Molecular Species Identification and Genotyping of Free-Living Amoebae in Soil of Recreational Mountain Areas in the Babiogórski National Park and Surroundings, Southern Poland
Abstract
1. Introduction
2. Results
2.1. Results of Amoebae Cultures, Thermal Tolerance Test, PCRs, and Sequencing
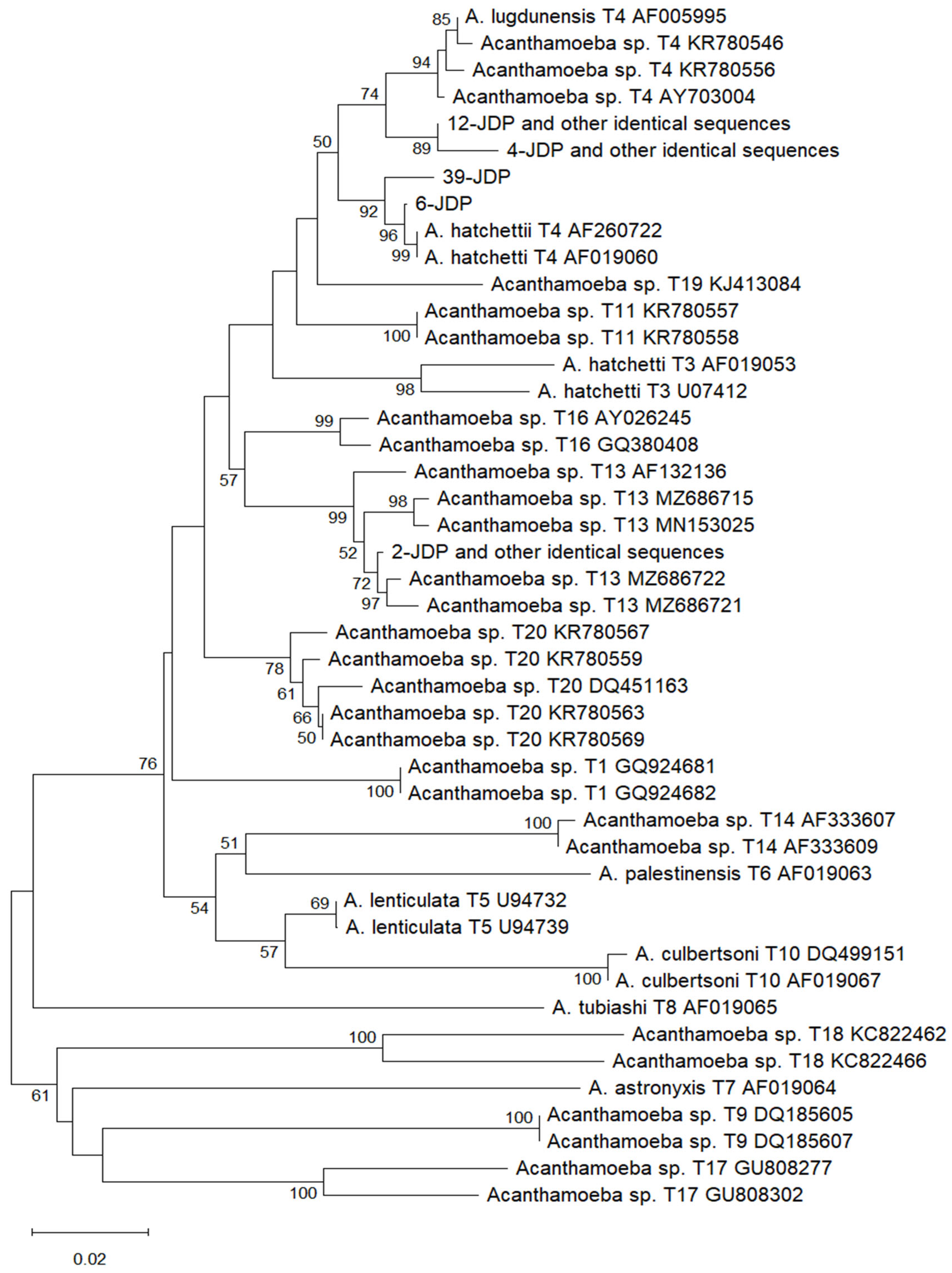
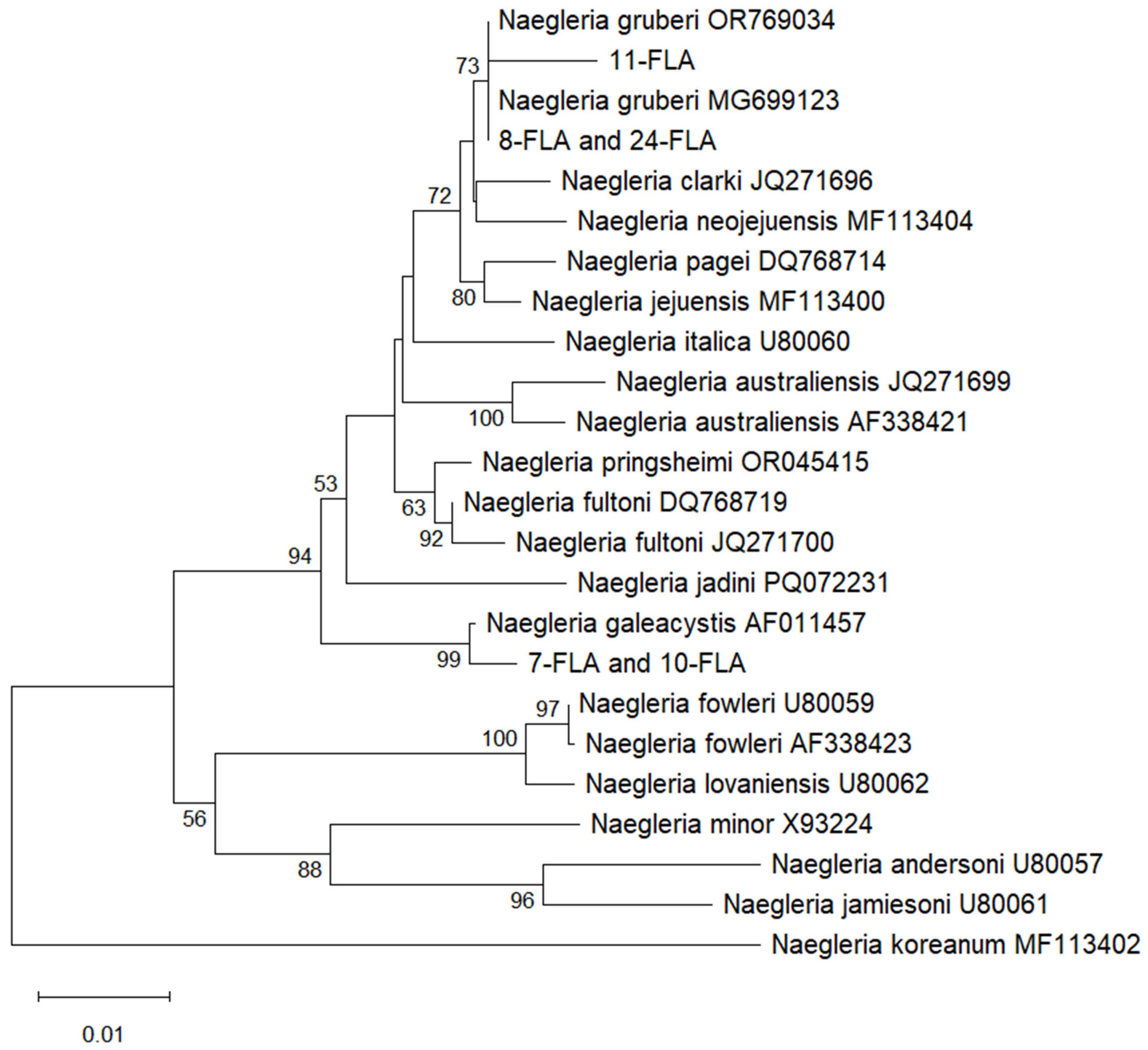
2.2. Results of Sequence Comparison and Phylogenetic Analysis
2.3. Results of Statistical Analysis
3. Discussion
4. Materials and Methods
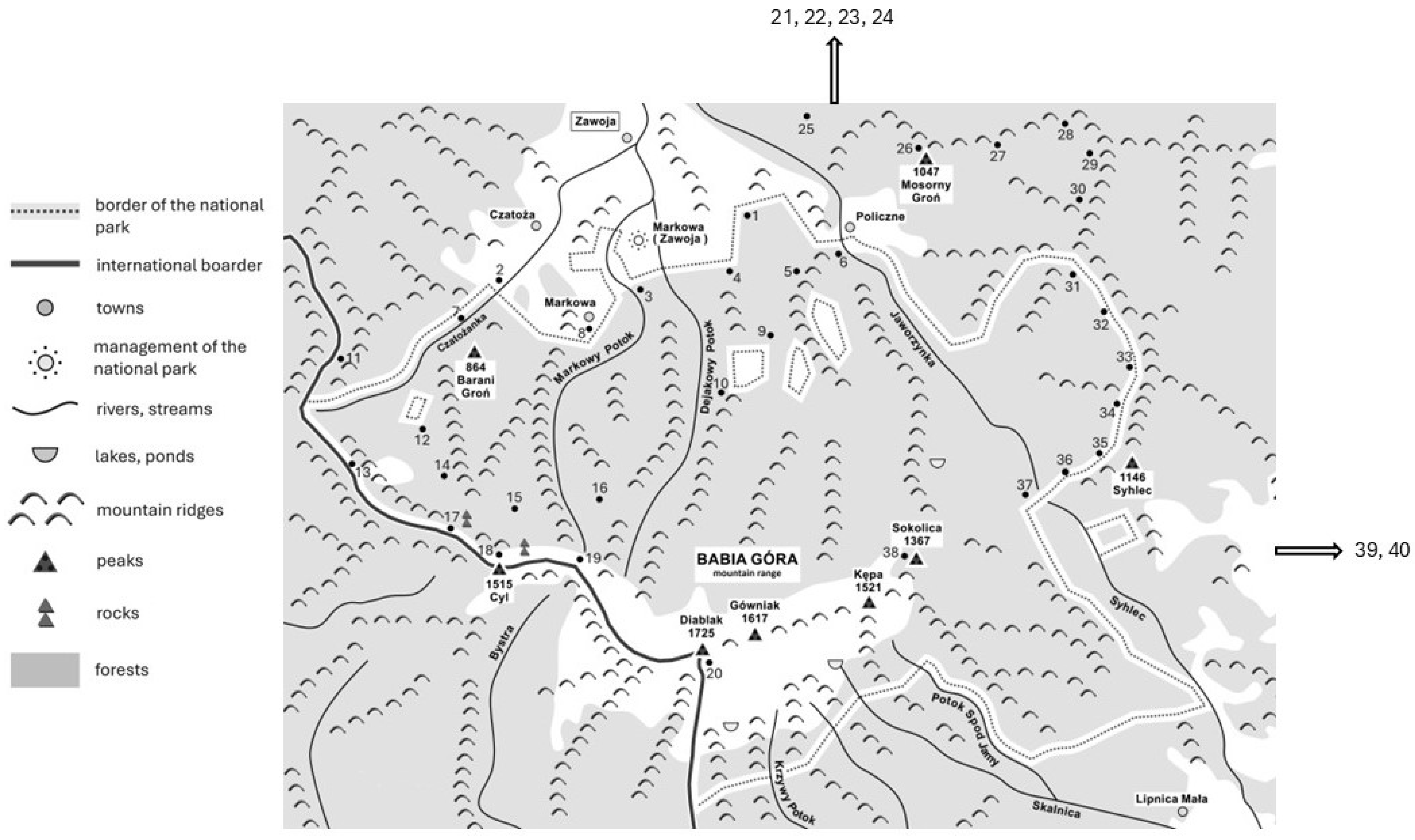
4.1. Study Area and Soil Sampling
4.2. Amoebae Cultures and Thermal Tolerance Test
4.3. DNA Extraction from Soil and Cultures, and PCR Protocols
4.4. Sequencing, Genotyping, and Phylogenetic Analysis
4.5. Statistical Analysis
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, M.; Rice, C.A.; Byrne, A.W.; Paré, P.E.; Beauvais, W. Modelling dynamics between free-living amoebae and bacteria. Environ. Microbiol. 2024, 26, e16623. [Google Scholar] [CrossRef]
- Otero-Ruiz, A.; Gonzalez-Zuñiga, L.D.; Rodriguez-Anaya, L.Z.; Lares-Jiménez, L.F.; Gonzalez-Galaviz, J.R.; Lares-Villa, F. Distribution and current state of molecular genetic characterization in pathogenic free-living amoebae. Pathogens 2022, 11, 1199. [Google Scholar] [CrossRef]
- Siddiqui, R.; Makhlouf, Z.; Khan, N.A. The increasing importance of Vermamoeba vermiformis. J. Eukaryot. Microbiol. 2021, 68, e12857. [Google Scholar] [CrossRef] [PubMed]
- Dinda, S.K.; Hazra, S.; De, A.; Datta, A.; Das, L.; Pattanayak, S.; Kumar, K.; Dey, M.D.; Basu, A.; Manna, D. Amoebae: Beyond pathogens-exploring their benefits and future potential. Front. Cell. Infect. Microbiol. 2024, 14, 1518925. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Shen, Y.; Qian, L. Social life of free-living amoebae in aquatic environment-comprehensive insights into interactions of free-living amoebae with neighboring microorganisms. Front. Microbiol. 2024, 15, 1382075. [Google Scholar] [CrossRef] [PubMed]
- Aykur, M.; Selver, O.B.; Dagci, H.; Palamar, M. Vermamoeba vermiformis as the ethiological agent in a patient with suspected non-Acanthamoeba keratitis. Parasitol. Res. 2024, 123, 323. [Google Scholar] [CrossRef]
- Salazar-Ardiles, C.; Valencia, K.P.; Andrade, D.C. Amoebas: The omnipotent organism and silent assassin. Mol. Biol. Rep. 2025, 52, 160. [Google Scholar] [CrossRef]
- Cardoso, I.R.; de Lima, C.S.; dos Reis, R.B.; Pinto, A.C.A.; Pissinatti, T.; Kugelmeier, T.; da Costa Neto, S.F.; da Silva, F.A.; Santos, H.L.C. Occurrence of free-living amoebae in non-human primate gut. Trop. Med. Infect. Dis. 2024, 9, 108. [Google Scholar] [CrossRef]
- Zurita-Artaloitia, J.M.; Riviera, J.; Vinuesa, P. Extensive cryptic diversity and ecological associations uncovered among Mexican and global collections of Naegleria and Vermamoeba species by 18S ribosomal DNA, internal transcribed spacer, and cytochrome oxidase subunit I sequence analysis. Microbiol. Spectr. 2023, 11, e03795-22. [Google Scholar] [CrossRef]
- Kahraman, M.; Polat, Z.A. Are thermotolerant and osmotolerant characteristics of Acanthamoeba species an indicator of pathogenicity? Türkiye Parazitol. Derg. 2024, 48, 15–20. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef]
- Chaúque, B.J.M.; da Silva, T.C.B.; dos Santos, D.L.; Benitez, G.B.; Chaúque, L.G.H.; Benetti, A.D.; Zanette, R.A.; Rott, M.B. Global prevalence of free-living amoebae in solid matrices—A systematic review with meta-analysis. Acta Trop. 2023, 247, 107006. [Google Scholar] [CrossRef]
- Bass, D.; Stentiford, G.D.; Littlewood, D.T.J.; Hartikainen, H. Diverse applications of environmental DNA methods in parasitology. Trends Parasitol. 2015, 31, 499–513. [Google Scholar] [CrossRef]
- Sengupta, M.E.; Lynggaard, C.; Mukaratriwa, S.; Vennervald, B.J.; Stensgaard, A.S. Environmental DNA in human and veterinary parasitology-current applications and future prospects for monitoring and control. Food Waterborne Parasitol. 2022, 29, e00183. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.; Nicola, A.M.; Almeida, D.; Magnabosco, G.; da Silveira Derengowski, L.; Crisóstomo, L.S.; Xavier, L.C.G.; de Oliveira Frazão, S.; Guilhelmelli, F.; de Oliveira, M.A.; et al. A hidden battle in dirt: Soil amoebae interactions with Paracoccidioides spp. PLoS Neglected Trop. Dis. 2019, 13, e0007742. [Google Scholar] [CrossRef]
- Hendiger-Rizo, E.B.; Chmielewska-Jeznach, M.; Poreda, K.; Liendo, A.R.; Koryszewska-Bagińska, A.; Olędzka, G.; Padzik, M. Potentially pathogenic free-living amoebae isolated from soil samples from Warsaw parks and squares. Pathogens 2024, 13, 895. [Google Scholar] [CrossRef]
- Pérez-Pérez, P.; Reyes-Batlle, M.; Morchón, R.; Piñero, J.E.; Lorenzo-Morales, J. Isolation and molecular identification of pathogenic free-living amoebae from environmental samples in Tenerife, Canary Islands. ACS EST Water 2025, 5, 2861–2869. [Google Scholar] [CrossRef]
- Sousa-Ramos, D.; Reyes-Batlle, M.; Bellini, N.K.; Rodriguez-Expósito, R.; Piñero, J.E.; Lorenzo-Morales, J. Free-living amoebae in soil samples from Santiago Island, Cape Verde. Parasitol. Res. 2021, 121, 2399–2404. [Google Scholar] [CrossRef]
- Sousa-Ramos, D.; Reyes-Batlle, M.; Bellini, N.K.; Rodriguez-Expósito, R.; Martin-Real, C.; Piñero, J.E.; Lorenzo-Morales, J. Pathogenic free-living amoebae from water sources in Cape Verde. Microorganisms 2022, 9, 1460. [Google Scholar] [CrossRef]
- Reyes-Batlle, M.; Wagner, C.; Zamora-Herrera, J.; Vargas-Mesa, A.; Sifaoui, I.; González, A.C.; López-Arencibia, A.; Valladares, B.; Martinez-Carretero, E.; Piñero, J.E.; et al. Isolation and molecular identification of Vermamoeba vermiformis strains from soil sources in El Hierro Island, Canary Islands, Spain. Curr. Microbiol. 2016, 73, 104–107. [Google Scholar] [CrossRef]
- Reyes-Batlle, M.; Diaz, F.J.; Sifaoui, I.; Rodriguez-Expósito, R.L.; Rizo-Liendo, A.; Piñero, J.E.; Lorenzo-Morales, J. Free living amoebae isolation in irrigation waters and soils of an insular arid agroecosystem. Sci. Total Environ. 2021, 753, 141833. [Google Scholar] [CrossRef]
- Denet, E.; Coupat-Goutaland, B.; Nazaret, S.; Pélandakis, M.; Favre-Bonté, S. Diversity of free-living amoebae in soils and their associated human opportunistic bacteria. Parasitol. Res. 2017, 116, 3151–3162. [Google Scholar] [CrossRef]
- Aykur, M.; Dagci, H. Molecular identification of Acanthamoeba spp., Balamuthia mandrillaris and Naegleria fowleri in soil samples using quantitative real-time PCR assay in Turkey; Hidden danger in the soil! Acta Trop. 2023, 244, 106956. [Google Scholar] [CrossRef]
- Pazoki, H.; Niyyati, M.; Javanmard, E.; Iasjerdi, Z.; Spotin, A.; Mirjalali, H.; Behravan, M.R. Isolation and phylogenetic analysis of free-living amoebae (Acanthamoeba, Naegleria, and Vermamoeba) in the farmland soils and recreational places in Iran. Acta Parasitol. 2020, 65, 36–43. [Google Scholar] [CrossRef]
- Pérez-Pérez, P.; Artigas, P.; Reyes-Batlle, M.; Córdoba-Lanús, E.; Rodriguez-Expósito, R.L.; Cuervo, P.F.; Dominguez-de Barros, A.; Garcia-Pérez, O.; Valero, M.A.; De Elias, A.; et al. Potentially pathogenic free-living amoebae at very high altitude: Detection by multiplex qPCR in the Northern Altiplano fascioliasis hyperendemic area in Bolivia. One Health 2025, 20, 100985. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, Y.; Ducat, C.; Talarmin, A.; Marcelino, I. Cartography of free-living amoebae in soil in Guadeloupe (French West Indies) using DNA metabarcoding. Pathogens 2020, 9, 440. [Google Scholar] [CrossRef]
- Tanzifi, A.; Moghaddam, Y.; Dodangeh, S.; Daryani, A.; Shahabeddin, S.; Gholami, S.; Hosseini, S.A.; Chegeni, T.N.; Hosseininejad, Z. Detection and molecular characterization of potentially pathogenic free-living amoebae from recreational and public soils in Mazandaran, Northern Iran. Iran. J. Parasitol. 2021, 16, 295–304. [Google Scholar] [CrossRef]
- Noinarin, P.; Chareonsudjai, P.; Wangsomnuk, P.; Wongratanacheewin, S.; Chareonsudjai, S. Environmental free-living amoebae isolated from soil in Khon Kaen, Thailand, antagonize Burkholderia pseudomallei. PLoS ONE 2016, 11, e0167355. [Google Scholar] [CrossRef]
- Watson, P.M.; Sorrell, S.C.; Brown, M.W. Ptolemeba n. gen., a novel genus of Hartmannellid amoebae (Tubulinea, Amoebozoa); with an emphasis on the taxonomy of Saccamoeba. J. Eukaryot. Microbiol. 2014, 61, 611–619. [Google Scholar] [CrossRef]
- Kudryavtsev, A.; Volkova, E.; Parshukov, A. Ptolemeba bulliensis Watson et al. 2014 (Amoebozoa, Tubulinea) from freshwater NGD-affected rainbow trout (Oncorhynchus mykiss Walbaum, 1792) gills tolerates brackish water conditions. J. Fish. Dis. 2025, 10, e14132. [Google Scholar] [CrossRef]
- Brown, S.; De Jonckheere, J.F. Isolation of a new valkhampfiid amoeba from soil: Paravahlkampfia lenta n. sp. Eur. J. Protistol. 2004, 40, 289–294. [Google Scholar] [CrossRef]
- McLaughlin, G.L.; Brandt, F.H.; Visvesvara, G.S. Restriction fragment length polymorphisms of the DNA of selected Naegleria and Acanthamoeba amebae. J. Clin. Microbiol. 1988, 26, 1655–1658. [Google Scholar] [CrossRef]
- Cholewiński, M.; Solarczyk, P.; Derda, M.; Wojtkowiak-Giera, A.; Hadaś, E. Presence of potential pathogenic genotypes of free-living amoebae isolated from sandboxes in children’s playgrounds. Folia Parasitol. 2015, 62, 064. [Google Scholar] [CrossRef]
- Orosz, E.; Posta, K. Genotyping of Acanthamoeba spp. from rizosphere in Hungary. Acta Microbiol. Immunol. Hung. 2020, 67, 171–175. [Google Scholar] [CrossRef]
- Schmitz-Esser, S.; Toenschoff, E.R.; Haider, S.; Heinz, E.; Hoenninger, V.M.; Wagner, M.; Horn, M. Diversity of bacterial endosymbionts of environmental Acanthamoeba isolates. Appl. Environ. Microbiol. 2008, 74, 5822–5831. [Google Scholar] [CrossRef]
- Geisen, S.; Fiore-Donno, A.M.; Walochnik, J.; Bonkowski, M. Acanthamoeba everywhere: High diversity of Acanthamoeba in soils. Parasitol. Res. 2014, 113, 3151–3158. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Zhao, Y.; Ju, X.; Wang, L.; Jin, L.; Fine, R.D.; Li, M. Biological characteristics and pathogenicity of Acanthamoeba. Front. Microbiol. 2023, 14, 11470077. [Google Scholar] [CrossRef]
- Garcia, A.; Goñi, P.; Clavel, A.; Lobez, S.; Fernandez, M.T.; Ormad, M.P. Potentially pathogenic free-living amoebae (FLA) isolated in Spanish wastewater treatment plants. Environ. Biol. Rep. 2011, 3, 622–626. [Google Scholar] [CrossRef]
- Ozkoc, S.; Tuncay, S.; Delibas, S.B.; Akisu, C.; Ozbek, Z.; Durak, I.; Walochnik, J. Identification of Acanthamoeba genotype T4 and Paravahlkampfia sp. from two clinical isolates. J. Med. Microbiol. 2008, 57, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Visvesvara, G.S.; Sriram, R.; Qvarnstrom, Y.; Bandyopadhyay, K.; da Silva, A.J.; Pieniążek, N.J.; Cabral, G.A. Paravahlkampfia francinae n. sp. masquerading as an agent of primary amoebic meningocephalitis. J. Eukaryot. Microbiol. 2009, 56, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Adamska, M.; Leońska-Duniec, A.; Łanocha, N.; Skotarczak, B. Thermophilic, potentially pathogenic amoebae isolated from natural water bodies in Poland and their molecular characterization. Acta Parasitol. 2014, 59, 433–441. [Google Scholar] [CrossRef]
- Schroeder, J.M.; Booton, G.C.; Hay, J.; Niszl, I.A.; Seal, D.V.; Markus, M.B.; Fuerst, P.A.; Byers, T.J. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification and Acanthamoebae from humans with keratitis and from sewage sludge. J. Clin. Microbiol. 2001, 39, 1903–1911. [Google Scholar] [CrossRef]
- Scheikl, U.; Sommer, R.; Kirschner, A.; Rameder, A.; Schrammel, B.; Zweimüller, I.; Wesner, W.; Hinker, M.; Walochnik, J. Free-living amoebae (FLA) co-occurring with legionellae in industrial waters. Eur. J. Protistol. 2014, 50, 422–429. [Google Scholar] [CrossRef]
- Tsvetkova, N.; Schild, M.; Panaiotov, S.; Kurdova-Mintcheva, R.; Gottstein, B.; Walochnik, J.; Aspöck, H.; Lucas, M.S.; Müller, N. The identification of free-living environmental isolates of amoebae from Bulgaria. Parasitol. Res. 2004, 92, 405–413. [Google Scholar] [CrossRef]
- Thomas, V.; Herrera-Rimann, K.; Blanc, D.S.; Greub, G. Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl. Environ. Microbiol. 2006, 72, 2428–2438. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.-Y.; Ji, D.-R.; Hsia, K.-T.; Hung, C.-C.; Sheng, W.-H.; Hsu, B.-M.; Chen, J.-S.; Wu, M.-H.; Lai, C.-H.; Ji, D.-D. Isolation and identification of Acanthamoeba species related to amoebic encephalitis and nonpathogenic free-living amoeba species from the rice field. J. Appl. Microbiol. 2010, 109, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis Version 12 for Adaptive and Green Computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
| Sample Number | Culture at 42 °C | PCR and Sequencing Results with Individual Primer Pairs | ||||
|---|---|---|---|---|---|---|
| JDP1/JDP2 | FLA-F/FLA-R | Ami6F1/Ami9R | AmeF977/ AmeR1534 | HARTfor/ HARTrev | ||
| 1. | Naegleria sp. | |||||
| 2. | + | Acanthamoeba T13 | V. vermiformis | |||
| 4. | Acanthamoeba T4 | Paravahlkampfia sp. | ||||
| 6. | + | Acanthamoeba T4 | ||||
| 7. | N. galeacystis | N. galeacystis | ||||
| 8. | + | N. gruberi | N. gruberi | |||
| 10. | Acanthamoeba T13 | N. galeacystis | N. galeacystis | |||
| 11. | N. gruberi | N. gruberi | Naegleria sp. | |||
| 12. | Acanthamoeba T4 | |||||
| 15. | + | Acanthamoeba T4 | ||||
| 19. | + | Acanthamoeba T4 | V. vermiformis | V. vermiformis | V. vermiformis | |
| 21. | + | Acanthamoeba T4 | ||||
| 23. | Acanthamoeba T13 | |||||
| 24. | + | N. gruberi | N. gruberi | Naegleria sp. | ||
| 27. | Acanthamoeba T4 | |||||
| 28. | Acanthamoeba T13 | P. bulliensis | ||||
| 29. | + | Acanthamoeba T4 | ||||
| 31. | + | Acanthamoeba T4 | ||||
| 32. | + | Acanthamoeba T13 | V. vermiformis | |||
| 33. | + | Acanthamoeba T13 | ||||
| 35. | + | Acanthamoeba T13 | ||||
| 37. | Acanthamoeba T4 | |||||
| 39. | Acanthamoeba T4 | |||||
| 40. | Acanthamoeba T4 | |||||
| The Primer Set | Detecting FLAs | The Amplifying Fragment of the 18S rRNA Gene and the Product Size | Hybridization Temperature | References |
|---|---|---|---|---|
| JDP1: 5′-GGCCCAGATCG- TTTACCGTGAA-3′ JDP2: 5′-TCTCACAAGCT- GCTAGGGAGTCA-3′ | Acanthamoeba spp. | 897–1358 bp of A. castellani sequence (U07400); 462 bp | 55 °C | [16,42] |
| HARTfor: 5′-GGAGGGCAAGT- CTGGTGCC-3′ HARTrev: 5′-GCCCGGAGAGTCATCCATG-3′ | Former genus Hartmannella | 562–1095 bp of V. vermiformis sequence (EU137741); 534 bp | 58 °C | [43] |
| FLA-F: 5′-CGCGGTAATTCC- AGCTCCAATAGC-3′ FLA-R: 5′-CAGGTTAAGGT- CTCGTTCGTTAAC-3′ | All FLAs except Balamuthia and Sappinia | 631–1614 bp of A. castellani sequence (U07400); 984 bp | 55 °C | [16,44] |
| Ami6F1: 5′-CCAGCTCCAATAGCGTATATT-3′ Ami9R: 5′-GTTGAGTCGA- ATTAAGCCGC-3′ | All amoebae | 641–1468 bp of A. castellani sequence (U07400); 828 bp | 55 °C | [45] |
| AmeF977: 5′-GATYAGATACCGTCGTAGTC-3′ AmeR1534: 5′-TCTAAGRGCAT- CACAGACCTG-3′ | All amoebae | 1179–1829 bp of A. castellani sequence (U07400); 651 bp | 60 °C | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamska, M. Molecular Species Identification and Genotyping of Free-Living Amoebae in Soil of Recreational Mountain Areas in the Babiogórski National Park and Surroundings, Southern Poland. Int. J. Mol. Sci. 2025, 26, 8160. https://doi.org/10.3390/ijms26178160
Adamska M. Molecular Species Identification and Genotyping of Free-Living Amoebae in Soil of Recreational Mountain Areas in the Babiogórski National Park and Surroundings, Southern Poland. International Journal of Molecular Sciences. 2025; 26(17):8160. https://doi.org/10.3390/ijms26178160
Chicago/Turabian StyleAdamska, Małgorzata. 2025. "Molecular Species Identification and Genotyping of Free-Living Amoebae in Soil of Recreational Mountain Areas in the Babiogórski National Park and Surroundings, Southern Poland" International Journal of Molecular Sciences 26, no. 17: 8160. https://doi.org/10.3390/ijms26178160
APA StyleAdamska, M. (2025). Molecular Species Identification and Genotyping of Free-Living Amoebae in Soil of Recreational Mountain Areas in the Babiogórski National Park and Surroundings, Southern Poland. International Journal of Molecular Sciences, 26(17), 8160. https://doi.org/10.3390/ijms26178160







