Lactoferrin and Thioredoxin in Rheumatoid Arthritis Are Associated with Fibrinogen but Not with Other Acute Phase Proteins
Abstract
1. Introduction
2. Results
3. Discussion
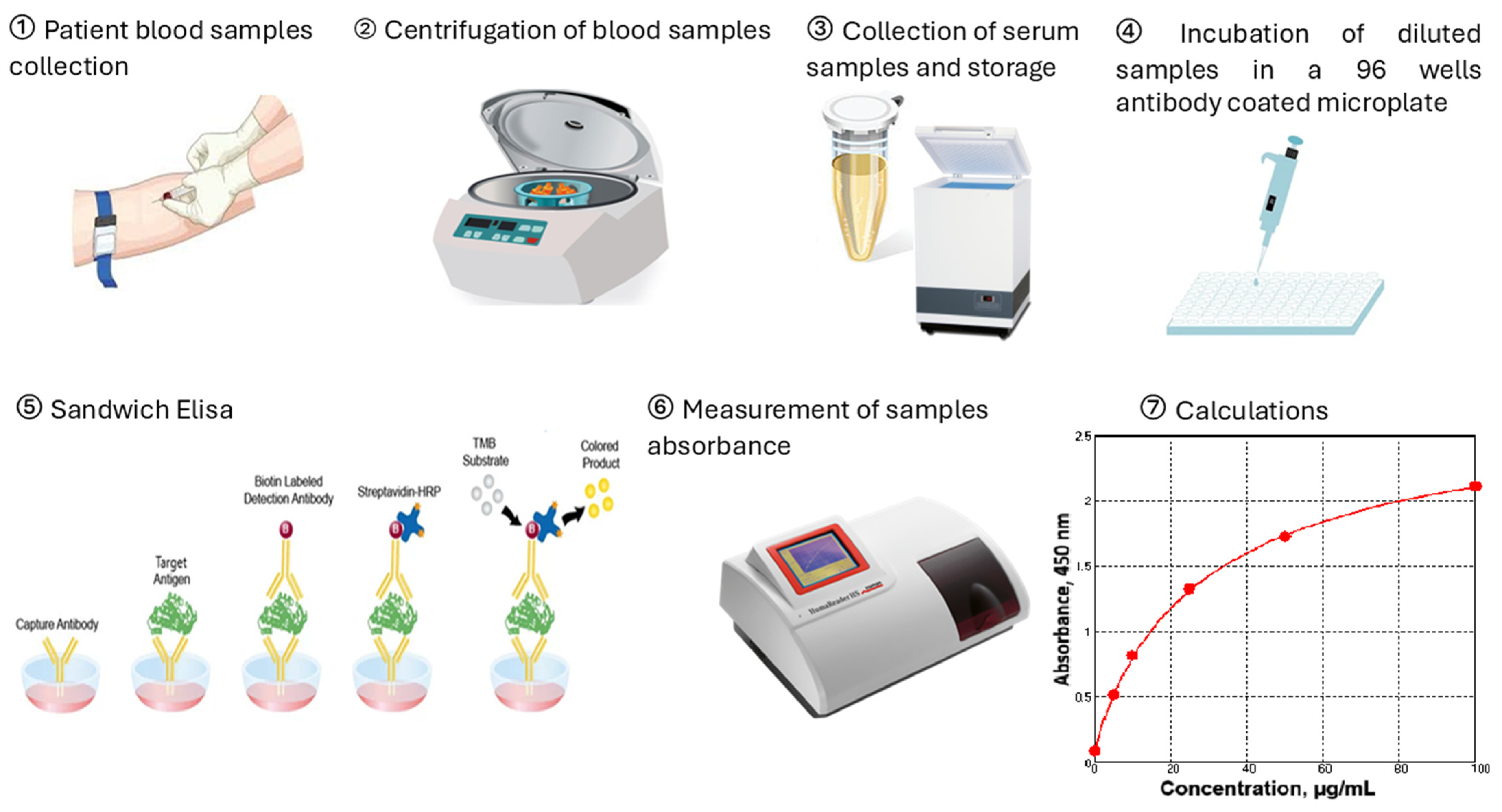
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, L.; Shi, Y.; Subatijang, P.; Zhang, L.; Gao, J.; Sun, R.; Jiang, K. Global research trends and hotspots in rheumatoid arthritis joint replacement:Bibliometric analysis and visualization study. J. Orthop. 2024, 61, 72–84. [Google Scholar] [CrossRef]
- Tański, W.; Chabowski, M.; Jankowska-Polańska, B.; Jankowska, E.A. Anaemia and iron deficiency in patients with rheumatoid arthritis and other chronic diseases. Postępy Hig. Med. Dośw. 2021, 75, 143–151. [Google Scholar] [CrossRef]
- Delcheva, G.; Stefanova, K.; Stankova, T. Serum sRAGE, sRANKL and osteoprotegerin in subgroups of rheumatoid arthritis patients: Biomarkers associated with iron and disease status. Biotechnol. Biotec. Eq. 2023, 37, 2256428. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef]
- Lin, Y.J.; Anzaghe, M.; Schülke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Li, Y.; Liu, Y.; Chu, C.Q. Preclinical RA: How to halt its progression. Best Pract. Res. Clin. Rheumatol. 2025, 39, 102030. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, A.; Emery, P. Rheumatoid arthritis: A review of the key clinical features and ongoing challenges of the disease. Panminerva Med. 2024, 66, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Petrovská, N.; Prajzlerová, K.; Vencovský, J.; Šenolt, L.; Filková, M. The pre-clinical phase of rheumatoid arthritis: From risk factors to prevention of arthritis. Autoimmun. Rev. 2021, 20, 102797. [Google Scholar] [CrossRef]
- Fujii, T.; Murata, K.; Kohjitani, H.; Onishi, A.; Murakami, K.; Tanaka, M.; Yamamoto, W.; Nagai, K.; Yoshikawa, A.; Etani, Y.; et al. Predicting rheumatoid arthritis progression from seronegative undifferentiated arthritis using machine learning: A deep learning model trained on the KURAMA cohort and externally validated with the ANSWER cohort. Arthritis Res. Ther. 2025, 27, 65. [Google Scholar] [CrossRef]
- Radu, A.-F.; Negru, P.A.; Radu, A.; Tarce, A.G.; Bungau, S.G.; Bogdan, M.A.; Tit, D.M.; Uivaraseanu, B. Simulation-Based Research on Phytoconstituents of Embelia ribes Targeting Proteins with Pathophysiological Implications in Rheumatoid Arthritis. Life 2023, 13, 1467. [Google Scholar] [CrossRef]
- Mohamed, W.A.; Schaalan, M.F. Antidiabetic efficacy of lactoferrin in type 2 diabetic pediatrics; controlling impact on PPAR-γ, SIRT-1, and TLR4 downstream signaling pathway. Diabetol. Metab. Syndr. 2018, 10, 89. [Google Scholar] [CrossRef]
- Farnaud, S.; Evans, R.W. Lactoferrin—A multifunctional protein with antimicrobial properties. Mol. Immunol. 2003, 40, 395–405. [Google Scholar] [CrossRef]
- Cui, S.; Lv, X.; Sun, G.; Wu, W.; Xu, H.; Li, Y.; Liu, Y.; Li, J.; Du, G.; Wang, M.; et al. Recent advances and prospects in purification and heterologous expression of lactoferrin. Food Bioeng. 2022, 1, 58–67. [Google Scholar] [CrossRef]
- Notari, S.; Gambardella, G.; Vincenzoni, F.; Desiderio, C.; Castagnola, M.; Bocedi, A.; Ricci, G. The unusual properties of lactoferrin during its nascent pase. Nat. Portf. Sci. Rep. 2023, 13, 14113. [Google Scholar] [CrossRef]
- Available online: https://www.recombinant-hsa.com/sale-8774689-antibacterial-greater-than-95-purity-recombinant-lactoferrin-lyophiized-with-inorganic-salt.html (accessed on 8 April 2025).
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Delcheva, G.; Stefanova, K.; Stankova, T.; Maneva, A. Serum thioredoxin and lactoferrin in rheumatoid arthritis and their association with rheumatoid factor. J. Med. Biochem. 2025, 44, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Francis, N.; Chahal, H.; Raza, K.; Salmon, M.; Scheel-Toellner, D.; Lord, J.M. Lactoferrin is a survival factor for neutrophils in rheumatoid synovial fluid. Rheumatology 2009, 48, 39–44. [Google Scholar] [CrossRef]
- Cross, A.; Barnes, T.; Bucknall, R.C.; Edwards, S.W.; Moots, R.J. Neutrophil apoptosis in rheumatoid arthritis is regulated by local oxygen tensions within joints. J. Leukoc. Biol. 2006, 80, 521–528. [Google Scholar] [CrossRef]
- Kasahara, Y.; Iwai, K.; Yachie, A.; Ohta, K.; Konno, A.; Seki, H.; Miyawaki, T.; Taniguchi, N. Involvement of reactive oxygen intermediates in spontaneous and CD95 (Fas/APO-1)-mediated apoptosis of neutrophils. Blood 1997, 89, 1748–1753. [Google Scholar] [CrossRef]
- Rollet-Labelle, E.; Grange, M.J.; Elbim, C.; Marquetty, C.; Gougerot-Pocidalo, M.A.; Pasquier, C. Hydroxyl radical as a potential intracellular mediator of polymorphonuclear neutrophil apoptosis. Free Radic. Biol. Med. 1998, 24, 563–572. [Google Scholar] [CrossRef]
- Scheel-Toellner, D.; Wang, K.; Craddock, R.; Webb, P.R.; McGettrick, H.M.; Assi, L.K.; Parkes, N.; Clough, L.E.; Gulbins, E.; Salmon, M.; et al. Reactive oxygen species limit neutrophil life span by activating death receptor signaling. Blood 2004, 104, 2557–2564. [Google Scholar] [CrossRef]
- Holmgren, A.; Lu, J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem. Biophys. Res. Commun. 2010, 396, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Yodoi, J. Extracellular thioredoxin: A therapeutic tool to combat inflammation. Cytokine Growth Factor Rev. 2013, 24, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Yan, F.; Deng, R.; Gu, R.; Zhang, X.; Bai, J. Thioredoxin-1 Rescues MPP+/MPTP-Induced Ferroptosis by Increasing Glutathione Peroxidase 4. Mol. Neurobiol. 2021, 58, 3187–3197. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.; Lane, D.; Nguyen, T.P.M.; Bush, A.; Ayton, S. In defence of ferroptosis. Signal Transduct. Target Ther. 2025, 10, 2. [Google Scholar] [CrossRef]
- Dobson, D.A.; Fish, R.J.; de Vries, P.S.; Morrison, A.C.; Neerman-Arbez, M.; Wolberg, A.S. Regulation of fibrinogen synthesis. Thromb. Res. 2024, 242, 109134. [Google Scholar] [CrossRef]
- Amrani, D.L. Regulation of fibrinogen biosynthesis: Glucocorticoid and interleukin-6 control. Blood Coagul. Fibrinolysis 1990, 1, 443–446. [Google Scholar] [CrossRef]
- Güven, B.; Can, M. Fibrinogen: Structure, abnormalities and laboratory assays. Adv. Clin. Chem. 2024, 120, 117–143. [Google Scholar] [CrossRef]
- So, A.K.; Varisco, P.A.; Kemkes-Matthes, B.; Herkenne-Morard, C.; Chobaz-Péclat, V.; Gerster, J.C.; Busso, N. Arthritis is linked to local and systemic activation of coagulation and fibrinolysis pathways. J. Thromb. Haemost. 2003, 1, 2510–2515. [Google Scholar] [CrossRef]
- Dijkshoorn, B.; Hansildaar, R.; Vedder, D.; Soutari, N.; Rudin, A.; Nordström, D.; Gudbjornsson, B.; Lend, K.; Uhlig, T.; Haavardsholm, E.A.; et al. Impaired coagulation parameters in early RA are restored by effective antirheumatic therapy: A prospective pilot study. RMD Open 2024, 10, e004838. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in Aseptic and Septic Inflammation. Molecules 2019, 24, 1323. [Google Scholar] [CrossRef]
- Miyauchi, S.; Umekita, K.; Hidaka, T.; Umeki, K.; Aratake, Y.; Takahashi, N.; Sawaguchi, A.; Nakatake, A.; Morinaga, I.; Morishita, K.; et al. Increased plasma lactoferrin levels in leucocytapheresis therapy in patients with rheumatoid arthritis. Rheumatology 2014, 53, 1966–1972. [Google Scholar] [CrossRef]
- Zhuravleva, Y.; Gusev, E. Relationship between systemic inflammatory response and hypercoagulation in patients with immuno-inflammatory rheumatic diseases. Med. Immunol. 2023, 25, 1059–1064. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Jikimoto, T.; Nishikubo, Y.; Koshiba, M.; Kanagawa, S.; Morinobu, S.; Morinobu, A.; Saura, R.; Mizuno, K.; Kondo, S.; Toyokuni, S.; et al. Thioredoxin as a biomarker for oxidative stress in patients with rheumatoid arthritis. Mol. Immunol. 2002, 38, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Ramani, S.; Pathak, A.; Dalal, V.; Paul, A.; Biswas, S. Oxidative Stress in Autoimmune Diseases: An Under Dealt Malice. Curr. Protein Pept. Sci. 2020, 21, 611–621. [Google Scholar] [CrossRef]
- Nagar, M.; Tilvawala, R.; Thompson, P.R. Thioredoxin Modulates Protein Arginine Deiminase 4 (PAD4)-Catalyzed Citrullination. Front. Immunol. 2019, 10, 244. [Google Scholar] [CrossRef]
- Kurowska, W.; Kuca-Warnawin, E.H.; Radzikowska, A.; Maśliński, W. The role of anti-citrullinated protein antibodies (ACPA) in the pathogenesis of rheumatoid arthritis. Cent. Eur. J. Immunol. 2017, 42, 390–398. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, M.; Choi, J.Y.; Kim, J.Y.; Kim, J.Y.; Song, J.-S.; Ivashkiv, L.B.; Lee, E.Y. Implication of the Association of Fibrinogen Citrullination and Osteoclastogenesis in Bone Destruction in Rheumatoid Arthritis. Cells 2020, 9, 2720. [Google Scholar] [CrossRef]
- Raijmakers, R.; van Beers, J.J.; El-Azzouny, M.; Visser, N.F.; Božič, B.; Pruijn, G.J.; Heck, A.J. Elevated levels of fibrinogen-derived endogenous citrullinated peptides in synovial fluid of rheumatoid arthritis patients. Arthritis Res. Ther. 2012, 14, R114. [Google Scholar] [CrossRef]
- Pretorius, E.; Akeredolu, O.O.; Soma, P.; Kell, D.B. Major involvement of bacterial components in rheumatoid arthritis and its accompanying oxidative stress, systemic inflammation and hypercoagulability. Exp. Biol. Med. 2017, 242, 355–373. [Google Scholar] [CrossRef]
- Li, S.; Zhou, C.; Li, W.; Kang, L.; Mu, H. The effects of coagulation factors on the risk of autoimmune diseases: A Mendelian randomization study. Medicine 2024, 103, e40893. [Google Scholar] [CrossRef]

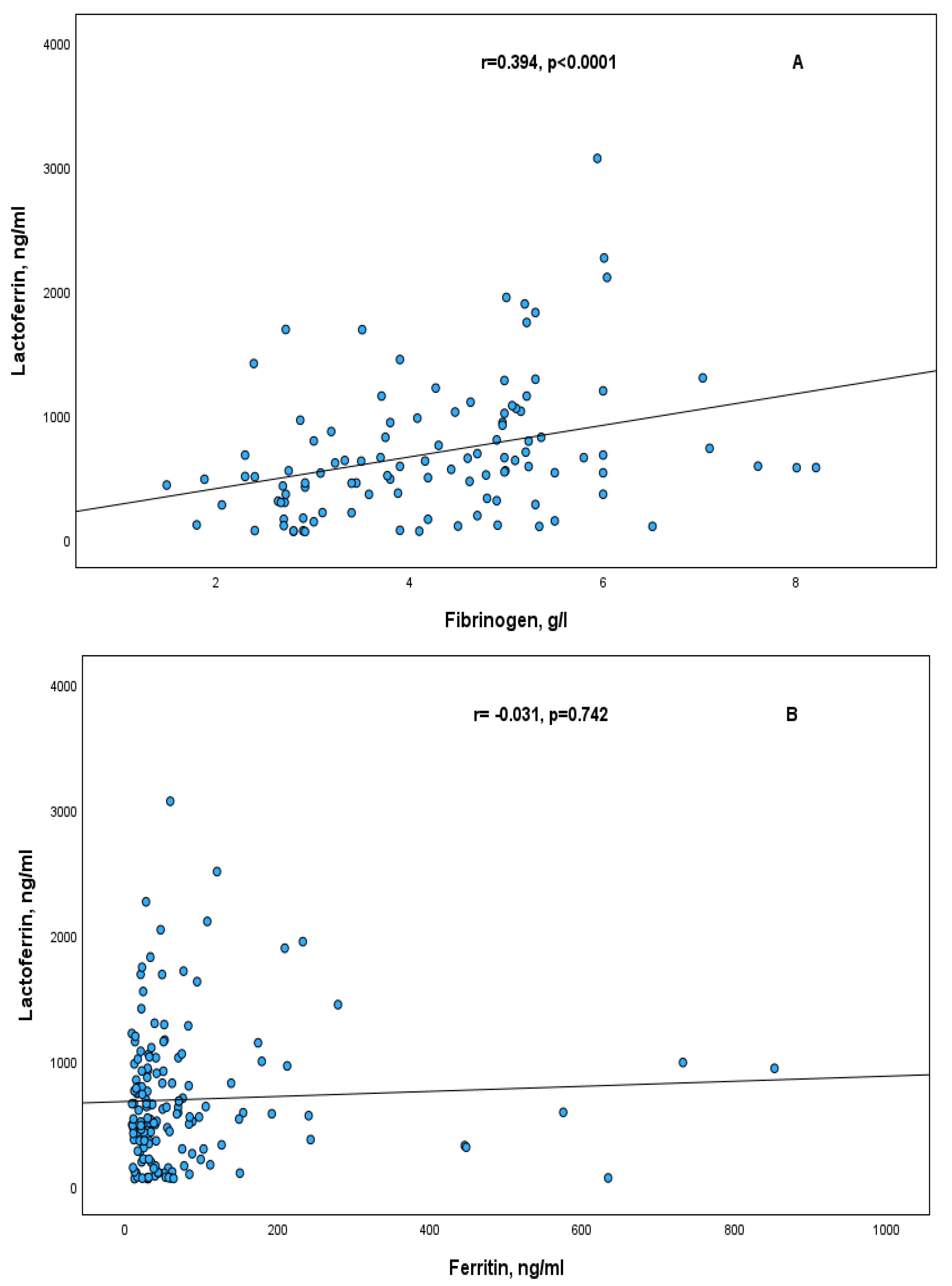



| Characteristics | |
|---|---|
| Mean age, years | 58 ± 10 years |
| Sex (M/F), n (%) | 16 (14%)/98 (86%) |
| DAS28 | 5.7 ± 1.2, n = 114 |
| CRP, µg/mL | 2.7 (1.9–10.3), n = 114 |
| ESR, mm/h | 36 (28–58), n = 114 |
| Lactoferrin, ng/mL | 579.6 (312.8–947.5) |
| Thioredoxin, ng/mL | 36.4 (29.6–40.2) |
| Fibrinogen, g/L | 4.25 ± 1.38, n = 108 |
| RF, U/mL | 56 (20–198), n = 107 |
| Anti-CCP antibodies, U/mL | 140 (54–246), n = 67 |
| Medication regime of the patients | |
| NSAIDs and DMARDs | n = 83 |
| NSAIDs, DMARDs, and corticosteroids | n = 10 |
| NSAIDs, biological agents, DMARDs, and corticosteroids | n = 4 |
| NSAIDs and corticosteroids | n = 2 |
| NSAIDs, biological agents, and DMARDs | n = 11 |
| NSAIDs, biological agents, and corticosteroids | n = 3 |
| Parameters | r | p | r | p |
|---|---|---|---|---|
| Males | Females | |||
| Lactoferrin/Fibrinogen | NS | 0.375 | <0.0001 | |
| Thioredoxin/Fibrinogen | NS | 0.447 | 0.001 | |
| DAS28 ≤ 5.1 | DAS28 > 5.1 | |||
| Lactoferrin/Fibrinogen | 0.689 | <0.0001 | NS | |
| Thioredoxin/Fibrinogen | 0.604 | 0.001 | NS | |
| CRP ≤ 8 | CRP > 8 | |||
| Lactoferrin/Fibrinogen | 0.488 | <0.0001 | NS | |
| Thioredoxin/Fibrinogen | 0.414 | 0.005 | NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delcheva, G.; Stefanova, K.; Selimov, P.; Stankova, T. Lactoferrin and Thioredoxin in Rheumatoid Arthritis Are Associated with Fibrinogen but Not with Other Acute Phase Proteins. Int. J. Mol. Sci. 2025, 26, 8211. https://doi.org/10.3390/ijms26178211
Delcheva G, Stefanova K, Selimov P, Stankova T. Lactoferrin and Thioredoxin in Rheumatoid Arthritis Are Associated with Fibrinogen but Not with Other Acute Phase Proteins. International Journal of Molecular Sciences. 2025; 26(17):8211. https://doi.org/10.3390/ijms26178211
Chicago/Turabian StyleDelcheva, Ginka, Katya Stefanova, Pavel Selimov, and Teodora Stankova. 2025. "Lactoferrin and Thioredoxin in Rheumatoid Arthritis Are Associated with Fibrinogen but Not with Other Acute Phase Proteins" International Journal of Molecular Sciences 26, no. 17: 8211. https://doi.org/10.3390/ijms26178211
APA StyleDelcheva, G., Stefanova, K., Selimov, P., & Stankova, T. (2025). Lactoferrin and Thioredoxin in Rheumatoid Arthritis Are Associated with Fibrinogen but Not with Other Acute Phase Proteins. International Journal of Molecular Sciences, 26(17), 8211. https://doi.org/10.3390/ijms26178211







