Comparative Analysis of Gene and MicroRNA Expression in Subcutaneous Adipose Tissue in Metabolically Healthy and Unhealthy Obesity
Abstract
1. Introduction
2. Results
2.1. Participants’ Characteristics
2.2. Differential Gene and miRNA Expression Profiles in Obesity: Associations with Metabolic Dysregulation
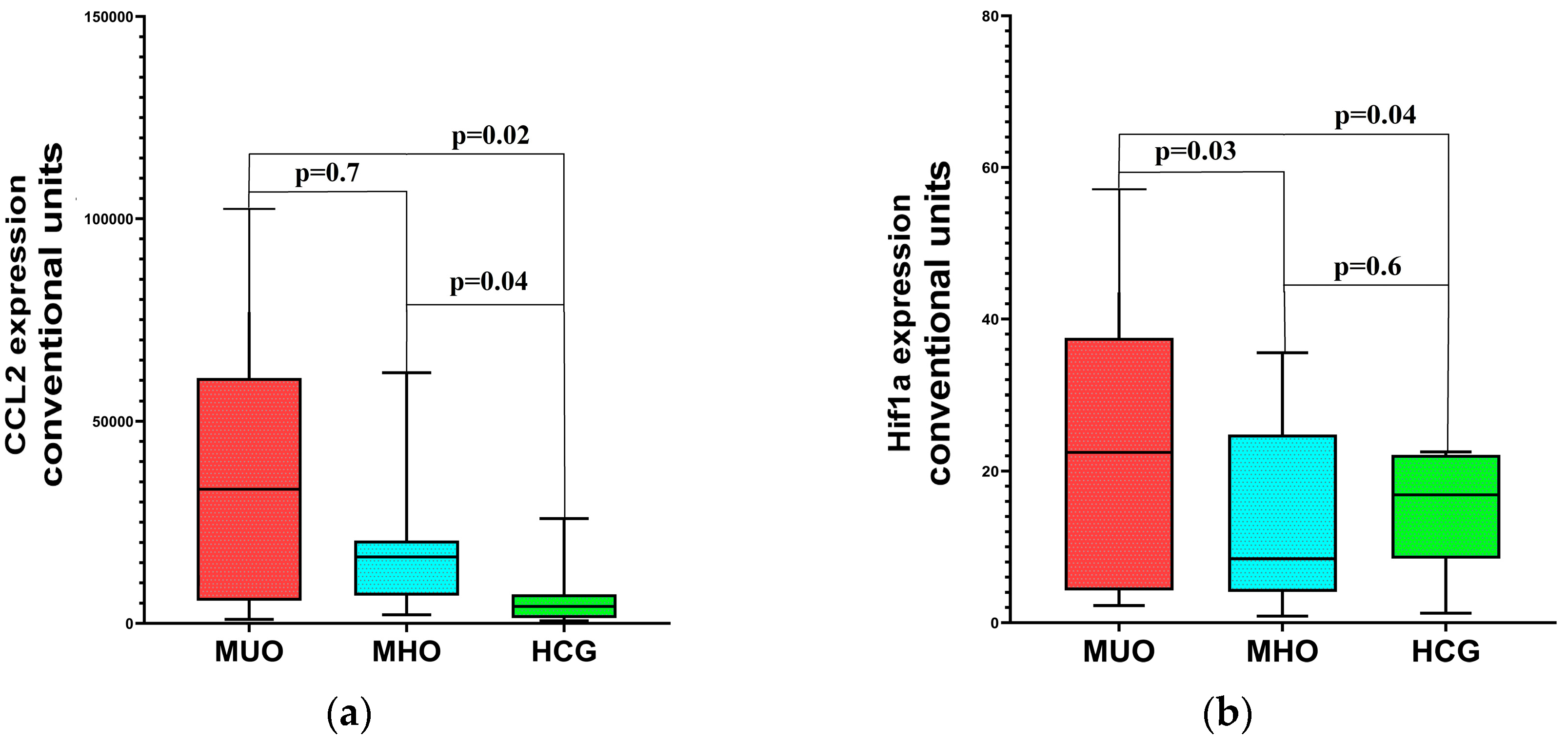
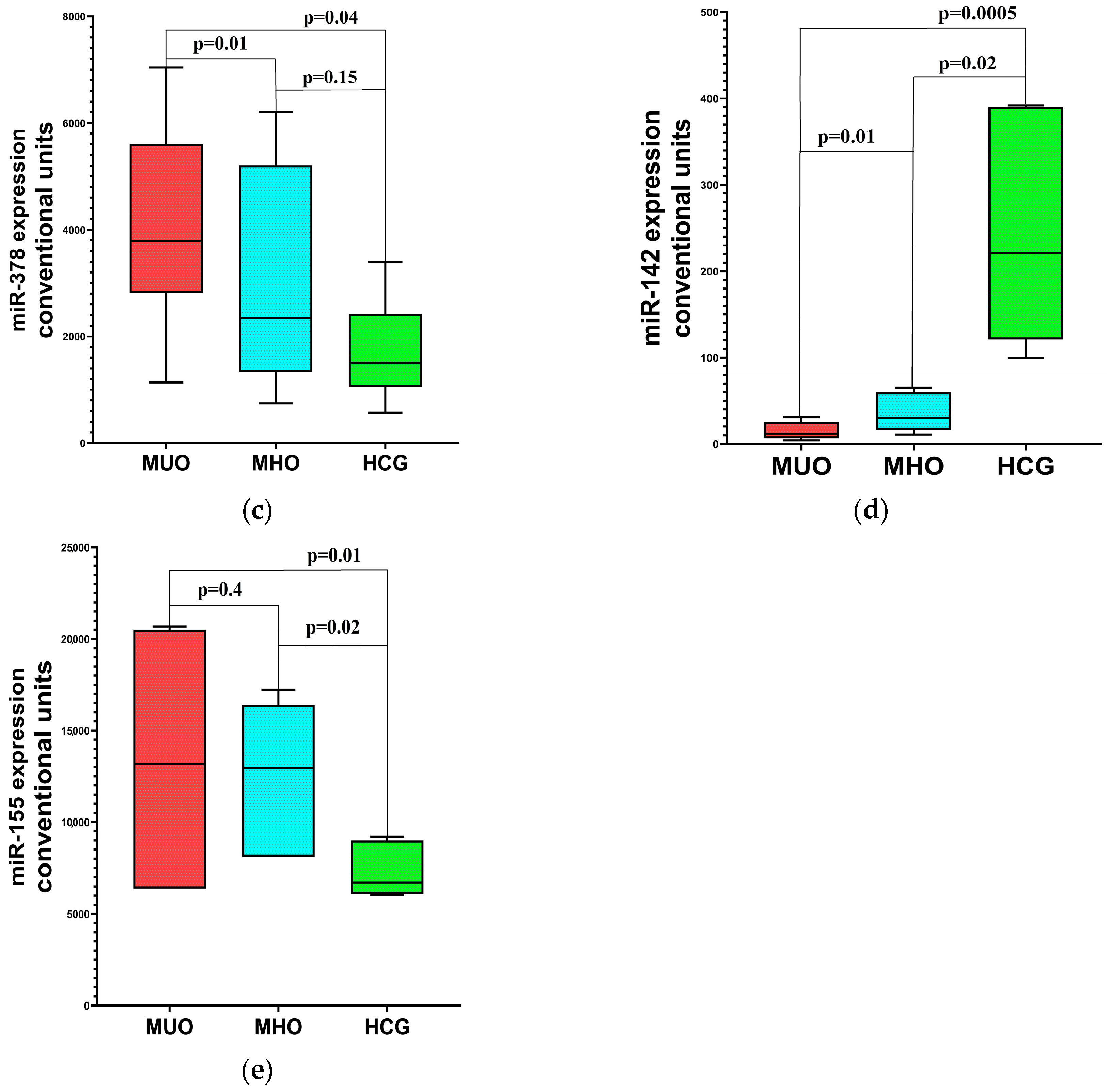
2.3. Distinct Molecular Patterns in MHO and MUO: Inflammation, Hypoxia, and Metabolic Correlations
3. Discussion
4. Materials and Methods
4.1. Clinical Characteristics of the Examined Patients
4.2. Laboratory Methods
4.3. Instrumental Methods
4.4. Statistical Analysis
4.5. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tchkonia, T.; Thomou, T.; Zhu, Y.; Karagiannides, I.; Pothoulakis, C.; Jensen, M.D.; Kirkland, J.L. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013, 17, 644–656. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macotela, Y.; Emanuelli, B.; Mori, M.A.; Gesta, S.; Schulz, T.J.; Tseng, Y.H.; Kahn, C.R. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes 2012, 61, 1691–1699. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meigs, J.B.; Wilson, P.W.; Fox, C.S.; Vasan, R.S.; Nathan, D.M.; Sullivan, L.M.; D'Agostino, R.B. Body mass index, metabolic syndrome, and risk of type 2 diabetes and cardiovascular disease. J. Clin. Endocrinol. Metab. 2006, 91, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Heyn, G.S.; Corrêa, L.H.; Magalhães, K.G. The Impact of Adipose Tissue-Derived miRNAs in Metabolic Syndrome, Obesity, and Cancer. Front. Endocrinol. 2020, 11, 563816. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Youssef, E.M.; Elfiky, A.M.; BanglySoliman Abu-Shahba, N.; Elhefnawi, M.M. Expression profiling and analysis of some miRNAs in subcutaneous white adipose tissue during development of obesity. Genes Nutr. 2020, 15, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ortega, F.J.; Mercader, J.M.; Catalán, V.; Moreno-Navarrete, J.M.; Pueyo, N.; Sabater, M.; Gómez-Ambrosi, J.; Anglada, R.; Fernández-Formoso, J.A.; Ricart, W.; et al. Targeting the circulating microRNA signature of obesity. Clin. Chem. 2013, 59, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Karkeni, E.; Astier, J.; Tourniaire, F.; El Abed, M.; Romier, B.; Gouranton, E.; Wan, L.; Borel, P.; Salles, J.; Walrand, S.; et al. Obesity-associated Inflammation Induces microRNA-155 Expression in Adipocytes and Adipose Tissue: Outcome on Adipocyte Function. J. Clin. Endocrinol. Metab. 2016, 101, 1615–1626. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.; Song, Y.; Wu, Y.; Kumar, V.; Mahato, R.I.; Su, Q. Activation of dsRNA-Dependent Protein Kinase R by miR-378 Sustains Metabolic Inflammation in Hepatic Insulin Resistance. Diabetes 2021, 70, 710–719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, T.; Hu, J.; Wang, X.; Zhao, X.; Li, Z.; Niu, J.; Steer, C.J.; Zheng, G.; Song, G. MicroRNA-378 promotes hepatic inflammation and fibrosis via modulation of the NF-κB-TNFα pathway. J. Hepatol. 2019, 70, 87–96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurylowicz, A. microRNAs in Human Adipose Tissue Physiology and Dysfunction. Cells 2021, 10, 3342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gerin, I.; Bommer, G.T.; McCoin, C.S.; Sousa, K.M.; Krishnan, V.; MacDougald, O.A. Roles for miRNA-378/378* in adipocyte gene expression and lipogenesis. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E198–E206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Talebi, F.; Ghorbani, S.; Chan, W.F.; Boghozian, R.; Masoumi, F.; Ghasemi, S.; Vojgani, M.; Power, C.; Noorbakhsh, F. MicroRNA-142 regulates inflammation and T cell differentiation in an animal model of multiple sclerosis. J. NeuroInflamm. 2017, 14, 55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Ouyang, M.; Wang, Q.; Jian, Z. MicroRNA-142-3p inhibits hypoxia/reoxygenation-induced apoptosis and fibrosis of cardiomyocytes by targeting high mobility group box 1. Int. J. Mol. Med. 2016, 38, 1377–1386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Q.; Zheng, Q.; He, J.; Li, L.; Xie, X.; Liang, H. Hsa-miR-142-3p reduces collagen I in human scleral fibroblasts by targeting TGF-β1 in high myopia. Exp. Eye Res. 2022, 219, 109023. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Qin, X.; Kang, X.; Zhou, H.; Wang, S.; Wei, D. MiR-142-3p inhibits adipogenic differentiation and autophagy in obesity through targeting KLF9. Mol. Cell. Endocrinol. 2020, 518, 111028. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, Q.; Yan, L.; Jiao, Y.; Su, Q.; Li, X.; Liu, C.; Zhao, F. MiRNA-128 and MiRNA-142 Regulate Tumorigenesis and EMT in Oral Squamous Cell Carcinoma Through HOXA10. Cancer Manag. Res. 2020, 12, 9987–9997. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matveev, G.A.; Khromova, N.V.; Zasypkin, G.G.; Kononova, Y.A.; Vasilyeva, E.Y.; Babenko, A.Y.; Shlyakhto, E.V. Tissue and Circulating MicroRNAs 378 and 142 as Biomarkers of Obesity and Its Treatment Response. Int. J. Mol. Sci. 2023, 24, 13426. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, C.; Zhang, J.; Chen, X.; Zhang, J.; Ding, X.; You, X.; Fan, L.; Chen, C.; Zhou, Y. MicroRNA-155 Mediates Obesity-Induced Renal Inflammation and Dysfunction. Inflammation 2019, 42, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Jankauskas, S.S.; Gambardella, J.; Sardu, C.; Lombardi, A.; Santulli, G. Functional Role of miR-155 in the Pathogenesis of Diabetes Mellitus and Its Complications. Noncoding RNA 2021, 7, 39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Velázquez, K.T.; Enos, R.T.; Carson, M.S.; Cranford, T.L.; Bader, J.E.; Sougiannis, A.T.; Pritchett, C.; Fan, D.; Carson, J.A.; Murphy, E.A. miR155 deficiency aggravates high-fat diet-induced adipose tissue fibrosis in male mice. Physiol. Rep. 2017, 5, e13412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bodo, M.J.; Jimenez, E.Y.; Conn, C.; Dye, A.; Pomo, P.; Kolkmeyer, D.; Orlando, R.; Kong, A.S. Association between circulating CCL2 levels and modifiable behaviors in overweight and obese adolescents: A cross-sectional pilot study. J. Pediatr. Endocrinol. Metab. 2016, 29, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Amano, S.U.; Cohen, J.L.; Vangala, P.; Tencerova, M.; Nicoloro, S.M.; Yawe, J.C.; Shen, Y.; Czech, M.P.; Aouadi, M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014, 19, 162–171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luck, H.; Khan, S.; Kim, J.H.; Copeland, J.K.; Revelo, X.S.; Tsai, S.; Chakraborty, M.; Cheng, K.; Tao Chan, Y.; Nøhr, M.K.; et al. Gut-associated IgA+ immune cells regulate obesity-related insulin resistance. Nat. Commun. 2019, 10, 3650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, C.; Drummer C4th Virtue, A.; Gao, T.; Wu, S.; Hernandez, M.; Singh, L.; Wang, H.; Yang, X.F. Increased Expression of Resistin in MicroRNA-155-Deficient White Adipose Tissues May Be a Possible Driver of Metabolically Healthy Obesity Transition to Classical Obesity. Front. Physiol. 2018, 9, 1297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Florijn, B.W.; Bijkerk, R.; van der Veer, E.P.; van Zonneveld, A.J. Gender and cardiovascular disease: Are sex-biased microRNA networks a driving force behind heart failure with preserved ejection fraction in women? Cardiovasc. Res. 2018, 114, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.B.; Riopel, M.; Cabrales, P.; Huh, J.Y.; Bandyopadhyay, G.K.; Andreyev, A.Y.; Murphy, A.N.; Beeman, S.C.; Smith, G.I.; Klein, S.; et al. Knockdown of Ant2 Reduces Adipocyte Hypoxia and Improves Insulin Resistance in Obesity. Nat. Metab. 2019, 1, 86–97. [Google Scholar] [CrossRef]
- Rakib, A.; Kiran, S.; Mandal, M.; Singh, U.P. MicroRNAs: A crossroad that connects obesity to immunity and aging. Immun. Ageing 2022, 19, 64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guiot, J.; Cambier, M.; Boeckx, A.; Henket, M.; Nivelles, O.; Gester, F.; Louis, E.; Malaise, M.; Dequiedt, F.; Louis, R.; et al. Macrophage-derived exosomes attenuate fibrosis in airway epithelial cells through delivery of antifibrotic miR-142-3p. Thorax 2020, 75, 870–881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, W.H.; Ahn, J.; Um, M.Y.; Jung, C.H.; Jung, S.E.; Ha, T.Y. Circulating microRNA expression profiling in young obese Korean women. Nutr. Res. Pract. 2020, 14, 412–422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Korac, A.; Srdic-Galic, B.; Kalezic, A.; Stancic, A.; Otasevic, V.; Korac, B.; Jankovic, A. Adipokine signatures of subcutaneous and visceral abdominal fat in normal-weight and obese women with different metabolic profiles. Arch. Med. Sci. 2021, 17, 323–336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vasilieva, L.B.; Artemyeva, M.S.; Ma, I.; Kondratov, K.A.; Anopova, A.D.; Neimark, A.E.; Babenko, A.Y.; Fedorov, A.V. The effect of obesity, impaired carbohydrate metabolism and bariatric surgery on adiponectin and leptin mRNA levels in different adipose tissue depots. Arter. Gipertenz. (Arter. Hypertens.) 2019, 25, 568–576. [Google Scholar] [CrossRef]
| Criteria | Aguilar-Salinas |
|---|---|
| FPG, mg/dL | <126 and no treatment |
| BP, mmHg | <140/90 and no treatment |
| TGs, mg/dL | ≥150 |
| HDL, mg/dL | ≥40 |
| MHO criteria | All of the above |
| BMI, kg/m2 | ≥30 |
| Groups | Age, Years | M/F (%) | BMI (kg/m2) | FGL, mmol/L | SBP, mmHg | DBP, mmHg | HDL-C, mmol/L | TGs, mmol/L |
|---|---|---|---|---|---|---|---|---|
| Obesity, n = 39 | 42.0 (30.0–53.0) | 23.1/76.9 | 35.71 (32.2–40.3) | 5.47 (5.16–5.67) | 124.8 (113.4–133.2) | 71.3 (70.0–82.4) | 1.42 (1.0–1.68) | 1.37 (1.0–1.76) |
| Healthy control, n = 10 | 38.9 (29–49) | 18.2/81.8 | 23.3 (22.1–24.9) | 4.71 (4.4–5.4) | 116.8 (111.3–128.8) | 77.3 (71.1–85.4) | 1.4 (1.3–1.51) | 1.29 (1.01–1.68) |
| p | >0.05 | >0.05 | ≤0.001 | ≤0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
| Assessed Parameters | Obese Patients n = 39 | Reference or Target Intervals, Where Applicable | |
|---|---|---|---|
| Age, years | 42.0 (30.0–53.0) | NA | |
| Body weight, kg | 102.0 (91.0–116.0) | NA | |
| BMI, kg/m2 | 35.71 (32.2–40.3) | <25 | |
| WC, cm | Male and female | 108.0 (100.0–118.0) | |
| Male | 118.0 (117–120.0) | <90 | |
| Female | 107.0 (96.0–114.0) | <84 | |
| HC, cm | Male and female | 119.0 (112.0–126.0) | |
| Male | 114.0 (108.0–121.0) | NA | |
| Female | 121.0 (112.0–128.0) | NA | |
| Fasting glucose level, mmol/L | 5.47 (5.16–5.67) | 3.3–6.1 | |
| Fasting insulin level, pmol/L | 141.05 (80.9–182.8) | 17.8–173.0 | |
| HOMA-IR | 4.72 (2.69–7.85) | <2.77 | |
| HOMA-B | 202.0 (132.9–288.9) | >100 | |
| TC, mmol/L | 5.38 (4.3–5.78) | <4.5 | |
| HDL-C, mmol/L | Male and female | 1.42 (1.0–1.68) | |
| Male | 1.12 (0.99–1.43) | >1.0 | |
| Female | 1.47 (1.08–1.68) | >1.2 | |
| LDL-C, mmol/L | 2.89 (2.23–3.61) | <1.8 | |
| TGs, mmol/L | 1.37 (1.0–1.76) | <1.7 | |
| SBP, mmHg | 124.8 (113.4–133.2) | <140 | |
| DBP, mmHg | 71.3 (70.0–82.4) | <85 | |
| Indicant | Obesity Before Treatment, n = 39 | Healthy Control, n = 10 | p |
|---|---|---|---|
| ADIPOQ | 3.01 (2.18–3.89) | 6.305 (2.3–7.68) | 0.039 |
| HIF1a | 12.95 (4.39–30.2) | 20.95 (11.62–22.550) | 0.63 |
| CCL2 | 13,829 (6861–50,704) | 4200 (1397–5302) | 0.004 |
| miR-155 | 12,960 (6385–19,960) | 6304 (6039–8623) | 0.017 |
| miR-142 | 3805 (675–16,300) | 21,400 (11,035–39,100) | 0.008 |
| miR-378 | 3360 (1650–5600) | 1820 (1131–2620) | 0.04 |
| Parameter | MHO (n = 20) | M3O (n = 19) | p |
|---|---|---|---|
| Age, years | 36 (25–47) | 39 (30.5–49) | 0.1 |
| Duration of obesity, years | 9.32 (6.3–12.8) | 8,97 (7.7–11.6) | 0.5 |
| % male | 20.6 | 21.4 | >0.05 |
| BMI, kg/m2 | 34.8 (32.8–39.8) | 35.9 (31.2–41.6) | 0.2 |
| Glucose, mmol/L | 5.6 (5.4–6.0) | 5.36 (5.12–5.6) | 0.05 |
| Insulin, pmol/L | 160.0(122.1–254.4) | 102.3 (63.3–155.3) | 0.01 |
| HOMA-IR | 5.7 (5.16–5.97) | 3.57 (2.2–5.4) | 0.02 |
| TC, mmol/L | 5.65 (3.96–6.0) | 5.26 (4.42–5.78) | 0.3 |
| HDL-C, mmol/L | 1.0 (0.91–1.49) | 1.53 (1.41–1.8) | 0.001 |
| TGs, mmol/L | 1.8 (1.2–2.34) | 1.15 (0.85–1.38) | 0.001 |
| SBP, mmHg | 128.3 (112–136) | 126.4 (109–132.1) | 0.6 |
| DBP, mmHg | 73 (70–80) | 73.3 (70–81.4) | 0.1 |
| CRP, mg/L | 3.61 (2.0–10.5) | 2.49 (0.72–6.1) | 0.04 |
| Parameters | CCL2 in the MUO Group | CCL2 in the MHO Group | ||
|---|---|---|---|---|
| r | p | r | p | |
| Glucose | 0.63 | 0.001 | −0.48 | 0.26 |
| TC | 0.45 | 0.03 | −0.02 | 0.9 |
| TGs | 0.52 | 0.01 | 0.3 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markina, N.O.; Matveev, G.A.; Zasypkina, K.A.; Khromova, N.V.; Babenko, A.Y.; Shlyakhto, E.V. Comparative Analysis of Gene and MicroRNA Expression in Subcutaneous Adipose Tissue in Metabolically Healthy and Unhealthy Obesity. Int. J. Mol. Sci. 2025, 26, 8212. https://doi.org/10.3390/ijms26178212
Markina NO, Matveev GA, Zasypkina KA, Khromova NV, Babenko AY, Shlyakhto EV. Comparative Analysis of Gene and MicroRNA Expression in Subcutaneous Adipose Tissue in Metabolically Healthy and Unhealthy Obesity. International Journal of Molecular Sciences. 2025; 26(17):8212. https://doi.org/10.3390/ijms26178212
Chicago/Turabian StyleMarkina, Natalia O., Georgy A. Matveev, Ksenia A. Zasypkina, Natalia V. Khromova, Alina Yu. Babenko, and Evgeny V. Shlyakhto. 2025. "Comparative Analysis of Gene and MicroRNA Expression in Subcutaneous Adipose Tissue in Metabolically Healthy and Unhealthy Obesity" International Journal of Molecular Sciences 26, no. 17: 8212. https://doi.org/10.3390/ijms26178212
APA StyleMarkina, N. O., Matveev, G. A., Zasypkina, K. A., Khromova, N. V., Babenko, A. Y., & Shlyakhto, E. V. (2025). Comparative Analysis of Gene and MicroRNA Expression in Subcutaneous Adipose Tissue in Metabolically Healthy and Unhealthy Obesity. International Journal of Molecular Sciences, 26(17), 8212. https://doi.org/10.3390/ijms26178212






