Abstract
Although cytoplasmic vacuoles are instantly recognizable on bone marrow smears, their etiologic significance remains a diagnostic dilemma in everyday practice. We conducted a PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) aligned systematic review with narrative synthesis registered in PROSPERO and refrained from meta-analysis owing to high between-study heterogeneity (I2 > 80%). Across 22 studies, we identified 818 unique adults with vacuolated marrow cytopenias. A stepwise diagnostic algorithm—serum copper/ceruloplasmin followed, when non-diagnostic, by UBA1 hotspot sequencing in adults meeting clinical “red flags”—correctly classified 97% of cases within <5 days at a median laboratory cost of ~USD 173, reserving broad myeloid next-generation sequencing for the atypical minority. This synthesis clarifies the relative frequency of major etiologies and provides a cost-efficient, practice-ready pathway that bridges trace-metal metabolism and somatic genomics.
Keywords:
vacuoles; cytopenia; copper deficiency; VEXAS; UBA1; systematic review; diagnostic algorithm 1. Introduction
Although cytoplasmic vacuoles are instantly recognizable under the microscope, their etiologic significance remains a diagnostic dilemma in everyday practice. A recent state-of-the-art review [1] stresses that the classic literature links large, lipid-poor vacuoles to copper deficiency, alcohol misuse, or drug toxicity, yet these causes explain only a fraction of post-2020 cases [2,3]. The 2020 discovery of somatic UBA1-mutant VEXAS (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) syndrome further reshaped the differential: this late-onset autoinflammatory disease features prominent vacuoles and often presents with otherwise unexplained cytopenia [4]. More than 700 patients have since been reported, but data remain fragmented across small series, impeding reliable prevalence estimates and rational test ordering.
Clinicians thus face a practical dilemma: should they begin with serum copper studies, order targeted UBA1 sequencing, proceed directly to broad myeloid next-generation sequencing (NGS), or pursue cytogenetics when confronted with vacuolated cytopenia? Without an evidence-weighted pathway, reversible etiologies such as copper deficiency may be overlooked, whereas costly or invasive investigations may be overused.
Here, we consolidate current knowledge by two complementary approaches. First, we performed a PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) aligned systematic review to quantify the relative frequencies, clinical profiles, and morphologic hallmarks of vacuolated marrow cytopenias in adults. Second, guided by the evolving literature—including the insights of the recent review by Elbadry and Mabed [1]—we integrate these data with post-2020 pathobiology to propose a pragmatic four-step diagnostic algorithm that balances diagnostic yield, turnaround time, and cost. This narrative synthesis aims to guide daily hematology practice while highlighting areas where prospective studies are urgently needed.
2. Methods
2.1. Framework and Registration
We embedded a systematic review within a narrative review, following the PRISMA 2020 statement [5]. The protocol was registered prospectively in PROSPERO (CRD420251082738) [6], and the full search strings, checklist, and protocol are reproduced in Supplementary Protocol S1.
We performed a PRISMA-aligned systematic review with narrative synthesis, registered in PROSPERO, and prespecified that no quantitative meta-analysis would be undertaken given substantial between-study heterogeneity (I2 > 80%). Wherever available, we extracted individual-level data and summarized simple proportions with exact binomial confidence intervals.
2.2. Eligibility Criteria
We considered reports that analyzed adults (≥18 years) with single-lineage or multilineage cytopenia in whom cytoplasmic vacuoles were demonstrated in at least one hematopoietic precursor compartment on light microscopy. Acceptable study designs were cohort studies, cross-sectional analyses, or case series that enrolled three or more patients and provided primary diagnostic or outcome data. Single-case reports, narrative reviews, conference abstracts without full texts, pediatric studies, and non-English publications were excluded. We queried PubMed, Web of Science Core Collection, and CENTRAL on 31 May 2025 using a search window that began 31 December 2020, the day prior to the first description of VEXAS. To capture the earlier literature on copper-deficiency cytopenia, we performed backward citation-chasing and keyword hand-searching (2000–2020), which yielded three additional cohort reports not indexed by the electronic filters.
2.3. Information Sources and Search Strategy
With support from a medical librarian, we searched PubMed, Web of Science Core Collection, and Cochrane Central Register of Controlled Trials (CENTRAL) on 31 May 2025 (Supplementary Table S1). The core PubMed string was:
(“vacuole”[Title/Abstract] OR “vacuolated”[Title/Abstract]) AND (“bone marrow”[Title/Abstract] OR “marrow”[Title/Abstract]) AND (cytopenia OR anemia OR neutropenia OR thrombocytopenia) OR “VEXAS” OR “copper deficiency”
Filters were set to humans, English language, adults (≥18 years), and publication dates 31 December 2020–31 May 2025. Equivalent concept blocks adapted to Web of Science and CENTRAL retrieved additional citations. The backward citation-chasing and consultation with content experts added three copper-deficiency series published between 2000 and 2020 that were not captured electronically. Full search syntax and filter details are provided in Supplementary Protocol S1.
2.4. Study Selection
All records were imported into Covidence, where duplicates were removed and two reviewers (A.T. and K.U.) independently screened titles and abstracts before assessing full texts. Disagreements were resolved by discussion or, when necessary, adjudication by a third reviewer. Inter-rater agreement was quantified with Cohen’s κ.
To avoid double-counting in the VEXAS literature, we undertook an explicit overlap check. Specifically, all VEXAS series were cross-checked for shared (i) recruiting centers, (ii) study periods, and (iii) first or last authors. When potential overlap was identified (e.g., among three French cohorts) and individual-level linkage was not available, we applied a conservative rule: we retained the most comprehensive cohort for quantitative synthesis and excluded overlapping cohorts from pooled counts, citing them qualitatively as needed. A leave-one-cohort-out sensitivity analysis was conducted to assess the robustness of pooled estimates (Supplementary Methods S1).
A PRISMA flow diagram summarizes the process in Figure 1.
Figure 1.
PRISMA 2020 study selection diagram. Electronic searches of PubMed, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL) yielded 271 records, of which 58 were duplicates. Five additional records were identified through hand searching, three of which dated from 2000–2020 and described copper-deficiency cytopenia. After a full-text review of 44 articles, 22 studies met all the inclusion criteria.
2.5. Data Extraction
We constructed a piloted REDCap form to record bibliographic details, study design, patient demographics, cytopenia pattern, confirmatory tests (serum copper, ceruloplasmin, UBA1 sequencing, cytogenetics, myeloid NGS), treatments, and hematologic or inflammatory outcomes. When critical data were missing, corresponding authors were contacted once for clarification.
2.6. Risk of Bias Appraisal
Methodological quality was judged with ROBINS-I [7], rating each study across seven domains and assigning an overall level of low, moderate, serious, or critical risk of bias. The full assessment appears in Supplementary Table S2.
2.7. Data Synthesis
Because both clinical context and methodology varied widely—reflected in I2 values exceeding 80% for key prevalence estimates—we refrained from formal meta-analysis. Instead, we pooled simple counts with exact 95% confidence intervals, repeated the calculations after excluding studies deemed at serious risk of bias, compared molecular and morphologic signatures qualitatively, and incorporated turnaround time and Medicare fee schedule costs to build the four-step diagnostic algorithm.
Terminology was standardized (e.g., “post-2020” instead of “contemporary”) to ensure temporal clarity across cohorts.
2.8. Statistical Analysis
All the analyses were run in EZR version 1.68 [8]. p values are two-sided and reported solely for exploratory purposes, with p < 0.05 considered nominally significant.
3. Results and Discussion
We identified 22 eligible studies (Table 1) after screening 218 full texts; the PRISMA flow diagram appears in Figure 1. Collectively, these reports describe 818 adults with at least one cytopenia accompanied by light-microscopic marrow vacuolization. Etiology was overwhelmingly skewed toward two disorders: UBA1-mutant VEXAS syndrome accounted for 727 cases (89%), copper-deficiency cytopenia for 70 (9%), and a single institutional cohort contributed 21 cases (2%) of vacuolated cell myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML).

Table 1.
Characteristics of the 22 studies describing cytoplasmic vacuolization with cytopenia.
3.1. Demographic and Hematologic Landscape
Across 22 independent series, we identified 818 unique adults with vacuolated marrow cytopenias after applying explicit overlap-avoidance rules for VEXAS cohorts (centers, periods, and author lists cross-checked).
Pooling 818 adults from 22 studies, we observed a distinct age-and-sex gradient across etiologies. Copper-deficiency cytopenia occurred at a median age of 57.5 years and showed a near gender balance (41% male), whereas VEXAS patients were older (median 68.0 years) and almost exclusively male (98%), reflecting the X-linked biology of UBA1 (Figure 2 and Table 2). The small vacuolated cell MDS/AML cohort sat between these poles (median 66.0 years; 71% male). Anemia was nearly universal in both copper deficiency (92%) and VEXAS (91%); neutropenia predominated in copper deficiency (61%), whereas thrombocytopenia was the most frequent deficit in VEXAS (40%). Notably, 76% of copper-deficient patients had at least one reversible predisposing factor—zinc excess, proton-pump inhibitor use, bariatric surgery, or malabsorption [29]—summarized in Table 3.
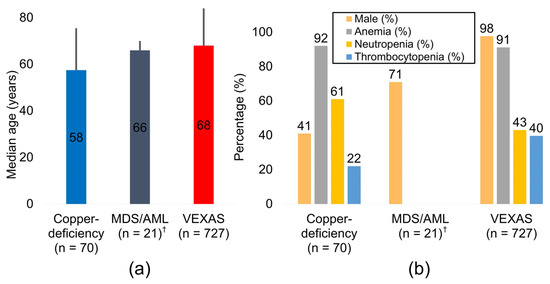
Figure 2.
Clinical and hematologic profiles by etiology. (a) Pooled median age for copper deficiency (n = 70), vacuolated cell MDS/AML (n = 21) †, and VEXAS syndrome (n = 727). Central numerals denote the median; vertical error bars indicate the 95% confidence interval. (b) Prevalence of male sex, anemia, neutropenia, and thrombocytopenia in the same three diagnostic groups. † Estimates for the MDS/AML group derive from a single institutional cohort and should be interpreted cautiously. Abbreviations: AML, acute myeloid leukemia; CI, confidence interval; MDS, myelodysplastic syndrome.

Table 2.
Pooled demographic and hematologic data by diagnostic group.

Table 3.
Common causes and risk factors for acquired copper deficiency.
3.2. Molecular and Morphologic Correlates
Pathogenic UBA1 p.Met41 variants were consistently identified in all VEXAS cohorts and were absent from copper deficiency and vacuolated cell MDS/AML series. The latter group instead carried high-risk genomic alterations, including monosomy 7 and TP53 mutations [9]. Quantitative analysis of vacuole morphology failed to distinguish between etiologies; median diameters ranged from 3 to 6 µm, and the typical number of vacuoles per precursor was two to five, observed across copper deficiency, VEXAS, drug toxicity, and MDS/AML. Both erythroid and myeloid precursors were affected in all these disease entities, emphasizing that morphology alone is nondiscriminatory (Supplementary Figure S1). Mechanistically, three partially distinct pathways were evident: trace-element depletion in copper deficiency, clonal ubiquitylation defects in VEXAS, and genomic instability with impaired autophagy in vacuolated cell MDS/AML [30,31,32,33]. Drug-induced or toxic vacuolization, primarily linked to linezolid, disulfiram, or alcohol exposure, was rare and is summarized in Table 4. Therefore, accurate diagnosis requires integration of molecular testing with clinical context, rather than reliance on morphology alone.

Table 4.
Clinical conditions that produce vacuolization of hematopoietic precursors.
3.3. Algorithm Performance
In adults meeting clinical “red flags”, Step 1–2 (serum copper/ceruloplasmin followed, when non-diagnostic, by UBA1 hotspot sequencing) correctly classified 97% of cases within <5 days at a median laboratory cost of ~USD 173; broad myeloid next-generation sequencing was reserved for the UBA1-negative minority.
When the four-step pathway (Figure 3) was applied to every case in the literature, Step 1—evaluation for reversible causes and serum copper/ceruloplasmin assays (Table 4)—followed, when normal, by Step 2—hot-spot UBA1 sequencing limited to patients ≥ 50 years of age who exhibit ≥ 1 VEXAS clinical “red flag”. (Table 5) correctly classified 97% of vacuolated-marrow cytopenias. The combined laboratory charge for Steps 1–2 was approximately USD 173 with a median turnaround of five days, about one-fifteenth the cost and two weeks faster than reflex broad myeloid NGS (Step 3) [46,47,48,49]. Only UBA1–wild-type patients with persistent cytopenia underwent morphology-guided cytogenetics and targeted NGS; Step 4 was rated serious ROBINS-I risk did not alter the 97% yield underscoring the robustness of the sequence. A concise comparison of first-line therapy, steroid-sparing strategies, transfusion dependence, and two-year overall survival across copper deficiency, VEXAS, and vacuolated cell MDS/AML is provided in Table 6.

Figure 3.
Four-step diagnostic algorithm for adult cytopenia with vacuolated marrow precursors. Step 1: Rule out reversible causes—zinc excess, proton pump inhibitor use, bariatric surgery, malabsorption—and measure serum copper and ceruloplasmin (Table 4). Step 2: Sequence UBA1 in any patient aged ≥ 50 years who has ≥ 1 VEXAS clinical “red flag” (Table 5). Step 3: If UBA1 is wild type, perform morphology-guided cytogenetics and targeted myeloid next-generation sequencing (NGS). Step 4: Investigate rarer mimics (drug/toxin exposure, inherited marrow failure syndromes, immune or infectious etiologies).

Table 5.
Clinical and laboratory “red flags” that should prompt UBA1 mutation testing in suspected VEXAS syndrome.

Table 6.
Outcomes and representative management strategies for vacuolated marrow cytopenias.
3.4. Risk of Bias Overview
Most studies were judged low-to-moderate risk of bias by ROBINS-I; exclusion of serious-risk studies did not alter the principal classification result.
ROBINS-I appraisal assigned an overall moderate risk of bias to 24 studies and a serious risk to 3; no study reached the critical-risk tier (Supplementary Table S2). Serious judgments were driven by substantial missing outcome data or non-validated outcome measurements in two VEXAS cohorts and one copper-deficiency series, all of which were retrospective case series without prespecified protocols. The remaining studies were classified as moderate risk chiefly because of incomplete follow-up or selective outcome reporting, typical limitations of retrospective designs. Repeating the diagnostic algorithm analysis after excluding the three serious-risk studies reproduced the same 97% classification rate indicating that our principal findings are robust despite underlying methodological heterogeneity.
3.5. Summary of Key Findings
In modern hematology practice, two readily recognizable conditions—copper deficiency and VEXAS syndrome—account for 97% of adult cytopenias marked by vacuolated marrow precursors. A straightforward, cost-conscious sequence that begins with reversible-cause screening and serum copper/ceruloplasmin testing, followed by focused UBA1 hotspot sequencing, correctly classifies nearly all patients within a few days while reserving invasive or high-cost investigations for the small minority of atypical cases. These data form the empirical backbone of the pragmatic diagnostic algorithm in Figure 3 and provide a clear rationale for prospective validation studies.
3.6. What This Review Adds
By aggregating 818 adults across 22 studies, we demonstrate that copper-deficiency cytopenia and UBA1-mutant VEXAS syndrome explain 97% of modern cases of cytopenia with vacuolated marrow precursors, whereas vacuolated cell MDS or AML and other mimics together account for only 3%. This quantitative clarification moves the field beyond anecdote and provides an empirical foundation for a streamlined, mechanism-oriented diagnostic pathway.
3.7. Mechanistic Perspectives on Vacuole Biology
Marrow vacuoles arise through at least three biologically distinct yet light-microscopically indistinguishable routes.
Copper deficiency disrupts cupro-oxidase activity, stalls mitochondrial respiration, and traps iron, producing lipid-poor vacuoles that disappear within weeks of supplementation [33]. Retrospective re-analysis of “refractory anemia” cohorts has revealed that a fraction represented occult copper deficiency [2,11].
VEXAS syndrome follows a proteotoxic pathway. Somatic UBA1 p.Met41 variants eliminate the cytoplasmic E1 isoform, abolish K48-linked polyubiquitination, and arrest macro-autophagy. Single-cell proteomics and ultrastructural studies reveal ribosome-lined, LC3-positive pre-autophagosomal structures, swollen rough endoplasmic reticulum cisternae, and disrupted mitochondrial cristae [21,53,54]. A CRISPR knock-in model reproduces these vacuoles, whereas lentiviral expression of wild-type UBA1 rescues them [55]. Consistent with this mechanism, Lacombe et al. [51] showed that non-canonical splice-site and catalytic-domain UBA1 mutations can generate a comparable, albeit sometimes milder, vacuolar phenotype.
Vacuolated-cell MDS/AML appears to involve a third mechanism. High-risk lesions such as monosomy 7 and TP53 disruption impair autophagosome maturation and trigger p53-dependent metabolic stress, yielding larger, irregular vacuoles and a rapid leukemic trajectory [37,38,40,56].
Historical copper-deficiency series were never sequenced for UBA1, and early VEXAS reports often lacked trace-element data; diagnostic overlap therefore persists. Because light microscopy cannot differentiate these vacuole types, the laboratory context is decisive: serum copper and ceruloplasmin identify reversible deficiency; targeted UBA1 sequencing confirms or excludes VEXAS; and cytogenetics with focused myeloid NGS uncovers high-risk clonal disease. Prospective studies pairing deep UBA1 sequencing with copper profiling in newly diagnosed MDS or unexplained cytopenia will be essential to resolve residual overlap and to determine whether vacuolization is causal or merely a marker of upstream injury.
3.8. Clinical Implications
A two-step screen—serum copper/ceruloplasmin followed, when normal, by UBA1 hotspot sequencing—classifies nearly all patients within five days for a median laboratory cost of USD 173, a fraction of the expense and turnaround associated with broad myeloid NGS panels. Early recognition of copper deficiency permits curative supplementation, whereas prompt confirmation of VEXAS redirects management toward durable immunomodulation or clinical trial enrollment rather than empiric cytoreduction. Cytogenetics and 20-gene NGS are reserved for the UBA1-negative minority, aligning resource use with diagnostic yield and minimizing incidental findings.
3.9. Limitations and Potential Biases
Female low-level UBA1 mosaicism may be under-recognized in the existing literature; prospective validation and formal health economic modeling are warranted.
Interpretation is tempered by several constraints. First, the vacuolated-cell MDS/AML category rests on a single 21-patient cohort [9]; its observed prevalence of 2% almost certainly underestimates the true burden. Second, many copper-deficiency reports pre-date VEXAS and lacked UBA1 testing, whereas marginally low copper levels in older VEXAS cases may have inflated the nutritional category. Third, systematic ultrastructural data are scarce; only 7% of cases underwent electron microscopy, limiting insight into vacuole biogenesis. Fourth, the virtual exclusivity of male subjects introduces ascertainment bias and suggests that low-level UBA1 mosaicism in women or non-binary individuals is under-recognized; recent work by Lacombe et al. [51] documents such non-canonical presentations. Fifth, all the included studies were retrospective, methodologically heterogeneous, and often incomplete in outcome reporting, leaving residual confounding despite sensitivity analyses. Finally, publication bias likely favored dramatic or diagnostically challenging cases, skewing the spectrum toward VEXAS and high-risk neoplasia while under-representing routine copper deficiency that resolves with supplementation.
3.10. Future Directions
Prospective, multicenter implementation of the four-step diagnostic algorithm will be essential to verify its diagnostic accuracy, turnaround time, and cost-effectiveness, while systematically combining serum copper and ceruloplasmin assays with targeted UBA1 sequencing in all adults presenting with unexplained cytopenia. These studies should specifically include women with low-level UBA1 mosaicism, an underrecognized and likely underdiagnosed population [19]. Given the aging global population, the increasing prevalence of malnutrition, prior gastrointestinal resections, and the widespread promotion of zinc supplementation, the incidence of acquired copper deficiency-related cytopenias is expected to rise in the coming decade [29]. A concurrent research priority is the deployment of advanced transmission electron microscopy with correlative immunogold labeling to dissect vacuole biogenesis across nutritional, inflammatory, and clonal etiologies. In clonal myeloid diseases, vacuolated blasts may signal high-risk biology and predict inferior survival [37], warranting systematic evaluation in future MDS/AML cohorts that integrate copper profiling and deep UBA1 sequencing. Randomized or registry-based trials are needed to compare IL-6 or JAK–STAT blockade, hypomethylating therapy, and ubiquitin-pathway modulators in VEXAS [14,15,50], with one planned study prospectively evaluating ruxolitinib versus azacitidine as a steroid-sparing strategy. Finally, formal health economic modeling across diverse healthcare systems will be essential to confirm that the proposed pathway delivers value proportionate to its biological rationale and helps prevent delayed or missed diagnoses of reversible or treatable vacuolated marrow cytopenias.
4. Conclusions
Marrow vacuoles are no longer an enigmatic microscopic finding. An evidence-weighted diagnostic sequence that begins with inexpensive, high-yield tests can rapidly lead to diagnoses that are either readily reversible or require targeted immunomodulation. Routine measurement of serum copper and focused UBA1 hotspot testing can spare most patients from invasive, costly investigations, reserving advanced genomics for the few who truly need it. The pathway presented here offers a pragmatic template for current practice and a platform for prospective validation and therapeutic innovation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26168044/s1, Supplementary Protocol S1 including PRISMA 2020 Checklist and PROSPERO Protocol; Supplementary Table S1: Search strategies used for PubMed, Web of Science, and CENTRAL; Supplementary Table S2: Risk of bias summary for the 24 included studies; Supplementary Methods S1: Overlap assessment and sensitivity analysis for VEXAS Cohorts; Supplementary Figure S1: Representative photomicrographs of cytoplasmic vacuoles in three etiologic categories.
Author Contributions
Conceptualization, A.T.; formal analysis, A.T. and K.U.; investigation, A.T., K.U., and J.L.E.; resources, M.T., S.S., Y.K., and M.E.; data curation, M.T., S.S., Y.K., and M.E.; writing—original draft preparation, A.T.; writing—review and editing, A.T., K.U., M.T., S.S., J.L.E., and M.E.; project administration, A.T.; funding acquisition, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by a Grant-in-Aid for Scientific Research (KAKENHI 24K11527) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by a Health and Labour Sciences Research Grant (23KC2009) from the Ministry of Health, Labour and Welfare of Japan. The funding agencies had no role in study design, collection, analysis, interpretation of data, or manuscript preparation.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Aichi Medical University Institutional Review Board (IRB; approval No. AMU-2021-022, dated 25 May 2021).
Informed Consent Statement
Because only de-identified data were analyzed, the IRB granted a waiver of informed consent.
Data Availability Statement
The data that support the findings of this review—including the extraction sheets and the decision-tree workbook—are available from the corresponding author without undue reservation.
Acknowledgments
We would like to thank all the clinicians and administrative staff who contributed to this study. We are also deeply grateful to Michiko Ichikawa, librarian at Aichi Medical University, for her invaluable assistance with the comprehensive literature search.
Conflicts of Interest
A.T. has received research funding from AIR WATER, lecture fees from Novartis Pharmaceuticals, and donation funding from Chugai Pharmaceutical and Kyowa Kirin. The remaining authors declare no competing financial or non-financial interests.
References
- Elbadry, M.I.; Mabed, M. Bone Marrow Vacuolization at the Crossroads of Specialties: Molecular Insights and Diagnostic Challenges. Eur. J. Haematol. 2025, 115, 204–217. [Google Scholar] [CrossRef]
- Huff, J.D.; Keung, Y.K.; Thakuri, M.; Beaty, M.W.; Hurd, D.D.; Owen, J.; Molnar, I. Copper deficiency causes reversible myelodysplasia. Am. J. Hematol. 2007, 82, 625–630. [Google Scholar] [CrossRef]
- Gregg, X.T.; Reddy, V.; Prchal, J.T. Copper deficiency masquerading as myelodysplastic syndrome. Blood 2002, 100, 1493–1495. [Google Scholar] [CrossRef] [PubMed]
- Beck, D.B.; Ferrada, M.A.; Sikora, K.A.; Ombrello, A.K.; Collins, J.C.; Pei, W.; Balanda, N.; Ross, D.L.; Ospina Cardona, D.; Wu, Z.; et al. Somatic Mutations in UBA1 and Severe Adult-Onset Autoinflammatory Disease. N. Engl. J. Med. 2020, 383, 2628–2638. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- NIHR. PROSPERO: International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/PROSPERO/home (accessed on 1 June 2025).
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Gurnari, C.; Pagliuca, S.; Durkin, L.; Terkawi, L.; Awada, H.; Kongkiatkamon, S.; Zawit, M.; Hsi, E.D.; Carraway, H.E.; Rogers, H.J.; et al. Vacuolization of hematopoietic precursors: An enigma with multiple etiologies. Blood 2021, 137, 3685–3689. [Google Scholar] [CrossRef]
- Uchino, K.; Quang, L.V.; Enomoto, M.; Nakano, Y.; Yamada, S.; Matsumura, S.; Kanasugi, J.; Takasugi, S.; Nakamura, A.; Horio, T.; et al. Cytopenia associated with copper deficiency. EJHaem 2021, 2, 729–737. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Kumar, N.; Hogan, W.J.; Murray, J.A. Copper deficiency in celiac disease. J. Clin. Gastroenterol. 2009, 43, 162–164. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Kumar, N.; Li, C.Y.; Phyliky, R.L.; Hogan, W.J. Hematological manifestations of copper deficiency: A retrospective review. Eur. J. Haematol. 2008, 80, 523–531. [Google Scholar] [CrossRef]
- Vitale, A.; Leone, F.; Caggiano, V.; Hinojosa-Azaola, A.; Martin-Nares, E.; Guaracha-Basanez, G.A.; Torres-Ruiz, J.; Ayumi Kawakami-Campos, P.; Hissaria, P.; Callisto, A.; et al. Efficacy and safety of conventional disease-modifying antirheumatic drugs in VEXAS syndrome: Real-world data from the international AIDA network. Front. Pharmacol. 2025, 16, 1539756. [Google Scholar] [CrossRef]
- Johansen, M.M.; El Fassi, D.; Nielsen, C.T.H.; Krintel, S.B.; Graudal, N.; Hansen, J.W. Treatment experiences with focus on IL-6R inhibition in patients with VEXAS syndrome and a case of remission with azacytidine treatment. Rheumatology 2025, 64, 826–830. [Google Scholar] [CrossRef]
- Hadjadj, J.; Nguyen, Y.; Mouloudj, D.; Bourguiba, R.; Heiblig, M.; Aloui, H.; McAvoy, C.; Lacombe, V.; Ardois, S.; Campochiaro, C.; et al. Efficacy and safety of targeted therapies in VEXAS syndrome: Retrospective study from the FRENVEX. Ann. Rheum. Dis. 2024, 83, 1358–1367. [Google Scholar] [CrossRef]
- Maeda, A.; Tsuchida, N.; Uchiyama, Y.; Horita, N.; Kobayashi, S.; Kishimoto, M.; Kobayashi, D.; Matsumoto, H.; Asano, T.; Migita, K.; et al. Efficient detection of somatic UBA1 variants and clinical scoring system predicting patients with variants in VEXAS syndrome. Rheumatology 2024, 63, 2056–2064. [Google Scholar] [CrossRef]
- Kusne, Y.; Ghorbanzadeh, A.; Dulau-Florea, A.; Shalhoub, R.; Alcedo, P.E.; Nghiem, K.; Ferrada, M.A.; Hines, A.; Quinn, K.A.; Panicker, S.R.; et al. Venous and arterial thrombosis in patients with VEXAS syndrome. Blood 2024, 143, 2190–2200. [Google Scholar] [CrossRef]
- Wolff, L.; Caratsch, L.; Lotscher, F.; Seitz, L.; Seitz, P.; Coattrenec, Y.; Seebach, J.; Vilinovszki, O.; Balabanov, S.; Nilsson, J.; et al. VEXAS syndrome: A Swiss national retrospective cohort study. Swiss Med. Wkly. 2024, 155, 3879. [Google Scholar] [CrossRef] [PubMed]
- Beck, D.B.; Bodian, D.L.; Shah, V.; Mirshahi, U.L.; Kim, J.; Ding, Y.; Magaziner, S.J.; Strande, N.T.; Cantor, A.; Haley, J.S.; et al. Estimated Prevalence and Clinical Manifestations of UBA1 Variants Associated With VEXAS Syndrome in a Clinical Population. JAMA 2023, 329, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Mascaro, J.M.; Rodriguez-Pinto, I.; Poza, G.; Mensa-Vilaro, A.; Fernandez-Martin, J.; Caminal-Montero, L.; Espinosa, G.; Hernandez-Rodriguez, J.; Diaz, M.; Rita-Marques, J.; et al. Spanish cohort of VEXAS syndrome: Clinical manifestations, outcome of treatments and novel evidences about UBA1 mosaicism. Ann. Rheum. Dis. 2023, 82, 1594–1605. [Google Scholar] [CrossRef] [PubMed]
- Hines, A.S.; Koster, M.J.; Bock, A.R.; Go, R.S.; Warrington, K.J.; Olteanu, H.; Lasho, T.L.; Patnaik, M.M.; Reichard, K.K. Targeted testing of bone marrow specimens with cytoplasmic vacuolization to identify previously undiagnosed cases of VEXAS syndrome. Rheumatology 2023, 62, 3947–3951. [Google Scholar] [CrossRef]
- Islam, S.; Cullen, T.; Sumpton, D.; Damodaran, A.; Heath, D.; Bosco, A.; Doo, N.W.; Kidson-Gerber, G.; Cheong, A.; Lawford, R.; et al. VEXAS syndrome: Lessons learnt from an early Australian case series. Intern. Med. J. 2022, 52, 658–662. [Google Scholar] [CrossRef]
- Mekinian, A.; Zhao, L.P.; Chevret, S.; Desseaux, K.; Pascal, L.; Comont, T.; Maria, A.; Peterlin, P.; Terriou, L.; D’Aveni Piney, M.; et al. A Phase II prospective trial of azacitidine in steroid-dependent or refractory systemic autoimmune/inflammatory disorders and VEXAS syndrome associated with MDS and CMML. Leukemia 2022, 36, 2739–2742. [Google Scholar] [CrossRef]
- Georgin-Lavialle, S.; Terrier, B.; Guedon, A.F.; Heiblig, M.; Comont, T.; Lazaro, E.; Lacombe, V.; Terriou, L.; Ardois, S.; Bouaziz, J.D.; et al. Further characterization of clinical and laboratory features in VEXAS syndrome: Large-scale analysis of a multicentre case series of 116 French patients. Br. J. Dermatol. 2022, 186, 564–574. [Google Scholar] [CrossRef]
- Comont, T.; Heiblig, M.; Riviere, E.; Terriou, L.; Rossignol, J.; Bouscary, D.; Rieu, V.; Le Guenno, G.; Mathian, A.; Aouba, A.; et al. Azacitidine for patients with Vacuoles, E1 Enzyme, X-linked, Autoinflammatory, Somatic syndrome (VEXAS) and myelodysplastic syndrome: Data from the French VEXAS registry. Br. J. Haematol. 2022, 196, 969–974. [Google Scholar] [CrossRef]
- Ferrada, M.A.; Savic, S.; Cardona, D.O.; Collins, J.C.; Alessi, H.; Gutierrez-Rodrigues, F.; Kumar, D.B.U.; Wilson, L.; Goodspeed, W.; Topilow, J.S.; et al. Translation of cytoplasmic UBA1 contributes to VEXAS syndrome pathogenesis. Blood 2022, 140, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, N.; Kunishita, Y.; Uchiyama, Y.; Kirino, Y.; Enaka, M.; Yamaguchi, Y.; Taguri, M.; Yamanaka, S.; Takase-Minegishi, K.; Yoshimi, R.; et al. Pathogenic UBA1 variants associated with VEXAS syndrome in Japanese patients with relapsing polychondritis. Ann. Rheum. Dis. 2021, 80, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Ferrada, M.A.; Sikora, K.A.; Luo, Y.; Wells, K.V.; Patel, B.; Groarke, E.M.; Ospina Cardona, D.; Rominger, E.; Hoffmann, P.; Le, M.T.; et al. Somatic Mutations in UBA1 Define a Distinct Subset of Relapsing Polychondritis Patients With VEXAS. Arthritis Rheumatol. 2021, 73, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Takami, A.; Uchino, K. Discovering the hidden link: Hematological disorders caused by copper deficiency. Int. J. Hematol. 2025. [Google Scholar] [CrossRef]
- Watson, A.S.; Mortensen, M.; Simon, A.K. Autophagy in the pathogenesis of myelodysplastic syndrome and acute myeloid leukemia. Cell Cycle 2011, 10, 1719–1725. [Google Scholar] [CrossRef]
- Pierro, F.; Fazio, M.; Murdaca, G.; Stagno, F.; Gangemi, S.; Allegra, A. Oxidative Stress and Mitochondrial Dysfunction in Myelodysplastic Syndrome: Roles in Development, Diagnosis, Prognosis, and Treatment. Int. J. Mol. Sci. 2025, 26, 6415. [Google Scholar] [CrossRef]
- Savy, C.; Bourgoin, M.; Cluzeau, T.; Jacquel, A.; Robert, G.; Auberger, P. VEXAS, Chediak-Higashi syndrome and Danon disease: Myeloid cell endo-lysosomal pathway dysfunction as a common denominator? Cell Mol. Biol. Lett. 2025, 30, 12. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.L.; Gonzalez-Ibanez, A.M.; Mendoza, P.; Ruiz, L.M.; Riedel, C.A.; Simon, F.; Schuringa, J.J.; Elorza, A.A. Copper deficiency-induced anemia is caused by a mitochondrial metabolic reprograming in erythropoietic cells. Metallomics 2019, 11, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Chetty-Raju, N.; Cook, R.; Erber, W.N. Vacuolated neutrophils in ethanol toxicity. Br. J. Haematol. 2004, 127, 478. [Google Scholar] [CrossRef] [PubMed]
- Liapis, K.; Vrachiolias, G.; Spanoudakis, E.; Kotsianidis, I. Vacuolation of early erythroblasts with ring sideroblasts: A clue to the diagnosis of linezolid toxicity. Br. J. Haematol. 2020, 190, 809. [Google Scholar] [CrossRef]
- Rosenbach, L.M.; Caviles, A.P.; Mitus, W.J. Chloramphenicol toxicity. Reversible vacuolization of erythroid cells. N. Engl. J. Med. 1960, 263, 724–728. [Google Scholar] [CrossRef]
- Ballo, O.; Stratmann, J.; Serve, H.; Steffen, B.; Finkelmeier, F.; Brandts, C. Blast vacuolization in AML patients indicates adverse-risk AML and is associated with impaired survival after intensive induction chemotherapy. PLoS ONE 2019, 14, e0223013. [Google Scholar] [CrossRef]
- Gao, J.; Chen, Y.H.; Mina, A.; Altman, J.K.; Kim, K.Y.; Zhang, Y.; Lu, X.; Jennings, L.; Sukhanova, M. Unique morphologic and genetic characteristics of acute myeloid leukemia with chromothripsis: A clinicopathologic study from a single institution. Hum. Pathol. 2020, 98, 22–31. [Google Scholar] [CrossRef]
- Ballard, H.S. The hematological complications of alcoholism. Alcohol. Health Res. World 1997, 21, 42–52. [Google Scholar] [CrossRef]
- Song, J.; Shang, B.; Pei, Y.; Shi, M.; Niu, X.; Dou, L.; Drokow, E.K.; Xu, F.; Bai, Y.; Sun, K. A higher percentage of leukemic blasts with vacuoles predicts unfavorable outcomes in patients with acute myeloid leukemia. Leuk. Res. 2021, 109, 106638. [Google Scholar] [CrossRef]
- Khambatta, S.; Nguyen, D.L.; Beckman, T.J.; Wittich, C.M. Kearns-Sayre syndrome: A case series of 35 adults and children. Int. J. Gen. Med. 2014, 7, 325–332. [Google Scholar] [CrossRef]
- Bellanne-Chantelot, C.; Schmaltz-Panneau, B.; Marty, C.; Fenneteau, O.; Callebaut, I.; Clauin, S.; Docet, A.; Damaj, G.L.; Leblanc, T.; Pellier, I.; et al. Mutations in the SRP54 gene cause severe congenital neutropenia as well as Shwachman-Diamond-like syndrome. Blood 2018, 132, 1318–1331. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Guhde, G.; Suter, A.; Eskelinen, E.L.; Hartmann, D.; Lullmann-Rauch, R.; Janssen, P.M.; Blanz, J.; von Figura, K.; Saftig, P. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 2000, 406, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Zanelli, M.; Ruggeri, L.; Sanguedolce, F.; Zizzo, M.; Martino, G.; Genua, A.; Ascani, S. Parvovirus B19 Infection in a Patient with Common Variable Immunodeficiency. Mediterr. J. Hematol. Infect. Dis. 2021, 13, e2021026. [Google Scholar] [CrossRef] [PubMed]
- Inai, K.; Noriki, S.; Iwasaki, H.; Naiki, H. Risk factor analysis for bone marrow histiocytic hyperplasia with hemophagocytosis: An autopsy study. Virchows Arch. 2014, 465, 109–118. [Google Scholar] [CrossRef]
- CMS. 25CLABQ2. Available online: https://www.cms.gov/medicare/payment/fee-schedules/clinical-laboratory-fee-schedule-clfs/files/25CLABQ2 (accessed on 3 July 2025).
- UNC. UBA1Q Mutation Quantitative Detection, DDPCR (VEXAS Syndrome). Available online: https://www.uncmedicalcenter.org/mclendon-clinical-laboratories/available-tests/uba1q-mutation-quantitative-detection-ddpcr-vexas-syndrome/ (accessed on 3 July 2025).
- University, J.H. Myeloid Panel, NGS, Blood. Available online: https://pathology.jhu.edu/test-directory/leukemia-panel-ngs-blood-2 (accessed on 27 June 2025).
- Vianna, P.G.; Press, R.D.; Stehr, H.; Yang, F.; Gojenola, L.; Zehnder, J.L.; Gotlib, J. Clinical Utility of a Multi-Gene Next-Generation Sequencing Myeloid Panel in an Academic Hematology Practice. Blood 2019, 134, 1408. [Google Scholar] [CrossRef]
- Koster, M.J.; Lasho, T.L.; Olteanu, H.; Reichard, K.K.; Mangaonkar, A.; Warrington, K.J.; Patnaik, M.M. VEXAS syndrome: Clinical, hematologic features and a practical approach to diagnosis and management. Am. J. Hematol. 2024, 99, 284–299. [Google Scholar] [CrossRef]
- Lacombe, V.; Hadjadj, J.; Georgin-Lavialle, S.; Lavigne, C.; Genevieve, F.; Kosmider, O. Vacuoles in bone marrow progenitors: VEXAS syndrome and beyond. Lancet Haematol. 2024, 11, e160–e167. [Google Scholar] [CrossRef]
- Al-Hakim, A.; Trikha, R.; Phyu Htut, E.E.; Chowdhury, O.; MacLennan, C.A.; Chee, A.; Kaul, A.; Poulter, J.A.; Cargo, C.; Wason, J.M.S.; et al. Treatment outcomes in patients with VEXAS syndrome: A retrospective cohort study. Lancet Rheumatol. 2025, 7, e472–e484. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, S.; Gao, Q.; Patel, B.A.; Groarke, E.M.; Feng, X.; Manley, A.L.; Li, H.; Ospina Cardona, D.; Kajigaya, S.; et al. Early activation of inflammatory pathways in UBA1-mutated hematopoietic stem and progenitor cells in VEXAS. Cell Rep. Med. 2023, 4, 101160. [Google Scholar] [CrossRef]
- Cardoneanu, A.; Rezus, I.I.; Burlui, A.M.; Richter, P.; Bratoiu, I.; Mihai, I.R.; Macovei, L.A.; Rezus, E. Autoimmunity and Autoinflammation: Relapsing Polychondritis and VEXAS Syndrome Challenge. Int. J. Mol. Sci. 2024, 25, 2261. [Google Scholar] [CrossRef]
- Chiaramida, A.; Obwar, S.G.; Nordstrom, A.E.H.; Ericsson, M.; Saldanha, A.; Ivanova, E.V.; Griffin, G.K.; Khan, D.H.; Belizaire, R. Sensitivity to targeted UBA1 inhibition in a myeloid cell line model of VEXAS syndrome. Blood Adv. 2023, 7, 7445–7456. [Google Scholar] [CrossRef]
- Lin, J.F.; Chi, C.W.; Huang, Y.C.; Tsai, T.H.; Chen, Y.J. Anti-Cancer Effects of Oxygen-Atom-Modified Derivatives of Wasabi Components on Human Leukemia Cells. Int. J. Mol. Sci. 2023, 24, 6823. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).