The Role of GCH1 Deficiency and Tetrahydrobiopterin in Mental Health
Abstract
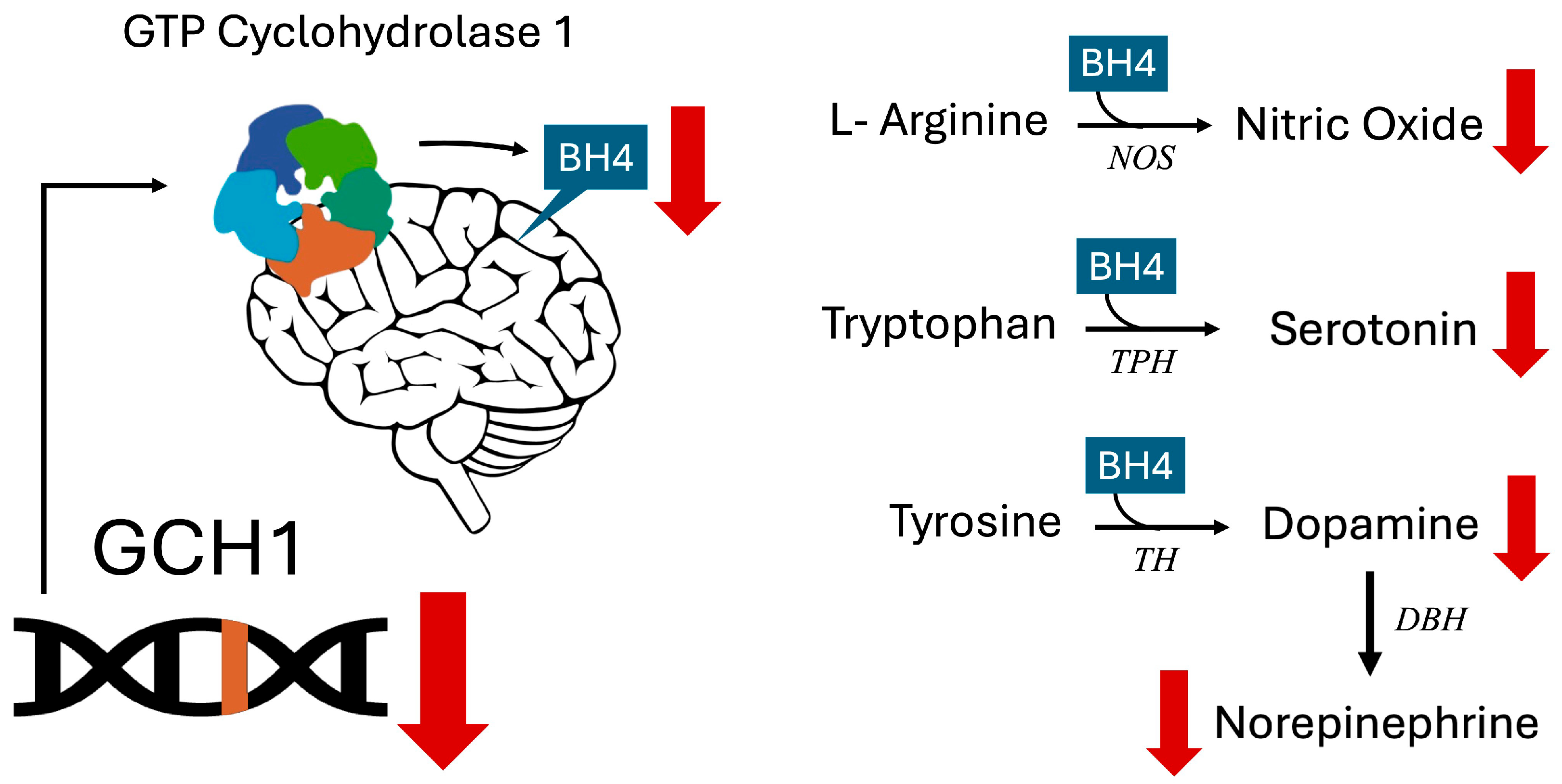
1. Introduction
2. Results
2.1. Case 1: Patient A
2.1.1. Patient Background
2.1.2. Genomic Results and Interpretation
- GCH1 (GTP Cyclohydrolase 1) rs841 homozygous: This SNP is associated with a lower GCH1 expression and BH4 production, which in turn impairs serotonin production and contributes to anxiety [4,8,9]. The rs841 is also associated with a decreased response to SSRIs [11], an issue this patient was experiencing with fluoxetine. Given that infections deplete nitric oxide and increase NO requirements [15], and that BH4 is a cofactor required for NO production [1], the BH4 impairments seen with this SNP shed light on why she became markedly pale with exacerbated anxiety symptoms when ill.
- GSTO1 (Glutathione S-Transferase Omega 1) homozygous: GSTO1 is needed for the recycling of vitamin C [16], a cofactor required for norepinephrine synthesis [17], and variants have been associated with a lower GSTO expression in the brain of autopsied subjects [16]. Impaired vitamin C recycling, along with insufficient BH4 due her lower functioning GCH1, likely resulted in further reductions in norepinephrine that impacted the patient’s mood [18,19,20].
2.1.3. Intervention and Post-Treatment Improvements
2.2. Case 2: Patient B
2.2.1. Patient Background
2.2.2. Genomic Results and Interpretation
- FOLR1 (Folate Receptor Alpha, FR-α) heterozygous: Patient B had a rare FOLR1 variant that added strain to her dysregulated neurotransmitter production triggered by GCH1. This variant is known to decrease FR-α transport capabilities, creating a relative cerebral folate deficiency when methylfolate is used as the folate source [28,29].
- MTHFR (Methylenetetrahydrofolate Reductase) C677T homozygous: Associated with an approximate 70% reduction in enzymatic activity, MTHFR converts folic acid into methylfolate, which is the form of folate that is most able to cross the blood–brain barrier. Methylfolate or a bioactive folate that can enter the brain is crucial for making serotonin, norepinephrine, and dopamine in the brain [30,31,32]. The combination of FOLR1 [33] and the MTHFR variants [34] could compound the problems caused by a GCH1-induced BH4 deficiency and have additive effects on downstream neurotransmitters [1].
- ESR2 (Estrogen Receptor Beta) homozygous: Decreased ESR2 signaling seen with this SNP. This variant has been associated with mood disorders during times of low estrogen exposure, including anxiety [35] and severe depression among postmenopausal women not on hormone therapy [36]. This variant, combined with the natural drop in estrogen that occurs before menstruation [37], helped explain why her mood symptoms peaked during that part of her cycle.
- IL10 (Interleukin 10) homozygous: Patient B’s well-studied IL10-lowering SNP may have further contributed to her inflammation and premenstrual symptoms, as IL10 is anti-inflammatory [38]. ESR2 also normally modulates inflammation via IL10, and thus the combination of low ESR2 signaling and low IL10 may compound each other’s effects [39].
2.2.3. Intervention and Post-Treatment Improvements
2.2.4. Case 2.2: Patient B2, Patient B’s Son
2.3. Case 3: Patient C
2.3.1. Patient Background
2.3.2. Genomic Results and Interpretation
- MTHFR (Methylenetetrahydrofolate Reductase) C677T homozygous: As discussed above, methylfolate is the form of folate that most easily crosses the blood–brain barrier and is a critical cofactor, along with BH4, for synthesizing serotonin from tryptophan, dopamine from tyrosine, and norepinephrine from dopamine [1,32]. This MTHFR variant is a strong contributing factor to ASD [31]. Additionally, MTHFR C677T can contribute to an increased risk of developing folate receptor antibodies [44], which this patient had.
- DBH (Dopamine Beta Hydroxylase) homozygous: DBH is the enzyme that converts dopamine to norepinephrine. Low functioning DBH variants are significant contributors to ADHD [47]. Increased dopamine and decreased norepinephrine have also been associated with irritability and increased ASD severity [48].
2.3.3. Intervention and Post-Treatment Improvements
2.4. Case 4: Patient D
2.4.1. Patient Background
2.4.2. Genomic Results and Interpretation
- GC (Vitamin D Binding Protein) homozygous: Patient D carries two copies of a variant strongly associated with low vitamin D levels and impaired transport of active vitamin D metabolites [54,55]. Low vitamin D may have worsened her depression, as vitamin D is essential for serotonin synthesis [56] and is linked to both depression and anxiety [57].
- GSTO1 and GSTO2 (Glutathione S-Transferase Omega 1 and 2) haplotype: These enzymes support detox pathways, and the variants are associated with decreased enzyme activity and a reduction in the body’s ability to recycle vitamin C [16,60], which is crucial for norepinephrine synthesis—a neurotransmitter important for energy and concentration [17].
2.4.3. Intervention and Post-Treatment Improvements
2.5. Case 5: Patient E
2.5.1. Patient Background
2.5.2. Genomic Results and Interpretation
- CCL2 (C-C Motif Chemokine Ligand 2) homozygous: Patient E had two copies of a SNP found in roughly 9% of the population that is associated with increased CCL2 [62]. Increased activity of this chemotactic factor can translate into more brain inflammation and tissue destruction at the area of inflammation [63].
- CUBN (Cubilin) homozygous: Low B12 absorption in gut due to lower intrinsic factor, thus increasing B12 needs [66].
- MTRR (Methionine Synthase Reductase) homozygous: Increased need for B vitamins, also a significant contributor to ADHD [67].
- HTR1A (5-Hydroxytryptamine Receptor 1A) homozygous: Low serotonin synthesis due to serotonin dysregulation [68].
- GSTP1 (Glutathione S-Transferase Pi 1) homozygous: Reduced detox capacity and less ability to process and remove glyphosates [69].
2.5.3. Intervention and Post-Treatment Improvements
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BH4 | Tetrahydrobiopterin |
| 5-HT | Serotonin |
| DA | Dopamine |
| NE | Norepinephrine |
| NO | Nitric Oxide |
| CSF | Cerebrospinal Fluid |
| GCH1 | GTP Cyclohydrolase 1 |
| SNP | Single Nucleotide Polymorphism |
| SSRIs | Selective Serotonin Reuptake Inhibitors |
| ADHD | Attention-Deficit/Hyperactivity Disorder |
| PMDD | Premenstrual Dysphoric Disorder |
| ASD | Autism Spectrum Disorder |
| MTHFR | Methylenetetrahydrofolate Reductase |
| IXXD | IntellxxDNA™ |
| CDS | Clinical Decision Support Tool |
| ATEC | Autism Treatment Evaluation Checklist |
| NAC | N-AcetylCysteine |
| GABA | Gamma-Aminobutyric Acid (GABA) |
| OCD | Obsessive–Compulsive Disorder |
| OCPs | Oral Contraceptive Pills |
| eNOS | Endothelial Nitric Oxide Synthase |
| GSTO1 | Glutathione S-Transferase Omega 1 |
| GSTO2 | Glutathione S-Transferase Omega 2 |
| DRD2 | Dopamine Receptor D2 |
| HTR1A | 5-Hydroxytryptamine Receptor 1A |
| HgA1c | Hemoglobin A1c |
| FOLR1 | Folate Receptor Alpha |
| ESR2 | Estrogen Receptor Beta |
| IL10 | Interleukin 10 |
| PPCDC | Phosphopantothenoylcysteine Decarboxylase |
| DBH | Dopamine Beta Hydroxylase |
| HTR1B | 5-Hydroxytryptamine Receptor 1B |
| P5P | Pyridoxal 5′-Phosphate |
| HTR2A | 5-Hydroxytryptamine Receptor 2A |
| CCL2 | C-C Motif Chemokine Ligand 2 |
| IL1B | Interleukin 1 Beta |
| CUBN | Cubilin |
| MTRR | Methionine Synthase Reductase |
| GSTP1 | Glutathione S-Transferase Pi 1 |
References
- Fanet, H.; Capuron, L.; Castanon, N.; Calon, F.; Vancassel, S. Tetrahydrobiopterin (BH4) Pathway: From Metabolism to Neuropsychiatry. Curr. Neuropharmacol. 2021, 19, 591–609. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Guo, W. Emotional Roles of Mono-Aminergic Neurotransmitters in Major Depressive Disorder and Anxiety Disorders. Front. Psychol. 2018, 9, 2201. [Google Scholar] [CrossRef]
- Sekhon, S.; Gupta, V. Mood Disorder. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK558911/ (accessed on 19 March 2025).
- Vancassel, S.; Capuron, L.; Castanon, N. Brain Kynurenine and BH4 Pathways: Relevance to the Pathophysiology and Treatment of Inflammation-Driven Depressive Symptoms. Front. Neurosci. 2018, 12, 499. [Google Scholar] [CrossRef]
- Filho, C.C.; Melfior, L.; Ramos, S.L.; Pizi, M.S.O.; Taruhn, L.F.; Muller, M.E.; Nunes, T.K.; Schmitt, L.d.O.; Gaspar, J.M.; de Oliveira, M.d.A.; et al. Tetrahydrobiopterin and Autism Spectrum Disorder: A Systematic Review of a Promising Therapeutic Pathway. Brain Sci. 2025, 15, 151. [Google Scholar] [CrossRef]
- Clot, F.; Grabli, D.; Cazeneuve, C.; Roze, E.; Castelnau, P.; Chabrol, B.; Landrieu, P.; Nguyen, K.; Ponsot, G.; Abada, M.; et al. Exhaustive Analysis of BH4 and Dopamine Biosynthesis Genes in Patients with Dopa-Responsive Dystonia. Brain 2009, 132, 1753–1763. [Google Scholar] [CrossRef]
- Frye, R.E.; Huffman, L.C.; Elliott, G.R. Tetrahydrobiopterin as a Novel Therapeutic Intervention for Autism. Neurotherapeutics 2010, 7, 241–249. [Google Scholar] [CrossRef]
- Kouhsar, S.S.; Bigdeli, M.; Shakiba, Y.; Sadeghniiat, K. GCH1 (rs841) Polymorphism in the Nitric Oxide-Forming Pathway Has Protective Effects on Obstructive Sleep Apnea. Sci. Rep. 2019, 9, 18664. [Google Scholar] [CrossRef]
- Zhang, L.; Rao, F.; Zhang, K.; Khandrika, S.; Das, M.; Vaingankar, S.M.; Bao, X.; Rana, B.K.; Smith, D.W.; Wessel, J.; et al. Discovery of Common Human Genetic Variants of GTP Cyclohydrolase 1 (GCH1) Governing Nitric Oxide, Autonomic Activity, and Cardiovascular Risk. J. Clin. Investig. 2007, 117, 2658–2671. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, Y.; Hashimoto, R.; Ohi, K.; Yamamori, H.; Fujimoto, M.; Umeda-Yano, S.; Fujino, H.; Fukunaga, M.; Horiguchi, M.; Takeda, M.; et al. A Functional Polymorphism of the GTP Cyclohydrolase 1 Gene Predicts Attention Performance. Neurosci. Lett. 2014, 566, 46–49. [Google Scholar] [CrossRef]
- Kishi, T.; Ichinose, H.; Yoshimura, R.; Fukuo, Y.; Kitajima, T.; Inada, T.; Kunugi, H.; Kato, T.; Yoshikawa, T.; Ujike, H.; et al. GTP Cyclohydrolase 1 Gene Haplotypes as Predictors of SSRI Response in Japanese Patients with Major Depressive Disorder. J. Affect. Disord. 2012, 142, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Sadahiro, R.; Suzuki, A.; Matsumoto, Y.; Shibuya, N.; Enokido, M.; Kamata, M.; Goto, K.; Otani, K. Functional Polymorphism of the GTP Cyclohydrolase 1 Gene Affects the Personality Trait of Novelty Seeking in Healthy Subjects. Neurosci. Lett. 2011, 503, 220–223. [Google Scholar] [CrossRef]
- Maruf, A.A.; Poweleit, E.A.; Brown, L.C.; Strawn, J.R.; Bousman, C.A. Systematic Review and Meta-Analysis of L-Methylfolate Augmentation in Depressive Disorders. Pharmacopsychiatry 2021, 54, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Junger, I.; Caixeta, L.F.; Noll, M.; Oliveira, C.; Silveira, É.A. The Effects of Methylfolate on Cognitive Decline and Dementia: A Protocol for Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3075. [Google Scholar] [CrossRef]
- Lisi, F.; Zelikin, A.N.; Chandrawati, R. Nitric Oxide to Fight Viral Infections. Adv. Sci. 2021, 8, 2003895. [Google Scholar] [CrossRef]
- Allen, M.; Zou, F.; Chai, H.; Younkin, C.S.; Miles, R.; Nair, A.A.; Crook, J.E.; Pankratz, V.; Carrasquillo, M.M.; Rowley, C.N.; et al. Glutathione S-Transferase Omega Genes in Alzheimer and Parkinson Disease Risk, Age-at-Diagnosis and Brain Gene Expression: An Association Study with Mechanistic Implications. Mol. Neurodegener. 2012, 7, 13. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Bozonet, S.M.; Vissers, M.C.M. High Vitamin C Status Is Associated with Elevated Mood in Male Tertiary Students. Antioxidants 2018, 7, 91. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.; Meredith, M.E. Mechanisms of Ascorbic Acid Stimulation of Norepinephrine Synthesis in Neuronal Cells. Biochem. Biophys. Res. Commun. 2012, 426, 148–152. [Google Scholar] [CrossRef]
- Takeshita, N.; Kawade, N.; Suzuki, W.; Hara, S.; Horio, F.; Ichinose, H. Deficiency of Ascorbic Acid Decreases the Contents of Tetrahydrobiopterin in the Liver and the Brain of ODS Rats. Neurosci. Lett. 2020, 715, 134656. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Horiguchi, M.; Yamaguti, K.; Nakatomi, Y.; Kuratsune, H.; Ichinose, H.; Watanabe, Y. Association of Monoamine-Synthesizing Genes with the Depression Tendency and Personality in Chronic Fatigue Syndrome Patients. Life Sci. 2012, 92, 183–186. [Google Scholar] [CrossRef]
- Roetker, N.S.; Yonker, J.A.; Lee, C.; Chang, V.; Basson, J.; Roan, C.L.; Hauser, T.S.; Hauser, R.A.; Atwood, C.S. Multigene Interactions and the Prediction of Depression in the Wisconsin Longitudinal Study. BMJ Open 2012, 2, e000944. [Google Scholar] [CrossRef] [PubMed]
- Sanwald, S.; Montag, C.; Kiefer, M. Cumulative Genetic Score of DRD2 Polymorphisms Is Associated with Impulsivity and Masked Semantic Priming. J. Mol. Neurosci. 2022, 72, 1682–1694. [Google Scholar] [CrossRef]
- Molina, E.; Cervilla, J.; Rivera, M.; Torres, F.; Bellón, J.Á.; Moreno, B.; King, M.; Nazareth, I.; Gutiérrez, B. Polymorphic Variation at the Serotonin 1-A Receptor Gene Is Associated with Comorbid Depression and Generalized Anxiety. Psychiatr. Genet. 2011, 21, 195–201. [Google Scholar] [CrossRef]
- Lee, D.K.; Lipner, S.R. The Potential of N-Acetylcysteine for Treatment of Trichotillomania, Excoriation Disorder, Onychophagia, and Onychotillomania: An Updated Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 6370. [Google Scholar] [CrossRef]
- Jeon, Y.K.; Ha, C.H. The Effect of Exercise Intensity on Brain Derived Neurotrophic Factor and Memory in Adolescents. Environ. Health Prev. Med. 2017, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Abudukadier, A.; Fujita, Y.; Obara, A.; Ohashi, A.; Fukushima, T.; Sato, Y.; Ogura, M.; Nakamura, Y.; Fujimoto, S.; Hosokawa, M.; et al. Tetrahydrobiopterin Has a Glucose-Lowering Effect by Suppressing Hepatic Gluconeogenesis in an Endothelial Nitric Oxide Synthase–Dependent Manner in Diabetic Mice. Diabetes 2013, 62, 3033–3043. [Google Scholar] [CrossRef]
- Shah, N.R.; Jones, J.B.; Aperi, J.; Shemtov, R.; Karne, A.; Borenstein, J. Selective Serotonin Reuptake Inhibitors for Premenstrual Syndrome and Premenstrual Dysphoric Disorder. Obstet. Gynecol. 2008, 111, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Böttiger, A.K.; Hagnelius, N.-O.; Nilsson, T.K. Mutations in Exons 2 and 3 of the FOLR1 Gene in Demented and Non-Demented Elderly Subjects. Int. J. Mol. Med. 2007, 20, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Saha, T.; Sinha, S.; Rajamma, U.; Mukhopadhyay, K. Autistic Traits and Components of the Folate Metabolic System: An Explorative Analysis in the Eastern Indian ASD Subjects. Nutr. Neurosci. 2019, 23, 860–867. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Cerebral Folate Deficiency, Folate Receptor Alpha Autoantibodies and Leucovorin (Folinic Acid) Treatment in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 1141. [Google Scholar] [CrossRef]
- Wan, L.; Li, Y.; Zhang, Z.; Sun, Z.; He, Y.; Li, R. Methylenetetrahydrofolate Reductase and Psychiatric Diseases. Transl. Psychiatry 2018, 8, 242. [Google Scholar] [CrossRef]
- Shelton, R.C.; Manning, J.S.; Barrentine, L.W.; Tipa, E.V. Assessing Effects of L-Methylfolate in Depression Management: Results of a Real-World Patient Experience Trial. Prim. Care Companion CNS Disord. 2013, 15, PCC.13m01520. [Google Scholar] [CrossRef]
- Goldman, I.D. FOLR1-Related Cerebral Folate Transport Deficiency. In GeneReviews® [Internet]; University of Washington: Seattle, WA, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK599286/ (accessed on 19 March 2025).
- Khan, S.; Naeem, A.; Fritts, A.; Cummins, M.; Kayes, C.; Fang, W. Discovery of Methylenetetrahydrofolate Reductase (MTHFR) Deficiency in Individuals with Common Psychiatric Comorbidities: A Retrospective Case Review. Cureus 2024, 16, e58122. [Google Scholar] [CrossRef]
- Ryan, J.; Scali, J.; Carrière, I.; Scarabin, P.-Y.; Ritchie, K.; Ancelin, M.-L. Estrogen Receptor Gene Variants Are Associated with Anxiety Disorders in Older Women. Psychoneuroendocrinology 2011, 36, 1582–1586. [Google Scholar] [CrossRef]
- Ryan, J.; Ancelin, M.-L. Polymorphisms of Estrogen Receptors and Risk of Depression. Drugs 2012, 72, 1725–1738. [Google Scholar] [CrossRef]
- Reed, B.G.; Carr, B.R. The Normal Menstrual Cycle and the Control of Ovulation. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279054/ (accessed on 20 March 2025).
- Zheng, Z.; Huang, G.; Gao, T.; Huang, T.; Zou, M.; Zou, Y.; Duan, S. Epigenetic Changes Associated with Interleukin-10. Front. Immunol. 2020, 11, 1105. [Google Scholar] [CrossRef]
- Kassi, E.; Moutsatsou, P. Estrogen Receptor Signaling and Its Relationship to Cytokines in Systemic Lupus Erythematosus. J. Biomed. Biotechnol. 2010, 2010, 317452. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, M. L-Methylfolate in Antidepressant Non-Responders: The Impact of Body Weight and Inflammation. Front. Psychiatry 2022, 13, 840116. [Google Scholar] [CrossRef] [PubMed]
- Lobato, T.B.; Santos, E.S.S.; Iser-Bem, P.N.; Falcão, H.S.; Gimenes, G.M.; Pauferro, J.R.B.; Rodrigues, G.T.; Correa, I.S.; Pereira, A.C.G.; Passos, M.E.P.; et al. Omega-3 Fatty Acids Weaken Lymphocyte Inflammatory Features and Improve Glycemic Control in Nonobese Diabetic Goto-Kakizaki Rats. Nutrients 2024, 16, 4106. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C—Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesan, P.; Kabbani, A.; Vyshedskiy, A. Parent-Reported Assessment Scores Reflect the ASD Severity Level in 2- to 7-Year-Old Children. Children 2022, 9, 701. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, L.; Lei, Y.; Yang, N.; Cabrera, R.M.; Finnell, R.H.; Ren, A. Gene Variants in the Folate Pathway Are Associated with Increased Levels of Folate Receptor Autoantibodies. Birth Defects Res. 2018, 110, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.M.; Zhu, G.; Dy, V.; Heath, A.C.; Madden, P.A.F.; Kemp, J.P.; McMahon, G.; St Pourcain, B.; Timpson, N.J.; Golding, J.; et al. Genome-Wide Association Study Identifies Loci Affecting Blood Copper, Selenium and Zinc. Hum. Mol. Genet. 2013, 22, 3998–4006. [Google Scholar] [CrossRef]
- Kaczmarek, K.A.; Dobrzyńska, M.; Drzymała-Czyż, S. Iron, Magnesium, Zinc and Selenium—The Most Common Elemental Deficiencies in Children with Autism Spectrum Disorder. Res. Autism Spectr. Disord. 2023, 110, 102288. [Google Scholar] [CrossRef]
- Barrie, E.S.; Pinsonneault, J.K.; Sadee, W.; Hollway, J.A.; Handen, B.L.; Smith, T.; Arnold, L.E.; Butter, E.; Hansen-Kiss, E.; Herman, G.E.; et al. Testing Genetic Modifiers of Behavior and Response to Atomoxetine in Autism Spectrum Disorder with ADHD. J. Dev. Phys. Disabil. 2018, 30, 355–371. [Google Scholar] [CrossRef]
- For, S. Dopamine Excess and/or Norepinephrine and Epinephrine Deficiency in Autistic Patients Due to Prenatal and/or Postnatal Deficiency of Dopamine Beta-Hydroxylase. ISOM 2021, 36, 1. Available online: https://isom.ca/article/dopamine-excess-and-or-norepinephrine-and-epinephrine-deficiency-in-autistic-patients-due-to-prenatal-and-or-postnatal-deficiency-of-dopamine-beta-hydroxylase/ (accessed on 12 April 2025).
- Bidwell, L.C.; Gray, J.C.; Weafer, J.; Palmer, A.A.; de Wit, H.; MacKillop, J. Genetic Influences on ADHD Symptom Dimensions: Examination of a Priori Candidates, Gene-Based Tests, Genome-Wide Variation, and SNP Heritability. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2017, 174, 458–466. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, D.; Hartman, C.A.; van Donkelaar, M.; Bralten, J.; von Rhein, D.; Hakobjan, M.; Franke, B.; Heslenfeld, D.J.; Oosterlaan, J.; Rommelse, N.; et al. Variation in Serotonin Neurotransmission Genes Affects Neural Activation During Response Inhibition in Adolescents and Young Adults with ADHD and Healthy Controls. World J. Biol. Psychiatry 2015, 16, 625–634. [Google Scholar] [CrossRef]
- García-Minguillán, C.J.; Fernandez-Ballart, J.D.; Ceruelo, S.; Ríos, L.; Bueno, O.; Berrocal-Zaragoza, M.I.; Molloy, A.M.; Ueland, P.M.; Meyer, K.; Murphy, M.M. Riboflavin Status Modifies the Effects of Methylenetetrahydrofolate Reductase (MTHFR) and Methionine Synthase Reductase (MTRR) Polymorphisms on Homocysteine. Genes Nutr. 2014, 9, 435. [Google Scholar] [CrossRef]
- Wang, J.; Huang, H.; Liu, C.; Zhang, Y.; Wang, W.; Zou, Z.; Yang, L.; He, X.; Wu, J.; Ma, J.; et al. Research Progress on the Role of Vitamin D in Autism Spectrum Disorder. Front. Behav. Neurosci. 2022, 16, 859151. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-M.; Lee, K.-M.; Lee, C.-Y.; Lee, H.-C.; Tam, K.-W.; Loh, E.-W. Effectiveness of N-Acetylcysteine in Autism Spectrum Disorders: A Meta-Analysis of Randomized Controlled Trials. Aust. N. Z. J. Psychiatry 2020, 55, 196–206. [Google Scholar] [CrossRef]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common Genetic Determinants of Vitamin D Insufficiency: A Genome-Wide Association Study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Didriksen, A.; Grimnes, G.; Hutchinson, M.S.; Kjærgaard, M.; Svartberg, J.; Joakimsen, R.M.; Jorde, R. The Serum 25-Hydroxyvitamin D Response to Vitamin D Supplementation Is Related to Genetic Factors, BMI, and Baseline Levels. Eur. J. Endocrinol. 2013, 169, 559–567. [Google Scholar] [CrossRef]
- Sabir, M.S.; Haussler, M.R.; Mallick, S.; Kaneko, I.; Lucas, D.A.; Haussler, C.A.; Whitfield, G.K.; Jurutka, P.W. Optimal Vitamin D Spurs Serotonin: 1,25-Dihydroxyvitamin D Represses Serotonin Reuptake Transport (SERT) and Degradation (MAO-A) Gene Expression in Cultured Rat Serotonergic Neuronal Cell Lines. Genes Nutr. 2018, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Akpınar, Ş.; Karadağ, M.G. Is Vitamin D Important in Anxiety or Depression? What Is the Truth? Curr. Nutr. Rep. 2022, 11, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Jiang, M.-Y.; Kan, Z.-M.; Chu, Y. Influence of 5-HTR2A Genetic Polymorphisms on the Efficacy of Antidepressants in the Treatment of Major Depressive Disorder: A Meta-Analysis. J. Affect. Disord. 2014, 168, 430–438. [Google Scholar] [CrossRef]
- Guiard, B.P.; Di Giovanni, G. Central Serotonin-2A (5-HT2A) Receptor Dysfunction in Depression and Epilepsy: The Missing Link? Front. Pharmacol. 2015, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, Y.; Gao, Y.; Zhao, L.; Feng, H.; Wei, W.; Qiu, C.; He, Q.; Zhang, Y.; Fu, S.; et al. Association Between Arsenic Metabolism Gene Polymorphisms and Arsenic-Induced Skin Lesions in Individuals Exposed to High-Dose Inorganic Arsenic in Northwest China. Sci. Rep. 2018, 8, 6436. [Google Scholar] [CrossRef]
- Okada, M.; Fukuyama, K.; Kawano, Y.; Shiroyama, T.; Suzuki, D.; Ueda, Y. Effects of Acute and Sub-Chronic Administrations of Guanfacine on Catecholaminergic Transmissions in the Orbitofrontal Cortex. Neuropharmacology 2019, 156, 107547. [Google Scholar] [CrossRef]
- Shen, R.; Lin, S.; He, L.; Zhu, X.; Zhou, Z.; Chen, S.; Wang, Y.; Ding, J. Association of Two Polymorphisms in CCL2 with Parkinson’s Disease: A Case-Control Study. Front. Neurol. 2019, 10, 35. [Google Scholar] [CrossRef]
- Conductier, G.; Blondeau, N.; Guyon, A.; Nahon, J.-L.; Rovère, C. The Role of Monocyte Chemoattractant Protein MCP1/CCL2 in Neuroinflammatory Diseases. J. Neuroimmunol. 2010, 224, 93–100. [Google Scholar] [CrossRef]
- Krakowiak, P.; Goines, P.E.; Tancredi, D.J.; Ashwood, P.; Hansen, R.L.; Hertz-Picciotto, I.; Van de Water, J. Neonatal Cytokine Profiles Associated with Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 442–451. [Google Scholar] [CrossRef]
- Toma, F.M.; Kalam, K.T.; Haque, M.A.; Reza, S.; Akter, R.; Islam, M.S.; Islam, M.R.; Nahar, Z. Interleukin-1β rs16944 and rs1143627 Polymorphisms and Risk of Developing Major Depressive Disorder: A Case-Control Study among Bangladeshi Population. PLoS ONE 2025, 20, e0317665. [Google Scholar] [CrossRef]
- Surendran, S.; Adaikalakoteswari, A.; Saravanan, P.; Shatwaan, I.A.; Lovegrove, J.A.; Vimaleswaran, K.S. An Update on Vitamin B12-Related Gene Polymorphisms and B12 Status. Genes Nutr. 2018, 13, 2. [Google Scholar] [CrossRef]
- Saha, T.; Chatterjee, M.; Verma, D.; Ray, A.; Sinha, S.; Rajamma, U.; Mukhopadhyay, K. Genetic Variants of the Folate Metabolic System and Mild Hyperhomocysteinemia May Affect ADHD Associated Behavioral Problems. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 84, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, Z.R.; Piel, D.A.; Santos, T.L.; Richardson-Jones, J.; Leonardo, E.D.; Beck, S.G.; Champagne, F.A.; Hen, R. Developmental Effects of Serotonin 1A Autoreceptors on Anxiety and Social Behavior. Neuropsychopharmacology 2013, 39, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Bocedi, A.; Noce, A.; Marrone, G.; Noce, G.; Cattani, G.; Gambardella, G.; Di Lauro, M.; Di Daniele, N.; Ricci, G. Glutathione Transferase P1-1: An Enzyme Useful in Biomedicine and as Biomarker in Clinical Practice and in Environmental Pollution. Nutrients 2019, 11, 1741. [Google Scholar] [CrossRef] [PubMed]
- Tuplin, E.W.; Holahan, M.R. Aripiprazole, a Drug That Displays Partial Agonism and Functional Selectivity. Curr. Neuropharmacol. 2017, 15, 1192–1203. [Google Scholar] [CrossRef]
- Bertollo, A.G.; Puntel, C.F.; da Silva, B.V.; Martins, M.; Bagatini, M.D.; Ignácio, Z.M. Neurobiological Relationships Between Neurodevelopmental Disorders and Mood Disorders. Brain Sci. 2025, 15, 307. [Google Scholar] [CrossRef]
- Krasner, H.; Ong, C.V.; Hewitt, P.; Vida, T.A. From Stress to Synapse: The Neuronal Atrophy Pathway to Mood Dysregulation. Int. J. Mol. Sci. 2025, 26, 3219. [Google Scholar] [CrossRef]
- Sanford, M.; Keating, G.M. Sapropterin. Drugs 2009, 69, 461–476. [Google Scholar] [CrossRef]
- Wilson, S.K.; Thomas, J. BH4 as a Therapeutic Target for ADHD: Relevance to Neurotransmitters and Stress-Driven Symptoms. J. Atten. Disord. 2024, 28, 161–167. [Google Scholar] [CrossRef]
- Godfrey, P.S.A.; Toone, B.K.; Bottiglien, T.; Laundy, M.; Reynolds, E.H.; Carney, M.W.P.; Flynn, T.G.; Chanarin, I. Enhancement of recovery from psychiatric illness by methylfolate. Lancet 1990, 336, 392–395. [Google Scholar] [CrossRef]
- Lam, N.S.K.; Long, X.X.; Li, X.; Saad, M.; Lim, F.; Doery, J.C.; Griffin, R.C.; Galletly, C. The Potential Use of Folate and Its Derivatives in Treating Psychiatric Disorders: A Systematic Review. Biomed. Pharmacother. 2022, 146, 112541. [Google Scholar] [CrossRef]
- Ashe, K.; Kelso, W.; Farrand, S.; Panetta, J.; Fazio, T.; De Jong, G.; Walterfang, M. Psychiatric and Cognitive Aspects of Phenylketonuria: The Limitations of Diet and Promise of New Treatments. Front. Psychiatry 2019, 10, 561. [Google Scholar] [CrossRef]
- Cavaleri, D.; Bartoli, F.; Capogrosso, C.A.; Guzzi, P.; Moretti, F.; Riboldi, I.; Misiak, B.; Kishi, T.; Rubin, R.T.; Fuchs, D.; et al. Blood Concentrations of Neopterin and Biopterin in Subjects with Depression: A Systematic Review and Meta-Analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 120, 110633. [Google Scholar] [CrossRef]
- Cacabelos, R.; Naidoo, V.; Martínez-Iglesias, O.; Corzo, L.; Cacabelos, N.; Pego, R.; Carril, J.C. Personalized Management and Treatment of Alzheimer’s Disease. Life 2022, 12, 460. [Google Scholar] [CrossRef]
- Way, H.; Williams, G.; Hausman-Cohen, S.; Reeder, J. Genomics as a Clinical Decision Support Tool: Successful Proof of Concept for Improved ASD Outcomes. J. Pers. Med. 2021, 11, 596. [Google Scholar] [CrossRef] [PubMed]
- Gray, I.D.; Kross, A.R.; Renfrew, M.E.; Wood, P. Precision Medicine in Lifestyle Medicine: The Way of the Future? Am. J. Lifestyle Med. 2019, 13, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Milić, J.; Zeković, J.; Stankić, D.; Henčić, B.; Jančić, J.; Samardžić, J. Cognition-enhancing drugs and applications to aging. In Assessments, Treatments and Modeling in Aging and Neurological Disease; Martin, C.R., Preedy, V.R., Rajendram, R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 367–378. [Google Scholar] [CrossRef]
- Goldberg, J.F. Does Pharmacogenomic Testing Meaningfully Improve Antidepressant Treatment Outcomes When Looking Only at Patients Taking Phase I Hepatically Metabolized Drugs? A Little. J. Clin. Psychiatry 2019, 80, 14236. Available online: https://www.psychiatrist.com/jcp/pharmacogenic-testing-antidepressant-treatment-outcomes-hepatically-metabolized-drugs/ (accessed on 2 July 2025). [CrossRef] [PubMed]
- Zagorski, N. Treatment-Resistant Depression Consumes Billions of Dollars. Psychiatr. News 2014, 49, 17. [Google Scholar] [CrossRef]
- Shah, D.; Allen, L.; Zheng, W.; Madhavan, S.S.; Wei, W.; LeMasters, T.J.; Sambamoorthi, U. Economic Burden of Treatment-Resistant Depression among Adults with Chronic Non-Cancer Pain Conditions and Major Depressive Disorder in the US. Pharmacoeconomics 2021, 39, 639–651. [Google Scholar] [CrossRef] [PubMed]
| Case Study Patient | Age, Sex, Weight, and Primary Concern(s) | GCH1 rs841 Genotype | BH4 Dose | BH4 Dose by Weight |
|---|---|---|---|---|
| Patient A | 11, Female, 102 lbs, and Severe Anxiety | AA | 5 mg BID | 0.216 mg/kg/day |
| Patient B | 42, Female, 125 lbs, and PMDD | AA | 2.5 mg BID-TID | 0.088–0.132 mg/kg/day |
| Patient B2 | 9, Male, 52 lbs, and Mild ADHD Behaviors | AA | 2.5 mg QD | 0.106 mg/kg/day |
| Patient C | 10, Male, 66 lbs, ASD (Level 1), and ADHD | AA | 2.5 mg BID | 0.167 mg/kg/day |
| Patient D | 57, Female, 151 lbs, Depression, Anxiety, and Chronic Insomnia | AA | 7–10 mg BID | 0.204–0.292 mg/kg/day |
| Patient E | 8, Male, 55 lbs, and Complex Behavioral Issues (Diagnosed Anxiety with OCD traits, ADHD, and Major Depressive Disorder) | AA | 2.5 mg BID | 0.2 mg/kg/day |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, G.E.; Hausman-Cohen, S.; Sotos, M.; Gutierrez, E.; Bilich, C.; Mueller, F.W.; Jagshi, S. The Role of GCH1 Deficiency and Tetrahydrobiopterin in Mental Health. Int. J. Mol. Sci. 2025, 26, 8030. https://doi.org/10.3390/ijms26168030
Williams GE, Hausman-Cohen S, Sotos M, Gutierrez E, Bilich C, Mueller FW, Jagshi S. The Role of GCH1 Deficiency and Tetrahydrobiopterin in Mental Health. International Journal of Molecular Sciences. 2025; 26(16):8030. https://doi.org/10.3390/ijms26168030
Chicago/Turabian StyleWilliams, Grant E., Sharon Hausman-Cohen, Maryelaine Sotos, Emily Gutierrez, Carol Bilich, Francis W. Mueller, and Shaun Jagshi. 2025. "The Role of GCH1 Deficiency and Tetrahydrobiopterin in Mental Health" International Journal of Molecular Sciences 26, no. 16: 8030. https://doi.org/10.3390/ijms26168030
APA StyleWilliams, G. E., Hausman-Cohen, S., Sotos, M., Gutierrez, E., Bilich, C., Mueller, F. W., & Jagshi, S. (2025). The Role of GCH1 Deficiency and Tetrahydrobiopterin in Mental Health. International Journal of Molecular Sciences, 26(16), 8030. https://doi.org/10.3390/ijms26168030





