Atrial Myopathy and Heart Failure: Immunomolecular Mechanisms and Clinical Implications
Abstract
1. Introduction
2. Definition, Classification, and Clinical Overview of Heart Failure
3. Atrial Remodeling in the Pathophysiology of Heart Failure
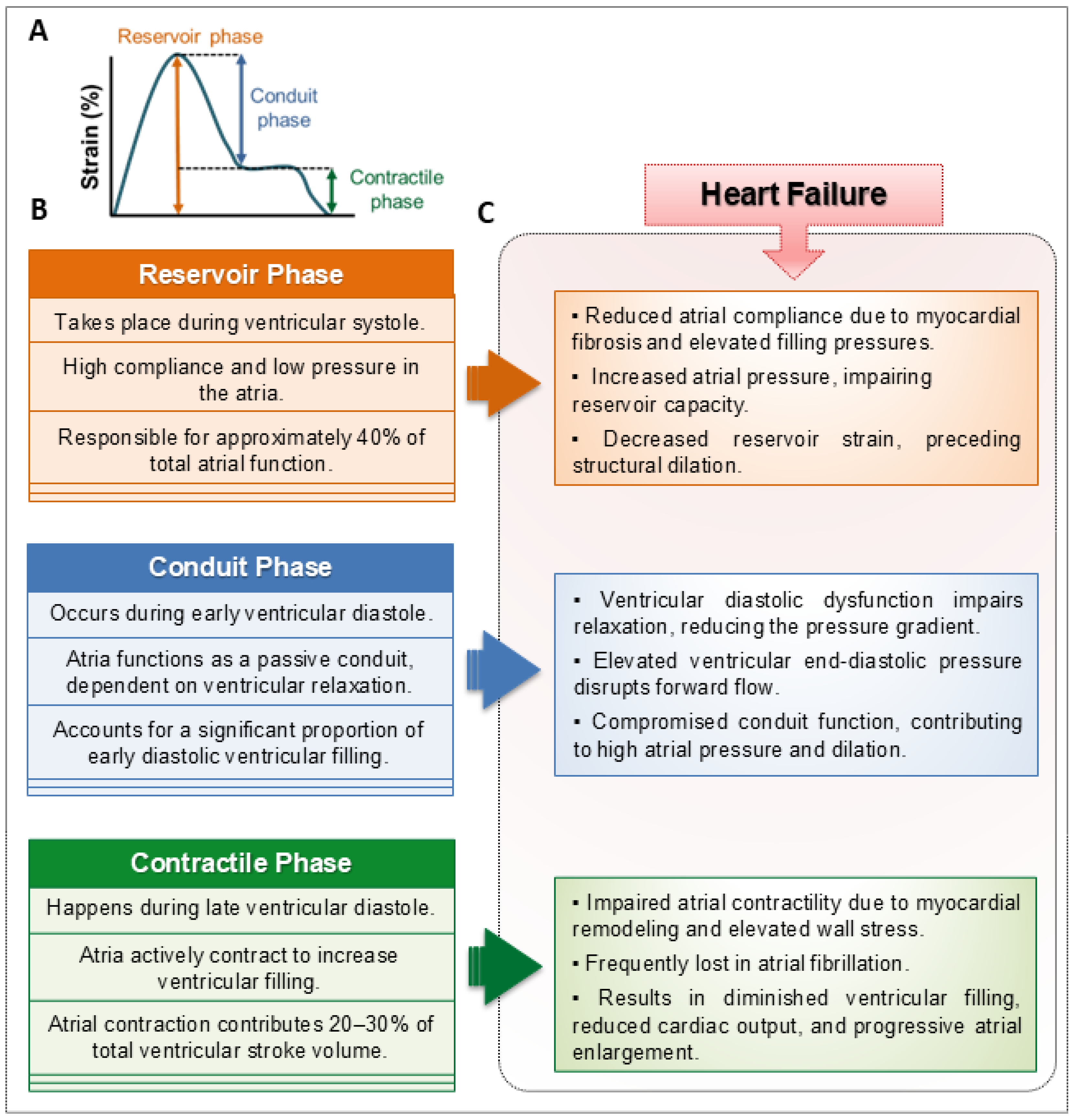
3.1. Structural Remodeling of the Atria
3.2. Functional Remodeling of the Atria
3.3. Electrical Remodeling of the Atria
3.4. Metabolic Remodeling of the Atria
3.5. Neurohormonal Remodeling of the Atria
4. Innate Immune Response and Inflammation in Heart Failure-Associated Atrial Myopathy
4.1. Pattern Recognition Receptors: Innate Immune Sensors in Atrial Remodeling
4.2. Toll-like and NOD-like Receptors in the Failing Heart
4.3. Inflammatory and Pro-Resolving Mediators Involved in Heart Failure and Atrial Myopathy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AERP | Atrial effective refractory period |
| AF | Atrial fibrillation |
| AIM2 | Absent in melanoma 2 |
| AMPK | AMP-activated protein kinase |
| Ang-II | Angiotensin II |
| ANP | Atrial natriuretic peptide |
| APD | Action potential duration |
| BNP | Brain natriuretic peptide |
| CASP1 | Caspase-1 |
| CLR | C-type lectin receptor |
| Cx40 | Connexin 40 |
| DAMPs | Damage-associated molecular patterns |
| ECM | Extracellular matrix |
| HF | Heart failure |
| HFmrEF | Heart failure with mildly reduced ejection fraction |
| HFpEF | Heart failure with preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| ICa,L | L-type calcium current |
| IK1 | Inward rectifier potassium current |
| IL-18 | Interleukin-18 |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| INa | Sodium current |
| LA | Left atrium |
| LV | Left ventricle |
| LVEF | Left ventricular ejection fraction |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor kappa-B |
| NLR | NOD-like receptor |
| PAMPs | Pathogen-associated molecular patterns |
| PRR | Pattern recognition receptor |
| RA | Right atrium |
| RAAS | Renin–angiotensin–aldosterone system |
| RIG-I | Retinoic acid-inducible gene I |
| ROS | Reactive oxygen species |
| RyR2 | Ryanodine receptor type 2 |
| TGF-β | Transforming growth factor-beta |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor-alpha |
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC)—Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- Shahim, B.; Kapelios, C.J.; Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure: An Updated Review. Card. Fail. Rev. 2023, 9, e11. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.B.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Tromp, J.; Sanchez, C.; Njoroge, J.; et al. Disproportionate Left Atrial Myopathy in Heart Failure with Preserved Ejection Fraction among Participants of the PROMIS-HFpEF Study. Sci. Rep. 2021, 11, 4885. [Google Scholar] [CrossRef]
- Hanna, N.; Cardin, S.; Leung, T.K.; Nattel, S. Differences in Atrial versus Ventricular Remodeling in Dogs with Ventricular Tachypacing-Induced Congestive Heart Failure. Cardiovasc. Res. 2004, 63, 236–244. [Google Scholar] [CrossRef]
- Patel, R.B.; Vaduganathan, M.; Shah, S.J.; Butler, J. Atrial Fibrillation in Heart Failure with Preserved Ejection Fraction: Insights into Mechanisms and Therapeutics. Pharmacol. Ther. 2017, 176, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, D.; Nakao, M.; Nagai, T.; Mizuguchi, Y.; Yokota, I.; Koya, T.; Tada, A.; Ishizaka, S.; George, F.; Kato, Y.; et al. Clinical Phenotyping and Treatment Response in Patients with Chronic Heart Failure. JACC Adv. 2025, 4, 101972. [Google Scholar] [CrossRef]
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal Trends and Patterns in Heart Failure Incidence: A Population-Based Study of 4 Million Individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef]
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7. [Google Scholar] [CrossRef]
- Braunwald, E. Heart Failure. JACC Heart Fail. 2013, 1, 1–20. [Google Scholar] [CrossRef]
- Halade, G.V.; Lee, D.H. Inflammation and Resolution Signaling in Cardiac Repair and Heart Failure. EBioMedicine 2022, 79, 103992. [Google Scholar] [CrossRef]
- Beghini, A.; Sammartino, A.M.; Papp, Z.; von Haehling, S.; Biegus, J.; Ponikowski, P.; Adamo, M.; Falco, L.; Lombardi, C.M.; Pagnesi, M.; et al. 2024 Update in Heart Failure. ESC Heart Fail. 2025, 12, 8–42. [Google Scholar] [CrossRef] [PubMed]
- Kittleson, M.M.; Panjrath, G.S.; Amancherla, K.; Davis, L.L.; Deswal, A.; Dixon, D.L.; Januzzi, J.L.; Yancy, C.W. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure with Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2023, 81, 1835–1878. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Rev. Esp. Cardiol. 2022, 75, 523. [Google Scholar] [CrossRef]
- Maddox, T.M.; Januzzi, J.L.; Allen, L.A.; Breathett, K.; Brouse, S.; Butler, J.; Davis, L.L.; Fonarow, G.C.; Ibrahim, N.E.; Lindenfeld, J.A.; et al. 2024 ACC Expert Consensus Decision Pathway for Treatment of Heart Failure with Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2024, 83, 1444–1488. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Solomon, S.D. Classification of Heart Failure According to Ejection Fraction: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 3217–3225. [Google Scholar] [CrossRef]
- Savarese, G.; Stolfo, D.; Sinagra, G.; Lund, L.H. Heart Failure with Mid-Range or Mildly Reduced Ejection Fraction. Nat. Rev. Cardiol. 2022, 19, 100–116. [Google Scholar] [CrossRef]
- Zhang, M.J.; Ji, Y.; Wang, W.; Norby, F.L.; Parikh, R.; Eaton, A.A.; Inciardi, R.M.; Alonso, A.; Soliman, E.Z.; Mosley, T.H.; et al. Association of Atrial Fibrillation with Stroke and Dementia Accounting for Left Atrial Function and Size. JACC Adv. 2023, 2, 100408. [Google Scholar] [CrossRef]
- Barbier, P.; Solomon, S.B.; Schiller, N.B.; Glantz, S.A. Left Atrial Relaxation and Left Ventricular Systolic Function Determine Left Atrial Reservoir Function. Circulation 1999, 100, 427–436. [Google Scholar] [CrossRef]
- Chen, Y.C.; Voskoboinik, A.; Gerche, A.L.; Marwick, T.H.; McMullen, J.R. Prevention of Pathological Atrial Remodeling and Atrial Fibrillation: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 2846–2864. [Google Scholar] [CrossRef]
- Cameli, M.; Pastore, M.C.; Henein, M.Y.; Mondillo, S. The Left Atrium and the Right Ventricle: Two Supporting Chambers to the Failing Left Ventricle. Heart Fail. Rev. 2019, 24, 661–669. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Pieske, B.; Butler, J.; Parissis, J.; Giamouzis, G.; Skoularigis, J.; Brutsaert, D.; Boudoulas, H. Global Left Atrial Failure in Heart Failure. Eur. J. Heart Fail. 2016, 18, 1307–1320. [Google Scholar] [CrossRef]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE Expert Consensus on Atrial Cardiomyopathies: Definition, Characterization, and Clinical Implication. Europace 2016, 18, 1455. [Google Scholar] [CrossRef]
- Peigh, G.; Shah, S.J.; Patel, R.B. Left Atrial Myopathy in Atrial Fibrillation and Heart Failure: Clinical Implications, Mechanisms, and Therapeutic Targets. Curr. Heart Fail. Rep. 2021, 18, 85–98. [Google Scholar] [CrossRef]
- Casaclang-Verzosa, G.; Gersh, B.J.; Tsang, T.S.M. Structural and Functional Remodeling of the Left Atrium. Clinical and Therapeutic Implications for Atrial Fibrillation. J. Am. Coll. Cardiol. 2008, 51, 986. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.S.M.; Barnes, M.E.; Gersh, B.J.; Bailey, K.R.; Seward, J.B. Left Atrial Volume as a Morphophysiologic Expression of Left Ventricular Diastolic Dysfunction and Relation to Cardiovascular Risk Burden. Am. J. Cardiol. 2002, 90, 1284–1289. [Google Scholar] [CrossRef]
- Theall, B.; Alcaide, P. The Heart under Pressure: Immune Cells in Fibrotic Remodeling. Curr. Opin. Physiol. 2022, 25, 100484. [Google Scholar] [CrossRef]
- Shen, M.J.; Arora, R.; Jalife, J. Atrial Myopathy. JACC Basic Transl. Sci. 2019, 4, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Kume, O.; Teshima, Y.; Abe, I.; Ikebe, Y.; Oniki, T.; Kondo, H.; Saito, S.; Fukui, A.; Yufu, K.; Miura, M.; et al. Role of Atrial Endothelial Cells in the Development of Atrial Fibrosis and Fibrillation in Response to Pressure Overload. Cardiovasc. Pathol. 2017, 27, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fontaine, G.H.; Fan, S.; Yan, Y.; Bode, P.K.; Duru, F.; Frank, R.; Saguner, A.M. Right Atrial Pathology in Arrhythmogenic Right Ventricular Dysplasia. Cardiol. J. 2019, 26, 736–743. [Google Scholar] [CrossRef]
- Nattel, S. How Does Fibrosis Promote Atrial Fibrillation Persistence: In Silico Findings, Clinical Observations, and Experimental Data. Cardiovasc. Res. 2016, 110, 295–297. [Google Scholar] [CrossRef]
- Carlisle, M.A.; Fudim, M.; DeVore, A.D.; Piccini, J.P. Heart Failure and Atrial Fibrillation, Like Fire and Fury. JACC Heart Fail. 2019, 7, 447–456. [Google Scholar] [CrossRef]
- Dobrev, D.; Aguilar, M.; Heijman, J.; Guichard, J.-B.; Nattel, S. Postoperative Atrial Fibrillation: Mechanisms, Manifestations and Management. Nat. Rev. Cardiol. 2019, 16, 417–436. [Google Scholar] [CrossRef]
- Nattel, S.; Heijman, J.; Zhou, L.; Dobrev, D. Molecular Basis of Atrial Fibrillation Pathophysiology and Therapy: A Translational Perspective. Circ. Res. 2020, 127, 51–72. [Google Scholar] [CrossRef] [PubMed]
- van Staveren, L.N.; de Groot, N.M.S. Exploring Refractoriness as an Adjunctive Electrical Biomarker for Staging of Atrial Fibrillation. J. Am. Heart Assoc. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Workman, A.J.; Kane, K.A.; Rankin, A.C. Cellular Bases for Human Atrial Fibrillation. Heart Rhythm 2008, 5, S1–S6. [Google Scholar] [CrossRef]
- Dobrev, D.; Voigt, N.; Wehrens, X.H.T. The Ryanodine Receptor Channel as a Molecular Motif in Atrial Fibrillation: Pathophysiological and Therapeutic Implications. Cardiovasc. Res. 2011, 89, 734. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, D.; Nattel, S. Calcium Handling Abnormalities in Atrial Fibrillation as a Target for Innovative Therapeutics. J. Cardiovasc. Pharmacol. 2008, 52, 293–299. [Google Scholar] [CrossRef]
- Dobrev, D. Electrical Remodeling in Atrial Fibrillation. Herz 2006, 31, 108–112. [Google Scholar] [CrossRef]
- Zhou, J.B.; Qian, L.L.; Wu, D.; Wang, R.X. The Role of Ferroptosis in Atrial Fibrillation: A Promising Future. Rev. Cardiovasc. Med. 2024, 25, 127. [Google Scholar] [CrossRef]
- Chung, Y.J.; Luo, A.; Park, K.C.; Loonat, A.A.; Lakhal-Littleton, S.; Robbins, P.A.; Swietach, P. Iron-Deficiency Anemia Reduces Cardiac Contraction by Downregulating RyR2 Channels and Suppressing SERCA Pump Activity. JCI Insight 2019, 4, e125618. [Google Scholar] [CrossRef]
- Othon-Martínez, D.; Fernandez-Betances, O.A.; Málaga-Espinoza, B.X.; Torres-Perez, M.E.; Cobos, E.; Gutierrez-Martinez, C. Iron and Cardiovascular Health: A Review. J. Investig. Med. 2024, 72, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Alnuwaysir, R.I.S.; Hoes, M.F.; van Veldhuisen, D.J.; van der Meer, P.; Beverborg, N.G. Iron Deficiency in Heart Failure: Mechanisms and Pathophysiology. J. Clin. Med. 2021, 11, 125. [Google Scholar] [CrossRef]
- Sousa, L.; Oliveira, M.M.; Pessôa, M.T.C.; Barbosa, L.A. Iron Overload: Effects on Cellular Biochemistry. Clin. Chim. Acta 2020, 504, 180–189. [Google Scholar] [CrossRef]
- Shen, J.; Fu, H.; Ding, Y.; Yuan, Z.; Xiang, Z.; Ding, M.; Huang, M.; Peng, Y.; Li, T.; Zha, K.; et al. The Role of Iron Overload and Ferroptosis in Arrhythmia Pathogenesis. Int. J. Cardiol. Heart Vasc. 2024, 52, 101414. [Google Scholar] [CrossRef]
- Rose, R.A.; Sellan, M.; Simpson, J.A.; Izaddoustdar, F.; Cifelli, C.; Panama, B.K.; Davis, M.; Zhao, D.; Markhani, M.; Murphy, G.G.; et al. Iron Overload Decreases CaV1.3-Dependent L-Type Ca2+ Currents Leading to Bradycardia, Altered Electrical Conduction, and Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2011, 4, 733–742. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Zhou, W.; Men, H.; Bao, T.; Sun, Y.; Wang, Q.; Tan, Y.; Keller, B.B.; Tong, Q.; et al. Ferroptosis Is Essential for Diabetic Cardiomyopathy and Is Prevented by Sulforaphane via AMPK/NRF2 Pathways. Acta Pharm. Sin. B 2021, 12, 708. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Wu, L.; Yang, G.; Zhang, C.; Liu, X.; Sun, X.; Chen, X.; Wang, N. The Role of Iron Metabolism in Chronic Diseases Related to Obesity. Mol. Med. 2022, 28, 130. [Google Scholar] [CrossRef]
- Ru, Q.; Li, Y.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron Homeostasis and Ferroptosis in Human Diseases: Mechanisms and Therapeutic Prospects. Signal Transduct. Target. Ther. 2024, 9, 271. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F. Systemic Inflammation and Arrhythmic Risk: Lessons from Rheumatoid Arthritis. Eur. Heart J. 2017, 38, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Chen, Y.J.; Lin, Y.J.; Chen, S.A. Inflammation and the Pathogenesis of Atrial Fibrillation. Nat. Rev. Cardiol. 2015, 12, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.M.; Dzeja, P.P.; Shen, W.K.; Jahangir, A.; Hart, C.Y.T.; Terzic, A.; Redfield, M.M. Failing Atrial Myocardium: Energetic Deficits Accompany Structural Remodeling and Electrical Instability. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1313–H1320. [Google Scholar] [CrossRef]
- Barger, P.M.; Kelly, D.P. Fatty Acid Utilization in the Hypertrophied and Failing Heart: Molecular Regulatory Mechanisms. Am. J. Med. Sci. 1999, 318, 36. [Google Scholar] [CrossRef]
- Nattel, S. Electrophysiologic Remodeling: Are Ion Channels Static Players or Dynamic Movers? J. Cardiovasc. Electrophysiol. 1999, 10, 1553–1556. [Google Scholar] [CrossRef]
- Ausma, J.; Coumans, W.A.; Duimel, H.; Van Der Vusse, G.J.; Allessie, M.A.; Borgers, M. Atrial High Energy Phosphate Content and Mitochondrial Enzyme Activity during Chronic Atrial Fibrillation. Cardiovasc. Res. 2000, 47, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Reyat, J.S.; Sommerfeld, L.C.; O’Reilly, M.; Roth Cardoso, V.; Thiemann, E.; Khan, A.O.; O’Shea, C.; Harder, S.; Müller, C.; Barlow, J.; et al. PITX2 Deficiency Leads to Atrial Mitochondrial Dysfunction. Cardiovasc. Res. 2024, 120, 1907–1923. [Google Scholar] [CrossRef]
- Li, D.; Liu, Y.; Li, C.; Zhou, Z.; Gao, K.; Bao, H.; Yang, J.; Xue, G.; Yin, D.; Zhao, X.; et al. Spexin Diminishes Atrial Fibrillation Vulnerability by Acting on Galanin Receptor 2. Circulation 2024, 150, 111–127. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of Reactive Oxygen Species and NF-κB Signaling. Cell Res. 2010, 21, 103. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, Y.; Zheng, Q. Metabolic Inflexibility as a Pathogenic Basis for Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 8291. [Google Scholar] [CrossRef]
- Tao, S.M.; Yang, M. Immune Regulation in Atrial Cardiomyopathy. Rev. Cardiovasc. Med. 2025, 26, 26897. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Tian, R.; Cai, S. The Role of Innate Immune Cells in Cardiac Injury and Repair: A Metabolic Perspective. Curr. Cardiol. Rep. 2023, 25, 1. [Google Scholar] [CrossRef]
- Muszyński, P.; Bonda, T.A. Mitochondrial Dysfunction in Atrial Fibrillation—Mechanisms and Pharmacological Interventions. J. Clin. Med. 2021, 10, 2385. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Chen, Z.; Huang, W.; Zhang, J.; Zou, L.; Wang, H. Communications between Macrophages and Cardiomyocytes. Cell Commun. Signal 2023, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Theofilis, P.; Vlachakis, P.K.; Ktenopoulos, N.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Atrial Cardiomyopathy in Atrial Fibrillation: Mechanistic Pathways and Emerging Treatment Concepts. J. Clin. Med. 2025, 14, 3250. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Huiskes, F.G.; de Groot, N.M.S.; Brundel, B.J.J.M. The Role of Immune Cells Driving Electropathology and Atrial Fibrillation. Cells 2024, 13, 311. [Google Scholar] [CrossRef]
- Tang, Y.; Jiao, Y.; An, X.; Tu, Q.; Jiang, Q. Neutrophil Extracellular Traps and Cardiovascular Disease: Associations and Potential Therapeutic Approaches. Biomed. Pharmacother. 2024, 180, 117476. [Google Scholar] [CrossRef]
- Sovari, A.A.; Dudley, S.C. Reactive Oxygen Species-Targeted Therapeutic Interventions for Atrial Fibrillation. Front. Physiol. 2012, 3, 311. [Google Scholar] [CrossRef]
- Lim, T.K.; Ashrafian, H.; Dwivedi, G.; Collinson, P.O.; Senior, R. Increased Left Atrial Volume Index Is an Independent Predictor of Raised Serum Natriuretic Peptide in Patients with Suspected Heart Failure but Normal Left Ventricular Ejection Fraction: Implication for Diagnosis of Diastolic Heart Failure. Eur. J. Heart Fail. 2006, 8, 38–45. [Google Scholar] [CrossRef]
- Inoue, S.-I.; Murakami, Y.; Sano, K.; Katoh, H.; Shimada, T. Atrium as a Source of Brain Natriuretic Polypeptide in Patients with Atrial Fibrillation. J. Card. Fail. 2000, 6, 92–96. [Google Scholar] [CrossRef]
- Weber, K.T.; Brilla, C.G.; Campbell, S.E.; Guarda, E.; Zhou, G.; Sriram, K. Myocardiasl Fibrosis: Role of Angiotensin II and Aldosterone. Basic. Res. Cardiol. 1993, 88, 107–124. [Google Scholar] [CrossRef]
- Verheule, S.; Sat, T.; Everett IV, T.; Engle, S.K.; Otten, D.; Rubart-Von Der Lohe, M.; Nakajima, H.O.; Nakajima, H.; Field, L.J.; Olgin, J.E. Increased Vulnerability to Atrial Fibrillation in Transgenic Mice with Selective Atrial Fibrosis Caused by Overexpression of TGF-Β1. Circ. Res. 2004, 94, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Joca, H.C.; Santos-Miranda, A.; Joviano-Santos, J.V.; Maia-Joca, R.P.M.; Brum, P.C.; Williams, G.S.B.; Cruz, J.S. Chronic Sympathetic Hyperactivity Triggers Electrophysiological Remodeling and Disrupts Excitation-Contraction Coupling in Heart. Sci. Rep. 2020, 10, 8001. [Google Scholar] [CrossRef]
- Val-Blasco, A.; Piedras, M.J.G.M.; Ruiz-Hurtado, G.; Suarez, N.; Prieto, P.; Gonzalez-Ramos, S.; Gómez-Hurtado, N.; Delgado, C.; Pereira, L.; Benito, G.; et al. Role of NOD1 in Heart Failure Progression via Regulation of Ca2+ Handling. J. Am. Coll. Cardiol. 2017, 69, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L. Innate Immunity and the Failing Heart. Circ. Res. 2015, 116, 1254–1268. [Google Scholar] [CrossRef]
- Rai, V.; Mathews, G.; Agrawal, D.K. Translational and Clinical Significance of DAMPs, PAMPs, and PRRs in Trauma-Induced Inflammation. Arch. Clin. Biomed. Res. 2022, 6, 673. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate Immune Pattern Recognition: A Cell Biological Perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef]
- Mathur, S.; Walley, K.R.; Wang, Y.; Indrambarya, T.; Boyd, J.H. Extracellular Heat Shock Protein 70 Induces Cardiomyocyte Inflammation and Contractile Dysfunction via TLR2. Circ. J. 2011, 75, 2445–2452. [Google Scholar] [CrossRef]
- Monnerat-Cahli, G.; Alonso, H.; Gallego, M.; Alarcón, M.L.; Bassani, R.A.; Casis, O.; Medei, E. Toll-like Receptor 4 Activation Promotes Cardiac Arrhythmias by Decreasing the Transient Outward Potassium Current (Ito) through an IRF3-Dependent and MyD88-Independent Pathway. J. Mol. Cell Cardiol. 2014, 76, 70–75. [Google Scholar] [CrossRef]
- Jaén, R.I.; Val-Blasco, A.; Prieto, P.; Gil-Fernández, M.; Smani, T.; López-Sendón, J.L.; Delgado, C.; Boscá, L.; Fernández-Velasco, M. Innate Immune Receptors, Key Actors in Cardiovascular Diseases. JACC Basic Transl. Sci. 2020, 5, 735–749. [Google Scholar] [CrossRef]
- Zhang, C.; Mo, M.; Ding, W.; Liu, W.; Yan, D.; Deng, J.; Luo, X.; Liu, J. High-Mobility Group Box 1 (HMGB1) Impaired Cardiac Excitation-Contraction Coupling by Enhancing the Sarcoplasmic Reticulum (SR) Ca2+ Leak through TLR4-ROS Signaling in Cardiomyocytes. J. Mol. Cell Cardiol. 2014, 74, 260–273. [Google Scholar] [CrossRef]
- Wang, J.; Xue, L.; Cao, H.; Cui, F.; Dai, T.; Chen, Y. TLR2 Was Overexpressed Independent of IL-6 in Patients with Valvular Atrial Fibrillation. J. Biomed. Res. 2011, 25, 178. [Google Scholar] [CrossRef]
- Gungor, B.; Ekmekci, A.; Arman, A.; Ozcan, K.S.; Ucer, E.; Alper, A.T.; Calik, N.; Yilmaz, H.; Tezel, T.; Coker, A.; et al. Assessment of Interleukin-1 Gene Cluster Polymorphisms in Lone Atrial Fibrillation: New Insight into the Role of Inflammation in Atrial Fibrillation. PACE—Pacing Clin. Electrophysiol. 2013, 36, 1220–1227. [Google Scholar] [CrossRef]
- Luan, Y.; Guo, Y.; Li, S.; Yu, B.; Zhu, S.; Li, S.; Li, N.; Tian, Z.; Peng, C.; Cheng, J.; et al. Interleukin-18 among Atrial Fibrillation Patients in the Absence of Structural Heart Disease. Europace 2010, 12, 1713–1718. [Google Scholar] [CrossRef]
- Karakasis, P.; Pamporis, K.; Theofilis, P.; Milaras, N.; Vlachakis, P.K.; Grigoriou, K.; Patoulias, D.; Karamitsos, T.; Antoniadis, A.P.; Fragakis, N. Inflammasome Signaling in Cardiac Arrhythmias: Linking Inflammation, Fibrosis, and Electrical Remodeling. Int. J. Mol. Sci. 2025, 26, 5954. [Google Scholar] [CrossRef]
- Yao, C.; Veleva, T.; Scott, L.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Pan, X.; Alsina, K.M.; Abu-Taha, I.; et al. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227. [Google Scholar] [CrossRef]
- Ridker, P.M. Anticytokine Agents: Targeting Interleukin Signaling Pathways for the Treatment of Atherothrombosis. Circ. Res. 2019, 124, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Castillo, E.C.; Vázquez-Garza, E.; Yee-Trejo, D.; García-Rivas, G.; Torre-Amione, G. What Is the Role of the Inflammation in the Pathogenesis of Heart Failure? Curr. Cardiol. Rep. 2020, 22, 139. [Google Scholar] [CrossRef] [PubMed]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the Role of Inflammation in Heart Failure. Nat. Rev. Cardiol. 2020, 17, 269–285. [Google Scholar] [CrossRef]
- Heijman, J.; Muna, A.P.; Veleva, T.; Molina, C.E.; Sutanto, H.; Tekook, M.; Wang, Q.; Abu-Taha, I.H.; Gorka, M.; Künzel, S.; et al. Atrial Myocyte NLRP3/CaMKII Nexus Forms a Substrate for Post-Operative Atrial Fibrillation. Circ. Res. 2020, 127, 1036. [Google Scholar] [CrossRef]
- Dobrev, D.; Heijman, J.; Hiram, R.; Li, N.; Nattel, S. Inflammatory Signalling in Atrial Cardiomyocytes: A Novel Unifying Principle in Atrial Fibrillation Pathophysiology. Nat. Rev. Cardiol. 2022, 20, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Laghi-Pasini, F.; Acampa, M.; Srivastava, U.; Bertolozzi, I.; Giabbani, B.; Finizola, F.; Vanni, F.; Dokollari, A.; Natale, M.; et al. Systemic Inflammation Rapidly Induces Reversible Atrial Electrical Remodeling: The Role of Interleukin-6-Mediated Changes in Connexin Expression. J. Am. Heart Assoc. 2019, 8. [Google Scholar] [CrossRef]
- Hanna, A.; Frangogiannis, N.G. Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure. Cardiovasc. Drugs Ther. 2020, 34, 849–863. [Google Scholar] [CrossRef]
- Saba, S.; Janczewski, A.M.; Baker, L.C.; Shusterman, V.; Gursoy, E.C.; Feldman, A.M.; Salama, G.; McTiernan, C.F.; London, B. Atrial Contractile Dysfunction, Fibrosis, and Arrhythmias in a Mouse Model of Cardiomyopathy Secondary to Cardiac-Specific Overexpression of Tumor Necrosis Factor-α. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1456–H1467. [Google Scholar] [CrossRef]
- Lakin, R.; Polidovitch, N.; Yang, S.; Guzman, C.; Gao, X.; Wauchop, M.; Burns, J.; Izaddoustdar, F.; Backx, P.H. Inhibition of Soluble TNFα Prevents Adverse Atrial Remodeling and Atrial Arrhythmia Susceptibility Induced in Mice by Endurance Exercise. J. Mol. Cell Cardiol. 2019, 129, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Halade, G.V.; Kain, V.; Serhan, C.N. Immune Responsive Resolvin D1 Programs Myocardial Infarction–Induced Cardiorenal Syndrome in Heart Failure. FASEB J. 2018, 32, 3717–3729. [Google Scholar] [CrossRef] [PubMed]
- Fredman, G.; Serhan, C.N. Specialized Pro-Resolving Mediators in Vascular Inflammation and Atherosclerotic Cardiovascular Disease. Nat. Rev. Cardiol. 2024, 21, 808–823. [Google Scholar] [CrossRef] [PubMed]
- Jaén, R.I.; Sánchez-García, S.; Fernández-Velasco, M.; Boscá, L.; Prieto, P. Resolution-Based Therapies: The Potential of Lipoxins to Treat Human Diseases. Front. Immunol. 2021, 12, 143–170. [Google Scholar] [CrossRef]
- Val-Blasco, A.; Prieto, P.; Jaén, R.I.; Gil-Fernández, M.; Pajares, M.; Domenech, N.; Terrón, V.; Tamayo, M.; Jorge, I.; Vázquez, J.; et al. Specialized Proresolving Mediators Protect Against Experimental Autoimmune Myocarditis by Modulating Ca2+ Handling and NRF2 Activation. JACC Basic. Transl. Sci. 2022, 7, 544–560. [Google Scholar] [CrossRef]
- Chiurchiu, V.; Leuti, A.; Saracini, S.; Fontana, D.; Finamore, P.; Giua, R.; Padovini, L.; Incalzi, R.A.; MacCarrone, M. Resolution of Inflammation Is Altered in Chronic Heart Failure and Entails a Dysfunctional Responsiveness of T Lymphocytes. FASEB J. 2019, 33, 909–916. [Google Scholar] [CrossRef]
- Hiram, R.; Xiong, F.; Naud, P.; Xiao, J.; Sosnowski, D.K.; Le Quilliec, E.; Saljic, A.; Abu-Taha, I.H.; Kamler, M.; LeBlanc, C.A.; et al. An Inflammation Resolution–Promoting Intervention Prevents Atrial Fibrillation Caused by Left Ventricular Dysfunction. Cardiovasc. Res. 2024, 120, 345–359. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Muela-Zarzuela, I.; Suarez-Rivero, J.M.; Boy-Ruiz, D.; López-Pérez, J.; Sotelo-Montoro, M.; del Mar Navarrete-Alonso, M.; Collado, I.G.; Botubol-Ares, J.M.; Sanz, A.; Cordero, M.D. The NLRP3 Inhibitor Dapansutrile Improves the Therapeutic Action of Lonafarnib on Progeroid Mice. Aging Cell 2024, 23, e14272. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Santana, B.; Saldaña-García, J.; Jurado-Román, A.; Cantolla-Pablo, P.; Gil-Fernández, M.; López-Sendón, J.; Tardif, J.C.; Moreno, R.; Fernández-Velasco, M. Early Anti-Inflammatory Therapy in Acute Myocardial Infarction: A Network Meta-Analysis of Timing-Dependent Effects in 23 Randomized Trials and 28,220 Patients. Atherosclerosis 2025, 408, 120443. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil Fernández, M.; Bueno Sen, A.; Cantolla Pablo, P.; Val Blasco, A.; Ruiz Hurtado, G.; Delgado, C.; Cubillos, C.; Boscá, L.; Fernández Velasco, M. Atrial Myopathy and Heart Failure: Immunomolecular Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2025, 26, 8210. https://doi.org/10.3390/ijms26178210
Gil Fernández M, Bueno Sen A, Cantolla Pablo P, Val Blasco A, Ruiz Hurtado G, Delgado C, Cubillos C, Boscá L, Fernández Velasco M. Atrial Myopathy and Heart Failure: Immunomolecular Mechanisms and Clinical Implications. International Journal of Molecular Sciences. 2025; 26(17):8210. https://doi.org/10.3390/ijms26178210
Chicago/Turabian StyleGil Fernández, Marta, Andrea Bueno Sen, Paula Cantolla Pablo, Almudena Val Blasco, Gema Ruiz Hurtado, Carmen Delgado, Carolina Cubillos, Lisardo Boscá, and María Fernández Velasco. 2025. "Atrial Myopathy and Heart Failure: Immunomolecular Mechanisms and Clinical Implications" International Journal of Molecular Sciences 26, no. 17: 8210. https://doi.org/10.3390/ijms26178210
APA StyleGil Fernández, M., Bueno Sen, A., Cantolla Pablo, P., Val Blasco, A., Ruiz Hurtado, G., Delgado, C., Cubillos, C., Boscá, L., & Fernández Velasco, M. (2025). Atrial Myopathy and Heart Failure: Immunomolecular Mechanisms and Clinical Implications. International Journal of Molecular Sciences, 26(17), 8210. https://doi.org/10.3390/ijms26178210






