Pinealectomy-Induced Neuroinflammation Varies with Age in Rats
Abstract
1. Introduction
2. Results
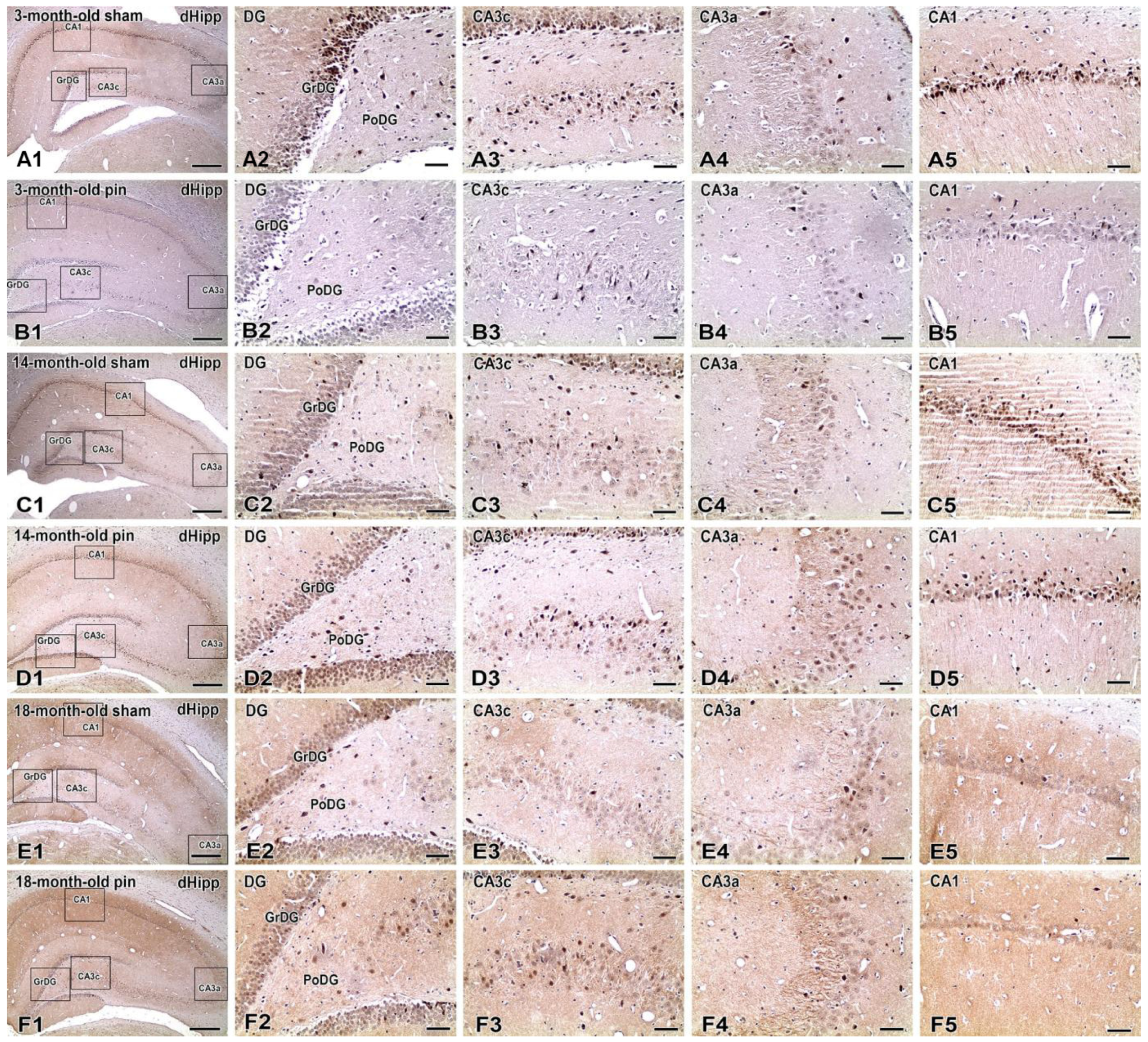
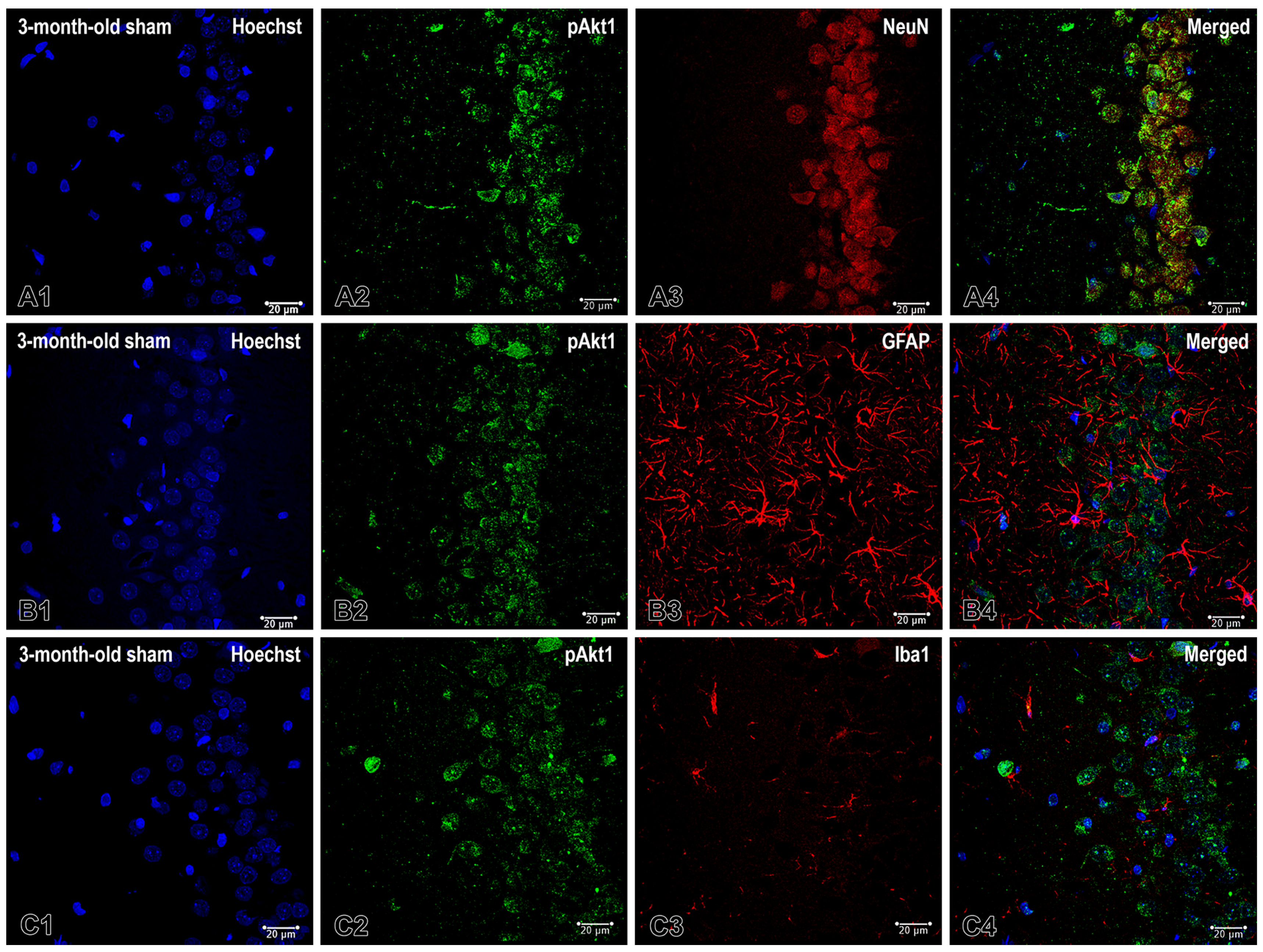
2.1. Effects of Pinealectomy and Aging on Phosphorylated Form of Akt (pAkt1) Expression in Different Hippocampal Regions
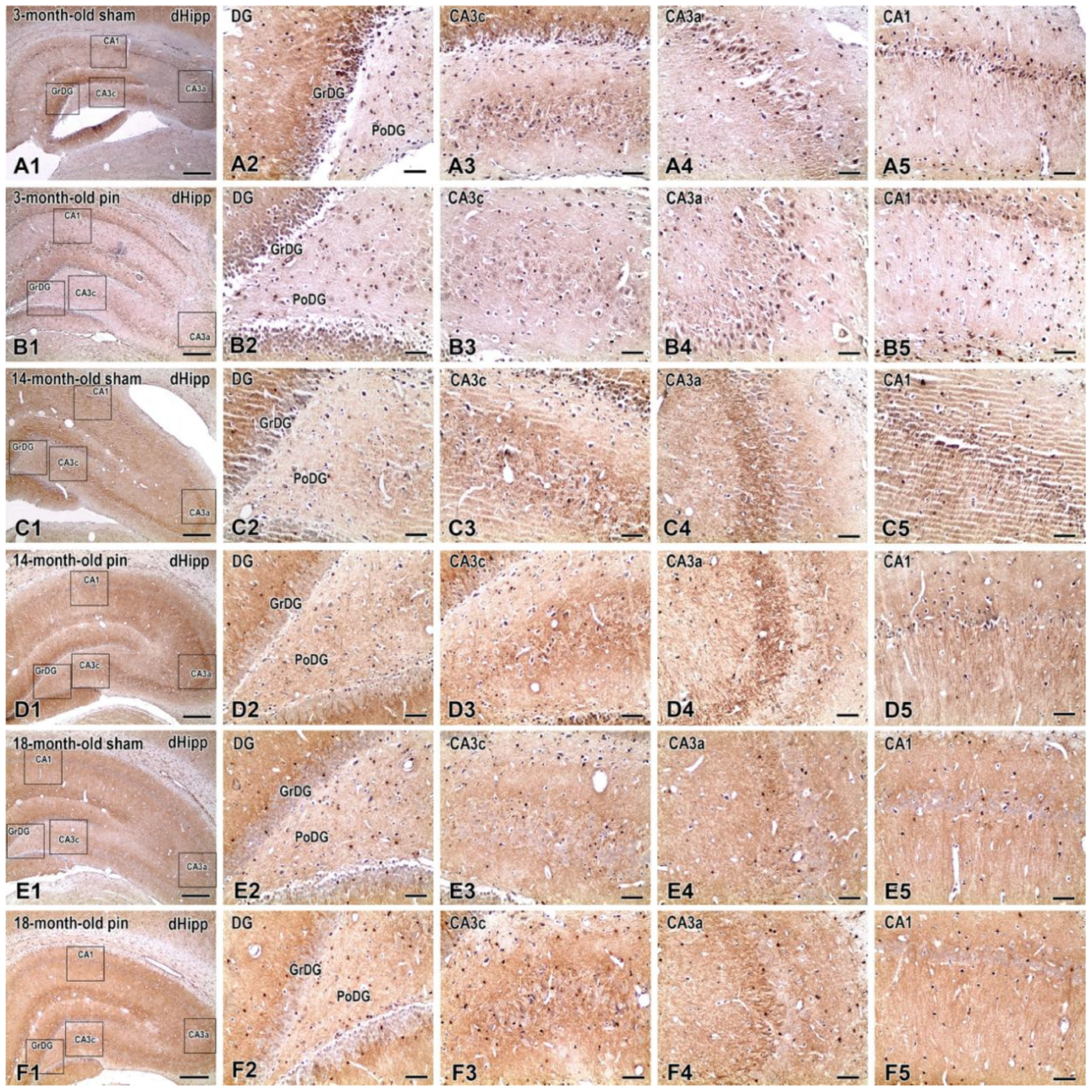
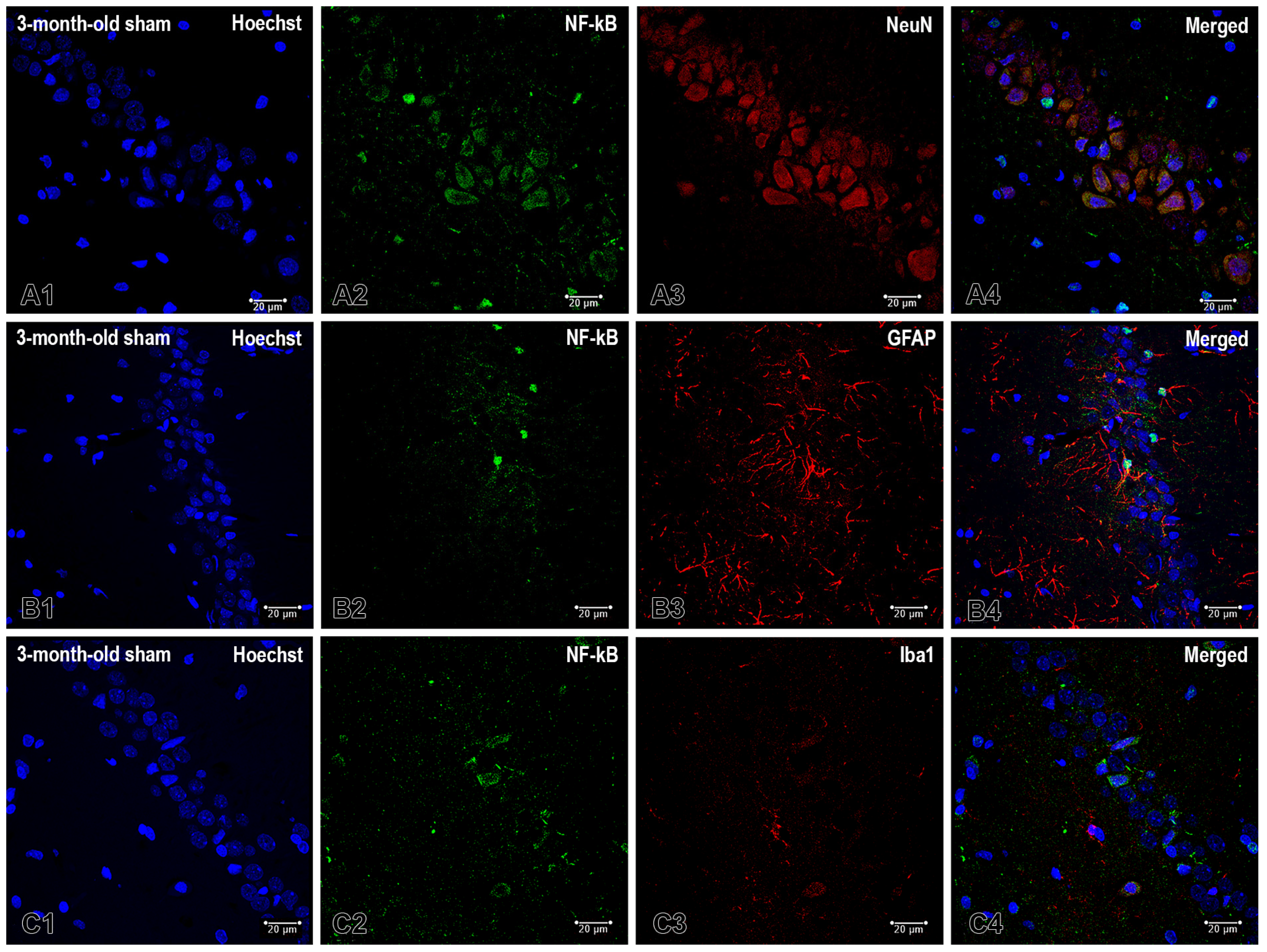
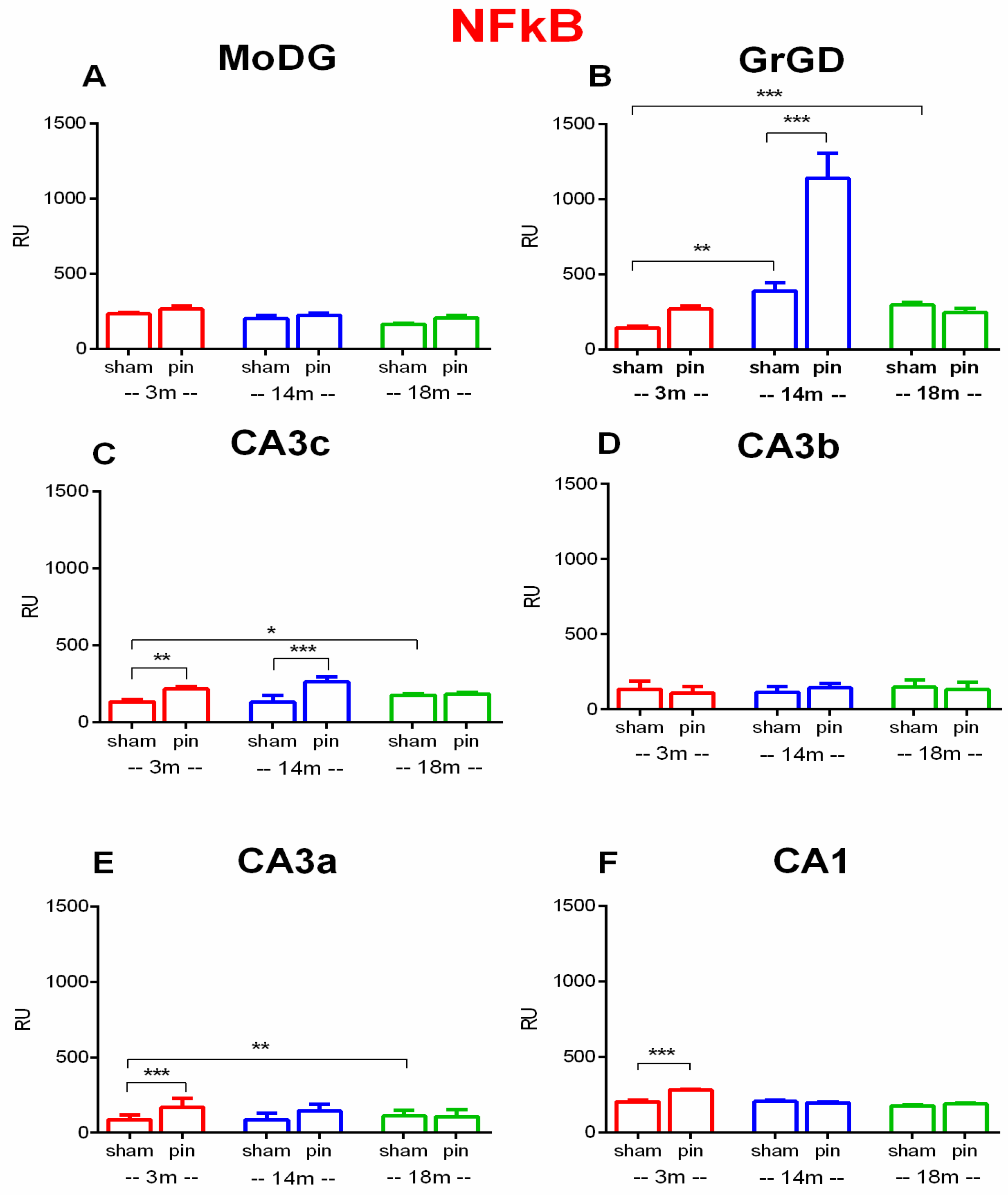
2.2. Effects of Pinealectomy and Aging on NF-kB Expression in Different Hippocampal Regions
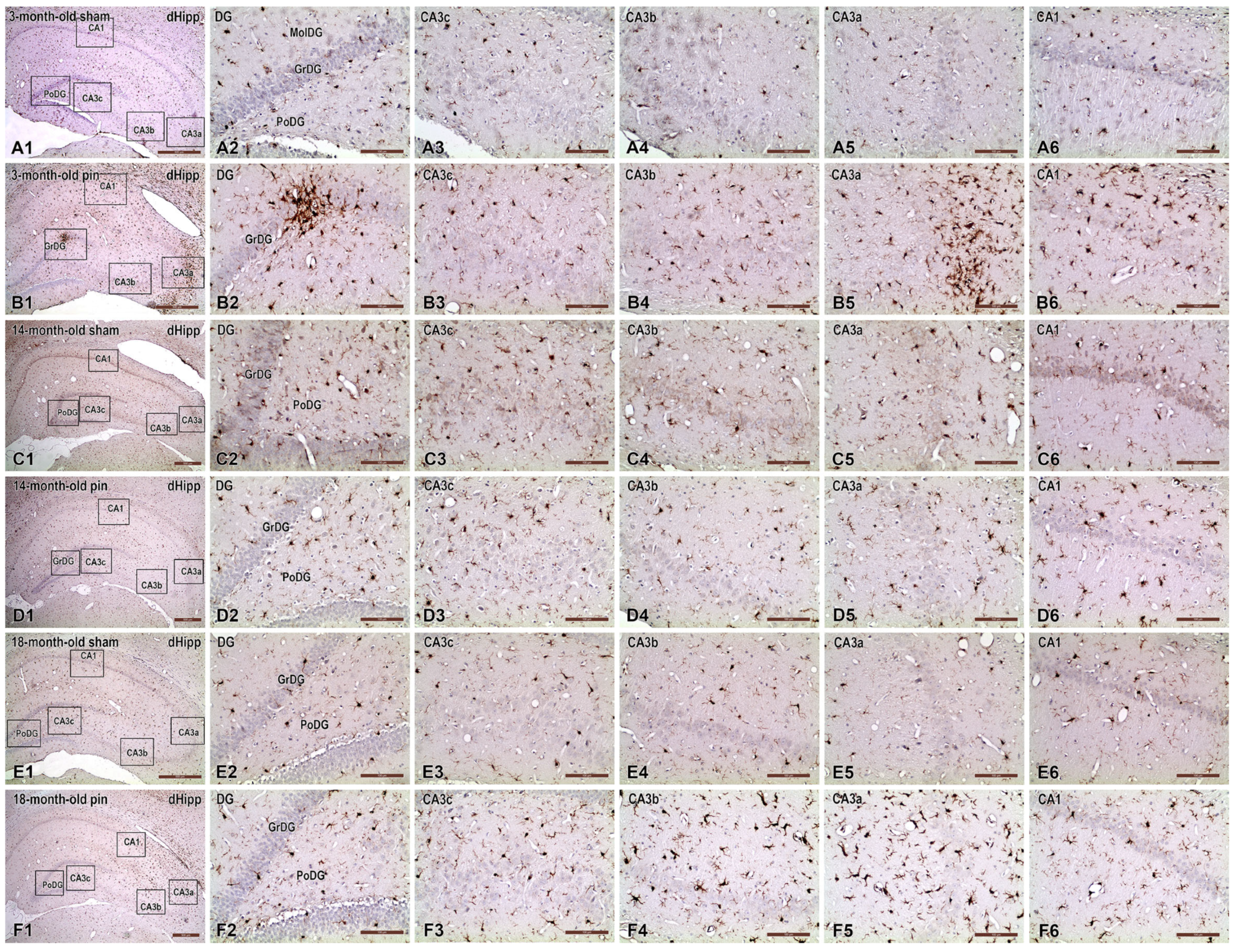
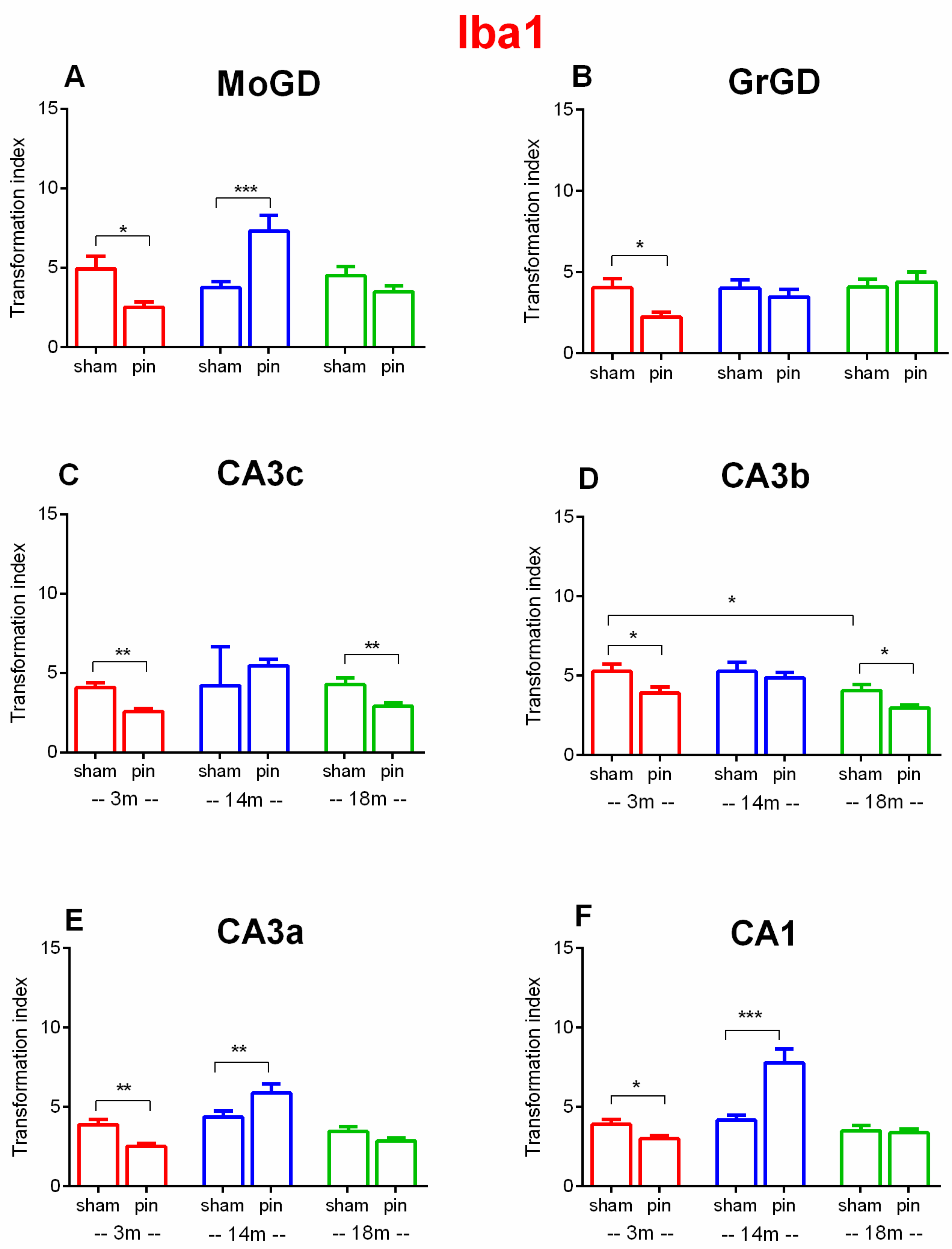
2.3. Effects of Pinealectomy and Aging on Microglia Activation in Different Hippocampal Regions
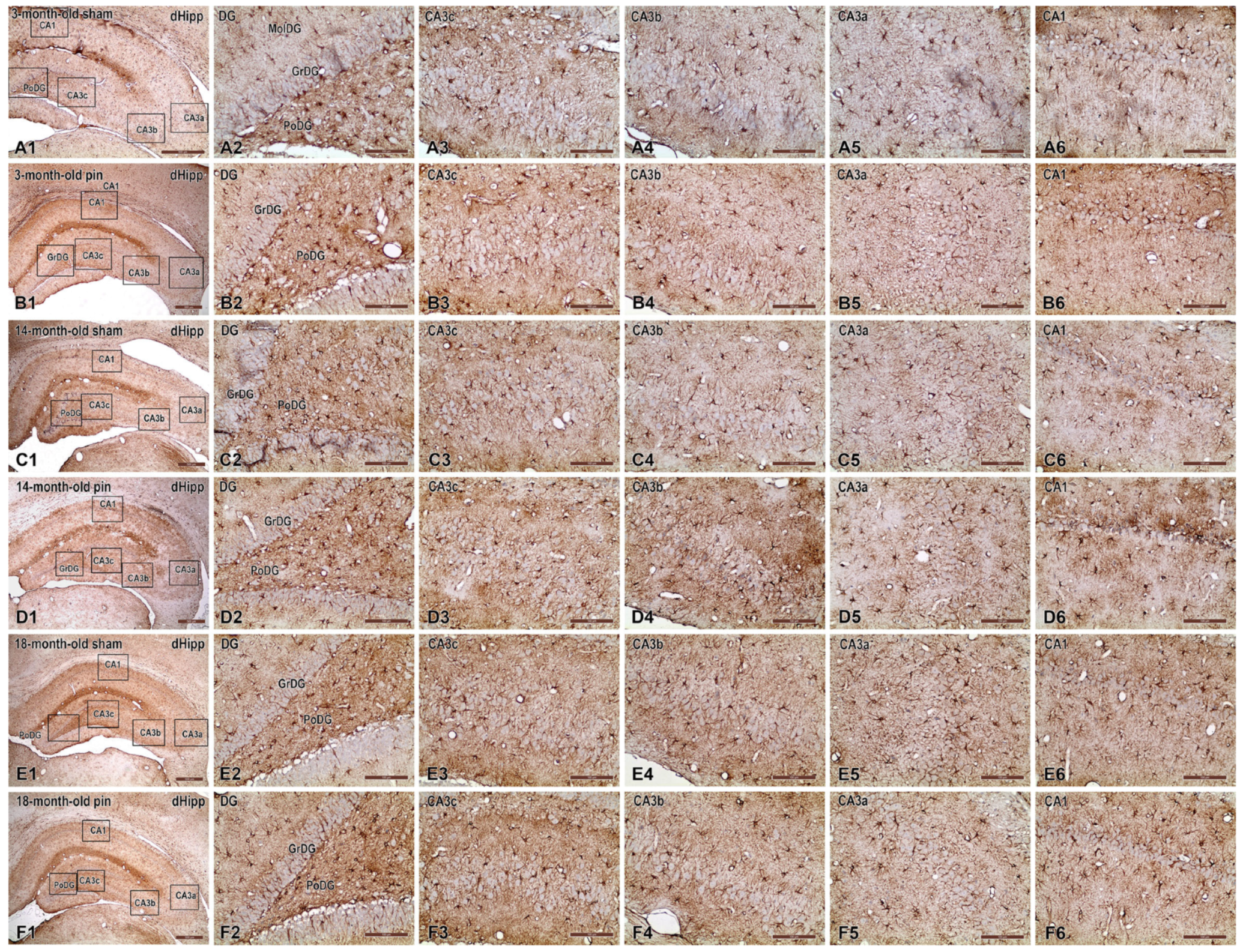
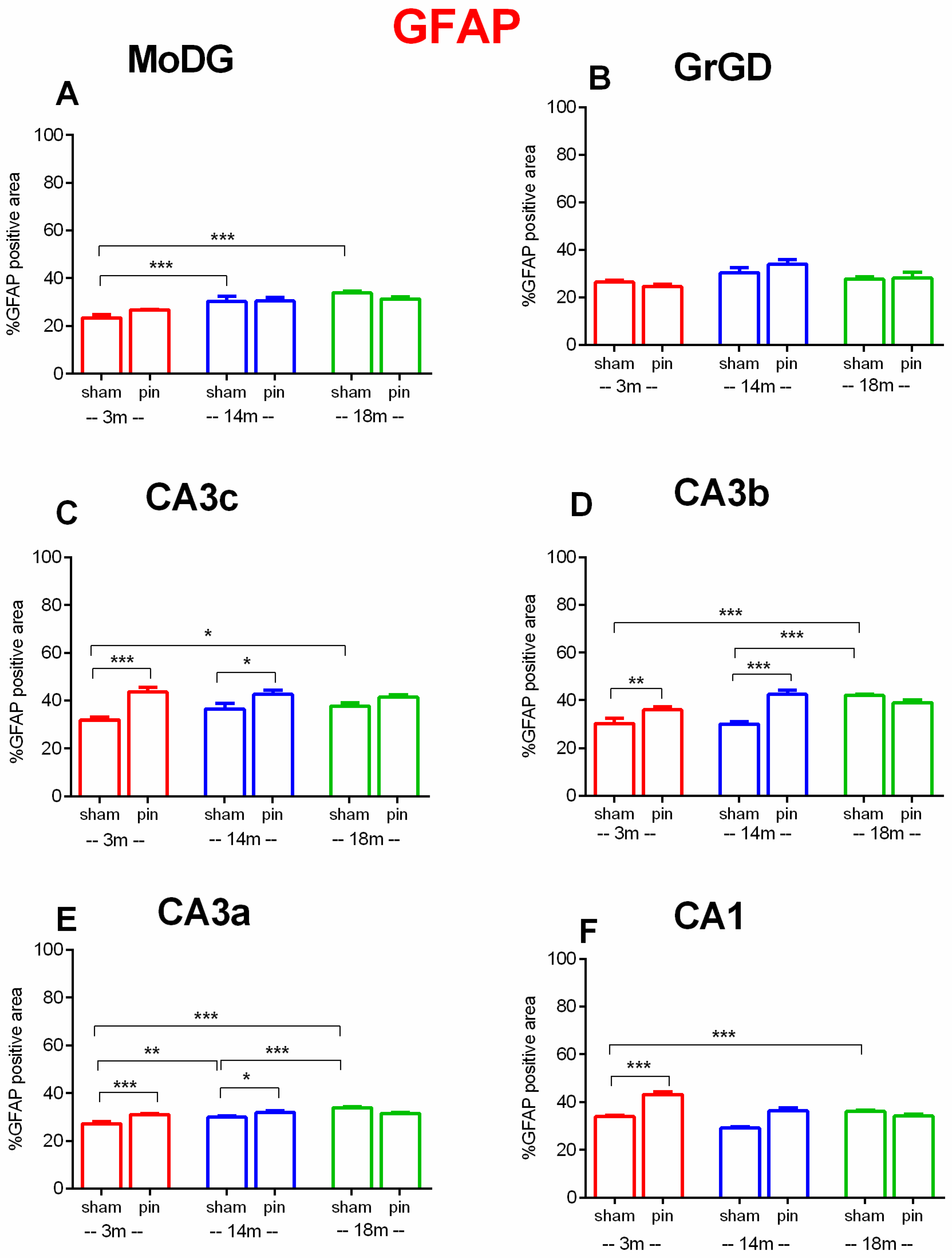
2.4. Effects of Pinealectomy and Aging on GFAP Expression in Different Hippocampal Regions
3. Discussion
3.1. Aging and Pinealectomy in Young Adult Rats Reduce pAkt1 Expression in dHipp, While Melatonin Deficiency in Older Rats Increases It
3.2. Pinelectomy Induced Age- and Structure-Dependent Increased Expression of NF-κB in the dHipp
3.3. Pinealectomy Increased the Activation of Microglia in the Hippocampus of Young Adult and Aged Rats, but Made Middle-Aged Rats Resistant to Activation
3.4. Age- and Pinealectomy-Induced Vulnerability to Astrocytes Activation Is Region-Specific for Young Adult, Middle-Aged and Old Rats
4. Materials and Methods
4.1. The Animals
4.2. Surgery
4.3. Immunohistochemistry and Immunofluorescence
4.3.1. Photodocumentation and Image Analysis
4.3.2. Transformation Index
4.3.3. GFAP-Positive Area
4.4. Statistical Analysis
5. Limitation and Future Direction
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | Protein Kinase B (also referred to as AKT1 when specifying isoform) |
| ANOVA | Analysis of Variance |
| BDNF | Brain-Derived Neurotrophic Factor |
| CA1, CA2, CA3a, CA3b, CA3c | Cornu Ammonis areas 1, 2, and 3 (hippocampal subregions) |
| CCR7 | C-C Chemokine Receptor Type 7 |
| CREB | cAMP Response Element-Binding Protein |
| DAB | 3,3′-Diaminobenzidine |
| DG | Dentate Gyrus |
| dHipp | Dorsal Hippocampus |
| ERK1/2 | Extracellular signal-Regulated Kinases 1 and 2 |
| EU | European Union (context: Council Directive 2010/63/EU) |
| F | F-statistic (from ANOVA) |
| FC | Frontal Cortex |
| GFAP | Glial Fibrillary Acidic Protein |
| GrDG | Granular layer of the Dentate Gyrus |
| HRP | Horseradish Peroxidase |
| Hsp | Heat Shock Protein |
| Hsp70 | Heat Shock Protein 70 |
| Hsp90 | Heat Shock Protein 90 |
| Iba1 | Ionized calcium-binding adapter molecule 1 (also known as AIF1–Allograft Inflammatory Factor 1) |
| IHC | Immunohistochemistry |
| IkB | Inhibitor of NF-κB |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| MAPK | Mitogen-Activated Protein Kinase |
| MoDG | Molecular layer of the Dentate Gyrus |
| MT1/MT2 | Melatonin Receptors 1 and 2 |
| MyD88 | Myeloid Differentiation Primary Response 88 |
| n | Sample size (number of animals per group) |
| NF-kB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| p | Probability value (used in statistical significance testing) |
| p65/p50 | Subunits of NF-κB |
| pAkt1 | Phosphorylated form of Akt1 |
| PB | Phosphate Buffer |
| PBS | Phosphate-Buffered Saline |
| PFA | Paraformaldehyde |
| PI3K | Phosphoinositide 3-Kinase (also: Phosphatidylinositol 3-Kinase) |
| pIkB | Phosphorylated IkB |
| PoDG | Polymorphic layer of the Dentate Gyrus |
| ROI | Region of Interest |
| ROS | Reactive Oxygen Species |
| S.E.M./SEM | Standard Error of the Mean |
| Ser473 | Serine at position 473 (phosphorylation site on Akt1) |
| SIRT1 | Sirtuin 1 |
| TBST | Tris-Buffered Saline with Tween-20 |
| Thr308 | Threonine at position 308 (phosphorylation site on Akt1) |
| TIF | Tagged Image File Format |
| TLR4 | Toll-Like Receptor 4 |
| TNF-α | Tumor Necrosis Factor-alpha |
References
- Songkiatisak, P.; Rahman, S.M.T.; Aqdas, M.; Sung, M.-H. NF-κB, a Culprit of Both Inflamm-Ageing and Declining Immunity? Immun. Ageing 2022, 19, 20. [Google Scholar] [CrossRef]
- Sameri, S.; Samadi, P.; Dehghan, R.; Salem, E.; Fayazi, N.; Amini, R. Stem Cell Aging in Lifespan and Disease: A State-of-the-Art Review. Curr. Stem Cell Res. Ther. 2020, 15, 362–378. [Google Scholar] [CrossRef]
- García-García, V.A.; Alameda, J.P.; Page, A.; Casanova, M.L. Role of NF-κB in Ageing and Age-Related Diseases: Lessons from Genetically Modified Mouse Models. Cells 2021, 10, 1906. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Y.; Hsieh, T.; Wang, C.; Cheng, K.; Wang, L.; Lin, T.; Cheung, C.H.A.; Wu, C.; Chiang, H. Aging-induced Akt Activation Involves in Aging-related Pathologies and Aβ-induced Toxicity. Aging Cell 2019, 18, e12989. [Google Scholar] [CrossRef] [PubMed]
- Unger, J.W. Glial Reaction in Aging and Alzheimer’s Disease. Microsc. Res. Tech. 1998, 43, 24–28. [Google Scholar] [CrossRef]
- Norden, D.M.; Muccigrosso, M.M.; Godbout, J.P. Microglial Priming and Enhanced Reactivity to Secondary Insult in Aging, and Traumatic CNS Injury, and Neurodegenerative Disease. Neuropharmacology 2015, 96, 29–41. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, L.; Lu, A.; Han, Y.; Colangelo, D.; Bukata, C.; Scibetta, A.; Yousefzadeh, M.J.; Li, X.; Gurkar, A.U.; et al. ATM Is a Key Driver of NF-κB-Dependent DNA-Damage-Induced Senescence, Stem Cell Dysfunction and Aging. Aging 2020, 12, 4688–4710. [Google Scholar] [CrossRef]
- Buffolo, F.; Petrosino, V.; Albini, M.; Moschetta, M.; Carlini, F.; Floss, T.; Kerlero De Rosbo, N.; Cesca, F.; Rocchi, A.; Uccelli, A.; et al. Neuroinflammation Induces Synaptic Scaling through IL-1β-Mediated Activation of the Transcriptional Repressor REST/NRSF. Cell Death Dis. 2021, 12, 180. [Google Scholar] [CrossRef]
- Dash, P.K.; Karl, K.A.; Colicos, M.A.; Prywes, R.; Kandel, E.R. cAMP Response Element-Binding Protein Is Activated by Ca2+/Calmodulin- as Well as cAMP-Dependent Protein Kinase. Proc. Natl. Acad. Sci. USA 1991, 88, 5061–5065. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Kareem, O.; Khushtar, M.; Akbar, M.; Haque, M.R.; Iqubal, A.; Haider, M.F.; Pottoo, F.H.; Abdulla, F.S.; Al-Haidar, M.B.; et al. Neuroinflammation: A Potential Risk for Dementia. Int. J. Mol. Sci. 2022, 23, 616. [Google Scholar] [CrossRef]
- Martín Giménez, V.M.; De Las Heras, N.; Lahera, V.; Tresguerres, J.A.F.; Reiter, R.J.; Manucha, W. Melatonin as an Anti-Aging Therapy for Age-Related Cardiovascular and Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 888292. [Google Scholar] [CrossRef] [PubMed]
- Karasek, M. Melatonin, Human Aging, and Age-Related Diseases. Exp. Gerontol. 2004, 39, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Waldhauser, F.; Ková, J.; Reiter, E. Age-Related Changes in Melatonin Levels in Humans and Its Potential Consequences for Sleep Disorders. Exp. Gerontol. 1998, 33, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Begemann, K.; Rawashdeh, O.; Olejniczak, I.; Pilorz, V.; De Assis, L.V.M.; Osorio-Mendoza, J.; Oster, H. Endocrine Regulation of Circadian Rhythms. npj Biol. Timing Sleep. 2025, 2, 10. [Google Scholar] [CrossRef]
- Buijink, M.R.; Michel, S. A Multi-level Assessment of the Bidirectional Relationship between Aging and the Circadian Clock. J. Neurochem. 2021, 157, 73–94. [Google Scholar] [CrossRef]
- Sánchez-Hidalgo, M.; Guerrero Montávez, J.M.; Carrascosa-Salmoral, M.D.P.; Naranjo Gutierrez, M.D.C.; Lardone, P.J.; De La Lastra Romero, C.A. Decreased MT1 and MT2 Melatonin Receptor Expression in Extrapineal Tissues of the Rat during Physiological Aging. J. Pineal Res. 2009, 46, 29–35. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.; Álvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J. Melatonin: Buffering the Immune System. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef]
- Withyachumnarnkul, B.; Nonaka, K.O.; Santana, C.; Attia, A.M.; Reiter, R.J. Interferon-γ Modulates Melatonin Production in Rat Pineal Glands in Organ Culture. J. Interferon Res. 1990, 10, 403–411. [Google Scholar] [CrossRef]
- Pontes, G.N.; Cardoso, E.C.; Carneiro-Sampaio, M.M.S.; Markus, R.P. Pineal Melatonin and the Innate Immune Response: The TNF-α Increase after Cesarean Section Suppresses Nocturnal Melatonin Production. J. Pineal Res. 2007, 43, 365–371. [Google Scholar] [CrossRef]
- Alghamdi, B.S. The Neuroprotective Role of Melatonin in Neurological Disorders. J. Neurosci. Res. 2018, 96, 1136–1149. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and the Theories of Aging: A Critical Appraisal of Melatonin’s Role in Antiaging Mechanisms. J. Pineal Res. 2013, 55, 325–356. [Google Scholar] [CrossRef] [PubMed]
- Won, E.; Na, K.-S.; Kim, Y.-K. Associations between Melatonin, Neuroinflammation, and Brain Alterations in Depression. Int. J. Mol. Sci. 2021, 23, 305. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Park, H.Y.; Ali, T.; Kim, M.O. Melatonin as a Potential Regulator of Oxidative Stress, and Neuroinflammation: Mechanisms and Implications for the Management of Brain Injury-Induced Neurodegeneration. J. Inflamm. Res. 2021, 14, 6251–6264. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Ikram, M.; Ullah, N.; Alam, S.; Park, H.; Badshah, H.; Choe, K.; Ok Kim, M. Neurological Enhancement Effects of Melatonin against Brain Injury-Induced Oxidative Stress, Neuroinflammation, and Neurodegeneration via AMPK/CREB Signaling. Cells 2019, 8, 760. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Hrischev, P.; Ivanova, P.; Boyadjiev, N.; Georgieva, K. Metabolic Footprint in Young, Middle-Aged and Elderly Rats with Melatonin Deficit. Physiol. Behav. 2022, 250, 113786. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Nenchovska, Z.; Kortenska, L.; Uzunova, V.; Georgieva, I.; Tzoneva, R. Impact of Melatonin Deficit on Emotional Status and Oxidative Stress-Induced Changes in Sphingomyelin and Cholesterol Level in Young Adult, Mature, and Aged Rats. Int. J. Mol. Sci. 2022, 23, 2809. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Krushovlieva, D.; Ivanova, P.; Nenchovska, Z.; Toteva, G.; Atanasova, M. The Role of Melatonin Deficiency Induced by Pinealectomy on Motor Activity and Anxiety Responses in Young Adult, Middle-Aged and Old Rats. Behav. Brain Funct. 2024, 20, 3. [Google Scholar] [CrossRef]
- Pierpaoli, W. The Pineal Gland as Ontogenetic Scanner of Reproduction, Immunity, and Aging The Aging Clock. Ann. N.Y. Acad. Sci. 1994, 741, 46–49. [Google Scholar] [CrossRef]
- Pierpaoli, W.; Bulian, D. The Pineal Aging and Death Program. I. Grafting of Old Pineals in Young Mice Accelerates Their Aging. J. Anti Aging Med. 2001, 4, 31–37. [Google Scholar] [CrossRef]
- Pierpaoli, W.; Bulian, D. The Pineal Aging and Death Program: Life Prolongation in Pre-aging Pinealectomized Mice. Ann. N.Y. Acad. Sci. 2005, 1057, 133–144. [Google Scholar] [CrossRef]
- Ikeyama, S.; Kokkonen, G.; Shack, S.; Wang, X.-T.; Holbrook, N.J. Loss in Oxidative Stress Tolerance with Aging Linked to Reduced Extracellular Signal-regulated Kinase and Akt Kinase Activities. FASEB J. 2002, 16, 1–16. [Google Scholar] [CrossRef]
- Jackson, T.C.; Rani, A.; Kumar, A.; Foster, T.C. Regional Hippocampal Differences in AKT Survival Signaling across the Lifespan: Implications for CA1 Vulnerability with Aging. Cell Death Differ. 2009, 16, 439–448. [Google Scholar] [CrossRef]
- Guillot, F.; Kemppainen, S.; Levasseur, G.; Miettinen, P.O.; Laroche, S.; Tanila, H.; Davis, S. Brain-Specific Basal and Novelty-Induced Alternations in PI3K-Akt and MAPK/ERK Signaling in a Middle-Aged AβPP/PS1 Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 51, 1157–1173. [Google Scholar] [CrossRef] [PubMed]
- Balasuriya, N.; Davey, N.E.; Johnson, J.L.; Liu, H.; Biggar, K.K.; Cantley, L.C.; Li, S.S.-C.; O’Donoghue, P. Phosphorylation-Dependent Substrate Selectivity of Protein Kinase B (AKT1). J. Biol. Chem. 2020, 295, 8120–8134. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Wang, J.; Li, B.-M. Role of the Phosphoinositide 3-Kinase-Akt-Mammalian Target of the Rapamycin Signaling Pathway in Long-Term Potentiation and Trace Fear Conditioning Memory in Rat Medial Prefrontal Cortex. Learn. Mem. 2008, 15, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Bruel-Jungerman, E.; Davis, S.; Rampon, C.; Laroche, S. Long-Term Potentiation Enhances Neurogenesis in the Adult Dentate Gyrus. J. Neurosci. 2006, 26, 5888–5893. [Google Scholar] [CrossRef]
- Wu, H.; Lu, D.; Jiang, H.; Xiong, Y.; Qu, C.; Li, B.; Mahmood, A.; Zhou, D.; Chopp, M. Simvastatin-Mediated Upregulation of VEGF and BDNF, Activation of the PI3K/Akt Pathway, and Increase of Neurogenesis Are Associated with Therapeutic Improvement after Traumatic Brain Injury. J. Neurotrauma 2008, 25, 130–139. [Google Scholar] [CrossRef]
- Zhang, T.; Ding, H.; Wang, Y.; Yuan, Z.; Zhang, Y.; Chen, G.; Xu, Y.; Chen, L. Akt3-mTOR Regulates Hippocampal Neurogenesis in Adult Mouse. J. Neurochem. 2021, 159, 498–511. [Google Scholar] [CrossRef]
- Nie, K.; Yu, J.-C.; Fu, Y.; Cheng, H.-Y.; Chen, F.-Y.; Qu, Y.; Han, J.-X. Age-Related Decrease in Constructive Activation of Akt/PKB in SAMP10 Hippocampus. Biochem. Biophys. Res. Commun. 2009, 378, 103–107. [Google Scholar] [CrossRef]
- Hardeland, R. Brain Inflammaging: Roles of Melatonin, Circadian Clocks and Sirtuins. J. Clin. Cell Immunol. 2018, 9, 1000543. [Google Scholar] [CrossRef]
- Cecon, E.; Oishi, A.; Jockers, R. Melatonin Receptors: Molecular Pharmacology and Signalling in the Context of System Bias. Br. J. Pharmacol. 2018, 175, 3263–3280. [Google Scholar] [CrossRef]
- Wang, L.; Xu, M.; Wang, Y.; Wang, F.; Deng, J.; Wang, X.; Zhao, Y.; Liao, A.; Yang, F.; Wang, S.; et al. Melatonin Improves Synapse Development by PI3K/Akt Signaling in a Mouse Model of Autism Spectrum Disorder. Neural Regen. Res. 2024, 19, 1618–1624. [Google Scholar] [CrossRef]
- Kilic, U.; Caglayan, A.B.; Beker, M.C.; Gunal, M.Y.; Caglayan, B.; Yalcin, E.; Kelestemur, T.; Gundogdu, R.Z.; Yulug, B.; Yılmaz, B.; et al. Particular Phosphorylation of PI3K/Akt on Thr308 via PDK-1 and PTEN Mediates Melatonin’s Neuroprotective Activity after Focal Cerebral Ischemia in Mice. Redox Biol. 2017, 12, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Ye, L.; Ding, Z.; Gao, F.; Yang, S.; Fang, B.; Liu, Z.; Xi, J. Melatonin Protects Against Ischemic Brain Injury by Modulating PI3K/AKT Signaling Pathway via Suppression of PTEN Activity. ASN Neuro 2021, 13, 17590914211022888. [Google Scholar] [CrossRef] [PubMed]
- Crampton, S.J.; O’Keeffe, G.W. NF-κB: Emerging Roles in Hippocampal Development and Function. Int. J. Biochem. Cell Biol. 2013, 45, 1821–1824. [Google Scholar] [CrossRef] [PubMed]
- Bortolotto, V.; Cuccurazzu, B.; Canonico, P.L.; Grilli, M. NF-κB Mediated Regulation of Adult Hippocampal Neurogenesis: Relevance to Mood Disorders and Antidepressant Activity. BioMed Res. Int. 2014, 2014, 612798. [Google Scholar] [CrossRef]
- Terai, K.; Matsuo, A.; McGeer, P.L. Enhancement of Immunoreactivity for NF-κB in the Hippocampal Formation and Cerebral Cortex of Alzheimer’s Disease. Brain Res. 1996, 735, 159–168. [Google Scholar] [CrossRef]
- Yin, C.; Zhang, M.; Cheng, L.; Ding, L.; Lv, Q.; Huang, Z.; Zhou, J.; Chen, J.; Wang, P.; Zhang, S.; et al. Melatonin Modulates TLR4/MyD88/NF-κB Signaling Pathway to Ameliorate Cognitive Impairment in Sleep-Deprived Rats. Front. Pharmacol. 2024, 15, 1430599. [Google Scholar] [CrossRef]
- Tan, D.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One Molecule, Many Derivatives: A Never-ending Interaction of Melatonin with Reactive Oxygen and Nitrogen Species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Mayo, J.C.; Sainz, R.M.; Tan, D.-X.; Hardeland, R.; Leon, J.; Rodriguez, C.; Reiter, R.J. Anti-Inflammatory Actions of Melatonin and Its Metabolites, N1-Acetyl-N2-Formyl-5-Methoxykynuramine (AFMK) and N1-Acetyl-5-Methoxykynuramine (AMK), in Macrophages. J. Neuroimmunol. 2005, 165, 139–149. [Google Scholar] [CrossRef]
- Martín, V.; Sainz, R.M.; Antolín, I.; Mayo, J.C.; Herrera, F.; Rodríguez, C. Several Antioxidant Pathways Are Involved in Astrocyte Protection by Melatonin. J. Pineal Res. 2002, 33, 204–212. [Google Scholar] [CrossRef]
- Korkmaz, A.; Reiter, R.J.; Topal, T.; Manchester, L.C.; Oter, S.; Tan, D.-X. Melatonin: An Established Antioxidant Worthy of Use in Clinical Trials. Mol. Med. 2009, 15, 43–50. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, L.; Xiao, F.; Wu, L.; Guo, Y.; Zhang, Z.; Xiao, Y.; Sun, G.; Yang, Q.; Guo, H. Melatonin-Driven NLRP3 Inflammation Inhibition Via Regulation of NF-κB Nucleocytoplasmic Transport: Implications for Postoperative Cognitive Dysfunction. Inflammation 2023, 46, 1471–1492. [Google Scholar] [CrossRef]
- Chatterjee, M.; Andrulis, M.; Stuhmer, T.; Muller, E.; Hofmann, C.; Steinbrunn, T.; Heimberger, T.; Schraud, H.; Kressmann, S.; Einsele, H.; et al. The PI3K/Akt Signaling Pathway Regulates the Expression of Hsp70, Which Critically Contributes to Hsp90-Chaperone Function and Tumor Cell Survival in Multiple Myeloma. Haematologica 2013, 98, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Wawrzyniuk, M.; Rutten, V.P.M.G.; Van Eden, W.; Sijts, A.J.A.M.; Broere, F. Hsp70 and NF-kB Mediated Control of Innate Inflammatory Responses in a Canine Macrophage Cell Line. Int. J. Mol. Sci. 2020, 21, 6464. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Ghosal, I.; Das, D.; Chakraborty, S.B. Melatonin Ameliorates H2O2-Induced Oxidative Stress through Modulation of Erk/Akt/NFkB Pathway. Biol. Res. 2018, 51, 17. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Atanasova, D.; Krushovlieva, D.; Barbutska, D.; Atanasova, M.; Rashev, P.; Nenchovska, Z.; Mourdjeva, M.; Koeva, Y. Age-Related Memory Decline Is Accelerated by Pinealectomy in Young Adult and Middle-Aged Rats via BDNF/ERK/CREB Signalling in the Hippocampus 2024. Neurochem. Int. 2025, 185, 105960. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Ivanova, P.; Krushovlieva, D.; Kortenska, L.; Angelova, V.T. Protective Effect of the Novel Melatonin Analogue Containing Donepezil Fragment on Memory Impairment via MT/ERK/CREB Signaling in the Hippocampus in a Rat Model of Pinealectomy and Subsequent Aβ1-42 Infusion. Int. J. Mol. Sci. 2024, 25, 1867. [Google Scholar] [CrossRef]
- Chen, B.; Liu, J.; Ho, T.-T.; Ding, X.; Mo, Y.-Y. ERK-Mediated NF-κB Activation through ASIC1 in Response to Acidosis. Oncogenesis 2016, 5, e279. [Google Scholar] [CrossRef]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The Role of the Transcription Factor CREB in Immune Function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef]
- Hiramoto, K.; Imai, M.; Tanaka, S.; Ooi, K. Dementia Is Induced via the AGEs/Iba1/iNOS Pathway in Aged KK-Ay/Tajcl Mice. Life 2023, 13, 1540. [Google Scholar] [CrossRef]
- Ahn, J.H.; Park, J.H.; Lee, T.; Yang, G.E.; Shin, M.C.; Cho, J.H.; Won, M.; Lee, C. Age-dependent Alterations in the Immunoreactivity of Macrophage Inflammatory Protein-3α and Its Receptor CCR6 in the Gerbil Hippocampus. Mol. Med. Rep. 2020, 22, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Suo, Z.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin Modulates Neuroinflammatory Response and Microglial Activation in Mice Exposed to Dim Blue Light at Night. Front. Pharmacol. 2024, 15, 1416350. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Luo, K.; Hu, Y.; Niu, Y.; Zhu, X.; Li, S.; Zhang, H. Melatonin Alleviates Chronic Stress-Induced Hippocampal Microglia Pyroptosis and Subsequent Depression-like Behaviors by Inhibiting Cathepsin B/NLRP3 Signaling Pathway in Rats. Transl. Psychiatry 2024, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Merlo, S.; Luaces, J.P.; Spampinato, S.F.; Toro-Urrego, N.; Caruso, G.I.; D’Amico, F.; Capani, F.; Sortino, M.A. SIRT1 Mediates Melatonin’s Effects on Microglial Activation in Hypoxia: In Vitro and In Vivo Evidence. Biomolecules 2020, 10, 364. [Google Scholar] [CrossRef]
- Arioz, B.I.; Tastan, B.; Tarakcioglu, E.; Tufekci, K.U.; Olcum, M.; Ersoy, N.; Bagriyanik, A.; Genc, K.; Genc, S. Melatonin Attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 Inflammasome Activation Through the SIRT1/Nrf2 Pathway. Front. Immunol. 2019, 10, 1511. [Google Scholar] [CrossRef]
- Labarta-Bajo, L.; Allen, N.J. Astrocytes in Aging. Neuron 2025, 113, 109–126. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, P.; Ren, L.; Hu, C.; Bi, J. Protective Effect of Melatonin on Soluble Aβ1–42-Induced Memory Impairment, Astrogliosis, and Synaptic Dysfunction via the Musashi1/Notch1/Hes1 Signaling Pathway in the Rat Hippocampus. Alzheimer’s Res. Ther. 2016, 8, 40. [Google Scholar] [CrossRef]
- Gáll, Z.; Boros, B.; Kelemen, K.; Urkon, M.; Zolcseak, I.; Márton, K.; Kolcsar, M. Melatonin Improves Cognitive Dysfunction and Decreases Gliosis in the Streptozotocin-Induced Rat Model of Sporadic Alzheimer’s Disease. Front. Pharmacol. 2024, 15, 1447757. [Google Scholar] [CrossRef]
- Hoffman, R.A.; Reiter, R.J. Rapid pinealectomy in hamsters and other small rodents. Anat. Record. 1965, 24, 83–89. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Nenchovska, Z.; Atanasova, D.; Atanasova, M.; Kortenska, L.; Stefanova, M.; Alova, L.; Lazarov, N. Consequences of Long-Term Treatment with Agomelatine on Depressive-like Behavior and Neurobiological Abnormalities in Pinealectomized Rats. Behav. Brain Res. 2016, 302, 11–28. [Google Scholar] [CrossRef]
- Ghorbandaiepour, T.; Sadroddiny, E.; Zahmatkesh, M.; Hassanzadeh, G. Inhibition of Hippocampal Melatonin Synthesis by siRNA Induced Learning and Memory Deficits in Male Rats. Horm. Behav. 2024, 164, 105599. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.S.; Santos, A.A.; Ribeiro-Paz, E.E.; Córdoba-Moreno, M.; Trevisan, I.L.; Caldeira, W.; Muxel, S.M.; Sousa, K.D.; Markus, R.P. Melatonin Synthesized by Activated Microglia Orchestrates the Progression of Microglia from a Pro-Inflammatory to a Recovery/Repair Phenotype. Melatonin Res. 2022, 5, 55–67. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuang, J.; Zhu, H.; Shen, Y.; Tan, Z.; Zhou, J. Cultured Rat Cortical Astrocytes Synthesize Melatonin: Absence of a Diurnal Rhythm. J. Pineal Res. 2007, 43, 232–238. [Google Scholar] [CrossRef] [PubMed]

| Inflammatory Signaling | % Change Pin 3-Months | % Change Pin 14-Months | % Change Pin 18-Months | Glial Markers | % Change Pin 3-Months | % Change Pin 14-Months |
|---|---|---|---|---|---|---|
| Akt | Iba1 | |||||
| MoDG | 81%↓ | 358%↑ | ─ | MoDG | 49%↓ | 95%↑ |
| GrDG | 73%↓ | 84%↑ | ─ | GrDG | 45%↓ | ─ |
| CA3c | 79%↓ | 350%↑ | ─ | CA3c | 37%↓ | ─ |
| CA3b | 66%↓ | 307%↑ | ─ | CA3b | 26%↓ | ─ |
| CA3a | 70%↓ | ─ | ─ | CA3a | 35%↓ | 34%↑ |
| CA1 | 83%↓ | ─ | ─ | CA1 | 23%↓ | 87%↑ |
| NFkB | GFAP | |||||
| MoDG | ─ | ─ | ─ | MoDG | ─ | ─ |
| GrDG | ─ | 193%↑ | ─ | GrDG | ─ | ─ |
| CA3c | 62%↑ | 99%↑ | ─ | CA3c | 37%↑ | 17%↑ |
| CA3b | ─ | ─ | ─ | CA3b | 18%↑ | 41%↑ |
| CA3a | 98%↑ | ─ | ─ | CA3a | 14%↑ | 6%↑ |
| CA1 | 38%↑ | ─ | ─ | CA1 | 27%↑ | ─ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atanasova, D.; Krushovlieva, D.; Rashev, P.; Mourdjeva, M.; Pupaki, D.; Tchekalarova, J. Pinealectomy-Induced Neuroinflammation Varies with Age in Rats. Int. J. Mol. Sci. 2025, 26, 8093. https://doi.org/10.3390/ijms26168093
Atanasova D, Krushovlieva D, Rashev P, Mourdjeva M, Pupaki D, Tchekalarova J. Pinealectomy-Induced Neuroinflammation Varies with Age in Rats. International Journal of Molecular Sciences. 2025; 26(16):8093. https://doi.org/10.3390/ijms26168093
Chicago/Turabian StyleAtanasova, Dimitrinka, Desislava Krushovlieva, Pavel Rashev, Milena Mourdjeva, Despina Pupaki, and Jana Tchekalarova. 2025. "Pinealectomy-Induced Neuroinflammation Varies with Age in Rats" International Journal of Molecular Sciences 26, no. 16: 8093. https://doi.org/10.3390/ijms26168093
APA StyleAtanasova, D., Krushovlieva, D., Rashev, P., Mourdjeva, M., Pupaki, D., & Tchekalarova, J. (2025). Pinealectomy-Induced Neuroinflammation Varies with Age in Rats. International Journal of Molecular Sciences, 26(16), 8093. https://doi.org/10.3390/ijms26168093







