Relationship Between High Serum Levels of Follistatin with Impaired Physical Function, and Severe Disease Activity in Rheumatoid Arthritis
Abstract
1. Introduction
2. Results
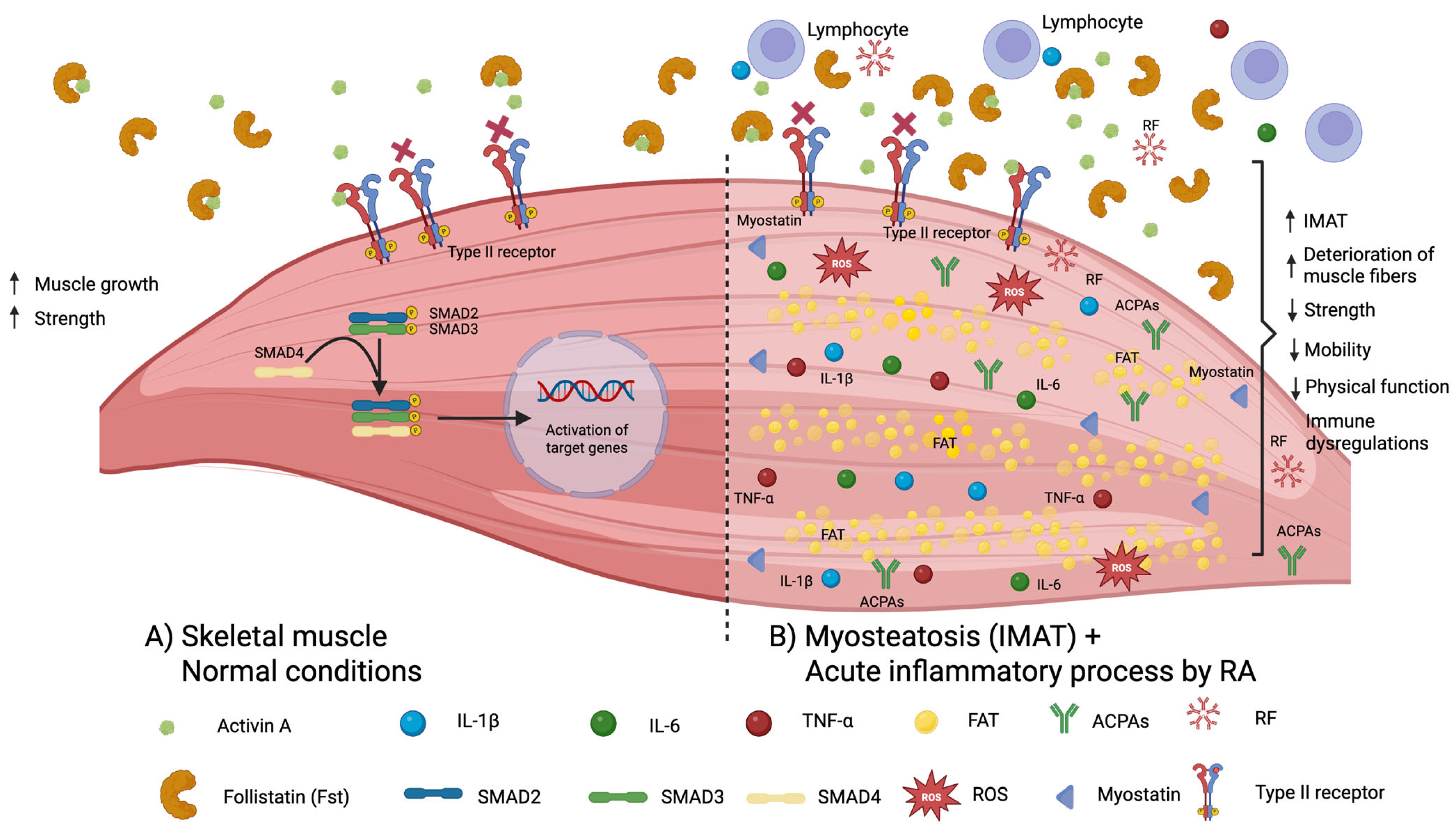
3. Discussion
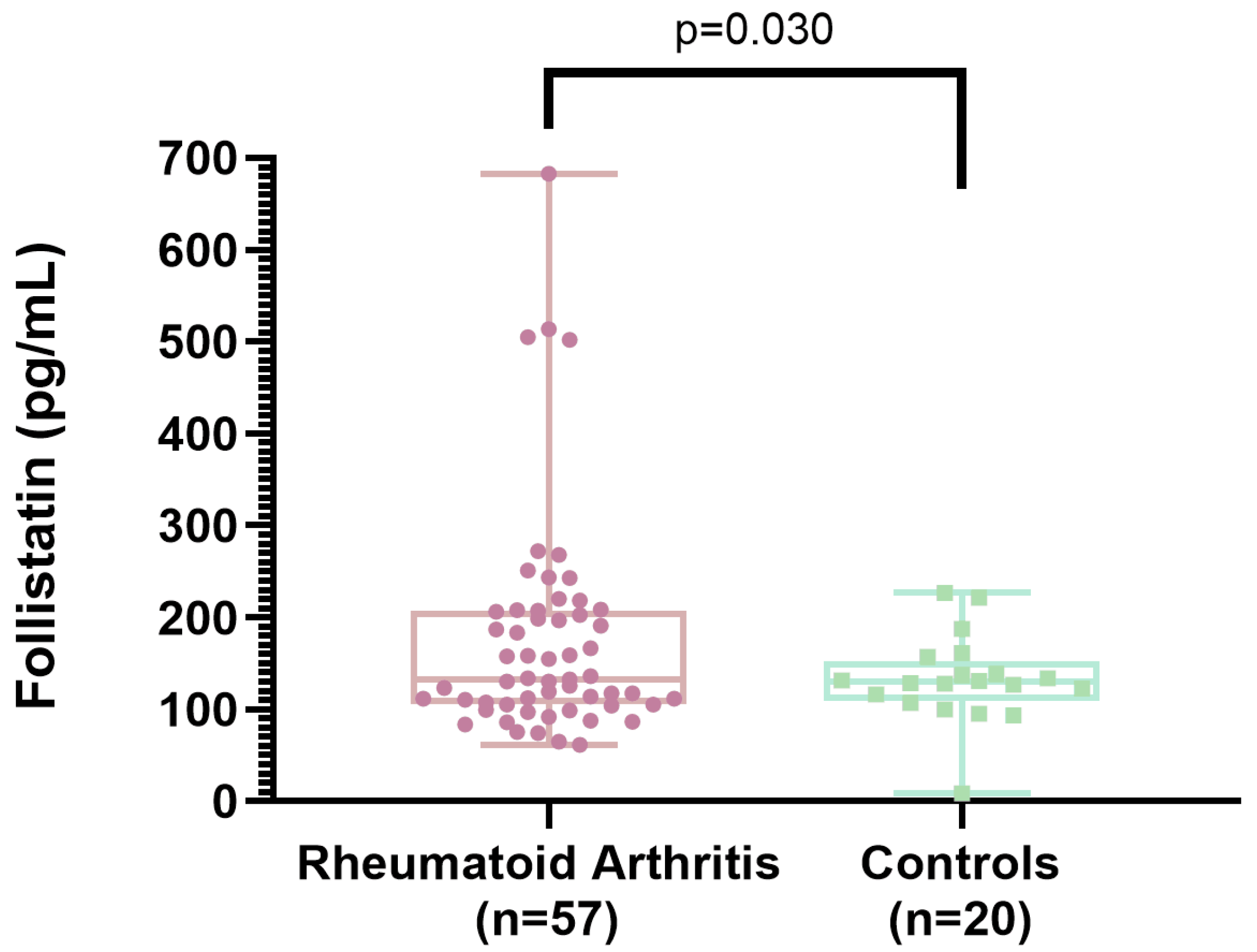
3.1. Follistatin Levels Between Rheumatoid Arthritis Patients and Controls
3.2. Correlation of Serum Follistatin Levels with Clinical Variables
3.3. Correlation of HAQ-DI Score with Clinical Variables
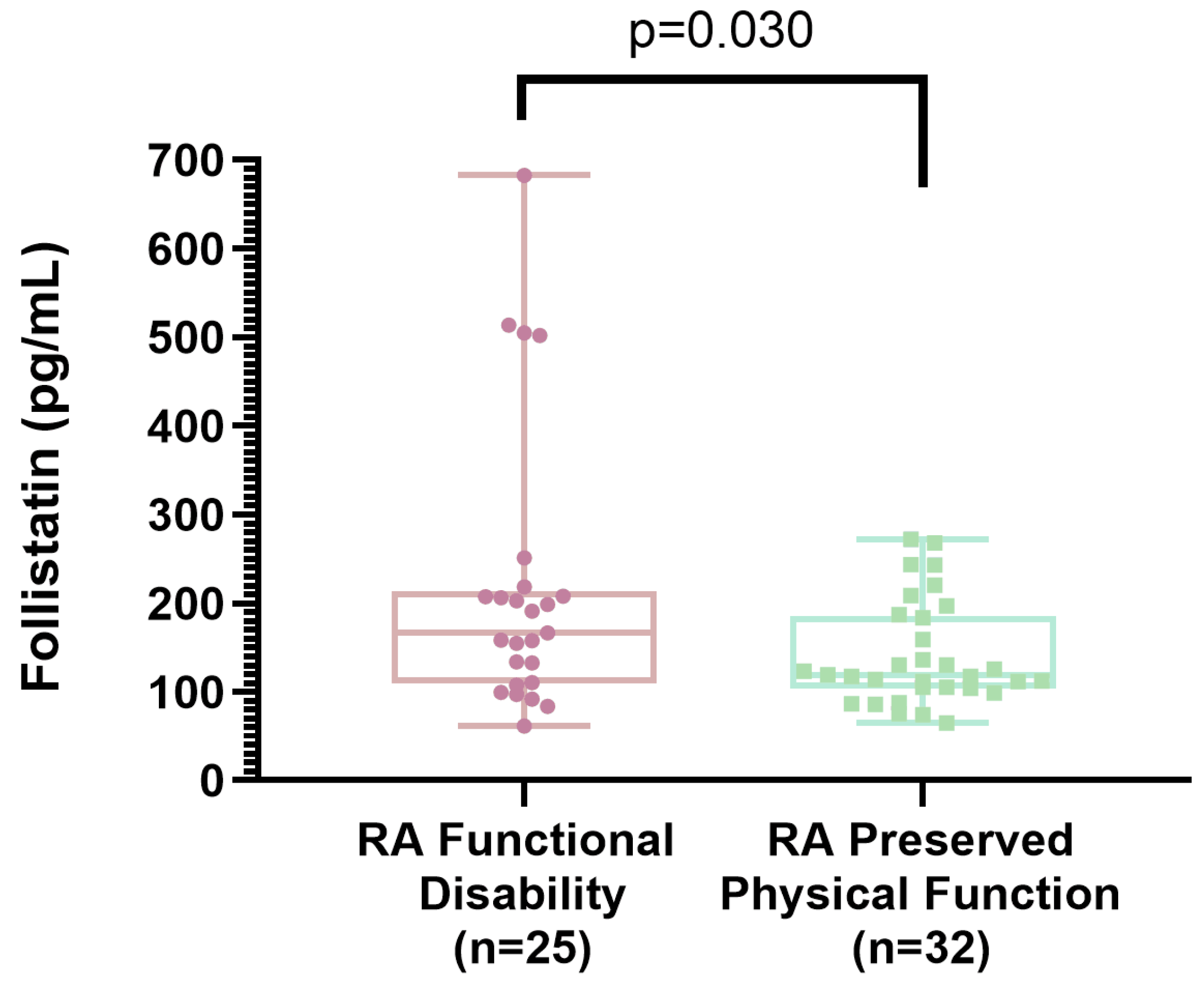
3.4. Association Between Functional Disability in Rheumatoid Arthritis and Clinical Variables
3.5. Strengths of the Present Study
3.6. Study Limitations
4. Materials and Methods
4.1. Type of Study
4.2. Study Population
4.3. Assessments of RA Patients
4.3.1. Assessment of Functional Disability
4.3.2. Evaluation for Active Disease
4.4. Inclusion Control Subjects
4.5. Clinical Evaluation for RA and Controls
4.5.1. Grip Strength
4.5.2. Physical Performance
4.6. Serum Follistatin Determination
4.7. Statistical Analysis
4.8. Ethical Approval
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACR | American College of Rheumatology |
| ActRIIA/ActRIIB | Activin Receptor Type IIA/B |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| BMI | Body Mass Index |
| CKD | Chronic Kidney Disease |
| DXA | Dual-Energy X-ray Absorptiometry |
| DMARDs | Disease-Modifying Anti-Rheumatic Drugs |
| DAS28-ESR | Disease Activity Score of 28 Joints—Erythrocyte Sedimentation Rate |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ESR | Erythrocyte Sedimentation Rate |
| HAQ-DI | Health Assessment Questionnaire-Disability Index |
| IL-6 | Interleukin 6 |
| IL-β | Interleukin 1β |
| IMAT | Intermuscular Adipose Tissue |
| INTEC | Instituto de Terapeutica Experimental y Clinica |
| MyHC | Myosin Heavy-Chain |
| RA | Rheumatoid Arthritis |
| SMMI | Skeletal Muscle Mass Index |
| TGFβ | Transforming Growth Factor Beta |
| TNF-α | Tumor Necrosis Factor-α |
References
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. Corretion in 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid Arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Wen, L.; He, B.; Ding, Y.; Liu, M.; Tang, F.; Wang, L.; Wu, J.; Deng, X.; et al. Health-Related Quality of Life Profiles in Patients with Rheumatoid Arthritis: A Latent Profile Analysis. Front. Public Health 2024, 12, 1478376. [Google Scholar] [CrossRef] [PubMed]
- Santo, R.C.D.E.; Baker, J.F.; Dos Santos, L.P.; Silva, J.M.S.; Filippin, L.I.; Portes, J.K.S.; Brenol, C.V.; Chakr, R.M.D.S.; Xavier, R.M. Changes in Physical Function over Time in Rheumatoid Arthritis Patients: A Cohort Study. PLoS ONE 2023, 18, e0280846. [Google Scholar] [CrossRef]
- Cardiel, M.H.; Abello-Banfi, M.; Ruiz-Mercado, R.; Alarcon-Segovia, D. How to Measure Health Status in Rheumatoid Arthritis in Non-English Speaking Patients: Validation of a Spanish Version of the Health Assessment Questionnaire Disability Index (Spanish HAQ-DI). Clin. Exp. Rheumatol. 1993, 11, 117–121. [Google Scholar]
- Teuwen, M.M.H.; van Wissen, M.A.T.; Peter, W.F.; van Schaardenburg, D.; van den Ende, C.H.M.; Gademan, M.G.J.; van Weely, S.F.E. The Extent and Nature of Functional Limitations According to the Health Assessment Questionnaire Disability Index in Patients with Rheumatoid Arthritis and Severe Functional Disability. J. Clin. Med. 2024, 13, 379. [Google Scholar] [CrossRef] [PubMed]
- Coppers, B.; Heinrich, S.; Tascilar, K.; Phutane, U.; Kleyer, A.; Simon, D.; Bräunig, J.; Penner, J.; Vossiek, M.; Schönau, V.; et al. Sensor-Assessed Grasping Time as a Biomarker of Functional Impairment in Rheumatoid Arthritis. Sci. Rep. 2025, 15, 6018. [Google Scholar] [CrossRef]
- Jin, H.; Wang, G.; Lu, Q.; Rawlins, J.; Chen, J.; Kashyap, S.; Charlesworth, O.; Xu, D.; Dai, L.; Zhu, S.; et al. Pathophysiology of Myopenia in Rheumatoid Arthritis. Bone Res. 2025, 13, 64. [Google Scholar] [CrossRef]
- Baig, M.H.; Ahmad, K.; Moon, J.S.; Park, S.-Y.; Ho Lim, J.; Chun, H.J.; Qadri, A.F.; Hwang, Y.C.; Jan, A.T.; Ahmad, S.S.; et al. Myostatin and Its Regulation: A Comprehensive Review of Myostatin Inhibiting Strategies. Front. Physiol. 2022, 13, 876078. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.Q.; Iskenderian, A.; Ehmann, D.; Jasper, P.; Zhang, Z.; Rong, H.; Welty, D.; Narayanan, R. Leveraging Quantitative Systems Pharmacology Approach into Development of Human Recombinant Follistatin Fusion Protein for Duchenne Muscular Dystrophy. CPT Pharmacomet. Syst. Pharmacol. 2020, 9, 342–352. [Google Scholar] [CrossRef]
- Wang, S.; Fang, L.; Cong, L.; Chung, J.P.W.; Li, T.C.; Chan, D.Y.L. Myostatin: A Multifunctional Role in Human Female Reproduction and Fertility—A Short Review. Reprod. Biol. Endocrinol. 2022, 20, 96. [Google Scholar] [CrossRef]
- Wu, C.; Borné, Y.; Gao, R.; López Rodriguez, M.; Roell, W.C.; Wilson, J.M.; Regmi, A.; Luan, C.; Aly, D.M.; Peter, A.; et al. Elevated Circulating Follistatin Associates with an Increased Risk of Type 2 Diabetes. Nat. Commun. 2021, 12, 6486. [Google Scholar] [CrossRef]
- Stefan, N.; Schick, F.; Birkenfeld, A.L.; Häring, H.-U.; White, M.F. The Role of Hepatokines in NAFLD. Cell Metab. 2023, 35, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Correa-de-Araujo, R.; Addison, O.; Miljkovic, I.; Goodpaster, B.H.; Bergman, B.C.; Clark, R.V.; Elena, J.W.; Esser, K.A.; Ferrucci, L.; Harris-Love, M.O.; et al. Myosteatosis in the Context of Skeletal Muscle Function Deficit: An Interdisciplinary Workshop at the National Institute on Aging. Front. Physiol. 2020, 11, 963. [Google Scholar] [CrossRef]
- Chang, K.-V.; Wu, W.-T.; Chen, Y.-H.; Chen, L.-R.; Hsu, W.-H.; Lin, Y.-L.; Han, D.-S. Enhanced Serum Levels of Tumor Necrosis Factor-α, Interleukin-1β, and -6 in Sarcopenia: Alleviation through Exercise and Nutrition Intervention. Aging (Albany NY) 2023, 15, 13471. [Google Scholar] [CrossRef]
- Dondero, K.; Friedman, B.; Rekant, J.; Landers-Ramos, R.; Addison, O. The Effects of Myosteatosis on Skeletal Muscle Function in Older Adults. Physiol. Rep. 2024, 12, e16042. [Google Scholar] [CrossRef]
- Andonian, B.J.; Huffman, K.M. Skeletal Muscle Disease in Rheumatoid Arthritis: The Center of Cardiometabolic Comorbidities? Curr. Opin. Rheumatol. 2020, 32, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Xu, C.; Shi, H.; Jiang, X.; Tang, W.; Wu, X.; Chen, M.; Li, H.; Zhang, X.; Cheng, Q. Serum Concentrations of Oxytocin, DHEA and Follistatin Are Associated with Osteoporosis or Sarcopenia in Community-Dwelling Postmenopausal Women. BMC Geriatr. 2021, 21, 542. [Google Scholar] [CrossRef]
- Fife, E.; Kostka, J.; Kroc, Ł.; Guligowska, A.; Pigłowska, M.; Sołtysik, B.; Kaufman-Szymczyk, A.; Fabianowska-Majewska, K.; Kostka, T. Relationship of Muscle Function to Circulating Myostatin, Follistatin and GDF11 in Older Women and Men. BMC Geriatr. 2018, 18, 200. [Google Scholar] [CrossRef]
- Liaw, F.-Y.; Kao, T.-W.; Fang, W.-H.; Han, D.-S.; Chi, Y.-C.; Yang, W.-S. Increased Follistatin Associated with Decreased Gait Speed among Old Adults. Eur. J. Clin. Investig. 2016, 46, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Pazokian, F.; Amani-Shalamzari, S.; Rajabi, H. Corretion in Effects of Functional Training with Blood Occlusion on the Irisin, Follistatin, and Myostatin Myokines in Elderly Men. Eur. Rev. Aging Phys. Act. 2022, 19, 22. [Google Scholar] [CrossRef]
- Miyamoto, T.; Carrero, J.J.; Qureshi, A.R.; Anderstam, B.; Heimbürger, O.; Bárány, P.; Lindholm, B.; Stenvinkel, P. Circulating Follistatin in Patients with Chronic Kidney Disease: Implications for Muscle Strength, Bone Mineral Density, Inflammation, and Survival. Clin. J. Am. Soc. Nephrol. 2011, 6, 1001–1008. [Google Scholar] [CrossRef]
- Echeverria, I.; Besga, A.; Sanz, B.; Amasene, M.; Hervás, G.; Barroso, J.; Rodriguez-Larrad, A.; Irazusta, J. Identification of Frailty and Sarcopenia in Hospitalised Older People. Eur. J. Clin. Investig. 2021, 51, e13420. [Google Scholar] [CrossRef]
- Kurose, S.; Onishi, K.; Miyauchi, T.; Takahashi, K.; Kimura, Y. Serum Follistatin Levels Are Independently Associated with Exercise Tolerance in Patients with Obesity. Endocr. Res. 2023, 48, 120–128. [Google Scholar] [CrossRef]
- Kerschan-Schindl, K.; Ebenbichler, G.; Föeger-Samwald, U.; Leiss, H.; Gesslbauer, C.; Herceg, M.; Stummvoll, G.; Marculescu, R.; Crevenna, R.; Pietschmann, P. Rheumatoid Arthritis in Remission: Decreased Myostatin and Increased Serum Levels of Periostin. Wien. Klin. Wochenschr. 2019, 131, 1–7. [Google Scholar] [CrossRef]
- van Groen, M.M.; ten Klooster, P.M.; Taal, E.; van de Laar, M.A.F.J.; Glas, C.A.W. Application of the Health Assessment Questionnaire Disability Index to Various Rheumatic Diseases. Qual. Life Res. 2010, 19, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- de Sordi, C.M.; Dos Reis-Neto, E.T.; Keppeke, G.D.; Shinjo, S.K.; Sato, E.I. Serum Myostatin and Follistatin Levels in Patients with Dermatomyositis and Polymyositis. J. Clin. Rheumatol. 2022, 28, 33–37. [Google Scholar] [CrossRef]
- Samaan, S.F.; Taha, S.I. The Impact of Metabolic Syndrome on Quality of Life Among Individuals with Knee Osteoarthritis Living in Egypt. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2022, 15, 11795441221097361. [Google Scholar] [CrossRef] [PubMed]
- Son, K.M.; Kang, S.H.; Seo, Y.I.; Kim, H.A. Association of Body Composition with Disease Activity and Disability in Rheumatoid Arthritis. Korean J. Intern. Med. 2021, 36, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Melikoğlu, M.A. Presarcopenia and Its Impact on Disability in Female Patients with Rheumatoid Arthritis. Arch. Rheumatol. 2017, 32, 53–59. [Google Scholar] [CrossRef]
- Lopes, A.J.; Justo, A.C.; Ferreira, A.S.; Guimaraes, F.S. Systemic Sclerosis: Association between Physical Function, Handgrip Strength and Pulmonary Function. J. Bodyw. Mov. Ther. 2017, 21, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Özsoy, Z.; Hafızoğlu, M.; Öztürk, Z.; Şahiner, Z.; Karaduman, D.; Uzun, G.S.; Ünaldı, E.; Tahıllıoğlu, Y.; Halil, M.G.; Özsoy, Z.; et al. Improvement in Rheumatoid Sarcopenia with Biological Therapy; Muscle Ultrasound Study. J. Turk. Soc. Rheumatol. 2024, 16, 113–120. [Google Scholar] [CrossRef]
- Hammer, H.B.; Jensen Hansen, I.M.; Järvinen, P.; Leirisalo-Repo, M.; Ziegelasch, M.; Agular, B.; Terslev, L. Rheumatoid Arthritis Patients with Predominantly Tender Joints Rarely Achieve Clinical Remission despite Being in Ultrasound Remission. Rheumatol. Adv. Pr. 2021, 5, rkab030. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yu, L.; Fang, Y.; Zhang, X.; Li, S.; Dou, L. Correlation between Disease Activity and Patient-Reported Health-Related Quality of Life in Rheumatoid Arthritis: A Cross-Sectional Study. BMJ Open 2024, 14, e082020. [Google Scholar] [CrossRef]
- Hulmi, J.J.; Nissinen, T.A.; Penna, F.; Bonetto, A. Targeting the Activin Receptor Signaling to Counteract the Multi-Systemic Complications of Cancer and Its Treatments. Cells 2021, 10, 516. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of Muscle Atrophy and Hypertrophy: Implications in Health and Disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Iyer, C.C.; Chugh, D.; Bobbili, P.J.; Blatnik, A.J.; Crum, A.E.; Yi, A.F.; Kaspar, B.K.; Meyer, K.C.; Burghes, A.H.M.; Arnold, W.D. Follistatin-Induced Muscle Hypertrophy in Aged Mice Improves Neuromuscular Junction Innervation and Function. Neurobiol. Aging 2021, 104, 32–41. [Google Scholar] [CrossRef]
- Kramerova, I.; Marinov, M.; Owens, J.; Lee, S.-J.; Becerra, D.; Spencer, M.J. Myostatin Inhibition Promotes Fast Fibre Hypertrophy but Causes Loss of AMP-Activated Protein Kinase Signalling and Poor Exercise Tolerance in a Model of Limb-Girdle Muscular Dystrophy R1/2A. J. Physiol. 2020, 598, 3927–3939. [Google Scholar] [CrossRef]
- Rybalka, E.; Timpani, C.A.; Debruin, D.A.; Bagaric, R.M.; Campelj, D.G.; Hayes, A. The Failed Clinical Story of Myostatin Inhibitors against Duchenne Muscular Dystrophy: Exploring the Biology behind the Battle. Cells 2020, 9, 2657. [Google Scholar] [CrossRef]
- Agrawal, S.; Chakole, S.; Shetty, N.; Prasad, R.; Lohakare, T.; Wanjari, M. Exploring the Role of Oxidative Stress in Skeletal Muscle Atrophy: Mechanisms and Implications. Cureus 2023, 15, e42178. [Google Scholar] [CrossRef]
- Escalante, A.; Haas, R.W.; del Rincón, I. Measurement of Global Functional Performance in Patients with Rheumatoid Arthritis Using Rheumatology Function Tests. Arthritis Res. Ther. 2004, 6, R315–R325. [Google Scholar] [CrossRef] [PubMed]
- Thyberg, I.; Hass, U.A.M.; Nordenskiöld, U.; Gerdle, B.; Skogh, T. Activity Limitation in Rheumatoid Arthritis Correlates with Reduced Grip Force Regardless of Sex: The Swedish TIRA Project. Arthritis Rheum. 2005, 53, 886–896. [Google Scholar] [CrossRef]
- Meng, C.F.; Lee, Y.; Schieir, O.; Valois, M.-F.; Butler, M.; Boire, G.; Hazlewood, G.; Hitchon, C.; Keystone, E.; Tin, D.; et al. Having More Tender Than Swollen Joints Is Associated with Worse Function and Work Impairment in Patients with Early Rheumatoid Arthritis. ACR Open Rheumatol. 2024, 6, 347–355. [Google Scholar] [CrossRef]
- Krause, M.L.; Crowson, C.S.; Bongartz, T.; Matteson, E.L.; Michet, C.J.; Mason, T.G.; Persellin, S.T.; Gabriel, S.E.; Davis, J.M. Determinants of Disability in Rheumatoid Arthritis: A Community-Based Cohort Study. Open Rheumatol. J. 2015, 9, 88–93. [Google Scholar] [CrossRef]
- Pfeiffer, B.M.; Krenzer, S.; Dockhorn, R.; Schwenke, R.; Schwenke, H.; Waehrisch, J.; Kraus, E. Impact of Modified-Release Prednisone on Functional Ability in Patients with Rheumatoid Arthritis. Rheumatol. Int. 2013, 33, 1447–1454. [Google Scholar] [CrossRef]
- Maska, L.; Anderson, J.; Michaud, K. Measures of Functional Status and Quality of Life in Rheumatoid Arthritis: Health Assessment Questionnaire Disability Index (HAQ), Modified Health Assessment Questionnaire (MHAQ), Multidimensional Health Assessment Questionnaire (MDHAQ), Health Assessment Questionnaire II (HAQ-II), Improved Health Assessment Questionnaire (Improved HAQ), and Rheumatoid Arthritis Quality of Life (RAQoL). Arthritis Care Res. (Hoboken) 2011, 63 (Suppl. 11), S4–S13. [Google Scholar] [PubMed]
- Prevoo, M.L.; van ’t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified Disease Activity Scores That Include Twenty-Eight-Joint Counts. Development and Validation in a Prospective Longitudinal Study of Patients with Rheumatoid Arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef]
- Fransen, J.; van Riel, P.L.C.M. The Disease Activity Score and the EULAR Response Criteria. Clin. Exp. Rheumatol. 2005, 23 (Suppl. 39), S93–S99. [Google Scholar] [CrossRef]
- Dougados, M.; Brault, Y.; Logeart, I.; van der Heijde, D.; Gossec, L.; Kvien, T. Defining Cut-off Values for Disease Activity States and Improvement Scores for Patient-Reported Outcomes: The Example of the Rheumatoid Arthritis Impact of Disease (RAID). Arthritis Res. Ther. 2012, 14, R129. [Google Scholar] [CrossRef]
- Garrow, J.S. Obesity and Related Diseases; Churchill Livingstone: Edinburgh, UK, 1988; Available online: https://www.scirp.org/reference/referencespapers?referenceid=888123 (accessed on 18 July 2025).
- Megnien, J.L.; Denarie, N.; Cocaul, M.; Simon, A.; Levenson, J. Predictive Value of Waist-to-Hip Ratio on Cardiovascular Risk Events. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Binkley, N.; Morgan, S.L.; Shuhart, C.R.; Camargos, B.M.; Carey, J.J.; Gordon, C.M.; Jankowski, L.G.; Lee, J.-K.; Leslie, W.D. Best Practices for Dual-Energy X-Ray Absorptiometry Measurement and Reporting: International Society for Clinical Densitometry Guidance. J. Clin. Densitom. 2016, 19, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M. Assessment of Lean Mass and Physical Performance in Sarcopenia. J. Clin. Densitom. 2015, 18, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Yamada, M.; Yoshida, T.; Miyachi, M.; Arai, H. Validating Muscle Mass Cutoffs of Four International Sarcopenia-working Groups in Japanese People Using DXA and BIA. J. Cachexia Sarcopenia Muscle 2021, 12, 1000–1010. [Google Scholar] [CrossRef]
- Spijkerman, D.C.; Snijders, C.J.; Stijnen, T.; Lankhorst, G.J. Standardization of Grip Strength Measurements. Effects on Repeatability and Peak Force. Scand. J. Rehabil. Med. 1991, 23, 203–206. [Google Scholar] [CrossRef]
- Alley, D.E.; Shardell, M.D.; Peters, K.W.; McLean, R.R.; Dam, T.-T.L.; Kenny, A.M.; Fragala, M.S.; Harris, T.B.; Kiel, D.P.; Guralnik, J.M.; et al. Grip Strength Cutpoints for the Identification of Clinically Relevant Weakness. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 559–566. [Google Scholar] [CrossRef]
- Boshnjaku, A.; Krasniqi, E. Diagnosing Sarcopenia in Clinical Practice: International Guidelines vs. Population-Specific Cutoff Criteria. Front. Med. 2024, 11, 1405438. [Google Scholar]
| Variables | RA (n = 57) | Controls (n = 20) | p-Value |
|---|---|---|---|
| Age, mean ± SD | 57 ± 8 | 56.5 ± 8 | 0.781 |
| Menopause, frequency (%) | 43 (77) | 15 (75) | 1.000 |
| Diabetes mellitus 2, frequency (%) | 7 (12) | 2 (10) | 1.000 |
| Hypertension, frequency (%) | 20 (35) | 4 (20) | 0.210 |
| Body mass index (kg/m2), mean ± SD | 27.3 ± 4.9 | 27.0 ± 4.5 | 0.788 |
| Waist circumference, mean ± SD | 90.2 ± 12.6 | 89.3 ± 9.5 | 0.783 |
| Waist-to-hip ratio, mean ± SD | 0.89 ± 0.07 | 0.86 ± 0.06 | 0.274 |
| Skeletal muscle mass index (Kg/m2) | 6.1 ± 1.0 | 6.2 ± 1.1 | 0.485 |
| Grip strength, mean ± SD | 11.6 ± 5.9 | 22.2 ± 8.0 | <0.001 |
| Decreased grip strength ≤ 16 kg, frequency (%) | 45 (79) | 4 (20) | <0.001 |
| Gait speed, mean ± SD | 0.86 ± 0.21 | 1.03 ± 0.16 | 0.001 |
| Decreased gait speed < 1.0 m/s, frequency (%) | 39 (68) | 7 (35) | 0.009 |
| Physical performance, mean ± SD | 7.5 ± 2.2 | 5.9 ± 0.95 | <0.001 |
| Functional disability (HAQ-DI ≥ 0.6) frequency (%) | 25 (44) | ----- | ------ |
| RA disease activity (DAS28 > 2.6), frequency (%) | 27 (47) | ----- | ------ |
| Treatments: | |||
| Methotrexate, frequency (%) | 52 (92) | ----- | ----- |
| Sulfasalazine, frequency (%) | 41 (72) | ----- | ----- |
| Leflunomide, frequency (%) | 29 (51) | ----- | ----- |
| Chloroquine, frequency (%) | 35 (61) | ----- | ----- |
| Anti-TNF agents *, frequency (%) | 19 (33) | ----- | ----- |
| Prednisone ≤ 10 mg/day, frequency (%) | 27 (47) | ||
| Follistatin (pg/mL), mean ± SD | 175 ± 119 | 133 ± 47 | 0.030 |
| Follistatin | Disability Score | |||
|---|---|---|---|---|
| Variable, n = 57 | r | p-Value | r | p-Value |
| Age | 0.088 | 0.515 | 0.009 | 0.947 |
| Body mass index | 0.126 | 0.349 | 0.165 | 0.220 |
| Waist circumference | 0.199 | 0.142 | 0.338 | 0.011 |
| Waist-to-hip ratio | 0.122 | 0.370 | 0.386 | 0.003 |
| Skeletal muscle mass index (kg/m2) | 0.077 | 0.569 | 0.032 | 0.814 |
| Grip strength | −0.173 | 0.197 | −0.546 | <0.001 |
| Gait speed | −0.124 | 0.357 | −0.307 | 0.020 |
| Physical performance | 0.114 | 0.398 | 0.285 | 0.032 |
| RA disease duration (years) | 0.015 | 0.919 | 0.170 | 0.239 |
| Tender joints | 0.105 | 0.435 | 0.356 | 0.007 |
| Swollen joints | 0.217 | 0.105 | 0.302 | 0.022 |
| HAQ-DI | 0.491 | <0.001 | ------- | ------- |
| DAS28-ESR | 0.344 | 0.009 | 0.471 | <0.001 |
| Erythrocyte sedimentation rate | 0.398 | 0.002 | 0.318 | 0.016 |
| Follistatin (pg/mL) | ------- | ------- | 0.491 | <0.001 |
| Variables | RA + Functional Disability (HAQ-DI ≥ 0.6) N = 25 | RA + Preserved Physical Function (HAQ-DI < 0.6) N = 32 | p-Value |
|---|---|---|---|
| Age, mean ± SD | 57 ± 10 | 57 ± 8 | 0.867 |
| Smoking, frequency (%) | 9 (36) | 8 (25) | 0.368 |
| Menopause, frequency (%) | 18 (75) | 25 (78) | 0.784 |
| Diabetes mellitus 2, frequency (%) | 5 (20) | 2 (6) | 0.221 |
| Hypertension, frequency (%) | 11 (44) | 9 (28) | 0.213 |
| Body mass index (kg/m2), mean ± SD | 28.1 ± 5.6 | 26.8 ± 4.4 | 0.326 |
| Waist circumference, mean ± SD | 94.5 ± 12.8 | 87.1 ± 11.6 | 0.029 |
| Waist-to-hip ratio, mean ± SD | 0.92 ± 0.06 | 0.86 ± 0.08 | 0.006 |
| Skeletal muscle mass index (kg/m2) | 6.0 ± 1.0 | 6.2 ± 1.0 | 0.473 |
| Grip strength, mean ± SD | 7.9 ± 4.6 | 14.5 ± 5.1 | <0.001 |
| Decreased grip strength, frequency (%) | 24 (96) | 21 (66) | 0.005 |
| Gait speed, mean ± SD | 0.77 ± 0.20 | 0.92 ± 0.20 | 0.010 |
| Decreased gait speed, frequency (%) | 20 (80) | 19 (59) | 0.096 |
| Physical performance, mean ± SD | 8.3 ± 2.4 | 6.9 ± 1.8 | 0.014 |
| RA Characteristics: | |||
| RA disease duration, mean ± SD | 17.9 ± 12 | 15.2 ± 10 | 0.377 |
| Tender joints count, mean ± SD | 3.7 ± 4.6 | 1.9 ± 3.9 | 0.112 |
| Tender joints count ≥ 4, frequency (%) | 12 (48) | 5 (16) | 0.008 |
| Swollen joints count, mean ± SD | 2.2 ± 3.0 | 1.2 ± 2.6 | 0.180 |
| Swollen joints count ≥ 4, frequency (%) | 6 (24) | 4 (13) | 0.308 |
| DAS28-ESR, mean ± SD | 3.8 ± 1.5 | 2.8 ± 1.2 | 0.008 |
| RA with active disease, frequency (%) | 16 (64) | 11 (34) | 0.026 |
| Erythrocyte sedimentation rate, mean ± SD | 21.5 ± 6.9 | 18.4 ± 6.9 | 0.096 |
| Follistatin (pg/mL), mean ± SD | 218 ± 159 | 141 ± 59 | 0.030 |
| Prednisone ≤ 10 mg/day, frequency (%) | 16 (64) | 11 (35) | 0.025 |
| Methotrexate, frequency (%) | 22 (88) | 30 (94) | 0.645 |
| Sulfasalazine, frequency (%) | 18 (72) | 23 (72) | 0.992 |
| Leflunomide, frequency (%) | 13 (52) | 16 (50) | 0.881 |
| Chloroquine, frequency (%) | 17 (68) | 18 (56) | 0.366 |
| Anti-TNF agents, frequency (%) | 7 (28) | 12 (38) | 0.450 |
| Drug | n (%) | Follistatin Levels, Mean ± SD | p-Value |
|---|---|---|---|
| Methotrexate users | 52 (92) | 181.2 ± 122.8 | 0.19 |
| Non-methotrexate users | 5 (8) | 107.8 ± 30.3 | |
| Sulfasalazine users | 41 (72) | 182.0 ± 125.0 | 0.47 |
| Non-sulfasalazine users | 16 (28) | 156.3 ± 104.9 | |
| Leflunomide users | 29 (51) | 177.8 ± 108.0 | 0.85 |
| Non-leflunomide users | 28 (49) | 171.6 ± 132.0 | |
| Chloroquine users | 35 (61) | 190.2 ± 130.7 | 0.22 |
| Non-chloroquine users | 22 (39) | 150.3 ± 96.5 | |
| Anti-TNF users | 19 (33) | 194.6 ± 155.9 | 0.38 |
| Non-anti-TNF users | 38 (67) | 164.9 ± 97.1 | |
| Prednisone users | 27 (47) | 180.9 ± 107.7 | 0.72 |
| Non-prednisone users | 30 (53) | 169.3 ± 130.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Ponce, F.; Gamez-Nava, J.I.; Jacobo-Cuevas, H.; Ponce-Guarneros, J.M.; Valdivia-Tangarife, E.R.; Nava-Valdivia, C.A.; Rodriguez-Jimenez, N.A.; Ramirez-Villafaña, M.; Gomez-Ramirez, E.E.; Gonzalez-Vazquez, S.A.; et al. Relationship Between High Serum Levels of Follistatin with Impaired Physical Function, and Severe Disease Activity in Rheumatoid Arthritis. Int. J. Mol. Sci. 2025, 26, 8232. https://doi.org/10.3390/ijms26178232
Gonzalez-Ponce F, Gamez-Nava JI, Jacobo-Cuevas H, Ponce-Guarneros JM, Valdivia-Tangarife ER, Nava-Valdivia CA, Rodriguez-Jimenez NA, Ramirez-Villafaña M, Gomez-Ramirez EE, Gonzalez-Vazquez SA, et al. Relationship Between High Serum Levels of Follistatin with Impaired Physical Function, and Severe Disease Activity in Rheumatoid Arthritis. International Journal of Molecular Sciences. 2025; 26(17):8232. https://doi.org/10.3390/ijms26178232
Chicago/Turabian StyleGonzalez-Ponce, Fabiola, Jorge Ivan Gamez-Nava, Heriberto Jacobo-Cuevas, Juan Manuel Ponce-Guarneros, Edgar Ricardo Valdivia-Tangarife, Cesar Arturo Nava-Valdivia, Norma Alejandra Rodriguez-Jimenez, Melissa Ramirez-Villafaña, Eli Efrain Gomez-Ramirez, Sergio Antonio Gonzalez-Vazquez, and et al. 2025. "Relationship Between High Serum Levels of Follistatin with Impaired Physical Function, and Severe Disease Activity in Rheumatoid Arthritis" International Journal of Molecular Sciences 26, no. 17: 8232. https://doi.org/10.3390/ijms26178232
APA StyleGonzalez-Ponce, F., Gamez-Nava, J. I., Jacobo-Cuevas, H., Ponce-Guarneros, J. M., Valdivia-Tangarife, E. R., Nava-Valdivia, C. A., Rodriguez-Jimenez, N. A., Ramirez-Villafaña, M., Gomez-Ramirez, E. E., Gonzalez-Vazquez, S. A., Brambila-Tapia, A. J. L., Olivas-Flores, E. M., Totsuka-Sutto, S., Cardona-Muñoz, E. G., & Gonzalez-Lopez, L., on behalf Group for the Assessment of Prognosis Biomarkers in Autoimmune Disorders. (2025). Relationship Between High Serum Levels of Follistatin with Impaired Physical Function, and Severe Disease Activity in Rheumatoid Arthritis. International Journal of Molecular Sciences, 26(17), 8232. https://doi.org/10.3390/ijms26178232






