Accumulation of Phenolic Compounds in Microshoot Cultures of Rhododendron tomentosum Harmaja (Ledum palustre L.)
Abstract
1. Introduction
2. Results and Discussion
2.1. Qualitative and Quantitative Analysis of Phenolic Compounds in Wild-Grown Plants and In Vitro Cultures of Rhododendron tomentosum
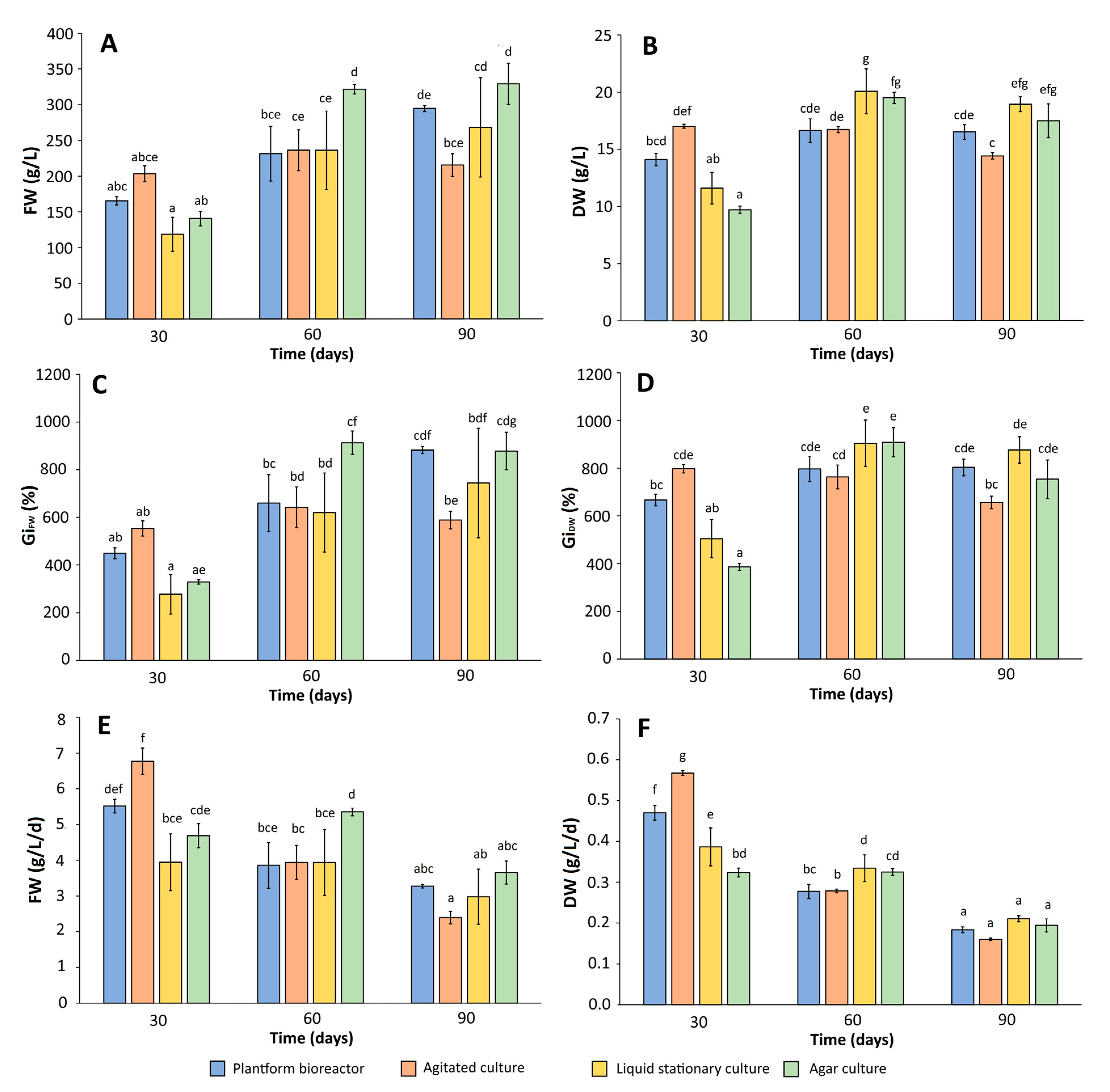
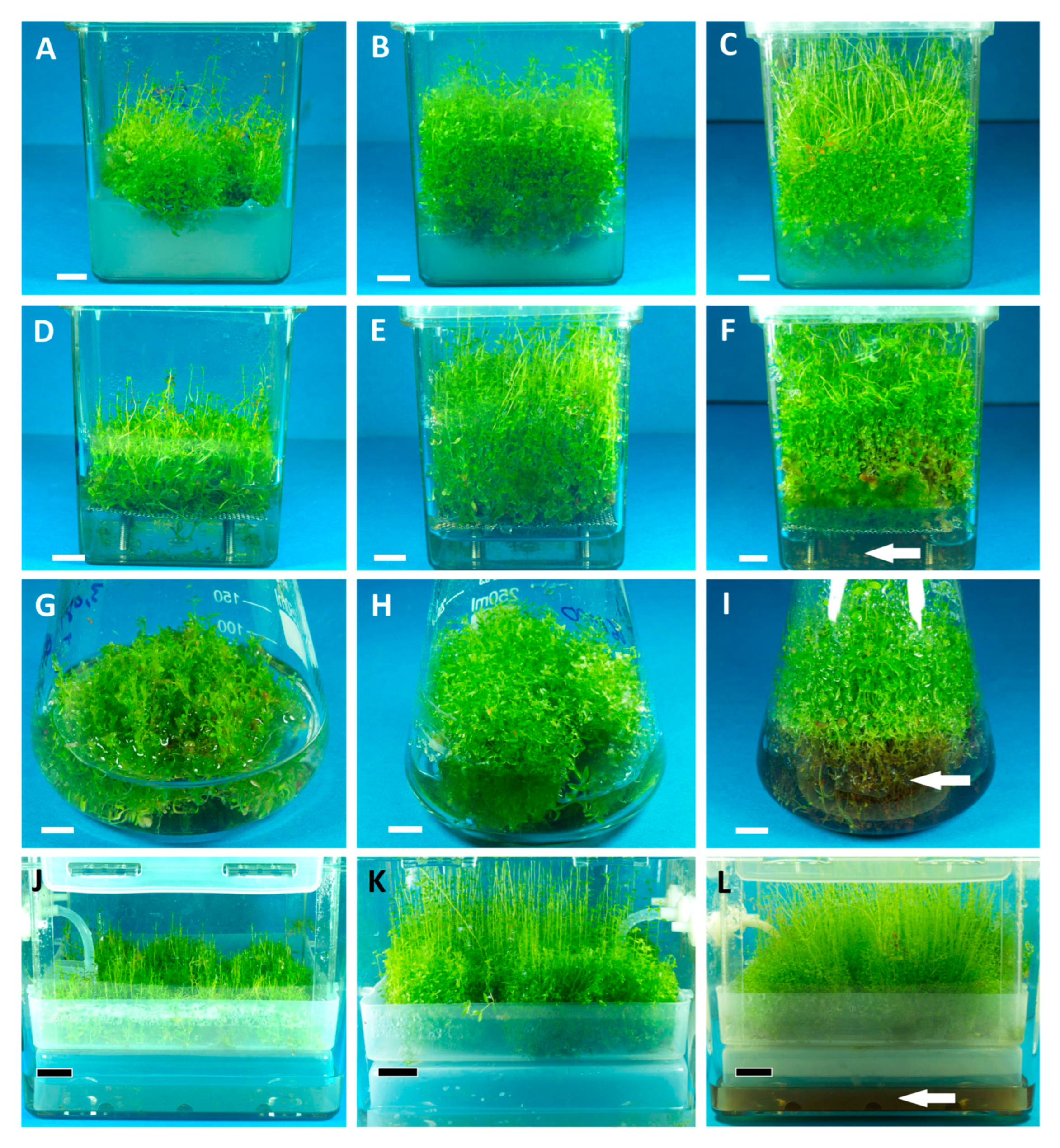
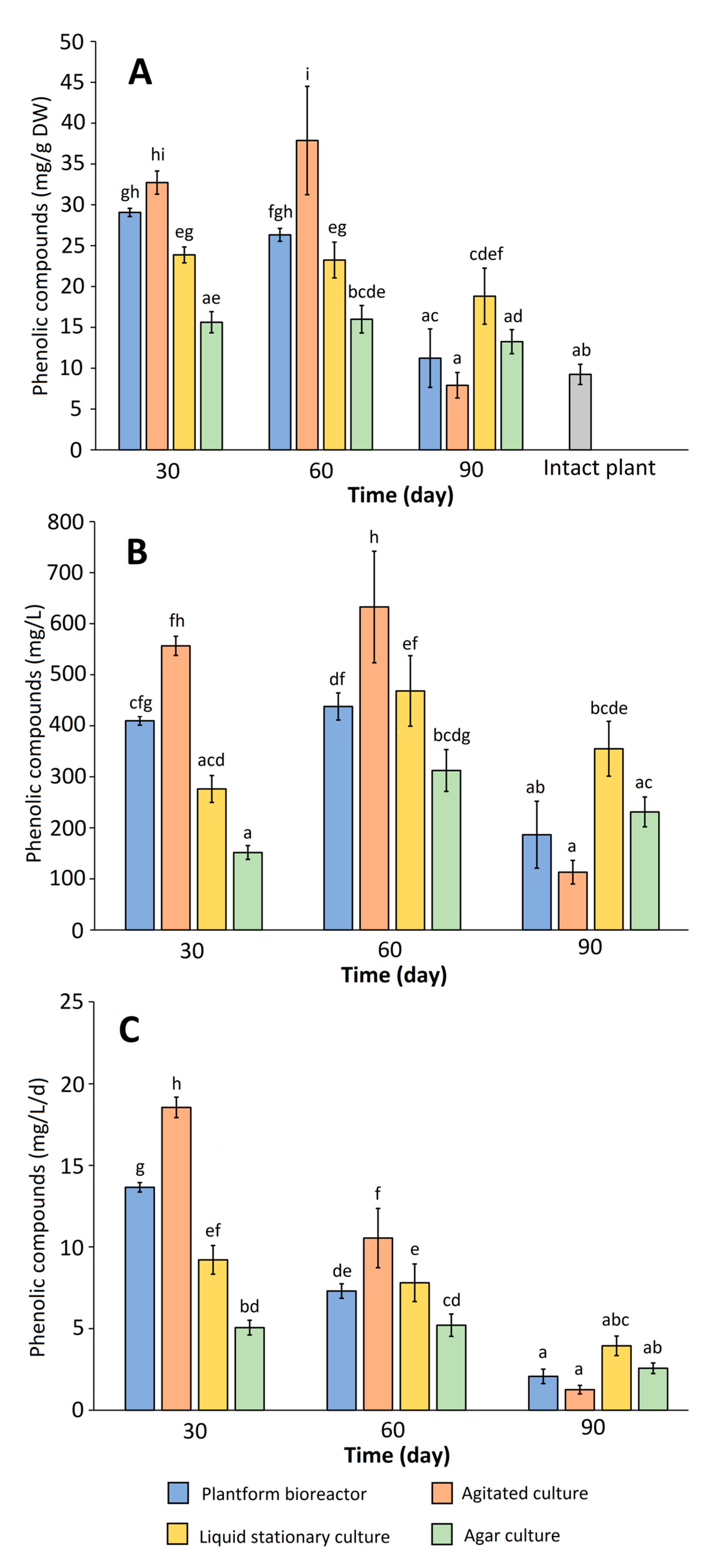
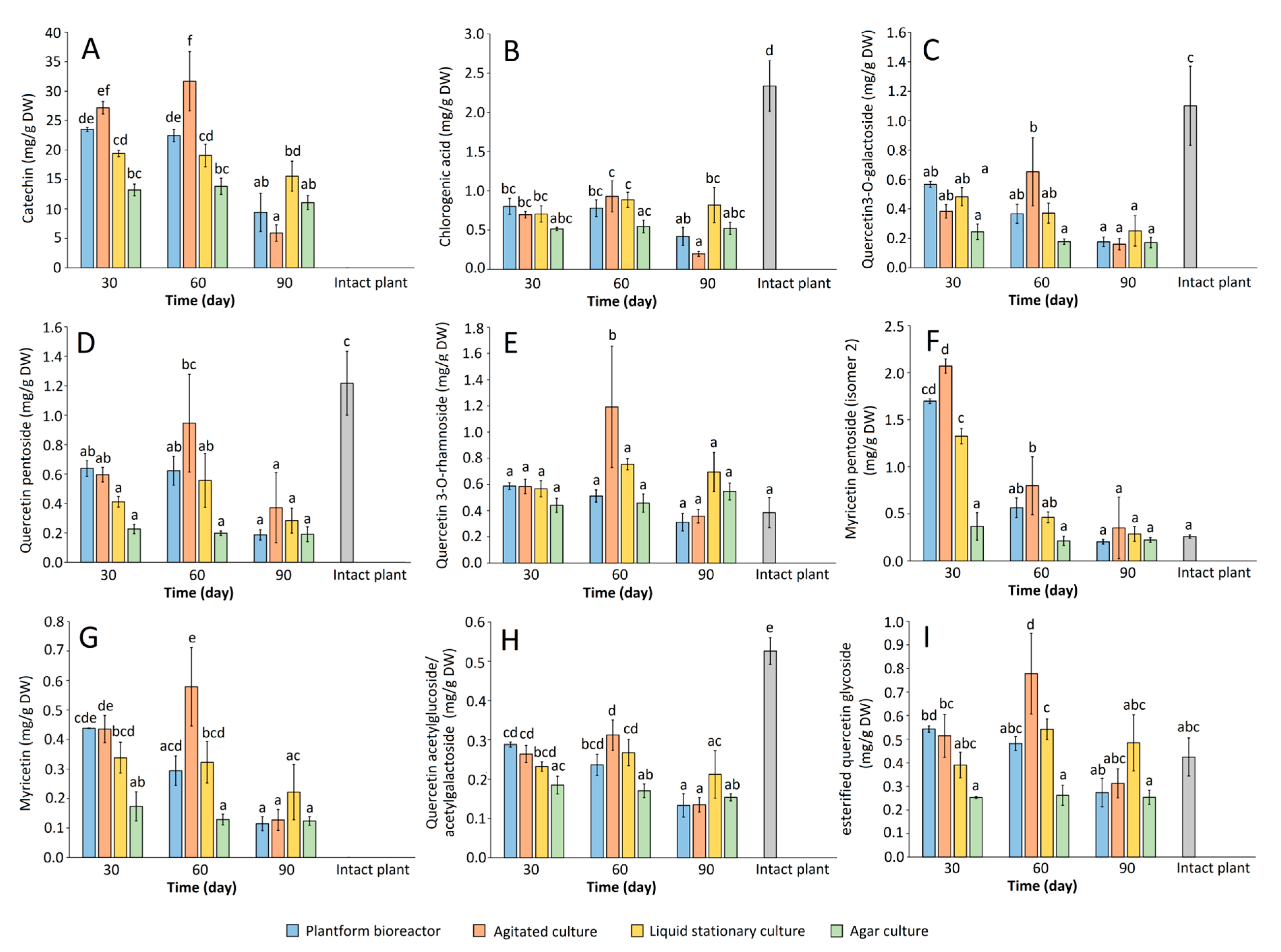
2.2. Biomass Growth and Phenolics Accumulation in Rhododendron tomentosum Microshoot Cultures Maintained in Different In Vitro Systems
3. Materials and Methods
3.1. Reagents
3.2. Plant Material
3.3. Experimental In Vitro Cultures
3.4. Extract Preparation
3.5. HPLC Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SH | Schenk–Hildebrandt |
| 2iP | 6-(γ,γ-Dimethylallylamino)purine |
| FW | Fresh weight |
| DW | Dried weight |
| GiFW | Growth index for fresh biomass |
| GiDW | Growth index for dry biomass |
| LC-DAD-ESI-MS | Liquid chromatography–diode array detection–electrospray ionization-mass spectrometry |
| HPLC | High-performance liquid chromatography |
| TIS | Temporary immersion system |
Appendix A

References
- Jesionek, A.; Kokotkiewicz, A.; Luczkiewicz, M. Biotechnological Approach to Cultivation of Rhododendron Tomentosum (Ledum palustre) as the Source of the Biologically Active Essential Oil. In Medicinal Plants: Domestication, Biotechnology and Regional Importance; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 583–604. [Google Scholar] [CrossRef]
- Krol, A.; Kokotkiewicz, A.; Szopa, A.; Ekiert, H.; Luczkiewicz, M. Bioreactor-Grown Shoot Cultures for the Secondary Metabolite Production. In Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2020; pp. 1–62. [Google Scholar] [CrossRef]
- Park, S.-Y.; Paek, K.-Y. Bioreactor Culture of Shoots and Somatic Embryos of Medicinal Plants for Production of Bioactive Compounds. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.-Y., Murthy, H.N., Zhong, J.-J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 337–368. [Google Scholar] [CrossRef]
- Steingroewer, J.; Bley, T.; Georgiev, V.; Ivanov, I.; Lenk, F.; Marchev, A.; Pavlov, A. Bioprocessing of differentiated plant in vitro systems. Eng. Life Sci. 2013, 13, 26–38. [Google Scholar] [CrossRef]
- Verpoorte, R.; Contin, A.; Memelink, J. Biotechnology for the production of plant secondary metabolites. Phytochem. Rev. 2002, 1, 13–25. [Google Scholar] [CrossRef]
- Jesionek, A.; Kokotkiewicz, A.; Mikosik-Roczynska, A.; Ciesielska-Figlon, K.; Luczkiewicz, P.; Bucinski, A.; Daca, A.; Witkowski, J.M.; Bryl, E.; Zabiegala, B.; et al. Chemical variability of Rhododendron tomentosum (Ledum palustre) essential oils and their pro-apoptotic effect on lymphocytes and rheumatoid arthritis synoviocytes. Fitoterapia 2019, 139, 104402. [Google Scholar] [CrossRef]
- Kukhtenko, H.; Bevz, N.; Konechnyi, Y.; Kukhtenko, O.; Jasicka-Misiak, I. Spectrophotometric and Chromatographic Assessment of Total Polyphenol and Flavonoid Content in Rhododendron tomentosum Extracts and Their Antioxidant and Antimicrobial Activity. Molecules 2024, 29, 1095. [Google Scholar] [CrossRef]
- Vengrytė, M.; Raudonė, L. Phytochemical Profiling and Biological Activities of Rhododendron Subsect. Ledum: Discovering the Medicinal Potential of Labrador Tea Species in the Northern Hemisphere. Plants 2024, 13, 901. [Google Scholar] [CrossRef]
- Popescu, R.; Kopp, B. The genus Rhododendron: An ethnopharmacological and toxicological review. J. Ethnopharmacol. 2013, 147, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Kimel, K.; Godlewska, S.; Krauze-Baranowska, M.; Pobłocka-Olech, L. HPLC-DAD-ESI/MS analysis of Arnica TM constituents. Acta Pol. Pharm. Drug Res. 2019, 76, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Karioti, A.; Chiarabini, L.; Alachkar, A.; Fawaz Chehna, M.; Vincieri, F.F.; Bilia, A.R. HPLC-DAD and HPLC-ESI-MS analyses of Tiliae flos and its preparations. J. Pharm. Biomed. Anal. 2014, 100, 205–214. [Google Scholar] [CrossRef]
- Black, P.; Saleem, A.; Dunford, A.; Guerrero-Analco, J.; Walshe-Roussel, B.; Haddad, P.; Cuerrier, A.; Arnason, J.T. Seasonal variation of phenolic constituents and medicinal activities of northern labrador tea, Rhododendron tomentosum ssp subarcticum, an Inuit and cree first nations traditional medicine. Planta Medica 2011, 77, 1655–1662. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Zakharenko, A.M.; Golokhvast, K.S. Investigation of the Supercritical CO2 Extracts of Wild Ledum palustre L. (Rhododendron Tomentosum Harmaja) and Identification of Its Metabolites by Tandem Mass Spectrometry. Russ. J. Bioorganic Chem. 2023, 49, 1645–1657. [Google Scholar] [CrossRef]
- Ganina, M.M.; Popova, O.I. Contents of Phenolic Compounds in Ledum Procumbens (Ledum Decumbens Lodd. Ex Steud) Shoots Growing on the Territory of the Yamalo-Nenets Autonomous Region. Pharm. Chem. J. 2015, 49, 467–469. [Google Scholar] [CrossRef]
- Rapinski, M.; Liu, R.; Saleem, A.; Arnason, J.T.; Cuerrier, A. Environmental trends in the variation of biologically active phenolic compounds in Labrador tea, Rhododendron groenlandicum, from northern Quebec, Canada. Botany 2014, 92, 783–794. [Google Scholar] [CrossRef]
- Saleem, A.; Harris, C.S.; Asim, M.; Cuerrier, A.; Martineau, L.; Haddad, P.S.; Arnason, J.T. A RP-HPLC-DAD-APCI/MSD method for the characterisation of medicinal Ericaceae used by the Eeyou Istchee Cree First Nations. Phytochem. Anal. 2010, 21, 328–339. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Leaf survey of flavonoids and simple phenols in the genus Rhododendron. Phytochemistry 1971, 10, 2727–2744. [Google Scholar] [CrossRef]
- Hussain, A.; Azam, S.; Maqsood, R.; Anwar, R.; Akash, M.S.H.; Hussain, H.; Wang, D.; Imran, M.; Kotwica-Mojzych, K.; Khan, S.; et al. Chemistry, biosynthesis, and theranostics of antioxidant flavonoids and polyphenolics of genus Rhododendron: An overview. Naunyn-Schmiedeberg Arch. Pharmacol. 2025, 398, 1171–1214. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; He, J.; Miao, X.; Guo, X.; Shang, X.; Wang, W.; Li, B.; Wang, Y.; Pan, H.; Zhang, J. Multiple Biological Activities of Rhododendron przewalskii Maxim. Extracts and UPLC-ESI-Q-TOF/MS Characterization of Their Phytochemical Composition. Front. Pharmacol. 2021, 12, 599778. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.Y.; Wang, S.L.; Niu, X.Y. Analysis of anthocyanins and flavonols in petals of 10 Rhododendron species from the Sygera Mountains in Southeast Tibet. Plant Physiol. Biochem. 2016, 104, 250–256. [Google Scholar] [CrossRef]
- Du, H.; Lai, L.; Wang, F.; Sun, W.; Zhang, L.; Li, X.; Wang, L.; Jiang, L.; Zheng, Y. Characterisation of flower colouration in 30 Rhododendron species via anthocyanin and flavonol identification and quantitative traits. Plant Biol. 2018, 20, 121–129. [Google Scholar] [CrossRef]
- Belousov, M.V.; Berezovskaya, T.P.; Komissarenko, N.F.; Tikhonova, L.A. Flavonoids of Siberian-Far-Eastern species of rhododendrons of the subspecies Rhodorastrum. Chem. Nat. Compd. 1998, 34, 510–511. [Google Scholar] [CrossRef]
- Zobayed, S.M.A.; Murch, S.J.; Rupasinghe, H.P.V.; De Boer, J.G.; Glickman, B.W.; Saxena, P.K. Optimized system for biomass production, chemical characterization and evaluation of chemo-preventive properties of Scutellaria baicalensis Georgi. Plant Sci. 2004, 167, 439–446. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Luczkiewicz, M.; Ekiert, H. Schisandra lignans production regulated by different bioreactor type. J. Biotechnol. 2017, 247, 11–17. [Google Scholar] [CrossRef]
- Krol, A.; Kokotkiewicz, A.; Gorniak, M.; Naczk, A.M.; Zabiegala, B.; Gebalski, J.; Graczyk, F.; Zaluski, D.; Bucinski, A.; Luczkiewicz, M. Evaluation of the yield, chemical composition and biological properties of essential oil from bioreactor-grown cultures of Salvia apiana microshoots. Sci. Rep. 2023, 13, 7141. [Google Scholar] [CrossRef]
- Schumann, A.; Berkov, S.; Claus, D.; Gerth, A.; Bastida, J.; Codina, C. Production of galanthamine by leucojum aestivum shoots grown in different bioreactor systems. Appl. Biochem. Biotechnol. 2012, 167, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Jesionek, A.; Kokotkiewicz, A.; Wlodarska, P.; Filipowicz, N.; Bogdan, A.; Ochocka, R.; Szreniawa-Sztajnert, A.; Zabiegała, B.; Bucinski, A.; Luczkiewicz, M. In Vitro Propagation of Rhododendron tomentosum—An Endangered Essential Oil Bearing Plant from Peatland. Acta Biol. Cracoviensia Bot. 2016, 58, 29–43. [Google Scholar] [CrossRef]
- Murthy, B.N.S.; Murch, S.J.; Saxena, P.K. Thidiazuron: A potent regulator of in vitro plant morphogenesis. Vitr. Cell. Dev. Biol. Plant 1998, 34, 267–275. [Google Scholar] [CrossRef]
- Tan, S.H.; Goa, R. Prospect and challenges in Rhododendron micropropagation. In Proceedings of the Acta Horticulturae, Bangkok, Thailand, 12–13 July 2021; pp. 207–216. [Google Scholar] [CrossRef]
- Welander, M.; Persson, J.; Asp, H.; Zhu, L.H. Evaluation of a new vessel system based on temporary immersion system for micropropagation. Sci. Hortic. 2014, 179, 227–232. [Google Scholar] [CrossRef]
- Carvalho, L.S.O.; Ozudogru, E.A.; Lambardi, M.; Paiva, L.V. Temporary immersion system for micropropagation of tree species: A bibliographic and systematic review. Not. Bot. Horti Agrobot. 2019, 47, 269–277. [Google Scholar] [CrossRef]
- De Carlo, A.; Tarraf, W.; Lambardi, M.; Benelli, C. Temporary immersion system for production of biomass and bioactive compounds from medicinal plants. Agronomy 2021, 11, 2414. [Google Scholar] [CrossRef]
- Jesionek, A.; Kokotkiewicz, A.; Wlodarska, P.; Zabiegala, B.; Bucinski, A.; Luczkiewicz, M. Bioreactor shoot cultures of Rhododendron tomentosum (Ledum palustre) for a large-scale production of bioactive volatile compounds. Plant Cell Tissue Organ Cult. 2017, 131, 51–64. [Google Scholar] [CrossRef]
- Vidal, N.; Sánchez, C. Use of bioreactor systems in the propagation of forest trees. Eng. Life Sci. 2019, 19, 896–915. [Google Scholar] [CrossRef]
- Raj, D.; Kokotkiewicz, A.; Luczkiewicz, M. Production of Therapeutically Relevant Indolizidine Alkaloids in Securinega suffruticosa In Vitro Shoots Maintained in Liquid Culture Systems. Appl. Biochem. Biotechnol. 2015, 175, 1576–1587. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Marzec-Wróblewska, U.; Bucinski, A.; Luczkiewicz, M.; Ekiert, H. Accumulation of dibenzocyclooctadiene lignans in agar cultures and in stationary and agitated liquid cultures of Schisandra chinensis (Turcz.) Baill. Appl. Microbiol. Biotechnol. 2016, 100, 3965–3977. [Google Scholar] [CrossRef]
- Sharma, V.; Goyal, S.; Ramawat, K.G. Increased puerarin biosynthesis during in vitro shoot formation in Pueraria tuberosa grown in growtek bioreactor with aeration. Physiol. Mol. Biol. Plants 2011, 17, 87–92. [Google Scholar] [CrossRef]
- Piatczak, E.; Wielanek, M.; Wysokinska, H. Liquid culture system for shoot multiplication and secoiridoid production in micropropagated plants of Centaurium erythraea Rafn. Plant Sci. 2005, 168, 431–437. [Google Scholar] [CrossRef]
- Polivanova, O.B.; Bedarev, V.A. Hyperhydricity in Plant Tissue Culture. Plants 2022, 11, 3313. [Google Scholar] [CrossRef]
- Mirzabe, A.H.; Hajiahmad, A.; Fadavi, A.; Rafiee, S. Temporary immersion systems (TISs): A comprehensive review. J. Biotechnol. 2022, 357, 56–83. [Google Scholar] [CrossRef]
- Hwang, H.D.; Kwon, S.H.; Murthy, H.N.; Yun, S.W.; Pyo, S.S.; Park, S.Y. Temporary Immersion Bioreactor System as an Efficient Method for Mass Production of In Vitro Plants in Horticulture and Medicinal Plants. Agronomy 2022, 12, 346. [Google Scholar] [CrossRef]
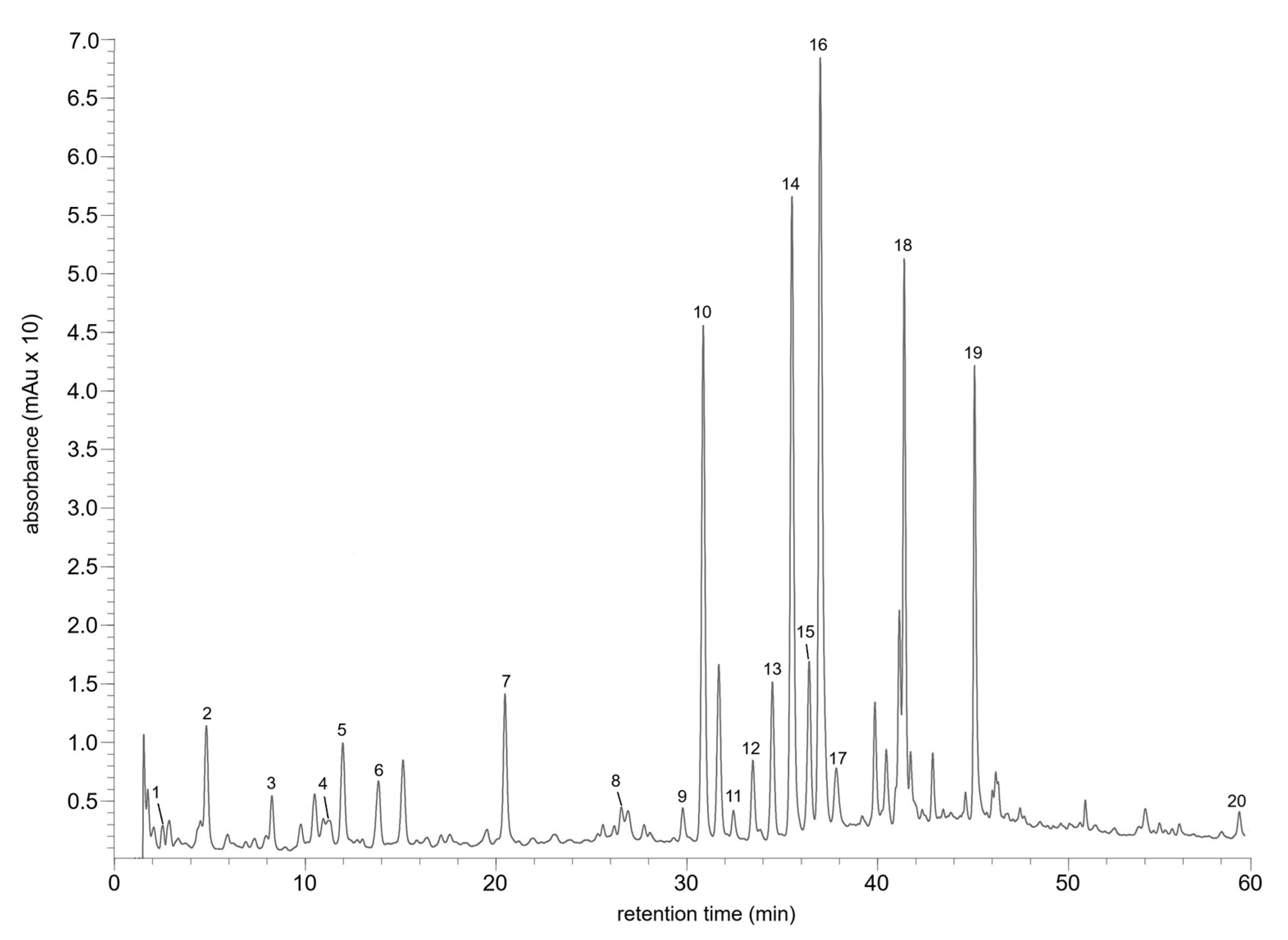
| No. | tR (min) | Λmax (nm) | [M + H]+ | [M − H]− | Compound | Wild-Grown Plant | Microshoot Culture | |
|---|---|---|---|---|---|---|---|---|
| Miszewko | Finland | |||||||
| 1 | 2.52 | 209, 269 | - | 169 | Gallic acid a | + | ND | ND |
| 2 | 4.82 | 207, 251, 294 | - | 153 | Protocatechuic acid a | + | + | ND |
| 3 | 8.26 | 204, 254 | - | - | p-Hydroxybenzoic acid a | + | ND | ND |
| 4 | 11.28 | 212, 278, 318 | 291 | 289 | Catechin a | + | + | + |
| 5 | 11.96 | 294sh, 325 | 355 | 353 | Chlorogenic acid a | + | + | + |
| 6 | 13.02 | 209, 229, 301sh, 338 | 209 | 369 | Chlorogenic acid methyl ether b | + | + | + |
| 7 | 20.46 | 197, 230sh, 289, 339sh | 305, 467 | 465 | Taxifolin hexoside b | + | + | + |
| 8 | 26.55 | 201, 226sh, 277 | 577 | 575 | Procyanidin A1/A2 b | + | + | + |
| 9 | 29.77 | 206, 257, 303sh, 351 | 451, 319 | 449 | Myricetin pentoside (isomer 1) b | + | ND | ND |
| 10 | 30.85 | 200, 252, 267sh, 300sh, 353 | 465, 303 | 463 | Hyperoside a | + | + | + |
| 11 | 32.45 | 206, 251, 367 | 481, 319 | 479 | Myricetin galactoside b | + | ND | ND |
| 12 | 33.46 | 204, 254, 266sh, 300sh, 352 | 435, 303 | - | Quercetin 3-O-xylopyranoside a | + | + | ND |
| 13 | 34.49 | 202, 255, 265sh, 300sh, 353 | 435, 303 | - | Quercetin 3-O-arabinoside a | + | + | + |
| 14 | 35.51 | 202, 254, 264sh, 350 | 435, 303 | - | Quercetin pentoside b | + | + | + |
| 15 | 36.40 | 202, 254, 264sh, 300sh, 346 | 449, 303 | 447 | Quercetin-3-rhamnoside b | + | + | + |
| 16 | 36.99 | 207, 252, 268sh, 300sh, 368 | 451, 319 | 449 | Myricetin pentoside (isomer 2) b | + | + | + |
| 17 | 37.82 | 204, 257, 300sh, 369 | 319 | 317 | Myricetin b | + | ND | + |
| 18 | 41.39 | 202, 254, 266sh, 300sh, 350 | 507, 303 | 505 | Quercetin acetylgalactoside/acetylglucoside b | + | + | + |
| 19 | 45.08 | 197, 256, 266sh, 314, 363sh | 611, 303 | - | Esterified quercetin glycoside b | + | + | + |
| 20 | 58.95 | 266, 333 | 285 | 283 | 6.7-dihydroxy-4′-methoxyisoflavone b | + | + | ND |
| No. | Compound | Plantform Bioreactor | Agitated Culture | Liquid Stationary Culture | Agar Culture | Intact Plant (Miszewko) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 d | 60 d | 90 d | 30 d | 60 d | 90 d | 30 d | 60 d | 90 d | 30 d | 60 d | 90 d | |||
| 2 | Protocatechuic acid | - | - | - | - | - | - | - | - | - | - | - | 1.97 | |
| 4 | Catechin | 23.51 | 22.47 | 9.41 | 27.18 | 31.68 | 5.90 | 19.42 | 19.08 | 15.56 | 13.22 | 13.84 | 11.07 | - |
| 5 | Chlorogenic acid | 0.80 | 0.78 | 0.42 | 0.70 | 0.93 | 0.20 | 0.71 | 0.89 | 0.82 | 0.51 | 0.55 | 0.52 | 2.34 |
| 7 | Taxifolin hexoside | - | - | - | - | - | - | - | - | - | - | - | - | 0.43 |
| 10 | Hyperoside (quercetin 3-O-galactoside) | 0.57 | 0.37 | 0.18 | 0.38 | 0.65 | 0.16 | 0.48 | 0.37 | 0.25 | 0.24 | 0.18 | 0.17 | 1.10 |
| 12 | Quercetin 3-O-xylopyranoside | - | - | - | - | - | - | - | - | - | - | - | - | 0.27 |
| 13 | Quercetin 3-O-arabinoside | - | - | - | - | - | - | - | - | - | - | - | - | 0.32 |
| 14 | Quercetin pentoside | 0.64 | 0.62 | 0.19 | 0.60 | 0.95 | 0.37 | 0.41 | 0.56 | 0.28 | 0.23 | 0.20 | 0.19 | 1.22 |
| 15 | Quercetin-3-rhamnoside | 0.59 | 0.51 | 0.31 | 0.58 | 1.19 | 0.36 | 0.57 | 0.75 | 0.69 | 0.44 | 0.46 | 0.55 | 0.38 |
| 16 | Myricetin pentoside (isomer 2) | 1.70 | 0.56 | 0.20 | 2.07 | 0.80 | 0.35 | 1.32 | 0.46 | 0.29 | 0.37 | 0.21 | 0.22 | 0.26 |
| 17 | Myricetin | 0.44 | 0.29 | 0.11 | 0.43 | 0.58 | 0.13 | 0.34 | 0.32 | 0.22 | 0.17 | 0.13 | 0.12 | - |
| 18 | Quercetin acetylgalactoside/ acetylglucoside | 0.29 | 0.24 | 0.13 | 0.26 | 0.31 | 0.13 | 0.23 | 0.27 | 0.21 | 0.19 | 0.17 | 0.15 | 0.53 |
| 19 | Quercetin glycoside | 0.54 | 0.48 | 0.27 | 0.51 | 0.78 | 0.31 | 0.39 | 0.54 | 0.48 | 0.25 | 0.26 | 0.25 | 0.42 |
| Sum | 29.07 | 26.32 | 11.23 | 32.72 | 37.87 | 7.91 | 23.87 | 23.24 | 18.82 | 15.63 | 15.99 | 13.25 | 9.24 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokotkiewicz, A.; Godlewska, S.; Sparzak-Stefanowska, B.; Panow, O.; Król, A.; Szopa, A.; Krauze-Baranowska, M.; Łuczkiewicz, M. Accumulation of Phenolic Compounds in Microshoot Cultures of Rhododendron tomentosum Harmaja (Ledum palustre L.). Int. J. Mol. Sci. 2025, 26, 7999. https://doi.org/10.3390/ijms26167999
Kokotkiewicz A, Godlewska S, Sparzak-Stefanowska B, Panow O, Król A, Szopa A, Krauze-Baranowska M, Łuczkiewicz M. Accumulation of Phenolic Compounds in Microshoot Cultures of Rhododendron tomentosum Harmaja (Ledum palustre L.). International Journal of Molecular Sciences. 2025; 26(16):7999. https://doi.org/10.3390/ijms26167999
Chicago/Turabian StyleKokotkiewicz, Adam, Sylwia Godlewska, Barbara Sparzak-Stefanowska, Oliwer Panow, Agata Król, Agnieszka Szopa, Mirosława Krauze-Baranowska, and Maria Łuczkiewicz. 2025. "Accumulation of Phenolic Compounds in Microshoot Cultures of Rhododendron tomentosum Harmaja (Ledum palustre L.)" International Journal of Molecular Sciences 26, no. 16: 7999. https://doi.org/10.3390/ijms26167999
APA StyleKokotkiewicz, A., Godlewska, S., Sparzak-Stefanowska, B., Panow, O., Król, A., Szopa, A., Krauze-Baranowska, M., & Łuczkiewicz, M. (2025). Accumulation of Phenolic Compounds in Microshoot Cultures of Rhododendron tomentosum Harmaja (Ledum palustre L.). International Journal of Molecular Sciences, 26(16), 7999. https://doi.org/10.3390/ijms26167999







