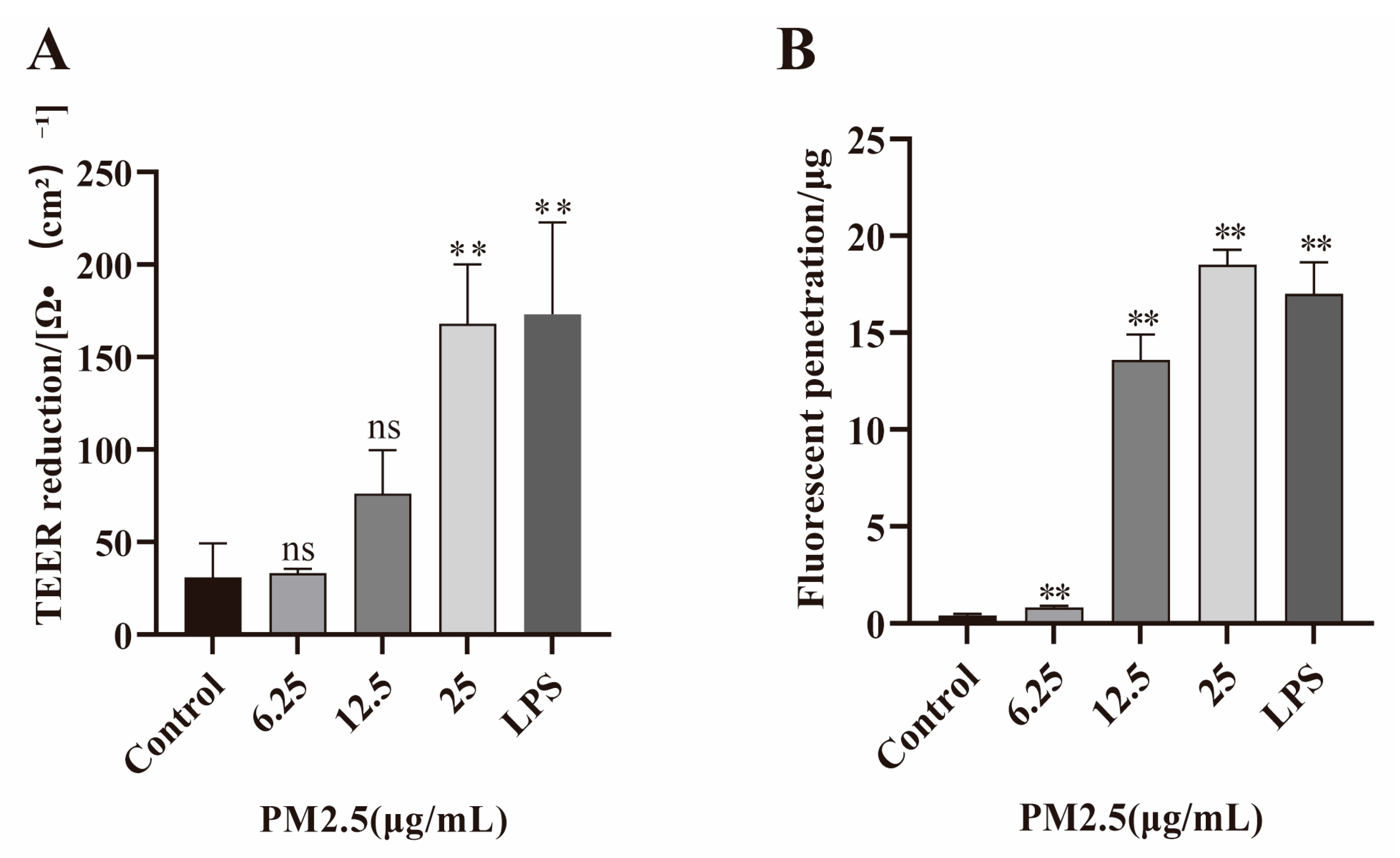
2.2. PM2.5 Increases Permeability of Caco-2 Cells
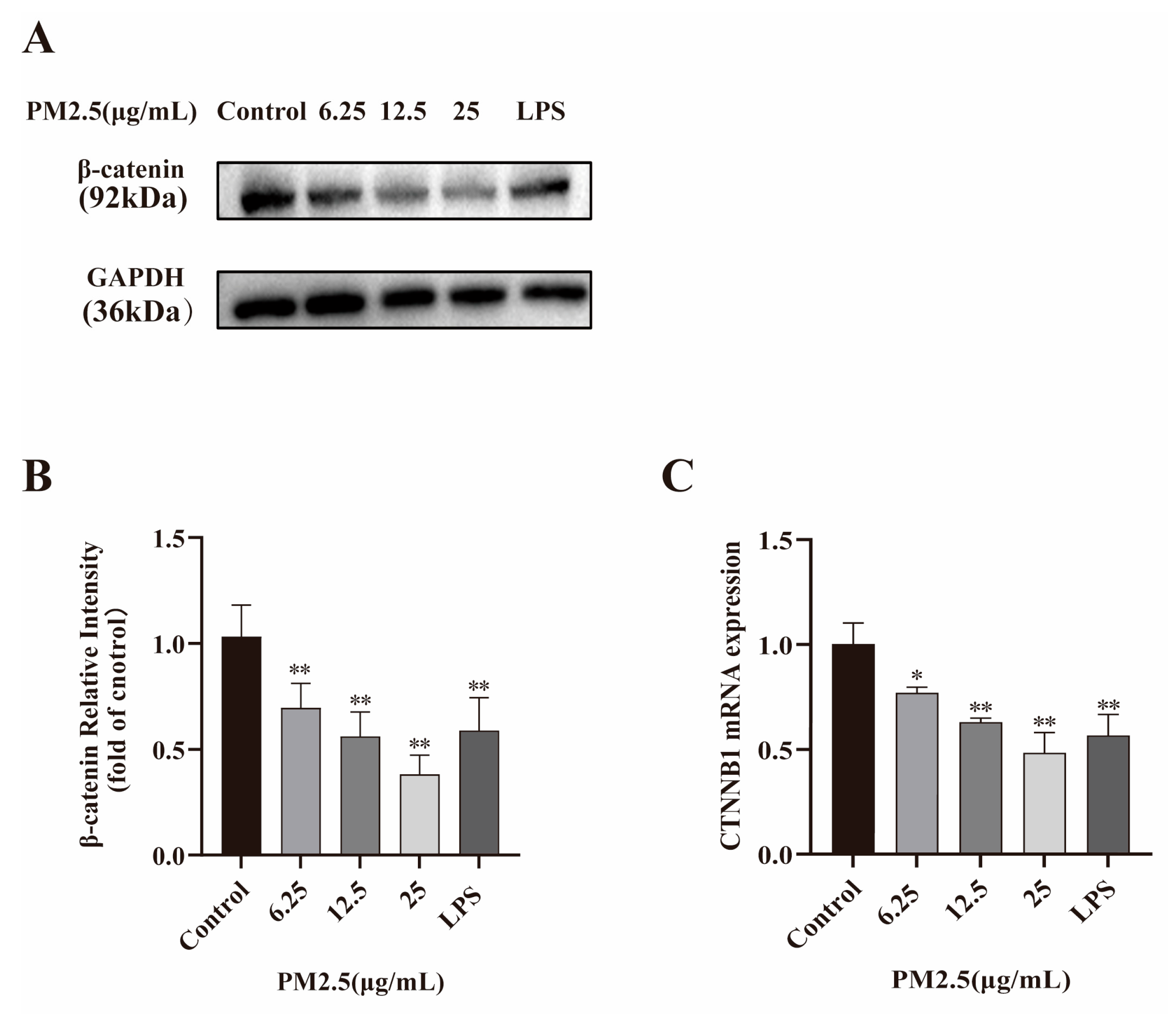
2.3. PM2.5 Reduces β-catenin and Its Encoding Gene CTNNB1 Expression
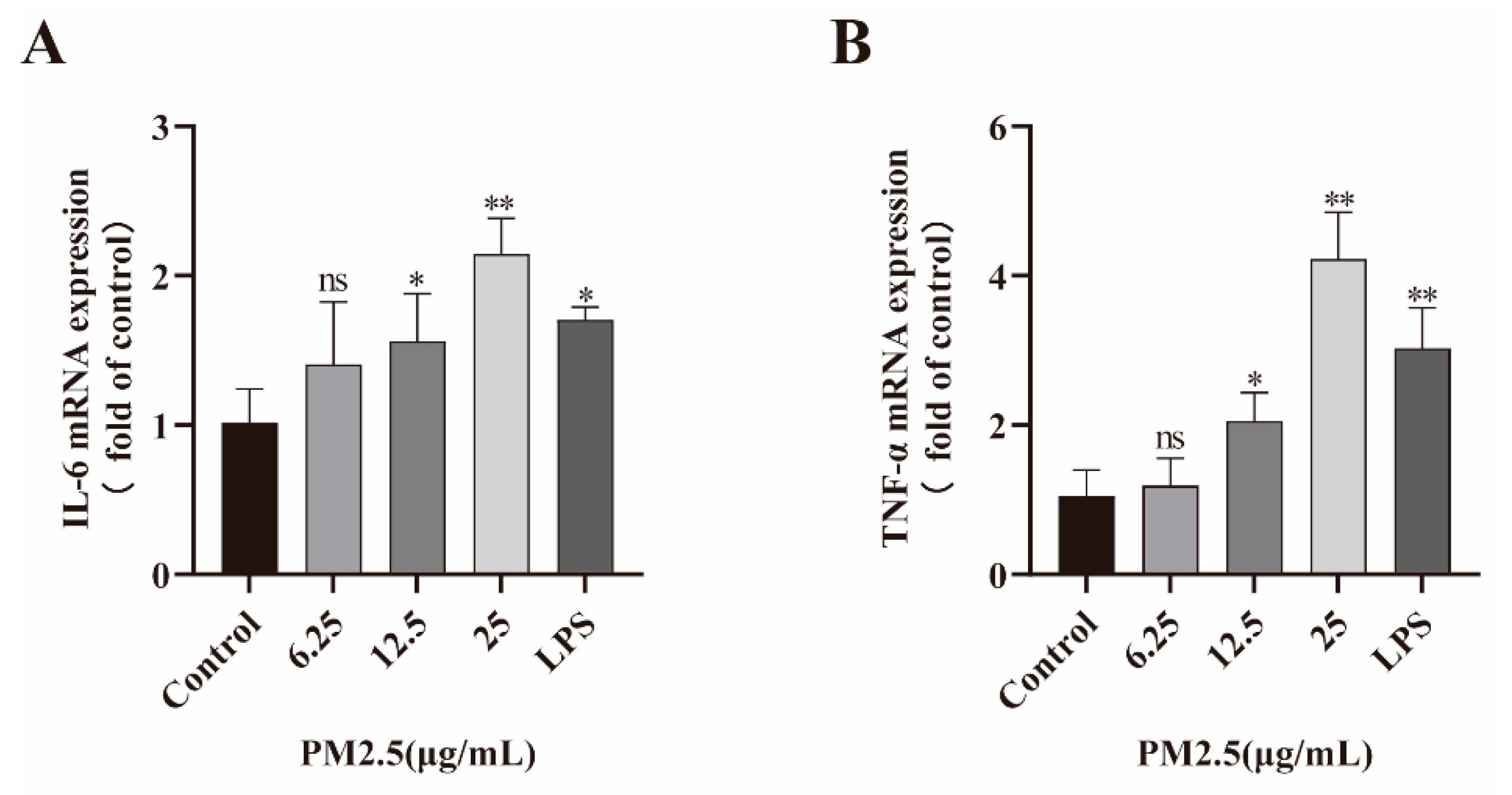
2.4. PM2.5 Induced Inflammatory Factor Expression
2.5. PM2.5 Activates PI3K/AKT Signaling Pathway
2.6. LY294002 Against PM2.5-Induced Intestinal Hyperpermeability
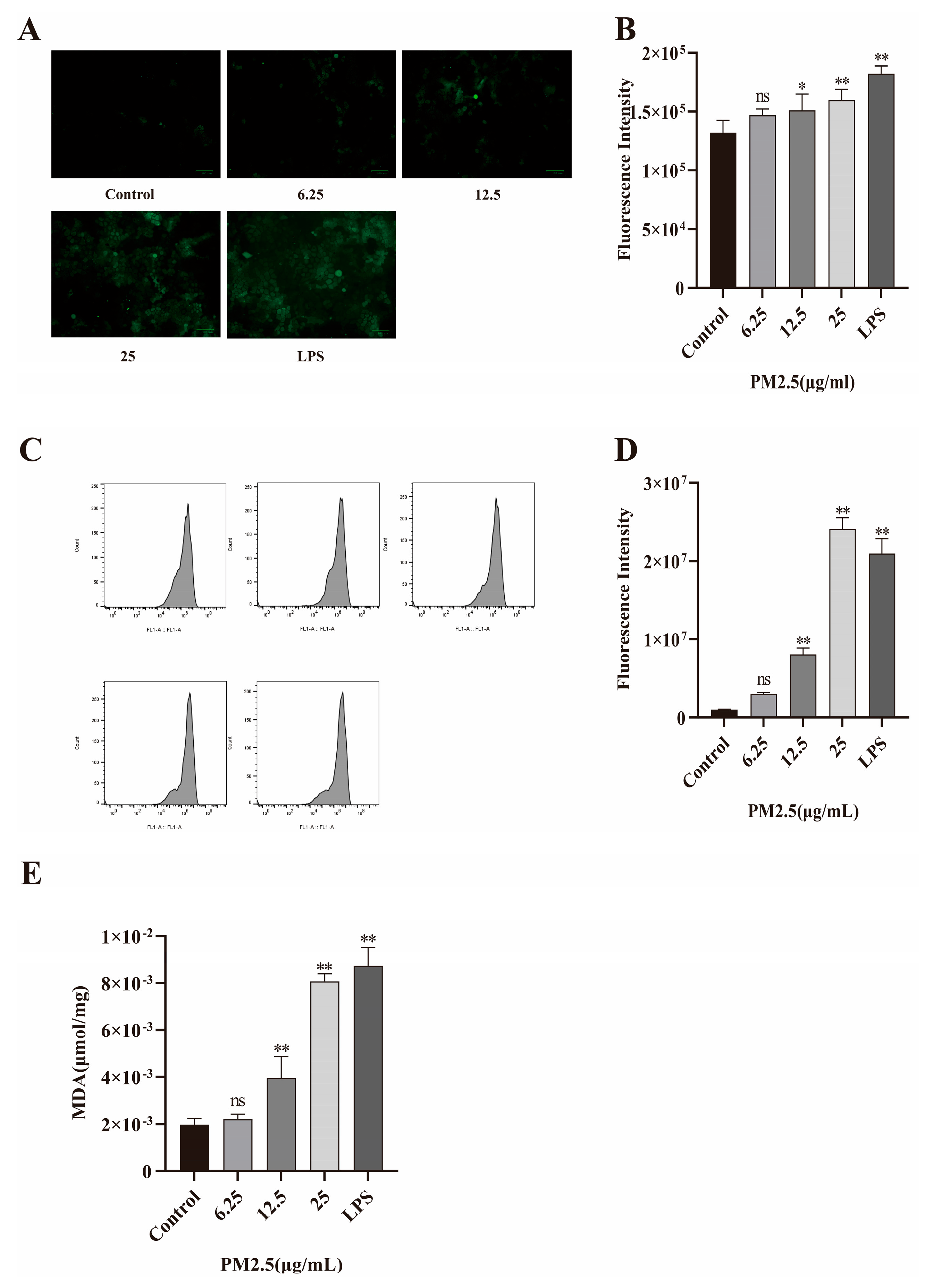
2.7. PM2.5-Induced Oxidative Stress in Caco-2 Cells
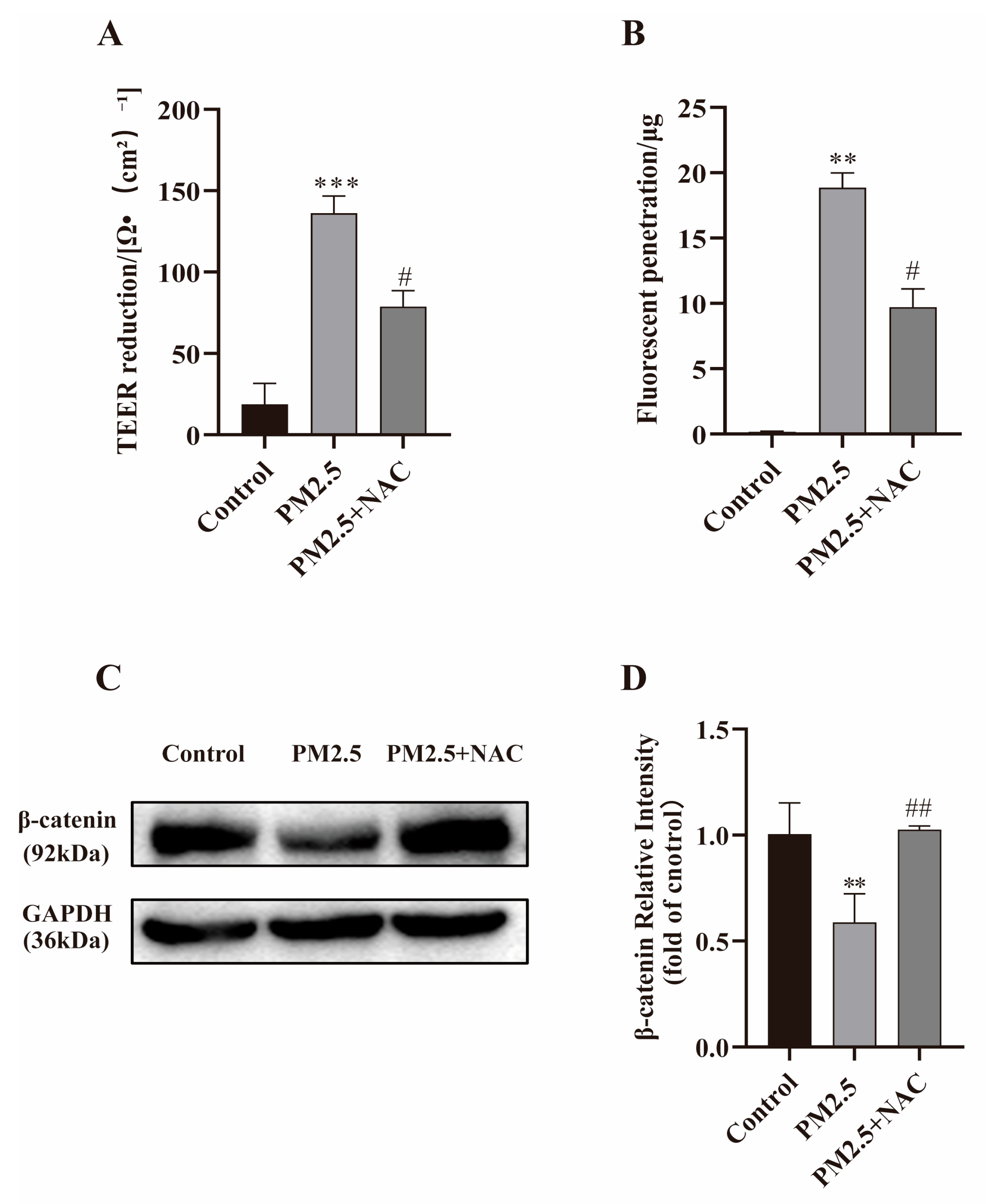
2.8. NAC Against PM2.5-Induced Barrier Dysfunction
3. Discussion
4. Materials and Methods
4.1. Preparation of PM2.5
4.2. Materials
4.3. Cell Culture and Viability Assay
4.4. Measuring TEER and the Penetration of FITC–Dextran
4.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.6. Western Blot
4.7. Determination of Reactive Oxygen Species Levels
4.8. Malondialdehyde (MDA) Detection
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, M.; Joshi, S.; Banjade, P.; Ghamande, S.A.; Surani, S. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023 Guidelines Reviewed. Open Respir. Med. J. 2024, 18, e18743064279064. [Google Scholar] [CrossRef]
- Korsiak, J.; Lavigne, E.; You, H.; Pollitt, K.; Kulka, R.; Hatzopoulou, M.; Evans, G.; Burnett, R.T.; Weichenthal, S. Air Pollution and Pediatric Respiratory Hospitalizations: Effect Modification by Particle Constituents and Oxidative Potential. Am. J. Respir. Crit. Care Med. 2022, 206, 1370–1378. [Google Scholar] [CrossRef]
- Bălă, G.P.; Timar, B.; Gorun, F.; Motisan, R.; Pescaru, C.; Tudorache, E.; Marc, M.; Manolescu, D.; Citu, C.; Oancea, C. The Impact of Air Pollution on Frequent Exacerbations among COPD Patients: An Observational Study on the Population of Western Romania. J. Clin. Med. 2022, 11, 4352. [Google Scholar] [CrossRef]
- Sahu, M.; Peipert, J.; Singhal, V.; Yadama, G.N.; Biswas, P. Evaluation of mass and surface area concentration of particle emissions and development of emissions indices for cookstoves in rural India. Environ. Sci. Technol. 2011, 45, 2428–2434. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, M.; Zhao, M.; Wang, Y.; Luo, J.; Lu, S.; Li, J.; Liu, Q. Pollution characteristics and human health risks of PM(2.5)-bound heavy metals: A 3-year observation in Suzhou, China. Environ. Geochem. Health 2023, 45, 5145–5162. [Google Scholar] [CrossRef]
- Li, J.; Liang, L.; Lyu, B.; Cai, Y.S.; Zuo, Y.; Su, J.; Tong, Z. Double trouble: The interaction of PM(2.5) and O(3) on respiratory hospital admissions. Environ. Pollut. 2023, 338, 122665. [Google Scholar] [CrossRef]
- Bai, R.; Liu, B.; Li, T.; Zhou, H.; Yue, X.; Liu, Y.; Shan, Y.; Li, Z.; Wei, Y.; Wu, J. The synergistic effects of PM(2.5) and high-fat diet on Th1/Th2 balance in model mice with asthma. J. Thorac. Dis. 2025, 17, 1502–1511. [Google Scholar] [CrossRef]
- Kazemi Moghadam, V.; Dickerson, A.S.; Shahedi, F.; Bazrafshan, E.; Seyedhasani, S.N.; Sarmadi, M. Association of the global distribution of multiple sclerosis with ultraviolet radiation and air pollution: An ecological study based on GBD data. Environ. Sci. Pollut. Res. Int. 2021, 28, 17802–17811. [Google Scholar] [CrossRef]
- Lutsey, P.L.; Misialek, J.R.; Young, M.T.; Berman, J.; Leiser, C.L.; Pope, Z.C.; Cushman, M.; Folsom, A.R.; Kaufman, J.D. Air pollution is associated with increased risk of venous thromboembolism: The Multi-Ethnic Study of Atherosclerosis. Blood 2025, 145, 1089–1096. [Google Scholar] [CrossRef]
- Hayes, R.B.; Lim, C.; Zhang, Y.; Cromar, K.; Shao, Y.; Reynolds, H.R.; Silverman, D.T.; Jones, R.R.; Park, Y.; Jerrett, M.; et al. PM2.5 air pollution and cause-specific cardiovascular disease mortality. Int. J. Epidemiol. 2020, 49, 25–35. [Google Scholar] [CrossRef]
- Duan, C.; Talbott, E.O.; Broadwin, R.; Brooks, M.; Matthews, K.; Barinas-Mitchell, E. Residential Exposure to PM(2.5) and Ozone and Progression of Subclinical Atherosclerosis Among Women Transitioning Through Menopause: The Study of Women’s Health Across the Nation. J. Womens Health 2019, 28, 802–811. [Google Scholar] [CrossRef]
- Cai, C.; Zhu, S.; Qin, M.; Li, X.; Feng, C.; Yu, B.; Dai, S.; Qiu, G.; Li, Y.; Ye, T.; et al. Long-term exposure to PM(2.5) chemical constituents and diabesity: Evidence from a multi-center cohort study in China. Lancet Reg. Health West Pac. 2024, 47, 101100. [Google Scholar] [CrossRef]
- Suo, D.; Zeng, S.; Zhang, J.; Meng, L.; Weng, L. PM2.5 induces apoptosis, oxidative stress injury and melanin metabolic disorder in human melanocytes. Exp. Ther. Med. 2020, 19, 3227–3238. [Google Scholar] [CrossRef]
- Ji, Y.; Su, X.; Zhang, F.; Huang, Z.; Zhang, X.; Chen, Y.; Song, Z.; Li, L. Impacts of short-term air pollution exposure on appendicitis admissions: Evidence from one of the most polluted cities in mainland China. Front. Public Health 2023, 11, 1144310. [Google Scholar] [CrossRef]
- Kaplan, G. Air pollution and the inflammatory bowel diseases. Inflamm. Bowel Dis. 2011, 17, 1146–1148. [Google Scholar] [CrossRef]
- Ye, Q.; Fu, J.F.; Mao, J.H.; Shen, H.Q.; Chen, X.J.; Shao, W.X.; Shang, S.Q.; Wu, Y.F. Haze is an important medium for the spread of rotavirus. Environ. Pollut. 2016, 216, 324–331. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, M. Invisible Foes: How Air Pollution and Lifestyle Conspire in the Rise of Colorectal Cancer. QJM 2025. online ahead of print. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Dixon, E.; Panaccione, R.; Fong, A.; Chen, L.; Szyszkowicz, M.; Wheeler, A.; MacLean, A.; Buie, W.D.; Leung, T.; et al. Effect of ambient air pollution on the incidence of appendicitis. CMAJ 2009, 181, 591–597. [Google Scholar] [CrossRef]
- Okafor, P.N.; Dahlen, A.; Youssef, M.; Olayode, A.; Sonu, I.; Neshatian, L.; Nguyen, L.; Charu, V. Environmental Pollutants Are Associated With Irritable Bowel Syndrome in a Commercially Insured Cohort of California Residents. Clin. Gastroenterol. Hepatol. 2023, 21, 1617–1626.e1619. [Google Scholar] [CrossRef]
- Chen, J.; Dan, L.; Sun, Y.; Yuan, S.; Liu, W.; Chen, X.; Jiang, F.; Fu, T.; Zhang, H.; Deng, M.; et al. Ambient Air Pollution and Risk of Enterotomy, Gastrointestinal Cancer, and All-Cause Mortality among 4,708 Individuals with Inflammatory Bowel Disease: A Prospective Cohort Study. Environ. Health Perspect. 2023, 131, 77010. [Google Scholar] [CrossRef]
- Fu, C.; Wang, Q.; Chen, Y.; Zhang, Y. Exploring the causal relationship between airborne particulate matter and ulcerative colitis: A two-sample mendelian randomization study. PLoS ONE 2024, 19, e0300066. [Google Scholar] [CrossRef]
- Möller, W.; Häussinger, K.; Winkler-Heil, R.; Stahlhofen, W.; Meyer, T.; Hofmann, W.; Heyder, J. Mucociliary and long-term particle clearance in the airways of healthy nonsmoker subjects. J. Appl. Physiol. 2004, 97, 2200–2206. [Google Scholar] [CrossRef]
- Pambianchi, E.; Pecorelli, A.; Valacchi, G. Gastrointestinal tissue as a “new” target of pollution exposure. IUBMB Life 2022, 74, 62–73. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, C.; Zhao, J.; Li, D.; Chen, J. Exposure to concentrated ambient PM(2.5) (CAPM) induces intestinal disturbance via inflammation and alternation of gut microbiome. Environ. Int. 2022, 161, 107138. [Google Scholar] [CrossRef]
- He, W.; Wang, Y.; Li, X.; Ji, Y.; Yuan, J.; Yang, W.; Yan, S.; Yan, J. Sealing the Pandora’s vase of pancreatic fistula through entrapping the digestive enzymes within a dextrorotary (D)-peptide hydrogel. Nat. Commun. 2024, 15, 7235. [Google Scholar] [CrossRef]
- Xu, T.; Sun, R.; Zhang, Y.; Zhang, C.; Wang, Y.; Wang, Z.A.; Du, Y. Recent Research and Application Prospect of Functional Oligosaccharides on Intestinal Disease Treatment. Molecules 2022, 27, 7622. [Google Scholar] [CrossRef]
- Ozawa, M.; Ringwald, M.; Kemler, R. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc. Natl. Acad. Sci. USA 1990, 87, 4246–4250. [Google Scholar] [CrossRef]
- Huber, A.H.; Stewart, D.B.; Laurents, D.V.; Nelson, W.J.; Weis, W.I. The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin. A possible mechanism for regulating cadherin turnover. J. Biol. Chem. 2001, 276, 12301–12309. [Google Scholar] [CrossRef]
- Xia, W.; Lin, H.; Zhang, J.; Bai, Y.; Wei, Z.; Zhao, H.; Xia, Y.; Dai, Y. Hyperoside promotes autophagy of colonic epithelial cells to protect intestinal barrier function in ulcerative colitis. Food Funct. 2025, 16, 5543–5555. [Google Scholar] [CrossRef]
- Mehta, S.; Nijhuis, A.; Kumagai, T.; Lindsay, J.; Silver, A. Defects in the adherens junction complex (E-cadherin/ β-catenin) in inflammatory bowel disease. Cell Tissue Res. 2015, 360, 749–760. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Z.; Chen, Y.; Dong, Y. Restraint Stress Disrupted Intestinal Homeostasis via 5-HT/HTR7/Wnt/β-Catenin/NF-kB Signaling. Int. J. Mol. Sci. 2025, 26, 4021. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Zhang, Y.G.; Xia, Y.; Sun, J. Loss of intestinal endosome associated protein sorting nexin 27 disrupts epithelial barrier and promotes inflammation. bioRxiv 2025. online ahead of print. [Google Scholar] [CrossRef]
- Gaowa, A.; Leangpanich, S.; Park, E.J.; Kawamoto, E.; Shimaoka, M. Irisin promotes intestinal epithelial cell proliferation via Wnt/β-catenin and focal adhesion kinase signaling pathways. Sci. Rep. 2024, 14, 25702. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.; Wu, J.; Zhong, Y.; Shen, X.; Petrov, B.; Cai, W. Maternal Vitamin D Deficiency Increases Intestinal Permeability and Programs Wnt/β-Catenin Pathway in BALB/C Mice. JPEN J. Parenter. Enter. Nutr. 2021, 45, 102–114. [Google Scholar] [CrossRef]
- Das, P.; Goswami, P.; Das, T.K.; Nag, T.; Sreenivas, V.; Ahuja, V.; Panda, S.K.; Gupta, S.D.; Makharia, G.K. Comparative tight junction protein expressions in colonic Crohn’s disease, ulcerative colitis, and tuberculosis: A new perspective. Virchows Arch. 2012, 460, 261–270. [Google Scholar] [CrossRef]
- Jangula, A.; Murphy, E.J. Lipopolysaccharide-induced blood brain barrier permeability is enhanced by alpha-synuclein expression. Neurosci. Lett. 2013, 551, 23–27. [Google Scholar] [CrossRef]
- Lv, S.; Song, H.L.; Zhou, Y.; Li, L.X.; Cui, W.; Wang, W.; Liu, P. Tumour necrosis factor-alpha affects blood-brain barrier permeability and tight junction-associated occludin in acute liver failure. Liver Int. 2010, 30, 1198–1210. [Google Scholar] [CrossRef]
- Blamire, A.M.; Anthony, D.C.; Rajagopalan, B.; Sibson, N.R.; Perry, V.H.; Styles, P. Interleukin-1beta -induced changes in blood-brain barrier permeability, apparent diffusion coefficient, and cerebral blood volume in the rat brain: A magnetic resonance study. J. Neurosci. 2000, 20, 8153–8159. [Google Scholar] [CrossRef]
- Xian, M.; Ma, S.; Wang, K.; Lou, H.; Wang, Y.; Zhang, L.; Wang, C.; Akdis, C.A. Particulate Matter 2.5 Causes Deficiency in Barrier Integrity in Human Nasal Epithelial Cells. Allergy Asthma Immunol. Res. 2020, 12, 56–71. [Google Scholar] [CrossRef]
- Wiley, J.W.; Zong, Y.; Zheng, G.; Zhu, S.; Hong, S. Histone H3K9 methylation regulates chronic stress and IL-6-induced colon epithelial permeability and visceral pain. Neurogastroenterol. Motil. 2020, 32, e13941. [Google Scholar] [CrossRef]
- Lin, P.Y.; Stern, A.; Peng, H.H.; Chen, J.H.; Yang, H.C. Redox and Metabolic Regulation of Intestinal Barrier Function and Associated Disorders. Int. J. Mol. Sci. 2022, 23, 14463. [Google Scholar] [CrossRef]
- Liu, D.Y.; Lou, W.J.; Zhang, D.Y.; Sun, S.Y. ROS Plays a Role in the Neonatal Rat Intestinal Barrier Damages Induced by Hyperoxia. Biomed. Res. Int. 2020, 2020, 8819195. [Google Scholar] [CrossRef]
- Aviello, G.; Knaus, U.G. NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immunol. 2018, 11, 1011–1023. [Google Scholar] [CrossRef]
- Li, B.; Alli, R.; Vogel, P.; Geiger, T.L. IL-10 modulates DSS-induced colitis through a macrophage-ROS-NO axis. Mucosal Immunol. 2014, 7, 869–878. [Google Scholar] [CrossRef]
- Balmus, I.M.; Cojocariu, R.O.; Ciobica, A.; Strungaru, S.; Strungaru-Jijie, R.; Cantemir, A.; Galatanu, C.; Gorgan, L. Preliminary Study on the Tears Oxidative Stress Status and Sleep Disturbances in Irritable Bowel Syndrome Patients. Oxid. Med. Cell. Longev. 2020, 2020, 4690713. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, H.; Zhang, F.; Hao, L.; Guo, J. Active Ingredients and Potential Mechanisms of the Gan Jiang-Huang Qin-Huang Lian-Ren Shen Decoction against Ulcerative Colitis: A Network Pharmacology and Molecular Docking-Based Study. Evid. Based Complement. Alternat. Med. 2021, 2021, 1925718. [Google Scholar] [CrossRef]
- Lin, C.; Zhou, Z.; Zhang, L.; Wang, H.; Lu, J.; Wang, X.; An, R. Gegen Qinlian Decoction Relieves Ulcerative Colitis via Adjusting Dysregulated Nrf2/ARE Signaling. Evid. Based Complement. Alternat. Med. 2022, 2022, 2934552. [Google Scholar] [CrossRef]
- Chu, J.; Yuan, C.; Zhou, L.; Zhao, Y.; Wu, X.; Yan, Y.; Liu, Y.; Liu, X.; Jing, L.; Dong, T.; et al. JianPiTongLuo (JPTL) Recipe regulates anti-apoptosis and cell proliferation in colorectal cancer through the PI3K/AKT signaling pathway. Heliyon 2024, 10, e35490. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, F.; Li, Y.; Fan, N.; Zhao, K.; Zhang, A.; Kang, J.; Lin, Y.; Xue, X.; Jiang, X. Blockade of PI3K/AKT signaling pathway by Astragaloside IV attenuates ulcerative colitis via improving the intestinal epithelial barrier. J. Transl. Med. 2024, 22, 406. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, J.; Fu, R.; Zhu, C.; Fan, D. The ginsenoside Rk3 exerts anti-esophageal cancer activity in vitro and in vivo by mediating apoptosis and autophagy through regulation of the PI3K/Akt/mTOR pathway. PLoS ONE 2019, 14, e0216759. [Google Scholar] [CrossRef]
- Yang, C.Z.; Wang, S.H.; Zhang, R.H.; Lin, J.H.; Tian, Y.H.; Yang, Y.Q.; Liu, J.; Ma, Y.X. Neuroprotective effect of astragalin via activating PI3K/Akt-mTOR-mediated autophagy on APP/PS1 mice. Cell Death Discov. 2023, 9, 15. [Google Scholar] [CrossRef]
- Bai, D.; Ueno, L.; Vogt, P.K. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int. J. Cancer 2009, 125, 2863–2870. [Google Scholar] [CrossRef]
- Joshi, L.; Agnihotri, P.; Saquib, M.; Chakraborty, D.; Sarkar, A.; Choudhary, B.; Kumar, V.; Biswas, S. The role of ITIH4 in regulating the PI3K/Akt signaling in rheumatoid arthritis. Rheumatology 2025. online ahead of print. [Google Scholar] [CrossRef]
- Xie, D.; Bu, C. TIGAR Alleviates Acute Pancreatitis by Suppressing Glycolysis Through the LAMP2/PI3K/Akt Axis. Biotechnol. Appl. Biochem. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Zheng, D.; Ma, L.; Xin, X.; Gao, Y.; Dong, S.; Huang, Y.; Wu, J.; Huo, J.; Li, S. Ovotransferrin ameliorated intestinal barrier dysfunction in DSS-induced ulcerative colitis mice via inhibition of PI3K-Akt/MAPK signaling pathways and modulation of tissue metabolism. Int. J. Biol. Macromol. 2025, 321, 146274. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Xu, X.; Li, M.; Gao, W.; Li, Y.; Yang, Y.; Yin, D. Platycodon grandiflorus polysaccharides combined with hesperidin exerted the synergistic effect of relieving ulcerative colitis in mice by modulating PI3K/AKT and JAK2/STAT3 signaling pathways. Chin. J. Nat. Med. 2025, 23, 848–862. [Google Scholar] [CrossRef]
- Li, Q.; Wu, S.S.; Chen, B.Z.; Li, M.H.; Wang, T.S.; Li, C. Bioactive components from Pericarpium Citri Reticulatae ‘Chachi’ alleviates ulcerative colitis by PI3K/AKT signaling pathway: Network pharmacology, molecular docking and experimental verification. Fitoterapia 2025, 185, 106736. [Google Scholar] [CrossRef]
- Li, N.; Neu, J. Glutamine deprivation alters intestinal tight junctions via a PI3-K/Akt mediated pathway in Caco-2 cells. J. Nutr. 2009, 139, 710–714. [Google Scholar] [CrossRef]
- Akbari, P.; Braber, S.; Varasteh, S.; Alizadeh, A.; Garssen, J.; Fink-Gremmels, J. The intestinal barrier as an emerging target in the toxicological assessment of mycotoxins. Arch. Toxicol. 2017, 91, 1007–1029. [Google Scholar] [CrossRef]
- Press, B.; Di Grandi, D. Permeability for intestinal absorption: Caco-2 assay and related issues. Curr. Drug Metab. 2008, 9, 893–900. [Google Scholar] [CrossRef]
- Xiong, F.; Yao, H.L.; Wu, Y.H.; Hu, W.H.; Ou, Y.H.; Li, H.J.; Lan, W.J. Sulfation and carboxymethylation of Citrus medica ‘Fingered’ polysaccharides: Physicochemical properties and protective effects against LPS-induced intestinal barrier injury in Caco-2 cells. Int. J. Biol. Macromol. 2025, 321 Pt 1, 146211. [Google Scholar] [CrossRef]
- Ichikawa, M.; Akamine, H.; Murata, M.; Ito, S.; Takayama, K.; Mizuguchi, H. Generation of tetracycline-controllable CYP3A4-expressing Caco-2 cells by the piggyBac transposon system. Sci. Rep. 2021, 11, 11670. [Google Scholar] [CrossRef]
- De Munck, T.J.I.; Xu, P.; Verwijs, H.J.A.; Masclee, A.A.M.; Jonkers, D.; Verbeek, J.; Koek, G.H. Intestinal permeability in human nonalcoholic fatty liver disease: A systematic review and meta-analysis. Liver Int. 2020, 40, 2906–2916. [Google Scholar] [CrossRef]
- Ran, Y.; Lei, J.; Li, L.; Wang, L.; Sun, Y.; Mei, L.; Ye, F.; Dai, F. Particulate matter exposure may increase the risk of irritable bowel syndrome: A large-scale prospective study based on the UK Biobank. Environ. Sci. Nano 2024, 11, 846–854. [Google Scholar] [CrossRef]
- Fu, P.; Li, R.; Sze, S.C.W.; Yung, K.K.L. Associations between fine particulate matter and colorectal cancer: A systematic review and meta-analysis. Rev. Environ. Health 2024, 39, 447–457. [Google Scholar] [CrossRef]
- Ran, Z.; Yang, J.; Liu, L.; Wu, S.; An, Y.; Hou, W.; Cheng, T.; Zhang, Y.; Zhang, Y.; Huang, Y.; et al. Chronic PM (2.5) exposure disrupts intestinal barrier integrity via microbial dysbiosis-triggered TLR2/5-MyD88-NLRP3 inflammasome activation. Environ. Res. 2024, 258, 119415. [Google Scholar] [CrossRef]
- Vandenbroucke, R.E.; Dejonckheere, E.; Van Hauwermeiren, F.; Lodens, S.; De Rycke, R.; Van Wonterghem, E.; Staes, A.; Gevaert, K.; López-Otin, C.; Libert, C. Matrix metalloproteinase 13 modulates intestinal epithelial barrier integrity in inflammatory diseases by activating TNF. EMBO Mol. Med. 2013, 5, 1000–1016. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Guo, Z.; Zhang, R.; Deng, C.; Xu, J.; Dong, W.; Hong, Z.; Yu, H.; Situ, H.; Liu, C.; et al. Nasal epithelial barrier disruption by particulate matter ≤2.5 μm via tight junction protein degradation. J. Appl. Toxicol. 2018, 38, 678–687. [Google Scholar] [CrossRef]
- Lessey, L.R.; Robinson, S.C.; Chaudhary, R.; Daniel, J.M. Adherens junction proteins on the move-From the membrane to the nucleus in intestinal diseases. Front. Cell Dev. Biol. 2022, 10, 998373. [Google Scholar] [CrossRef]
- Dong, Y.; Fan, H.; Zhang, Z.; Jiang, F.; Li, M.; Zhou, H.; Guo, W.; Zhang, Z.; Kang, Z.; Gui, Y.; et al. Berberine ameliorates DSS-induced intestinal mucosal barrier dysfunction through microbiota-dependence and Wnt/β-catenin pathway. Int. J. Biol. Sci. 2022, 18, 1381–1397. [Google Scholar] [CrossRef]
- Pope, C.A., 3rd; Bhatnagar, A.; McCracken, J.P.; Abplanalp, W.; Conklin, D.J.; O’Toole, T. Exposure to Fine Particulate Air Pollution Is Associated With Endothelial Injury and Systemic Inflammation. Circ. Res. 2016, 119, 1204–1214. [Google Scholar] [CrossRef]
- Lu, Y.; Qiu, W.; Liao, R.; Cao, W.; Huang, F.; Wang, X.; Li, M.; Li, Y. Subacute PM2.5 Exposure Induces Hepatic Insulin Resistance Through Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 812. [Google Scholar] [CrossRef]
- Wang, H.; Song, L.; Ju, W.; Wang, X.; Dong, L.; Zhang, Y.; Ya, P.; Yang, C.; Li, F. The acute airway inflammation induced by PM(2.5) exposure and the treatment of essential oils in Balb/c mice. Sci. Rep. 2017, 7, 44256. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, M. PM2.5 induces autophagy and apoptosis through endoplasmic reticulum stress in human endothelial cells. Sci. Total Environ. 2020, 710, 136397. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Deng, B.; Lin, A.; Zhang, G.; Ma, M.; Wang, Y.; Yang, Y.; Kang, X. The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: Mechanisms and therapeutic opportunities. Cell Prolif. 2022, 55, e13275. [Google Scholar] [CrossRef]
- Cianciulli, A.; Calvello, R.; Porro, C.; Trotta, T.; Salvatore, R.; Panaro, M.A. PI3k/Akt signalling pathway plays a crucial role in the anti-inflammatory effects of curcumin in LPS-activated microglia. Int. Immunopharmacol. 2016, 36, 282–290. [Google Scholar] [CrossRef]
- Khan, M.W.; Keshavarzian, A.; Gounaris, E.; Melson, J.E.; Cheon, E.C.; Blatner, N.R.; Chen, Z.E.; Tsai, F.N.; Lee, G.; Ryu, H.; et al. PI3K/AKT signaling is essential for communication between tissue-infiltrating mast cells, macrophages, and epithelial cells in colitis-induced cancer. Clin. Cancer Res. 2013, 19, 2342–2354. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Y.; Li, S.; Du, R.; Shi, J.; Jiang, C.; Wang, R.; Zhu, Y. Novel Small-Molecule miR-124 Inducer Acts as “a Physiological Brake” of Inflammation in Ulcerative Colitis by Targeting the PIK3R2/PI3K/Akt Axis. J. Med. Chem. 2025, 68, 14114–14126. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Dai, J.; Song, B.; Zhang, Y.; Yang, T.; Xu, H.; Xu, X.; Gao, Y.; Yan, T.; Shen, W.; et al. Connexin 43 Prevents Radiation-Induced Intestinal Damage via the Ca2+-Dependent PI3K/Akt Signaling Pathway. Radiat. Res. 2024, 201, 294–303. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, J.; Sun, J.; Xin, L. PM(2.5) induces inflammatory responses via oxidative stress-mediated mitophagy in human bronchial epithelial cells. Toxicol. Res. 2022, 11, 195–205. [Google Scholar] [CrossRef]
- Kurlawala, Z.; Singh, P.; Hill, B.G.; Haberzettl, P. Fine Particulate Matter (PM2.5)-Induced Pulmonary Oxidative Stress Contributes to Changes in the Plasma Lipidome and Liver Transcriptome in Mice. Toxicol. Sci. 2023, 192, 209–222. [Google Scholar] [CrossRef]
- Du, Z.; Lin, L.; Li, Y.; Sun, M.; Liang, Q.; Sun, Z.; Duan, J. Combined exposure to PM(2.5) and high-fat diet facilitates the hepatic lipid metabolism disorders via ROS/miR-155/PPARγ pathway. Free Radic. Biol. Med. 2022, 190, 16–27. [Google Scholar] [CrossRef]
- He, X.; Zhang, L.; Hu, L.; Liu, S.; Xiong, A.; Wang, J.; Xiong, Y.; Li, G. PM2.5 Aggravated OVA-Induced Epithelial Tight Junction Disruption Through Fas Associated via Death Domain-Dependent Apoptosis in Asthmatic Mice. J. Asthma Allergy 2021, 14, 1411–1423. [Google Scholar] [CrossRef]
- Cristaldi, A.; Oliveri Conti, G.; Pellitteri, R.; La Cognata, V.; Copat, C.; Pulvirenti, E.; Grasso, A.; Fiore, M.; Cavallaro, S.; Dell’Albani, P.; et al. In vitro exposure to PM(2.5) of olfactory Ensheathing cells and SH-SY5Y cells and possible association with neurodegenerative processes. Environ. Res. 2024, 241, 117575. [Google Scholar] [CrossRef]
- Ma, S.; Xian, M.; Wang, Y.; Wang, C.; Zhang, L. Budesonide repairs decreased barrier integrity of eosinophilic nasal polyp epithelial cells caused by PM(2.5). Clin. Transl. Allergy 2021, 11, e12019. [Google Scholar] [CrossRef]
- Hong, Z.; Guo, Z.; Zhang, R.; Xu, J.; Dong, W.; Zhuang, G.; Deng, C. Airborne Fine Particulate Matter Induces Oxidative Stress and Inflammation in Human Nasal Epithelial Cells. Tohoku J. Exp. Med. 2016, 239, 117–125. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Chakravarthi, S. Oxidant-Sensitive Inflammatory Pathways and Male Reproductive Functions. Adv. Exp. Med. Biol. 2022, 1358, 165–180. [Google Scholar] [CrossRef]
- Moustakli, E.; Stavros, S.; Katopodis, P.; Skentou, C.; Potiris, A.; Panagopoulos, P.; Domali, E.; Arkoulis, I.; Karampitsakos, T.; Sarafi, E.; et al. Oxidative Stress and the NLRP3 Inflammasome: Focus on Female Fertility and Reproductive Health. Cells 2025, 14, 36. [Google Scholar] [CrossRef]
- Karrasch, T.; Jobin, C. NF-kappaB and the intestine: Friend or foe? Inflamm. Bowel Dis. 2008, 14, 114–124. [Google Scholar] [CrossRef]
- Mikuda, N.; Schmidt-Ullrich, R.; Kärgel, E.; Golusda, L.; Wolf, J.; Höpken, U.E.; Scheidereit, C.; Kühl, A.A.; Kolesnichenko, M. Deficiency in IκBα in the intestinal epithelium leads to spontaneous inflammation and mediates apoptosis in the gut. J. Pathol. 2020, 251, 160–174. [Google Scholar] [CrossRef]
- de Souza Xavier Costa, N.; Ribeiro Júnior, G.; Dos Santos Alemany, A.A.; Belotti, L.; Frota Cavalcante, M.; Ribeiro, S.; Matera Veras, M.; Kallás, E.G.; Saldiva, P.H.N.; Dolhnikoff, M.; et al. LPS Response Is Impaired by Urban Fine Particulate Matter. Int. J. Mol. Sci. 2022, 23, 3913. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Y.; Zhu, L.; Lin, H.; Zhao, X.; Liu, C.; Lv, Y.; Wang, Z.; Zuo, Z.; Wang, J.; et al. Fuzi Lizhong Pill inhibited inflammatory response and promoted colon mucosal healing in dextran sulfate sodium-induced ulcerative colitis mice by down-regulating PI3K/AKT/NF-κB signaling pathway. J. Ethnopharmacol. 2025, 343, 119483. [Google Scholar] [CrossRef] [PubMed]
- Slomiany, B.L.; Slomiany, A. Role of LPS-elicited signaling in triggering gastric mucosal inflammatory responses to H. pylori: Modulatory effect of ghrelin. Inflammopharmacology 2017, 25, 415–429. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Zhou, T.; Wu, J.; Feng, F.; Wang, S.; Chi, Q.; Sha, Y.; Zha, S.; Shu, S.; et al. Gut microbiota-derived butyric acid regulates calcific aortic valve disease pathogenesis by modulating GAPDH lactylation and butyrylation. iMeta 2025, 4, e70048. [Google Scholar] [CrossRef]
- Bae, Y.J.; Park, K.Y.; Han, H.S.; Kim, Y.S.; Hong, J.Y.; Han, T.Y.; Seo, S.J. Effects of Particulate Matter in a Mouse Model of Oxazolone-Induced Atopic Dermatitis. Ann. Dermatol. 2020, 32, 496–507. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Liang, L.M.; Cheng, P.P.; Xiong, L.; Wang, M.; Song, L.J.; Yu, F.; He, X.L.; Xiong, L.; Wang, X.R.; et al. VEGF/Src signaling mediated pleural barrier damage and increased permeability contributes to subpleural pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L990–L1004. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| β-actin | GGACTTCGAGCAAGAGATGG | GGACTTCGAGCAAGAGATGG |
| CTNNB1 | AAAGCGGCTGTTAGTCACTGG | CGAGTCATTGCATACTGTCCAT |
| IL-6 | GCCTTCGGTCCAGTTGCCTTC | GTTCTGAAGAGGTGAGTGGCTGTC |
| TNF-α | CAATGGCGTGGAGCTGAGAGATAAC | TCTGGTAGGAGACGGCGATGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, R.; Zhang, Q.; Lu, Y.; Huang, F.; Cao, W.; Li, M.; Zhou, L.; Li, Y. Fine Particulate Matter (PM2.5) Disrupts Intestinal Barrier Function by Inducing Oxidative Stress and PI3K/AKT-Mediated Inflammation in Caco-2 Cells. Int. J. Mol. Sci. 2025, 26, 8271. https://doi.org/10.3390/ijms26178271
Liao R, Zhang Q, Lu Y, Huang F, Cao W, Li M, Zhou L, Li Y. Fine Particulate Matter (PM2.5) Disrupts Intestinal Barrier Function by Inducing Oxidative Stress and PI3K/AKT-Mediated Inflammation in Caco-2 Cells. International Journal of Molecular Sciences. 2025; 26(17):8271. https://doi.org/10.3390/ijms26178271
Chicago/Turabian StyleLiao, Ruiwei, Qianwen Zhang, Yao Lu, Feifei Huang, Wenjuan Cao, Ming Li, Lin Zhou, and Yan Li. 2025. "Fine Particulate Matter (PM2.5) Disrupts Intestinal Barrier Function by Inducing Oxidative Stress and PI3K/AKT-Mediated Inflammation in Caco-2 Cells" International Journal of Molecular Sciences 26, no. 17: 8271. https://doi.org/10.3390/ijms26178271
APA StyleLiao, R., Zhang, Q., Lu, Y., Huang, F., Cao, W., Li, M., Zhou, L., & Li, Y. (2025). Fine Particulate Matter (PM2.5) Disrupts Intestinal Barrier Function by Inducing Oxidative Stress and PI3K/AKT-Mediated Inflammation in Caco-2 Cells. International Journal of Molecular Sciences, 26(17), 8271. https://doi.org/10.3390/ijms26178271







