Abstract
Down syndrome (DS) is the most common survivable chromosome trisomy, with an incidence of about 1 in 600–700 births. Consequences of chromosome 21 trisomy include developmental delays, congenital cardiac abnormalities, skeletal abnormalities, and age-related dementia of the Alzheimer’s disease (AD) type. Up to 90% of individuals with DS develop dementia symptoms in their 40s or 50s. Because the biological mechanisms involved in DS-related developmental and age-related pathology are less known, animal models consisting of both lower-order and higher-order animals have been developed. We here review the most pertinent and well-studied DS animal models including models developed in C. elegans, Drosophila, zebrafish, and mice. Molecular pathways involved in DS morbidity that were discovered in animal models will also be discussed.
1. Introduction
Down syndrome (DS) is the most common survivable trisomy, with a complete or partial triplication of human chromosome 21 (Hsa21) [,]. This condition leads to developmental delays and intellectual disabilities as well as age-related Alzheimer’s disease (AD). Individuals with DS have a range of co-morbidities that can shorten lifespan, including thyroid dysfunction, congenital heart malformations, and sometimes attention deficit/hyperactivity disorder (ADHD) [,] or autism spectrum disorder []. While between 80 and 90% of individuals with DS develop dementia symptoms [], 28% to 54% have thyroid dysfunctions, with hypothyroidism the most common, and around 50% have congenital heart conditions, mostly ventricular or atrial septal defects []. In terms of autism spectrum disorders and ADHD, it is estimated that 30–40% of those with DS have these co-morbidities []. The most common causes of death in individuals with DS are AD, heart disease, and respiratory infections including pneumonia []. Hsa21 contains more than 500 genes, and the triplication of this chromosome leads to complex genetic dysfunctions with significant pathological consequences. It is therefore beneficial to use several animal models to fully explore the multiple consequences of aneuploidy of Hsa21. For example, invertebrates have a simple genomic makeup, which is easy to manipulate, so they are suitable for examining specific gene effects during development or aging. Mice, on the other hand, have a more complex genome but are more suitable for behavioral studies including learning and memory.
Hsa21 is the smallest chromosome and was fully sequenced by an international team of scientists []. This was the second human chromosome to be fully sequenced, following chromosome 22. The short arm of the chromosome, Hsa21p, encodes largely repetitive DNA and does therefore not contribute significantly to the DS phenotype. The long arm, Hsa21q, contains about 500 functional genes, of which 164 are protein-coding. It also contains at least 5 microRNAs (miRNAs), including miR-99a, let-7c, miR-125b-2, miR-155, and miR-802. Several of these miRNAs contribute to the inflammatory phenotype of DS and/or aggregation of beta-amyloid (Aβ) associated with the early onset AD phenotype of DS [,,,,].
Investigators using invertebrate as well as vertebrate animal models have revealed novel molecular pathways that are regulated by Hsa21, by studying orthologous genes. These models have been used for decades to examine novel drug targets, molecular pathways of disease, and behavioral consequences of gene dysregulation and provide increased scientific rigorwhen biological functions are validated at several different levels [,,,,]. However, barriers exist that may hinder the effectiveness of existing animal models as complete representations of DS in humans. DS and other chromosomal variations represent complex genetic changes that affect both behavior and physiology. Thus, complete models of this human condition are difficult to replicate in animal models. Lower-order or higher-order animal models for DS must accommodate studies of both developmental abnormalities, on one hand, and age-related conditions such as hearing loss, vision loss, or AD, on the other hand. Barriers for existing animal DS models include incomplete genetic representation, phenotype variability (animal models may only partially replicate these phenotypes), and differences in timing of development or neurodegeneration between any animal model and the human brain. New animal models include, e.g., humanized transgenes for DS-related AD, but their success in directly translating to human therapies is still debated. AD is primarily known as a human condition, with few animal species naturally developing AD pathology, providing a major barrier in examining the physiological consequences of this condition using animal models. This review will highlight the benefits of both invertebrate and mammalian models for DS and their individual contributions as well as their shortfalls for understanding the underpinnings of DS-related pathology, particularly in the central nervous system (CNS).
2. Introduction to Animal Models for DS
2.1. Invertebrate and Lower-Order Animal Models for Trisomy 21
Although mouse models have been the most commonly used animal models for DS, lower-order models have their place, due to their relatively simple genetic makeup, shorter lifespan, and easy gene manipulation strategies (Figure 1). As discussed below, these models have unique benefits and drawbacks, but there is no doubt that these lower-order models have contributed to current knowledge, especially in terms of examining gene expression results on specific pathways that are orthologous to the genes on the human Hsa21.
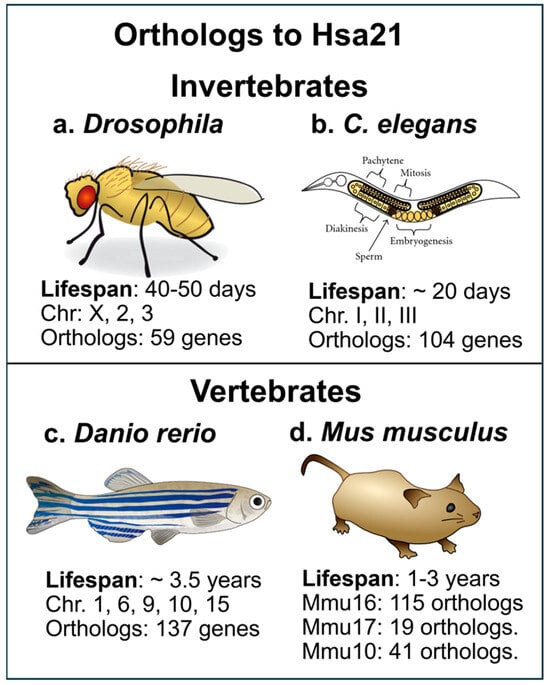
Figure 1.
(a) The fruit fly, Drosophila melanogaster, has a lifespan of 40–50 days and has been used to investigate pathways that are orthologous to Hsa21 changes, for example, the calcineurin pathway; (b) The C. elegans nematode has also been used for examining specific alterations in molecular pathways caused by Hsa21 trisomy in humans. Developmental and age-related molecular and behavioral changes in genes dispersed over chromosomes can be tested in this invertebrate; (c) The zebrafish (Danio rerio), a ray-finned fish with translucent fry which are often used in developmental studies, since the entire fish embryo can be imaged and show distribution of dysregulated gene products; (d) The mouse, Mus musculus, is the most common lab animal and has been easier to manipulate than the rat. The mouse has an average lifespan of 1–3 years, with Hsa21 orthologs dispersed over MMU16, 17, and 10. From: (a): https://commons.m.wikimedia.org/wiki/File:Drosophila-drawing.svg (accessed on 2 May 2025); (b): https://commons.wikimedia.org/wiki/File:C-elegans-Schematic-drawing-of-the-two-gonads-and-uterus-of-C.jpg (accessed on 2 May 2025); (c): https://commons.wikimedia.org/wiki/File:201108_zebrafish.png (accessed on 2 May 2025); (d): https://commons.wikimedia.org/wiki/File:Mouse.svg (accessed on 2 May 2025).
2.1.1. Drosophila Models of Trisomy
Drosophila fruit flies (Drosophila melanogaster) have been used for decades to study the biological effects of genetic manipulations (Figure 1a and []). The relatively simple genomics of the fly makes it easy for studies of trisomic conditions. Orthologous genes to the Hsa21 are dispersed on chromosomes X, 2, and 3 in Drosophila []. Trisomic flies with an extra fourth chromosome survive at rates equivalent to disomic flies and exhibit phenotypic differences, while triplication of the second or third chromosome in Drosophila is rarely survivable. Interestingly, Drosophila has a built-in dosage compensation. Thus, male and female flies exhibit similar levels of X-linked gene expression, leading to males having a two-fold upregulation of the X chromosome to match the expression levels in females, who have two X chromosomes. It is challenging to create a Drosophila model that has a complete or near-complete trisomy of the Hsa21 orthologous genes since the orthologs in the fly genome are dispersed over several chromosomes, as mentioned above. Drosophila models have provided valuable information to the field regarding functional consequences of specific molecular pathways altered by chromosomal triplication [].
Studies have shown similarities in over-expression of certain genes in Drosophila that correlate with similar changes in fetal tissue with Hsa21 trisomy. One example of a pathway studied in Drosophila is the calcineurin signaling pathway [], which is also dysregulated in other animal models for DS, such as the Ts65Dn mouse model []. The calcineurin pathway is also dysregulated in humans with DS via over-expression of genes on Hsa21 including Regulator of Calcineurin 1 (RCAN1) and Down syndrome critical region 1 (DSCR1). Over-expression of these two genes leads to suppression of calcineurin activity, resulting in developmental problems. There is a feedback loop where calcineurin regulates the transcription of the DSCR1 protein, providing a fine-tuned regulation of this important pathway. The over-expression of the RCAN1 protein leads to a downregulation of calcineurin signaling in DS and is thought to directly contribute to intellectual disability and learning deficits. Calcineurin inhibitors are immunosuppressants that have been proposed for clinical use in transplantation and autoimmune diseases. Since calcineurin is also implicated in AD, it is possible that calcineurin inhibitors could prevent AD in DS as well []. The Drosophila model played an important role in revealing the dysfunction of the calcineurin pathway in DS, since the Nebula gene in Drosophila is an ortholog of the human DSCR1 gene and is involved in learning and memory in the fly model []. The Drosophila fly has a lifespan of 40–50 days (Figure 1a), making aging studies relatively simple compared to higher-order animal models. The Drosophila genome has been sequenced in its entirety, and this invertebrate was one of the first eukaryotic genomes to be sequenced, providing significant genetic tractability []. However, Hsa21 gene orthologs are dispersed in several chromosomes of the Drosophila genome, and conserved regions are short (see Figure 1a) as detailed elsewhere []. A barrier with the Drosophila model is that gene-gene interactions may be better examined with other models and complex cognitive studies cannot be conducted using this model. Fruit flies and humans are evolutionarily distant and therefore display significant differences in molecular function and physiology, lessening the translatability of findings to the human condition.
2.1.2. C. elegans Models for Trisomy 21
Similarly to Drosophila, the C. elegans nematode (Caenorhabditis elegans) has a relatively simple genome and a well-defined genetic system, making it easier to manipulate and study than many other animal models []. Despite the simplistic genome, many fundamental biological processes, including meiosis and chromosome segregation, are conserved between C. elegans and higher organisms, including humans. C. elegans can be used to model trisomy, particularly autosomal trisomy. Specifically, C. elegans with an extra X chromosome (trisomy for the X chromosome) has been shown to be viable and fertile, contrary to trisomy of the X chromosome in Drosophila []. Additionally, some studies have explored autosomal trisomy in C. elegans, demonstrating that it can be corrected during oocyte meiosis, leading to a higher frequency of euploid offspring than expected. Researchers have used this model to examine the impact of over-expressing or disrupting gene homologs to those on Hsa21. About 40% of human genes associated with disease have worm orthologues (Figure 1b). Nordquist and collaborators studied loss-of-function mutations of 10 different Hsa21 orthologs in C. elegans and their importance for neuromuscular function []. They found that three of the 10 orthologs to Hsa21 were required for acetylcholine transmitter release, demonstrating the ability to systematically examine loss—or gain-of function for specific Hsa21 genes, and thus being able to dissect which of the 164 protein-encoding genes on Hsa21 that are crucial for normal development or age-related function. C. elegans have a lifespan of approximately 20 days (Figure 1b), and the genetic makeup has been sequenced, making this nematode particularly useful for genetic studies and high-throughput genetic screening. C. elegans was the first multicellular organism for which the entire genome was sequenced, with several new additions []. The genome consists of approximately 100 million base pairs which are organized into six pairs of chromosomes in hermaphrodites or five pairs of autosomes with an XO chromosome in males []. Approximately 52% of Hsa21 genes have orthologs in C. elegans; about 104 have corresponding genes in C. elegans. An example is NCAM2, located on Hsa21. The C. elegans ortholog is NCAM1. Both genes are cell adhesion molecules, and the NCAM-1 role in axonal outgrowth has been studied in C. elegans []. A shortfall with the C elegans model is that they lack a circulatory system and organs including kidneys, liver, and lungs, which limit studies of systemic effects in DS as well as interactions between the CNS and peripheral organs.
2.1.3. Zebrafish Models for DS
Zebrafish (Danio rerio) models for trisomy have been developed by manipulating genes orthologous to crucial genes on Hsa21 []. This is a ray-finned fish, and while it is a vertebrate animal, the genetic makeup is less complex in terms of development and some features compared to higher-order mammals. The zebrafish represents another lower-order animal model that can be used to examine human disorders due to their short lifespan, transparent embryos, and a simple gene makeup, similar to the invertebrate models described above. Zebrafish models have, for example, been used to examine developmental effects of the DYRK1A gene [], which is highly conserved in the zebrafish and plays an important role in deficits occurring in DS in humans [,,]. The zebrafish can live up to 3.5 years in captivity (Figure 1c) but typically live 1–2 years in the wild, and undergo progressive cellular and behavioral senescence similar to the aging process in humans []. The age-related neurodegeneration observed in zebrafish includes gradual neuronal loss and therefore represents a valuable model for studies of age-related neurodegeneration []. There is anatomical and molecular homology between the zebrafish and mammal neurotransmitter systems, allowing detailed studies of neuropharmacology in this model []. The zebrafish genome is also known in its entirety, and was mapped relatively late (in 2013), compared to other models []. Zebrafish have approximately 137 genes that are orthologs to the protein-coding genes on Hsa21, and 35 of those have more than one zebrafish ortholog (Figure 1c). These orthologs represent about 77% of the protein-coding genes on Hsa21. The zebrafish genome also contains duplicated genes, some of which are orthologs of Hsa21 genes.
In sum, a significant benefit of the Drosophila, C. elegans, and zebrafish models is that they have a relatively short lifespan, and therefore can be generated in large numbers, allowing for efficient screening and analysis of phenotypes related to Hsa21 trisomy. Since many cellular functions and pathways are preserved compared to higher-order species, and orthologs to many genes and pathways in humans are known, these are viable models to explore specific pathway dysregulations as well as to test novel treatment paradigms for individuals with DS. As mentioned above, the genome of each of these three lower-order models has been sequenced, allowing sophisticated gene manipulations. Invertebrates do not have a chromosome that is syntenic to Hsa21, the ortholog genes are spread out over several chromosomes, making it more difficult to mimic the DS trisomy in invertebrates or fish.
2.2. Mouse Models for DS
2.2.1. Comparison Between Mouse and Human Pathology
Mice have genes that are preserved within mammals [], but also genes that are not; for example, the human gene for insulin and the mouse gene for insulin evolved from a common ancestral gene. Mouse chromosomes (Mmu) 10, 16, and 17 contain genes that are also found on Hsa21 and are therefore relevant for studying DS. Mmu16 contains the “Down syndrome critical region” (DSCR) including the Cbr3, Dscr1/RCAN1, Erg, Jam2, Pttg1ip, and Tiam1 genes []. Mice live 1–3 years in captivity but only 12–18 months in the wild (Figure 1d, []) allowing a relatively rapid progression of disease phenotype or normal aging. The first mouse model for DS was the Ts65Dn mouse, developed by Muriel Davisson at Jackson Laboratories, and was accomplished by X-ray irradiation of a male mouse [], with an extra copy of the distal region of the murine Chr. 16, which is partially homologous to the Hsa21. As shown in Figure 2 and in [,,,,,,,,,,,,], the Ts65Dn mouse develops many molecular and physiological features observed in humans with DS; both during development and aging. The Ts65Dn mice exhibit progressively more neuroinflammation and glial activation with aging (Figure 2c,f, [,]), as seen in humans with DS [,,,], along with age-related loss of basal forebrain cholinergic neurons (BFCNs), locus coeruleus noradrenergic neurons (LC-NE), and subpopulations of hippocampal neurons []. Ts65Dn mice have increased APP and Aβ levels and increased intracellular phospho-Tau [,], but do not form neurofibrillary tangles (NFTs) or amyloid plaques (Figure 2g,h).
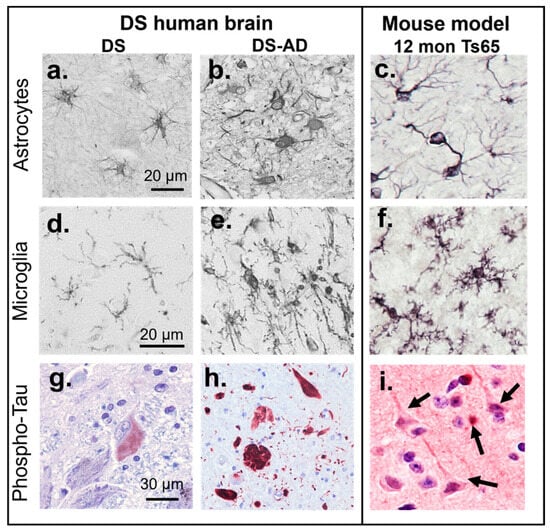
Figure 2.
Comparison of pathology in the human brain with DS (a,d,g) or DS-AD (b,e,h) and Ts65Dn mice (c,f,i). Astrocytes in the hippocampus gray matter exhibit a resting morphology in a 25-year-old (YO) with DS without dementia (a), compared to the activation and increased immunostaining with antibodies directed against glial fibrillary acidic protein (GFAP) in an individual with DS-AD (b) [] and the increased GFAP staining in hippocampus of a 12-month-old Ts65Dn mouse (c) []. Microglial activation (stained with Iba1 antibodies) was not observed in a 37-year-old male with DS and no dementia (d) but was frequently observed in DS-AD cases (e) [] and in the 12-month-old Ts65Dn mouse (f) []. The locus coeruleus (LC) in a young (25 YO) individual with DS did not exhibit frank neurofibrillary tangles (NFTs), but a few neurons stained for an oligomeric Tau antibody (TOC1, red chromophore, (g) []. In DS-AD, frequent AT8-positive tangles and fibers were observed throughout the brain (here a 54-year-old male with DS-AD, red chromophore, (h). The mouse brain does not naturally develop NFTs, unless a human mutated tau transgene is introduced, or after injections with human p-Tau (i). Tangle-like p-Tau inclusions (see arrows) in large cortical neurons adjacent to an exosome injection from a DS-AD participant’s plasma [].
We have demonstrated that the progressive accumulation of p-Tau containing NFTs seen in the human brain with DS-AD (Figure 2h, [,]) can be mimicked in DS mouse models by stereotaxic intracranial injection of neuron-derived extracellular vesicles (NDEVs) that contain p-Tau “seeds” from humans with DS-AD (Figure 2i and []). Others have shown that exosomes or p-Tau extracted from AD brain tissue also gives rise to the spreading of Tau pathology in the mouse when injected [,,,,,]. This experimental design may represent a new way to induce protein aggregation and AD pathology in DS models, instead of the addition of human transgenes to the mouse genome which could have its own challenges []. Challenges with transgenic animals include the potential that the disease phenotype varies depending on the mouse strain background, potential off-target effects, and the risk that species-specific cell functions could affect the interpretation of experimental outcomes. An example of this is that human versus murine microglial cells exhibit different responses to a common trigger despite having many features in common []. This could represent a confounding factor. In addition, mice and humans have different chromosomal structures, therefore adding a third copy of Hsa21 to a mouse genome may not replicate the pathophysiology of human DS.
Mouse models are crucial for understanding the role of specific genes and/or gene-gene interactions in the developmental and cognitive deficits associated with DS. In mice, the Mmu16 chromosome contains the largest region of Hsa21 synteny, with approximately 115 orthologs of Hsa21 genes, while the Mmu17 contains around 19 orthologs and the Mmu10 contains roughly 41 orthologs []. Sophisticated behavioral and physiological traits could not be studied using lower animals such as the Drosophila or C elegans.
The most common DS mouse models have a segmental trisomy of overlapping fragments of mouse chromosome 16 (MMU16) that is syntenic to Hsa21. The Ts65Dn model (Ts(1716)65Dn/J) carries an additional mini chromosome with the Mir155 to Zbtb21 region of mouse chromosome 16, homologous to Hsa21. This region includes approximately 90 genes, fused to the centromeric part of mouse chromosome 17 from Pisd-ps2/Scaf8 to Pde10a, which includes 46 genes that are not homologous to Hsa21 and could therefore complicate the interpretation of results in this model, although it was the only mouse model for a long time that recapitulated DS human phenotypes [], and was therefore used extensively for behavioral, pharmacological, and molecular DS studies [,,]. However, derivations of frozen embryos of the Ts65Dn mouse line have shown significant variability in Ts65Dn lines across generations caused by genetic drifting [], which reduces the current quality of the model for studying aspects of neurological development in DS. Numerous other models for DS have been developed that may reflect a more accurate genetic representation of Hsa21 (Table 1). There are benefits with having multiple models for DS; this allows us to examine genotype-phenotype relationships by using a combination of transgene and murine trisomy models. We can also identify driver genes in multiple vertebrate and invertebrate animal models and therefore gain rigorous proofs of concept for therapeutics by comparing their effectiveness and potential toxicity in several models. This approach is highly recommended, especially when examining novel biological pathways or novel therapeutic interventions.

Table 1.
Summary of mouse models for Down syndrome. Each of these models has specific advantages based on the purpose of the study and whether developmental or age-related behaviors or physiological processes are targeted.
Table 1 above shows a summary of the currently developed mouse models and a summarized genetic over-expression and/or chromosomal trisomy theyentail [,] and benefits of each model. The specific genes involved in each mouse model have been described elsewhere []. With so many DS models it may be difficult to determine which strain to use for a specific experimental question, and this may be limited by the availability of some DS models as well as a lack of studies that demonstrate age-related cognitive dysfunction and neuronal loss in some of the newer models. Although the Ts65Dn mouse described in detail above is known to exhibit age-related learning and memory deficits [,,,], other DS mouse models may not show these impairments. Thus, the variability of cognitive and neurodegeneration phenotypes in these different models may limit their use for drug therapy or mechanisms associated with cognitive loss with aging, even though they are valuable for mechanistic studies. For example, the adult Dp16 mouse shows cognitive deficits and cholinergic cell loss but lacks the prenatal brain defects seen in humans with DS and in other DS mouse models [] and, thus, does not fully recapitulate DS in humans.
2.2.2. Comparisons Between Different DS Mouse Models
Several research groups have performed comparison studies of the phenotypes between different DS models, to determine which model is best suited for their studies. An interesting study by Arima-Yoshida et al. [] showed significant differences between the Ts1Cje, Ts2Cje, and Ts1Rhr mice (see Table 1) in terms of electrophysiological parameters of GABAb receptor function during tetanic stimulation. The Ts2Cje mouse has a slightly larger segmental trisomy of MMU16 compared to the Ts1Cje mice, leading to more severe learning deficits and BFCN degeneration compared to Ts1Cje []. This research group was able to demonstrate that a dysfunction caused by the Carbonyl Reductase 1 (Cbr1) gene could be rectified when one copy of the Cbr1 gene was deleted (Ts1Cje; Cbr1+/+/−), demonstrating the usefulness of studying several models in parallel and combining the trisomy models with targeted gene deletions. These studies demonstrated the importance of correcting the Cbr1 over-expression which can lead to low blood pressure and regulate sympathetic tone. New druggable targets for humans with DS can come out of this research. Low blood pressure in DS affects exercise tolerance and quality of life, and therefore correction of over-expression of this gene can have meaningful translational effects. Another research group examined differences between the Ts65Dn mouse and the Ts1Cje model showing that the lack of age-related degeneration of BFCNs in the latter model was due to the shorter triplication of MMU16 in Ts1Cje mice, excluding the APP gene and demonstrating therefore that APP and Aβ contribute to cholinergic loss in the Ts65Dn mice and most likely also in humans with DS [].
Reeves et al. [] developed the Ts1Rhr and Ms1Rhr mouse models that are trisomic and monosomic, respectively, for a region on MMU16 that is homologous to 5.3 Mb of Hsa21; the Down syndrome critical region (DSCR). Utilizing these two models, they found that trisomy for the DSCR alone is not sufficient to produce the structural and functional features of hippocampal impairment that were previously described in the Ts65Dn mouse and in humans with DS. On the contrary, when the DSCR was returned to normal gene dosage (Ms1Rhr/Ts65Dn mice, see Table 1), the performance in spatial reference memory tasks was normalized, thus proposing that the DSCR was critical at least for spatial reference learning and memory. They concluded that even if the critical region hypothesis was disproven by these experimental mouse models, they helped define the contribution of this gene region of Hsa21 and its influence on learning and memory [].
In a study by Siegel et al. [], they examined three different DS mouse models for visual discrimination learning as well as inhibitory control. The models were the Dp(16)1/Yey, Ts65Dn, and Ts1Cje models (Table 1). They found that the Dp(16)1/Yey and Ts1Cje models lacked learning deficits during early pre-training, while the Ts1Cje mice had significant learning delays in the late pre-training session, which would suggest frontal cortex pathology []. One of the most important findings with this study was that the mouse background strains (which were different between the mouse lines) had significant effects on behavior, pointing out that the background strain is very important when performing behavioral studies and can cause confounding effects. This manuscript points out the importance of selecting the best model depending on which cognitive tasks are proposed and whether developmental or age-related deficits will be examined. Genetic drifting and different genetic background strains can affect the interpretation of morphological, physiological, or behavioral outcomes, since different mouse strains have distinct behavioral traits also when it comes to specific reactions to an introduced mutation or transgene [,]. These are examples of the usefulness of studying several DS models in parallel to explore gene-specific influences on DS developmental and age-related phenotypic pathological traits.
2.2.3. The Latest DS Mouse Models
The Ts66Yah mouse model [] is based on the existing Ts65Dn model and was generated with CRISPR/Cas9 technology [] removing a specific region of mouse chromosome 17 (Mmu17) on the additional mini-chromosome that is not homologous to the Hsa21 genome. The Ts65Dn mouse carries a freely segregating mini chromosome that includes 13.4 Mb of distal Mmu16 which is fused with 10 Mb of the centromeric part of Mmu17. The Ts66Yah Mouse only has an extra copy of the Mir155 to Zbtb21 region of Mmu16, while the Mmu17 portion is removed. It was created by the Duchon/Herault research groups and was first described in 2022 []. CRISPR/Cas9 technology was used to delete a specific 6.2 Mb region of DNA on the mini chromosome, removing genes not homologous to Hsa21. The deletion was performed in vivo, with sperm from fertile males of the Ts65Dn ‘1924’ line and WT F1B6C3B oocytes. One founder carrying the recombined mini chromosome, with the deletion of the centromeric part of Mmu17, was selected and crossed with C57BL/6NCrl females to start this new cohort of mice. It is generally thought that this version of Ts65Dn mice have a milder cognitive phenotype than the Ts65Dn mouse, and that they do not exhibit age-related neuronal loss as some of the other models. On the other hand, this model may be truer to the genes involved in the Hsa21 trisomy. This mouse model presents us with an important conundrum: if a more specific representation of orthologs to Hsa21 protein-encoding genes is generated, using, e.g., the CRISPR/Cas9 methods in the Ts66Yah mouse, and this leads to a milder phenotype with only mild learning and memory impairment and no BFCN or other neuronal loss, where does that leave us? It suggests that the severe learning deficits often seen in individuals with DS, and age-related BFCN loss and cognitive impairment associated with AD pathology cannot be accurately capitulated with a limited specific trisomy of syntenic genes in the mouse. Maybe this is because the mouse is using additional or different genes and/or gene-gene interactions to accomplish the sophisticated learning and memory tasks that scientists have designed. The question is whether it is most important to have a model that does have severe neurodegeneration and cognitive loss or if it is more important to have a genetically accurate model.
The TcMAC21 mouse model of DS was first described in 2020 by Kazuki, Gao, and Reeves et al. []. This model was created in a different manner, by incorporating a nearly complete copy of the long arm of Hsa21 (Hsa21q) into a mouse artificial chromosome (MAC); hence the TcMAC21 name []. They used microneedle-mediated chromosome transfer, injecting embryonic stem cells, which were then used to generate chimeric mice, where the MAC contributed to brain development. These mice, although less studied than, e.g., the Ts65Dn mouse, exhibit many phenotypes of DS including growth retardation, heart abnormalities, skeletal abnormalities, and neuronal developmental delays []. However, studies have not yet been completed regarding the developmental or aging phenotype of the TcMAC21 mice yet (see Table 1). There is hope that the TcMAC21 mouse model will provide a closer genetic model for DS. It has already been shown that TcMAC21 mice undergo age-related BFCN loss (as do the Ts65Dn mice, see above), learning and memory deficits, and elevated levels of APP and its cleavage products, Aβ40 and Aβ42, which contribute to the BFCN degeneration []. Cardiac septal defects were found in many of the fetuses, along with cerebellar hypoplasia, and abnormal craniofacial features. Future studies will reveal whether this is the most accurate DS model.
In sum, there have been comprehensive reviews of the mouse models for DS (see, e.g., [,]). Therefore, the current review is intended to provide an overview of the most common and available DS mouse models and their basic phenotypes and is not a complete list of all mouse models for DS.
2.3. Deficits in Specific Pathways Identified and Examined in Animal Models
Several major pathways that are implicated in DS in humans have been successfully mimicked and studied in lower-order animals including C. elegans, zebrafish, and Drosophila as well as mice. These include the MAP kinase pathway, the TGFβ pathway, and the JAK/STAT pathway (which led to a clinical trial in DS, see below). Below is a summary of some of the most important pathways that have been examined using the animal models described in this review.
- The APP/Aβ pathway plays a crucial role in early onset AD in individuals with DS (see, e.g., []). While some of the DS mouse models have elevated levels of APP and/or Aβ, transgenes for Aβ have been used in both C. elegans [] and Drosophila models to further characterize their physiological and molecular role in the brain. In C. elegans, a transgene expression of human Aβ mutations gives rise to behavioral deficits and shortened lifespans [], mimicking the effects of this toxic product of APP in humans with DS. Although lower-order animals are suitable tools for screening approaches in terms of anti-amyloid treatments, the molecular effects of Aβ on neurons could not be elucidated utilizing these strains, at least not initially []. A series of elegant studies using Ts1Cje, Ts2Cje, and Ts65Dn mice showed that APP is necessary but not sufficient to give rise to age-related cognitive impairment and BFCN degeneration [], most likely via an amyloid disruption of nerve growth factor mechanisms by binding to NGF receptors [].
- The Akt/mTOR/Insulin pathway shows an aberrant activation, leading to dysregulation of downstream important pathways in the hippocampus of Ts1Cje mice [], as well as in Ts65Dn mice and humans with DS []. Further, studies in Drosophila have shown that increased activity of the mTOR pathway can lead to neurodevelopmental defects and accumulation of AD pathology [], which mimics aberrant signaling in this pathway in individuals with DS. Both insulin and mTOR signaling pathways are dysregulated early in life in individuals with DS [,]. The mTORC1/insulin signaling pathways are key regulators of cell growth and proliferation []; this has been shown in multiple animal models. Dysregulation of mTOR and associated signaling can affect autophagy and oxidative stress, which are known to contribute to AD pathology (see, e.g., []). In Drosophila, the mTORC1 pathway is involved in organ size control and longevity. Patterson and collaborators administered Rapamycin, an mTOR inhibitor, to Ts65Dn mice, giving rise to increased lifespan and health span [], which was corroborated by several other research groups [,]. Thus, the mTOR/Akt/Insulin pathways are highly conserved between different animal models, and the clinical field has benefited significantly from these animal studies.
- The JAK/STAT pathway. The JAK/STAT pathway has been extensively investigated in different DS models, especially as it relates to brain development and to leukemia [], which has an increased incidence in children with DS []. In 1992, the laboratories of Darnell, Kerr, and Stark discovered the JAK/STAT signaling pathway when exploring the cellular response to interferon []. JAK/STAT signaling is implicated in neuroinflammation and activation of astrocytes, making it a potential target for therapeutic interventions in the chronically hyper-inflamed DS brain. The connection between impaired JAK/STAT signaling and cognitive function was first described in mouse models, where the JAK/STAT pathway was examined using traditional gene knockout studies []. Mechanistic studies of small molecules that can act as inhibitors in this pathway have been carried out in several animal models and have led to clinical trials using the JAK inhibitor tofacitinib, to assess its effects on individuals with DS []. Hyperactivation of the interferon pathway and downstream JAK/STAT signaling has been linked to developmental and cognitive deficits [,,].
- The DYRK1A pathway. DYRK1A is a kinase located in the DSCR and is therefore over-expressed in DS. In Drosophila, the homolog of DYRK1A is Mini brain (mnb), which is involved in neurogenesis and migration of neurons. By studying the mnb pathway in Drosophila, researchers have gained insights into the role of DYRK1A in DS neurodevelopment. Calcium signaling, which is crucial for angiogenesis, has been shown to be dysregulated in DYRK1A-deficient zebrafish, mimicking vascular defects seen in DS in humans []. On the other hand, over-expression of DYRK1A in zebrafish leads to enhanced Wnt signaling and inhibited TGFβ signaling [,]; similar to the imbalance in these two pathways seen in neuronal progenitors in humans with DS []. By suppressing calcineurin signaling in zebrafish and then co-treating with DYRK1A inhibitors, the potential therapeutic benefits and risks with these interventions were studied in zebrafish []. DYRK1A over-expression has also been studied heavily in mouse models, leading to the development of inhibitors that can be used for clinical prevention of cognitive deficits in humans with DS. The DYRK1A inhibitor Leucettinib is currently in Phase 1 clinical trial for both AD and DS [].
- The Notch pathway. Notch signaling is crucial during development, involved in cell fate determination, neurogenesis, and tissue organization []. Altered Notch signaling has been implicated in DS, especially during development (Figure 3). For example, dysregulation of the Notch pathway has been proposed to be involved in the gliogenic shift observed in the DS brain []. Interestingly, DYRK1A (which is located on Hsa21 and over-expressed in the DS brain) is co-expressed with Notch and over-expression of DYRK1A (as in the DS brain) leads to inhibition of Notch signaling [,], altering the fate of neural progenitor cell proliferation as well as neuronal migration that could cause some of the developmental delays seen in children with DS []. Studies in Drosophila have been instrumental in understanding the mechanisms of Notch signaling and its role in developmental processes, and continued studies using the Drosophila model have unearthed new findings regarding this important pathway for neural development [,,], see also Figure 3.
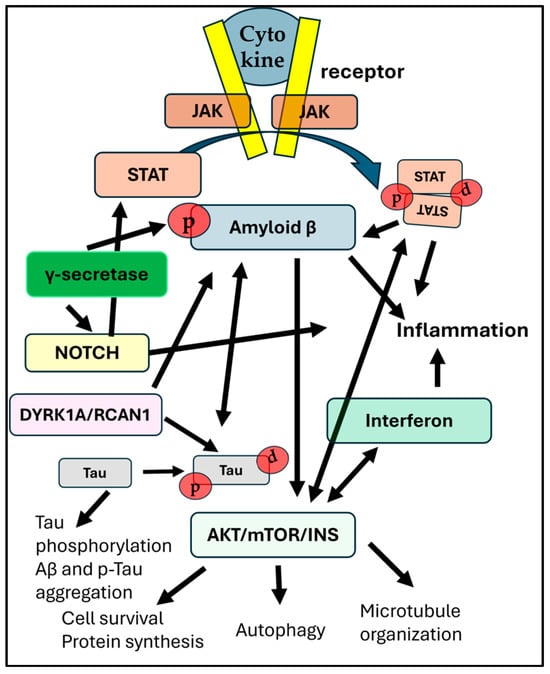 Figure 3. The complex interaction between pathways that are affected by trisomy 21. Arrows signify pathway cross-talks or one-directional influences between distinct pathways. The JAK/STAT pathway is activated by cytokine binding to the receptor and can influence the expression and activity of BACE1 (β-secretase), which is involved in the amyloidogenic pathway. mTOR hyperphosphorylation is linked to increased p-Tau aggregation. Both Notch and APP are processed by γ-secretase. While γ-secretase inhibitors can reduce Aβ production, they also inhibit Notch signaling, which can negatively influence important cellular processes. The interferon (IFN) and Notch pathways interact to influence immune response. The over-production of interferon receptors in DS may have consequences for cell survival and growth factor function, thus interacting also with the mTORC1 or mTORC2 complexes. Dyrk1A and RCAN 1 (both located on Hsa21) can affect amyloid production by phosphorylating APP and are also involved in hyperphosphorylation of tau. Finally, the Notch signaling pathway is involved in neuroinflammation, influencing microglia activation, cytokine release, and the integrity of the BBB. P-tau can promote amyloid-beta (Aβ) production, and Aβ, in turn, can drive tau phosphorylation. Modulating the JAK/STAT pathway is explored as a potential therapeutic strategy for AD, aiming to reduce neuroinflammation, restore neuronal function, and potentially impact amyloid pathology.
Figure 3. The complex interaction between pathways that are affected by trisomy 21. Arrows signify pathway cross-talks or one-directional influences between distinct pathways. The JAK/STAT pathway is activated by cytokine binding to the receptor and can influence the expression and activity of BACE1 (β-secretase), which is involved in the amyloidogenic pathway. mTOR hyperphosphorylation is linked to increased p-Tau aggregation. Both Notch and APP are processed by γ-secretase. While γ-secretase inhibitors can reduce Aβ production, they also inhibit Notch signaling, which can negatively influence important cellular processes. The interferon (IFN) and Notch pathways interact to influence immune response. The over-production of interferon receptors in DS may have consequences for cell survival and growth factor function, thus interacting also with the mTORC1 or mTORC2 complexes. Dyrk1A and RCAN 1 (both located on Hsa21) can affect amyloid production by phosphorylating APP and are also involved in hyperphosphorylation of tau. Finally, the Notch signaling pathway is involved in neuroinflammation, influencing microglia activation, cytokine release, and the integrity of the BBB. P-tau can promote amyloid-beta (Aβ) production, and Aβ, in turn, can drive tau phosphorylation. Modulating the JAK/STAT pathway is explored as a potential therapeutic strategy for AD, aiming to reduce neuroinflammation, restore neuronal function, and potentially impact amyloid pathology.
3. Discussion
As discussed in this review, animal models have significant value both for discovery of new pathways involved in pathology and for testing of new treatment avenues for comorbidities associated with DS. These could be pathways involved in development as well as aging. Above, we provided examples of crucial pathways that were discovered and studied in animal models first, followed by confirmatory studies in humans. One of the first genes on Hsa21 that was found to be important for the DS phenotype was the DYRK1A gene [,,,,,]. Diligent work by several research groups has now led to a clinical trial with the DYRK1A inhibitor Leucettinib-21 (see above). Although we now have a complete map of the protein-encoding genes on this chromosome, the molecular mechanisms involved in the downstream actions of many long non-coding RNAs and miRNAs located on Hsa21 are still not known. Work remains to be carried out—hopefully including all levels of animal models as well as human tissue—before we have a complete understanding of the developmental and age-related impairments in the DS brain.
Some mouse models for DS have pathological features in common with the human DS brain, while other pathophysiological traits are not developing in the murine system to the same extent as in humans. For example, mice do not develop neurofibrillary tangles (NFTs), nor do they exhibit extracellular amyloid plaques, like those seen in the human brain with AD or DS-AD unless a human transgene is introduced. It is thought that this is due to differences in the murine and human amyloid-beta (Aβ) protein sequence []; the three amino acid substitutions in the mouse Aβ sequence are believed to prevent the formation of Aβ plaques in the natural aging process. For the development of NFTs, a crucial component of AD pathology in humans, it is well-known that mice have a different version of the MAPT gene (microtubule-associated protein tau), encoding the tau protein. In humans, the MAPT gene can be spliced into different isoforms, including 3R and 4R, while adult mice only express the 4R isoform. Species-specific differences in phosphorylation of tau in the mouse vs. human brain are also involved []. This genetic difference in tau structure affects the tendency of the Tau protein to misfold and aggregate into NFTs []. Although these differences may defer studies of protein aggregation in murine models, they still exhibit over-expression of Aβ and some p-Tau isoforms; at least in some mouse strains including the Ts65Ds and the Dp(16)1Yey/+ mouse models [].
Interestingly, normalizing APP gene copy number in Ts65Dn mice fails to rescue plasticity in this model but can restore the integrity of the basal forebrain cholinergic system [,]. Another interesting way to utilize the different models is to differentiate behavioral or morphological differences between them to distinguish the importance of specific genes for DS-related phenotypic changes. For example, Ts65Dn mice demonstrate an abnormality in olfactory system connectivity, but Ts1Rhr mice do not exhibit the same deficits, suggesting that distinct genes or sets of genes that are different between these two strains of mice underlie visual and olfactory system phenotypes, according to William et al. []. Buck and collaborators [] showed that restoring the RCAN1 gene to two copies in the Dp(16)1Yey/+ (Dp16) mouse model for DS reduced wheel running activity and rhythmicity in both light-entrained and free-running young Dp16 mice. Critically, these diurnal and circadian deficits were rescued in part or entirely by reducing RCAN1 to two copies in Dp16 mice, see Buck et al., []. RCAN1 and DYRK1A, both genes located on Hsa21, act in concert to regulate signaling pathways that contribute to DS pathophysiology. DYRK1A over-expression phosphorylates RCAN1, which in turn inhibits the calcineurin pathway which downstream can lead to an increased phosphorylation of Tau []. Thus, gene-gene interactions are important to examine, and not only the genes located on Hsa21; there are of course cross-chromosomal interactions that are yet unknown.
Despite significant efforts to model DS and/or AD in various animal models, there are methodological limitations and barriers in translating findings to human health and disease. As mentioned above, neither neurofibrillary tangles nor amyloid plaques can be appropriately modeled in mice unless human transgenes are introduced. The transgenic methods are not without problems and can introduce limitations []. Overexpression of human APP or components of the gamma-secretase complex including presenilin transgenes can lead to unphysiological interactions with cellular proteins, which can skew results and reduce translatability. Indeed, many proposed AD drugs that look promising in transgenic AD models have failed to show efficacy in humans with AD or DS-AD []. These failures illustrate the difficulty in translating findings in animal models to human medicine. Examples of these failures include BACE1 inhibitors, γ-secretase inhibitors, as well as immunotherapies targeting amyloid oligomers or fibrils. Indeed, Aβ antibodies including aducanumab demonstrated significant effects in transgenic AD models [], but have modest cognitive effects in humans, with significant potentially dangerous side effects. Reasons include that mouse models may not exhibit the entire spectrum of human AD pathology or due to different lifespans of mice and humans, such that temporal components of the disease progression cannot be mimicked in mice. Other potential problems with translation include that some drugs may not cross the BBB in humans, even though they do so in the mouse brain. Finally, AD is a very heterogenous disease including etiology and progression; this cannot be mimicked in mice which are often inbred and of close to identical genetic origin.
In sum, the different DS animal models may exhibit significant differences in terms of developmental or age-related phenotypes and can therefore be used to determine which specific genes and/or proteins that are sufficient to produce cognitive or physiological deficits associated with the DS pathological phenotype in humans. Lower-order animals such as those mentioned here have their specific value, for example the relatively simple genome and the shorter lifespan, allowing rapid and high-throughput studies, while mouse models represent a relatively intelligent mammal that can perform complicated behavioral tasks that are more congruent with human cognitive behavior []. These findings show that trisomy manifests as a highly specific modification of the transcriptome within distinct cell types in the brain. All the different models are needed and complement each other in terms of the search for pathophysiological mechanisms as well as therapeutics. Future goals of the DS research community should be developed to improve the utilization of models and to develop additional models that more accurately model human conditions associated with DS and AD. A better understanding of all aspects of brain deficits during development in DS, as well as understanding different facets of AD pathology would improve the usage of correct models for the question at hand. Studies of human brain in DS during development and aging were difficult to undertake previously due to a lack of well-characterized human brain tissues. However, this has been remedied with the development of brain repositories focused on DS [] and will now allow important parallel studies of human vs. mouse DS-related pathologies.
4. Conclusions
Down syndrome is a complex condition that leads to both developmental and age-related deficits and the phenotype of this condition is often complicated by multiple possible comorbidities [,,,]. The modeling of DS in lower-order animals and mammals has provided unprecedented benefits for individuals with DS, both in terms of understanding the pathophysiological effects of trisomy 21 and for testing a myriad of different therapeutics. As the models have evolved, they have unearthed multiple important biological pathways that can be targeted for successful intervention; both to minimize developmental issues and for combating age-related dementia and AD pathology in individuals with DS. Due to species-specific variations in gene expression and posttranslational modifications (PMTs), a complete translation of findings in animal models to the human condition cannot be obtained, and treatments that were effective in these models have not always translated well to humans. This is because the chromosomes that make up the genomes do not align across species.
The DS population has tripled their lifespan just during the last 30 years, due to modern medicine and heightened knowledge regarding cardiac malformations and metabolic dysregulations. Because of this increased lifespan, it has become evident that most individuals with DS will develop AD pathology and dementia if they live long enough. Future studies need to consider species-specific protein function to design appropriate interventions. It is still debated in the field which model is more suitable for specific studies and depends on the scope of work as well as investment in previous models. Many scientists recommend using a couple of different models in parallel experiments to perform thorough studies, but this is not always feasible due to access or costs. While the Ts65Dn mouse model is most widely used and was recommended by the NIH, new models highlighted here (such as the Ts66Yah model) might be more accurate when appropriately characterized.
5. Summarization in Molecular Aspects
This review covered only a small number of the many pathways that are likely involved in development and aging of the DS brain. The following molecular pathways involved in DS pathology were discussed, as well as interactions between them:
- The APP/Aβ pathway;
- The Akt/mTOR/Insulin pathway;
- The JAK/STAT pathway;
- The DYRK1A pathway;
- The Notch pathway.
Funding
This research was funded by NIH grants (R01AG071228-02 and R01AG061566), a grant from the Lejeune Foundation (GRT-2023b/2277), and a BrightFocus Foundation grant (CA2018010).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MAPT | Microtubule-associated protein tau |
| AD | Alzheimer’s disease |
| DS | Down syndrome |
| DYRK1A | Dual-specificity tyrosine phosphorylation-regulated kinase 1A |
| RCAN1 | Regulator of Calcineurin 1 |
| MMMU | Mouse chromosome |
| MAC | Mammalian artificial chromosome |
| Hsa21 | Human chromosome 21 |
| ADHD | Attention deficit and hyperactivity disorder |
| ASD | Autism spectrum disorder |
| NGF | Nerve growth factor |
| NFT | Neurofibrillary tangles |
| DSCR | Down syndrome critical region |
References
- Antonarakis, S.E.; Skotko, B.G.; Rafii, M.S.; Strydom, A.; Pape, S.E.; Bianchi, D.W.; Sherman, S.L.; Reeves, R.H. Down syndrome. Nat. Rev. Dis. Primers 2020, 6, 9. [Google Scholar] [CrossRef]
- Antonaros, F.; Zenatelli, R.; Guerri, G.; Bertelli, M.; Locatelli, C.; Vione, B.; Catapano, F.; Gori, A.; Vitale, L.; Pelleri, M.C.; et al. The transcriptome profile of human trisomy 21 blood cells. Hum. Genom. 2021, 15, 25. [Google Scholar] [CrossRef]
- Capone, G.T.; Brecher, L.; Bay, M. Guanfacine Use in Children with Down Syndrome and Comorbid Attention-Deficit Hyperactivity Disorder (ADHD) with Disruptive Behaviors. J. Child Neurol. 2016, 31, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Mason, G.M.; Spano, G.; Edgin, J. Symptoms of attention-deficit/hyperactivity disorder in Down syndrome: Effects of the dopamine receptor D4 gene. Am. J. Intellect. Dev. Disabil. 2015, 120, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, J.A.; Amon, A.; Abbeduto, L.; Agiovlasitis, S.; Alsaied, T.; Anderson, H.A.; Bain, L.J.; Baumer, N.; Bhattacharyya, A.; Bogunovic, D.; et al. Opportunities, barriers, and recommendations in down syndrome research. Transl. Sci. Rare Dis. 2021, 5, 99–129. [Google Scholar] [CrossRef] [PubMed]
- Benhaourech, S.; Drighil, A.; Hammiri, A.E. Congenital heart disease and Down syndrome: Various aspects of a confirmed association. Cardiovasc. J. Afr. 2016, 27, 287–290. [Google Scholar] [CrossRef]
- Sterling, A.; Lorang, E.; Reis, K.; Elmquist, M. The Impact of Autistic Traits on Joint Attention in Young Children with Down Syndrome During Mother-Child and Father-Child Interactions. Am. J. Speech Lang. Pathol. 2025, 34, 834–844. [Google Scholar] [CrossRef]
- Landes, S.D.; Stevens, J.D.; Turk, M.A. Cause of death in adults with Down syndrome in the United States. Disabil. Health J. 2020, 13, 100947. [Google Scholar] [CrossRef]
- Hattori, M.; Fujiyama, A.; Taylor, T.D.; Watanabe, H.; Yada, T.; Park, H.S.; Toyoda, A.; Ishii, K.; Totoki, Y.; Choi, D.K.; et al. The DNA sequence of human chromosome 21. Nature 2000, 405, 311–319. [Google Scholar] [CrossRef]
- Rastogi, M.; Bartolucci, M.; Nanni, M.; Aloisio, M.; Vozzi, D.; Petretto, A.; Contestabile, A.; Cancedda, L. Integrative multi-omic analysis reveals conserved cell-projection deficits in human Down syndrome brains. Neuron 2024, 112, 2503–2523.e2510. [Google Scholar] [CrossRef]
- Kadkhoda, S.; Eslami, S.; Mahmud Hussen, B.; Ghafouri-Fard, S. A review on the importance of miRNA-135 in human diseases. Front. Genet. 2022, 13, 973585. [Google Scholar] [CrossRef]
- Liu, H.; Chen, S.; Sun, Q.; Sha, Q.; Tang, Y.; Jia, W.; Chen, L.; Zhao, J.; Wang, T.; Sun, X. Let-7c increases BACE2 expression by RNAa and decreases Abeta production. Am. J. Transl. Res. 2022, 14, 899–908. [Google Scholar]
- Mahernia, S.; Hassanzadeh, M.; Adib, M.; Peytam, F.; Haghighijoo, Z.; Iraji, A.; Mahdavi, M.; Edraki, N.; Amanlou, M. The possible effect of microRNA-155 (miR-155) and BACE1 inhibitors in the memory of patients with down syndrome and Alzheimer’s disease: Design, synthesis, virtual screening, molecular modeling and biological evaluations. J. Biomol. Struct. Dyn. 2022, 40, 5803–5814. [Google Scholar] [CrossRef]
- Perez-Villarreal, J.M.; Avina-Padilla, K.; Beltran-Lopez, E.; Guadron-Llanos, A.M.; Lopez-Bayghen, E.; Magana-Gomez, J.; Meraz-Rios, M.A.; Varela-Echavarria, A.; Angulo-Rojo, C. Profiling of circulating chromosome 21-encoded microRNAs, miR-155, and let-7c, in down syndrome. Mol. Genet. Genomic. Med. 2022, 10, e1938. [Google Scholar] [CrossRef]
- Andrade-Talavera, Y.; Benito, I.; Casanas, J.J.; Rodriguez-Moreno, A.; Montesinos, M.L. Rapamycin restores BDNF-LTP and the persistence of long-term memory in a model of Down’s syndrome. Neurobiol. Dis. 2015, 82, 516–525. [Google Scholar] [CrossRef]
- Chang, K.T.; Shi, Y.J.; Min, K.T. The Drosophila homolog of Down’s syndrome critical region 1 gene regulates learning: Implications for mental retardation. Proc. Natl. Acad. Sci. USA 2003, 100, 15794–15799. [Google Scholar] [CrossRef]
- Costa, A.C.; Stasko, M.R.; Schmidt, C.; Davisson, M.T. Behavioral validation of the Ts65Dn mouse model for Down syndrome of a genetic background free of the retinal degeneration mutation Pde6b(rd1). Behav. Brain Res. 2010, 206, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, E.; Morabito, S.; Henningfield, C.M.; Das, S.; Rahimzadeh, N.; Shabestari, S.K.; Michael, N.; Emerson, N.; Reese, F.; Shi, Z.; et al. Spatial and single-nucleus transcriptomic analysis of genetic and sporadic forms of Alzheimer’s disease. Nat. Genet. 2024, 56, 2704–2717. [Google Scholar] [CrossRef] [PubMed]
- Moyer, A.J.; Gardiner, K.; Reeves, R.H. All Creatures Great and Small: New Approaches for Understanding Down Syndrome Genetics. Trends Genet. 2021, 37, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Atas-Ozcan, H.; Brault, V.; Duchon, A.; Herault, Y. Dyrk1a from Gene Function in Development and Physiology to Dosage Correction across Life Span in Down Syndrome. Genes 2021, 12, 1833. [Google Scholar] [CrossRef]
- Costa, A.C.; Scott-McKean, J.J.; Stasko, M.R. Acute injections of the NMDA receptor antagonist memantine rescue performance deficits of the Ts65Dn mouse model of Down syndrome on a fear conditioning test. Neuropsychopharmacology 2008, 33, 1624–1632. [Google Scholar] [CrossRef]
- Dohl, J.; Treadwell, Z.; Norris, C.; Head, E. Calcineurin inhibition may prevent Alzheimer disease in people with Down syndrome. Alzheimers Dement. 2025, 21, e70034. [Google Scholar] [CrossRef]
- Shpak, M.; Ghanavi, H.R.; Lange, J.D.; Pool, J.E.; Stensmyr, M.C. Genomes from historical Drosophila melanogaster specimens illuminate adaptive and demographic changes across more than 200 years of evolution. PLoS Biol. 2023, 21, e3002333. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Fernandez, H.R.; Donohue, R.C.; Li, J.; Cheng, J.; Birchler, J.A. Male-specific lethal complex in Drosophila counteracts histone acetylation and does not mediate dosage compensation. Proc. Natl. Acad. Sci. USA 2013, 110, E808–E817. [Google Scholar] [CrossRef] [PubMed]
- Nordquist, S.K.; Smith, S.R.; Pierce, J.T. Systematic Functional Characterization of Human 21st Chromosome Orthologs in Caenorhabditis elegans. G3 (Bethesda) 2018, 8, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.L. A new reference genome sequence for Caenorhabditis elegans? Lab Anim. 2019, 48, 267–268. [Google Scholar] [CrossRef]
- Blaise, B.J.; Giacomotto, J.; Triba, M.N.; Toulhoat, P.; Piotto, M.; Emsley, L.; Segalat, L.; Dumas, M.E.; Elena, B. Metabolic profiling strategy of Caenorhabditis elegans by whole-organism nuclear magnetic resonance. J. Proteome Res. 2009, 8, 2542–2550. [Google Scholar] [CrossRef]
- Crouzier, L.; Richard, E.M.; Sourbron, J.; Lagae, L.; Maurice, T.; Delprat, B. Use of Zebrafish Models to Boost Research in Rare Genetic Diseases. Int. J. Mol. Sci. 2021, 22, 13356. [Google Scholar] [CrossRef]
- Chapman, L.R.; Ramnarine, I.V.P.; Zemke, D.; Majid, A.; Bell, S.M. Gene Expression Studies in Down Syndrome: What Do They Tell Us about Disease Phenotypes? Int. J. Mol. Sci. 2024, 25, 2968. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Wang, J.; Ren, C.; Chen, H.; Zhang, J. DYRK1A inhibitors for disease therapy: Current status and perspectives. Eur. J. Med. Chem. 2022, 229, 114062. [Google Scholar] [CrossRef]
- Tlili, A.; Hoischen, A.; Ripoll, C.; Benabou, E.; Badel, A.; Ronan, A.; Touraine, R.; Grattau, Y.; Stora, S.; van Bon, B.; et al. BDNF and DYRK1A are variable and inversely correlated in lymphoblastoid cell lines from Down syndrome patients. Mol. Neurobiol. 2012, 46, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Slack, B.E.; Uchiyama, J.; Zhdanova, I.V. Zebrafish as a genetic model in biological and behavioral gerontology: Where development meets aging in vertebrates--a mini-review. Gerontology 2009, 55, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Evsiukova, V.S.; Sorokin, I.E.; Kulikov, P.A.; Kulikov, A.V. Alterations in the brain serotonin system and serotonin-regulated behavior during aging in zebrafish males and females. Behav. Brain Res. 2024, 466, 115000. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Ericsson, A.C.; Crim, M.J.; Franklin, C.L. A brief history of animal modeling. Mo. Med. 2013, 110, 201–205. [Google Scholar]
- Farrell, C.; Mumford, P.; Wiseman, F.K. Rodent Modeling of Alzheimer’s Disease in Down Syndrome: In vivo and ex vivo Approaches. Front. Neurosci. 2022, 16, 909669. [Google Scholar] [CrossRef]
- Kawakami, K.; Matsuo, H.; Kajitani, N.; Yamada, T.; Matsumoto, K.I. Comparison of survival rates in four inbred mouse strains under different housing conditions: Effects of environmental enrichment. Exp. Anim. 2022, 71, 150–160. [Google Scholar] [CrossRef]
- Shaw, P.R.; Klein, J.A.; Aziz, N.M.; Haydar, T.F. Longitudinal neuroanatomical and behavioral analyses show phenotypic drift and variability in the Ts65Dn mouse model of Down syndrome. Dis. Models Mech. 2020, 13, dmm046243. [Google Scholar] [CrossRef]
- Alldred, M.J.; Lee, S.H.; Petkova, E.; Ginsberg, S.D. Expression profile analysis of hippocampal CA1 pyramidal neurons in aged Ts65Dn mice, a model of Down syndrome (DS) and Alzheimer’s disease (AD). Brain Struct. Funct. 2015, 220, 2983–2996. [Google Scholar] [CrossRef]
- Bimonte-Nelson, H.A.; Hunter, C.L.; Nelson, M.E.; Granholm, A.C. Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav. Brain Res. 2003, 139, 47–57. [Google Scholar] [CrossRef]
- Demas, G.E.; Nelson, R.J.; Krueger, B.K.; Yarowsky, P.J. Spatial memory deficits in segmental trisomic Ts65Dn mice. Behav. Brain Res. 1996, 82, 85–92. [Google Scholar] [CrossRef]
- Fortress, A.M.; Hamlett, E.D.; Vazey, E.M.; Aston-Jones, G.; Cass, W.A.; Boger, H.A.; Granholm, A.C. Designer receptors enhance memory in a mouse model of Down syndrome. J. Neurosci. 2015, 35, 1343–1353. [Google Scholar] [CrossRef]
- Granholm, A.C.; Ford, K.A.; Hyde, L.A.; Bimonte, H.A.; Hunter, C.L.; Nelson, M.; Albeck, D.; Sanders, L.A.; Mufson, E.J.; Crnic, L.S. Estrogen restores cognition and cholinergic phenotype in an animal model of Down syndrome. Physiol. Behav. 2002, 77, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Guidi, S.; Stagni, F.; Bianchi, P.; Ciani, E.; Ragazzi, E.; Trazzi, S.; Grossi, G.; Mangano, C.; Calza, L.; Bartesaghi, R. Early pharmacotherapy with fluoxetine rescues dendritic pathology in the Ts65Dn mouse model of down syndrome. Brain Pathol. 2013, 23, 129–143. [Google Scholar] [CrossRef]
- Hamlett, E.D.; Boger, H.A.; Ledreux, A.; Kelley, C.M.; Mufson, E.J.; Falangola, M.F.; Guilfoyle, D.N.; Nixon, R.A.; Patterson, D.; Duval, N.; et al. Cognitive Impairment, Neuroimaging, and Alzheimer Neuropathology in Mouse Models of Down Syndrome. Curr. Alzheimer Res. 2016, 13, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Granholm, A.C.; Sanders, L.A.; Crnic, L.S. Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down’s syndrome. Exp. Neurol. 2000, 161, 647–663. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; Block, A.; Tong, S.; Davisson, M.T.; Gardiner, K.J. Age exacerbates abnormal protein expression in a mouse model of Down syndrome. Neurobiol. Aging 2017, 57, 120–132. [Google Scholar] [CrossRef]
- Driscoll, L.L.; Carroll, J.C.; Moon, J.; Crnic, L.S.; Levitsky, D.A.; Strupp, B.J. Impaired sustained attention and error-induced stereotypy in the aged Ts65Dn mouse: A mouse model of Down syndrome and Alzheimer’s disease. Behav. Neurosci. 2004, 118, 1196–1205. [Google Scholar] [CrossRef]
- Escorihuela, R.M.; Vallina, I.F.; Martinez-Cue, C.; Baamonde, C.; Dierssen, M.; Tobena, A.; Florez, J.; Fernandez-Teruel, A. Impaired short- and long-term memory in Ts65Dn mice, a model for Down syndrome. Neurosci. Lett. 1998, 247, 171–174. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Johnson, N.R.; Boyd, T.D.; Coughlan, C.; Chial, H.J.; Potter, H. Innate Immune System Activation and Neuroinflammation in Down Syndrome and Neurodegeneration: Therapeutic Targets or Partners? Front. Aging Neurosci. 2021, 13, 718426. [Google Scholar] [CrossRef]
- Hamlett, E.D.; Hjorth, E.; Ledreux, A.; Gilmore, A.; Schultzberg, M.; Granholm, A.C. RvE1 treatment prevents memory loss and neuroinflammation in the Ts65Dn mouse model of Down syndrome. Glia 2020, 68, 1347–1360. [Google Scholar] [CrossRef]
- Alldred, M.J.; Martini, A.C.; Patterson, D.; Hendrix, J.; Granholm, A.C. Aging with Down Syndrome-Where Are We Now and Where Are We Going? J. Clin. Med. 2021, 10, 4687. [Google Scholar] [CrossRef]
- Head, E.; Lott, I.T.; Wilcock, D.M.; Lemere, C.A. Aging in Down Syndrome and the Development of Alzheimer’s Disease Neuropathology. Curr. Alzheimer Res. 2016, 13, 18–29. [Google Scholar] [CrossRef]
- Martini, A.C.; Helman, A.M.; McCarty, K.L.; Lott, I.T.; Doran, E.; Schmitt, F.A.; Head, E. Distribution of microglial phenotypes as a function of age and Alzheimer’s disease neuropathology in the brains of people with Down syndrome. Alzheimers Dement. 2020, 12, e12113. [Google Scholar] [CrossRef]
- Wilcock, D.M.; Griffin, W.S. Down’s syndrome, neuroinflammation, and Alzheimer neuropathogenesis. J. Neuroinflamm. 2013, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Lockrow, J.P.; Fortress, A.M.; Granholm, A.C. Age-related neurodegeneration and memory loss in down syndrome. Curr. Gerontol. Geriatr. Res. 2012, 2012, 463909. [Google Scholar] [CrossRef] [PubMed]
- Aldecoa, I.; Barroeta, I.; Carroll, S.L.; Fortea, J.; Gilmore, A.; Ginsberg, S.D.; Guzman, S.J.; Hamlett, E.D.; Head, E.; Perez, S.E.; et al. Down Syndrome Biobank Consortium: A perspective. Alzheimers Dement. 2024, 20, 2262–2272. [Google Scholar] [CrossRef]
- Di Domenico, F.; Tramutola, A.; Barone, E.; Lanzillotta, C.; Defever, O.; Arena, A.; Zuliani, I.; Foppoli, C.; Iavarone, F.; Vincenzoni, F.; et al. Restoration of aberrant mTOR signaling by intranasal rapamycin reduces oxidative damage: Focus on HNE-modified proteins in a mouse model of down syndrome. Redox Biol. 2019, 23, 101162. [Google Scholar] [CrossRef] [PubMed]
- Granholm, A.E.; Englund, E.; Gilmore, A.; Head, E.; Yong, W.H.; Perez, S.E.; Guzman, S.J.; Hamlett, E.D.; Mufson, E.J. Neuropathological findings in Down syndrome, Alzheimer’s disease and control patients with and without SARS-CoV: Preliminary findings. Acta Neuropathol. 2024, 147, 92. [Google Scholar] [CrossRef]
- Saternos, H.; Hamlett, E.D.; Guzman, S.; Head, E.; Granholm, A.C.; Ledreux, A. Unique Pathology in the Locus Coeruleus of Individuals with Down Syndrome. J. Alzheimers Dis. 2024, 101, 541–561. [Google Scholar] [CrossRef]
- Ledreux, A.; Thomas, S.; Hamlett, E.D.; Trautman, C.; Gilmore, A.; Rickman Hager, E.; Paredes, D.A.; Margittai, M.; Fortea, J.; Granholm, A.C. Small Neuron-Derived Extracellular Vesicles from Individuals with Down Syndrome Propagate Tau Pathology in the Wildtype Mouse Brain. J. Clin. Med. 2021, 10, 3931. [Google Scholar] [CrossRef]
- Di Domenico, F.; Tramutola, A.; Foppoli, C.; Head, E.; Perluigi, M.; Butterfield, D.A. mTOR in Down syndrome: Role in Ass and tau neuropathology and transition to Alzheimer disease-like dementia. Free Radic. Biol. Med. 2018, 114, 94–101. [Google Scholar] [CrossRef]
- Granholm, A.C.; Hamlett, E.D. The Role of Tau Pathology in Alzheimer’s Disease and Down Syndrome. J. Clin. Med. 2024, 13, 1338. [Google Scholar] [CrossRef]
- Winston, C.N.; Aulston, B.; Rockenstein, E.M.; Adame, A.; Prikhodko, O.; Dave, K.N.; Mishra, P.; Rissman, R.A.; Yuan, S.H. Neuronal Exosome-Derived Human Tau is Toxic to Recipient Mouse Neurons in vivo. J. Alzheimers Dis. 2019, 67, 541–553. [Google Scholar] [CrossRef]
- Bennett, R.E.; DeVos, S.L.; Dujardin, S.; Corjuc, B.; Gor, R.; Gonzalez, J.; Roe, A.D.; Frosch, M.P.; Pitstick, R.; Carlson, G.A.; et al. Enhanced Tau Aggregation in the Presence of Amyloid beta. Am. J. Pathol. 2017, 187, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Furman, J.L.; Vaquer-Alicea, J.; White, C.L., 3rd; Cairns, N.J.; Nelson, P.T.; Diamond, M.I. Widespread tau seeding activity at early Braak stages. Acta Neuropathol. 2017, 133, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Eisenberg, D.S.; Crowther, R.A. Propagation of Tau Aggregates and Neurodegeneration. Annu. Rev. Neurosci. 2017, 40, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Guix, F.X.; Corbett, G.T.; Cha, D.J.; Mustapic, M.; Liu, W.; Mengel, D.; Chen, Z.; Aikawa, E.; Young-Pearse, T.; Kapogiannis, D.; et al. Detection of Aggregation-Competent Tau in Neuron-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2018, 19, 663. [Google Scholar] [CrossRef]
- Guo, J.L.; Lee, V.M. Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J. Biol. Chem. 2011, 286, 15317–15331. [Google Scholar] [CrossRef]
- Haruyama, N.; Cho, A.; Kulkarni, A.B. Overview: Engineering transgenic constructs and mice. Curr. Protoc. Cell Biol. 2009, 42, 19-10. [Google Scholar] [CrossRef]
- Ogaki, A.; Ikegaya, Y.; Koyama, R. Replacement of Mouse Microglia with Human Induced Pluripotent Stem Cell (hiPSC)-Derived Microglia in Mouse Organotypic Slice Cultures. Front. Cell. Neurosci. 2022, 16, 918442. [Google Scholar] [CrossRef]
- Duchon, A.; Del Mar Muniz Moreno, M.; Chevalier, C.; Nalesso, V.; Andre, P.; Fructuoso-Castellar, M.; Mondino, M.; Po, C.; Noblet, V.; Birling, M.C.; et al. Ts66Yah, a mouse model of Down syndrome with improved construct and face validity. Dis. Model Mech. 2022, 15. [Google Scholar] [CrossRef]
- Emili, M.; Stagni, F.; Guidi, S.; Russo, C.; Chevalier, C.; Duchon, A.; Herault, Y.; Bartesaghi, R. Dendritic phenotype and proliferation potency in the hippocampal dentate gyrus of the Ts66Yah model of Down syndrome. Neurosci. Lett. 2025, 850, 138156. [Google Scholar] [CrossRef]
- Moreau, M.; Madani, A.; Dard, R.; Romero, N.; Ringot, M.; d’Ortho, M.P.; Bokov, P.; Janel, N.; Matrot, B. Neonatal obstructive sleep apneas in a mouse model of Down syndrome. J. Neurophysiol. 2025, 133, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Goodliffe, J.W.; Olmos-Serrano, J.L.; Aziz, N.M.; Pennings, J.L.; Guedj, F.; Bianchi, D.W.; Haydar, T.F. Absence of Prenatal Forebrain Defects in the Dp(16)1Yey/+ Mouse Model of Down Syndrome. J. Neurosci. 2016, 36, 2926–2944. [Google Scholar] [CrossRef] [PubMed]
- Lana-Elola, E.; Cater, H.; Watson-Scales, S.; Greenaway, S.; Muller-Winkler, J.; Gibbins, D.; Nemes, M.; Slender, A.; Hough, T.; Keskivali-Bond, P.; et al. Comprehensive phenotypic analysis of the Dp1Tyb mouse strain reveals a broad range of Down syndrome-related phenotypes. Dis. Model Mech. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dyakin, V.V.; Branch, C.A.; Ardekani, B.; Yang, D.; Guilfoyle, D.N.; Peterson, J.; Peterhoff, C.; Ginsberg, S.D.; Cataldo, A.M.; et al. In vivo MRI identifies cholinergic circuitry deficits in a Down syndrome model. Neurobiol. Aging 2009, 30, 1453–1465. [Google Scholar] [CrossRef]
- Siegel, A.E.; Bianchi, D.W.; Guedj, F. Visual discrimination and inhibitory control deficits in mouse models of Down syndrome: A pilot study using rodent touchscreen technology. J. Neurosci. Res. 2023, 101, 492–507. [Google Scholar] [CrossRef]
- Troca-Marin, J.A.; Casanas, J.J.; Benito, I.; Montesinos, M.L. The Akt-mTOR pathway in Down’s syndrome: The potential use of rapamycin/rapalogs for treating cognitive deficits. CNS Neurol. Disord. Drug Targets 2014, 13, 34–40. [Google Scholar] [CrossRef]
- Olson, L.E.; Roper, R.J.; Sengstaken, C.L.; Peterson, E.A.; Aquino, V.; Galdzicki, Z.; Siarey, R.; Pletnikov, M.; Moran, T.H.; Reeves, R.H. Trisomy for the Down syndrome ‘critical region’ is necessary but not sufficient for brain phenotypes of trisomic mice. Hum. Mol. Genet. 2007, 16, 774–782. [Google Scholar] [CrossRef]
- Arima-Yoshida, F.; Raveau, M.; Shimohata, A.; Amano, K.; Fukushima, A.; Watanave, M.; Kobayashi, S.; Hattori, S.; Usui, M.; Sago, H.; et al. Impairment of spatial memory accuracy improved by Cbr1 copy number resumption and GABA(B) receptor-dependent enhancement of synaptic inhibition in Down syndrome model mice. Sci. Rep. 2020, 10, 14187. [Google Scholar] [CrossRef]
- Kazuki, Y.; Gao, F.J.; Li, Y.; Moyer, A.J.; Devenney, B.; Hiramatsu, K.; Miyagawa-Tomita, S.; Abe, S.; Kazuki, K.; Kajitani, N.; et al. A non-mosaic transchromosomic mouse model of down syndrome carrying the long arm of human chromosome 21. Elife 2020, 9, e56223. [Google Scholar] [CrossRef]
- Herault, Y.; Delabar, J.M.; Fisher, E.M.C.; Tybulewicz, V.L.J.; Yu, E.; Brault, V. Rodent models in Down syndrome research: Impact and future opportunities. Dis. Model Mech. 2017, 10, 1165–1186. [Google Scholar] [CrossRef]
- Demas, G.E.; Nelson, R.J.; Krueger, B.K.; Yarowsky, P.J. Impaired spatial working and reference memory in segmental trisomy (Ts65Dn) mice. Behav. Brain Res. 1998, 90, 199–201. [Google Scholar] [CrossRef]
- Rueda, N.; Florez, J.; Martinez-Cue, C. Mouse models of Down syndrome as a tool to unravel the causes of mental disabilities. Neural. Plast. 2012, 2012, 584071. [Google Scholar] [CrossRef]
- Salehi, A.; Delcroix, J.D.; Belichenko, P.V.; Zhan, K.; Wu, C.; Valletta, J.S.; Takimoto-Kimura, R.; Kleschevnikov, A.M.; Sambamurti, K.; Chung, P.P.; et al. Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron 2006, 51, 29–42. [Google Scholar] [CrossRef]
- Troublesome variability in mouse studies. Nat. Neurosci. 2009, 12, 1075. [CrossRef] [PubMed]
- Casellas, J. Inbred mouse strains and genetic stability: A review. Animal 2011, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.G.; Marfil, V.; Li, C. Use of Caenorhabditis elegans as a model to study Alzheimer’s disease and other neurodegenerative diseases. Front. Genet. 2014, 5, 279. [Google Scholar] [CrossRef] [PubMed]
- Gallrein, C.; Iburg, M.; Michelberger, T.; Kocak, A.; Puchkov, D.; Liu, F.; Ayala Mariscal, S.M.; Nayak, T.; Kaminski Schierle, G.S.; Kirstein, J. Novel amyloid-beta pathology C. elegans model reveals distinct neurons as seeds of pathogenicity. Prog. Neurobiol. 2021, 198, 101907. [Google Scholar] [CrossRef]
- Kokkali, M.; Karali, K.; Thanou, E.; Papadopoulou, M.A.; Zota, I.; Tsimpolis, A.; Efstathopoulos, P.; Calogeropoulou, T.; Li, K.W.; Sidiropoulou, K.; et al. Multimodal beneficial effects of BNN27, a nerve growth factor synthetic mimetic, in the 5xFAD mouse model of Alzheimer’s disease. Mol. Psychiatry 2025, 30, 2265–2283. [Google Scholar] [CrossRef]
- Perluigi, M.; Picca, A.; Montanari, E.; Calvani, R.; Marini, F.; Matassa, R.; Tramutola, A.; Villani, A.; Familiari, G.; Domenico, F.D.; et al. Aberrant crosstalk between insulin signaling and mTOR in young Down syndrome individuals revealed by neuronal-derived extracellular vesicles. Alzheimers Dement. 2022, 18, 1498–1510. [Google Scholar] [CrossRef]
- Wohlfert, A.J.; Phares, J.; Granholm, A.C. The mTOR Pathway: A Common Link Between Alzheimer’s Disease and Down Syndrome. J. Clin. Med. 2024, 13, 6183. [Google Scholar] [CrossRef]
- Caldwell, A.L.M.; Sancho, L.; Deng, J.; Bosworth, A.; Miglietta, A.; Diedrich, J.K.; Shokhirev, M.N.; Allen, N.J. Aberrant astrocyte protein secretion contributes to altered neuronal development in multiple models of neurodevelopmental disorders. Nat. Neurosci. 2022, 25, 1163–1178. [Google Scholar] [CrossRef]
- Duval, N.; Vacano, G.N.; Patterson, D. Rapamycin Treatment Ameliorates Age-Related Accumulation of Toxic Metabolic Intermediates in Brains of the Ts65Dn Mouse Model of Down Syndrome and Aging. Front. Aging Neurosci. 2018, 10, 263. [Google Scholar] [CrossRef]
- Grimm, J.; Bhayadia, R.; Gack, L.; Heckl, D.; Klusmann, J.H. Combining LSD1 and JAK-STAT inhibition targets Down syndrome-associated myeloid leukemia at its core. Leukemia 2022, 36, 1926–1930. [Google Scholar] [CrossRef]
- Hsu, C.J.; Schraw, J.M.; Desrosiers, T.A.; Janitz, A.E.; Kirby, R.S.; Nestoridi, E.; Nembhard, W.N.; Salemi, J.L.; Shumate, C.; Tanner, J.P.; et al. All genetic subtypes of B-cell acute lymphoblastic leukemia exhibit increased incidence rates in children with Down syndrome. Leukemia 2025, 39, 1516–1519. [Google Scholar] [CrossRef] [PubMed]
- Philips, R.L.; Wang, Y.; Cheon, H.; Kanno, Y.; Gadina, M.; Sartorelli, V.; Horvath, C.M.; Darnell, J.E., Jr.; Stark, G.R.; O’Shea, J.J. The JAK-STAT pathway at 30: Much learned, much more to do. Cell 2022, 185, 3857–3876. [Google Scholar] [CrossRef] [PubMed]
- Rachubinski, A.L.; Wallace, E.; Gurnee, E.; Estrada, B.A.E.; Worek, K.R.; Smith, K.P.; Araya, P.; Waugh, K.A.; Granrath, R.E.; Britton, E.; et al. JAK inhibition decreases the autoimmune burden in Down syndrome. medRxiv 2024. [Google Scholar] [CrossRef]
- Chung, H.; Green, P.H.R.; Wang, T.C.; Kong, X.F. Interferon-Driven Immune Dysregulation in Down Syndrome: A Review of the Evidence. J. Inflamm. Res. 2021, 14, 5187–5200. [Google Scholar] [CrossRef]
- Espinosa, J.M. Down Syndrome and COVID-19: A Perfect Storm? Cell Rep. Med. 2020, 1, 100019. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.D.; Lewis, H.C.; Hill, A.A.; Pandey, A.; Jackson, L.P.; Cabral, J.M.; Smith, K.P.; Liggett, L.A.; Gomez, E.B.; Galbraith, M.D.; et al. Trisomy 21 consistently activates the interferon response. Elife 2016, 5, e16220. [Google Scholar] [CrossRef] [PubMed]
- Head, E.; Phelan, M.J.; Doran, E.; Kim, R.C.; Poon, W.W.; Schmitt, F.A.; Lott, I.T. Cerebrovascular pathology in Down syndrome and Alzheimer disease. Acta Neuropathol. Commun. 2017, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, Z.; Peng, Y.; Jiang, Y.; Zhang, X.; Zhu, H.; Zhang, L.; Chen, J.; Shu, X.; Luo, M.; et al. Embryonic organizer formation disorder leads to multiorgan dysplasia in Down syndrome. Cell Death Dis. 2022, 13, 1054. [Google Scholar] [CrossRef]
- Giffin-Rao, Y.; Sheng, J.; Strand, B.; Xu, K.; Huang, L.; Medo, M.; Risgaard, K.A.; Dantinne, S.; Mohan, S.; Keshan, A.; et al. Altered patterning of trisomy 21 interneuron progenitors. Stem Cell Reports 2022, 17, 1366–1379. [Google Scholar] [CrossRef]
- Meijer, L.; Chretien, E.; Ravel, D. Leucettinib-21, a DYRK1A Kinase Inhibitor as Clinical Drug Candidate for Alzheimer’s Disease and Down Syndrome. J. Alzheimers Dis. 2024, 101, S95–S113, Erratum in J. Alzheimers Dis. 2025, 103, 975. [Google Scholar] [CrossRef]
- McLaren, M.; Butts, J. Notch signaling in neurogenesis. Development 2025, 152. [Google Scholar] [CrossRef]
- Yusof, H.H.; Lee, H.C.; Seth, E.A.; Wu, X.; Hewitt, C.A.; Scott, H.S.; Cheah, P.S.; Li, Y.M.; Chau, D.M.; Ling, K.H. Expression Profiling of Notch Signalling Pathway and Gamma-Secretase Activity in the Brain of Ts1Cje Mouse Model of Down Syndrome. J. Mol. Neurosci. 2019, 67, 632–642. [Google Scholar] [CrossRef]
- Hammerle, B.; Ulin, E.; Guimera, J.; Becker, W.; Guillemot, F.; Tejedor, F.J. Transient expression of Mnb/Dyrk1a couples cell cycle exit and differentiation of neuronal precursors by inducing p27KIP1 expression and suppressing NOTCH signaling. Development 2011, 138, 2543–2554. [Google Scholar] [CrossRef]
- Xu, C.; Ramos, T.B.; Marshall, O.J.; Doe, C.Q. Notch signaling and Bsh homeodomain activity are integrated to diversify Drosophila lamina neuron types. Elife 2024, 12, RP90136. [Google Scholar] [CrossRef]
- Andriatsilavo, M.; Barata, C.; Reifenstein, E.; Dumoulin, A.; Tao Griffin, T.; Dutta, S.B.; Stoeckli, E.T.; von Kleist, M.; Hiesinger, P.R.; Hassan, B.A. Sequential and independent probabilistic events regulate differential axon targeting during development in Drosophila melanogaster. Nat. Neurosci. 2025, 28, 998–1011. [Google Scholar] [CrossRef]
- Poon, C.H.; Wang, Y.; Fung, M.L.; Zhang, C.; Lim, L.W. Rodent Models of Amyloid-Beta Feature of Alzheimer’s Disease: Development and Potential Treatment Implications. Aging Dis. 2020, 11, 1235–1259. [Google Scholar] [CrossRef]
- McKean, N.E.; Handley, R.R.; Snell, R.G. A Review of the Current Mammalian Models of Alzheimer’s Disease and Challenges That Need to Be Overcome. Int. J. Mol. Sci. 2021, 22, 13168. [Google Scholar] [CrossRef]
- Ashe, K.H.; Zahs, K.R. Probing the biology of Alzheimer’s disease in mice. Neuron 2010, 66, 631–645. [Google Scholar] [CrossRef]
- William, C.M.; Saqran, L.; Stern, M.A.; Chiang, C.L.; Herrick, S.P.; Rangwala, A.; Albers, M.W.; Frosch, M.P.; Hyman, B.T. Activity-Dependent Dysfunction in Visual and Olfactory Sensory Systems in Mouse Models of Down Syndrome. J. Neurosci. 2017, 37, 9880–9888. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Buck, J.M.; Borski, C.; Pafford, J.T.; Keller, B.N.; Milstead, R.A.; Hanson, J.L.; Stitzel, J.A.; Hoeffer, C.A. RCAN1 knockout and overexpression recapitulate an ensemble of rest-activity and circadian disruptions characteristic of Down syndrome, Alzheimer’s disease, and normative aging. J. Neurodev. Disord. 2022, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.S.; Park, J.H.; Ryu, Y.S.; Choi, S.H.; Yoon, S.H.; Kwen, M.Y.; Oh, J.Y.; Song, W.J.; Chung, S.H. Regulation of RCAN1 protein activity by Dyrk1A protein-mediated phosphorylation. J. Biol. Chem. 2011, 286, 40401–40412. [Google Scholar] [CrossRef] [PubMed]
- Sasaguri, H.; Nilsson, P.; Hashimoto, S.; Nagata, K.; Saito, T.; De Strooper, B.; Hardy, J.; Vassar, R.; Winblad, B.; Saido, T.C. APP mouse models for Alzheimer’s disease preclinical studies. EMBO J. 2017, 36, 2473–2487. [Google Scholar] [CrossRef]
- Tai, L.M.; Maldonado Weng, J.; LaDu, M.J.; Brady, S.T. Relevance of transgenic mouse models for Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 2021, 177, 1–48. [Google Scholar] [CrossRef]
- Welikovitch, L.A.; Mate de Gerando, A.; Khasnavis, A.; Bhavsar, H.; Meltzer, J.C.; Buee, L.; Chibnik, L.B.; Bussiere, T.; Hyman, B.T. Tau, synapse loss and gliosis progress in an Alzheimer’s mouse model after amyloid-beta immunotherapy. Brain 2025, 148, 1316–1328. [Google Scholar] [CrossRef]
- Jaramillo, S.; Zador, A.M. Mice and rats achieve similar levels of performance in an adaptive decision-making task. Front. Syst. Neurosci. 2014, 8, 173. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).