The Effect of Fibulin-5 on Hydrocephalus After Subarachnoid Hemorrhage in Mice
Abstract
1. Introduction
2. Results
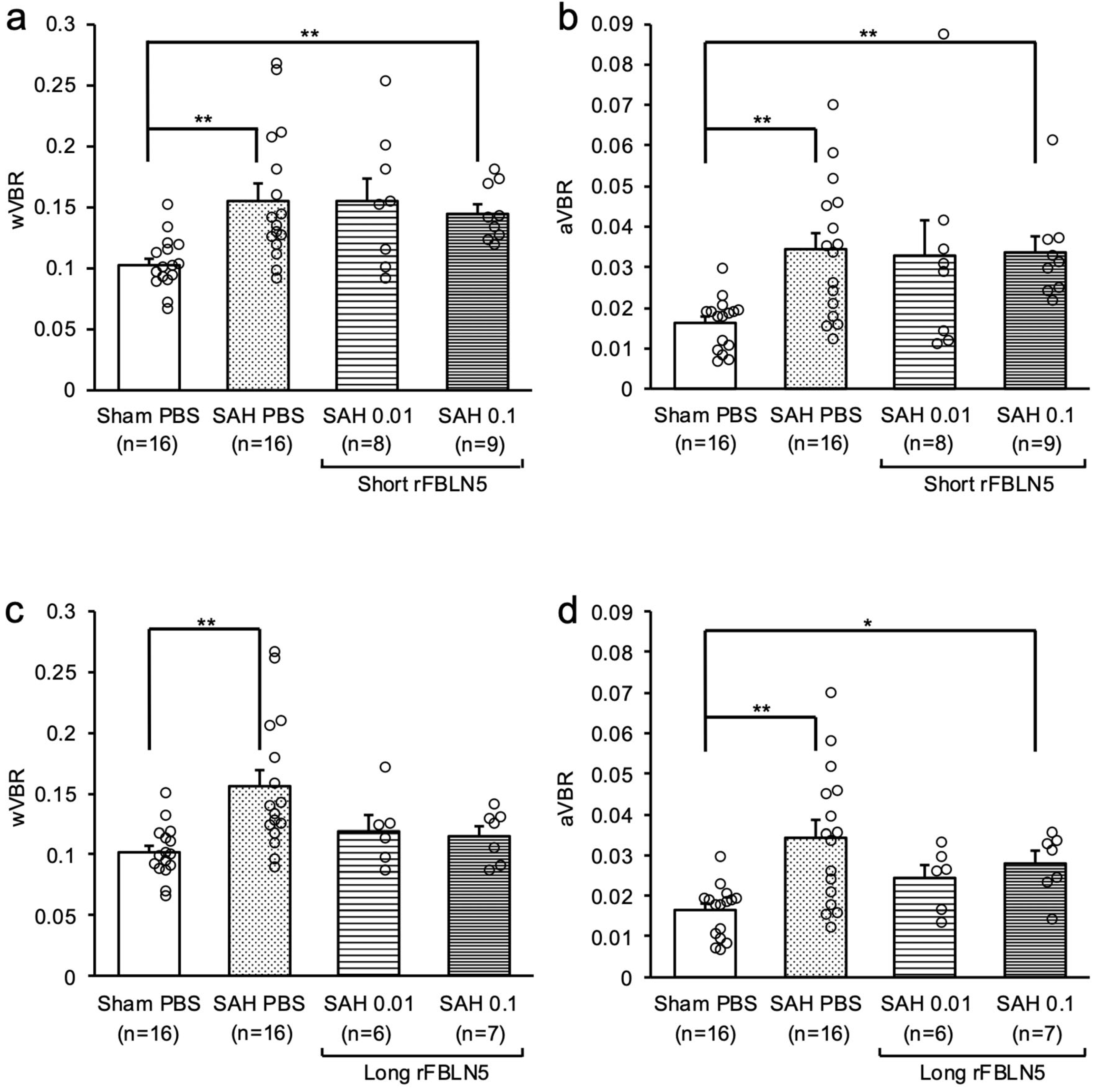
2.1. Effects of FBLN5 on Ventricular Enlargement at an Acute Phase
2.2. Inhibitory Effects of FBLN5 on Ventricular Enlargement at a Later Phase
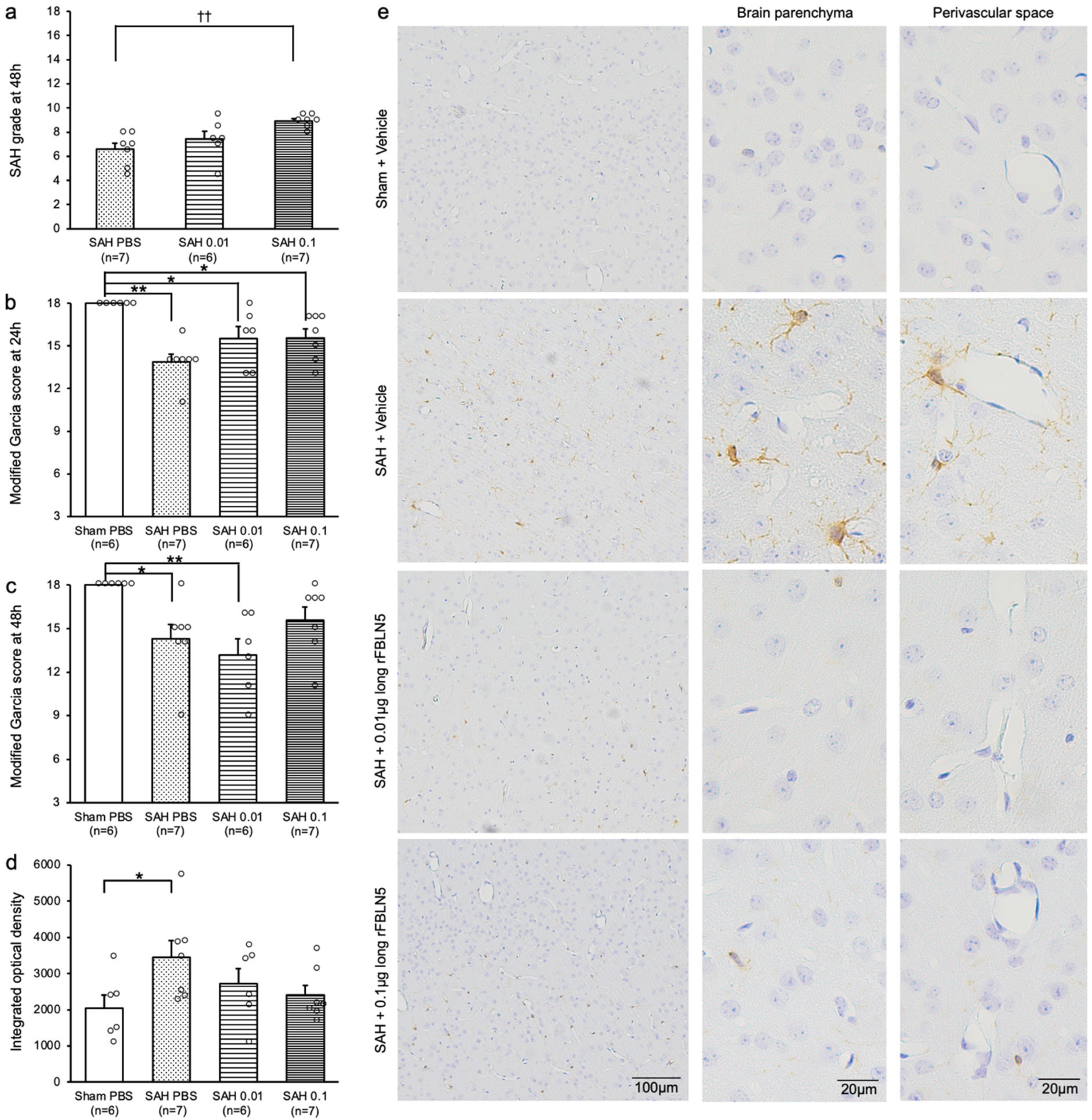
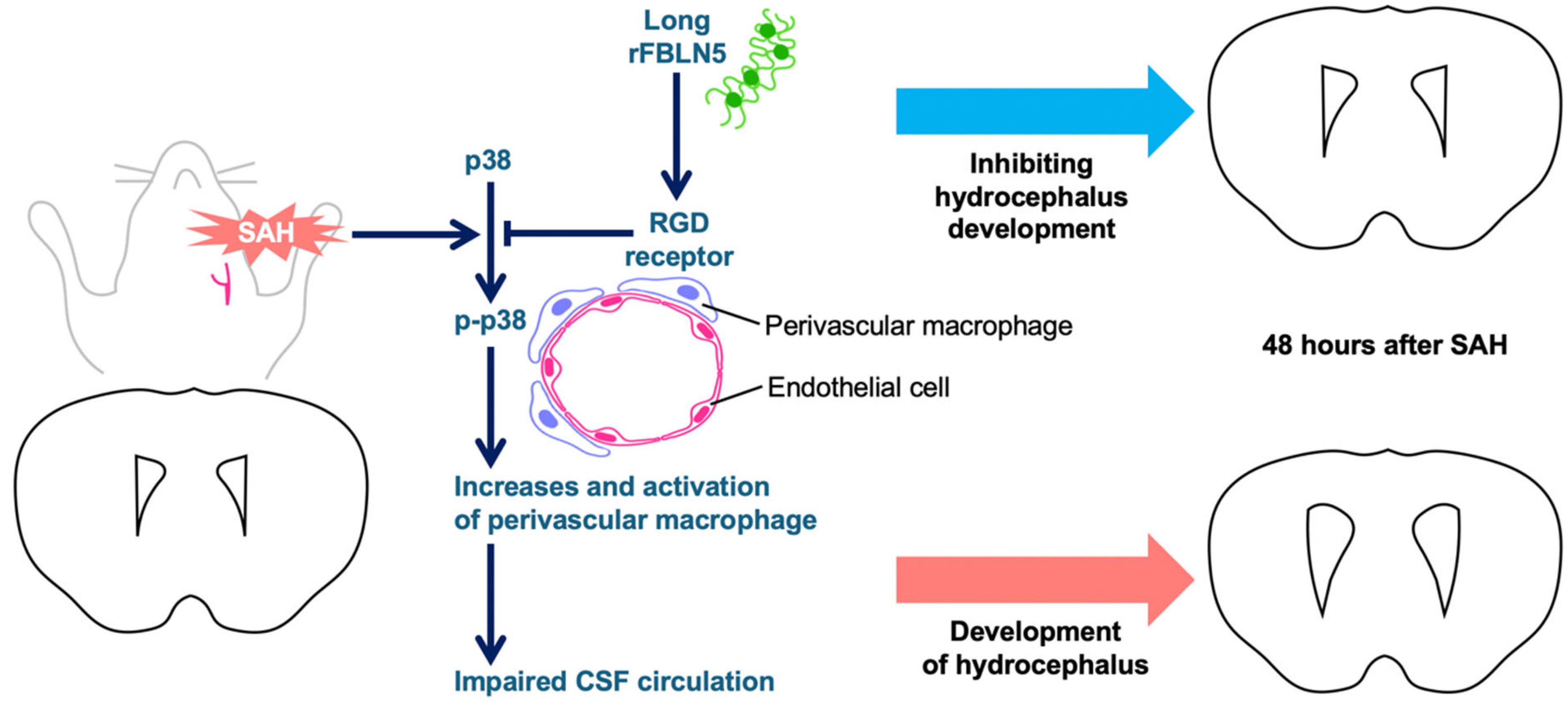
2.3. Exploring Mechanisms for LrFBLN5 to Prevent Post-SAH Ventricular Dilatation
2.4. Effects of LrFBLN5 on Ionized Calcium-Binding Adaptor Molecule 1 (Iba1)-Positive Cells in the Brain Parenchyma and Perivascular Space
3. Discussion
4. Materials and Methods
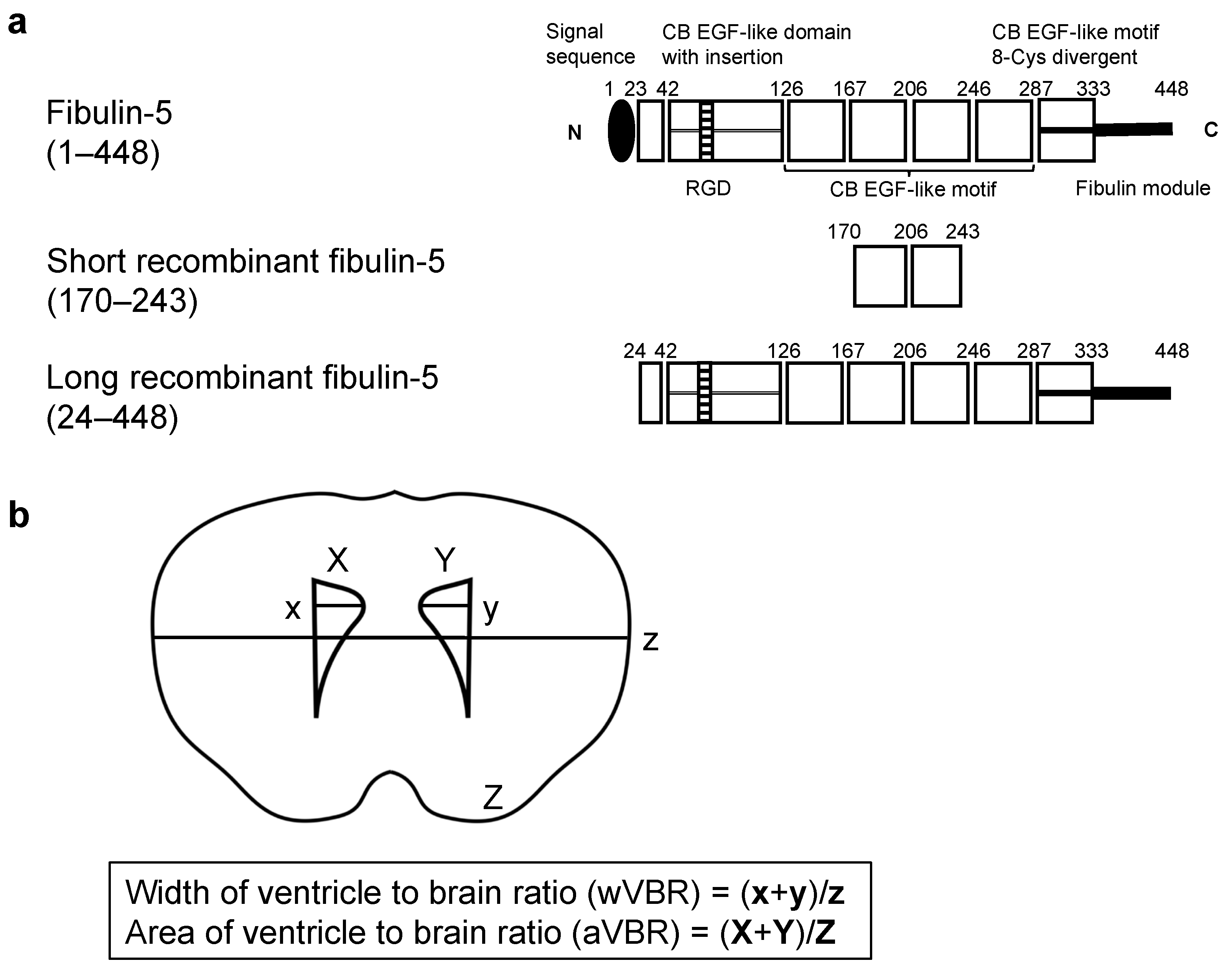
4.1. Study Protocols (Figure 1)
4.2. rFBLN5 (Figure 2a)
4.3. SAH Modeling
4.4. ICV
4.5. Neurobehavioral Test
4.6. SAH Grade and Exclusion Criteria
4.7. VBR (Figure 2b)
4.8. WB
4.9. Immunohistochemical Staining
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Macdonald, R.L.; Schweizer, T.A. Spontaneous subarachnoid haemorrhage. Lancet 2017, 389, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Neifert, S.N.; Chapman, E.K.; Martini, M.L.; Shuman, W.H.; Schupper, A.J.; Oermann, E.K.; Mocco, J.; Macdonald, R.L. Aneurysmal subarachnoid hemorrhage: The last decade. Transl. Stroke Res. 2021, 12, 428–446. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Alaraj, A.; Calderon, M.; Herrera, S.R.; Gao, W.; Ruland, S.; Roitberg, B.Z. Prediction of ventriculoperitoneal shunt dependency in patients with aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2009, 110, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Hu, X.; Zan, X.; Lin, S.; Li, H.; You, C. Predictors of shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage? A systematic review and meta-analysis. World Neurosurg. 2017, 106, 844–860.e6. [Google Scholar] [CrossRef]
- Germanwala, A.V.; Huang, J.; Tamargo, R.J. Hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurg. Clin. N. Am. 2010, 21, 263–270. [Google Scholar] [CrossRef]
- Winkler, E.A.; Burkhardt, J.K.; Rutledge, W.C.; Rick, J.W.; Partow, C.P.; Yue, J.K.; Birk, H.; Bach, A.M.; Raygor, K.P.; Lawton, M.T. Reduction of shunt dependency rates following aneurysmal subarachnoid hemorrhage by tandem fenestration of the lamina terminalis and membrane of Liliequist during microsurgical aneurysm repair. J. Neurosurg. 2018, 129, 1166–1172. [Google Scholar] [CrossRef]
- Paisan, G.M.; Ding, D.; Starke, R.M.; Crowley, R.W.; Liu, K.C. Shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage: Predictors and long-term functional outcomes. Neurosurgery 2018, 83, 393–402. [Google Scholar] [CrossRef]
- Haug Nordenmark, T.; Karic, T.; Sorteberg, W.; Sorteberg, A. Predictors of cognitive function in the acute phase after aneurysmal subarachnoid hemorrhage. Acta Neurochir. 2019, 161, 177–184. [Google Scholar] [CrossRef]
- Di Russo, P.; Di Carlo, D.T.; Lutenberg, A.; Morganti, R.; Evins, A.I.; Perrini, P. Shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. J. Neurosurg. Sci. 2020, 64, 181–189. [Google Scholar] [CrossRef]
- Zaidi, H.A.; Montoure, A.; Elhadi, A.; Nakaji, P.; McDougall, C.G.; Albuquerque, F.C.; Spetzler, R.F.; Zabramski, J.M. Long-term functional outcomes and predictors of shunt-dependent hydrocephalus after treatment of ruptured intracranial aneurysms in the BRAT trial: Revisiting the clip vs coil debate. Neurosurgery 2015, 76, 608–614. [Google Scholar] [CrossRef]
- Dóczi, T.; Nemessányi, Z.; Szegváry, Z.; Huszka, E. Disturbances of cerebrospinal fluid circulation during the acute stage of subarachnoid hemorrhage. Neurosurgery 1983, 12, 435–438. [Google Scholar] [CrossRef]
- Wilson, C.D.; Safavi-Abbasi, S.; Sun, H.; Kalani, M.Y.; Zhao, Y.D.; Levitt, M.R.; Hanel, R.A.; Sauvageau, E.; Mapstone, T.B.; Albuquerque, F.C.; et al. Meta-analysis and systematic review of risk factors for shunt dependency after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2017, 126, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Czorlich, P.; Ricklefs, F.; Reitz, M.; Vettorazzi, E.; Abboud, T.; Regelsberger, J.; Westphal, M.; Schmidt, N.O. Impact of intraventricular hemorrhage measured by Graeb and LeRoux score on case fatality risk and chronic hydrocephalus in aneurysmal subarachnoid hemorrhage. Acta Neurochir. 2015, 157, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Vara-Pérez, M.; Movahedi, K. Border-associated macrophages as gatekeepers of brain homeostasis and immunity. Immunity 2025, 58, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Drieu, A.; Du, S.; Storck, S.E.; Rustenhoven, J.; Papadopoulos, Z.; Dykstra, T.; Zhong, F.; Kim, K.; Blackburn, S.; Mamuladze, T.; et al. Parenchymal border macrophages regulate the flow dynamics of the cerebrospinal fluid. Nature 2022, 611, 585–593. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Schluterman, M.K.; Brekken, R.A. Fibulin-5, an integrin-binding matricellular protein: Its function in development and disease. J. Cell Commun. Signal. 2009, 3, 337–347. [Google Scholar] [CrossRef]
- Kowal, R.C.; Richardson, J.A.; Miano, J.M.; Olson, E.N. EVEC, a novel epidermal growth factor-like repeat-containing protein upregulated in embryonic and diseased adult vasculature. Circ. Res. 1999, 84, 1166–1176. [Google Scholar] [CrossRef]
- Nakamura, T.; Ruiz-Lozano, P.; Lindner, V.; Yabe, D.; Taniwaki, M.; Furukawa, Y.; Kobuke, K.; Tashiro, K.; Lu, Z.; Andon, N.L.; et al. DANCE, a novel secreted RGD protein expressed in developing, atherosclerotic, and balloon-injured arteries. J. Biol. Chem. 1999, 274, 22476–22483. [Google Scholar] [CrossRef]
- Suzuki, Y.; Okada, T.; Oinaka, H.; Nakajima, H.; Nampei, M.; Kawakita, F.; Suzuki, H.; pSEED group. Independent elevation of plasma fibulin-5 proceeding chronic hydrocephalus development after aneurysmal subarachnoid hemorrhage. Clin. Neurol. Neurosurg. 2024, 247, 108634. [Google Scholar] [CrossRef]
- Nakasaki, M.; Hwang, Y.; Xie, Y.; Kataria, S.; Gund, R.; Hajam, E.Y.; Samuel, R.; George, R.; Danda, D.; Mj, P.; et al. The matrix protein Fibulin-5 is at the interface of tissue stiffness and inflammation in fibrosis. Nat. Commun. 2015, 6, 8574. [Google Scholar] [CrossRef]
- Manders, D.B.; Kishore, H.A.; Gazdar, A.F.; Keller, P.W.; Tsunezumi, J.; Yanagisawa, H.; Lea, J.; Word, R.A. Dysregulation of fibulin-5 and matrix metalloproteases in epithelial ovarian cancer. Oncotarget 2018, 9, 14251–14267. [Google Scholar] [CrossRef]
- Orešković, D.; Klarica, M. Development of hydrocephalus and classical hypothesis of cerebrospinal fluid hydrodynamics: Facts and illusions. Prog. Neurobiol. 2011, 94, 238–258. [Google Scholar] [CrossRef] [PubMed]
- Strahle, J.; Garton, H.J.; Maher, C.O.; Muraszko, K.M.; Keep, R.F.; Xi, G. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl. Stroke Res. 2012, 3 (Suppl. 1), 25–38. [Google Scholar] [CrossRef] [PubMed]
- Uchikawa, H.; Uekawa, K.; Hasegawa, Y. Perivascular macrophages in cerebrovascular diseases. Exp. Neurol. 2024, 374, 114680. [Google Scholar] [CrossRef]
- Yang, T.; Guo, R.; Zhang, F. Brain perivascular macrophages: Recent advances and implications in health and diseases. CNS Neurosci. Ther. 2019, 25, 1318–1328. [Google Scholar] [CrossRef]
- Vergouwen, M.D.; Vermeulen, M.; Coert, B.A.; Stroes, E.S.; Roos, Y.B. Microthrombosis after aneurysmal subarachnoid hemorrhage: An additional explanation for delayed cerebral ischemia. J. Cereb. Blood Flow Metab. 2008, 28, 1761–1770. [Google Scholar] [CrossRef]
- McBride, D.W.; Blackburn, S.L.; Peeyush, K.T.; Matsumura, K.; Zhang, J.H. The role of thromboinflammation in delayed cerebral ischemia after subarachnoid hemorrhage. Front. Neurol. 2017, 8, 555. [Google Scholar] [CrossRef]
- Wan, H.; Brathwaite, S.; Ai, J.; Hynynen, K.; Macdonald, R.L. Role of perivascular and meningeal macrophages in outcome following experimental subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2021, 41, 1842–1857. [Google Scholar] [CrossRef]
- Lv, T.; Zhao, B.; Hu, Q.; Zhang, X. The glymphatic system: A novel therapeutic target for stroke treatment. Front. Aging Neurosci. 2021, 13, 689098. [Google Scholar] [CrossRef]
- Gerganova, G.; Riddell, A.; Miller, A.A. CNS border-associated macrophages in the homeostatic and ischaemic brain. Pharmacol. Ther. 2022, 240, 108220. [Google Scholar] [CrossRef]
- Nakamura, T.; Lozano, P.R.; Ikeda, Y.; Iwanaga, Y.; Hinek, A.; Minamisawa, S.; Cheng, C.F.; Kobuke, K.; Dalton, N.; Takada, Y.; et al. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature 2002, 415, 171–175. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Davis, E.C.; Starcher, B.C.; Ouchi, T.; Yanagisawa, M.; Richardson, J.A.; Olson, E.N. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 2002, 415, 168–171. [Google Scholar] [CrossRef]
- Peng, W.; Xie, Y.; Liu, Y.; Xu, J.; Yuan, F.; Li, C.; Qin, T.; Lu, H.; Duan, C.; Hu, J. Targeted delivery of CD163+ macrophage-derived small extracellular vesicles via RGD peptides promote vascular regeneration and stabilization after spinal cord injury. J. Control. Release 2023, 361, 750–765. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nampei, M.; Kawakita, F.; Oinaka, H.; Nakajima, H.; Suzuki, H. The effect of Fibulin-5 on early brain injury after subarachnoid hemorrhage in mice. Neurochem. Int. 2025, 187, 105989. [Google Scholar] [CrossRef] [PubMed]
- Douglas, M.R.; Daniel, M.; Lagord, C.; Akinwunmi, J.; Jackowski, A.; Cooper, C.; Berry, M.; Logan, A. High CSF transforming growth factor beta levels after subarachnoid haemorrhage: Association with chronic communicating hydrocephalus. J. Neurol. Neurosurg. Psychiatry 2009, 80, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, S.; Dimitriou, I. TGF beta1 and TGF beta2 and their role in posthemorrhagic hydrocephalus following SAH and IVH. J. Neurol. Surg. Part Cent. Eur. Neurosurg. 2013, 74, 279–284. [Google Scholar] [CrossRef]
- Dobolyi, A.; Vincze, C.; Pál, G.; Lovas, G. The neuroprotective functions of transforming growth factor beta proteins. Int. J. Mol. Sci. 2012, 13, 8219–8258. [Google Scholar] [CrossRef]
- Chen, H.; Chen, L.; Xie, D.; Niu, J. Protective effects of transforming growth factor-β1 knockdown in human umbilical cord mesenchymal stem cells against subarachnoid hemorrhage in a rat model. Cerebrovasc. Dis. 2020, 49, 79–87. [Google Scholar] [CrossRef]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef]
- Ji, H.; Tang, H.; Lin, H.; Mao, J.; Gao, L.; Liu, J.; Wu, T. Rho/Rock cross-talks with transforming growth factor-β/Smad pathway participates in lung fibroblast-myofibroblast differentiation. Biomed. Rep. 2014, 2, 787–792. [Google Scholar] [CrossRef]
- Lodyga, M.; Hinz, B. TGF-β1—A truly transforming growth factor in fibrosis and immunity. In Seminars in Cell & Developmental Biology; Academic Press: New York, NY, USA, 2020; Volume 101, pp. 123–139. [Google Scholar] [CrossRef]
- Runyan, C.E.; Schnaper, H.W.; Poncelet, A.C. Smad3 and PKCdelta mediate TGF-beta1-induced collagen I expression in human mesangial cells. Am. J. Physiol.-Ren. Physiol. 2003, 285, F413–F422. [Google Scholar] [CrossRef]
- Frangogiannis, N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Suzuki, H.; Kanamaru, K.; Shiba, M.; Fujimoto, M.; Imanaka-Yoshida, K.; Yoshida, T.; Taki, W. Cerebrospinal fluid tenascin-C in cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J. Neurosurg. Anesthesiol. 2011, 23, 310–317. [Google Scholar] [CrossRef]
- Suzuki, H.; Kanamaru, K.; Suzuki, Y.; Aimi, Y.; Matsubara, N.; Araki, T.; Takayasu, M.; Kinoshita, N.; Imanaka-Yoshida, K.; Yoshida, T.; et al. Tenascin-C is induced in cerebral vasospasm after subarachnoid hemorrhage in rats and humans: A pilot study. Neurol. Res. 2010, 32, 179–184. [Google Scholar] [CrossRef]
- Chiquet-Ehrismann, R.; Chiquet, M. Tenascins: Regulation and putative functions during pathological stress. J. Pathol. 2003, 200, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Chiovaro, F.; Chiquet-Ehrismann, R.; Chiquet, M. Transcriptional regulation of tenascin genes. Cell Adhes. Migr. 2015, 9, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Borel, C.O.; McKee, A.; Parra, A.; Haglund, M.M.; Solan, A.; Prabhakar, V.; Sheng, H.; Warner, D.S.; Niklason, L. Possible role for vascular cell proliferation in cerebral vasospasm after subarachnoid hemorrhage. Stroke 2003, 34, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, K.; Tada, T. Elevation of transforming growth factor-beta 1 level in cerebrospinal fluid of patients with communicating hydrocephalus after subarachnoid hemorrhage. Stroke 1994, 25, 1400–1404. [Google Scholar] [CrossRef]
- Deng, X.; Ding, J.; Liu, C.; Wang, Z.; Wang, J.; Duan, Q.; Li, W.; Chen, X.; Tang, X.; Zhao, L. Progressive histological and behavioral deterioration of a novel mouse model of secondary hydrocephalus after subarachnoid hemorrhage. Sci. Rep. 2024, 14, 31794. [Google Scholar] [CrossRef]
- Nakano, F.; Kanamaru, H.; Kawakita, F.; Liu, L.; Nakatsuka, Y.; Nishikawa, H.; Okada, T.; Suzuki, H. Epidermal growth factor receptor mediates neuronal apoptosis after subarachnoid hemorrhage in mice. Stroke 2023, 54, 1616–1626. [Google Scholar] [CrossRef]
- Kanamaru, H.; Kawakita, F.; Nishikawa, H.; Nakano, F.; Asadada, R.; Suzuki, H. Clarithromycin ameliorates early brain injury after subarachnoid hemorrhage via suppressing periostin-related pathway in mice. Neurotherapeutics 2021, 18, 1880–1890. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Ayer, R.; Jadhav, V.; Zhang, J.H. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J. Neurosci. Methods 2008, 167, 327–334. [Google Scholar] [CrossRef]
- Xu, H.; Miyajima, M.; Nakajima, M.; Ogino, I.; Kawamura, K.; Akiba, C.; Kamohara, C.; Sakamoto, K.; Karagiozov, K.; Nakamura, E.; et al. Ptpn20 deletion in H-Tx rats enhances phosphorylation of the NKCC1 cotransporter in the choroid plexus: An evidence of genetic risk for hydrocephalus in an experimental study. Fluids Barriers CNS 2022, 19, 39. [Google Scholar] [CrossRef]
- Shiba, M.; Fujimoto, M.; Imanaka-Yoshida, K.; Yoshida, T.; Taki, W.; Suzuki, H. Tenascin-C causes neuronal apoptosis after subarachnoid hemorrhage in rats. Transl. Stroke Res. 2014, 5, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fujimoto, M.; Nakano, F.; Nishikawa, H.; Okada, T.; Kawakita, F.; Imanaka-Yoshida, K.; Yoshida, T.; Suzuki, H. Deficiency of tenascin-C alleviates neuronal apoptosis and neuroinflammation after experimental subarachnoid hemorrhage in mice. Mol. Neurobiol. 2018, 55, 8346–8354. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, Y.; Nampei, M.; Kawakita, F.; Oinaka, H.; Nakajima, H.; Suzuki, H. The Effect of Fibulin-5 on Hydrocephalus After Subarachnoid Hemorrhage in Mice. Int. J. Mol. Sci. 2025, 26, 8259. https://doi.org/10.3390/ijms26178259
Suzuki Y, Nampei M, Kawakita F, Oinaka H, Nakajima H, Suzuki H. The Effect of Fibulin-5 on Hydrocephalus After Subarachnoid Hemorrhage in Mice. International Journal of Molecular Sciences. 2025; 26(17):8259. https://doi.org/10.3390/ijms26178259
Chicago/Turabian StyleSuzuki, Yume, Mai Nampei, Fumihiro Kawakita, Hiroki Oinaka, Hideki Nakajima, and Hidenori Suzuki. 2025. "The Effect of Fibulin-5 on Hydrocephalus After Subarachnoid Hemorrhage in Mice" International Journal of Molecular Sciences 26, no. 17: 8259. https://doi.org/10.3390/ijms26178259
APA StyleSuzuki, Y., Nampei, M., Kawakita, F., Oinaka, H., Nakajima, H., & Suzuki, H. (2025). The Effect of Fibulin-5 on Hydrocephalus After Subarachnoid Hemorrhage in Mice. International Journal of Molecular Sciences, 26(17), 8259. https://doi.org/10.3390/ijms26178259






