1. Introduction
Platycerium bifurcatum (Cav.) C. Chr. is a species of epiphytic fern that, due to its aesthetic value, is one of the most commonly cultivated ornamental fern species in the world [
1,
2]. Therefore, a detailed understanding of the mechanisms related to the reproduction of this species is particularly important due to the potential for optimizing production processes. This species originates from Australia and New Guinea [
3], where it is a valuable native flora species that is increasingly vulnerable to anthropopressure. At the same time,
P. bifurcatum can also be a potentially invasive species in urban ecosystems under subtropical climates [
4]. This feature is related to its ability to form colonies and its xeromorphic leaf structure, and consequently, relatively high resistance to abiotic stresses [
5]. Mature forms produce two morphologically and functionally differentiated types of leaves: sporotrophophylls and nest leaves, differentiated in morphological, anatomical, and functional terms [
6]. Sporotrophophyll has a dual function—the trophophilic part participates in photosynthesis, while the sporophilic part is responsible for reproduction through the production of spores. In turn, nest leaves in natural growth conditions (colonies) specialize in specific tasks. The top leaves are then responsible for the uptake of nutrients, while those in the lower sections specialize in water storage. Depending on the place of growth (trees, soil substrate), individuals of
P. bifurcatum may differ significantly in morphological and physiological terms. In the case of plants grown in the ground, not only an increase in the number of leaves was observed, but also more frequent sporulation than in natural epiphytic forms, which reproduce mainly vegetatively [
7].
Sporulation in ferns is a multi-stage process, including cell division, tissue differentiation, the formation of spore-protective structures, and their adaptation to long-term environmental resistance. Thin-spored ferns, which include P. bifurcatum, are one of the most morphologically and evolutionarily diverse groups of vascular plants. This diversity is also reflected in generative processes. Insight into the diversity of spore formation mechanisms and the processes that accompany this phenomenon enables a better understanding of adaptive strategies in various ecosystems.
At the cellular level, the process of sporulation involves a complex sequence of mitotic and meiotic divisions and structural changes, the course of which is regulated by both gene expression and hormonal signals [
8]. While most studies on hormones focus on seed plants, it is worth emphasizing the key role of phytohormones, including auxins, cytokinins, gibberellins, abscisic acid, and polyamines, in fern development. Gibberellins are involved in initiating spore germination and gametophyte development, especially under light conditions. For example, in
Dryopteris affinis, gibberellic acid (GA) enhances and supports morphogenetic reactions [
9]. Auxins, such as IAA (indole-3-acetic acid), play a key role in cell elongation and pattern formation in emerging gametophytes. Their exogenous administration may, depending on the concentration, support or inhibit the growth of the gametophyte [
10].
The initial stage of sporogenesis is the formation of sporangia in the sporophilic parts of sporotrophophyll leaves. Within the sporangium, archesporial cells differentiate, giving rise to sporogenous cells. In ferns, although the genetic sequences differ from those of seed plants, the mechanism of differentiation remains functionally similar [
11]. Sporogenous cells divide meiotically, leading to the formation of four haploid spores. A key aspect of sporogenous formation is the synthesis and deposition of a multilayered spore wall rich in biopolymers such as sporopollenin. This substance, partially synthesized in tapetum cells, provides spores with resistance to environmental factors such as UV radiation or dehydration [
12]. The final phase of sporulation is spore maturation, including their dehydration and the activation of protective mechanisms. This process depends on hormonal regulation, particularly high levels of abscisic acid (ABA) and other factors that protect against osmotic stress.
Mature spores of
P. bifurcatum are ellipsoidal and slightly asymmetrically flattened on one side. In the initial phase of development, they are characterized by a green color, which changes to light brown with maturation. The sporoderm consists of two layers: an outer exosporium and an inner endosporium [
13]. The surface of the spores exhibits papillary folds and spherical ornamental structures, which may play a role in their dispersal.
In
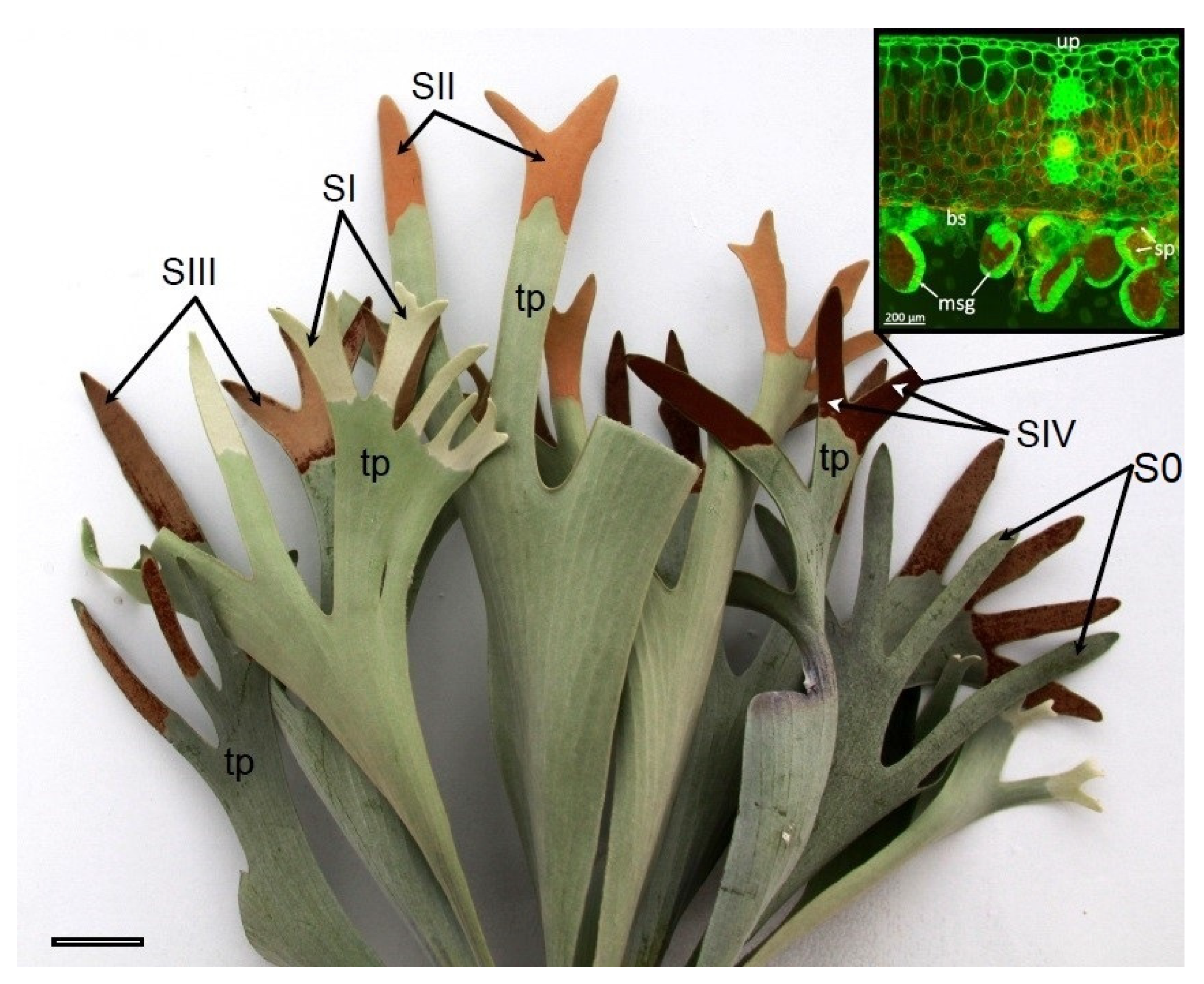
P. bifurcatum, sporangia are located in acrostichoids on the sporophilic parts of leaves, located at the dichotomously branched ends of the leaf blade. These structures have a single-layered cell wall and are located on the abaxial side of the leaves, near their axis [
14]. The specificity of the arrangement and spatial organization of sporangia makes this species a valuable experimental model in physiological studies. Due to the relatively large, clearly demarcated sporulating area, it is possible to independently measure metabolic parameters both in the zone of active sporulation and in non-sporulating leaf fragments. Such a configuration significantly facilitates the analysis of local physiological processes, which remains difficult in the case of most fern species, in which sporangia are concentrated in numerous, small sores with a small surface area.
Our previous studies on the photosynthetic process during spore formation have shown that the sporotrophophyll leaf is a heterogeneous structure, and the sporulation process in the lower sporophilic part leads to changes in energy absorption and transport in both the upper sporophilic and trophophilic parts of the leaf. Therefore, a deeper insight into the sporulation process of P. bifurcatum was conducted. The aim of this work was as follows: (i) assess anatomical changes occurring during the process of spore formation, especially in the sporulating parts; (ii) determine the metabolic activity of the different parts of the leaf during spore formation; (iii) analyze the spatial distribution of chemical compounds, including pigments, in P. bifurcatum leaves during sporogenesis; (iv) compare the content of selected phytohormones such as auxins, cytokinins, gibberellins, abscisic acid and jasmonates at individual stages of spore formation and in the trophophilic part of the leaf. For this purpose, in addition to imaging in a fluorescence microscope, isothermal microcalorimetry, Raman mapping, and ultrahigh performance liquid chromatography (UHPLC) coupled with a Triple Quad LC/MS equipped with an electrospray ionization (ESI) source were used.
3. Discussion
Four developmental stages can be distinguished in the sporulation process of
P. bifurcatum [
20]. This publication presents the morphological, anatomical, and physiological changes that occur from stage 0 (no sporulation features) to the release of spores in stage IV. The anatomical presence of a hadrocentric vascular bundle and extensive layers of sclerenchyma in the sporotrophophylls of
P. bifurcatum indicates conservative strategies of mechanical and vascular enhancement in different fern species [
21]. A typical feature of
P. bifurcatum is the presence of strongly developed trichomes at SI in the sporophilic parts located at the terminals of the sporotrophophylls, which protect the spores at the early stages of development. Such adaptations, although also found in other epiphytic ferns, are much rarer in terrestrial species [
22]. The dense covering of trichomes may play a role in the process of sporogenesis, protecting the tissue from water loss and excessive solar radiation and promoting microenvironmental stability around developing spores. These structures also have the effect of reducing transpiration and mechanically shielding the developing spores.
As we demonstrated earlier [
20], the onset of sporulation of
P. bifurcatum was associated with increased photosynthetic activity, especially in the sporophilic part of the leaves. The following stages of sporulation were accompanied by pigmentation changes in both the sporophilic and, to a lesser extent, the trophophilic parts of the leaf. Also, in other species such as
Adiantum pedatum or
Pteridium aquilinum, changes in the leaf pigment and thickening of the sporangial walls have been noted during sporulation [
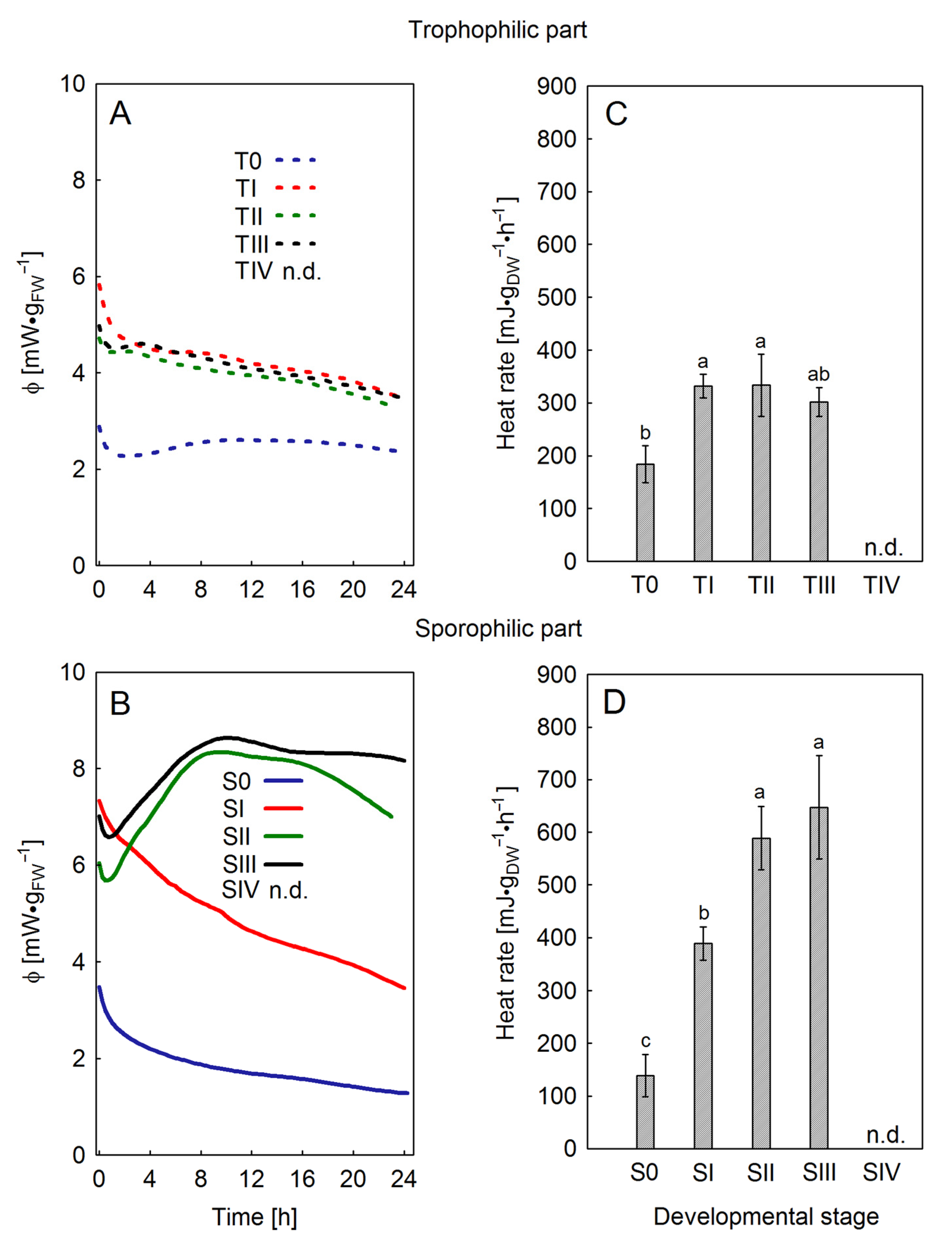
23]. Fluctuations in chlorophyll content were related to the photosynthetic activity of the leaf during sporogenesis. In the sporophilic part, an increase in Chl content was observed at the SII and SIII stages on the upper side of the leaf. At the same time, analysis of heat rate and power curves confirmed the increase in metabolic activity of the tissue. In the present study, the isothermal microcalorimetry method was employed for the first time to illustrate metabolic processes occurring during spore formation in ferns. The similarity of the curves and values of specific heat power in trophophilic and sporophilic parts of leaves at S0–SI stages suggests physiological homogeneity before the onset of sporogenesis. A significant increase in the value of specific heat power was recorded only in the sporophilic parts at stages SIII–SIV, which emphasizes the connection of increased metabolic activity with spore formation and maturation. Local thermogenesis may reflect increased energy requirements for processes such as sporoderm formation, lignification, or acquisition of dehydration resistance [
14].
In many fern species, the development of spores is localized in sori scattered on the underside of assimilatory leaves, which makes it challenging to study the sporulating and non-sporulating parts separately [
14,
24]. In contrast, in
P. bifurcatum, the spores are located in a large, compact area at the terminals of the sporotrophophylls, which allows them to be clearly distinguished by developmental stage and spatially separated from the rest of the leaf. These features allow for a more precise analysis of not only metabolic but also structural changes that occur during the formation, maturation, and release of spores.
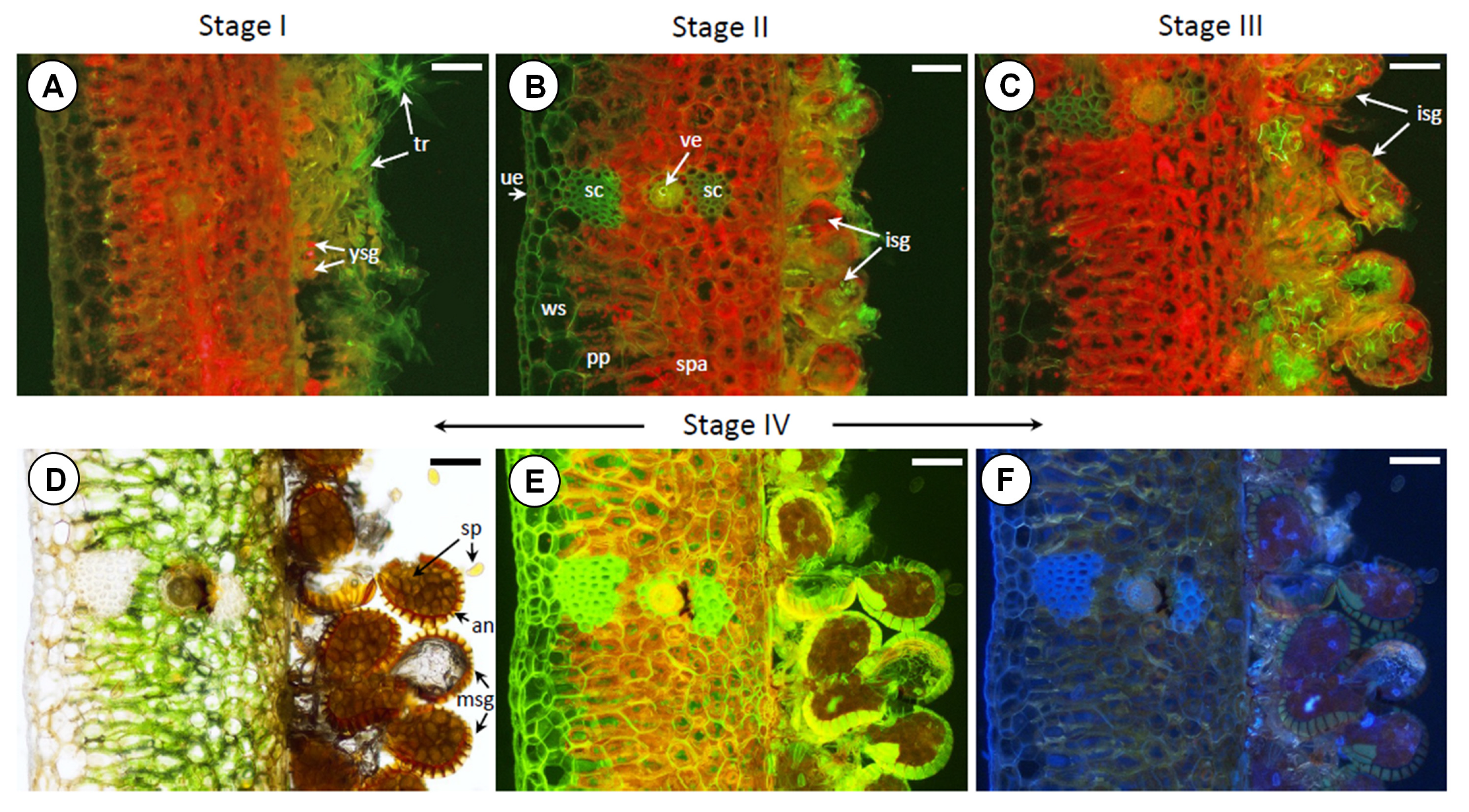
Anatomical cross-sections of selected leaf fragments (
Figure 2), combined with fluorescence imaging of the investigated plant tissues, provide strong corroborative evidence for the changes observed in the FT-Raman spectra (
Figure 6) and the Raman mapping results (
Figure 7). A comparative analysis of the fluorescence images (
Figure 2), which correspond to tissues sampled in stages SI, SII, SIII, and SIV, respectively, clearly confirmed dynamic alterations in the content, spatial distribution, and composition of pigments within the analyzed leaf tissues. A progressive shift in pigment content is apparent when comparing sections from stages SI to SIII (
Figure 2A–C). In stage SI, the emergence of a developing trichome layer is evident, while stage SII reveals the formation and early development of sporangia.
Figure 2C, corresponding to stage SIII, reflects compositional changes in the pigment profile, accompanied by an increase in Car accumulation. In contrast, stage SIV (
Figure 2D–F) exhibits only minimal fluorescence associated with Chl and Car, which can be attributed to tissue desiccation during the final stages of spore formation.
All these morphological and anatomical features, as well as changes in pigment composition and the process of photosynthesis in
P. bifurcatum, are under the control of endogenous phytohormones, whose levels differ in the sporophilic and trophophilic parts of a leaf during sporulation. The most influential phytohormones during sporulation and spore germination in ferns are gibberellins [
25,
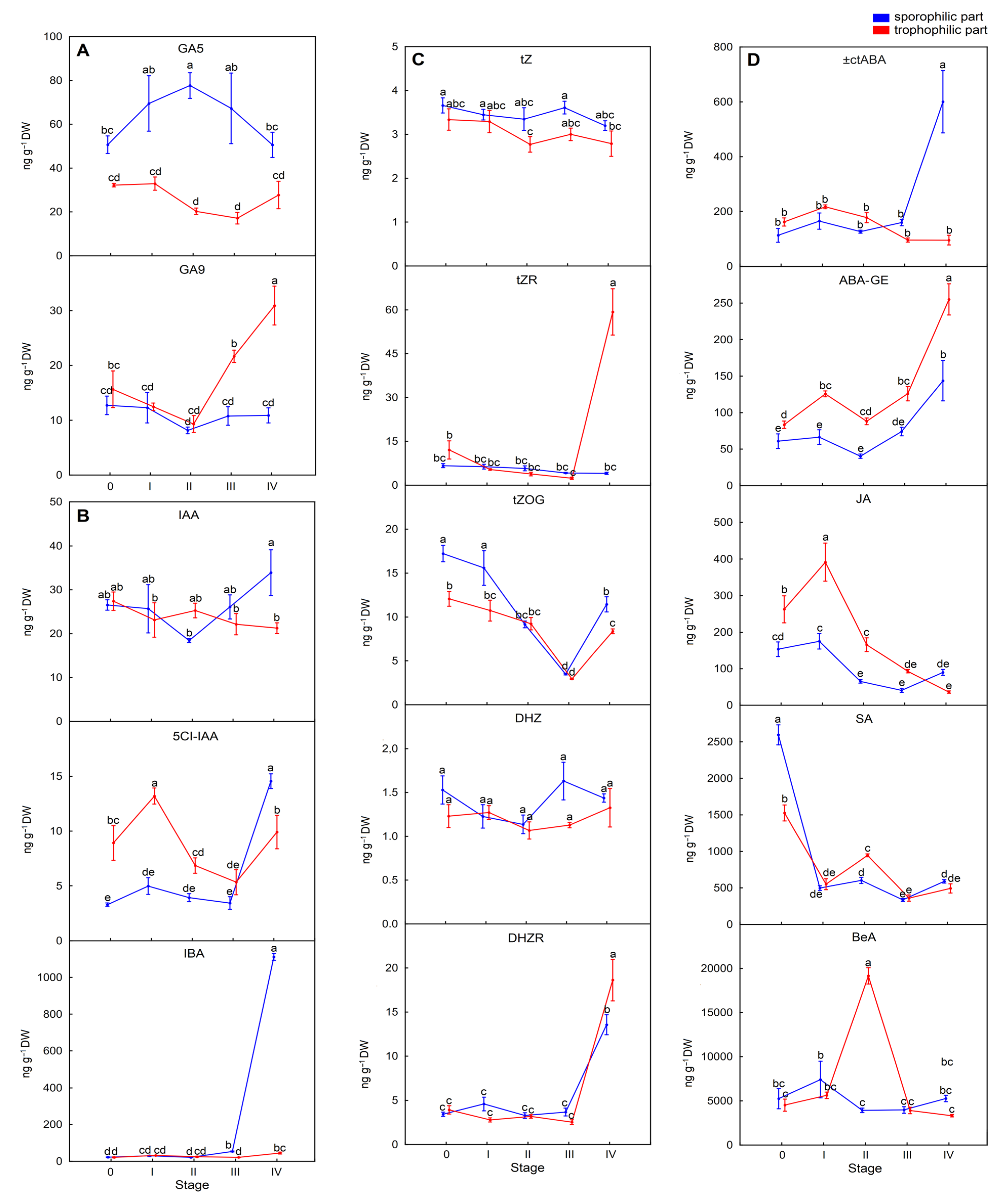
26]. In this manuscript, phytohormone profiling of sporotrophophylls confirmed that sporulation depends primarily on a gradual and progressive increase in total gibberellin content, resulting in an almost 80% increase at the SIV stage in the sporophilic part of a leaf. However, among the five gibberellins detected in the sporophilic part, only biologically active GAs GA3, GA4, and GA6 showed enhanced levels at the final mature spore stage (
Figure 4A,
Table S1). In sporophytes of the other epiphytic fern
Asplenium nidus, gibberellins GA3, GA4, and GA9 were also detected, with a particularly high level of GA4 [
27]. Moreover, the processes of spore maturation and release appear to rely also on increased levels of the auxin IBA, as well as ±ctABA and ABAGlc in
P. bifurcatum (
Figure 4B,D). Elevated levels of GA4 and ABA are consistent with previous research on phytohormone profiles in sporophytes of tree ferns such as
Cibotium glaucum and
Dicksonia antarctica [
28].
In contrast, in the trophophilic part of a leaf, responsible for extensive photosynthesis and nutrient supply for the sporophilic part, GA3 and GA6 were not detected in the case of most sporulation stages. However, GA
3 and increased GA9 levels were observed at the spore release stage (SIV) in this part of the leaf (
Table S1). Additionally, IBA and only ABAGlc levels significantly increased in the trophophilic part; however, their contents were not as elevated as in the sporophilic part (
Figure 4B,D). We speculated that ABAGlc, as it is a transported form of ABA, was transferred from the trophophilic part to the sporophilic part and hydrolyzed there to achieve a high level of ABA, supporting the process of spore dehydration and protecting them against osmotic stress [
29].
It has to be emphasized that although various types and forms of cytokinins were detected in both parts of sporotrophophylls, including tZ, cZ, DHZ, IP, and their ribosides, the total CK content significantly decreased below the control levels at all sporulation stages (
Figure 4C,
Table S1). This phenomenon suggests that cytokinins are not extensively involved in sporulation, particularly in spore maturation and release in
P. bifurcatum. The phytohormone profile of the fern-like vascular plant
Psilotum nudum (belonging to
Pteridophyta) revealed that from all detected cytokinins, only DHZR was increased at the final sporulation stage [
30], similar to sporotrophohylls of
P. bifurcatum. Moreover, an application of exogenous cytokinins retarded spore germination in
Dryopteris filix-mas [
31]. Reduced cytokinin contents, together with the elevated level of ABA, can be related to the initiation and progression of senescence and dying of the trophophilic part, which at the SIV stage is almost entirely dead, and to a lesser extent also of the sporophilic part of a leaf. Furthermore, ABA-dependent regulation may also be responsible for chlorophyll breakdown during the final stage of sporulation [
32]. All these catabolic processes were supported by a gradual, significant reduction of JA and SA levels observed in both parts of sporotrophophylls (
Figure 4D,
Table S1).
Raman spectroscopy offers a rapid, non-destructive, label-free, and often in-field applicable method for analyzing the biochemical composition of plants. Traditional analytical methods often require destructive sampling, solvent extraction, or chemical labeling, limiting temporal resolution and spatial precision. Raman spectroscopy bypasses these constraints, offering non-invasive molecular profiling of intact plant tissues. By capturing molecular fingerprints through vibrational spectra, it enables the real-time monitoring of physiological responses to environmental stressors, pigment composition, and protein profiles [
33,
34,
35].
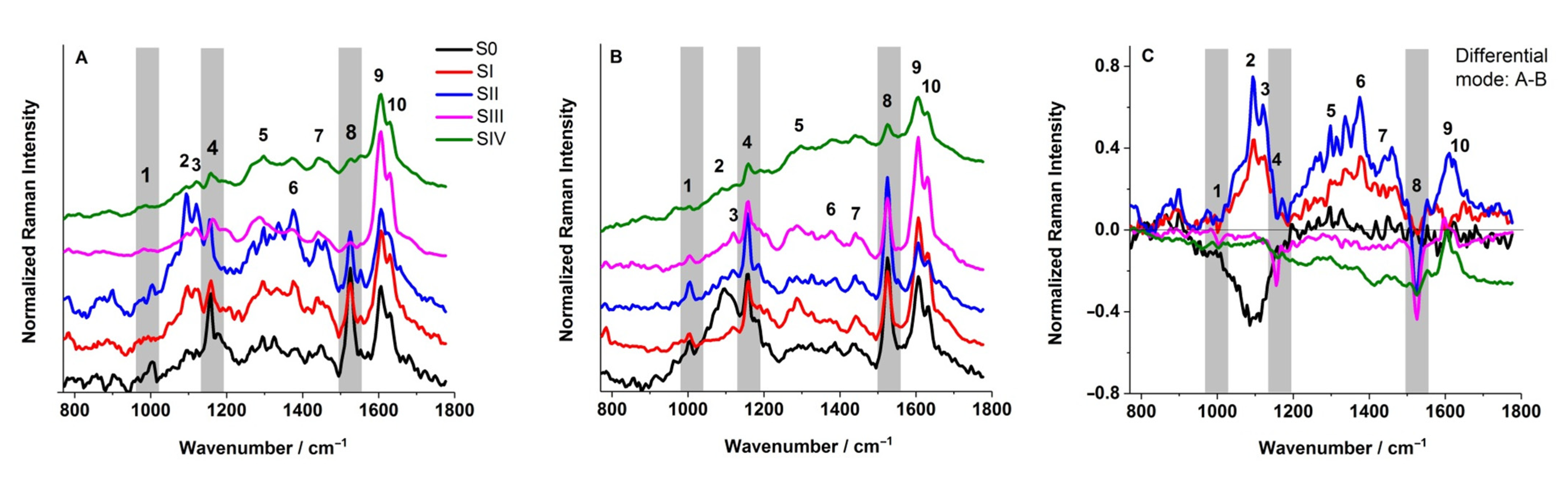
The FT-Raman spectra (
Figure 5), Raman imaging maps (
Figure 6), and fluorescence microscopy images (
Figure 2) collectively present a coherent dataset. The results obtained using these complementary techniques demonstrate internal consistency in most sporulation stages in
P. bifurcatum leaves. However, some discrepancies were observed between the FT-Raman spectra (collected from the abaxial leaf surface) and the corresponding 2D Raman images, particularly for developmental stage S0.
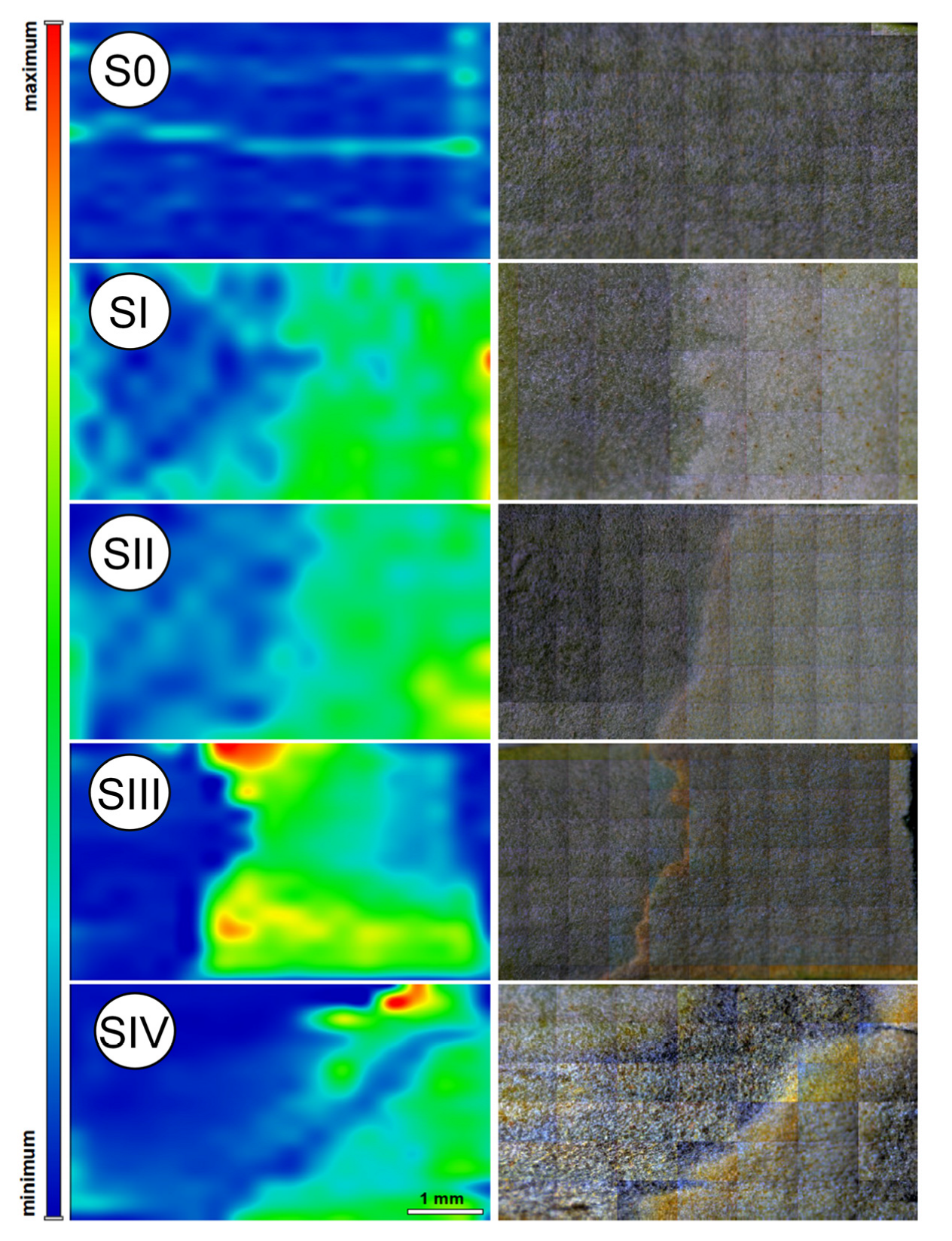
Figure 5A indicates a relatively high Car content not only in stages SI and SII, but also in the initial stage S0, as evidenced by the presence of characteristic peaks (bands 1, 4, and 8). In contrast, the 2D Raman image of the same stage (
Figure 6) reveals a uniformly low Car distribution across the mapped area, which is entirely represented in blue.
This discrepancy can be reasonably attributed to the fundamental differences in data acquisition methods. While the 2D Raman maps represent spatially resolved surface imaging, the FT-Raman spectra were acquired from discrete, localized points subjected to focused laser irradiation. It is therefore plausible that, in the stage S0, the point chosen for spectral acquisition coincided with a region exhibiting anomalously low Car concentrations. Another potential explanation involves sampling errors related to tissue type: in stage S0, the spectrum may have been inadvertently recorded from a trophophilic part, which typically contains higher levels of photosynthetic pigments, rather than the sporophyllous tissue. This is particularly likely given the absence of distinct morphological features that differentiate these tissue types at early sporulation stages. Despite these minor methodological discrepancies, all employed techniques consistently confirm that the content of photosynthetic pigments in the analyzed tissues is closely linked to the sporulation stage. This, in turn, reflects the progression of sporangium formation and spore maturation.
The decrease in Chl content in the trophophilic part (upper blade) in SIV is associated with a decrease in photosynthetic activity and chloroplast degradation, senescence, and drying of the leaf part directly adjacent to the sporophilic part. Similar trends associated with Chl loss were observed during sporangia maturation and leaf senescence in other fern species such as
Dryopteris affinis or
Pteridium aquilinum [
14,
36]. In the sporophilic part, an increase in Chl content (upper and lower side) was observed in stage II, which is a response to the increased demand for photosynthetic products during sporangia formation. Our studies confirm the presence of numerous, active chloroplasts in the perispore tissues (
Figure 2 and
Figure 7), which suggests their functional importance for spore formation in ferns. The greatest increase in Chl content in the sporophilic part occurred between SII and SIII in the upper part, while at the same time, a decrease in Chl was noted directly at the site of spore formation (bottom side). This may suggest a controlled degradation of Chl in the immediate vicinity of the sporangium and allocation of the degradation products to the upper part of the leaf, where increased Chl synthesis occurs. This is justified considering the changes occurring at this stage in the structure of the lower leaf surface (
Figure 1 and
Figure 2), associated with the development of dense tomentum, which hinders the absorption of light energy. Moreover, in seed plants, reproductive processes are often associated with changes in Chl metabolism and resource allocation, e.g., to generative organs [
37]. However, it should be emphasized that in ferns, sporophyll or sporotrophophyll leaves often retain photosynthetic functions for a relatively long time. In
P. bifurcatum, the complete loss of Chl associated with the aging of the sporophilic part and drying of the tissue took place only in the last stage of sporulation (SIV).
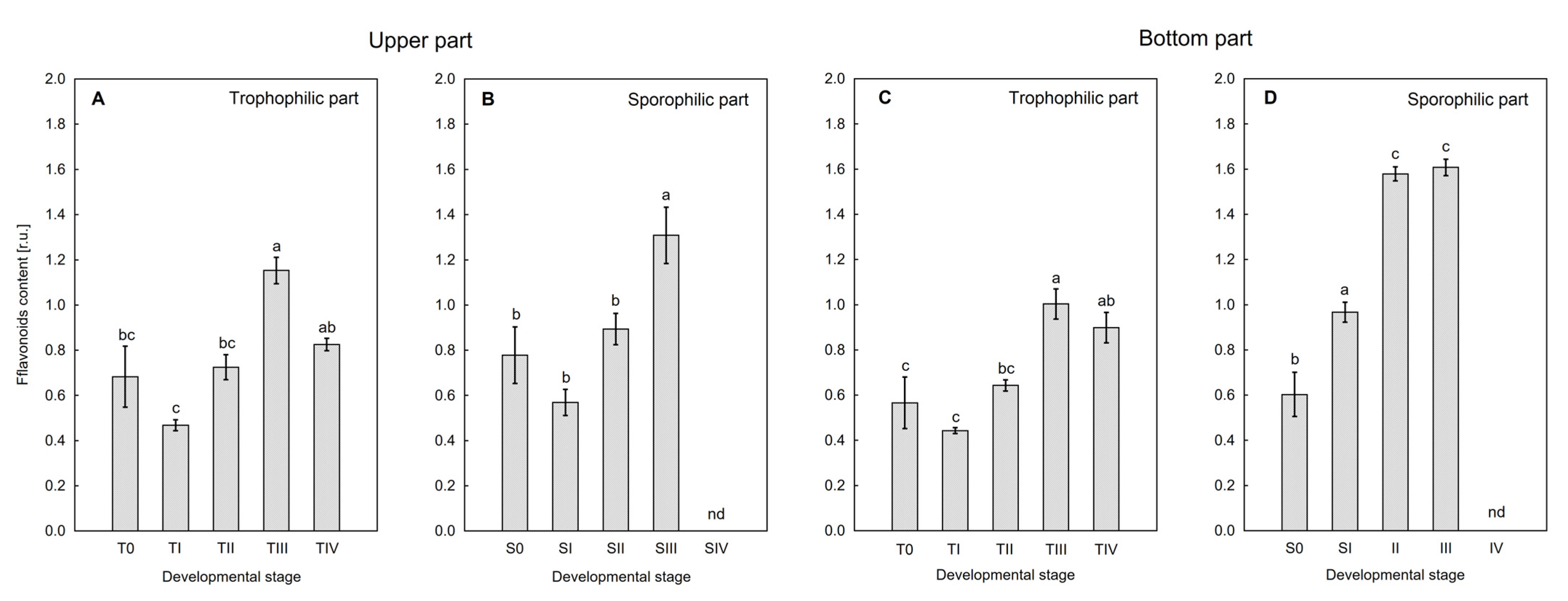
Figure 5 shows the dependence on the sporulation stage of flavonoid content in the tissue. The highest concentration of Flav was observed at the SII–SIII stage, on both the upper and lower surfaces of the sporotrophophyll, which indicates their participation in the process of spore formation and maturation. This is consistent with observations made on generative organs of seed plants, in which Flav content increases during meiosis [
38]. The increase in Flav content is likely related to a protective function for forming spores, which are exposed to UV-B and high temperature–stressors crucial for plant reproduction. Flavonols are also present in spore protoplasm, capture reactive oxygen species, thus contributing to increased resistance to abiotic stresses [
39]. The inability to determine Flav content at the SIV stage is due to the high degree of lignification of tissues, which prevents the detection of these compounds using nondestructive methods.
4. Materials and Methods
4.1. Plant Material
The study was conducted on 10-year-old sporophytes of
Platycerium bifurcatum (Cav.) C. Chr..). The plants came from the collection of the University of National Education Commission, Krakow, and were grown for 6 months before the analyses in a greenhouse climate chamber under a natural photoperiod and controlled temperature conditions (22 °C/16 °C—day/night) as well as relative humidity of 60% (±5%). All plants were watered with tap water to a constant mass every 7 days, and once a month with standard Steiner medium. Measurements were performed in the trophophilic (TI–TIV) and sporophilic part of the leaf in the four stages of sporulation (SI–SIV) described earlier by Skoczowski et al. [
20], in which the following were observed successively: juvenile sporangia during development (SI), maturing sporangia (SII, SIII), and mature sporangia releasing spores (SIV). Sporotrophophyll leaves without developed sporangia in the sporophilic part constituted the control–T0 and S0 (trophophilic and sporophilic part, respectively).
4.2. Anatomical Analysis
Cross sections were made manually in the sporophilic part of the leaf, approximately 2 cm from the apex. The anatomical analysis was performed in distilled water, representing cross-sections of the leaf lamina using a light microscope and a Nikon ECLIPSE Ni epifluorescence microscope (Nikon, Tokyo, Japan) equipped with Microscope Camera Digital Sight series DS-Fi1c and NIS Imaging, Nikon v. 4.11 software (Nikon, Tokyo, Japan). To induce autofluorescence, excitation in the UV and blue spectra was used.
4.3. Isothermal Calorimetry
Calorimetric measurements were performed on the leaves using an isothermal calorimetry (TAM III Thermometric 3101, TA Instruments, New Castle, DE, USA) at 20 °C, in the dark. The measurements were performed on 1 cm diameter discs cut from leaves at four development stages, both from the trophophilic and sporophilic parts. Due to the high degree of lignification of leaves at the SIV stage, calorimetric measurements were not performed there. The leaf discs were placed in measuring ampoules on paper discs moistened with distilled water. In the reference ampoule, water was contained in the same volume as that added to the experimental ampoules. Twenty-four-hour measurement of heat emission was carried out after 45 min of thermal stabilization. The results presented in the study are the mean of 8 independent biological replicates.
4.4. Analysis of Selected Plant Hormones
Selected phytohormones were analyzed following the method described by Płażek et al. [
40]. Freeze-dried leaf fragments separated from sporophilic and trophophilic parts were extracted using 1 cm
3 of a
P. bifurcatum extraction buffer (methanol/water/formic acid in a 15:4:1 volume ratio) after the addition of an internal standard solution. The extraction was performed for 5 min at 30 Hz (MM400, Retch, Haan, Germany). The samples were then centrifuged at 22,000×
g for 3 min (R32, Hettich, Tuttlingen, Germany), and the supernatant was collected. This extraction process was repeated two more times, and the resulting supernatants were combined, evaporated under nitrogen, and reconstituted in 5% methanol in 1 M formic acid. Cleanup was carried out using mixed-mode SPE cartridges (BondElutPlexa PCX, Agilent, Santa Clara, CA, USA), as outlined by Dziurka et al. [
41]. All results were expressed in femtomoles (fmol) or micromoles per gram of dry weight (µmol g
−1 DW).
Phytohormone analysis—including auxins, cytokinins, gibberellins, abscisic acid, and jasmonates—was performed using ultrahigh performance liquid chromatography (UHPLC) on an Agilent Infinity 1260 system coupled with a 6410 Triple Quad LC/MS equipped with an electrospray ionization (ESI) source (Agilent Technologies, Santa Clara, CA, USA). Separation was conducted on an Ascentis Express RP-Amide column (2.7 µm, 2.1 mm × 150 mm; Supelco, Bellefonte, PA, USA) with a linear gradient of water and acetonitrile containing 0.01% formic acid. The internal standards used were stable isotope-labeled versions of phytohormones, including: [15N4] dihydrozeatin, [15N4] kinetin, [2H2] gibberellin A4, A6, A5, [2H5] indole-3-acetic acid, and [2H6] cis,trans-abscisic acid (OlChemIm, Olomouc, Czech Republic).
Phytohormone profiling was performed using multiple reaction monitoring (MRM) and compared to pure and isotope-labeled standards of the target compounds. The identified hormone forms included: IAA, kinetin (KIN), zeatin (ZEA), active gibberellins (GA3, GA4, GA5, GA6, GA7), inactive gibberellins (GA8, GA9, GA20), active abscisic acid [(±)-cis,trans-ABA], inactive ABA glucosyl ester [(±)-cis,trans-ABA-glc], salicylic acid (SA), jasmonic acid (JA), and jasmonic acid methyl ester (JA-Met).
4.5. FT-Raman Spectroscopy Measurements
FT-Raman measurements were performed on both upper and bottom sides of sporophilic parts of leaves using a Nicolet NXR 9650 FT-Raman spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a Nd:YAG3+ laser emitting at 1064 nm and a germanium detector. All spectra were made at room temperature, at an aperture of 80 and a spectral resolution of 4 cm−1. They were collected in the range of 780–1800 cm−1, accumulated from 128 scans, and measured with the laser power of 0.4 W. Each presented spectrum was an average of 6–10 spectra. In order to extract the Raman signal from the registered results (containing the fluorescence background contribution originating from the intrinsic fluorescence of plant molecules), the baseline correction was performed. The spectra were normalized according to the most intense band, and the analysis was carried out using Omnic 8 and OriginPro 2017 software packages for Windows.
Two-dimensional Raman maps from the bottom parts of the tested leaves were obtained by moving the xy stage, where both x and y directions of the accessory were controlled by the software. The samples were irradiated with a focused laser beam of 100 mW and a diameter of about 0.1 mm. For all mapping measurements, the spectral resolution was constant and equal to 4 cm−1, and four scans were collected at each measured point. Raman mapping of leaves was performed at areas of 5.5 × 3.5 cm and with a step size of 100 µm. Bands related to -C=C- stretching vibration modes (1525 cm−1) were integrated and presented as 2-D maps colored according to Car content.
4.6. Chlorophyll and Flavonoid Content Measurement
The content of flavonoids and chlorophyll in the leaves was measured using a Dualex chlorophyllometer from FORCE-A, Orsay, France, at room temperature, according to the instructions for use issued by the manufacturer. Measurements were made on the leaves at four stages of development, on the upper and bottom sides of the leaf blade, in the sporophilic and trophophilic parts. The results presented in the study are the mean of 6 independent biological replicates.
4.7. Statistical Analysis
All results were analyzed using the Statistica 13 package (TIBCO Software, Palo Alto, CA, USA) using multivariate analysis of variance. Significance of differences was tested using Tukey’s test or Duncan’s test at a significance level of p ≤ 0.05.