Involvement of Ethylene in Adventitious Root Formation of Red-Stalked Rhubarb In Vitro
Abstract
1. Introduction
2. Results
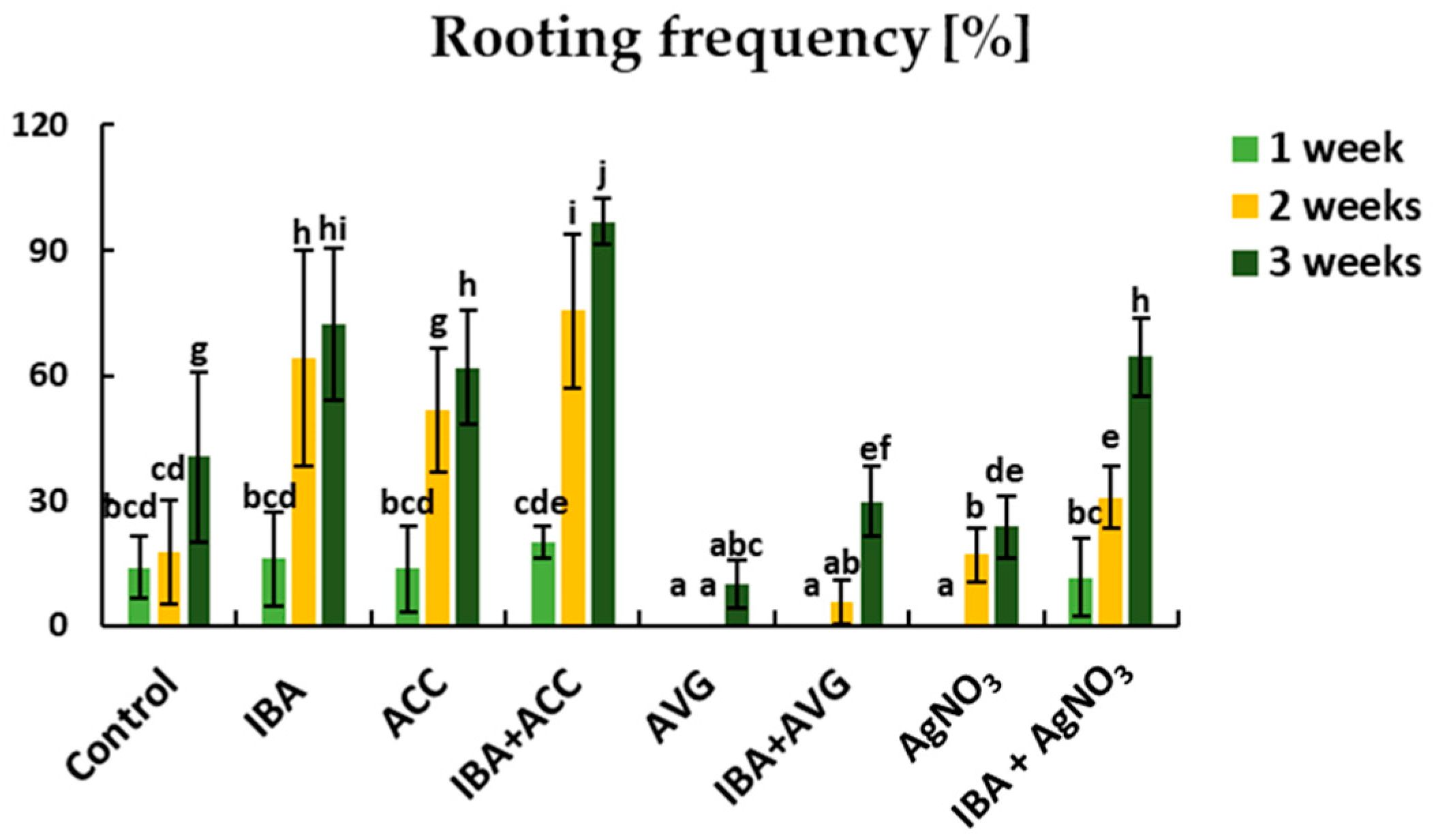
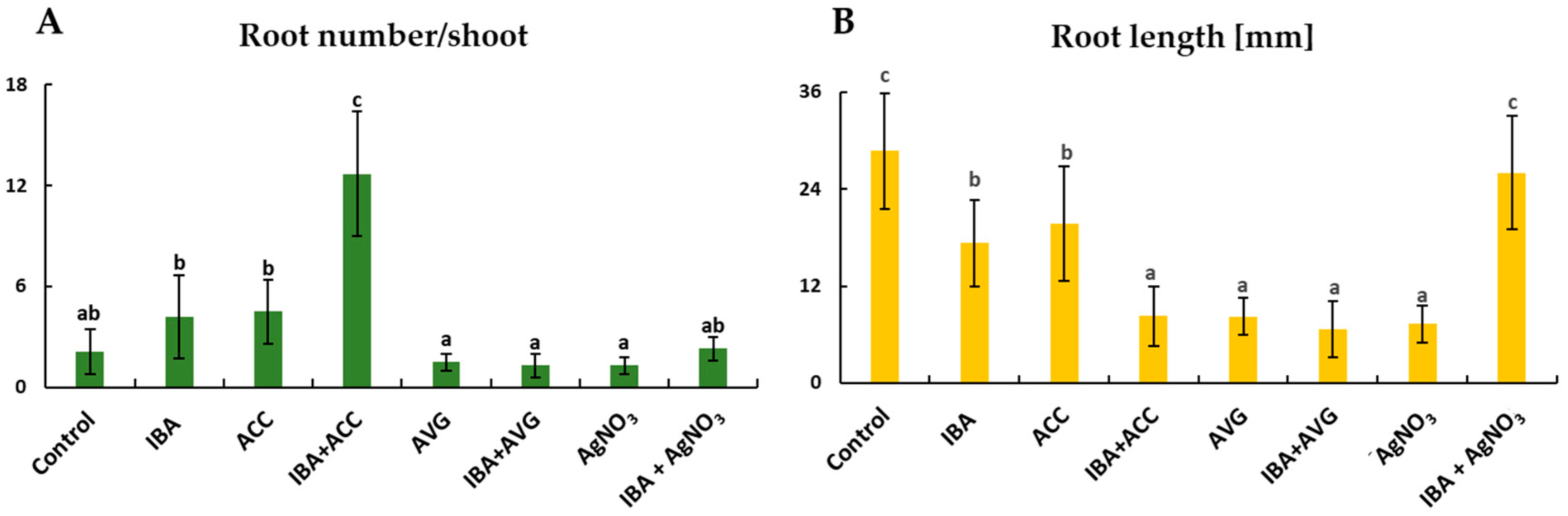
2.1. Adventitious Root Formation in Response to IBA and Ethylene
2.2. Changes in the Phytohormone Contents in Rhubarb Shoots
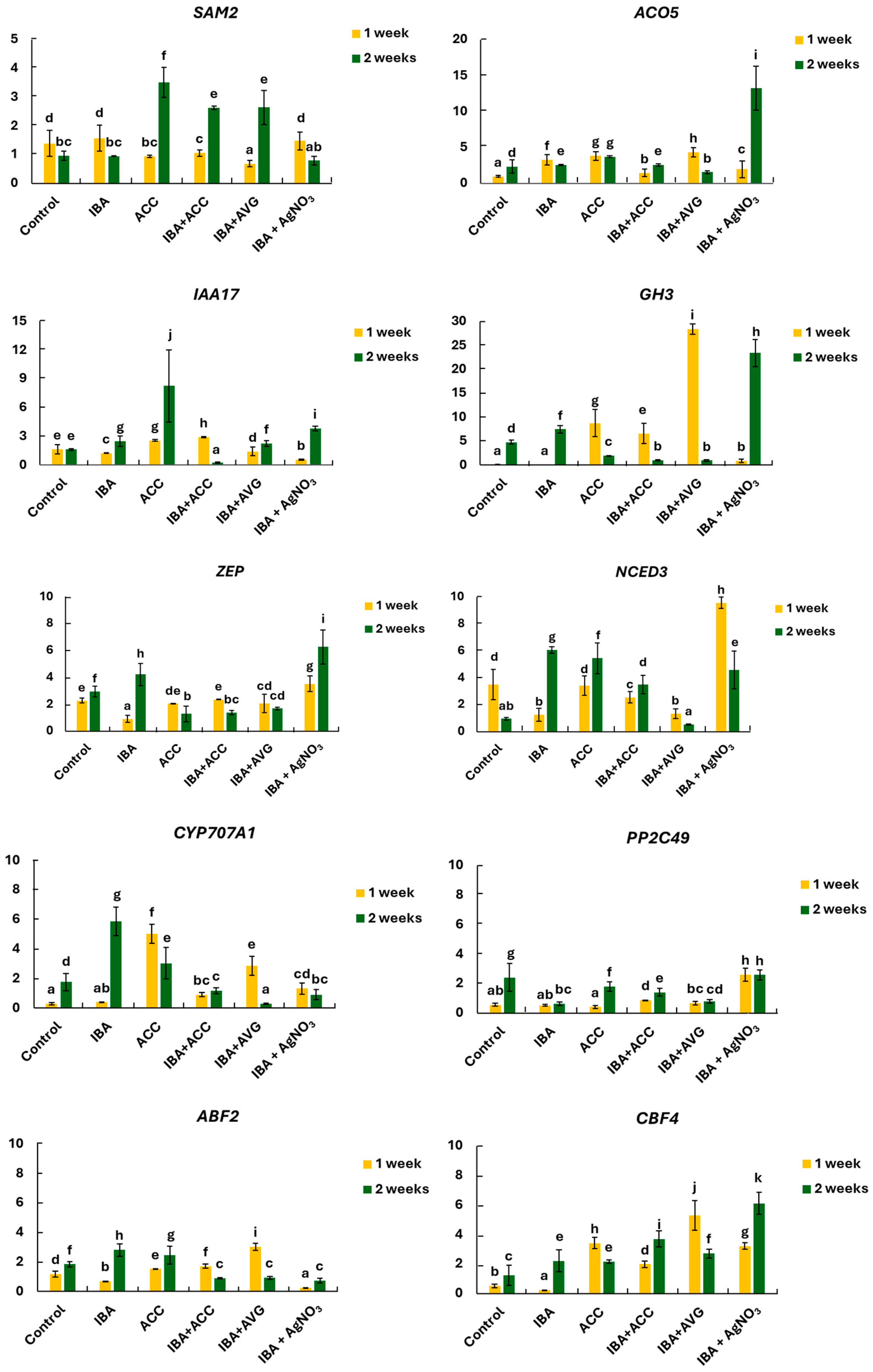
2.3. Changes in the Expression of Genes Related to Ethylene, Auxin, and ABA Metabolism
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Effect of Auxins and Ethylene on In Vitro Rooting
4.3. Quantification of Aux, ABA, and JA
4.4. Quantification of Cytokinins
4.5. Molecular Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalisz, S.; Oszmiański, J.; Kolniak-Ostek, J.; Grobelna, A.; Kieliszek, M.; Cendrowski, A. Effect of a variety of polyphenol compounds and antioxidant properties of rhubarb (Rheum rhabarbarum). Food Sci. Technol. 2020, 118, 108775. [Google Scholar] [CrossRef]
- Kołodziejczyk-Czepas, J.; Liudvytska, O. Rheum rhaponticum, and Rheum rhabarbarum: A review of phytochemistry, biological activities, and therapeutic potential. Phytochem. Rev. 2021, 20, 589–607. [Google Scholar] [CrossRef]
- Liudvytska, O.; Kolodziejczyk-Czepas, J. A Review on Rhubarb-Derived Substances as Modulators of Cardiovascular Risk Factors- A Special Emphasis on Anti-Obesity Action. Nutrients 2022, 14, 2053. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Korus, A.; Korus, J. Enhancing Nutritional Value of Rhubarb (Rheum rhaponticum L.) Products: The Role of Fruit and Vegetable Pomace. Agriculture 2024, 14, 1784. [Google Scholar] [CrossRef]
- Göksu, F.; Özlü, Z.; Bölek, S. Rhubarb powder: Potential uses as a functional bread. J. Food Sci. 2023, 89, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Bratsch, A.; Mainville, D. Specialty Crop Profile: Rhubarb; VirginiaTech: Blacksburg, VA, USA, 2009; Publication 438-110. [Google Scholar]
- Takeoka, G.R.; Dao, L.; Harden, L.; Pantoja, A.; Kuhl, J.C. Antioxidant activity, phenolic and anthocyanin contents of various rhubarb (Rheum spp.) varieties. Int. J. Food Sci. Technol. 2013, 48, 172–178. [Google Scholar] [CrossRef]
- Wojtania, A.; Waligórski, P. Optimizing the Micropropagation of Red-Stalked Rhubarb Selections: A Strategy for Mass Production of High-Quality Planting Material. Agronomy 2024, 15, 27. [Google Scholar] [CrossRef]
- Thomas, J. Virus Identification and Development of Long-Term Management Strategies for the Rhubarb Industry; Final Report Hal Project VG05053; Horticulture Australia Ltd.: Sydney, NSW, Australia, 2011; 93p. [Google Scholar]
- Wojtania, A.; Mieszczakowska-Frąc, M. In vitro propagation method for production of phenolic-rich planting material of culinary rhubarb ‘Malinowy’. Plants 2021, 10, 1768. [Google Scholar] [CrossRef]
- Guan, L.; Li, Y.; Huang, K.; Cheng, Z.M.M. Auxin regulation and MdPIN expression during adventitious root initiation in apple cuttings. Hortic. Res. 2020, 7, 143. [Google Scholar] [CrossRef]
- Geiss, G.; Gutierrez, L.; Bellini, C. Adventitious root formation: New insights and perspectives. Annu. Plant Rev. Root Dev. 2009, 37, 127–156. [Google Scholar]
- Li, S.-W. Molecular bases for the regulation of adventitious root generation in plants. Front. Plant Sci. 2021, 12, 614072. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, W.; Wan, S.; Fang, S. Insights into the hormone-regulating mechanism of adventitious root formation in softwood cuttings of Cyclocarya paliurus and optimization of the hormone-based formula for promoting rooting. Int. J. Mol. Sci. 2024, 25, 1343. [Google Scholar] [CrossRef]
- Pacurar, D.I.; Perrone, I.; Bellini, C. Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol. Plant. 2014, 151, 83–96. [Google Scholar] [CrossRef]
- Adem, M.; Sharma, L.; Shekhawat, G.S.; Šafranek, M.; Jásik, J. Auxin Signaling Transportation and Regulation during Adventitious Root Formation. Curr. Plant Biol. 2024, 40, 100385. [Google Scholar] [CrossRef]
- Wang, Q.; De Gernier, H.; Duan, X.; Xie, Y.; Geelen, D.; Hayashi, K.I.; Xuan, W.; Geisler, M.; Ten Tusscher, K.; Beeckman, T.; et al. GH3-mediated auxin inactivation attenuates multiple stages of lateral root development. New Phytol. 2023, 240, 1900–1912. [Google Scholar] [CrossRef] [PubMed]
- Wojtania, A.; Dziurka, M.; Skrzypek, E. In vitro rooting response of yellow-flowered magnolia in relation to the phenolic acids content. Agronomy 2020, 10, 1880. [Google Scholar] [CrossRef]
- Fu, Z.; Xu, M.; Wang, H.; Wang, E.; Li, Y.; Wang, L.; Gao, J.; Zhang, J.; Yuan, X.; Zhang, H. Analysis of the transcriptome and related physiological indicators of tree peony (Paeonia suffruticosa Andr.) plantlets before and after rooting in vitro. Plant Cell Tissue Organ Cult. 2021, 147, 529–543. [Google Scholar] [CrossRef]
- Qin, H.; He, L.; Huang, R. The coordination of ethylene and other hormones in primary root development. Front. Plant Sci. 2019, 10, 874. [Google Scholar] [CrossRef]
- Druege, U.; Hilo, A.; Pérez-Pérez, J.M.; Klopotek, Y.; Acosta, M.; Shahinnia, F.; Zerche, S.; Franken, P.; Hajirezaei, M. Molecular and physiological control of adventitious rooting in cuttings: Phytohormone action meets resource allocation. Ann. Bot. 2019, 123, 929–949. [Google Scholar] [CrossRef]
- Park, S.H.; Elhiti, M.; Wang, H.; Xu, A.; Brown, D.; Wang, A. Adventitious root formation of in vitro peach shoots is regulated by auxin and ethylene. Sci. Hortic. 2017, 226, 250–260. [Google Scholar] [CrossRef]
- Bai, T.; Dong, Z.; Zheng, X.; Song, S.; Jiao, J.; Wang, M.; Song, C. Auxin and its interaction with ethylene control adventitious root formation and development in apple rootstock. Front. Plant Sci. 2020, 11, 574881. [Google Scholar] [CrossRef]
- Druege, U.; Franken, P.; Lischewski, S.; Ahkami, A.H.; Zerche, S.; Hause, B.; Hajirezaei, M.R. Transcriptomic analysis reveals ethylene as stimulator and auxin as regulator of adventitious root formation in petunia cuttings. Front. Plant Sci. 2014, 5, 494. [Google Scholar] [CrossRef]
- Veloccia, A.; Fattorini, L.; Della Rovere, F.; Sofo, A.; D’Angeli, S.; Betti, C.; Falsca, G.; Altamura, M.M. Ethylene and auxin interaction in the control of adventitious rooting in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 6445–6458. [Google Scholar] [CrossRef]
- Castro-Camba, R.; Neves, M.; Correia, S.; Canhoto, J.; Vielba, J.M.; Sánchez, C. Ethylene action inhibition improves adventitious root induction in adult chestnut tissues. Plants 2024, 13, 738. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.; Akram, M.S.; Ali, S.; Sarker, P.K.; El-Sheikh, M.A. Deciphering morphological and biochemical modulations in mungbean by application of ethylene precursor and inhibitors under cadmium stress. Sci. Rep. 2025, 15, 15294. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.S.; Kieber, J.J. Eto Brute? Role of ACS turnover in regulating ethylene biosynthesis. Trends Plant Sci. 2005, 10, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Khan, M.I.R.; Thu, N.B.A.; Hoang, X.L.T.; Asgher, M.; Khan, N.A.; Tran, L.S.P. Role of ethylene and its cross talk with other signaling molecules in plant responses to heavy metal stress. Plant Physiol. 2015, 169, 73–84. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, D.; Meng, Y.; Li, K.; Wang, H.; Han, M. Inhibition of adventitious root development in apple rootstocks by cytokinin is based on its suppression of adventitious root primordia formation. Physiol. Plant 2019, 166, 663–676. [Google Scholar] [CrossRef]
- Guan, L.; Tayengwa, R.; Cheng, Z.; Peer, W.A.; Murphy, A.S.; Zhao, M. Auxin regulates adventitious root formation in tomato cuttings. BMC Plant Biol. 2019, 19, 435. [Google Scholar] [CrossRef]
- Villacorta-Martín, C.; Sánchez-García, A.B.; Villanova, J.; Cano, A.; van de Rhee, M.; de Haan, J.; Acosta, M.; Passarinho, P.; Pérez-Pérez, J.M. Gene expression profiling during adventitious root formation in carnation stem cuttings. BMC Genom. 2015, 16, 789. [Google Scholar] [CrossRef]
- Meng, F.; Xiang, D.; Zhu, J.; Li, Y.; Mao, C. Molecular mechanisms of root development in rice. Rice 2019, 12, 1. [Google Scholar] [CrossRef]
- Li, Y.Y.; Hao, Z.G.; Miao, S.; Zhang, X.; Li, J.Q.; Guo, S.X.; Lee, Y.I. Profiles of cytokinins metabolic genes and endogenous cytokinins dynamics during shoot multiplication in vitro of Phalaenopsis. Int. J. Mol. Sci. 2022, 23, 3755. [Google Scholar] [CrossRef] [PubMed]
- Montalbán, I.A.; Novák, O.; Rolčik, J.; Strnad, M.; Moncaleán, P. Endogenous cytokinin and auxin profiles during in vitro organogenesis from vegetative buds of Pinus radiata adult trees. Physiol. Plant 2013, 148, 214–231. [Google Scholar] [PubMed]
- Podwyszyńska, M.; Novák, O.; Doležal, K.; Strnad, M. Endogenous cytokinin dynamics in micropropagated tulips during bulb formation process influenced by TDZ and iP pretreatment. Plant Cell Tissue Organ Cult. 2014, 119, 331–346. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Węgrzynowicz-Lesiak, E.; Doležal, K.; Krekule, J.; Strnad, M.; Saniewski, M. New cytokinins-meta-methoxytopolins in micropropagation of Cotinus coggygria Scop. ‘Royal Purple’. Propag. Ornam. Plants 2012, 12, 220–228. [Google Scholar]
- Seliem, M.K.; Abdalla, N.; El-Mahrouk, M.E. Cytokinin potentials on in vitro shoot proliferation and subsequent rooting of Agave sisalana Perr. Syn. Horticulturae 2025, 11, 929. [Google Scholar] [CrossRef]
- Werbrouck, S.P.; Strnad, M.; Van Onckelen, H.A.; Debergh, P.C. Meta-topolin, an alternative to benzyladenine in tissue culture? Physiol. Plant 1996, 98, 291–297. [Google Scholar] [CrossRef]
- Chen, H.; Lei, Y.; Sun, J.; Ma, M.; Deng, P.; Quan, J.E.; Bi, H. Effects of different growth hormones on rooting and endogenous hormone content of two Morus alba L. cuttings. Horticulturae 2023, 9, 552. [Google Scholar] [CrossRef]
- Pál, M.; Janda, T.; Szalai, G. Interactions between plant hormones and thiol-related heavy metal chelators. Plant Growth Regul. 2018, 85, 173–185. [Google Scholar] [CrossRef]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between plant hormones and heavy metals responses. Genet. Mol. Biol. 2017, 40 (Suppl. S1), 373–386. [Google Scholar] [CrossRef]
- De Klerk, G.J.; Van Der Krieken, W.; de Jong, J.C. Review the formation of adventitious roots: New concepts, new possibilities. Vitr. Cell. Dev. Biol.-Plant 1999, 35, 189–199. [Google Scholar] [CrossRef]
- Damodaran, S.; Strader, L.C. Indole 3-butyric acid metabolism and transport in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 851. [Google Scholar] [CrossRef]
- Frick, E.M.; Strader, L.C. Roles for IBA-derived auxin in plant development. J. Exp. Bot. 2018, 69, 169–177. [Google Scholar] [CrossRef]
- Osterc, G.; Štampar, F. Differences in endo/exogenous auxin profile in cuttings of different physiological ages. J. Plant Physiol. 2011, 168, 2088–2092. [Google Scholar] [CrossRef]
- Staswick, P.E.; Serban, B.; Rowe, M.; Tiryaki, I.; Maldonado, M.T.; Maldonado, M.C.; Suza, W. Characterization of an Arabidopsis Enzyme Family That Conjugates Amino Acids to Indole-3-Acetic Acid. Plant Cell 2005, 17, 616–627. [Google Scholar] [CrossRef]
- Welander, M.; Geier, T.; Smolka, A.; Ahlman, A.; Fan, J.; Zhu, L.H. Origin, timing, and gene expression profile of adventitious rooting in Arabidopsis hypocotyls and stems. Am. J. Bot. 2014, 101, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Mellor, N.; Band, L.R.; Pěnčík, A.; Novák, O.; Rashed, A.; Holman, T.; Wilson, M.H.; Voβa, U.; Bishopp, A.; Kinga, J.R.; et al. Dynamic regulation of auxin oxidase and conjugating enzymes AtDAO1 and GH3 modulates auxin homeostasis. Proc. Natl. Acad. Sci. USA 2016, 113, 11022–11027. [Google Scholar] [CrossRef] [PubMed]
- Sherp, A.M.; Westfall, C.S.; Alvarez, S.; Jez, J.M. Arabidopsis thaliana GH3. 15-Acyl acid amido synthetase has a highly specific substrate preference for the auxin precursor indole-3-butyric acid. J. Biol. Chem. 2018, 293, 4277–4288. [Google Scholar] [CrossRef]
- Gutierrez, L.; Mongelard, G.; Floková, K.; Păcurar, D.I.; Novák, O.; Staswick, P.; Kowalczyk, M.; Pacurar, M.; Demailly, H.; Geiss, G.; et al. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 2012, 24, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Hosseini, S.A.; Hajirezaei, M.R.; Druege, U.; Geelen, D. Adventitious rooting declines with the vegetative to reproductive switch and involves a changed auxin homeostasis. J. Exp. Bot. 2015, 66, 1437–1452. [Google Scholar] [CrossRef]
- Fonseca, S.; Chico, J.M.; Solano, R. The jasmonate pathway: The ligand, the receptor and the core signalling module. Curr. Opin. Plant Biol. 2009, 12, 539–547. [Google Scholar] [CrossRef]
- Delfin, J.C.; Kanno, Y.; Seo, M.; Kitaoka, N.; Matsuura, H.; Tohge, T.; Shimizu, T. AtGH3.10 is another jasmonic acid-amido synthetase in Arabidopsis thaliana. Plant J. 2022, 110, 1082–1096. [Google Scholar] [CrossRef] [PubMed]
- Bouza, L.; Jacques, M.; Sotta, B.; Miginiac, E. The reactivation of tree peony (Paeonia suffruticosa Andr.) vitroplants by chilling is correlated with modifications of abscisic acid, auxin, and cytokinin levels. Plant Sci. 1994, 97, 153–160. [Google Scholar] [CrossRef]
- Wojtania, A.; Markiewicz, M.; Waligórski, P. Regulation of the bud dormancy development and release in micropropagated rhubarb ‘Malinowy’. Int. J. Mol. Sci. 2022, 23, 1480. [Google Scholar] [CrossRef]
- Bouza, L.; Sotta, B.; Bonnet, M.; Jacques, M.; Arnaud, Y. Hormone content and meristematic activity of Paeonia suffruticosa Andr. cv. “Madame de Vatry” vitroplants during in vitro rooting. Int. Symp. Sel. Breed. Woody Ornam. 1992, 320, 213–216. [Google Scholar] [CrossRef]
- Müller, M. Foes or friends: ABA and ethylene interaction under abiotic stress. Plants 2021, 10, 448. [Google Scholar] [CrossRef]
- Steffens, B.; Wang, J.; Sauter, M. Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 2006, 223, 604–612. [Google Scholar] [CrossRef]
- Yin, C.C.; Ma, B.; Collinge, D.P.; Pogson, B.J.; He, S.J.; Xiong, Q.; Duan, K.X.; Chen, H.; Yang, C.; Lu, X.; et al. Ethylene responses in rice roots and coleoptiles are differentially regulated by a carotenoid isomerase-mediated abscisic acid pathway. Plant Cell 2015, 27, 1061–1081. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Dobrev, P.I.; Kamınek, M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J. Chromatogr. A 2002, 950, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Stefancic, M.; Stampar, F.; Veberic, R.; Osterc, G. The levels of IAA, IAAsp and some phenolics in cherry rootstock ‘GiSelA 5′ leafy cuttings pretreated with IAA and IBA. Sci. Hortic. 2007, 112, 399–405. [Google Scholar] [CrossRef]
- Żur, I.; Dubas, E.; Krzewska, M.; Waligórski, P.; Dziurka, M.; Janowiak, F. Hormonal requirements for effective induction of microspore embryogenesis in triticale (× Triticosecale Wittm.) anther cultures. Plant Cell Rep. 2015, 34, 47–62. [Google Scholar] [CrossRef]
- Chang, S.; Puryear, J.; Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Mala, D.; Awasthi, S.; Sharma, N.K.; Swarnkar, M.K.; Shankar, R.; Kumar, S. Comparative transcriptome analysis of Rheum australe, an endangered medicinal herb, growing in its natural habitat and those grown in controlled growth chambers. Sci. Rep. 2021, 11, 3702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, X.; Shi, X.; Shao, L.; Xu, T.; Xia, Y.; Li, D.; Zhang, J. Chilling requirement validation and physiological and molecular responses of the bud endodormancy release in Paeonia lactiflora ‘Meiju’. Int. J. Mol. Sci. 2021, 22, 8382. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.S.; Singh, M.; Aggrawal, P.; Laxmi, A. Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS ONE 2009, 4, e4502. [Google Scholar] [CrossRef]
- Iwamoto, M.; Baba-Kasai, A.; Kiyota, S.; Hara, N.; Takano, M. ACO1, a gene for aminocyclopropane-1-carboxylate oxidase: Effects on internode elongation at the heading stage in rice. Plant Cell Environ. 2010, 33, 805–815. [Google Scholar] [CrossRef] [PubMed]


| Cytokinin (ng/g DM) | Time | Treatments | |||||
|---|---|---|---|---|---|---|---|
| Control | IBA | ACC | IBA + ACC | IBA + AVG | IBA + AgNO3 | ||
| free form * | |||||||
| trans-zeatin (tZ) | A | 1.4 ± 0.5 cd | 0.7 ± 0.2 ab | 2.4 ± 0.5 e | 0.6 ± 0.2 a | 0.4 ± 0.03 a | 0.5 ± 0.1 a |
| B | 0.7 ± 0.1 ab | 1.3 ± 0.2 bc | 2.0 ± 0.4 de | 0.5 ± 0.1 a | 2.0 ± 0.2 de | 5.0 ± 0.9 f | |
| izopentyladenine (IPA) | A | 3.7 ± 0.8 bc | 2.4 ± 1.1 ab | 4.1 ± 0.5 c | 3.7 ± 0.7 bc | 1.9 ± 0.5 a | 2.1 ± 0.4 a |
| B | 7.8 ± 2.1 de | 3.8 ± 0.8 bc | 2.4 ± 0.5 ab | 1.1 ± 0.3 a | 8.9 ± 0.4 e | 6.8 ± 0.9 d | |
| dihydroxyzeatin (DZ) | A | 0.2 ± 0.03 bc | 0.1 ± 0.03 a | 0.1 ± 0.03 a | 0.04 ± 0.03 a | 0.05 ± 0.00 a | 0.1 ± 0.02 a |
| B | 0.1 ± 0.02 a | 0.2 ± 0.1 b | 0.3 ± 0.07 c | 0.1 ± 0.01 a | 0.2 ± 0.05 bc | 0.1 ± 0.00 ab | |
| cis-zeatin (cZ) | A | 1.1 ± 0.4 ab | 1.2 ± 0.2 ab | 1.2 ± 0.3 ab | 0.7 ± 0.1 a | 1.4 ± 0.6 a–c | 1.4 ± 0.3 a–c |
| B | 1.3 ± 0.4 ab | 1.6 ± 0.4 ab | 1.0 ± 0.3 ab | 1.0 ± 0.1 ab | 1.0 ± 0.2 ab | 1.9 ± 0.2 c | |
| orto-Topolin (oT) | A | 0.4 ± 0.1 b | 1.0 ± 0.5 c | 0.3 ± 0.06 ab | 0.2 ± 0.06 ab | 0.1 ± 0.02 ab | 0.3 ± 0.09 ab |
| B | 0.4 ± 0.1 b | 0.2 ± 0.01 ab | 0.1 ± 0.03 ab | 0.02 ± 0.0 a | 0.02 ± 0.0 a | 0.1 ± 0.02 ab | |
| conjugated form * | |||||||
| trans-zeatin riboside (tZR) | A | 0.8 ± 0.2 a–c | 0.2 ± 0.03 a | 1.2 ± 0.4 cd | 0.3 ± 0.1 a | 0.4 ± 0.2 ab | 0.2 ± 0.05 a |
| B | 0.3 ± 0.2 ab | 1.6 ± 0.4 d | 3.9 ± 1.0 e | 1.0 ± 0.3 bd | 0.4 ± 0.1 ab | 0.3 ± 0.04 ab | |
| dihydroxyzeatin riboside (DZR) | A | 0.3 ± 0.1 a–c | 0.1 ± 0.05 a | 0.3 ±0.1 a–c | 0.2 ± 0.1 ac | 0.1 ± 0.0 a | 0.1 ± 0.00 a |
| B | 0.2 ± 0.04 ac | 0.4 ± 0.1 c | 1.0 ± 0.3 d | 0.4 ± 0.1 c | 0.1 ± 0.01 ab | 0.2 ± 0.03 a–c | |
| cis-zeatin riboside (cZR) | A | 5.5 ± 0.8 bc | 6.0 ± 1.6 bc | 3.2 ± 0.5 a | 6.2 ± 1.9 c | 3.9 ± 0.2 ab | 3.9 ± 0.3 ab |
| B | 4.6 ± 1.4 a–c | 4.4 ± 0.9 a–c | 3.0 ± 0.3 a | 5.8 ± 2.0 bc | 3.1 ± 0.3 a | 2.4 ± 0.5 a | |
| Total endogenous cytokinin | A | 13.4 ± 1.9 cd | 11.7 ± 2.0 bc | 12.6 ± 1.5 bcd | 11.7 ± 2.3 bc | 8.3 ± 1.4 a | 8.4 ± 3.0 a |
| B | 15.3 ± 2.8 cde | 13.1 ± 1.6 bcd | 13.7 ± 1.4 cde | 9.8 ± 1.9 ab | 15.7 ± 3.0 de | 16.9 ± 5.7 e | |
| meta-Topolin (mT) | A | 438 ± 85.2 f | 205 ± 17 de | 240 ± 65 e | 116 ± 19.9 ab | 227 ± 29.7 e | 81.9 ± 1.6 a |
| B | 129 ± 20 a–c | 159 ± 20 b–d | 112 ± 10 ab | 91 ± 18.2 a | 141 ± 19 a–d | 187 ± 3 b–e | |
| Total | A | 451.2 ± 88 g | 216.9 ± 21 ef | 252.8 ± 68 f | 128.0 ± 23 a–c | 235.6 ± 31 f | 90.4 ± 3.0 a |
| B | 143.9 ± 26 a–d | 171.6 ± 22 c–e | 126.0 ± 13 a–c | 100.4 ± 21 ab | 158 ± 21 b–e | 204 ± 5.7 d–f | |
| Gene | Sequence |
|---|---|
| ACO5 (according to Iwamoto et al. 2010 [69]) | 5′-CCGAAGGAGCTTCTTGATCGG-3′ |
| 5′-ATTTTGGCGCCTTGACGGCC-3′ | |
| SAM2 (according to Mala et al. 2021 [66]) | 5′-CATGCCCCTTAGCCACGTT-3′ |
| 5′-GGTCTTGCCATCAGGCCTTA-3′ | |
| IAA17 (according to Mishra et al. 2009 [68]) | 5′-CAAATCCAGATCAAAACACAGACAA-3′ |
| 5′-GGTGTTAATTGCTCTTTTTTTTCTTACG-3′ | |
| GH3 (according to Mishra et al. 2009 [68]) | 5′-CCCACAGTGAAAAAAAACGAGTAA-3′ |
| 5′-CTTGCTGGTGCTTTAGTTTTTCTTC-3′ | |
| ZEP (according to Mala et al. 2021 [66]) | 5′-GGCACAAGGGATCACGAACT-3′ |
| 5′-CCTTGGAGGAGAATCGAATGG-3′ | |
| NCED3 (according to Zhang et al. 2021 [67]) | 5′-TCGAAGCAGGGATGGTCAAC-3′ |
| 5′-CCTGAGACTTTAGGCCACGG-3′ | |
| CYP707A1 (according to Zhang et al. 2021 [67]) | 5′-CACTGAAGAGCAAGAGGCTATA-3′ |
| 5′-TTCTTGGTATCTGCCCAACTC-3′ | |
| PP2C49 (according to Mala et al. 2021 [66]) | 5′-GATCGACGACCTATCCATGCA-3′ |
| 5′-GGTCCTCCATGGCCATCA-3′ | |
| ABF2 (according to Zhang et al. 2021 [67]) | 5′-TCGTTGACTCTGCCTCGAAC-3′ |
| 5′-CCTGAGCCACCTGAGACAAG-3′ | |
| CBF4 (according to Zhang et al. 2021 [67]) | 5′-GATGATGAGGCGCTTTTGGG-3′ |
| 5′-TCACCCACTCCGTCAAAGTC-3′ | |
| GAPDH (according to Mala et al. 2021 [66]) | 5′-CTCAATGACGGCCACACAGA-3′ |
| 5′-ACCAGTGCTGCTGGGAATG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtania, A.; Waligórski, P.; Markiewicz, M. Involvement of Ethylene in Adventitious Root Formation of Red-Stalked Rhubarb In Vitro. Int. J. Mol. Sci. 2025, 26, 9429. https://doi.org/10.3390/ijms26199429
Wojtania A, Waligórski P, Markiewicz M. Involvement of Ethylene in Adventitious Root Formation of Red-Stalked Rhubarb In Vitro. International Journal of Molecular Sciences. 2025; 26(19):9429. https://doi.org/10.3390/ijms26199429
Chicago/Turabian StyleWojtania, Agnieszka, Piotr Waligórski, and Monika Markiewicz. 2025. "Involvement of Ethylene in Adventitious Root Formation of Red-Stalked Rhubarb In Vitro" International Journal of Molecular Sciences 26, no. 19: 9429. https://doi.org/10.3390/ijms26199429
APA StyleWojtania, A., Waligórski, P., & Markiewicz, M. (2025). Involvement of Ethylene in Adventitious Root Formation of Red-Stalked Rhubarb In Vitro. International Journal of Molecular Sciences, 26(19), 9429. https://doi.org/10.3390/ijms26199429





