Redefining Chemoresistance: Natural Bioactives as Molecular Modulators at the Cancer–Tumor Microenvironment Interface
Abstract
1. Introduction
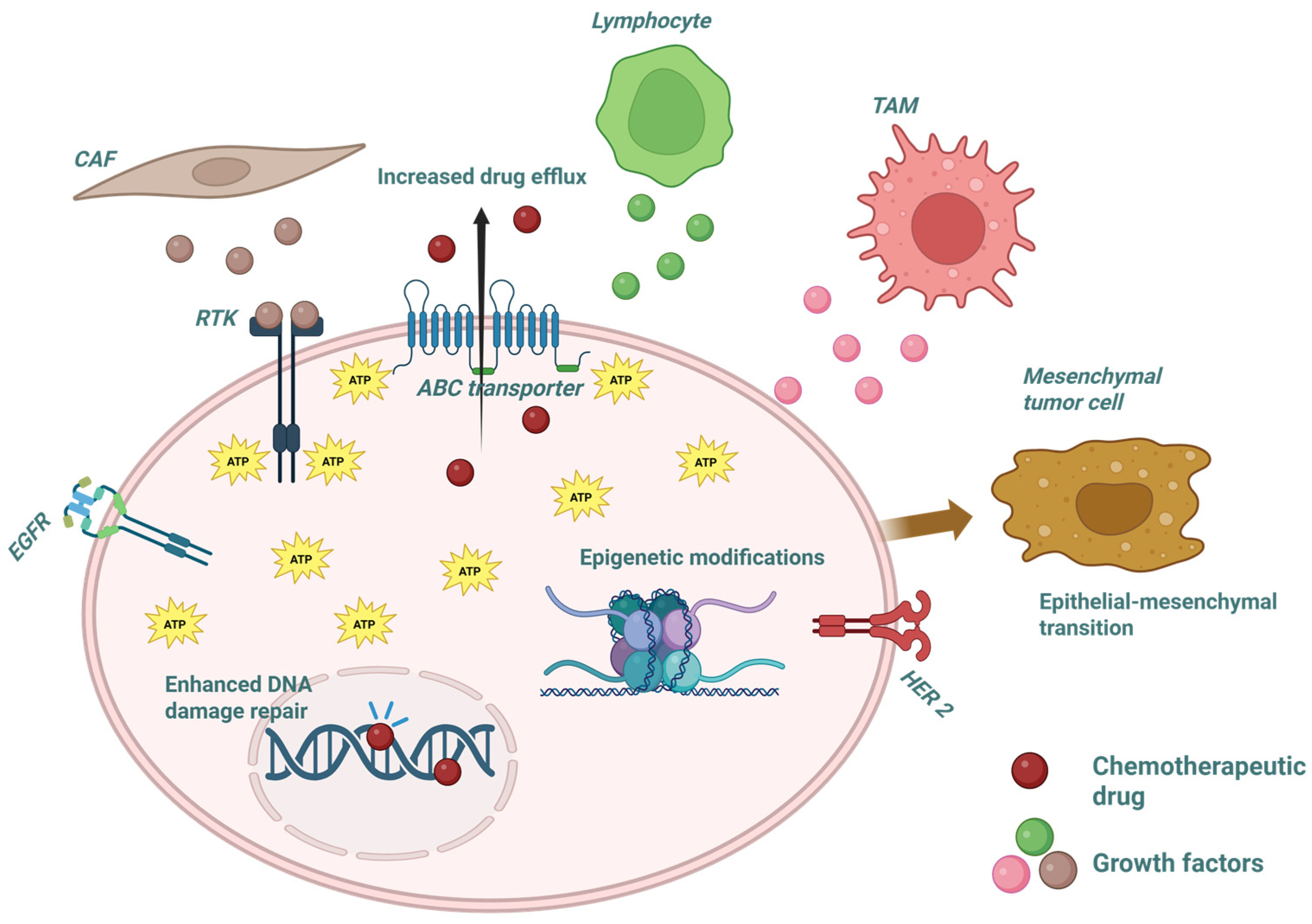
2. Key Molecular Mechanisms Underlying Therapeutic Resistance
3. The Tumor Microenvironment as a Co-Regulator of Resistance
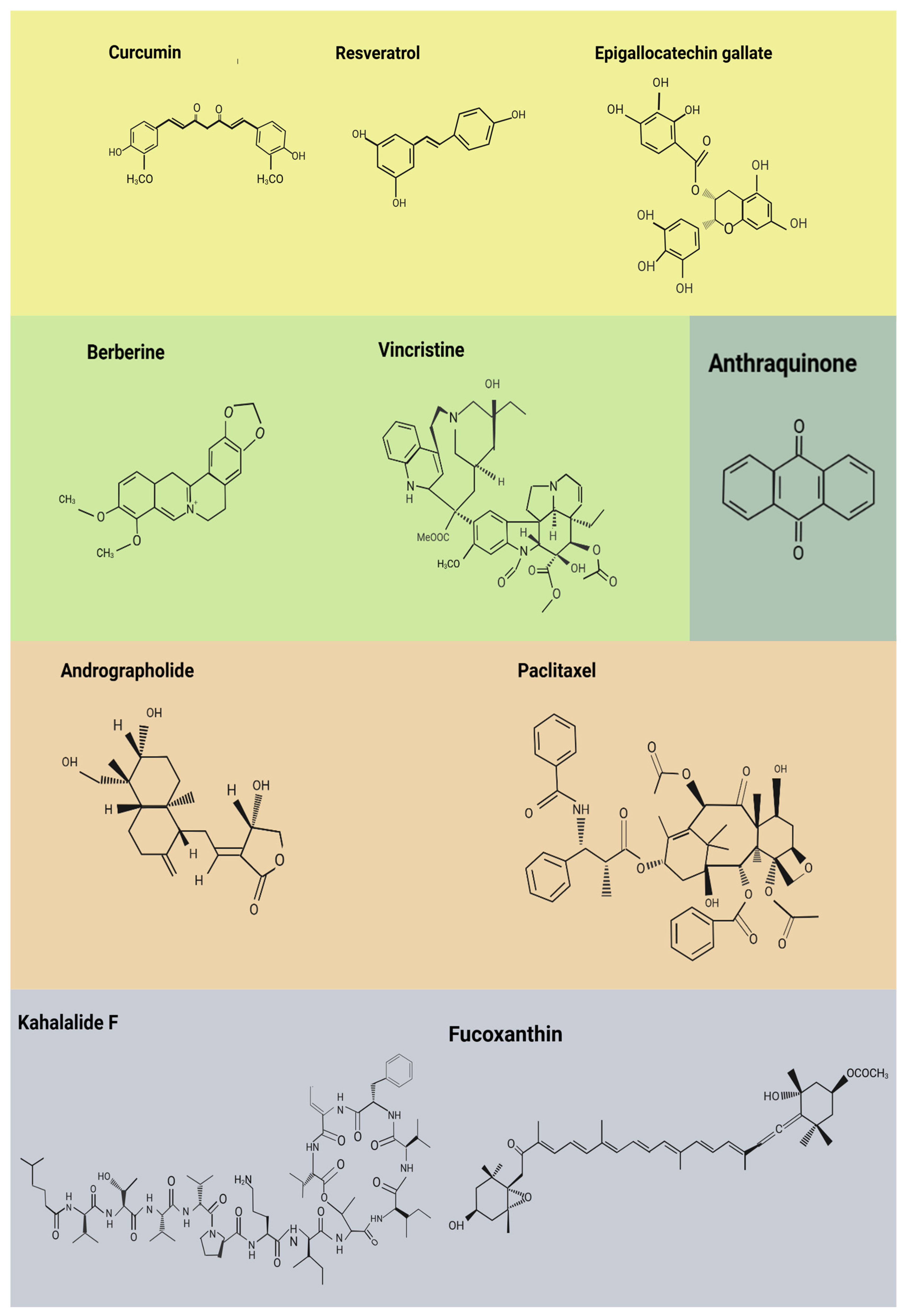
4. Natural Bioactive Compounds with Functional Evidence
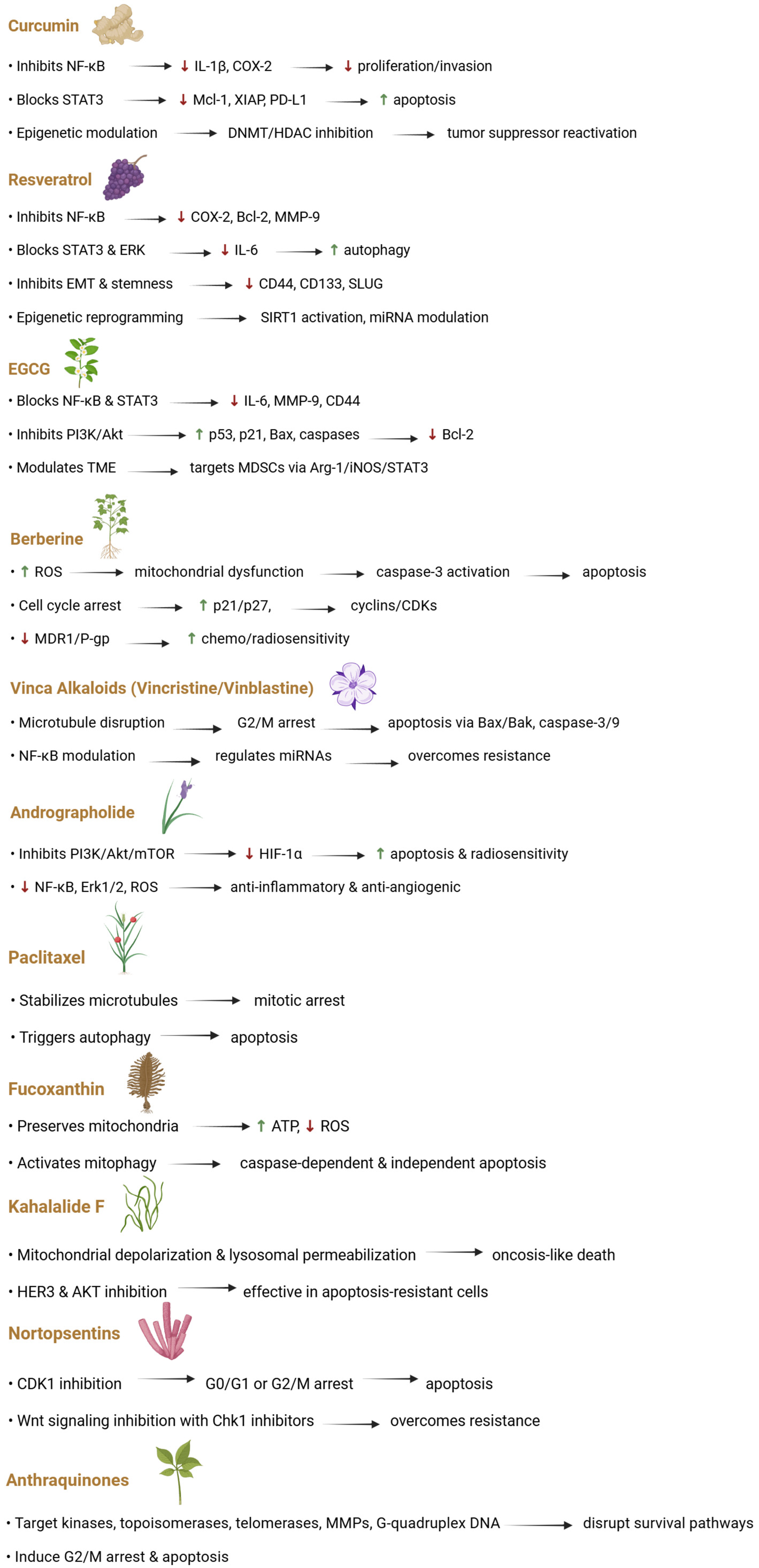
4.1. Polyphenols
4.2. Alkaloids
4.3. Terpenoids and Diterpenes
4.4. Bioactive Lipids and Marine-Derived Metabolites
4.5. Anthraquinones and Cobalamins
5. Emerging Strategies for Therapeutic Combination
| Type | Natural Compound | Chemotherapy Drug | Tissue and Origin | Mechanism |
|---|---|---|---|---|
| Polyphenol | Curcumin [226] | Doxorubicin | Breast, tumoral | Inhibits the ATPase function of ABCB4 while leaving its protein expression levels unchanged. |
| Resveratrol [227] | 5-fluorouracil | Colon, tumoral | Regulates the TNF-β signaling cascade, promotes apoptosis, and inhibits NFκB pathway activation. | |
| Resveratrol [228] | Cisplatin | Basal alveolar epithelial, tumoral | Triggers apoptosis by influencing autophagy-related cell death mechanisms. | |
| Urolithin A [229] | Oxaliplatin | Colon, tumoral | Stabilization of p53 and activation of its downstream target genes, leading to control of the cell cycle and suppression of glycolysis. | |
| Alkaloid | Neferine and isoliensinine [230] | Cisplatin | Colon, tumoral | Enhanced cellular absorption of Cisplatin and initiation of mitochondrial apoptosis through a ROS-dependent pathway. |
| Berberine [231] | Cisplatin | Ovarian, tumoral | Reduction in cell growth along with the promotion of both apoptosis and necroptosis as forms of cell death. | |
| Emetine [232] | Cisplatin | Ovarian, tumoral | Decreased cell survival capacity. | |
| Tetrandrine [233] | Cisplatin | Breast, tumoral | Initiation of apoptosis through a mechanism mediated by ROS. | |
| Piperlongumine [234] | Doxorubicin | Prostate, tumoral | Inhibitory effect on cell proliferation and promotion of apoptosis, marked by increased levels of cleaved poly (ADP-ribose) polymerase and caspase-3 proteins. | |
| Piperlongumine [235] | Paclitaxel | Intestinal, tumoral | Cell death triggered by ROS. | |
| Terpenoid | Oridonin [236] | Cisplatin | Bronchial epithelium cell, tumoral | Apoptosis initiation via activation of autophagosomes through the AMPK/Akt/mTOR signaling pathway. |
| Borneol [237] | Doxorubicin | Glioma cell, tumoral | Borneol increases the cellular absorption of doxorubicin and stimulates the generation of ROS. | |
| Vielanin k [238] | Doxorubicin | Breast and mammary cell, tumoral and non-tumoral | Triggering of endoplasmic reticulum stress and mitochondrial apoptosis through the IRE1α-TRAF2-JNK signaling pathway. | |
| Vielanin P [239] | Doxorubicin | Breast and myelegenous leukemia cell, tumoral | Promotion of doxorubicin buildup by decreasing MRP1 expression through the PI3K/Nrf2 signaling pathway. | |
| Ginkgolide B [240] | Gemcitabine | Pancreatic cell, tumoral | Inhibition of NFκB activity combined with an enhancement of antiproliferative effects. | |
| Pachymic acid and dehydrotumulosic acid [241] | Doxorubicin and Cisplatin | Liver, breast, and lung cells, tumoral | Blockage of P-glycoprotein (Pgp) activity leading to increased doxorubicin accumulation and amplification of its biological effects. |
6. Current Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| ABCB1 | ATP-binding cassette sub-family B member 1 |
| Akt | protein kinase B |
| AMPK | adenosine monophosphate-activated protein kinase |
| Arg-1 | arginase 1 |
| ATP | adenosine triphosphate |
| BAK | Bcl-2 antagonist/killer 1 |
| BAX | Bcl-2-associated X protein |
| BCL | B-cell lymphoma |
| CAAs | cancer-associated adipocyte |
| CAFs | cancer-associated fibroblasts |
| CSCs | cancer stem cells |
| CTL | cytotoxic T lymphocyte |
| DNMTs | DNA methyltransferases |
| ECM | extracellular matrix |
| EGCG | epigallocatechin gallate |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| EMT | epithelial-to-mesenchymal transition |
| ERK | extracellular signal-regulated kinase |
| FAK | focal adhesion kinase |
| FGF | fibroblast growth factor |
| FX | fucoxanthin |
| GADD153 | growth arrest and DNA damage-inducible protein 153 |
| HDACs | histone deacetylases |
| HER | human epidermal growth factor receptor |
| HIFs | hypoxia-inducible factors |
| IGF-1 | insulin-like growth factor-1 |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| JAK | Janus kinase |
| KF | kahalalide F |
| MAPK | mitogen-activated protein kinase |
| MCL | myeloid cell leukemia |
| MDR | multidrug resistance |
| MDR1 | multidrug resistance protein 1 |
| MDSCs | myeloid-derived suppressor cells |
| MHC | major histocompatibility complex |
| MMPs | matrix metalloproteinases |
| mTOR | mammalian target of rapamycin |
| mTORc | mammalian target of rapamycin complex |
| NPs | nanoparticles |
| NF-κB | nuclear factor-kappa B |
| NSCLC | non-small-cell lung cancer |
| P-gp | P-glycoprotein |
| PD-1 | programmed cell death protein 1 |
| PD-L1 | programmed cell death 1 ligand 1 |
| PDGF | platelet-derived growth factor |
| PI3K | phosphoinositide 3-kinase |
| Tregs | regulatory T cells |
| ROS | reactive oxygen species |
| RTK | receptor tyrosine kinase |
| STAT | signal transducer and activator of transcription |
| TAMs | tumor-associated macrophages |
| TGF-β | transforming growth factor beta |
| TME | tumor microenvironment |
| TNF-α | tumor necrosis factor alpha |
| TNF-β | tumor necrosis factor beta |
| VEGF | vascular endothelial growth factor |
| Wnt | wingless-type MMTV integration site family |
References
- Dakal, T.C.; Sharma, N.K.; Sharma, A. Editorial: Revisiting the challenges and opportunities in cancer drug resistance. Front. Mol. Biosci. 2024, 11, 1497754. [Google Scholar] [CrossRef]
- Shaham, S.H.; Vij, P.; Tripathi, M.K. Advances in Targeted and Chemotherapeutic Strategies for Colorectal Cancer: Current Insights and Future Directions. Biomedicines 2025, 13, 642. [Google Scholar] [CrossRef] [PubMed]
- Rosendo-Chalma, P.; Díaz-Landy, E.N.; Antonio-Véjar, V.; Ortiz Tejedor, J.G.; Reytor-González, C.; Simancas-Racines, D.; Bigoni-Ordóñez, G.D. Endometriosis: Challenges in Clinical Molecular Diagnostics and Treatment. Int. J. Mol. Sci. 2025, 26, 3979. [Google Scholar] [CrossRef]
- Cortesi, M.; Rossino, G.; Chakrabarty, A.; Rossi, D. Editorial: Tumor adaptation to cellular stresses: Mechanisms, biomarkers and therapeutic opportunities. Front. Med. 2023, 10, 1268976. [Google Scholar] [CrossRef]
- Oršolić, N.; Jazvinšćak Jembrek, M. Potential Strategies for Overcoming Drug Resistance Pathways Using Propolis and Its Polyphenolic/Flavonoid Compounds in Combination with Chemotherapy and Radiotherapy. Nutrients 2024, 16, 3741. [Google Scholar] [CrossRef]
- Fatima, S. Tumor Microenvironment: A Complex Landscape of Cancer Development and Drug Resistance. Cureus 2025, 17, e82090. [Google Scholar] [CrossRef]
- Yun, H.; Dong, F.; Wei, X.; Yan, X.; Zhang, R.; Zhang, X.; Wang, Y. Role and value of the tumor microenvironment in the progression and treatment resistance of gastric cancer (Review). Oncol. Rep. 2024, 53, 14. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Haque, A.; Brazeau, D.; Amin, A.R. Perspectives on natural compounds in chemoprevention and treatment of cancer: An update with new promising compounds. Eur. J. Cancer 2021, 149, 165–183. [Google Scholar] [CrossRef]
- Abuzahrah, S.S.; Elbehairi, S.E.I.; Bakhsh, T.; Atwa, A.; Juaid, N.; Mekky, R.H. Marine-derived secondary metabolites in oncology: A comprehensive review. Curr. Res. Biotechnol. 2025, 10, 100300. [Google Scholar] [CrossRef]
- Pongen, Y.L.; Thirumurugan, D.; Ramasubburayan, R.; Prakash, S. Harnessing actinobacteria potential for cancer prevention and treatment. Microb. Pathog. 2023, 183, 106324. [Google Scholar] [CrossRef] [PubMed]
- Asma, S.T.; Acaroz, U.; Imre, K.; Morar, A.; Shah, S.R.A.; Hussain, S.Z.; Arslan-Acaroz, D.; Demirbas, H.; Hajrulai-Musliu, Z.; Istanbullugil, F.R.; et al. Natural Products/Bioactive Compounds as a Source of Anticancer Drugs. Cancers 2022, 14, 6203. [Google Scholar] [CrossRef] [PubMed]
- Kaliaperumal, K.; Salendra, L.; Liu, Y.; Ju, Z.; Sahu, S.K.; Elumalai, S.; Subramanian, K.; Alotaibi, N.M.; Alshammari, N.; Saeed, M.; et al. Isolation of anticancer bioactive secondary metabolites from the sponge-derived endophytic fungi Penicillium sp. and in-silico computational docking approach. Front. Microbiol. 2023, 14, 1216928. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, W.; Zhang, H.; Guo, Q.; Yang, W.; Li, B.; Sun, Z.; Gao, S.; Cui, R. Modulation of Multiple Signaling Pathways of the Plant-Derived Natural Products in Cancer. Front. Oncol. 2019, 9, 1153. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Ozleyen, A.; Boyunegmez Tumer, T.; Oluwaseun Adetunji, C.; El Omari, N.; Balahbib, A.; Taheri, Y.; Bouyahya, A.; Martorell, M.; Martins, N.; et al. Natural Products and Synthetic Analogs as a Source of Antitumor Drugs. Biomolecules 2019, 9, 679. [Google Scholar] [CrossRef]
- Ahmed, K.R.; Rahman, M.d.M.; Islam, M.d.N.; Fahim, M.d.M.H.; Rahman, M.A.; Kim, B. Antioxidants activities of phytochemicals perspective modulation of autophagy and apoptosis to treating cancer. Biomed. Pharmacother. 2024, 174, 116497. [Google Scholar] [CrossRef]
- de Oliveira Júnior, R.G.; Christiane Adrielly, A.F.; da Silva Almeida, J.R.G.; Grougnet, R.; Thiéry, V.; Picot, L. Sensitization of tumor cells to chemotherapy by natural products: A systematic review of preclinical data and molecular mechanisms. Fitoterapia 2018, 129, 383–400. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Barakat, M.; Abu-Taha, M.I.; Mahmod, A.I. Targeting Drug Chemo-Resistance in Cancer Using Natural Products. Biomedicines 2021, 9, 1353. [Google Scholar] [CrossRef]
- Zou, J.-Y.; Chen, Q.-L.; Luo, X.-C.; Damdinjav, D.; Abdelmohsen, U.R.; Li, H.-Y.; Battulga, T.; Chen, H.-B.; Wang, Y.-Q.; Zhang, J.-Y. Natural products reverse cancer multidrug resistance. Front. Pharmacol. 2024, 15, 1348076. [Google Scholar] [CrossRef]
- Cruz-Martins, N. Advances in Plants-Derived Bioactives for Cancer Treatment. Cells 2023, 12, 1112. [Google Scholar] [CrossRef]
- Yadav, J.; El Hassani, M.; Sodhi, J.; Lauschke, V.M.; Hartman, J.H.; Russell, L.E. Recent developments in in vitro and in vivo models for improved translation of preclinical pharmacokinetics and pharmacodynamics data. Drug Metab. Rev. 2021, 53, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Ahmed, M.M.S.; Islam, M.A.; Hossain, N.; Chowdhury, M.A. Advances in nanoparticles in targeted drug delivery—A review. Results Surf. Interfaces. 2025, 19, 100529. [Google Scholar] [CrossRef]
- Ammar, M.M.; Ali, R.; Abd Elaziz, N.A.; Habib, H.; Abbas, F.M.; Yassin, M.T.; Maniah, K.; Abdelaziz, R. Nanotechnology in oncology: Advances in biosynthesis, drug delivery, and theranostics. Discov. Oncol. 2025, 16, 1172. [Google Scholar] [CrossRef]
- Manna, D.; Sarkar, D. Multifunctional Role of Astrocyte Elevated Gene-1 (AEG-1) in Cancer: Focus on Drug Resistance. Cancers 2021, 13, 1792. [Google Scholar] [CrossRef]
- Wium, M.; Ajayi-Smith, A.F.; Paccez, J.D.; Zerbini, L.F. The Role of the Receptor Tyrosine Kinase Axl in Carcinogenesis and Development of Therapeutic Resistance: An Overview of Molecular Mechanisms and Future Applications. Cancers 2021, 13, 1521. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Chang, M.; Lin, R.; Zhang, J.; Lu, Y. Updates on altered signaling pathways in tumor drug resistance. Vis. Cancer Med. 2024, 5, 6. [Google Scholar] [CrossRef]
- Garg, P.; Malhotra, J.; Kulkarni, P.; Horne, D.; Salgia, R.; Singhal, S.S. Emerging Therapeutic Strategies to Overcome Drug Resistance in Cancer Cells. Cancers 2024, 16, 2478. [Google Scholar] [CrossRef]
- Xue, X.; Liang, X.-J. Overcoming drug efflux-based multidrug resistance in cancer with nanotechnology. Chin. J. Cancer 2012, 31, 100–109. [Google Scholar] [CrossRef]
- Honeywell, R.J.; Kathmann, I.; Giovannetti, E.; Tibaldi, C.; Smit, E.F.; Rovithi, M.N.; Verheul, H.M.W.; Peters, G.J. Epithelial Transfer of the Tyrosine Kinase Inhibitors Erlotinib, Gefitinib, Afatinib, Crizotinib, Sorafenib, Sunitinib, and Dasatinib: Implications for Clinical Resistance. Cancers 2020, 12, 3322. [Google Scholar] [CrossRef]
- Szczygieł, M.; Markiewicz, M.; Szafraniec, M.J.; Hojda, A.; Fiedor, L.; Urbanska, K. Systemic Mobilization of Breast Cancer Resistance Protein in Response to Oncogenic Stress. Cancers 2022, 14, 313. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Anselmi, M.; Limonta, P. Molecular Mechanisms of Cancer Drug Resistance: Emerging Biomarkers and Promising Targets to Overcome Tumor Progression. Cancers 2022, 14, 1614. [Google Scholar] [CrossRef] [PubMed]
- Doldi, V.; El Bezawy, R.; Zaffaroni, N. MicroRNAs as Epigenetic Determinants of Treatment Response and Potential Therapeutic Targets in Prostate Cancer. Cancers 2021, 13, 2380. [Google Scholar] [CrossRef] [PubMed]
- Seborova, K.; Vaclavikova, R.; Rob, L.; Soucek, P.; Vodicka, P. Non-Coding RNAs as Biomarkers of Tumor Progression and Metastatic Spread in Epithelial Ovarian Cancer. Cancers 2021, 13, 1839. [Google Scholar] [CrossRef]
- Ruiz-Pozo, V.A.; Guevara-Ramírez, P.; Paz-Cruz, E.; Tamayo-Trujillo, R.; Cadena-Ullauri, S.; Frias-Toral, E.; Simancas-Racines, D.; Altuna-Roshkova, Y.; Reytor-González, C.; Zambrano, A.K. The role of the Mediterranean diet in prediabetes management and prevention: A review of molecular mechanisms and clinical outcomes. Food Agric. Immunol. 2024, 35, 2398042. [Google Scholar] [CrossRef]
- Kar, A.; Agarwal, S.; Singh, A.; Bajaj, A.; Dasgupta, U. Insights into molecular mechanisms of chemotherapy resistance in cancer. Transl. Oncol. 2024, 42, 101901. [Google Scholar] [CrossRef]
- Kinnel, B.; Singh, S.K.; Oprea-Ilies, G.; Singh, R. Targeted Therapy and Mechanisms of Drug Resistance in Breast Cancer. Cancers 2023, 15, 1320. [Google Scholar] [CrossRef]
- Gu, Y.; Yang, R.; Zhang, Y.; Guo, M.; Takehiro, K.; Zhan, M.; Yang, L.; Wang, H. Molecular mechanisms and therapeutic strategies in overcoming chemotherapy resistance in cancer. Mol. Biomed. 2025, 6, 2. [Google Scholar] [CrossRef]
- La Rocca, A.; De Gregorio, V.; Lagreca, E.; Vecchione, R.; Netti, P.A.; Imparato, G. Colorectal Cancer Bioengineered Microtissues as a Model to Replicate Tumor-ECM Crosstalk and Assess Drug Delivery Systems In Vitro. Int. J. Mol. Sci. 2023, 24, 5678. [Google Scholar] [CrossRef]
- Marzagalli, M.; Fontana, F.; Raimondi, M.; Limonta, P. Cancer Stem Cells—Key Players in Tumor Relapse. Cancers 2021, 13, 376. [Google Scholar] [CrossRef]
- Salemme, V.; Centonze, G.; Avalle, L.; Natalini, D.; Piccolantonio, A.; Arina, P.; Morellato, A.; Ala, U.; Taverna, D.; Turco, E.; et al. The role of tumor microenvironment in drug resistance: Emerging technologies to unravel breast cancer heterogeneity. Front. Oncol. 2023, 13, 1170264. [Google Scholar] [CrossRef]
- Proietto, M.; Crippa, M.; Damiani, C.; Pasquale, V.; Sacco, E.; Vanoni, M.; Gilardi, M. Tumor heterogeneity: Preclinical models, emerging technologies, and future applications. Front. Oncol. 2023, 13, 1164535. [Google Scholar] [CrossRef]
- Zhu, L.; Jiang, M.; Wang, H.; Sun, H.; Zhu, J.; Zhao, W.; Fang, Q.; Yu, J.; Chen, P.; Wu, S.; et al. A narrative review of tumor heterogeneity and challenges to tumor drug therapy. Ann. Transl. Med. 2021, 9, 1351. [Google Scholar] [CrossRef]
- Chen, S.; Wang, M.; Lu, T.; Liu, Y.; Hong, W.; He, X.; Cheng, Y.; Liu, J.; Wei, Y.; Wei, X. JMJD6 in tumor-associated macrophage regulates macrophage polarization and cancer progression via STAT3/IL-10 axis. Oncogene 2023, 42, 2737–2750. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhu, J.; Wang, Y.; Chen, W.; Fang, S.; Mao, W.; Xu, Z.; Yang, Y.; Weng, Q.; Zhao, Z.; et al. Targeted xCT-mediated Ferroptosis and Protumoral Polarization of Macrophages Is Effective against HCC and Enhances the Efficacy of the Anti-PD-1/L1 Response. Adv. Sci. 2023, 10, 2203973. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, Z.; Chai, Z.; Zhan, Y.; Zhang, Y.; Liu, Z.; Liu, Y.; Li, Z.; Lin, M.; Zhang, Z.; et al. Targeting MS4A4A on tumour-associated macrophages restores CD8+ T-cell-mediated antitumour immunity. Gut 2023, 72, 2307–2320. [Google Scholar] [CrossRef] [PubMed]
- Diaz Bessone, M.I.; Gattas, M.J.; Laporte, T.; Tanaka, M.; Simian, M. The Tumor Microenvironment as a Regulator of Endocrine Resistance in Breast Cancer. Front. Endocrinol. 2019, 10, 547. [Google Scholar] [CrossRef]
- Reytor-González, C.; Simancas-Racines, D.; Román-Galeano, N.M.; Campuzano-Donoso, M.; Carella, A.M.; Zambrano-Villacres, R.; Marinelli, T.; Coppola, L.; Marchetti, M.; Galasso, M.; et al. Obesity and breast cancer: Exploring the nexus of chronic inflammation, metabolic dysregulation, and nutritional strategies. Food Agric. Immunol. 2025, 36, 2521270. [Google Scholar] [CrossRef]
- Simancas-Racines, D.; Campuzano-Donoso, M.; Román-Galeano, N.M.; Zambrano-Villacres, R.; Memoli, P.; Verde, L.; Reytor-González, C.; Carbone, L. Obesity and endometrial cancer: Biological mechanisms, nutritional strategies, and clinical perspectives. Food Agric. Immunol. 2025, 36, 2510961. [Google Scholar] [CrossRef]
- Sarno, G.; Reytor-González, C.; Frias-Toral, E.; Campuzano-Donoso, M.; Katsanos, C.S.; Simancas-Racines, D. Navigating the weight: The impact of obesity on gastrointestinal cancer surgery and strategies for improved outcomes. Semin. Cancer Biol. 2025, 114, 138–149. [Google Scholar] [CrossRef]
- Sarno, G.; Simancas-Racines, D.; Gargiulo, A.; Tedesco, A.; Iacone, B.; Reytor-González, C.; Parise-Vasco, J.M.; Angamarca Iguago, J.; Sarno, S.; Frias-Toral, E.; et al. Impact of obesity on postoperative complications in colorectal cancer surgery: A systematic review and meta-analysis. Semin. Cancer Biol. 2025, 113, 176–189. [Google Scholar] [CrossRef]
- Simancas-Racines, D.; Annunziata, G.; Verde, L.; Fascì-Spurio, F.; Reytor-González, C.; Muscogiuri, G.; Frias-Toral, E.; Barrea, L. Nutritional Strategies for Battling Obesity-Linked Liver Disease: The Role of Medical Nutritional Therapy in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Management. Curr. Obes. Rep. 2025, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Reytor-González, C.; Annunziata, G.; Campuzano-Donoso, M.; Morales-López, T.; Basantes-Tituaña, C.; Fascì-Spurio, F.; Verde, L.; Muscogiuri, G.; Barrea, L.; Frias-Toral, E.; et al. Endocrinologist’s crucial role in metabolic dysfunction-associated steatotic liver disease: A comprehensive review. Minerva Endocrinol. 2025, 50, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Reytor-González, C.; Parise-Vasco, J.M.; González, N.; Simancas-Racines, A.; Zambrano-Villacres, R.; Zambrano, A.K.; Simancas-Racines, D. Obesity and periodontitis: A comprehensive review of their interconnected pathophysiology and clinical implications. Front. Nutr. 2024, 11, 1440216. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Gieniec, K.A.; Lannagan, T.R.M.; Wang, T.; Asai, N.; Mizutani, Y.; Iida, T.; Ando, R.; Thomas, E.M.; Sakai, A.; et al. The Origin and Contribution of Cancer-Associated Fibroblasts in Colorectal Carcinogenesis. Gastroenterology 2022, 162, 890–906. [Google Scholar] [CrossRef]
- Krisnawan, V.E.; Stanley, J.A.; Schwarz, J.K.; DeNardo, D.G. Tumor Microenvironment as a Regulator of Radiation Therapy: New Insights into Stromal-Mediated Radioresistance. Cancers 2020, 12, 2916. [Google Scholar] [CrossRef]
- Liang, L.; Li, W.; Li, X.; Jin, X.; Liao, Q.; Li, Y.; Zhou, Y. ‘Reverse Warburg effect’ of cancer-associated fibroblasts (Review). Int. J. Oncol. 2022, 60, 67. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, L.; Qin, G.; Qiao, Y.; Ren, F.; Shen, C.; Wang, S.; Liu, S.; Lian, J.; Wang, D.; et al. Cancer-associated fibroblasts induce monocytic myeloid-derived suppressor cell generation via IL-6/exosomal miR-21-activated STAT3 signaling to promote cisplatin resistance in esophageal squamous cell carcinoma. Cancer Lett. 2021, 518, 35–48. [Google Scholar] [CrossRef]
- Xiang, H.; Ramil, C.P.; Hai, J.; Zhang, C.; Wang, H.; Watkins, A.A.; Afshar, R.; Georgiev, P.; Sze, M.A.; Song, X.S.; et al. Cancer-Associated Fibroblasts Promote Immunosuppression by Inducing ROS-Generating Monocytic MDSCs in Lung Squamous Cell Carcinoma. Cancer Immunol. Res. 2020, 8, 436–450. [Google Scholar] [CrossRef]
- Wang, W.-Z.; Cao, X.; Bian, L.; Gao, Y.; Yu, M.; Li, Y.-T.; Xu, J.-G.; Wang, Y.-H.; Yang, H.-F.; You, D.-Y.; et al. Analysis of mRNA-miRNA interaction network reveals the role of CAFs-derived exosomes in the immune regulation of oral squamous cell carcinoma. BMC Cancer 2023, 23, 591. [Google Scholar] [CrossRef] [PubMed]
- Miaomiao, S.; Xiaoqian, W.; Yuwei, S.; Chao, C.; Chenbo, Y.; Yinghao, L.; Yichen, H.; Jiao, S.; Kuisheng, C. Cancer-associated fibroblast-derived exosome microRNA-21 promotes angiogenesis in multiple myeloma. Sci. Rep. 2023, 13, 9671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shang, J.; Yang, Q.; Dai, Z.; Liang, Y.; Lai, C.; Feng, T.; Zhong, D.; Zou, H.; Sun, L.; et al. Exosomes derived from human adipose mesenchymal stem cells ameliorate hepatic fibrosis by inhibiting PI3K/Akt/mTOR pathway and remodeling choline metabolism. J. Nanobiotechnol. 2023, 21, 29. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, C.; Wei, C.; Xia, S.; Qiao, Z.; Zhang, X.-W.; Yu, B.; Zhou, J.; Wang, R. Exosomal miR-625-3p secreted by cancer-associated fibroblasts in colorectal cancer promotes EMT and chemotherapeutic resistance by blocking the CELF2/WWOX pathway. Pharmacol. Res. 2022, 186, 106534. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, Z.; Zhang, Y.; Pradhan, R.N.; Ganguly, D.; Chandra, R.; Murimwa, G.; Wright, S.; Gu, X.; Maddipati, R.; et al. Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of regulatory T cells in pancreatic cancer. Cancer Cell 2022, 40, 656–673.e7. [Google Scholar] [CrossRef]
- Song, M.; He, J.; Pan, Q.-Z.; Yang, J.; Zhao, J.; Zhang, Y.-J.; Huang, Y.; Tang, Y.; Wang, Q.; He, J.; et al. Cancer-Associated Fibroblast-Mediated Cellular Crosstalk Supports Hepatocellular Carcinoma Progression. Hepatology 2021, 73, 1717–1735. [Google Scholar] [CrossRef]
- Feng, B.; Wu, J.; Shen, B.; Jiang, F.; Feng, J. Cancer-associated fibroblasts and resistance to anticancer therapies: Status, mechanisms, and countermeasures. Cancer Cell Int. 2022, 22, 166. [Google Scholar] [CrossRef]
- Yu, W.; Lei, Q.; Yang, L.; Qin, G.; Liu, S.; Wang, D.; Ping, Y.; Zhang, Y. Contradictory roles of lipid metabolism in immune response within the tumor microenvironment. J. Hematol. Oncol. 2021, 14, 187. [Google Scholar] [CrossRef]
- Kennel, K.B.; Bozlar, M.; De Valk, A.F.; Greten, F.R. Cancer-Associated Fibroblasts in Inflammation and Antitumor Immunity. Clin. Cancer Res. 2023, 29, 1009–1016. [Google Scholar] [CrossRef]
- Galbo, P.M.; Zang, X.; Zheng, D. Molecular Features of Cancer-associated Fibroblast Subtypes and their Implication on Cancer Pathogenesis, Prognosis, and Immunotherapy Resistance. Clin. Cancer Res. 2021, 27, 2636–2647. [Google Scholar] [CrossRef]
- Glabman, R.A.; Choyke, P.L.; Sato, N. Cancer-Associated Fibroblasts: Tumorigenicity and Targeting for Cancer Therapy. Cancers 2022, 14, 3906. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Hamberger, F.; Ravichandra, A.; Miller, M.; Nair, A.; Affo, S.; Filliol, A.; Chin, L.; Savage, T.M.; Yin, D.; et al. Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts. J. Clin. Investig. 2021, 131, e146987. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Z.; Peng, G.; Xiao, Y.; Guo, J.; Wu, B.; Li, X.; Zhou, W.; Li, J.; Li, Z.; et al. Single-cell RNA sequencing reveals a pro-invasive cancer-associated fibroblast subgroup associated with poor clinical outcomes in patients with gastric cancer. Theranostics 2022, 12, 620–638. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S. A concise review of VEGF, PDGF, FGF, Notch, angiopoietin, and HGF signalling in tumor angiogenesis with a focus on alternative approaches and future directions. Int. J. Biol. Macromol. 2022, 221, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Magar, A.G.; Morya, V.K.; Kwak, M.K.; Oh, J.U.; Noh, K.C. A Molecular Perspective on HIF-1α and Angiogenic Stimulator Networks and Their Role in Solid Tumors: An Update. Int. J. Mol. Sci. 2024, 25, 3313. [Google Scholar] [CrossRef] [PubMed]
- Malekan, M.; Ebrahimzadeh, M.A.; Sheida, F. The role of Hypoxia-Inducible Factor-1alpha and its signaling in melanoma. Biomed. Pharmacother. 2021, 141, 111873. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef]
- Luo, Z.; Tian, M.; Yang, G.; Tan, Q.; Chen, Y.; Li, G.; Zhang, Q.; Li, Y.; Wan, P.; Wu, J. Hypoxia signaling in human health and diseases: Implications and prospects for therapeutics. Signal Transduct. Target. Ther. 2022, 7, 218. [Google Scholar] [CrossRef]
- Lin, S.; Chai, Y.; Zheng, X.; Xu, X. The role of HIF in angiogenesis, lymphangiogenesis, and tumor microenvironment in urological cancers. Mol. Biol. Rep. 2024, 51, 14. [Google Scholar] [CrossRef]
- Manuelli, V.; Pecorari, C.; Filomeni, G.; Zito, E. Regulation of redox signaling in HIF-1-dependent tumor angiogenesis. FEBS J. 2022, 289, 5413–5425. [Google Scholar] [CrossRef]
- Bakleh, M.Z.; Al Haj Zen, A. The Distinct Role of HIF-1α and HIF-2α in Hypoxia and Angiogenesis. Cells 2025, 14, 673. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, H.; Wang, J.; Liu, Y.; Luo, T.; Hua, H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J. Hematol. Oncol. 2022, 15, 34. [Google Scholar] [CrossRef]
- Simancas-Racines, D.; Reytor-González, C.; Frias-Toral, E.; Katsanos, C.S.; Hidalgo, R. Weighty matters: Unraveling the impact of obesity on colorectal cancer and nutritional interventions. Semin. Cancer Biol. 2025, 114, 29–40. [Google Scholar] [CrossRef]
- Pandey, K.; Umar, S. Microbiome in drug resistance to colon cancer. Curr. Opin. Physiol. 2021, 23, 100472. [Google Scholar] [CrossRef]
- Veziant, J.; Villéger, R.; Barnich, N.; Bonnet, M. Gut Microbiota as Potential Biomarker and/or Therapeutic Target to Improve the Management of Cancer: Focus on Colibactin-Producing Escherichia coli in Colorectal Cancer. Cancers 2021, 13, 2215. [Google Scholar] [CrossRef] [PubMed]
- Belotti, D.; Pinessi, D.; Taraboletti, G. Alternative Vascularization Mechanisms in Tumor Resistance to Therapy. Cancers 2021, 13, 1912. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Qi, Y.; Huang, Y.; Hu, F.; Dong, F.; Shu, K.; Lei, T. Signal Pathways Involved in the Interaction Between Tumor-Associated Macrophages/TAMs and Glioblastoma Cells. Front. Oncol. 2022, 12, 822085. [Google Scholar] [CrossRef]
- Downs-Canner, S.M.; Meier, J.; Vincent, B.G.; Serody, J.S. B Cell Function in the Tumor Microenvironment. Annu. Rev. Immunol. 2022, 40, 169–193. [Google Scholar] [CrossRef]
- Chmiel, P.; Rychcik-Pazyrska, P.; Stec, R. Defining Tumor Microenvironment as a Possible Target for Effective GEP-NENs Immunotherapy—A Systematic Review. Cancers 2023, 15, 5232. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, Y.; He, Y.; Gu, W.; Zhou, Q.; Jin, B.; Chen, S.; Lin, H. Unraveling the immune evasion mechanisms in the tumor microenvironment of head and neck squamous cell carcinoma. Front. Immunol. 2025, 16, 1597202. [Google Scholar] [CrossRef]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef] [PubMed]
- Ghebremedhin, A.; Athavale, D.; Zhang, Y.; Yao, X.; Balch, C.; Song, S. Tumor-Associated Macrophages as Major Immunosuppressive Cells in the Tumor Microenvironment. Cancers 2024, 16, 3410. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.D.; Chorawala, M.R.; Raghani, N.R.; Patel, R.; Fareed, M.; Kashid, V.A.; Prajapati, B.G. Tumor microenvironment: Recent advances in understanding and its role in modulating cancer therapies. Med. Oncol. 2025, 42, 117. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, Z.; Wu, W.; Wang, Z.; Zhang, N.; Zhang, L.; Zeng, W.-J.; Liu, Z.; Cheng, Q. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. J. Exp. Clin. Cancer Res. 2021, 40, 184. [Google Scholar] [CrossRef]
- Wang, H.; Tian, T.; Zhang, J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int. J. Mol. Sci. 2021, 22, 8470. [Google Scholar] [CrossRef]
- Bigos, K.J.A.; Quiles, C.G.; Lunj, S.; Smith, D.J.; Krause, M.; Troost, E.G.C.; West, C.M.; Hoskin, P.; Choudhury, A. Tumour response to hypoxia: Understanding the hypoxic tumour microenvironment to improve treatment outcome in solid tumours. Front. Oncol. 2024, 14, 1331355. [Google Scholar] [CrossRef]
- Rømer, A.M.A.; Thorseth, M.-L.; Madsen, D.H. Immune Modulatory Properties of Collagen in Cancer. Front. Immunol. 2021, 12, 791453. [Google Scholar] [CrossRef]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 2020, 11, 940. [Google Scholar] [CrossRef]
- Zaghdoudi, S.; Decaup, E.; Belhabib, I.; Samain, R.; Cassant-Sourdy, S.; Rochotte, J.; Brunel, A.; Schlaepfer, D.; Cros, J.; Neuzillet, C.; et al. FAK activity in cancer-associated fibroblasts is a prognostic marker and a druggable key metastatic player in pancreatic cancer. EMBO Mol. Med. 2020, 12, e12010. [Google Scholar] [CrossRef]
- Whisner, C.M.; Athena Aktipis, C. The Role of the Microbiome in Cancer Initiation and Progression: How Microbes and Cancer Cells Utilize Excess Energy and Promote One Another’s Growth. Curr. Nutr. Rep. 2019, 8, 42–51. [Google Scholar] [CrossRef]
- Ilerhunmwuwa, N.P.; Abdul Khader, A.H.S.; Smith, C.; Cliff, E.R.S.; Booth, C.M.; Hottel, E.; Aziz, M.; Lee-Smith, W.; Goodman, A.; Chakraborty, R.; et al. Dietary interventions in cancer: A systematic review of all randomized controlled trials. JNCI J. Natl. Cancer Inst. 2024, 116, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Garay, C.; Djouder, N. Dietary interventions and precision nutrition in cancer therapy. Trends Mol. Med. 2023, 29, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Verde, L.; Frias-Toral, E.; Reytor-González, C.; Annunziata, G.; Proganò, M.; Savastano, S.; Simancas-Racines, D.; Colao, A.; Barrea, L. Weight loss, changes in body composition and inflammatory status after a very low-energy ketogenic therapy (VLEKT): Does gender matter? J. Transl. Med. 2024, 22, 949. [Google Scholar] [CrossRef] [PubMed]
- Reytor-González, C.; Zambrano, A.K.; Frias-Toral, E.; Campuzano-Donoso, M.; Simancas-Racines, D. Mediterranean diet and breast cancer: A narrative review. Medwave 2025, 25, e3027. [Google Scholar] [CrossRef]
- Simancas-Racines, D.; Reytor-González, C.; Zambrano, A.K.; Annunziata, G.; Carella, A.M.; Verde, L.; Frias-Toral, E.; Guerra, C.V.; Hidalgo, R. Unlocking the potential: Very-low-energy ketogenic therapy in obesity-related disorders. Food Agric. Immunol. 2025, 36, 2442368. [Google Scholar] [CrossRef]
- Reytor-González, C.; Simancas-Racines, D.; Campuzano-Donoso, M.; Castano Jimenez, J.; Román-Galeano, N.M.; Sarno, G.; Frias-Toral, E. Harnessing nutrition to combat MASLD: A comprehensive guide to food-based therapeutic strategies. Food Agric. Immunol. 2025, 36, 2496499. [Google Scholar] [CrossRef]
- Reytor-González, C.; Frias-Toral, E.; Nuñez-Vásquez, C.; Parise-Vasco, J.M.; Zambrano-Villacres, R.; Simancas-Racines, D.; Schiavo, L. Preventing and Managing Pre- and Postoperative Micronutrient Deficiencies: A Vital Component of Long-Term Success in Bariatric Surgery. Nutrients 2025, 17, 741. [Google Scholar] [CrossRef]
- Simancas-Racines, D.; Frias-Toral, E.; Campuzano-Donoso, M.; Ramos-Sarmiento, D.; Zambrano-Villacres, R.; Reytor-González, C.; Schiavo, L. Preoperative Nutrition in Bariatric Surgery: A Narrative Review on Enhancing Surgical Success and Patient Outcomes. Nutrients 2025, 17, 566. [Google Scholar] [CrossRef]
- Reytor-González, C.; Simancas-Racines, D.; Román-Galeano, N.M.; Annunziata, G.; Galasso, M.; Zambrano-Villacres, R.; Verde, L.; Muscogiuri, G.; Frias-Toral, E.; Barrea, L. Chrononutrition and Energy Balance: How Meal Timing and Circadian Rhythms Shape Weight Regulation and Metabolic Health. Nutrients 2025, 17, 2135. [Google Scholar] [CrossRef]
- Ubago-Guisado, E.; Rodríguez-Barranco, M.; Ching-López, A.; Petrova, D.; Molina-Montes, E.; Amiano, P.; Barricarte-Gurrea, A.; Chirlaque, M.-D.; Agudo, A.; Sánchez, M.-J. Evidence Update on the Relationship between Diet and the Most Common Cancers from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: A Systematic Review. Nutrients 2021, 13, 3582. [Google Scholar] [CrossRef]
- Ruban, M.; Pozhidaeva, E.; Bolotina, L.; Kaprin, A. The Role of Diet and Nutrition in Cancer Development and Management: From Molecular Mechanisms to Personalized Interventions. Foods 2025, 14, 1788. [Google Scholar] [CrossRef]
- Golonko, A.; Pienkowski, T.; Swislocka, R.; Orzechowska, S.; Marszalek, K.; Szczerbinski, L.; Swiergiel, A.H.; Lewandowski, W. Dietary factors and their influence on immunotherapy strategies in oncology: A comprehensive review. Cell Death Dis. 2024, 15, 254. [Google Scholar] [CrossRef]
- Bose, S.; Allen, A.E.; Locasale, J.W. The Molecular Link from Diet to Cancer Cell Metabolism. Mol. Cell 2020, 78, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Mercier, B.D.; Tizpa, E.; Philip, E.J.; Feng, Q.; Huang, Z.; Thomas, R.M.; Pal, S.K.; Dorff, T.B.; Li, Y.R. Dietary Interventions in Cancer Treatment and Response: A Comprehensive Review. Cancers 2022, 14, 5149. [Google Scholar] [CrossRef] [PubMed]
- Wyer, S.; Townsend, D.M.; Ye, Z.; Kourtidis, A.; Choo, Y.-M.; Branco de Barros, A.L.; Donia, M.S.; Hamann, M.T. Recent advances and limitations in the application of kahalalides for the control of cancer. Biomed. Pharmacother. 2022, 148, 112676. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.; Brockmüller, A.; Kubatka, P.; Shakibaei, M.; Büsselberg, D. Curcumin’s Beneficial Effects on Neuroblastoma: Mechanisms, Challenges, and Potential Solutions. Biomolecules 2020, 10, 1469. [Google Scholar] [CrossRef] [PubMed]
- Augimeri, G.; Montalto, F.I.; Giordano, C.; Barone, I.; Lanzino, M.; Catalano, S.; Andò, S.; De Amicis, F.; Bonofiglio, D. Nutraceuticals in the Mediterranean Diet: Potential Avenues for Breast Cancer Treatment. Nutrients 2021, 13, 2557. [Google Scholar] [CrossRef]
- Granja, A.; Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Therapeutic Potential of Epigallocatechin Gallate Nanodelivery Systems. Biomed. Res. Int. 2017, 2017, 5813793. [Google Scholar] [CrossRef]
- Bhavsar, P.; Jha, L.L.; Bera, K.; Patel, S. Phytoconstituents Loaded Liposomes Fabricated Based on Box Behnken Design for Metabolic Syndrome In Vitro and In Vivo Characterization. J. Nat. Remedies 2023, 23, 1035–1052. [Google Scholar] [CrossRef]
- Gündoğdu, Ö.; Şen, S.; Kishali, N. Synthesis of Bicyclic Pyrrolidine Derivatives and their Photoluminescent Properties. Biomed. J. Sci. Tech. Res. 2023, 49. [Google Scholar] [CrossRef]
- Lu, J.; Ma, Y.; Wu, J.; Huang, H.; Wang, X.; Chen, Z.; Chen, J.; He, H.; Huang, C. A review for the neuroprotective effects of andrographolide in the central nervous system. Biomed. Pharmacother. 2019, 117, 109078. [Google Scholar] [CrossRef]
- Principe, D.R.; Underwood, P.W.; Korc, M.; Trevino, J.G.; Munshi, H.G.; Rana, A. The Current Treatment Paradigm for Pancreatic Ductal Adenocarcinoma and Barriers to Therapeutic Efficacy. Front. Oncol. 2021, 11, 688377. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Production of anthraquinones from cell and organ cultures of Morinda species. Appl. Microbiol. Biotechnol. 2023, 107, 2061–2071. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.A.; Lin, C.H.; Chi, W.H.; Wang, C.Y.; Hsieh, Y.T.; Wei, Y.H.; Chen, Y.J. Resveratrol Impedes the Stemness, Epithelial-Mesenchymal Transition, and Metabolic Reprogramming of Cancer Stem Cells in Nasopharyngeal Carcinoma through p53 Activation. Evid.-Based Complement. Altern. Med. 2013, 2013, 590393. [Google Scholar] [CrossRef] [PubMed]
- Saiful Hakim, A.R.; Chee, C.F.; Wong, T.W.; Abu Kasim, N.H.; Nasruddin, N.S.; Yazid, F. Exploring resveratrol’s inhibitory potential on lung cancer stem cells: A scoping review of mechanistic pathways across cancer models. Med. Oncol. 2025, 42, 318. [Google Scholar] [CrossRef] [PubMed]
- Capasso, L.; De Masi, L.; Sirignano, C.; Maresca, V.; Basile, A.; Nebbioso, A.; Rigano, D.; Bontempo, P. Epigallocatechin Gallate (EGCG): Pharmacological Properties, Biological Activities and Therapeutic Potential. Molecules 2025, 30, 654. [Google Scholar] [CrossRef]
- Banyal, A.; Tiwari, S.; Sharma, A.; Chanana, I.; Patel, S.K.S.; Kulshrestha, S.; Kumar, P. Vinca alkaloids as a potential cancer therapeutics: Recent update and future challenges. 3 Biotech 2023, 13, 211. [Google Scholar] [CrossRef]
- Bozdaganyan, M.; Fedorov, V.; Kholina, E.; Kovalenko, I.; Gudimchuk, N.; Orekhov, P. Exploring tubulin-paclitaxel binding modes through extensive molecular dynamics simulations. Sci. Rep. 2025, 15, 8378. [Google Scholar] [CrossRef]
- Ferdous, K.A.; Jansen, J.; Amjad, E.; Pray, E.; Bloch, R.; Benoit, A.; Callahan, M.; Park, H.-A. Mitochondrial protective potential of fucoxanthin in brain disorders. J. Nutr. Sci. 2024, 13, e21. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Mendonca, P.; Elhag, R.; Soliman, K.F.A. Anticancer Effects of Fucoxanthin through Cell Cycle Arrest, Apoptosis Induction, Angiogenesis Inhibition, and Autophagy Modulation. Int. J. Mol. Sci. 2022, 23, 16091. [Google Scholar] [CrossRef]
- Pecoraro, C.; Terrana, F.; Panzeca, G.; Parrino, B.; Cascioferro, S.; Diana, P.; Giovannetti, E.; Carbone, D. Nortopsentins as Leads from Marine Organisms for Anticancer and Anti-Inflammatory Agent Development. Molecules 2023, 28, 6450. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zheng, L. A Review on Bioactive Anthraquinone and Derivatives as the Regulators for ROS. Molecules 2023, 28, 8139. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, X.-H.; Yu, X.; Cao, F.; Cai, X.; Chen, P.; Li, M.; Feng, Y.; Li, H.; Wang, X. Pharmacokinetics of Anthraquinones from Medicinal Plants. Front. Pharmacol. 2021, 12, 638993. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Sanchez, A.; Sahare, P.; Pathak, S.; Banerjee, A.; Estevez, M.; Duttaroy, A.K.; Luna-Bárcenas, G.; Paul, S. Evaluation of the synergistic effects of curcumin-resveratrol co-loaded biogenic silica on colorectal cancer cells. Front. Pharmacol. 2024, 15, 1341773. [Google Scholar] [CrossRef]
- Ciuca, M.D.; Racovita, R.C. Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions. Int. J. Mol. Sci. 2023, 24, 8874. [Google Scholar] [CrossRef]
- Shaharudin, N.S.; Surindar Singh, G.K.; Kek, T.; Sultan, S. Targeting signaling pathways with andrographolide in cancer therapy (Review). Mol. Clin. Oncol. 2024, 21, 81. [Google Scholar] [CrossRef]
- Simancas-Racines, D.; Román-Galeano, N.M.; Verde, L.; Annunziata, G.; Marchetti, M.; Matos, A.; Campuzano-Donoso, M.; Reytor-González, C.; Muscogiuri, G.; Barrea, L.; et al. Targeting Cytokine Dysregulation in Psoriasis: The Role of Dietary Interventions in Modulating the Immune Response. Int. J. Mol. Sci. 2025, 26, 2895. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Y.; Wan, T.; Song, D.; Palanisamy, C.P.; Geng, J.; Pei, J.; Özmen, S.; Abd El-Aty, A.M. Toward personalized cancer management: Role of precision nutrition–diet interventions. J. Funct. Foods 2024, 123, 106584. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Iordache, F.; Stanca, L.; Cimpeanu, C.; Furnaris, F.; Geicu, O.I.; Bilteanu, L.; Serban, A.I. Comprehensive and critical view on the anti-inflammatory and immunomodulatory role of natural phenolic antioxidants. Eur. J. Med. Chem. 2024, 265, 116075. [Google Scholar] [CrossRef]
- Jantan, I.; Haque, M.d.A.; Arshad, L.; Harikrishnan, H.; Septama, A.W.; Mohamed-Hussein, Z.-A. Dietary polyphenols suppress chronic inflammation by modulation of multiple inflammation-associated cell signaling pathways. J. Nutr. Biochem. 2021, 93, 108634. [Google Scholar] [CrossRef]
- Mileo, A.M.; Nisticò, P.; Miccadei, S. Polyphenols: Immunomodulatory and Therapeutic Implication in Colorectal Cancer. Front. Immunol. 2019, 10, 729. [Google Scholar] [CrossRef]
- Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G.A. The Role of Curcumin in Cancer Treatment. Biomedicines 2021, 9, 1086. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Y.; Tian, K.; Chen, X.; Zhang, R.; Mu, X.; Wu, Y.; Wang, D.; Wang, S.; Liu, F.; et al. Apigenin suppresses PD-L1 expression in melanoma and host dendritic cells to elicit synergistic therapeutic effects. J. Exp. Clin. Cancer Res. 2018, 37, 261. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, Y.; Yang, W.; Dong, J.; Li, L. Regulation of dietary polyphenols on cancer cell pyroptosis and the tumor immune microenvironment. Front. Nutr. 2022, 9, 974896. [Google Scholar] [CrossRef] [PubMed]
- Golmohammadi, M.; Zamanian, M.Y.; Al-Ani, A.M.; Jabbar, T.L.; Kareem, A.K.; Aghaei, Z.H.; Tahernia, H.; Hjazi, A.; Jissir, S.A.; Hakimizadeh, E. Targeting STAT3 signaling pathway by curcumin and its analogues for breast cancer: A narrative review. Anim. Model. Exp. Med. 2024, 7, 853–867. [Google Scholar] [CrossRef]
- Kötting, C.; Hofmann, L.; Lotfi, R.; Engelhardt, D.; Laban, S.; Schuler, P.J.; Hoffmann, T.K.; Brunner, C.; Theodoraki, M.N. Immune-Stimulatory Effects of Curcumin on the Tumor Microenvironment in Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 1335. [Google Scholar] [CrossRef]
- Ameer, S.F.; Mohamed, M.Y.; Elzubair, Q.A.; Sharif, E.A.M.; Ibrahim, W.N. Curcumin as a novel therapeutic candidate for cancer: Can this natural compound revolutionize cancer treatment? Front. Oncol. 2024, 14, 1438040. [Google Scholar] [CrossRef]
- Wu, R.; Wang, L.; Yin, R.; Hudlikar, R.; Li, S.; Kuo, H.D.; Peter, R.; Sargsyan, D.; Guo, Y.; Liu, X.; et al. Epigenetics/epigenomics and prevention by curcumin of early stages of inflammatory-driven colon cancer. Mol. Carcinog. 2020, 59, 227–236. [Google Scholar] [CrossRef]
- Ming, T.; Tao, Q.; Tang, S.; Zhao, H.; Yang, H.; Liu, M.; Ren, S.; Xu, H. Curcumin: An epigenetic regulator and its application in cancer. Biomed. Pharmacother. 2022, 156, 113956. [Google Scholar] [CrossRef]
- Cacciola, N.A.; Cuciniello, R.; Petillo, G.D.; Piccioni, M.; Filosa, S.; Crispi, S. An Overview of the Enhanced Effects of Curcumin and Chemotherapeutic Agents in Combined Cancer Treatments. Int. J. Mol. Sci. 2023, 24, 12587. [Google Scholar] [CrossRef]
- Qian, W.; Xiao, Q.; Wang, L.; Qin, T.; Xiao, Y.; Li, J.; Yue, Y.; Zhou, C.; Duan, W.; Ma, Q.; et al. Resveratrol slows the tumourigenesis of pancreatic cancer by inhibiting NFκB activation. Biomed. Pharmacother. 2020, 127, 110116. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Yazdi, M.; Bashiri Dezfouli, A.; Samani Sahraneshin, F.; Ebrahimi, S.M.; Hamidollah Ghaffari, S.; Yaghmaie, M.; Barin, A.; Shakibaei, M.; Shayan, P. Significant decrease in the viability and tumor stem cell marker expression in tumor cell lines treated with curcumin. J. Herb. Med. 2020, 22, 100339. [Google Scholar] [CrossRef]
- Hedayati, N.; Safari, M.H.; Milasi, Y.E.; Kahkesh, S.; Farahani, N.; Khoshnazar, S.M.; Dorostgou, Z.; Alaei, E.; Alimohammadi, M.; Rahimzadeh, P.; et al. Modulation of the PI3K/Akt signaling pathway by resveratrol in cancer: Molecular mechanisms and therapeutic opportunity. Discov. Oncol. 2025, 16, 669. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Liang, C.; Wang, R.; Yi, K.; Zhou, X.; Li, X.; Chen, Y.; Miao, D.; Zhong, C.; Zhu, J. Resveratrol suppresses lung cancer by targeting cancer stem-like cells and regulating tumor microenvironment. J. Nutr. Biochem. 2023, 112, 109211. [Google Scholar] [CrossRef]
- Thongchot, S.; Ferraresi, A.; Vidoni, C.; Salwa, A.; Vallino, L.; Kittirat, Y.; Loilome, W.; Namwat, N.; Isidoro, C. Preclinical evidence for preventive and curative effects of resveratrol on xenograft cholangiocarcinogenesis. Cancer Lett. 2024, 582, 216589. [Google Scholar] [CrossRef]
- Chitcholtan, K.; Singh, M.; Tino, A.; Garrill, A.; Sykes, P. Effects of Resveratrol on In Vivo Ovarian Cancer Cells Implanted on the Chorioallantoic Membrane (CAM) of a Chicken Embryo Model. Int. J. Mol. Sci. 2024, 25, 4374. [Google Scholar] [CrossRef]
- Guo, K.; Feng, Y.; Zheng, X.; Sun, L.; Wasan, H.S.; Ruan, S.; Chen, Y.-J. Resveratrol and Its Analogs: Potent Agents to Reverse Epithelial-to-Mesenchymal Transition in Tumors. Front. Oncol. 2021, 11, 644134. [Google Scholar] [CrossRef]
- Ribeiro, E.; Vale, N. The Role of Resveratrol in Cancer Management: From Monotherapy to Combination Regimens. Targets 2024, 2, 307–326. [Google Scholar] [CrossRef]
- Wang, Z.A.; Hsu, W.; Liu, W.R. Role of SIRT1 in Epigenetics. In Handbook of Nutrition, Diet, and Epigenetics; Springer International Publishing: Cham, Switzerland, 2019; pp. 311–329. [Google Scholar] [CrossRef]
- Marín, V.; Burgos, V.; Pérez, R.; Maria, D.A.; Pardi, P.; Paz, C. The Potential Role of Epigallocatechin-3-Gallate (EGCG) in Breast Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 10737. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, H.; Xu, P.; Yao, L.; Xie, Q.; Mao, L.; Wang, Y. Matcha green tea prevents obesity-induced hypothalamic inflammation via suppressing the JAK2/STAT3 signaling pathway. Food Funct. 2020, 11, 8987–8995. [Google Scholar] [CrossRef]
- Kciuk, M.; Alam, M.; Ali, N.; Rashid, S.; Głowacka, P.; Sundaraj, R.; Celik, I.; Yahya, E.B.; Dubey, A.; Zerroug, E.; et al. Epigallocatechin-3-Gallate Therapeutic Potential in Cancer: Mechanism of Action and Clinical Implications. Molecules 2023, 28, 5246. [Google Scholar] [CrossRef]
- Sheng, J.; Shi, W.; Guo, H.; Long, W.; Wang, Y.; Qi, J.; Celik, I.; Yahya, E.B.; Dubey, A.; Zerroug, E.; et al. The Inhibitory Effect of (−)-Epigallocatechin-3-Gallate on Breast Cancer Progression via Reducing SCUBE2 Methylation and DNMT Activity. Molecules 2019, 24, 2899. [Google Scholar] [CrossRef]
- Kamboj, N.; Sharma, S.; Kumar, R. Neuroprotective insights into epigallocatechin gallate (EGCG) for neurodegenerative disorders. Explor. Neurosci. 2025, 4, 100673. [Google Scholar] [CrossRef]
- Bontempo, P.; Capasso, L.; De Masi, L.; Nebbioso, A.; Rigano, D. Therapeutic Potential of Natural Compounds Acting through Epigenetic Mechanisms in Cardiovascular Diseases: Current Findings and Future Directions. Nutrients 2024, 16, 2399. [Google Scholar] [CrossRef]
- Xu, P.; Yan, F.; Zhao, Y.; Chen, X.; Sun, S.; Wang, Y.; Ying, L. Green Tea Polyphenol EGCG Attenuates MDSCs-mediated Immunosuppression through Canonical and Non-Canonical Pathways in a 4T1 Murine Breast Cancer Model. Nutrients 2020, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Reytor-González, C.; Simancas-Racines, D.; Jiménez-Flores, E.; Campuzano-Donoso, M.; Carella, A.M.; Coppola, L.; Marchetti, M.; Zambrano-Villacres, R.; Sarno, G. Oesophageal adenocarcinoma, obesity, and cancer: The role of nutrition in prevention and management. Food Agric. Immunol. 2025, 36, 2510951. [Google Scholar] [CrossRef]
- Nadalin, P.; Kim, Y.G.; Park, S.U. Recent Studies on Berberine and Its Biological and Pharmacological Activities. EXCLI J. 2023, 22, 315–328, Leibniz Research Centre for Working Environment and Human Factors. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Rahmani, A.H. Berberine: An Important Emphasis on Its Anticancer Effects through Modulation of Various Cell Signaling Pathways. Molecules 2022, 27, 5889. [Google Scholar] [CrossRef]
- Habtemariam, S. Recent Advances in Berberine Inspired Anticancer Approaches: From Drug Combination to Novel Formulation Technology and Derivatization. Molecules 2020, 25, 1426. [Google Scholar] [CrossRef]
- Sajeev, A.; Sailo, B.; Unnikrishnan, J.; Talukdar, A.; Alqahtani, M.S.; Abbas, M.; Alqahtani, A.; Sethi, G.; Kunnumakkara, A.B. Unlocking the potential of Berberine: Advancing cancer therapy through chemosensitization and combination treatments. Cancer Lett. 2024, 597, 217019. [Google Scholar] [CrossRef]
- Kou, Y.; Tong, B.; Wu, W.; Liao, X.; Zhao, M. Berberine Improves Chemo-Sensitivity to Cisplatin by Enhancing Cell Apoptosis and Repressing PI3K/AKT/mTOR Signaling Pathway in Gastric Cancer. Front. Pharmacol. 2020, 11, 616251. [Google Scholar] [CrossRef]
- Rozwadowska, P.; Bator, P.; Razik, M.; Ramian, J.; Rybak, J.; Magiera, B.; Magiera, K.; Razik, W. Anticancer properties of berberine—Analysis of the latest reports. J. Educ. Health Sport 2024, 61, 73–86. [Google Scholar] [CrossRef]
- Zhang, C.; Sheng, J.; Li, G.; Zhao, L.; Wang, Y.; Yang, W.; Yao, X.; Sun, L.; Zhang, Z.; Cui, R. Effects of Berberine and Its Derivatives on Cancer: A Systems Pharmacology Review. Front. Pharmacol. 2020, 10, 1461. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, J.; Sun, N.; Wang, X.-M.; Wei, Q.; Zhang, Y.; Huang, R.; Pu, Y.; Dai, H.; Ren, B.; et al. Berberine reverses multidrug resistance in Candida albicans by hijacking the drug efflux pump Mdr1p. Sci. Bull. 2021, 66, 1895–1905. [Google Scholar] [CrossRef]
- Goswami, S.; Ali, A.; Prasad, M.E.; Singh, P. Pharmacological significance of Catharanthus roseus in cancer management: A review. Pharmacol. Res.-Mod. Chin. Medicine. 2024, 11, 100444. [Google Scholar] [CrossRef]
- Taub, J.W.; Buck, S.A.; Xavier, A.C.; Edwards, H.; Matherly, L.H.; Ge, Y. The evolution and history of Vinca alkaloids: From the Big Bang to the treatment of pediatric acute leukemia. Pediatr. Blood Cancer 2024, 71, e31247. [Google Scholar] [CrossRef] [PubMed]
- Mendonce, K.C.; Palani, N.; Rajadesingu, S.; Radhakrishnan, K.; Ayyar, M.; Priya, L.S. Pharmacological potential of bioactive compounds in Catharanthus roseus extract: A comprehensive review. Toxicol. Rep. 2025, 14, 101998. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Chen, S.-R.; Chai, L.; Zhao, J.; Wang, Y.; Wang, Y. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Crit. Rev. Food Sci. Nutr. 2019, 59, S17–S29. [Google Scholar] [CrossRef]
- Paul, S.; Roy, D.; Pati, S.; Sa, G. The Adroitness of Andrographolide as a Natural Weapon Against Colorectal Cancer. Front. Pharmacol. 2021, 12, 731492. [Google Scholar] [CrossRef]
- Li, X.; Tian, R.; Liu, L.; Wang, L.; He, D.; Cao, K.; Ma, J.K.; Huang, C. Andrographolide enhanced radiosensitivity by downregulating glycolysis via the inhibition of the PI3K-Akt-mTOR signaling pathway in HCT116 colorectal cancer cells. J. Int. Med. Res. 2020, 48, 0300060520946169. [Google Scholar] [CrossRef]
- Tundis, R.; Patra, J.K.; Bonesi, M.; Das, S.; Nath, R.; Das Talukdar, A.; Das, G.; Loizzo, M.R. Anti-Cancer Agent: The Labdane Diterpenoid-Andrographolide. Plants 2023, 12, 1969. [Google Scholar] [CrossRef]
- Arsakhant, P.; Sirion, U.; Chairoungdua, A.; Suksen, K.; Piyachaturawat, P.; Suksamrarn, A.; Saeeng, R. Design and synthesis of C-12 dithiocarbamate andrographolide analogues as an anticancer agent. Bioorg. Med. Chem. Lett. 2020, 30, 127263. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Li, J.; Chen, C.; Wu, J.; Li, J.; Xue, X. Design, synthesis, and anticancer evaluation of novel andrographolide derivatives bearing an α,β-unsaturated ketone moiety. Bioorg. Chem. 2021, 112, 104941. [Google Scholar] [CrossRef] [PubMed]
- Beesetti, S.L.; Jayadev, M.; Subhashini, G.V.; Mansour, L.; Alwasel, S.; Harrath, A.H. Andrographolide as a therapeutic agent against breast and ovarian cancers. Open Life Sci. 2019, 14, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Vovchenko, M.; Alexandova, V.; Mustyatsa, V.; Gudimchuk, N. Study of the Microtubule-Paclitaxel Interaction in vitro and in silico. In Proceedings of the International Conference “Mathematical Biology and Bioinformatics”, Moscow, Russia, 12–19 October 2020; IMPB RAS: Moscow, Russia, 2020; Volume 8. [Google Scholar] [CrossRef]
- Paier, C.R.K.; Maranhão, S.S.; Carneiro, T.R.; Lima, L.M.; Rocha, D.D.; Santos, R.S.; Farias, K.M.; Moraes-Filho, M.O.; Pessoa, C. Natural products as new antimitotic compounds for anticancer drug development. Clinics 2018, 73, e813s. [Google Scholar] [CrossRef]
- Deng, S.; Shanmugam, M.K.; Kumar, A.P.; Yap, C.T.; Sethi, G.; Bishayee, A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer 2019, 125, 1228–1246. [Google Scholar] [CrossRef]
- Al-Bari, M.d.A.A.; Ito, Y.; Ahmed, S.; Radwan, N.; Ahmed, H.S.; Eid, N. Targeting Autophagy with Natural Products as a Potential Therapeutic Approach for Cancer. Int. J. Mol. Sci. 2021, 22, 9807. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Mao, J.-W.; Tan, X.-L. Research progress on the source, production, and anti-cancer mechanisms of paclitaxel. Chin. J. Nat. Med. 2020, 18, 890–897. [Google Scholar] [CrossRef]
- Lee, N.; Youn, K.; Yoon, J.-H.; Lee, B.; Kim, D.H.; Jun, M. The Role of Fucoxanthin as a Potent Nrf2 Activator via Akt/GSK-3β/Fyn Axis against Amyloid-β Peptide-Induced Oxidative Damage. Antioxidants 2023, 12, 629. [Google Scholar] [CrossRef]
- Elmorsy, E.M.; Al Doghaither, H.A.; Al-Ghafari, A.B.; Alyamani, S.A.; Mohammed, Z.M.S.; Ebrahim, N.A.; Elshopakey, G.E.; Shabana, S.M. Through its genoprotective, mitochondrial bioenergetic modulation, and antioxidant effects, Fucoxanthin and its metabolite minimize Ochratoxin A-induced nephrotoxicity in HK-2 human kidney cells. BMC Nephrol. 2025, 26, 379. [Google Scholar] [CrossRef]
- Teng, C.; Wu, J.; Zhang, Z.; Wang, J.; Yang, Y.; Dong, C.; Wu, L.; Lin, Z.; Hu, Y.; Wang, J.; et al. Fucoxanthin ameliorates endoplasmic reticulum stress and inhibits apoptosis and alleviates intervertebral disc degeneration in rats by upregulating Sirt1. Phytother. Res. 2024, 38, 2114–2127. [Google Scholar] [CrossRef] [PubMed]
- Dalisay, D.S.; Tenebro, C.P.; Sabido, E.M.; Suarez, A.F.L.; Paderog, M.J.V.; Reyes-Salarda, R.; Saludes, J.P. Marine-Derived Anticancer Agents Targeting Apoptotic Pathways: Exploring the Depths for Novel Cancer Therapies. Mar. Drugs 2024, 22, 114. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, P.; Aparna, V.; Sebastian, A.T.; Muneer, A.; Prabha, B.; Vipin, C.L.; Ijinu, T.P. Clinically tested marine mollusk-derived anticancer agents: Chemico-pharmacological aspects. Stud. Nat. Prod. Chem. 2024, 83, 95–131. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Hall, M.L.; Lach, J.; Clifford, J.; Chandrasena, K.; Canton, C.; Kontoyianni, M.; Choo, Y.-M.; Karan, D.; Hamann, M.T. RSK1 vs. RSK2 Inhibitory Activity of the Marine β-Carboline Alkaloid Manzamine A: A Biochemical, Cervical Cancer Protein Expression, and Computational Study. Mar. Drugs 2021, 19, 506. [Google Scholar] [CrossRef]
- Ryu, B.; Avalon, N.; Cuau, M.; Almaliti, J.; Din, M.O.; Brennan, C.; Glukhov, E.; Knight, R.; Gerwick, L.; Gerwick, W. Cyanobacteria Join the Kahalalide Conversation: Genome and Metabolite Evidence for Structurally Related Peptides. ChemRxiv 2025, 1–25. [Google Scholar] [CrossRef]
- Flores-Holguín, N.; Frau, J.; Glossman-Mitnik, D. Investigating the Chemical Reactivity of Kahalalides: A Promising Source of Therapeutic Peptides from Marine Natural Products Using Conceptual Density Functional Theory. ChemistrySelect 2023, 8, e202303207. [Google Scholar] [CrossRef]
- Malik, M.S.; Alsantali, R.I.; Jassas, R.S.; Alsimaree, A.A.; Syed, R.; Alsharif, M.A.; Kulkarni, K.; Morad, M.; Althagafi, I.I.; Ahmed, S. A Journey of anthraquinones as anticancer agents—A systematic review of recent literature. RSC Adv. 2021, 11, 35806–35827. [Google Scholar] [CrossRef]
- Mantareva, V.; Iliev, I.; Sulikovska, I.; Durmuş, M.; Angelov, I. Cobalamin (Vitamin B12) in Anticancer Photodynamic Therapy with Zn(II) Phthalocyanines. Int. J. Mol. Sci. 2023, 24, 4400. [Google Scholar] [CrossRef]
- Tolymbekova, A.; Lezina, L. CD320 Receptor and Vitamin B12 as Potential Targets for Anti-Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 5652. [Google Scholar] [CrossRef]
- Rachman, F.; Wibowo, J.T. Exploring Marine Rare Actinomycetes: Untapped Resources of Bioactive Compounds in Clinical Development. BIO Web Conf. 2024, 92, 02012. [Google Scholar] [CrossRef]
- Roy, A.; Datta, S.; Bhatia, K.S.; Bhumika Jha, P.; Prasad, R. Role of plant derived bioactive compounds against cancer. S. Afr. J. Bot. 2022, 149, 1017–1028. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, S.; Yu, M.; Shi, J.; Liu, J.; Li, X.; Chen, J.; Sun, X.; Huang, G.; Zheng, C. Natural Products from Marine-Derived Fungi with Anti-Inflammatory Activity. Mar. Drugs 2024, 22, 433. [Google Scholar] [CrossRef]
- Tamzi, N.N.; Rahman, M.M.; Das, S. Recent Advances in Marine-Derived Bioactives Towards Cancer Therapy. Int. J. Transl. Med. 2024, 4, 740–781. [Google Scholar] [CrossRef]
- Taylor, W.F.; Yanez, M.; Moghadam, S.E.; Moridi Farimani, M.; Soroury, S.; Ebrahimi, S.N.; Tabefam, M.; Jabbarzadeh, E. 7-epi-Clusianone, a Multi-Targeting Natural Product with Potential Chemotherapeutic, Immune-Modulating, and Anti-Angiogenic Properties. Molecules 2019, 24, 4415. [Google Scholar] [CrossRef] [PubMed]
- Chaachouay, N. Synergy, Additive Effects, and Antagonism of Drugs with Plant Bioactive Compounds. Drugs Drug Candidates 2025, 4, 4. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, Z.; Han, Y.; Wang, J.; Wang, Y.; Chen, X.; Shao, Y.; Cheng, Y.; Zhou, W.; Lu, X.; et al. A review on anti-cancer effect of green tea catechins. J. Funct. Foods 2020, 74, 104172. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G.; Ahmed, K.A. Curcumin Ameliorates Doxorubicin-Induced Cardiotoxicity and Hepatotoxicity Via Suppressing Oxidative Stress and Modulating iNOS, NF-κB, and TNF-α in Rats. Cardiovasc. Toxicol. 2022, 22, 152–166. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Hegde, M.; Parama, D.; Girisa, S.; Kumar, A.; Daimary, U.D.; Garodia, P.; Yenisetti, S.C.; Oommen, O.V.; Aggarwal, B.B. Role of Turmeric and Curcumin in Prevention and Treatment of Chronic Diseases: Lessons Learned from Clinical Trials. ACS Pharmacol. Transl. Sci. 2023, 6, 447–518. [Google Scholar] [CrossRef]
- Reytor-González, C.; Zambrano, A.K.; Montalvan, M.; Frias-Toral, E.; Simancas-Racines, A.; Simancas-Racines, D. Adherence to the Mediterranean Diet and its association with gastric cancer: Health benefits from a Planeterranean perspective. J. Transl. Med. 2024, 22, 483. [Google Scholar] [CrossRef]
- Boța, M.; Vlaia, L.; Jîjie, A.-R.; Marcovici, I.; Crişan, F.; Oancea, C.; Dehelean, C.A.; Mateescu, T.; Moacă, E.-A. Exploring Synergistic Interactions between Natural Compounds and Conventional Chemotherapeutic Drugs in Preclinical Models of Lung Cancer. Pharmaceuticals 2024, 17, 598. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; He, W.; Xia, S.; Jiang, X.; Li, X.; Bai, J.; Li, N.; Chen, L.; Yang, B. Apigenin Enhanced Antitumor Effect of Cisplatin in Lung Cancer via Inhibition of Cancer Stem Cells. Nutr. Cancer 2021, 73, 1489–1497. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Yao, Y.; Li, H.; Gao, C.; Sun, C.; Zhuang, J. Potential of cucurbitacin as an anticancer drug. Biomed. Pharmacother. 2023, 168, 115707. [Google Scholar] [CrossRef]
- Abdul Satar, N.; Ismail, M.N.; Yahaya, B.H. Synergistic Roles of Curcumin in Sensitising the Cisplatin Effect on a Cancer Stem Cell-Like Population Derived from Non-Small Cell Lung Cancer Cell Lines. Molecules 2021, 26, 1056. [Google Scholar] [CrossRef]
- Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; De Luca, M.; Garofalo, A.; Ragno, G.; Grande, F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules 2022, 27, 5436. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Li, W.; Li, Y.; Yin, S. Nanoparticle-based drug delivery systems targeting cancer cell surfaces. RSC Adv. 2023, 13, 21365–21382. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, S.; Sun, M.; Shi, J.; Zhang, H.; Yu, H.; Yang, Z. Recent advances and clinical translation of liposomal delivery systems in cancer therapy. Eur. J. Pharm. Sci. 2024, 193, 106688. [Google Scholar] [CrossRef]
- Amin, M.; Seynhaeve, A.L.B.; Sharifi, M.; Falahati, M.; ten Hagen, T.L.M. Liposomal Drug Delivery Systems for Cancer Therapy: The Rotterdam Experience. Pharmaceutics 2022, 14, 2165. [Google Scholar] [CrossRef]
- Ciftci, F.; Özarslan, A.C.; Kantarci, İ.C.; Yelkenci, A.; Tavukcuoglu, O.; Ghorbanpour, M. Advances in Drug Targeting, Drug Delivery, and Nanotechnology Applications: Therapeutic Significance in Cancer Treatment. Pharmaceutics 2025, 17, 121. [Google Scholar] [CrossRef]
- Wu, K.; Kwon, S.H.; Zhou, X.; Fuller, C.; Wang, X.; Vadgama, J.; Wu, Y. Overcoming Challenges in Small-Molecule Drug Bioavailability: A Review of Key Factors and Approaches. Int. J. Mol. Sci. 2024, 25, 13121. [Google Scholar] [CrossRef]
- Rastakhiz, S.; Yazdani, M.; Shariat, S.; Arab, A.; Momtazi-Borojeni, A.A.; Barati, N.; Mansourian, M.; Amin, M.; Abbasi, A.; Saberi, Z.; et al. Preparation of nanoliposomes linked to HER2/neu-derived (P5) peptide containing MPL adjuvant as vaccine against breast cancer. J. Cell Biochem. 2019, 120, 1294–1303. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, L.; Li, H.; Miao, F.; Zhang, Z.; Hu, C.; Yu, W.; Tang, Q.; Shao, G. Application of lipid nanovesicle drug delivery system in cancer immunotherapy. J. Nanobiotechnol. 2022, 20, 214. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Fu, L.; Huang, J.; Dai, Y.; Wang, B.; Xu, G.; Wu, L.; Zhou, H. Curcumin reverses doxorubicin resistance via inhibition the efflux function of ABCB4 in doxorubicin-resistant breast cancer cells. Mol. Med. Rep. 2019, 19, 5162–5168. [Google Scholar] [CrossRef]
- Buhrmann, C.; Shayan, P.; Brockmueller, A.; Shakibaei, M. Resveratrol Suppresses Cross-Talk between Colorectal Cancer Cells and Stromal Cells in Multicellular Tumor Microenvironment: A Bridge between In Vitro and In Vivo Tumor Microenvironment Study. Molecules 2020, 25, 4292. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, X.; Xu, R.; Ye, L.; Kong, H.; Zeng, X.; Wang, H.; Xie, W. The synergistic effect of resveratrol in combination with cisplatin on apoptosis via modulating autophagy in A549 cells. Acta Biochim. Biophys. Sin. 2016, 48, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Norden, E.; Heiss, E.H. Urolithin A gains in antiproliferative capacity by reducing the glycolytic potential via the p53/TIGAR axis in colon cancer cells. Carcinogenesis 2019, 40, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Manogaran, P.; Beeraka, N.M.; Huang, C.-Y.; Vijaya Padma, V. Neferine and isoliensinine enhance ‘intracellular uptake of cisplatin’ and induce ‘ROS-mediated apoptosis’ in colorectal cancer cells—A comparative study. Food Chem. Toxicol. 2019, 132, 110652. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fan, J.; Ai, G.; Liu, J.; Luo, N.; Li, C.; Cheng, Z. Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol. Res. 2019, 52, 37. [Google Scholar] [CrossRef]
- Alam, M.N.; Yu, J.Q.; Beale, P.; Huq, F. Cisplatin in combination with emetine and patulin showed dose and sequence dependent synergism against ovarian cancer. Synergy 2020, 10, 100060. [Google Scholar] [CrossRef]
- Bhagya, N.; Prabhu, A.; Rekha, P.D.; Chandrashekar, K.R. Combination of tetrandrine and cisplatin synergises cytotoxicity and apoptosis in triple negative breast cancer. Synergy 2020, 10, 100063. [Google Scholar] [CrossRef]
- Piska, K.; Koczurkiewicz, P.; Wnuk, D.; Karnas, E.; Bucki, A.; Wójcik-Pszczoła, K.; Jamrozik, M.; Michalik, M.; Kołaczkowski, M.; Pękala, E. Synergistic anticancer activity of doxorubicin and piperlongumine on DU-145 prostate cancer cells—The involvement of carbonyl reductase 1 inhibition. Chem. Biol. Interact. 2019, 300, 40–48. [Google Scholar] [CrossRef]
- Rawat, L.; Hegde, H.; Hoti, S.L.; Nayak, V. Piperlongumine induces ROS mediated cell death and synergizes paclitaxel in human intestinal cancer cells. Biomed. Pharmacother. 2020, 128, 110243. [Google Scholar] [CrossRef]
- Yang, H.; Gao, Y.; Fan, X.; Liu, X.; Peng, L.; Ci, X. Oridonin Sensitizes Cisplatin-Induced Apoptosis via AMPK/Akt/mTOR-Dependent Autophagosome Accumulation in A549 Cells. Front. Oncol. 2019, 9, 769. [Google Scholar] [CrossRef]
- Cao, W.; Li, Y.; Hou, Y.; Yang, M.; Fu, X.; Zhao, B.; Jiang, H.M.; Fu, X.Y. Enhanced anticancer efficiency of doxorubicin against human glioma by natural borneol through triggering ROS-mediated signal. Biomed. Pharmacother. 2019, 118, 109261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.N.; Xia, Y.Z.; Zhang, C.; Zhang, H.; Luo, J.G.; Yang, L.; Kong, L.Y. Vielanin K enhances doxorubicin-induced apoptosis via activation of IRE1α-TRAF2-JNK pathway and increases mitochondrial Ca2+ influx in MCF-7 and MCF-7/MDR cells. Phytomedicine 2020, 78, 153329. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-L.; Xia, Y.-Z.; Zhang, Y.-L.; Yang, L.; Kong, L.-Y. Vielanin P enhances the cytotoxicity of doxorubicin via the inhibition of PI3K/Nrf2-stimulated MRP1 expression in MCF-7 and K562 DOX-resistant cell lines. Phytomedicine 2019, 58, 152885. [Google Scholar] [CrossRef]
- Lou, C.; Lu, H.; Ma, Z.; Liu, C.; Zhang, Y. Ginkgolide B enhances gemcitabine sensitivity in pancreatic cancer cell lines via inhibiting PAFR/NF-κB pathway. Biomed. Pharmacother. 2019, 109, 563–572. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Lu, Y.; Chaurasiya, B.; Mi, G.; Shi, D.; Chen, D.; Webster, T.J.; Tu, J.; Shen, Y. Co-delivery of Poria cocos extract and doxorubicin as an ‘all-in-one’ nanocarrier to combat breast cancer multidrug resistance during chemotherapy. Nanomedicine 2020, 23, 102095. [Google Scholar] [CrossRef]
- Indorf, P.; Patzak, A.; Lichtenberger, F. Drug metabolism in animal models and humans: Translational aspects and chances for individual therapy. Acta Physiol. 2021, 233, e13734. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Q.; Yang, M.; Liu, D.; Hou, X.; Tang, L.; Wang, X.; Lyu, Y.; Chen, X.; Liu, K.; et al. Current trends in drug metabolism and pharmacokinetics. Acta Pharm. Sin. B. 2019, 9, 1113–1144. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.-I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin—An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Jakobušić Brala, C.; Karković Marković, A.; Kugić, A.; Torić, J.; Barbarić, M. Combination Chemotherapy with Selected Polyphenols in Preclinical and Clinical Studies—An Update Overview. Molecules 2023, 28, 3746. [Google Scholar] [CrossRef]
- Corsini, N.S.; Knoblich, J.A. Human organoids: New strategies and methods for analyzing human development and disease. Cell 2022, 185, 2756–2769. [Google Scholar] [CrossRef]
- Chaudhary, N.; La Ferlita, A.; Choudhary, B.S.; Jog, E.; Kazi, M.; Yahya, S.; Dalwai, A.; Ostwal, V.; Singh, S.; Redkar, S.; et al. Patient-Derived Organoids and Xenografts Uncover Therapeutic Vulnerabilities in Colorectal Signet Ring Cell Carcinomas. Clin. Cancer Res. 2025, 31, 1359–1373. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, X. Patient-derived xenografts or organoids in the discovery of traditional and self-assembled drug for tumor immunotherapy. Front. Oncol. 2023, 13, 1122322. [Google Scholar] [CrossRef]
- Aliu, T.B.; Obun, F.E.; Raji, H.; Badmus, K. Safety Evaluation and Concerns of Natural Products in Traditional Medicine. AROC Pharm. Biotechnol. 2025, 5, 9–17. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Simoben, C.V.; Babiaka, S.B.; Moumbock, A.F.A.; Namba-Nzanguim, C.T.; Eni, D.B.; Medina-Franco, J.L.; Günther, S.; Ntie-Kang, F.; Sippl, W. Challenges in natural product-based drug discovery assisted with in silico -based methods. RSC Adv. 2023, 13, 31578–31594. [Google Scholar] [CrossRef]
- Heinrich, M.; Jalil, B.; Abdel-Tawab, M.; Echeverria, J.; Kulić, Ž.; McGaw, L.J.; Pezzuto, J.M.; Potterat, O.; Wang, J.-B. Best Practice in the chemical characterisation of extracts used in pharmacological and toxicological research—The ConPhyMP—Guidelines12. Front. Pharmacol. 2022, 13, 953205. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Yu, L.; Jin, Y.; Song, M.; Zhao, Y.; Zhang, H. When Natural Compounds Meet Nanotechnology: Nature-Inspired Nanomedicines for Cancer Immunotherapy. Pharmaceutics 2022, 14, 1589. [Google Scholar] [CrossRef] [PubMed]
- Sabit, H.; Pawlik, T.M.; Radwan, F.; Abdel-Hakeem, M.; Abdel-Ghany, S.; Wadan, A.H.S.; Elzawahri, M.; El-Hashash, A.; Arneth, B. Precision nanomedicine: Navigating the tumor microenvironment for enhanced cancer immunotherapy and targeted drug delivery. Mol. Cancer 2025, 24, 160. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, W.; Xu, M.; Zhao, T.; Zhou, B.; Zhou, R.; Zhu, Z.; Chen, X.; Bao, Z.; Wang, K.; et al. Drug delivery systems based on mesoporous silica nanoparticles for the management of hepatic diseases. Acta Pharm. Sin. B 2025, 15, 809–833. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Loo, C.-Y.; Traini, D.; Young, P.M. Development and Evaluation of Paclitaxel and Curcumin Dry Powder for Inhalation Lung Cancer Treatment. Pharmaceutics 2020, 13, 9. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Mistry, K.J.; Suthar, S.F.; Wu, Z.-X.; Chen, Z.-S.; Hou, K. Nano-Drug Delivery Systems Entrapping Natural Bioactive Compounds for Cancer: Recent Progress and Future Challenges. Front. Oncol. 2022, 12, 867655. [Google Scholar] [CrossRef]
- Ghazal, H.; Waqar, A.; Yaseen, F.; Shahid, M.; Sultana, M.; Tariq, M.; Bashir, M.K.; Tahseen, H.; Raza, T.; Ahmad, F. Role of nanoparticles in enhancing chemotherapy efficacy for cancer treatment. Next Mater. 2024, 2, 100128. [Google Scholar] [CrossRef]
- Yang, L.; Han, T.; Liu, R.; Shi, S.; Luan, S.; Meng, S. Plant-derived natural compounds: A new frontier in inducing immunogenic cell death for cancer treatment. Biomed. Pharmacother. 2024, 177, 117099. [Google Scholar] [CrossRef]
- Lee, J.; Han, Y.; Wang, W.; Jo, H.; Kim, H.; Kim, S.; Yang, K.-M.; Kim, S.-J.; Dhanasekaran, D.N.; Song, Y.S. Phytochemicals in Cancer Immune Checkpoint Inhibitor Therapy. Biomolecules 2021, 11, 1107. [Google Scholar] [CrossRef]
- Ravindran Menon, D.; Li, Y.; Yamauchi, T.; Osborne, D.G.; Vaddi, P.K.; Wempe, M.F.; Zhai, Z.; Fujita, M. EGCG Inhibits Tumor Growth in Melanoma by Targeting JAK-STAT Signaling and Its Downstream PD-L1/PD-L2-PD1 Axis in Tumors and Enhancing Cytotoxic T-Cell Responses. Pharmaceuticals 2021, 14, 1081. [Google Scholar] [CrossRef]
- Fayyaz, A.; Haqqi, A.; Khan, R.; Irfan, M.; Khan, K.; Reiner, Ž.; Sharifi-Rad, J.; Calina, D. Revolutionizing cancer treatment: The rise of personalized immunotherapies. Discov. Oncol. 2024, 15, 756. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Acevedo, A.; Davidoff, E.J.; Timmins, L.M.; Marrero-Berrios, I.; Patel, M.; White, C.; Lowe, C.; Sherba, J.J.; Hartmanshenn, C.; et al. The growing role of precision and personalized medicine for cancer treatment. Technology 2018, 06, 79–100. [Google Scholar] [CrossRef]
- Zheng, C.-C.; Gao, L.; Sun, H.; Zhao, X.-Y.; Gao, Z.-Q.; Liu, J.; Guo, W. Advancements in enzymatic reaction-mediated microbial transformation. Heliyon 2024, 10, e38187. [Google Scholar] [CrossRef]
- Pant, A.; Maiti, T.K.; Mahajan, D.; Das, B. Human Gut Microbiota and Drug Metabolism. Microb. Ecol. 2023, 86, 97–111. [Google Scholar] [CrossRef]
- Kadam, O.; Dalai, S.; Chauhan, B.; Guru, R.R.; Mitra, S.; Raytekar, N.; Kumar, R. Nanobiotechnology Unveils the Power of Probiotics: A Comprehensive Review on the Synergistic Role of Probiotics and Advanced Nanotechnology in Enhancing Geriatric Health. Cureus 2025, 17, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Han, K.; Xu, J.; Moon, J.J. Novel strategies for modulating the gut microbiome for cancer therapy. Adv. Drug Deliv. Rev. 2024, 210, 115332. [Google Scholar] [CrossRef] [PubMed]
- Sholl, L.M.; Hirsch, F.R.; Hwang, D.; Botling, J.; Lopez-Rios, F.; Bubendorf, L.; Mino-Kenudson, M.; Roden, A.C.; Beasley, M.B.; Borczuk, A.; et al. The Promises and Challenges of Tumor Mutation Burden as an Immunotherapy Biomarker: A Perspective from the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2020, 15, 1409–1424. [Google Scholar] [CrossRef]
- Strickler, J.H.; Hanks, B.A.; Khasraw, M. Tumor Mutat. Burd. A Predict. Immunother. Response: Is. More Always Better? Clin. Cancer Res. 2021, 27, 1236–1241. [Google Scholar] [CrossRef]
- Cho, Y.A.; Lee, H.; Kim, D.G.; Kim, H.; Ha, S.Y.; Choi, Y.-L.; Jang, K.-T.; Kim, K.-M. PD-L1 Expression Is Significantly Associated with Tumor Mutation Burden and Microsatellite Instability Score. Cancers 2021, 13, 4659. [Google Scholar] [CrossRef]
- González-Méndez, I.; Sorroza-Martínez, K.; Cuétara-Guadarrama, F.; Vonlanthen, M.; Rivera, E. An overview of theranostic nanomedicine. In Theranostics Nanomaterials in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2025; pp. 1–10. [Google Scholar] [CrossRef]
- Al-Thani, A.N.; Jan, A.G.; Abbas, M.; Geetha, M.; Sadasivuni, K.K. Nanoparticles in cancer theragnostic and drug delivery: A comprehensive review. Life Sci. 2024, 352, 122899. [Google Scholar] [CrossRef]
- Puccetti, M.; Pariano, M.; Schoubben, A.; Giovagnoli, S.; Ricci, M. Biologics, theranostics, and personalized medicine in drug delivery systems. Pharmacol. Res. 2024, 201, 107086. [Google Scholar] [CrossRef]
- Manzari-Tavakoli, A.; Babajani, A.; Tavakoli, M.M.; Safaeinejad, F.; Jafari, A. Integrating natural compounds and nanoparticle-based drug delivery systems: A novel strategy for enhanced efficacy and selectivity in cancer therapy. Cancer Med. 2024, 13, e7010. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reytor-González, C.; Jiménez-Flores, E.; González, N.; Simancas-Racines, D. Redefining Chemoresistance: Natural Bioactives as Molecular Modulators at the Cancer–Tumor Microenvironment Interface. Int. J. Mol. Sci. 2025, 26, 8037. https://doi.org/10.3390/ijms26168037
Reytor-González C, Jiménez-Flores E, González N, Simancas-Racines D. Redefining Chemoresistance: Natural Bioactives as Molecular Modulators at the Cancer–Tumor Microenvironment Interface. International Journal of Molecular Sciences. 2025; 26(16):8037. https://doi.org/10.3390/ijms26168037
Chicago/Turabian StyleReytor-González, Claudia, Emilia Jiménez-Flores, Natalí González, and Daniel Simancas-Racines. 2025. "Redefining Chemoresistance: Natural Bioactives as Molecular Modulators at the Cancer–Tumor Microenvironment Interface" International Journal of Molecular Sciences 26, no. 16: 8037. https://doi.org/10.3390/ijms26168037
APA StyleReytor-González, C., Jiménez-Flores, E., González, N., & Simancas-Racines, D. (2025). Redefining Chemoresistance: Natural Bioactives as Molecular Modulators at the Cancer–Tumor Microenvironment Interface. International Journal of Molecular Sciences, 26(16), 8037. https://doi.org/10.3390/ijms26168037





