Bone Microstructural Deterioration and miR-155/RHOA-Mediated Osteoclastogenesis in Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Results
2.1. Histological Analysis
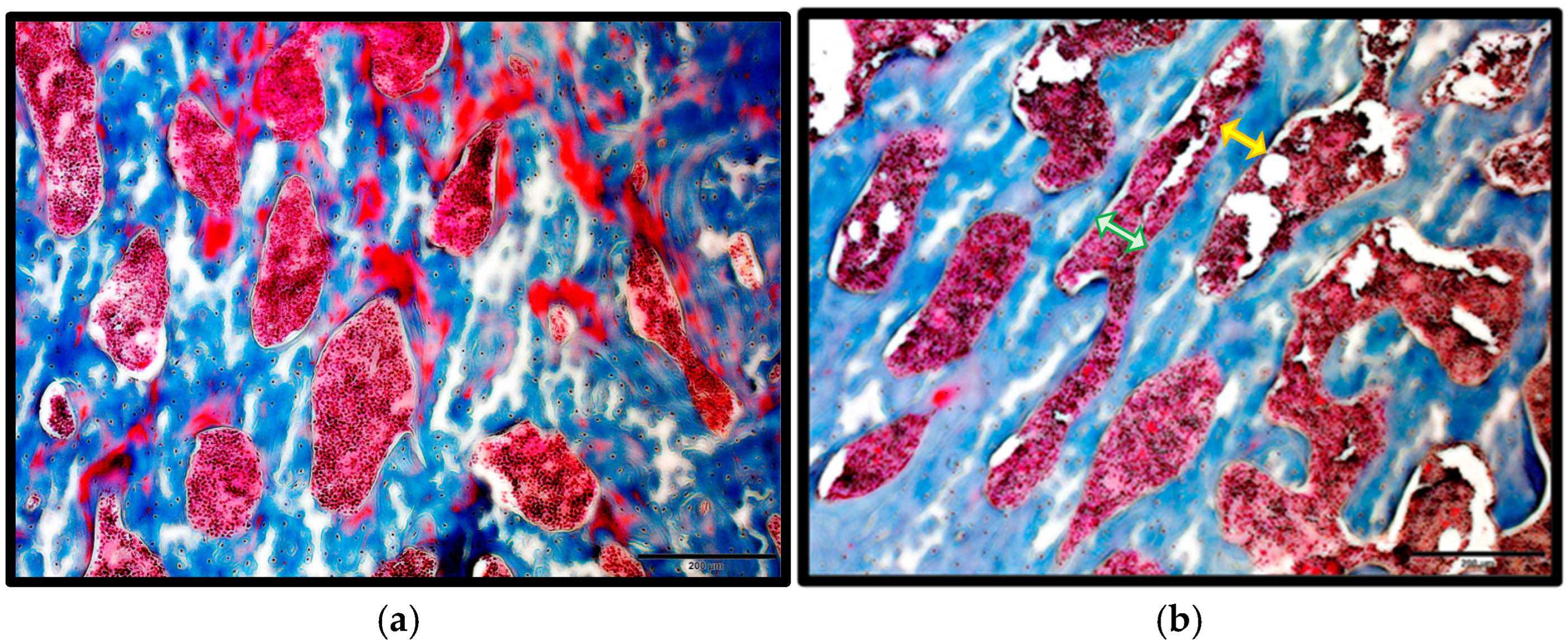
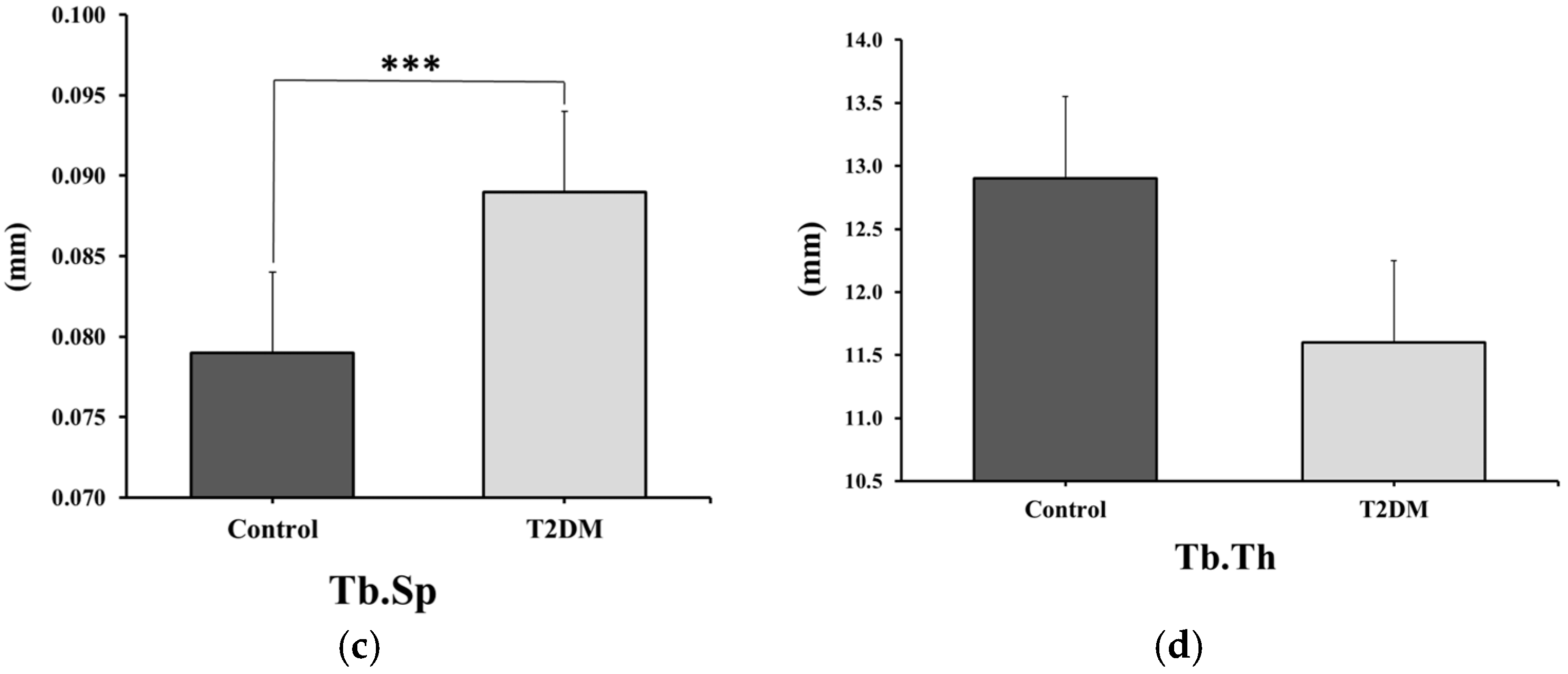
2.1.1. Masson’s Trichrome Staining for Trabecular Measurements
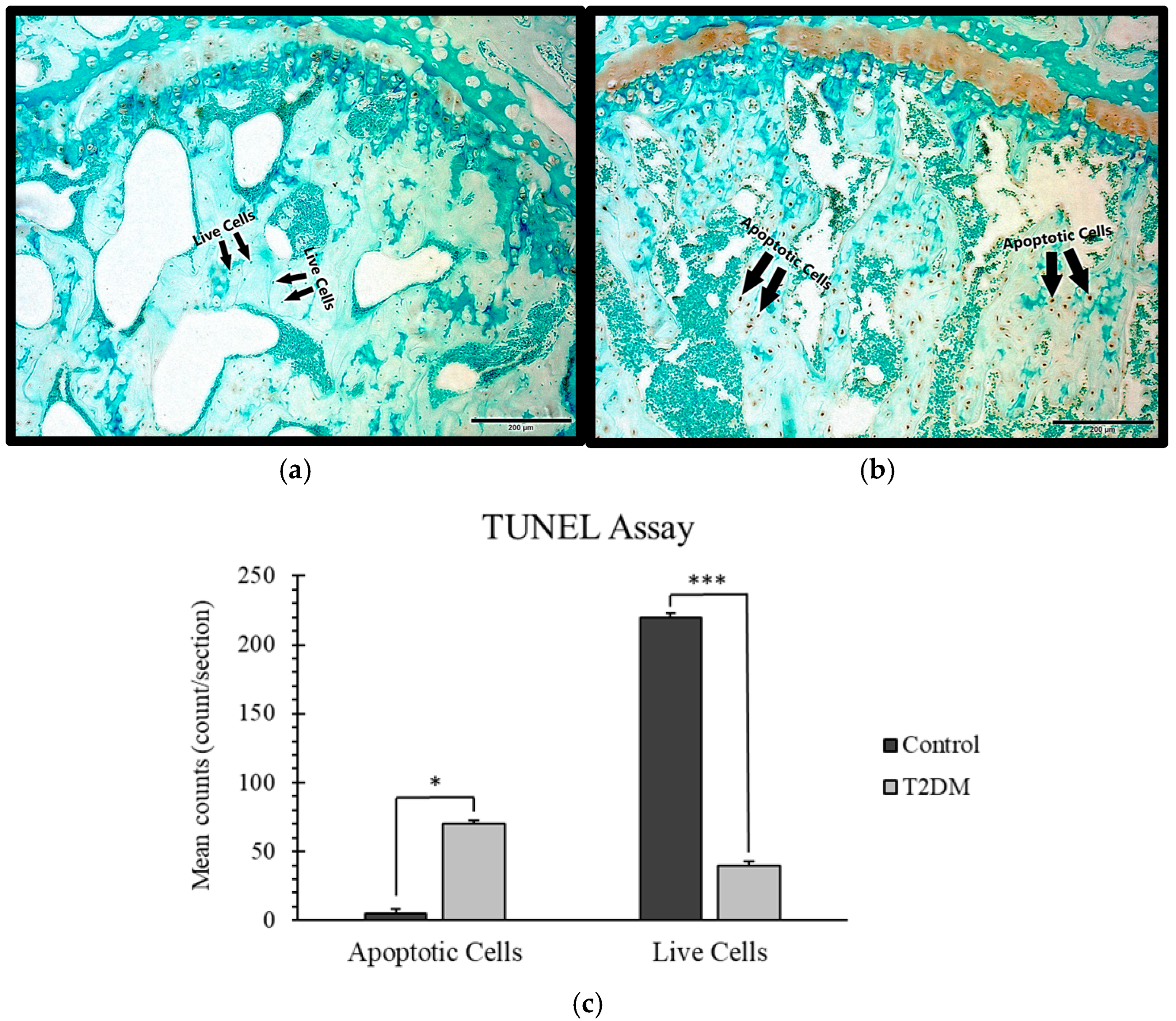
2.1.2. TUNEL Staining for the Estimation of Apoptosis
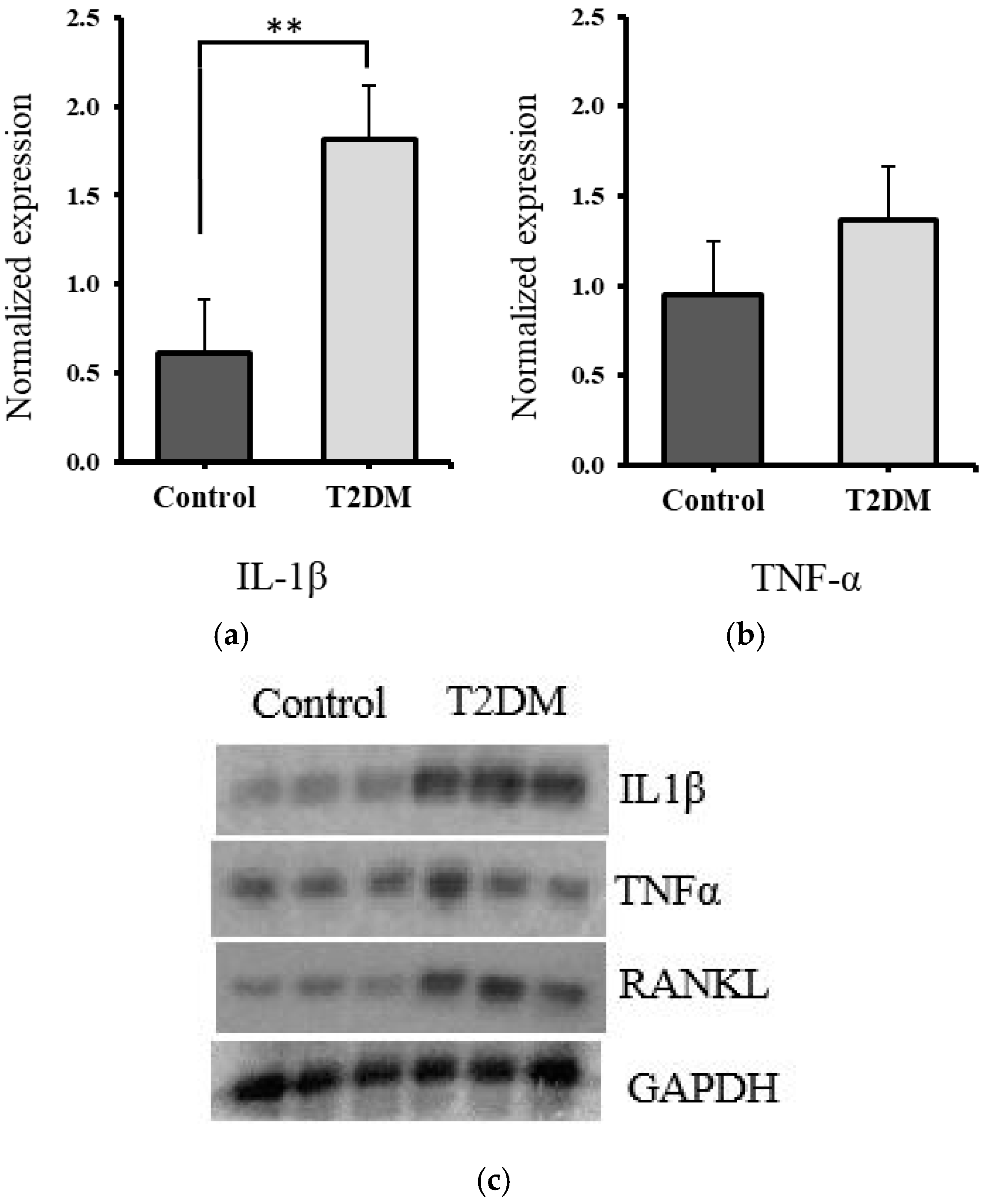
2.2. Estimation of Proinflammatory Cytokines
Significant Increase in IL-1β and Non-Significant Elevation in TNF-α in T2DM Rat Bones, Suggesting Elevated Inflammatory Activity
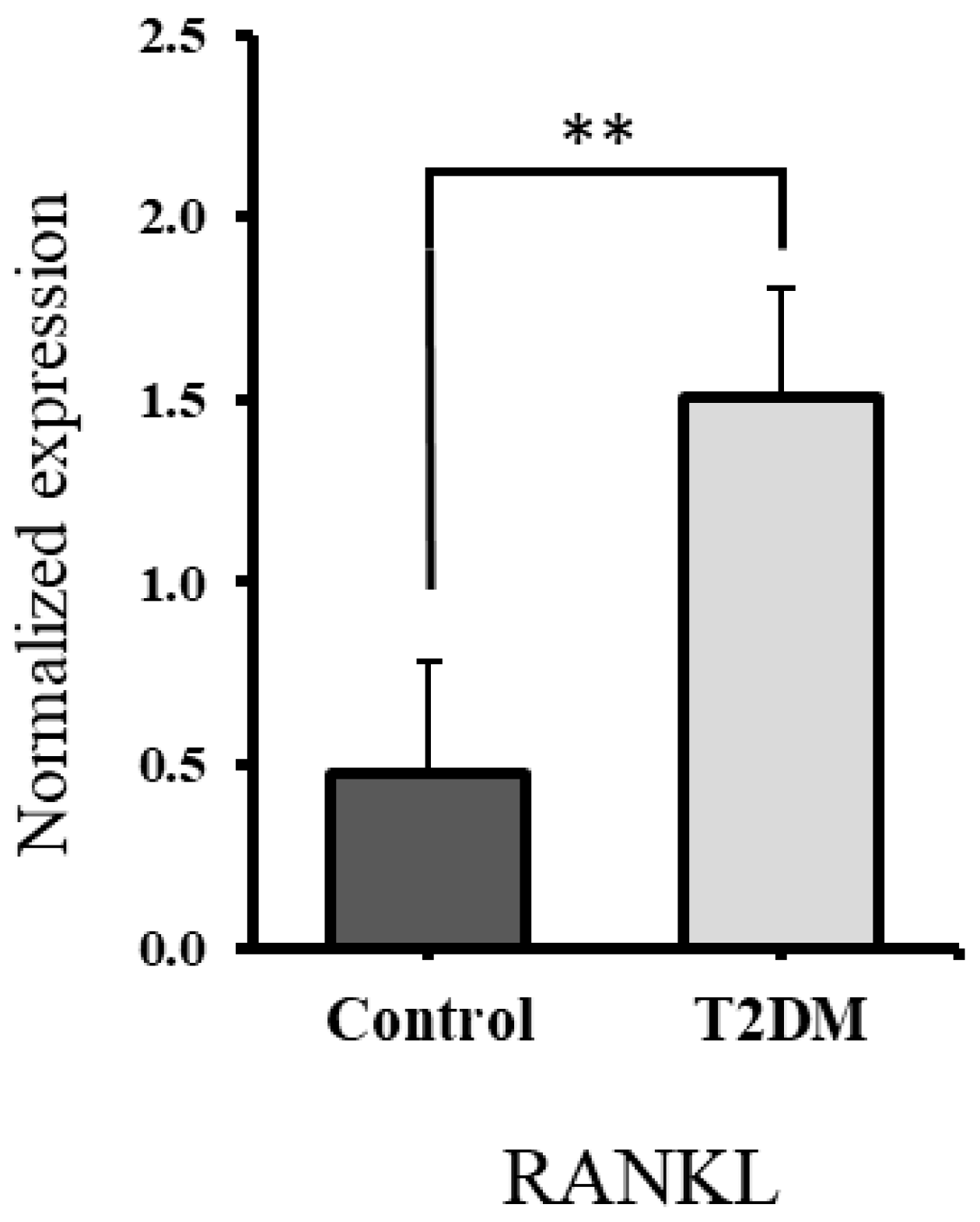
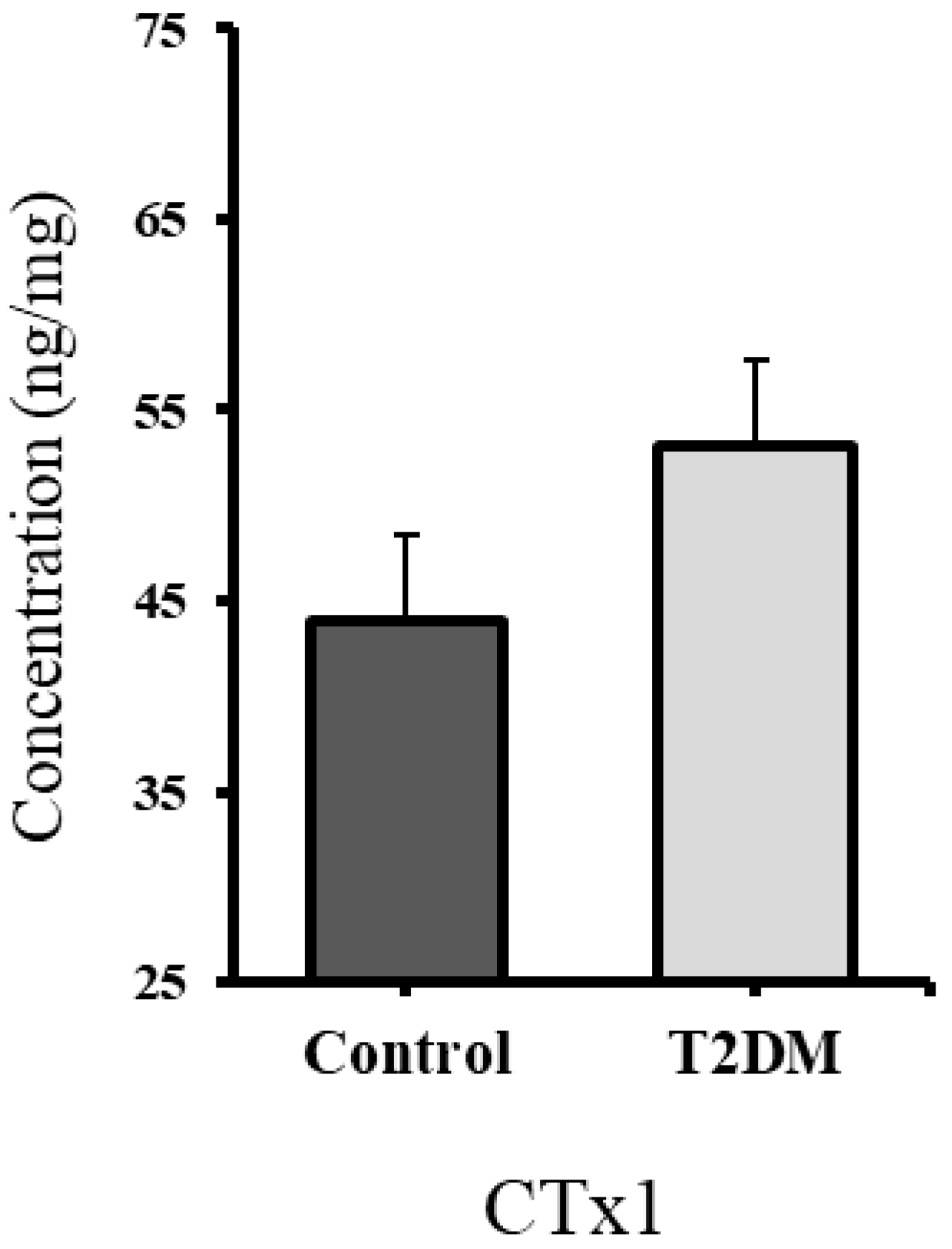
2.3. Significant Increase in RANKL and Non-Significant Elevation in CTx1, Suggesting Enhanced Bone Resorption in T2DM Rats
2.4. Profile Expression of MicroRNA-155 and Its Target Gene
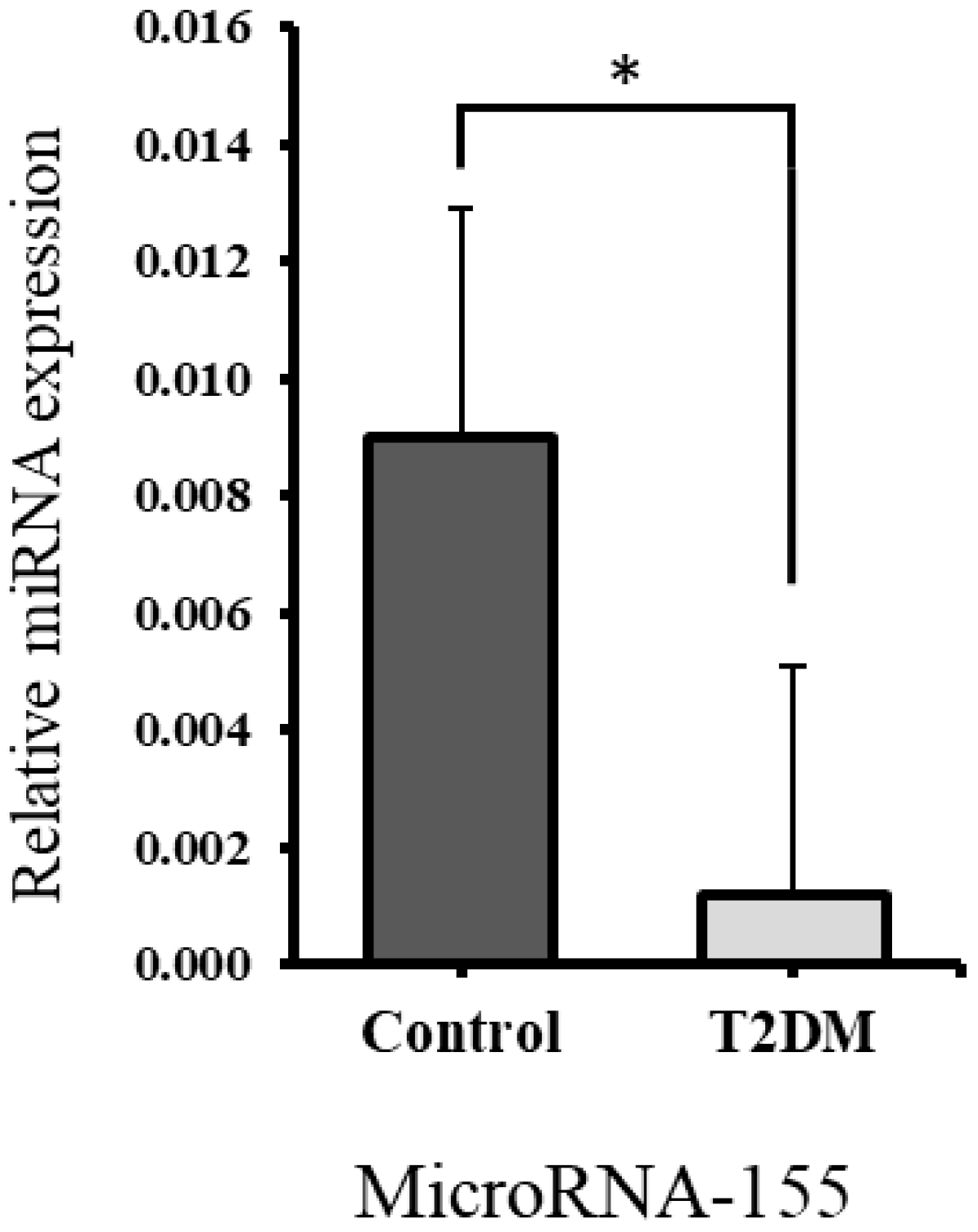
2.4.1. Significant Downregulation of miR-155 Expression in Bones of T2DM Rats Compared to Controls
2.4.2. Significant Upregulation of RHOA Gene Expression in Bones of T2DM Rats Correlates with Downregulated miR-155
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Masson’s Trichrome Stain
4.3. TUNEL Assay
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Western Blotting
4.6. Quantitative Real-Time PCR
4.7. Statistical Analysis
5. Conclusions
6. Limitation of the Study
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Ling, C.; Ronn, T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.B.; Zhang, N.; Van Der Pluijm, W. Global Prevalence of Type 2 Diabetes over the Next Ten Years (2018–2028). Diabetes 2018, 67 (Suppl. S1), 202-LB. [Google Scholar] [CrossRef]
- Meo, S.A.; Usmani, A.M.; Qalbani, E. Prevalence of type 2 diabetes in the Arab world: Impact of GDP and energy consumption. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1303–1312. [Google Scholar]
- Chiodini, I.; Catalano, A.; Gennari, L.; Gaudio, A. Osteoporosis and Fragility Fractures in Type 2 Diabetes. J. Diabetes Res. 2020, 2020, 9342696. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, G. Quantitative CT in the measurement of bone quantity and bone quality for assessing osteoporosis. Med. Eng. Phys. 1996, 18, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Ayati, S.; Ghasemi, S. Bone Quantity BT—Innovative Perspectives in Oral and Maxillofacial Surgery; Stevens, M.R., Ghasemi, S., Tabrizi, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 25–28. [Google Scholar] [CrossRef]
- Compston, J. Bone quality: What is it and how is it measured? Arq. Bras. De Endocrinol. Metabol. 2006, 50, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, S.; Kaimala, S.; Sunny, J.J.; Adeghate, E.; Brown, E.M. Type 2 diabetes mellitus increases the risk to hip fracture in postmenopausal osteoporosis by deteriorating the trabecular bone microarchitecture and bone mass. J. Diabetes Res. 2019, 2019, 3876957. [Google Scholar] [CrossRef]
- Eckhardt, B.A.; Rowsey, J.L.; Thicke, B.S.; Fraser, D.G.; O’gRady, K.L.; Bondar, O.P.; Hines, J.M.; Singh, R.J.; Thoreson, A.R.; Rakshit, K.; et al. Accelerated osteocyte senescence and skeletal fragility in mice with type 2 diabetes. JCI Insight 2020, 5, e135236. [Google Scholar] [CrossRef]
- Plotkin, L.I. Apoptotic osteocytes and the control of targeted bone resorption. Curr. Osteoporos. Rep. 2014, 12, 121–126. [Google Scholar] [CrossRef]
- Thomson, B.M.; Mundy, G.R.; Chambers, T.J. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J. Immunol. 1987, 138, 775–779. [Google Scholar] [CrossRef]
- Nguyen, L.; Dewhirst, F.E.; Hauschka, P.V.; Stashenko, P. Interleukin-1 beta stimulates bone resorption and inhibits bone formation in vivo. Lymphokine Cytokine Res. 1991, 10, 15–21. [Google Scholar] [PubMed]
- Steeve, K.T.; Marc, P.; Sandrine, T.; Dominique, H.; Yannick, F. IL-6, RANKL, TNF-alpha/IL-1: Interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004, 15, 49–60. [Google Scholar] [CrossRef]
- Eastell, R. Osteoporosis. Medicine 2013, 41, 586–591. [Google Scholar] [CrossRef]
- Calle, M.C.; Fernandez, M.L. Inflammation and type 2 diabetes. Diabetes Metab. 2012, 38, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Stephens, E.; Roy, M.; Bisson, M.; Nguyen, H.D.; Scott, M.S.; Boire, G.; Bouchard, L.; Roux, S. Osteoclast signaling-targeting miR-146a-3p and miR-155-5p are downregulated in Paget’s disease of bone. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165852. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, C.; Zhang, J.; Bao, Y.; Tang, Y.; Lv, X.; Ma, B.; Wu, X.; Mao, G. RhoA promotes osteoclastogenesis and regulates bone remodeling through mTOR-NFATc1 signaling. Mol. Med. 2023, 29, 49, Erratum in Mol. Med. 2023, 29, 102. [Google Scholar] [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. BioMed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef]
- Ruscitti, P.; Cipriani, P.; Carubbi, F.; Liakouli, V.; Zazzeroni, F.; Di Benedetto, P.; Berardicurti, O.; Alesse, E.; Giacomelli, R.; Ivanovska, N. The role of IL-1β in the bone loss during rheumatic diseases. Mediat. Inflamm. 2015, 2015, 782382. [Google Scholar] [CrossRef]
- Stashenko, P.; Dewhirst, F.E.; Peros, W.J.; Kent, R.L.; Ago, J.M. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J. Immunol. 1987, 138, 1464–1468. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.L. Regulation of Gene Expression. In Lehninger Principles of Biochemistry, 4th ed.; W.H. Freeman and Company: New York, NY, USA, 2005; pp. 1081–1119. [Google Scholar]
- Kong, W.; Yang, H.; He, L.; Zhao, J.J.; Coppola, D.; Dalton, W.S.; Cheng, J.Q. MicroRNA-155 Is Regulated by the Transforming Growth Factor β/Smad Pathway and Contributes to Epithelial Cell Plasticity by Targeting RhoA. Mol. Cell. Biol. 2008, 28, 6773–6784. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Kühne, C.A.; Viereck, V. The OPG/RANKL/RANK system in metabolic bone diseases. J. Musculoskelet. Neuronal Interact. 2004, 4, 268–275. [Google Scholar] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Alfadul, H.; Sabico, S.; Al-Daghri, N.M. The role of interleukin-1β in type 2 diabetes mellitus: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 901616. [Google Scholar] [CrossRef]
- Martín-Sánchez, F.; Diamond, C.; Zeitler, M.; I Gomez, A.; Baroja-Mazo, A.; Bagnall, J.; Spiller, D.; White, M.; Daniels, M.J.D.; Mortellaro, A.; et al. Inflammasome-dependent IL-1β release depends upon membrane permeabilisation. Cell Death Differ. 2016, 23, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Isaia, G.; Ardissone, P.; Di Stefano, M.; Ferrari, D.; Martina, V.; Porta, M.; Tagliabue, M.; Molinatti, G. Bone metabolism in type 2 diabetes mellitus. Acta Diabetol. 1999, 36, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Bal, H.; Desta, T.; Krothapalli, N.; Alyassi, M.; Luan, Q.; Graves, D. Diabetes enhances periodontal bone loss through enhanced resorption and diminished bone formation. J. Dent. Res. 2006, 85, 510–514. [Google Scholar] [CrossRef]
- Finkelstein, J.S.; Sowers, M.; Greendale, G.A.; Lee, M.L.T.; Neer, R.M.; Cauley, J.A.; Ettinger, B. Ethnic variation in bone turnover in pre-and early perimenopausal women: Effects of anthropometric and lifestyle factors. J. Clin. Endocrinol. Metab. 2002, 87, 3051–3056. [Google Scholar] [CrossRef]
- Reyes-García, R.; Rozas-Moreno, P.; López-Gallardo, G.; García-Martín, A.; Varsavsky, M.; Avilés-Perez, M.D.; Muñoz-Torres, M. Serum levels of bone resorption markers are decreased in patients with type 2 diabetes. Acta Diabetol. 2013, 50, 47–52. [Google Scholar] [CrossRef]
- Purnamasari, D.; Puspitasari, M.D.; Setiyohadi, B.; Nugroho, P.; Isbagio, H. Low bone turnover in premenopausal women with type 2 diabetes mellitus as an early process of diabetes-associated bone alterations: A cross-sectional study. BMC Endocr. Disord. 2017, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martín, A.; Reyes-Garcia, R.; García-Fontana, B.; Morales-Santana, S.; Coto-Montes, A.; Muñoz-Garach, M.; Rozas-Moreno, P.; Muñoz-Torres, M.; Kirchmair, R. Relationship of Dickkopf1 (DKK1) with Cardiovascular Disease and Bone Metabolism in Caucasian Type 2 Diabetes Mellitus. PLoS ONE 2014, 9, e111703, Erratum in PLoS ONE 2015, 10, e0117687. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, M.; Suzuki, K.; Matsubayashi, T.; Kikuyama, M.; Suzuki, H.; Takahashi, K.; Katsuta, H.; Mitsuhashi, J.; Nishida, S.; Yamaguchi, S.; et al. Increased bone resorption may play a crucial role in the occurrence of osteopenia in patients with type 2 diabetes: Possible involvement of accelerated polyol pathway in its pathogenesis. Diabetes Res. Clin. Pract. 2008, 82, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Bonaccorsi, G.; Cremonini, E.; Romani, A.; Fila, E.; Castaldini, M.C.; Ferrazzini, S.; Giganti, M.; Massari, L. Oxidative stress and bone resorption interplay as a possible trigger for postmenopausal osteoporosis. BioMed Res. Int. 2014, 2014, 569563. [Google Scholar] [CrossRef]
- The Chinese University of Hong Kong, “ID: MIRT000949,” miRTarBase. Available online: https://mirtarbase.cuhk.edu.cn/~miRTarBase/miRTarBase_2025/php/index.php (accessed on 1 May 2025).
- Zhao, W.; Zhang, W.; Yang, B.; Sun, J.; Yang, M. NIPA2 regulates osteoblast function via its effect on apoptosis pathways in type 2 diabetes osteoporosis. Biochem. Biophys. Res. Commun. 2019, 513, 883–890. [Google Scholar] [CrossRef]
- Hurtado, M.D.; Vella, A. What is type 2 diabetes? Medicine 2018, 47, 10–15. [Google Scholar] [CrossRef]
- Jakobsen, A.; Laurberg, P.; Vestergaard, P.; Andersen, S. Clinical risk factors for osteoporosis are common among elderly people in Nuuk, Greenland. Int. J. Circumpolar Health 2013, 72, 19596. [Google Scholar] [CrossRef]
- Loh, H.Y.; Lau, Y.Y.; Lai, K.S.; Osman, M.A. MicroRNAs in Bone Diseases: Progress and Prospects. In Transcriptional and Post-Transcriptional Regulation; Intechopen: London, UK, 2018; pp. 77–100. [Google Scholar]
- Bijkerk, R.; de Bruin, R.G.; van Solingen, C.; Duijs, J.M.; Kobayashi, K.; van der Veer, E.P.; Dijke, P.T.; Rabelink, T.J.; Goumans, M.J.; van Zonneveld, A.J. MicroRNA-155 functions as a negative regulator of RHOA signaling in TGF-β-induced endothelial to mesenchymal transition. MicroRNA 2012, 1, 2–10. [Google Scholar] [CrossRef]
- Porrelli, D.; Abrami, M.; Pelizzo, P.; Formentin, C.; Ratti, C.; Turco, G.; Grassi, M.; Canton, G.; Grassi, G.; Murena, L. Trabecular bone porosity and pore size distribution in osteoporotic patients–A low field nuclear magnetic resonance and microcomputed tomography investigation. J. Mech. Behav. Biomed. Mater. 2022, 125, 104933. [Google Scholar] [CrossRef]
- Mackey, D.C.; Lui, L.Y.; Cawthon, P.M.; Bauer, D.C.; Nevitt, M.C.; Cauley, J.A.; Hillier, T.A.; Lewis, C.E.; Barrett-Connor, E.; Cummings, S.R. High-Trauma Fractures and Low Bone Mineral Density in Older Women and Men. JAMA 2007, 298, 2381–2388. [Google Scholar] [CrossRef]
- Cvijanović, O.; Crnčević-Orlić, Ž; Kristofić, I.; Španjol, J.; Zoričić, S.; Marić, I.; Bobinac, D. Trabecular bone volume and bone mineral density correlation in lumbar spine. In 1st Joint Meeting of the International Bone and Mineral Society and the European Calcified Tissue Society; Elsevier: Amsterdam, The Netherlands, 2001; p. S173. [Google Scholar]
- Ru, J.; Wang, Y. Osteocyte apoptosis: The roles and key molecular mechanisms in resorption-related bone diseases. Cell Death Dis. 2020, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Kitaura, H.; Liu, L.; Mizoguchi, I.; Liu, S. The miR-155-5p inhibits osteoclast differentiation through targeting CXCR2 in orthodontic root resorption. J. Periodontal Res. 2021, 56, 761–773. [Google Scholar] [CrossRef]
- Ghasemi, A.; Jeddi, S.; Kashfi, K. The laboratory rat: Age and body weight matter. EXCLI J. 2021, 20, 1431. [Google Scholar] [PubMed]
- Lelovas, P.P.; Xanthos, T.T.; Thoma, S.E.; Lyritis, G.P.; Dontas, I.A. The laboratory rat as an animal model for osteoporosis research. Comp. Med. 2008, 58, 424–430. [Google Scholar]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lv, X.Y.; Li, J.; Xu, Z.G.; Chen, L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. J. Diabetes Res. 2008, 2008, 704045. [Google Scholar] [CrossRef] [PubMed]
- Yates, J.B.; Fountain, J.R. Fractures of the femur and tibial shaft. Surgery 2016, 34, 444–452. [Google Scholar] [CrossRef]

| Blood Glucose (mmol/L) | Body Weight (gm) | |
|---|---|---|
| Control | 6.34 ± 0.46 | 243.6 ± 26.2 |
| T2DM | 24.50 ± 2.90 | 203.5 ± 25.98 |
| Protein of Interest | Primary Antibodies | Secondary Antibodies |
|---|---|---|
| RANKL | Santa Cruz Biotechnology, Dallas, TX, USA Cat: Sc-377079 | Abcam, Cambridge, UK Cat: ab 205719 |
| TNF-α | Santa Cruz Biotechnology, Dallas, TX, USA Cat: Sc-52746 | Abcam, Cambridge, UK Cat: ab 205719 |
| IL-1β | Cloud-Clone Corp., Wuhan, China Cat: PAA563Ra01 | Cell Signaling Technology, Danvers, MA, USA Cat: 7074S |
| GAPDH | Santa Cruz Biotechnology, Dallas, TX, USA Cat: Sc-32233 | Abcam, Cambridge, UK Cat: ab 205719 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaleeli, M.M.; Kaimala, S.; Adeghate, E.; Mohsin, S. Bone Microstructural Deterioration and miR-155/RHOA-Mediated Osteoclastogenesis in Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2025, 26, 8159. https://doi.org/10.3390/ijms26178159
Alaleeli MM, Kaimala S, Adeghate E, Mohsin S. Bone Microstructural Deterioration and miR-155/RHOA-Mediated Osteoclastogenesis in Type 2 Diabetes Mellitus. International Journal of Molecular Sciences. 2025; 26(17):8159. https://doi.org/10.3390/ijms26178159
Chicago/Turabian StyleAlaleeli, Mouza M., Suneesh Kaimala, Ernest Adeghate, and Sahar Mohsin. 2025. "Bone Microstructural Deterioration and miR-155/RHOA-Mediated Osteoclastogenesis in Type 2 Diabetes Mellitus" International Journal of Molecular Sciences 26, no. 17: 8159. https://doi.org/10.3390/ijms26178159
APA StyleAlaleeli, M. M., Kaimala, S., Adeghate, E., & Mohsin, S. (2025). Bone Microstructural Deterioration and miR-155/RHOA-Mediated Osteoclastogenesis in Type 2 Diabetes Mellitus. International Journal of Molecular Sciences, 26(17), 8159. https://doi.org/10.3390/ijms26178159









