Intermediate Stage of Epileptogenesis Is Not Affected by Disrupting Memory Consolidation Processes
Abstract
1. Introduction
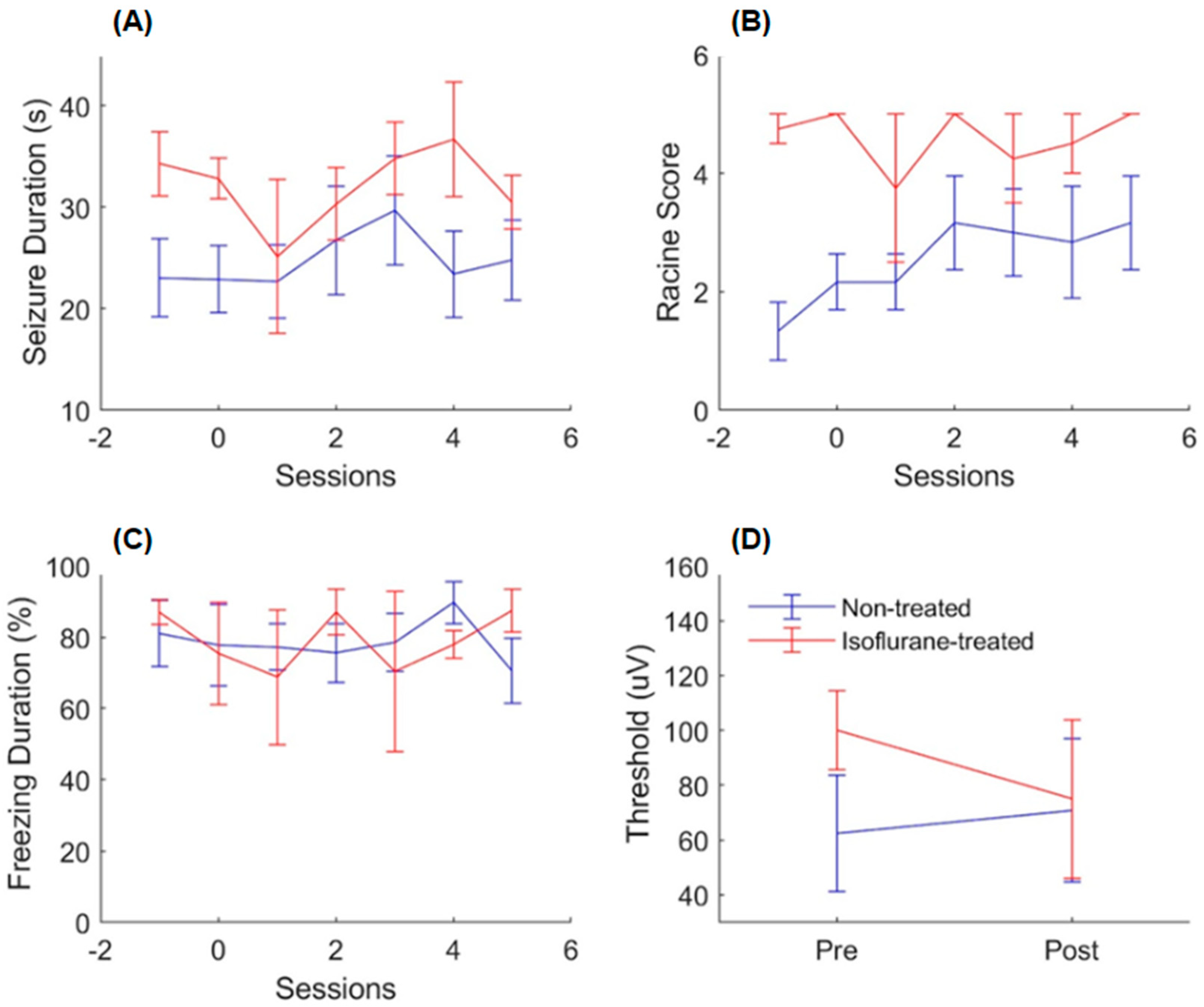
2. Results
2.1. Rapamycin Experiment
2.2. Isoflurane Experiment
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgery
4.3. Recording
4.4. Kindling and Sensory Stimulation
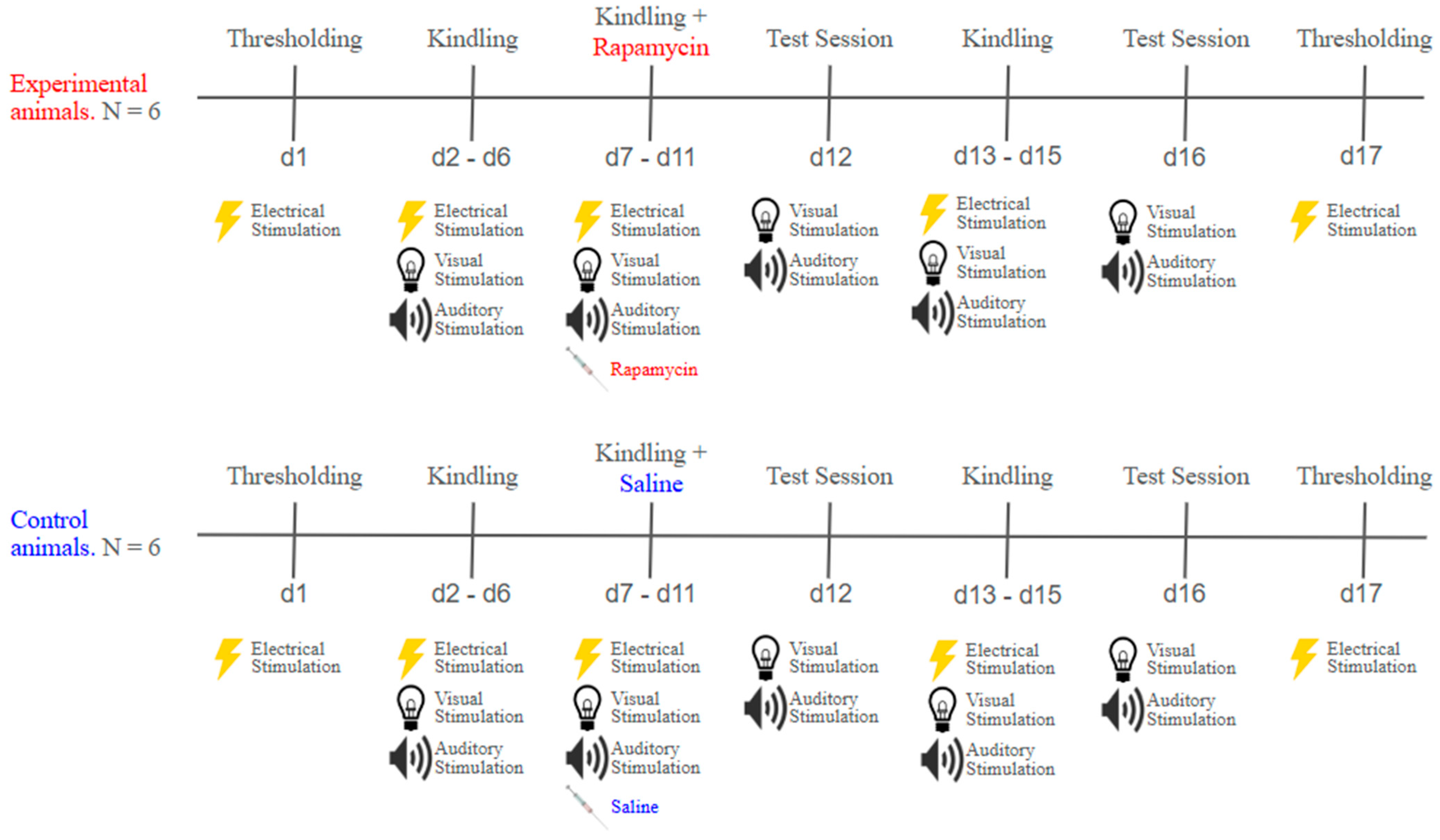
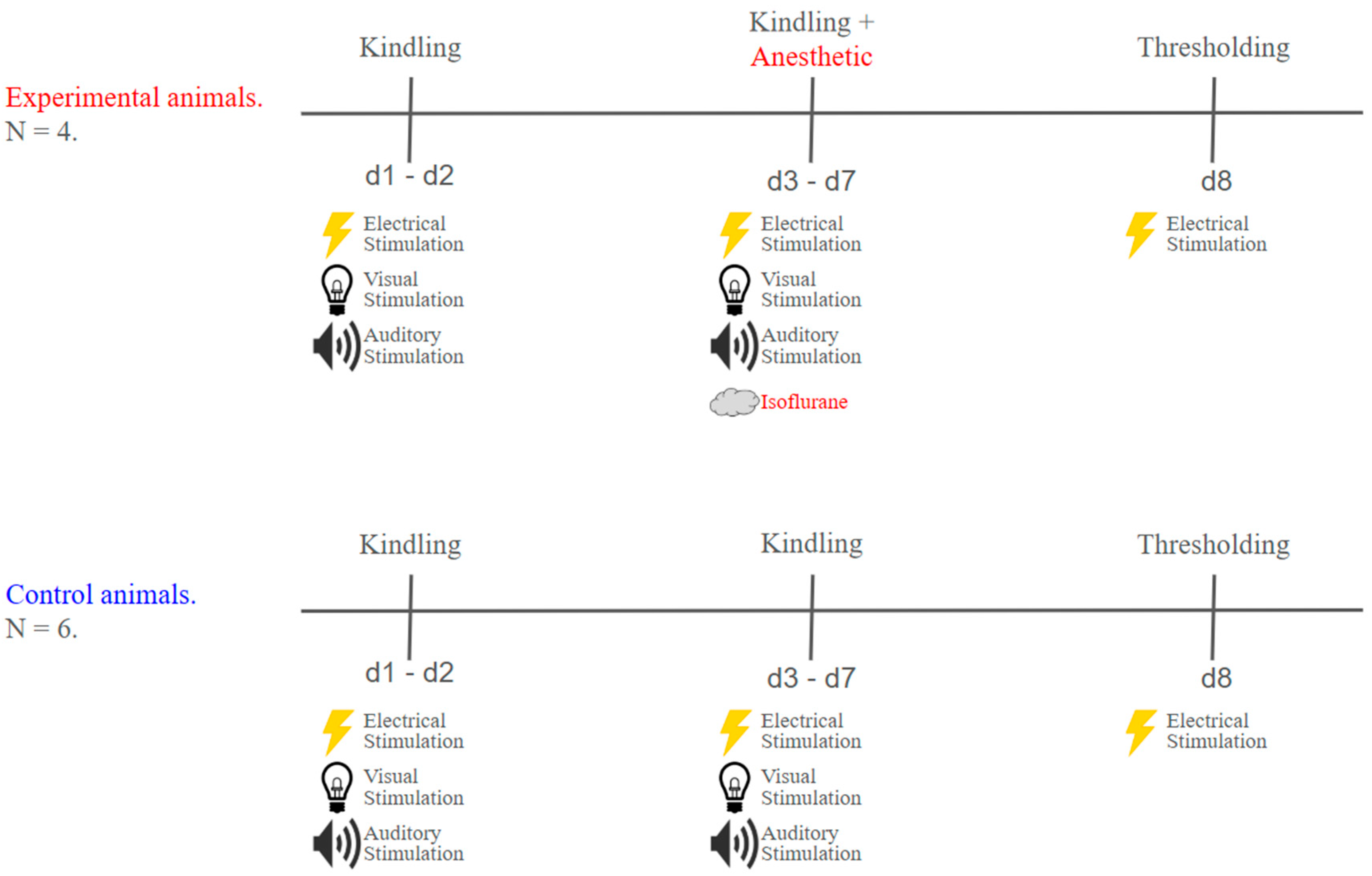
4.5. Experimental Timelines
4.5.1. Thresholding
4.5.2. Kindling
4.5.3. Rapamycin Treatment
4.5.4. Isoflurane Treatment
4.6. Analysis
4.6.1. Duration of Electrographic Seizure
4.6.2. Freezing Duration
4.6.3. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Łukasiuk, K.; Lasoń, W. Emerging molecular targets for anti-epileptogenic and epilepsy modifying drugs. Int. J. Mol. Sci. 2023, 24, 2928. [Google Scholar] [CrossRef]
- Beenhakker, M.P.; Huguenard, J.R. Neurons that fire together also conspire together:Is normal sleep circuitry hijacked to generate epilepsy? Neuron 2009, 62, 612–632. [Google Scholar] [CrossRef]
- Das, R.; Luczak, A. Epileptic seizures and link to memory processes. AIMS Neurosci. 2022, 9, 114–127. [Google Scholar] [CrossRef]
- Kucewicz, M.T.; Cimbalnik, J.; Garcia-Salinas, J.S.; Brazdil, M.; Worrell, G.A. High frequency oscillations in human memory and cognition: A neurophysiological substrate of engrams? Brain 2024, 147, 2966–2982. [Google Scholar] [CrossRef]
- Soula, M.; Maslarova, A.; Harvey, R.E.; Valero, M.; Brandner, S.; Hamer, H.; Fernández-Ruiz, A.; Buzsáki, G. Interictal epileptiform discharges affect memory in an Alzheimer’s disease mouse model. Proc. Natl. Acad. Sci. USA 2023, 120, e2302676120. [Google Scholar] [CrossRef]
- Squire, L.R.; Genzel, L.; Wixted, J.T.; Morris, R.G. Memory consolidation. Cold Spring Harb. Perspect. Biol. 2015, 7, a021766. [Google Scholar] [CrossRef]
- Farrell, J.S.; Wolff, M.D.; Teskey, G.C. Neurodegeneration and pathology in epilepsy: Clinical and basic perspectives. In Neurodegenerative Diseases: Pathology, Mechanisms, and Potential Therapeutic Targets; Springer Nature: Berlin/Heidelberg, Germany, 2017; pp. 317–334. [Google Scholar]
- Al-Redouan, A.; Busch, A.; Salaj, M.; Kubova, H.; Druga, R. Dorsal Striatum Is Compromised by Status Epilepticus Induced in Immature Developing Animal Experimental Model of Mesial Temporal Lobe Epilepsy. Int. J. Mol. Sci. 2025, 26, 3349. [Google Scholar] [CrossRef]
- Aniol, V.A.; Lazareva, N.A.; Moiseeva, Y.V.; Nedogreeva, O.A.; Novikova, M.R.; Kostryukov, P.A.; Onufriev, M.V.; Gulyaeva, N.V. Effects of Phenosanic Acid in Rat Seizure Models. Int. J. Mol. Sci. 2025, 26, 5668. [Google Scholar] [CrossRef]
- Hayashi, Y. Molecular mechanism of hippocampal long-term potentiation–Towards multiscale understanding of learning and memory. Neurosci. Res. 2022, 175, 3–15. [Google Scholar] [CrossRef]
- Jacobs, J.; Banks, S.; Zelmann, R.; Zijlmans, M.; Jones-Gotman, M.; Gotman, J. Spontaneous ripples in the hippocampus correlate with epileptogenicity and not memory function in patients with refractory epilepsy. Epilepsy Behav. 2016, 62, 258–266. [Google Scholar] [CrossRef]
- Bower, M.R.; Stead, M.; Bower, R.S.; Kucewicz, M.T.; Sulc, V.; Cimbalnik, J.; Brinkmann, B.H.; Vasoli, V.M.; St Louis, E.K.; Meyer, F.B.; et al. Evidence for consolidation of neuronal assemblies after seizures in humans. J. Neurosci. 2015, 35, 999–1010. [Google Scholar] [CrossRef]
- Del Felice, A.; Storti, S.F.; Manganotti, P. Sleep affects cortical source modularity in temporal lobe epilepsy: A high-density EEG study. Clin. Neurophysiol. 2015, 126, 1677–1683. [Google Scholar] [CrossRef]
- Neumann, A.R.; Raedt, R.; Steenland, H.W.; Sprengers, M.; Bzymek, K.; Navratilova, Z.; Mesina, L.; Xie, J.; Lapointe, V.; Kloosterman, F.; et al. Involvement of fast-spiking cells in ictal sequences during spontaneous seizures in rats with chronic temporal lobe epilepsy. Brain 2017, 140, 2355–2369. [Google Scholar] [CrossRef]
- Che, L.Q.; Qu, Z.Z.; Xie, T.; Zhang, Y.G.; Yuan, D.J.; Li, Q.; Jia, L.; Wang, W.P. Effect of low-frequency repetitive transcranial magnetic stimulation on cognitive function in rats with medial temporal lobe epilepsy. Acta Neurobiol. Exp. 2023, 83, 395–403. [Google Scholar] [CrossRef]
- Beroun, A.; Mitra, S.; Michaluk, P.; Pijet, B.; Stefaniuk, M.; Kaczmarek, L. MMPs in learning and memory and neuropsychiatric disorders. Cell. Mol. Life Sci. 2019, 76, 3207–3228. [Google Scholar] [CrossRef]
- Liguz-Lecznar, M.; Dobrzanski, G.; Kossut, M. Somatostatin and somatostatin-containing interneurons—From plasticity to pathology. Biomolecules 2022, 12, 312. [Google Scholar] [CrossRef]
- Blundell, J.; Kouser, M.; Powell, C.M. Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol. Learn. Mem. 2008, 90, 28–35. [Google Scholar] [CrossRef]
- Teskey, G. Kindling. In Oxford Research Encyclopedia of Psychology; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Mac Callum, P.E.; Hebert, M.; Adamec, R.E.; Blundell, J. Systemic inhibition of mTOR kinase via rapamycin disrupts consolidation and reconsolidation of auditory fear memory. Neurobiol. Learn. Mem. 2014, 112, 176–185. [Google Scholar] [CrossRef]
- Parsons, R.G.; Gafford, G.M.; Helmstetter, F.J. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J. Neurosci. 2006, 26, 12977–12983. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, S.; Bu, H.; Zhou, Y.; Chen, L.; Kang, Z.; Chen, L.; Yan, H.; Yang, C.; Yan, J.; et al. Disrupting reconsolidation by systemic inhibition of mTOR kinase via rapamycin reduces cocaine seeking behavior. Front. Pharmacol. 2021, 12, 652865. [Google Scholar] [CrossRef]
- Goddard, G.V.; Douglas, R.M. Does the engram of kindling model the engram of normal long term memory? Can. J. Neurol. Sci. 1975, 2, 385–394. [Google Scholar] [CrossRef][Green Version]
- Gelinas, J.N.; Khodagholy, D.; Thesen, T.; Devinsky, O.; Buzsáki, G. Interictal epileptiform discharges induce hippocampal–cortical coupling in temporal lobe epilepsy. Nat. Med. 2016, 22, 641–648. [Google Scholar] [CrossRef]
- Lambert, I.; Tramoni-Negre, E.; Lagarde, S.; Roehri, N.; Giusiano, B.; Trebuchon-Da Fonseca, A.; Carron, R.; Benar, C.; Felician, O.; Bartolomei, F. Hippocampal interictal spikes during sleep impact long-term memory consolidation. Ann. Neurol. 2020, 87, 976–987. [Google Scholar] [CrossRef]
- Zambrotta, M.E.; Houlé, J.D. Chapter 29—MicroRNA Regulation of mTOR Function. In MicroRNA in Regenerative Medicine; Sen, C.K., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 757–790. [Google Scholar]
- Swiech, L.; Perycz, M.; Malik, A.; Jaworski, J. Role of mTOR in physiology and pathology of the nervous system. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2008, 1784, 116–132. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One drug, many effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef]
- Zhao, W.; Xie, C.; Zhang, X.; Liu, J.; Liu, J.; Xia, Z. Advances in the mTOR signaling pathway and its inhibitor rapamycin in epilepsy. Brain Behav. 2023, 13, e2995. [Google Scholar] [CrossRef]
- Gericke, B.; Brandt, C.; Theilmann, W.; Welzel, L.; Schidlitzki, A.; Twele, F.; Kaczmarek, E.; Anjum, M.; Hillmann, P.; Löscher, W. Selective inhibition of mTORC1/2 or PI3K/mTORC1/2 signaling does not prevent or modify epilepsy in the intrahippocampal kainate mouse model. Neuropharmacology 2020, 162, 107817. [Google Scholar] [CrossRef]
- Cain, D.P.; Corcoran, M.E.; Staines, W.A. Effects of protein synthesis inhibition on kindling in the mouse. Exp. Neurol. 1980, 68, 409–419. [Google Scholar] [CrossRef]
- Jonec, V.; Wasterlain, C.G. Effect of inhibitors of protein synthesis on the development of kindled seizures in rats. Exp. Neurol. 1979, 66, 524–532. [Google Scholar] [CrossRef]
- French, J.A.; Lawson, J.A.; Yapici, Z.; Ikeda, H.; Polster, T.; Nabbout, R.; Curatolo, P.; de Vries, P.J.; Dlugos, D.J.; Berkowitz, N.; et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): A phase 3, randomised, double-blind, placebo-controlled study. Lancet 2016, 388, 2153–2163. [Google Scholar] [CrossRef]
- Hall, A.C.; Lieb, W.R.; Franks, N.P. Stereoselective and non-stereoselective actions of isoflurane on the GABAA receptor. Br. J. Pharmacol. 1994, 112, 906–910. [Google Scholar] [CrossRef]
- Bar-Klein, G.; Klee, R.; Brandt, C.; Bankstahl, M.; Bascuñana, P.; Töllner, K.; Dalipaj, H.; Bankstahl, J.P.; Friedman, A.; Löscher, W. Isoflurane prevents acquired epilepsy in rat models of temporal lobe epilepsy. Ann. Neurol. 2016, 80, 896–908. [Google Scholar] [CrossRef]
- Boroujeni, S.; Solaimanian, S.; Jafari, R.; Shafaroodi, O.; Maleki, A.; Mohammadi, P.; Karimi, E.; Dehpour, A. Opioidergic and nitrergic systems mediate the anticonvulsant effect of mefloquine and chloroquine on seizures induced by pentylenetetrazol and maximal electroshock in mice. Acta Neurobiol. Exp. 2022, 82, 157–169. [Google Scholar] [CrossRef]
- Ishii, T.; Kaya, M.; Muroi, Y. Oral Administration of Probiotic Bifidobacterium breve Ameliorates Tonic–Clonic Seizure in a Pentylenetetrazole-Induced Kindling Mouse Model via Integrin-Linked Kinase Signaling. Int. J. Mol. Sci. 2024, 25, 9259. [Google Scholar] [CrossRef]
- Schjetnan, A.G.P.; Luczak, A. Recording large-scale neuronal ensembles with silicon probes in the anesthetized rat. J. Vis. Exp. 2011, 56, e3282. [Google Scholar] [CrossRef]
- Wilson, M.A.; McNaughton, B.L. Reactivation of hippocampal ensemble memories during sleep. Science 1994, 265, 676–679. [Google Scholar] [CrossRef]
- Faraji, J.; Gomez-Palacio-Schjetnan, A.; Luczak, A.; Metz, G.A. Beyond the silence: Bilateral somatosensory stimulation enhances skilled movement quality and neural density in intact behaving rats. Behav. Brain Res. 2013, 253, 78–89. [Google Scholar] [CrossRef]
- Luczak, A. Measuring neuronal branching patterns using model-based approach. Front. Comput. Neurosci. 2010, 4, 135. [Google Scholar] [CrossRef]
- Ryait, H.; Bermudez-Contreras, E.; Harvey, M.; Faraji, J.; Mirza Agha, B.; Gomez-Palacio Schjetnan, A.; Gruber, A.; Doan, J.; Mohajerani, M.; Metz, G.A.S.; et al. Data-driven analyses of motor impairments in animal models of neurological disorders. PLoS Biol. 2019, 17, e3000516. [Google Scholar] [CrossRef]
- Torabi, R.; Jenkins, S.; Harker, A.; Whishaw, I.Q.; Gibb, R.; Luczak, A. A neural network reveals motoric effects of maternal preconception exposure to nicotine on rat pup behavior: A new approach for movement disorders diagnosis. Front. Neurosci. 2021, 15, 686767. [Google Scholar] [CrossRef]
- Tanabe, S.; Eckert, M.; Whishaw, I.; Tatsuno, M.; Luczak, A. Using Generative and Explainable Neural Networks to Investigate the Relationship Between Motor Cortex Activity and Animal Behavior During Motor Task Learning. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Racine, R.J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Kuerec, A.H.; Maier, A.B. Why is rapamycin not a rapalog? Gerontology 2023, 69, 657–659. [Google Scholar] [CrossRef]
- Drion, C.M.; Borm, L.E.; Kooijman, L.; Aronica, E.; Wadman, W.J.; Hartog, A.F.; van Vliet, E.A.; Gorter, J.A. Effects of rapamycin and curcumin treatment on the development of epilepsy after electrically induced status epilepticus in rats. Epilepsia 2016, 57, 688–697. [Google Scholar] [CrossRef]
- Das, R.; Howey, C.; McFetridge, A.; Lapointe, V.; Luczak, A. Associating sensory cues with incoming seizures: Developing an animal model of auras. Sci. Rep. 2024, 14, 20881. [Google Scholar] [CrossRef]
- Goddard, G.V.; McIntyre, D.C.; Leech, C.K. A permanent change in brain function resulting from daily electrical stimulation. Exp. Neurol. 1969, 25, 295–330. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howey, C.; McFetridge, A.; Luczak, A. Intermediate Stage of Epileptogenesis Is Not Affected by Disrupting Memory Consolidation Processes. Int. J. Mol. Sci. 2025, 26, 8364. https://doi.org/10.3390/ijms26178364
Howey C, McFetridge A, Luczak A. Intermediate Stage of Epileptogenesis Is Not Affected by Disrupting Memory Consolidation Processes. International Journal of Molecular Sciences. 2025; 26(17):8364. https://doi.org/10.3390/ijms26178364
Chicago/Turabian StyleHowey, Carlos, Autumn McFetridge, and Artur Luczak. 2025. "Intermediate Stage of Epileptogenesis Is Not Affected by Disrupting Memory Consolidation Processes" International Journal of Molecular Sciences 26, no. 17: 8364. https://doi.org/10.3390/ijms26178364
APA StyleHowey, C., McFetridge, A., & Luczak, A. (2025). Intermediate Stage of Epileptogenesis Is Not Affected by Disrupting Memory Consolidation Processes. International Journal of Molecular Sciences, 26(17), 8364. https://doi.org/10.3390/ijms26178364




