Author Contributions
Conceptualization, I.C.d.S., P.S.d.S.S., R.B.B.F., L.A.C., T.J.M.P. and M.C.M.; Data curation, I.C.d.S., K.C.N.L., L.H.H.G., N.T.M. and M.C.M.; Formal analysis, I.C.d.S., P.S.d.S.S., K.C.N.L., R.B.B.F., L.H.H.G., N.T.M. and M.C.M.; Funding acquisition, N.T.M. and M.C.M.; Investigation, I.C.d.S., P.S.d.S.S., K.C.N.L., R.B.B.F., A.L.P.d.S., A.C.d.S.M., G.Q.P., A.G.d.S.D., R.C.d.S.P., L.A.C., T.J.M.P., L.H.H.G. and M.C.M.; Methodology, P.S.d.S.S., K.C.N.L., R.B.B.F., A.L.P.d.S., A.C.d.S.M., G.Q.P., A.G.d.S.D., R.C.d.S.P., L.A.C., T.J.M.P. and M.C.M.; Project administration, N.T.M. and M.C.M.; Resources, N.T.M. and M.C.M.; Software, I.C.d.S., K.C.N.L., L.H.H.G., N.T.M. and M.C.M.; Supervisation, N.T.M. and M.C.M.; Validation, N.T.M. and M.C.M.; Visualization, K.C.N.L., N.T.M. and M.C.M.; Writing—original draft, I.C.d.S., P.S.d.S.S., R.B.B.F. and M.C.M.; Writing—review & editing, I.C.d.S., P.S.d.S.S., K.C.N.L., R.B.B.F., N.T.M. and M.C.M. All authors have read and agreed to the published version of the manuscript.
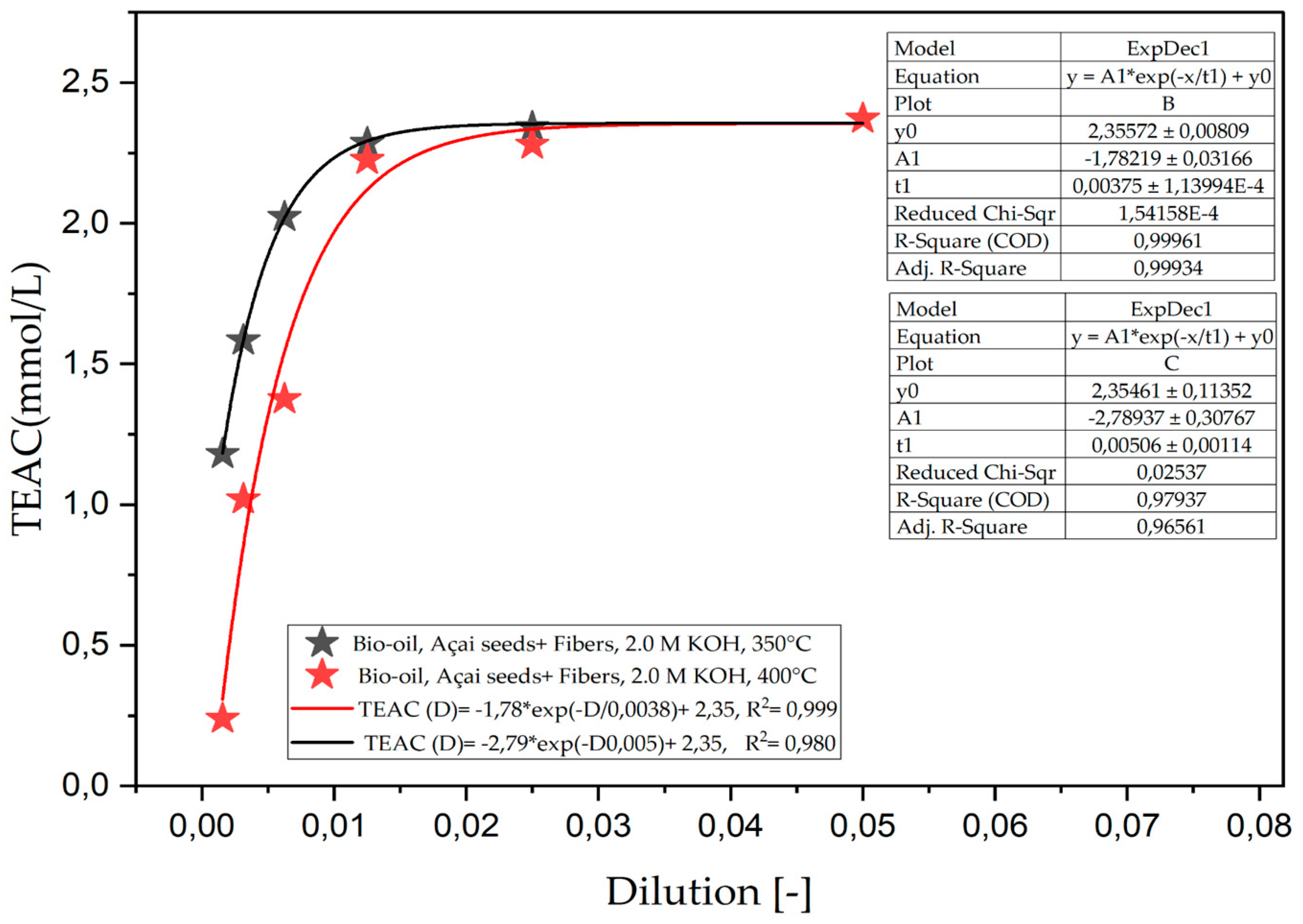
Figure 1.
Total antioxidant capacity (TEAC) of the organic phase (bio-oil) obtained via the pyrolysis of açaí seeds + fiber, chemically activated with 2.0 M KOH solutions at temperatures of 350 and 400 °C on a laboratory scale.
Figure 1.
Total antioxidant capacity (TEAC) of the organic phase (bio-oil) obtained via the pyrolysis of açaí seeds + fiber, chemically activated with 2.0 M KOH solutions at temperatures of 350 and 400 °C on a laboratory scale.
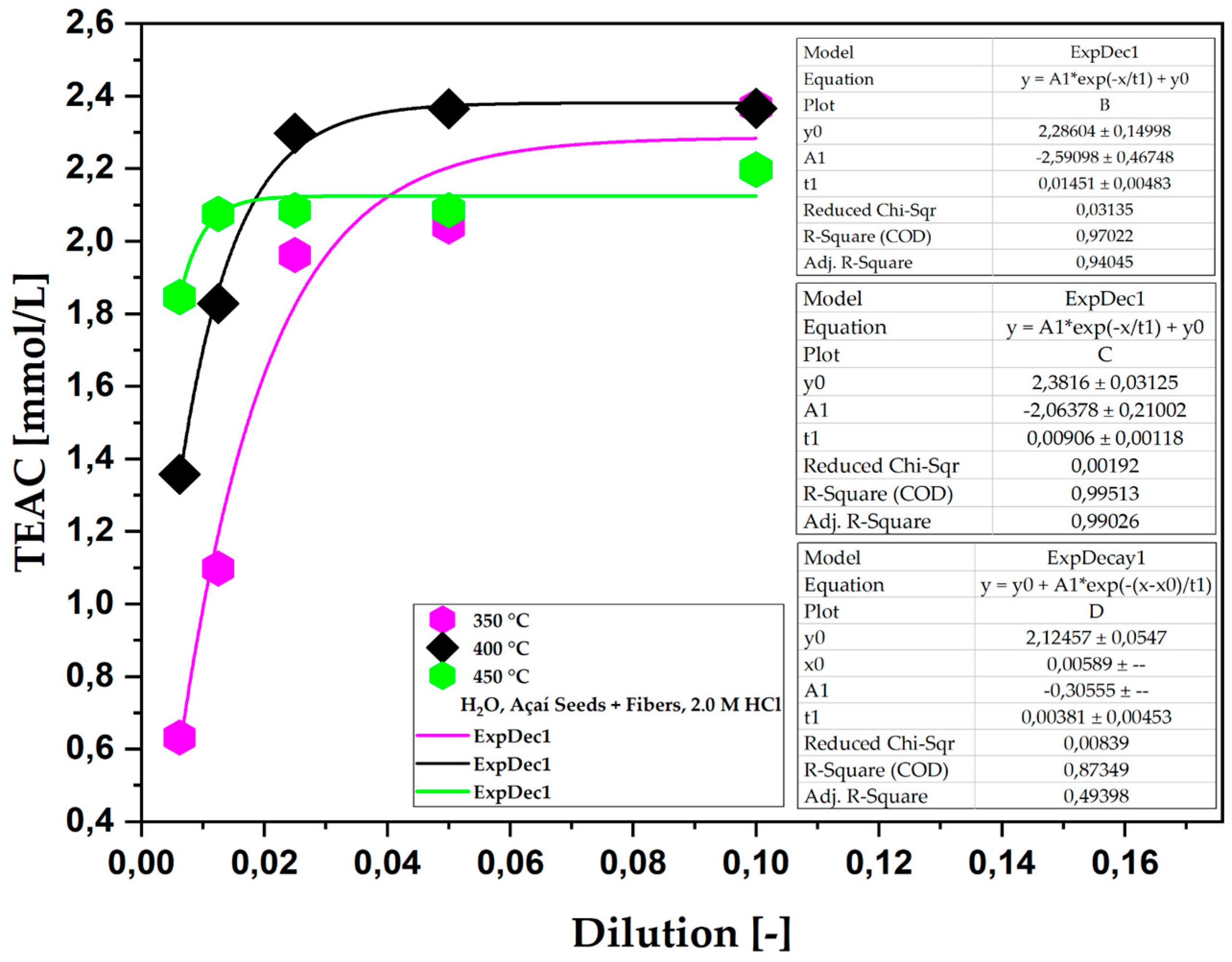
Figure 2.
TEAC of the aqueous phase obtained via the pyrolysis of açaí seeds + fiber, chemically activated with 2.0 M HCl solutions at temperatures of 350, 400, and 450 °C on a laboratory scale.
Figure 2.
TEAC of the aqueous phase obtained via the pyrolysis of açaí seeds + fiber, chemically activated with 2.0 M HCl solutions at temperatures of 350, 400, and 450 °C on a laboratory scale.
Figure 3.
Total antioxidant capacity (TEAC) of the organic phase (bio-oil) obtained via the pyrolysis of açaí seeds + fiber, chemically activated with 2.0 M KOH solutions at a temperature of 450 °C on a laboratory scale.
Figure 3.
Total antioxidant capacity (TEAC) of the organic phase (bio-oil) obtained via the pyrolysis of açaí seeds + fiber, chemically activated with 2.0 M KOH solutions at a temperature of 450 °C on a laboratory scale.
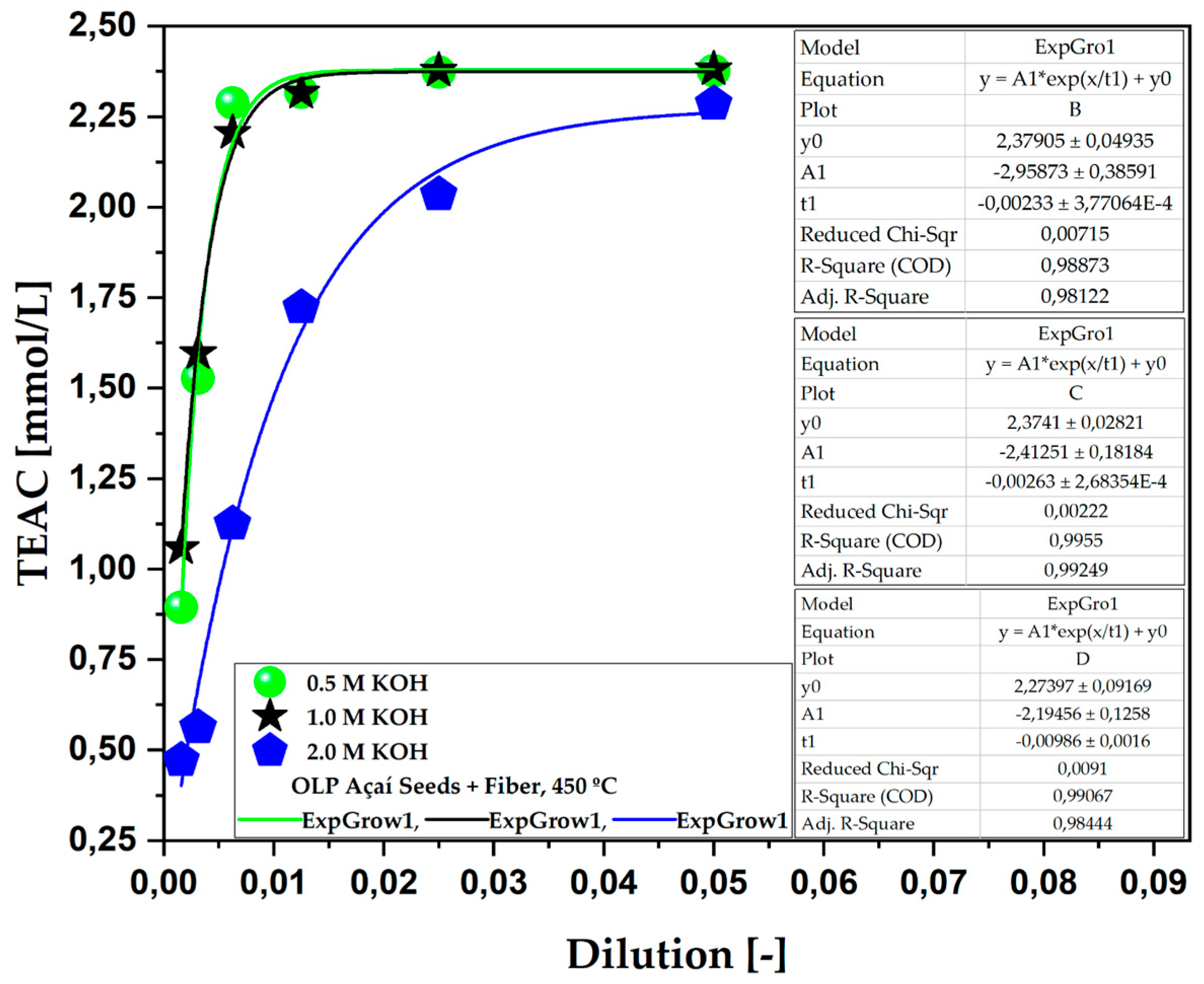
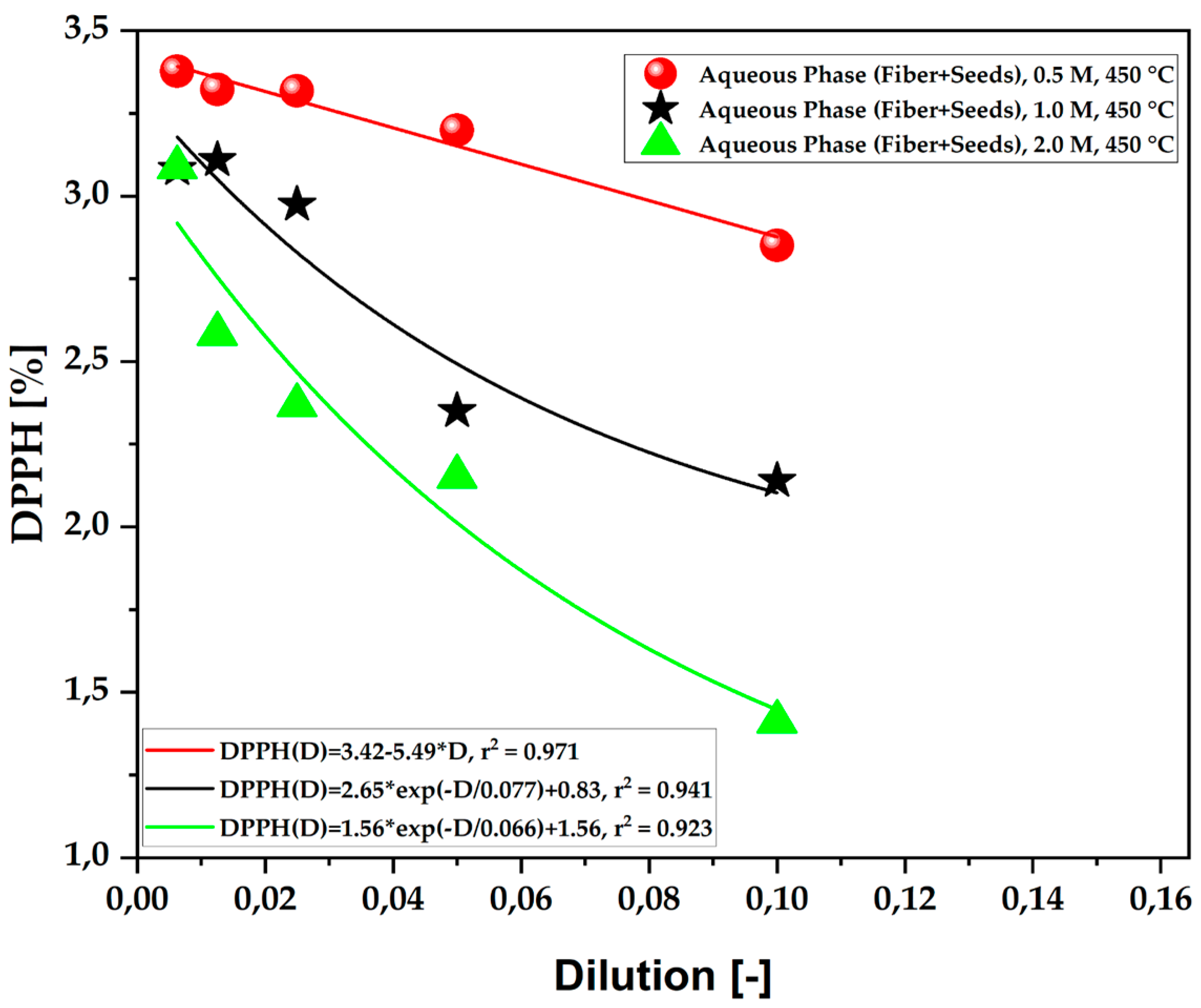
Figure 4.
TEAC of the aqueous phase obtained via the pyrolysis of açaí seeds + fiber, chemically activated with KOH solutions at 450 °C and molarities of 0.5 M, 1.0 M, and 2.0 M on a laboratory scale.
Figure 4.
TEAC of the aqueous phase obtained via the pyrolysis of açaí seeds + fiber, chemically activated with KOH solutions at 450 °C and molarities of 0.5 M, 1.0 M, and 2.0 M on a laboratory scale.
Figure 5.
Antioxidant capacity by the (DPPH•) method of the aqueous phase obtained via pyrolysis of açaí seeds + fiber, chemically activated with 2.0 M potassium hydroxide (KOH) solutions at temperatures of 350 °C and 400 °C.
Figure 5.
Antioxidant capacity by the (DPPH•) method of the aqueous phase obtained via pyrolysis of açaí seeds + fiber, chemically activated with 2.0 M potassium hydroxide (KOH) solutions at temperatures of 350 °C and 400 °C.
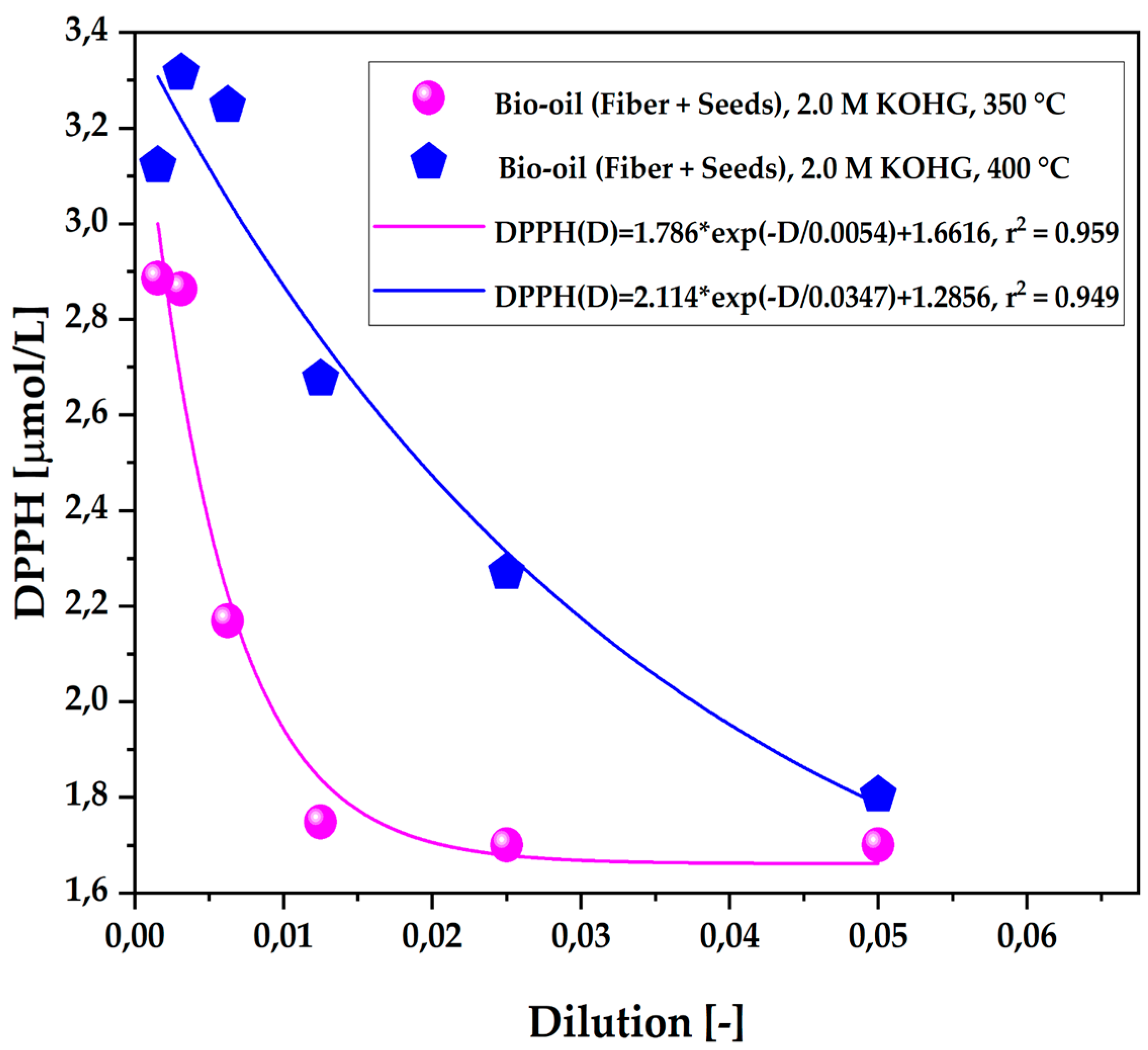
Figure 6.
Antioxidant capacity by the 1,1-diphenyl-2-picrylhydrazyl (DPPH•) method of the organic fraction of bio-oil obtained via pyrolysis of açaí seeds + fiber, chemically activated with 2.0 M KOH solutions at temperatures of 350 °C and 400 °C on a laboratory scale.
Figure 6.
Antioxidant capacity by the 1,1-diphenyl-2-picrylhydrazyl (DPPH•) method of the organic fraction of bio-oil obtained via pyrolysis of açaí seeds + fiber, chemically activated with 2.0 M KOH solutions at temperatures of 350 °C and 400 °C on a laboratory scale.
Figure 7.
Antioxidant capacity by the 1,1-diphenyl-2-picrylhydrazyl (DPPH•) method of the aqueous fraction of bio-oil obtained via the pyrolysis of açaí seeds + fiber, chemically activated with KOH solutions at different molarities (0.5 M–2.0 M) at a temperature of 450 °C on a laboratory scale.
Figure 7.
Antioxidant capacity by the 1,1-diphenyl-2-picrylhydrazyl (DPPH•) method of the aqueous fraction of bio-oil obtained via the pyrolysis of açaí seeds + fiber, chemically activated with KOH solutions at different molarities (0.5 M–2.0 M) at a temperature of 450 °C on a laboratory scale.
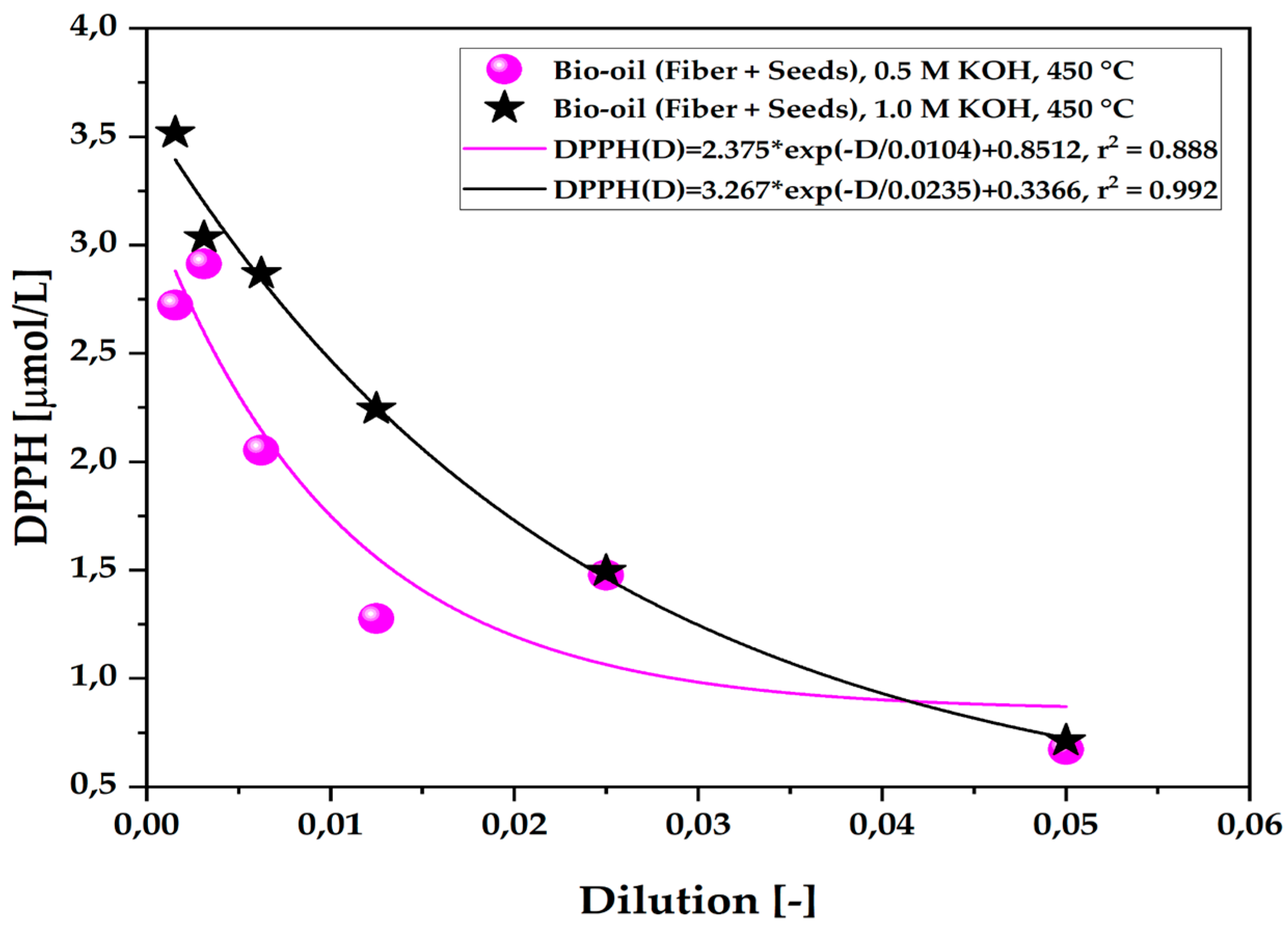
Figure 8.
Antioxidant capacity by the 1,1-diphenyl-2-picrylhydrazyl (DPPH•) method of the organic fraction of bio-oil obtained via the pyrolysis of açaí seeds + fiber, chemically activated with KOH solutions at different molarities (0.5 M–2.0 M) at a temperature of 450 °C on a laboratory scale.
Figure 8.
Antioxidant capacity by the 1,1-diphenyl-2-picrylhydrazyl (DPPH•) method of the organic fraction of bio-oil obtained via the pyrolysis of açaí seeds + fiber, chemically activated with KOH solutions at different molarities (0.5 M–2.0 M) at a temperature of 450 °C on a laboratory scale.
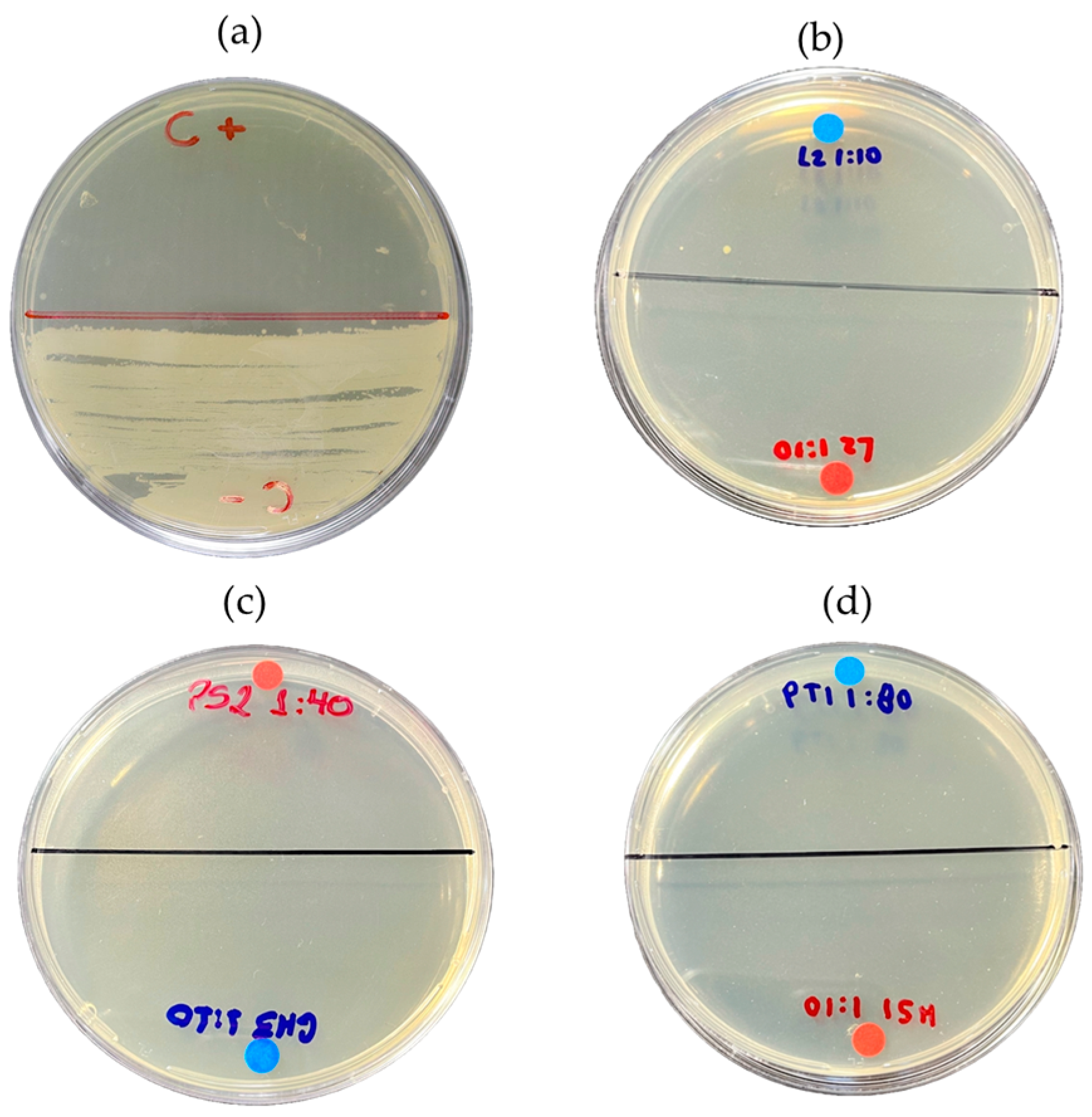
Figure 9.
Minimum bactericidal concentration (MBC): (a) positive control (E. coli) and negative control (chloramphenicol 5 mg/mL); (b) MBC corresponding to wash water; (c) aqueous fraction; and (d) organic fraction against E. coli strain.
Figure 9.
Minimum bactericidal concentration (MBC): (a) positive control (E. coli) and negative control (chloramphenicol 5 mg/mL); (b) MBC corresponding to wash water; (c) aqueous fraction; and (d) organic fraction against E. coli strain.
Figure 10.
Minimum bactericidal concentration (MBC): (a) positive control (S. aureus) and negative control (chloramphenicol 5 mg/mL); (b) MBC for wash water; (c) MBC for aqueous fraction; and (d) MBC for organic fraction against S. aureus strain.
Figure 10.
Minimum bactericidal concentration (MBC): (a) positive control (S. aureus) and negative control (chloramphenicol 5 mg/mL); (b) MBC for wash water; (c) MBC for aqueous fraction; and (d) MBC for organic fraction against S. aureus strain.
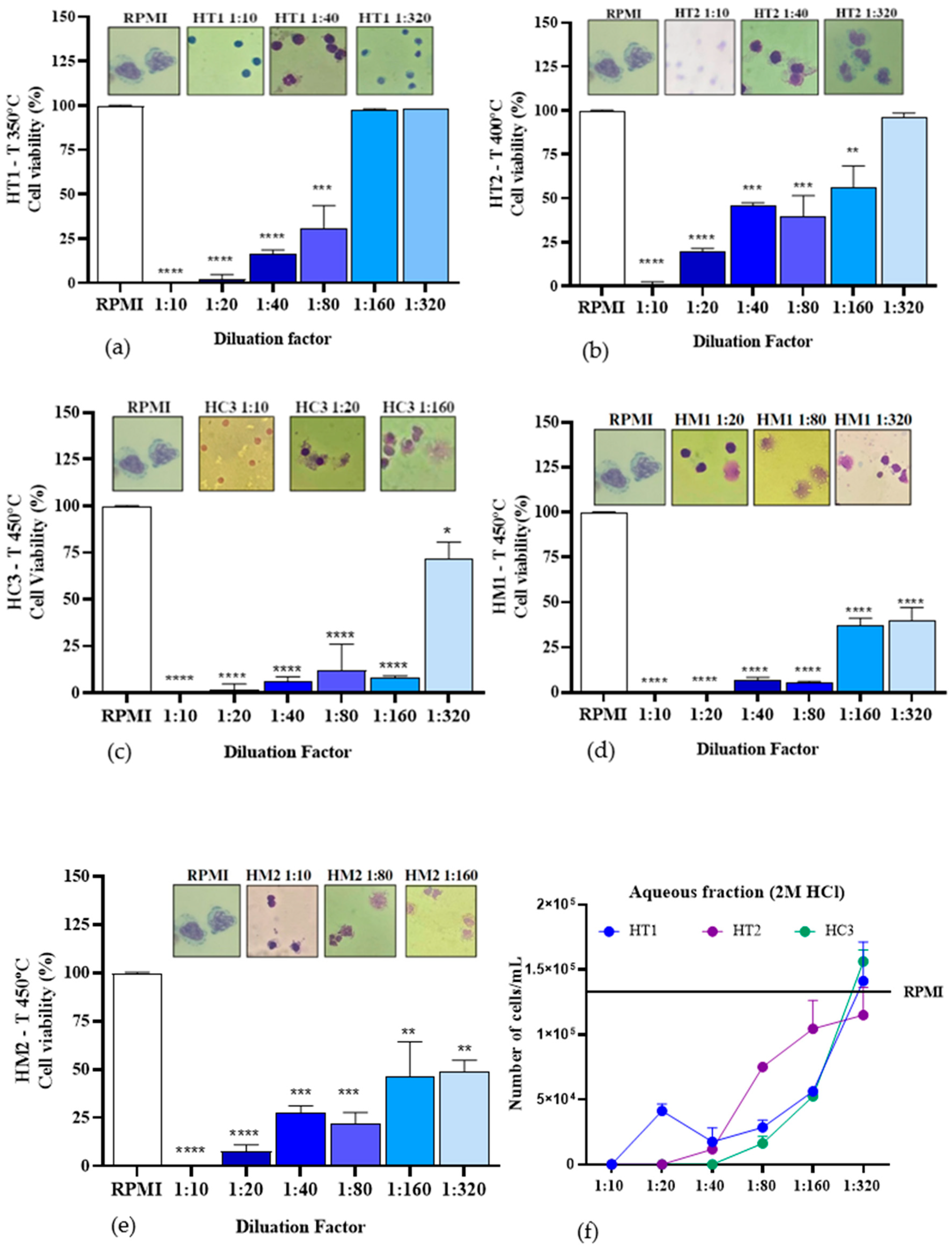
Figure 11.
Percentage cell viability of PBMC after incubation with aqueous fractions impregnated with or without HCl at different temperatures and dilutions: (a) aqueous fraction impregnated with 2.0 M HCl at 350 °C; (b) aqueous fraction impregnated with 2.0 M HCl at 400 °C; (c) aqueous fraction impregnated with 2.0 M HCl at 450 °C; (d) aqueous fraction impregnated with 0.50 M HCl at 450 °C; (e) aqueous fraction impregnated with 1.0 M HCl at 450 °C; and (f) number of cells/mL in aqueous fractions HT1, HT2, and HC3. * p ≤ 0.05 ** p < 0.01 *** p < 0.00001.
Figure 11.
Percentage cell viability of PBMC after incubation with aqueous fractions impregnated with or without HCl at different temperatures and dilutions: (a) aqueous fraction impregnated with 2.0 M HCl at 350 °C; (b) aqueous fraction impregnated with 2.0 M HCl at 400 °C; (c) aqueous fraction impregnated with 2.0 M HCl at 450 °C; (d) aqueous fraction impregnated with 0.50 M HCl at 450 °C; (e) aqueous fraction impregnated with 1.0 M HCl at 450 °C; and (f) number of cells/mL in aqueous fractions HT1, HT2, and HC3. * p ≤ 0.05 ** p < 0.01 *** p < 0.00001.
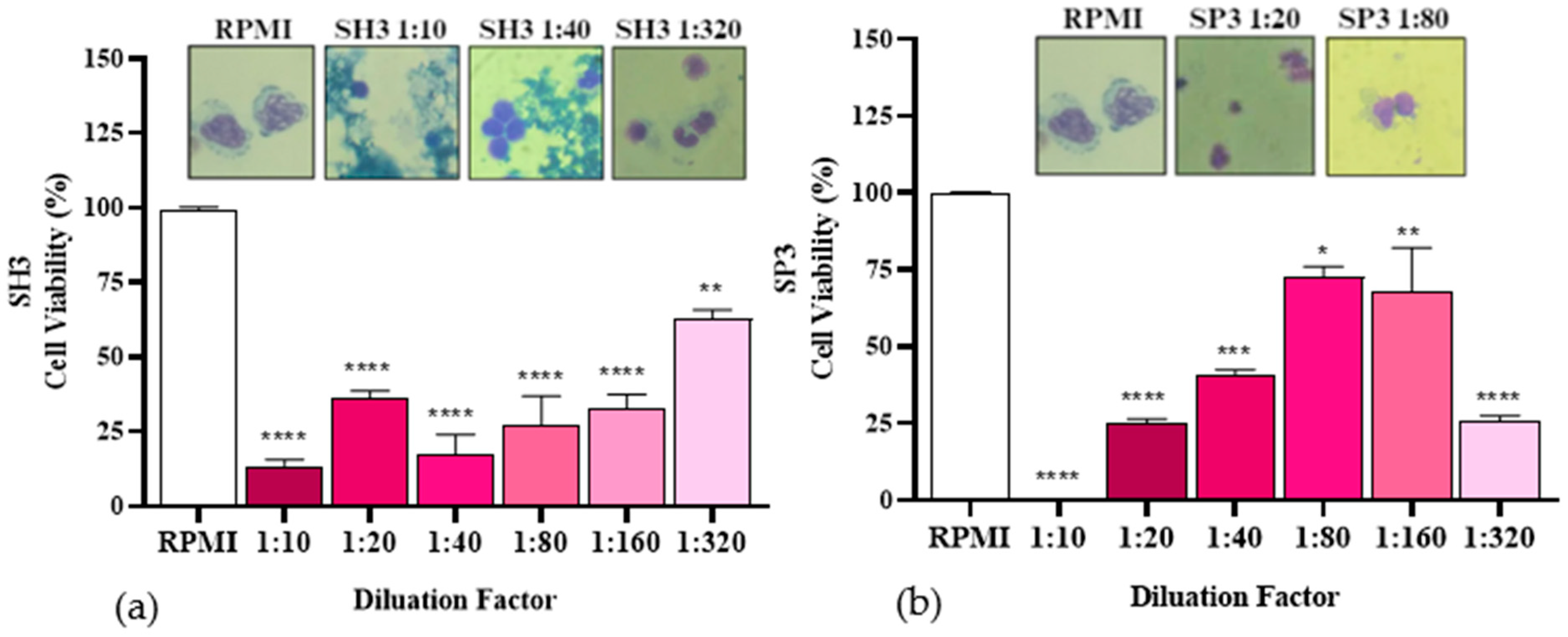
Figure 12.
Cell viability in percentage of PBMC after incubation with the non-impregnated organic fraction obtained via pyrolysis at 450 °C. * p ≤ 0.05 ** p < 0.01 *** p < 0.00001 (a,b).
Figure 12.
Cell viability in percentage of PBMC after incubation with the non-impregnated organic fraction obtained via pyrolysis at 450 °C. * p ≤ 0.05 ** p < 0.01 *** p < 0.00001 (a,b).
Table 1.
Chemical composition of bio-oil obtained through the pyrolysis of açaí seeds at 450 °C.
Table 1.
Chemical composition of bio-oil obtained through the pyrolysis of açaí seeds at 450 °C.
| Chemical Compound | Bio-Oil (Área%) |
|---|
| Linear hydrocarbons | 9.64 |
| Undecane | 1.12 |
| Tridecane | 2.48 |
| Pentadecane | 2.29 |
| Dodecane, 5,8-diethyl | 1.63 |
| 6-tridecene | 2.12 |
| Cyclic and aromatic hydrocarbons | 11.89 |
| Cyclohexene, 6-(2-butenylidene)-1,5,5-trimethyl | 1.85 |
| Naphthalene | 4.40 |
| Naphthalene, 1-methyl | 2.39 |
| 1H-Indene, 1-ethylidene | 3.25 |
| Carboxylic acids | 8.53 |
| Dodecanoicacid | 4.31 |
| Tetradecanoicacid | 4.22 |
| Esters | 4.07 |
| Undecanoicacid, 10-methyl-, methylester | 1.10 |
| Methyltetradecanoate | 2.97 |
| Ketones | 3.53 |
| 2-Pentanone, 4-hydroxy-4-methyl | 1.88 |
| 2-Cyclopenten-1-one, 2,3-dimethyl | 1.65 |
| Phenolic compounds | 55.70 |
| Phenol | 15.93 |
| Methylphenol | 20.53 |
| Dimethylphenol | 10.09 |
| Methoxyphenol | 4.58 |
| Ethylmethoxyphenol | 4.57 |
| Furans | 5.75 |
| Benzofuran, 2-methyl | 1.88 |
| Furan, 2-(2furanylmethyl)-5-methyl | 2.09 |
| Benzofuran, 4,7-dimethyl | 1.78 |
| Aldehydes | 0.91 |
| Cinnamaldehyde, β-methyl- | 0.91 |
| Total | 100 |
Table 2.
Chemical composition of the bio-oil and aqueous phase obtained through the pyrolysis of chemically activated açaí with a 2.0 M KOH solution at 450 °C.
Table 2.
Chemical composition of the bio-oil and aqueous phase obtained through the pyrolysis of chemically activated açaí with a 2.0 M KOH solution at 450 °C.
| Chemical Compound | Bio-Oil (Area%) | Aqueous Phase (Area%) |
|---|
| Alcohols | 10.43 | 26.61 |
| 2,3,4,5,6-Pentamethylbenzyl | 1.74 | - |
| 2-Furanmethanol | 1.58 | 8.94 |
| Benzenemethanol α-ethyl-4-methoxy Cyclohexanol | 1.80 | - |
| 5-methyl-2-(1-methylethyl)-, (1α,2α,5β) | 3.80 | - |
| 2,4-Dimethyl-2-oxazoline-4 methanol | - | 17.67 |
| 1-Hexadecanol, 2-methyl | 1.51 | - |
| Linear Hydrocarbons | 12.13 | 3.39 |
| Decane | 1.23 | - |
| Undecane | 1.59 | - |
| Tridecane | 2.60 | - |
| Tetradecane | 2.33 | - |
| 7-Tetradecane | 2.86 | 3.39 |
| Nonadecane | 1.52 | - |
| Cyclic and aromatic hydrocarbons | 13.59 | - |
| Bicyclo[4.2.0]octa-1,3,5-triene | 3.13 | - |
| Ethylbenzene | 2.21 | - |
| Toluene | 1.95 | - |
| 1,2,4,4 Tetramethylcyclopentene | 1.30 | - |
| 1,3-Cyclopentadiene, 5-(1-methylpropylidene | 0.89 | - |
| Cyclohexane | 3.25 | - |
| Cyclohexane, 1,2,4-tris(methylene) | 0.86 | - |
| Nitrogenated compounds | 4.38 | 13.05 |
| 4-(2,5-Dihydro-3 methoxyphenyl)butylamine | 2.44 | - |
| Tricyclo[3.1.0.0(2,4)] hex-3-ene 3-carbonitrile | 1.94 | - |
| N-Tert.-butyl-N-(2 propenyl)amine | - | 6.05 |
| 2-Propen-1-amine, N,N-bis(1-methylethyl) Aziridine | - | 3.62 |
| 2-(1,1-dimethylethyl)-1-ethyl 3-methyl-,trans- | - | 3.38 |
| Carboxylic acids | 0.97 | 9.23 |
| Butanoicacid,4-hydroxy | 0.97 | - |
| Butanedioic acid, methylene | - | 2.49 |
| Butanoicacid,4-hydroxy | - | 6.74 |
| Esters | 1.29 | 3.32 |
| Acetic acid, 7-hydroxy 1,3,4,5,6,7-hexahydro-2H naphthalen-4a-ylmethyl ester | 1.29 | - |
| Carbamicacid, phenyl ester | - | 3.32 |
| Ketones | 7.07 | 44.38 |
| 2-Cyclopenten-1-one, 2,3-dimethyl | 1.86 | - |
| 2-Cyclopenten-1-one, 2-methyl | 0.90 | - |
| 4-(3,7,7-Trimethyl-2 oxabicyclo[3.2.0]hept-3-en-1 yl)but-3-en-2-one | 3.02 | - |
| Spiro[2.3]hexan-5-one, 4,4-diethyl | 1.29 | - |
| 2-Pentanone, 4-amino-4-methyl | - | 32.54 |
| 2-Propanone, (1 methylethylidene)hydrazone | - | 2.29 |
| 4-Piperidinone, 2,2,6,6-tetramethy | - | 9.55 |
| Phenolic compounds | 42.98 | - |
| Phenol | 5.73 | - |
| Methyl phenol | 5.60 | - |
| Dimethyl phenol | 9.74 | - |
| Trimethyl phenol | 8.26 | - |
| Ethyl methyl phenol | 4.84 | - |
| Dimethoxy phenol | 2.90 | - |
| Ethyl phenol | 4.316 | - |
| Ethyl methoxy phenol | 2.94 | - |
| Non-identified fraction | 5.81 | - |
| Total | 100 | 100 |
Table 3.
Chemical composition of the compounds obtained by the pyrolysis of açaí (E. oleracea).
Table 3.
Chemical composition of the compounds obtained by the pyrolysis of açaí (E. oleracea).
| Chemical Composition Ci (Area%) | 2.0 M KOH |
|---|
| 350 °C | 400 °C | 450 °C |
|---|
| Alcohols | 2.34 | 20.74 | 26.62 |
| Carboxylic Acids | 4.05 | 15.02 | 9.23 |
| Ketones | 52.81 | 44.38 | 19.69 |
| Oxygenates | 40.80 | 19.86 | 44.46 |
| 100.00 | 100.00 | 100.00 |
| Acidity (mg KOH/g) | 118.9 | 26.8 | 17.9 |
Table 4.
Antioxidant capacity of bio-oils obtained by the pyrolysis of açaí seeds (E. oleracea) by the TEAC method.
Table 4.
Antioxidant capacity of bio-oils obtained by the pyrolysis of açaí seeds (E. oleracea) by the TEAC method.
| TEAC (mmol/L) |
|---|
| Dilution | 2.0 M KOH |
|---|
| 350 °C | 400 °C |
|---|
| 1:10 | 2.372 | 2.372 |
| 1:20 | 2.342 | 2.28 |
| 1:40 | 2.285 | 2.226 |
| 1:80 | 2.022 | 1.375 |
| 1:160 | 1.584 | 1.019 |
| 1:320 | 1.18 | 0.239 |
Table 5.
Antioxidant capacity of bio-oils obtained from the pyrolysis of açaí seeds (E. oleracea) chemically activated with 2.0 M HCl solutions at temperatures of 350, 400, and 450 °C on a laboratory scale.
Table 5.
Antioxidant capacity of bio-oils obtained from the pyrolysis of açaí seeds (E. oleracea) chemically activated with 2.0 M HCl solutions at temperatures of 350, 400, and 450 °C on a laboratory scale.
| TEAC (mmol/L) |
|---|
| Dilution | 2.0 M HCl |
|---|
| 350 °C | 400 °C | 450 °C |
|---|
| 1:10 | 2.34 | 2.32 | 2.31 |
| 1:20 | 2.25 | 2.23 | 2.08 |
| 1:40 | 1.22 | 1.19 | 1.15 |
| 1:80 | 0.96 | 0.94 | 0.90 |
| 1:160 | 0.93 | 0.90 | 0.87 |
Table 6.
Antioxidant capacity of bio-oils obtained by the pyrolysis of açaí seeds (E. oleracea) chemically activated with 0.5 M and 1.0 M KOH solutions at temperatures of 450 °C on a laboratory scale.
Table 6.
Antioxidant capacity of bio-oils obtained by the pyrolysis of açaí seeds (E. oleracea) chemically activated with 0.5 M and 1.0 M KOH solutions at temperatures of 450 °C on a laboratory scale.
| TEAC (mmol/L) |
|---|
| Dilution | KOH 450 °C |
|---|
| 0.5 M | 1.0 M |
|---|
| 1:10 | 2.357 | 2.292 |
| 1:20 | 2.272 | 2.28 |
| 1:40 | 2.203 | 2.252 |
| 1:80 | 2.163 | 1.513 |
| 1:160 | 1.664 | 0.885 |
| 1:320 | 0.56 | 0.57 |
Table 7.
Antioxidant capacity of bio-oils obtained by the pyrolysis of açaí seeds (E. oleracea), using different molarities (KOH solution of 0.5, 1.0, and 2.0 M), at 450 °C.
Table 7.
Antioxidant capacity of bio-oils obtained by the pyrolysis of açaí seeds (E. oleracea), using different molarities (KOH solution of 0.5, 1.0, and 2.0 M), at 450 °C.
| TEAC (mmol/L) |
|---|
| Dilution | KOH 450 °C |
|---|
| 0.5 M | 1.0 M | 2.0 M |
|---|
| 1:10 | 2.45 | 2.45 | 2.28 |
| 1:20 | 2.45 | 2.45 | 2.03 |
| 1:40 | 2.39 | 2.39 | 1.19 |
| 1:80 | 2.36 | 2.36 | 1.12 |
| 1:160 | 0.93 | 0.90 | 0.87 |
| 1:320 | 1.60 | 1.59 | 0.56 |
| 1:640 | 0.97 | 0.97 | 0.47 |
Table 8.
Antioxidant capacity of the aqueous phase of bio-oils obtained by the pyrolysis of Açaí seeds (E. oleracea), at different temperatures (350 °C and 400 °C), chemically activated with 2.0 M KOH on a laboratory scale.
Table 8.
Antioxidant capacity of the aqueous phase of bio-oils obtained by the pyrolysis of Açaí seeds (E. oleracea), at different temperatures (350 °C and 400 °C), chemically activated with 2.0 M KOH on a laboratory scale.
| DPPH (µM/L) |
|---|
| Dilution | 2.0 M KOH |
|---|
| 350 °C | 400 °C |
|---|
| 1:10 | 2.372 | 2.372 |
| 1:20 | 2.342 | 2.28 |
| 1:40 | 2.285 | 2.226 |
| 1:80 | 2.022 | 1.375 |
| 1:160 | 1.584 | 1.019 |
Table 9.
Antioxidant capacity of the organic fraction of bio-oils obtained by the pyrolysis of açaí seeds (E. oleracea), at different temperatures (350 °C and 400 °C), chemically activated with 2.0 M KOH on a laboratory scale.
Table 9.
Antioxidant capacity of the organic fraction of bio-oils obtained by the pyrolysis of açaí seeds (E. oleracea), at different temperatures (350 °C and 400 °C), chemically activated with 2.0 M KOH on a laboratory scale.
| DPPH (µM/L) |
|---|
| Dilution | 2.0 M KOH |
|---|
| 350° C | 400 °C |
|---|
| 1:20 | 2.145 | 1.805 |
| 1:40 | 1.701 | 2.271 |
| 1:80 | 1.748 | 2.675 |
| 1:160 | 2.170 | 3.248 |
| 1:320 | 2.864 | 3.315 |
| 1:640 | 2.886 | 3.121 |
Table 10.
Antioxidant capacity of the aqueous fraction of bio-oils obtained by the pyrolysis of açaí seeds (E. oleracea), chemically activated with 2.0 M KOH at different molarities (0.5 M–2.0 M) at 450 °C on a laboratory scale.
Table 10.
Antioxidant capacity of the aqueous fraction of bio-oils obtained by the pyrolysis of açaí seeds (E. oleracea), chemically activated with 2.0 M KOH at different molarities (0.5 M–2.0 M) at 450 °C on a laboratory scale.
| DPPH (µM/L) |
|---|
| Dilution | KOH 450 °C |
|---|
| 0.5 M | 1.0 M | 2.0 M |
|---|
| 1:10 | 0.206 | 0.256 | 0.714 |
| 1:20 | 0.199 | 0.278 | 1.111 |
| 1:40 | 0.365 | 0.362 | 1.694 |
| 1:80 | 0.402 | 0.583 | 2.618 |
| 1:160 | 0.893 | 1.185 | 2.598 |
Table 11.
Antioxidant capacity of the aqueous fraction of bio-oils obtained by the pyrolysis of açaí seeds (E. oleracea), chemically activated with 2.0 M KOH at different molarities (0.5 M–2.0 M) at 450 °C on a laboratory scale.
Table 11.
Antioxidant capacity of the aqueous fraction of bio-oils obtained by the pyrolysis of açaí seeds (E. oleracea), chemically activated with 2.0 M KOH at different molarities (0.5 M–2.0 M) at 450 °C on a laboratory scale.
| DPPH (µM/L) |
|---|
| Dilution | KOH 450 °C |
|---|
| 0.5 M | 1.0 M |
|---|
| 1:20 | 0.672 | 0.712 |
| 1:40 | 1.475 | 1.495 |
| 1:80 | 1.277 | 2.244 |
| 1:160 | 2.053 | 2.869 |
| 1:320 | 2.913 | 3.035 |
| 1:640 | 2.722 | 3.518 |
Table 12.
Minimum inhibitory and bactericidal concentrations of wash water from the pyrolysis of açaí seed impregnated with KOH against E. coli strain.
Table 12.
Minimum inhibitory and bactericidal concentrations of wash water from the pyrolysis of açaí seed impregnated with KOH against E. coli strain.
| Nature | Pretreatment | Temperature | Code | MIC | MBC |
|---|
| Wash water | KOH (0.5 M) | - | L1 | >1:10 | >1:10 |
| Wash water | KOH (1.0 M) | - | L2 | 1:40 | 1:40 |
| Wash water | KOH (2.0 M) | - | L3 | 1:40 | 1:40 |
Table 13.
Minimum inhibitory concentrations and bactericidal concentrations of the aqueous fraction of bio-oil from the pyrolysis of açaí seeds impregnated with KOH and HCl against E. coli strain.
Table 13.
Minimum inhibitory concentrations and bactericidal concentrations of the aqueous fraction of bio-oil from the pyrolysis of açaí seeds impregnated with KOH and HCl against E. coli strain.
| Nature | Pretreatment | Temperature | Code | MIC | MBC |
|---|
| Aqueous Phase | KOH (0.5 M) | 450 °C | HM1 | >1:10 | >1:10 |
| Aqueous Phase | KOH (1.0 M) | 450 °C | HM2 | 1:20 | 1:20 |
| Aqueous Phase | KOH (2.0 M) | 450 °C | HC3 | 1;40 | 1:40 |
| Aqueous Phase | HCL (0.5 M) | 450 °C | HM1 | 1:40 | 1:40 |
| Aqueous Phase | HCL (1.0 M) | 450 °C | HM2 | 1:40 | 1:40 |
| Aqueous Phase | HCL (2.0 M) | 450 °C | HC3 | >1:40 | >1:40 |
Table 14.
Minimum inhibitory concentrations and bactericidal concentrations of the organic fraction of bio-oil from the pyrolysis of açaí seeds impregnated with KOH against E. coli strain.
Table 14.
Minimum inhibitory concentrations and bactericidal concentrations of the organic fraction of bio-oil from the pyrolysis of açaí seeds impregnated with KOH against E. coli strain.
| Nature | Pretreatment | Temperature | Code | MIC | MBC |
|---|
| Organic Phase | KOH (0.5 M) | 450 °C | PM1 | 1:40 | 1:40 |
| Organic Phase | KOH (1.0 M) | 450 °C | PM2 | 1:80 | 1:80 |
| Organic Phase | KOH (2.0 M) | 350 °C | PT1 | >1:40 | >1:40 |
| Organic Phase | KOH (2.0 M) | 400 °C | PT2 | 1:80 | 1:80 |
Table 15.
Minimum inhibitory and bactericidal concentrations of wash water from açaí seed pyrolysis impregnated with KOH against the S. aureus strain.
Table 15.
Minimum inhibitory and bactericidal concentrations of wash water from açaí seed pyrolysis impregnated with KOH against the S. aureus strain.
| Nature | Pretreatment | Temperature | Code | MIC | MBC |
|---|
| Washing water | KOH (0.5 M) | - | L1 | 1:5 | 1:20 |
| Washing water | KOH (1.0 M) | - | L2 | >1:10 | 1:40 |
| Washing water | KOH(2.0 M) | - | L3 | >1:10 | 1:40 |
Table 16.
Minimum inhibitory and bactericidal concentrations of the aqueous fraction of bio-oil from açaí seed pyrolysis impregnated with KOH against the strain of S. aureus.
Table 16.
Minimum inhibitory and bactericidal concentrations of the aqueous fraction of bio-oil from açaí seed pyrolysis impregnated with KOH against the strain of S. aureus.
| Nature | Pretreatment | Temperature | Code | MIC | MBC |
|---|
| Aqueous Phase | KOH (2.0 M) | 350 °C | C.N/A | 1:40 | 1:40 |
| Aqueous Phase | KOH (2.0 M) | 400 °C | C.B | >1:40 | >1:40 |
| Aqueous Phase | KOH (2.0 M) | 450 °C | C.A | >1:40 | >1:40 |
Table 17.
Minimum inhibitory and bactericidal concentrations of the organic fraction of bio-oil from açaí seed pyrolysis impregnated with HCL against the strain of S. aureus.
Table 17.
Minimum inhibitory and bactericidal concentrations of the organic fraction of bio-oil from açaí seed pyrolysis impregnated with HCL against the strain of S. aureus.
| Nature | Pretreatment | Temperature | Code | MIC | MBC |
|---|
| Organic Phase | HCL (2.0 M) | 400 °C | PLO/B | 1:5 | 1:40 |
| Organic Phase | HCL (2.0 M) | 450 °C | PLO/A | >1:5 | >1:40 |