Functional and Structural Uterine Changes in PCOS
Abstract
1. Introduction
2. Materials and Methods
3. General Background
3.1. Polycystic Ovary Syndrome
3.1.1. Diagnosis
- I.
- II.
- Hyperandrogenism can be assessed in both clinical and biochemical manner—the clinical features include, e.g., hirsutism evaluated with the modified Ferriman–Gallwey score, alopecia, and severe acne. Biochemical indicators include the free androgen index and calculated free or bioavailable testosterone.
- III.
- Polycystic ovaries are described as more than 20 coexisting pre-antral follicles, measuring 2–9 mm in diameter, present in either ovary and/or an increased ovarian volume > 10 cm3. The number of follicles is an update of 12 reported in the original consensus from 2003 [23].
3.1.2. Clinical Features
3.1.3. Pathogenesis
3.1.4. Comorbidity
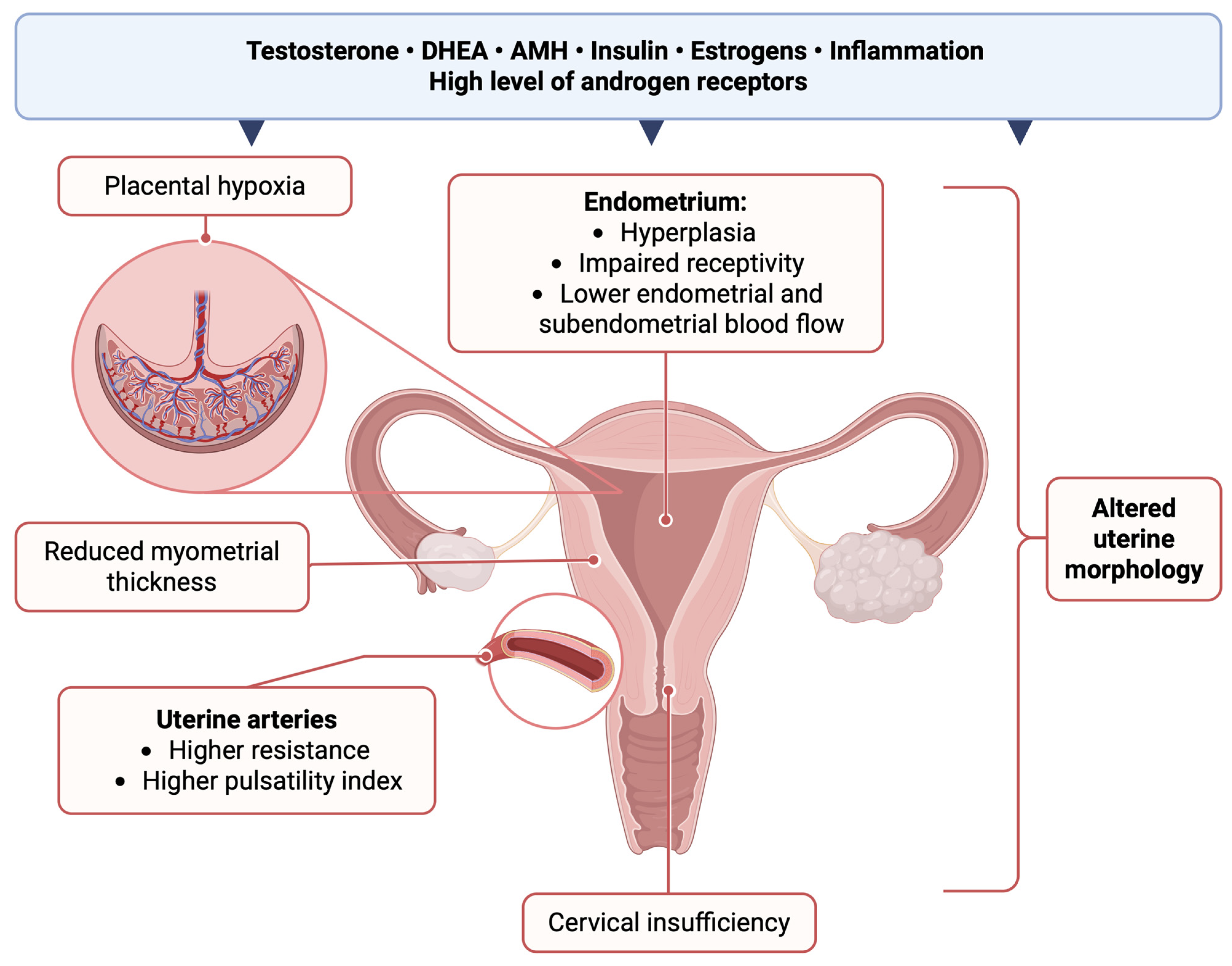
3.2. Uterus
4. PCOS in Clinical Trials
4.1. General Uterine Morphology Asessment
4.2. Fibroids
4.3. Endometrial Thickness
4.4. Myometrial Thickness
4.5. Uterine Arteries
5. PCOS in Animal Models
5.1. Uterine Morphology
5.2. Gravid Uterus in PCOS
6. The Impact of Hyperandrogenism on a Gravid Uterus
6.1. Endometrial Component of Subfertility in PCOS Patients
6.2. Cervix
6.3. Uterine Blood Flow in Pregnant Patients with PCOS
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCOS | Polycystic ovary syndrome |
| 2D/3D | Two- or three-dimensional |
| CI | Cervical insufficiency |
| TVUS | Transvaginal ultrasound |
| DHT | 5α-dihydrotestosterone |
| INS | insulin |
| ROS | Reactive oxygen species |
| BMI | Body mass index |
| DHEAS | Dehydroepiandrosterone sulfate |
| PI | Artery pulsatility index |
| RI | Resistance index |
| LH | Luteinizing hormone |
| FSH | Follicle-stimulating hormone |
| AMH | Anti-Müllerian Hormone |
| DES | Diethylstilbestrol |
| ASRM | American Society for Reproductive Medicine |
| ESHRE | European Society of Human Reproduction and Embryology |
| SHBG | Sex hormone-binding proteins |
References
- Palumbo, M.; Della Corte, L.; Colacurci, D.; Ascione, M.; D’Angelo, G.; Baldini, G.M.; Giampaolino, P.; Bifulco, G. PCOS and the Genome: Is the Genetic Puzzle Still Worth Solving? Biomedicines 2025, 13, 1912. [Google Scholar] [CrossRef]
- Sir-Petermann, T. Maternal Serum Androgens in Pregnant Women with Polycystic Ovarian Syndrome: Possible Implications in Prenatal Androgenization. Hum. Reprod. 2002, 17, 2573–2579. [Google Scholar] [CrossRef]
- Kahsar-Miller, M.D.; Nixon, C.; Boots, L.R.; Go, R.C.; Azziz, R. Prevalence of Polycystic Ovary Syndrome (PCOS) in First-Degree Relatives of Patients with PCOS. Fertil. Steril. 2001, 75, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B. The Prevalence and Phenotypic Features of Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef]
- Bayona, A.; Martínez-Vaello, V.; Zamora, J.; Nattero-Chávez, L.; Luque-Ramírez, M.; Escobar-Morreale, H.F. Prevalence of PCOS and Related Hyperandrogenic Traits in Premenopausal Women with Type 1 Diabetes: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2022, 28, 501–517. [Google Scholar] [CrossRef]
- Ma, L.; Cao, Y.; Ma, Y.; Zhai, J. Association between Hyperandrogenism and Adverse Pregnancy Outcomes in Patients with Different Polycystic Ovary Syndrome Phenotypes Undergoing in Vitro Fertilization/Intracytoplasmic Sperm Injection: A Systematic Review and Meta-Analysis. Gynecol. Endocrinol. 2021, 37, 694–701. [Google Scholar] [CrossRef]
- Grimbizis, G.F.; Gordts, S.; Di Spiezio Sardo, A.; Brucker, S.; De Angelis, C.; Gergolet, M.; Li, T.-C.; Tanos, V.; Brolmann, H.; Gianaroli, L.; et al. The ESHRE/ESGE Consensus on the Classification of Female Genital Tract Congenital Anomalies. Hum. Reprod. 2013, 28, 2032–2044. [Google Scholar] [CrossRef]
- Ludwin, A.; Ludwin, I.; Coelho Neto, M.A.; Nastri, C.O.; Bhagavath, B.; Lindheim, S.R.; Martins, W.P. Septate Uterus According to ESHRE/ESGE, ASRM and CUME Definitions: Association with Infertility and Miscarriage, Cost and Warnings for Women and Healthcare Systems. Ultrasound Obstet. Gynecol. 2019, 54, 800–814. [Google Scholar] [CrossRef]
- Pfeifer, S.M.; Attaran, M.; Goldstein, J.; Lindheim, S.R.; Petrozza, J.C.; Rackow, B.W.; Siegelman, E.; Troiano, R.; Winter, T.; Zuckerman, A.; et al. ASRM Müllerian Anomalies Classification 2021. Fertil. Steril. 2021, 116, 1238–1252. [Google Scholar] [CrossRef]
- Saravelos, S.H.; Jayaprakasan, K.; Ojha, K.; Li, T.-C. Assessment of the Uterus with Three-Dimensional Ultrasound in Women Undergoing ART. Hum. Reprod. Update 2016, 23, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Ludwin, A.; Coelho Neto, M.A.; Ludwin, I.; Nastri, C.O.; Costa, W.; Acién, M.; Alcazar, J.L.; Benacerraf, B.; Condous, G.; DeCherney, A.; et al. Congenital Uterine Malformation by Experts (CUME): Diagnostic Criteria for T-shaped Uterus. Ultrasound Obstet. Gynecol. 2020, 55, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Saravelos, S.H.; Cocksedge, K.A.; Li, T.-C. Prevalence and Diagnosis of Congenital Uterine Anomalies in Women with Reproductive Failure: A Critical Appraisal. Hum. Reprod. Update 2008, 14, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasan, K.; Chan, Y.Y.; Sur, S.; Deb, S.; Clewes, J.S.; Raine-Fenning, N.J. Prevalence of Uterine Anomalies and Their Impact on Early Pregnancy in Women Conceiving after Assisted Reproduction Treatment. Ultrasound Obstet. Gynecol. 2011, 37, 727–732. [Google Scholar] [CrossRef]
- Chen, W.; Pang, Y. Metabolic Syndrome and PCOS: Pathogenesis and the Role of Metabolites. Metabolites 2021, 11, 869. [Google Scholar] [CrossRef]
- Norman, R.J.; Dewailly, D.; Legro, R.S.; Hickey, T.E. Polycystic Ovary Syndrome. Lancet 2007, 370, 685–697. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations From the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2023, 108, 2447–2469. [Google Scholar] [CrossRef]
- Su, P.; Chen, C.; Sun, Y. Physiopathology of Polycystic Ovary Syndrome in Endocrinology, Metabolism and Inflammation. J. Ovarian Res. 2025, 18, 34. [Google Scholar] [CrossRef]
- Coviello, A.D.; Zhuang, W.V.; Lunetta, K.L.; Bhasin, S.; Ulloor, J.; Zhang, A.; Karasik, D.; Kiel, D.P.; Vasan, R.S.; Murabito, J.M. Circulating Testosterone and SHBG Concentrations Are Heritable in Women: The Framingham Heart Study. J. Clin. Endocrinol. Metab. 2011, 96, E1491–E1495. [Google Scholar] [CrossRef] [PubMed]
- Vink, J.M.; Sadrzadeh, S.; Lambalk, C.B.; Boomsma, D.I. Heritability of Polycystic Ovary Syndrome in a Dutch Twin-Family Study. J. Clin. Endocrinol. Metab. 2006, 91, 2100–2104. [Google Scholar] [CrossRef]
- Risal, S.; Pei, Y.; Lu, H.; Manti, M.; Fornes, R.; Pui, H.-P.; Zhao, Z.; Massart, J.; Ohlsson, C.; Lindgren, E.; et al. Prenatal Androgen Exposure and Transgenerational Susceptibility to Polycystic Ovary Syndrome. Nat. Med. 2019, 25, 1894–1904. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Lujan, M.E.; Chizen, D.R.; Pierson, R.A. Diagnostic Criteria for Polycystic Ovary Syndrome: Pitfalls and Controversies. J. Obstet. Gynaecol. Can. 2008, 30, 671–679. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef]
- Taylor, A.E.; McCourt, B.; Martin, K.A.; Anderson, E.J.; Adams, J.M.; Schoenfeld, D.; Hall, J.E. Determinants of Abnormal Gonadotropin Secretion in Clinically Defined Women with Polycystic Ovary Syndrome1. J. Clin. Endocrinol. Metab. 1997, 82, 2248–2256. [Google Scholar] [CrossRef]
- Stepto, N.K.; Cassar, S.; Joham, A.E.; Hutchison, S.K.; Harrison, C.L.; Goldstein, R.F.; Teede, H.J. Women with Polycystic Ovary Syndrome Have Intrinsic Insulin Resistance on Euglycaemic-Hyperinsulaemic Clamp. Hum. Reprod. 2013, 28, 777–784. [Google Scholar] [CrossRef]
- Ho, N.T.; Nguyen, D.L.; Ho, V.N.A.; Nguyen, N.T.; Pham, T.D.; Ly, L.D.; Tran, M.N.; Ho, T.M.; Vuong, L.N. O-129 Characteristics of serum anti-mullerian hormone levels in women with polycystic ovarian syndrome (PCOS) compared to those without PCOS: A propensity score matched study. Hum. Reprod. 2025, 40, deaf097.129. [Google Scholar] [CrossRef]
- Tal, R.; Seifer, D.B.; Khanimov, M.; Malter, H.E.; Grazi, R.V.; Leader, B. Characterization of Women with Elevated Antimüllerian Hormone Levels (AMH): Correlation of AMH with Polycystic Ovarian Syndrome Phenotypes and Assisted Reproductive Technology Outcomes. Am. J. Obstet. Gynecol. 2014, 211, 59-e1. [Google Scholar] [CrossRef]
- Codner, E.; Soto, N.; Lopez, P.; Trejo, L.; Ávila, A.; Eyzaguirre, F.C.; Íniguez, G.; Cassorla, F. Diagnostic Criteria for Polycystic Ovary Syndrome and Ovarian Morphology in Women with Type 1 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2006, 91, 2250–2256. [Google Scholar] [CrossRef]
- Peppard, H.R.; Marfori, J.; Iuorno, M.J.; Nestler, J.E. Prevalence of Polycystic Ovary Syndrome among Premenopausal Women with Type 2 Diabetes. Diabetes Care 2001, 24, 1050–1052. [Google Scholar] [CrossRef]
- Anttila, L.; Karjala, K.; Penttilä, R.A.; Ruutiainen, K.; Ekblad, U. Polycystic Ovaries in Women with Gestational Diabetes. Obstet. Gynecol. 1998, 92, 13–16. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Hoyos, L.R.; Chazenbalk, G.D.; Naik, R.; Padmanabhan, V.; Abbott, D.H. Mechanisms of Intergenerational Transmission of Polycystic Ovary Syndrome. Reproduction 2020, 159, R1–R13. [Google Scholar] [CrossRef]
- Tata, B.; Mimouni, N.E.H.; Barbotin, A.-L.; Malone, S.A.; Loyens, A.; Pigny, P.; Dewailly, D.; Catteau-Jonard, S.; Sundström-Poromaa, I.; Piltonen, T.T.; et al. Elevated Prenatal Anti-Müllerian Hormone Reprograms the Fetus and Induces Polycystic Ovary Syndrome in Adulthood. Nat. Med. 2018, 24, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.A.; Kay, A.R.; Navaratnarajah, R.; Iqbal, S.; Bamfo, J.E.A.K.; David, A.L.; Hines, M.; Hardiman, P.J. Umbilical Vein Testosterone in Female Infants Born to Mothers with Polycystic Ovary Syndrome Is Elevated to Male Levels. J. Obstet. Gynaecol. 2010, 30, 444–446. [Google Scholar] [CrossRef]
- Homburg, R.; Gudi, A.; Shah, A.; Layton, A.M. A Novel Method to Demonstrate That Pregnant Women with Polycystic Ovary Syndrome Hyper-Expose Their Fetus to Androgens as a Possible Stepping Stone for the Developmental Theory of PCOS. A Pilot Study. Reprod. Biol. Endocrinol. 2017, 15, 61. [Google Scholar] [CrossRef]
- Abbott, D.H.; Zhou, R.; Bird, I.M.; Dumesic, D.A.; Conley, A.J. Fetal Programming of Adrenal Androgen Excess: Lessons from a Nonhuman Primate Model of Polycystic Ovary Syndrome. In Disorders of the Human Adrenal Cortex; Karger: Basel, Switzerland, 2008; pp. 145–158. [Google Scholar]
- Abbott, D.H.; Barnett, D.K.; Bruns, C.M.; Dumesic, D.A. Androgen Excess Fetal Programming of Female Reproduction: A Developmental Aetiology for Polycystic Ovary Syndrome? Hum. Reprod. Update 2005, 11, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-Y.; Li, Z.-L.; Wu, C.-Y.; Liu, Y.-M.; Lin, H.; Wang, S.-H.; Xiao, W.-F. Endocrine Traits of Polycystic Ovary Syndrome in Prenatally Androgenized Female Sprague-Dawley Rats. Endocr. J. 2010, 57, 201–209. [Google Scholar] [CrossRef]
- Padmanabhan, V.; Veiga-Lopez, A. Sheep Models of Polycystic Ovary Syndrome Phenotype. Mol. Cell. Endocrinol. 2013, 373, 8–20. [Google Scholar] [CrossRef]
- Sir-Petermann, T.; Codner, E.; Maliqueo, M.; Echiburú, B.; Hitschfeld, C.; Crisosto, N.; PérEz-Bravo, F.; Recabarren, S.E.; Cassorla, F. Increased anti-Mullerian hormone serum concentrations in prepubertal daughters of women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 3105–3109. [Google Scholar] [CrossRef]
- Hart, R.; Sloboda, D.M.; Doherty, D.A.; Norman, R.J.; Atkinson, H.C.; Newnham, J.P.; Dickinson, J.E.; Hickey, M. Circulating Maternal Testosterone Concentrations at 18 Weeks of Gestation Predict Circulating Levels of Antimüllerian Hormone in Adolescence: A Prospective Cohort Study. Fertil. Steril. 2010, 94, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Weenen, C. Anti-Mullerian Hormone Expression Pattern in the Human Ovary: Potential Implications for Initial and Cyclic Follicle Recruitment. Mol. Hum. Reprod. 2004, 10, 77–83. [Google Scholar] [CrossRef]
- Pigny, P.; Merlen, E.; Robert, Y.; Cortet-Rudelli, C.; Decanter, C.; Jonard, S.; Dewailly, D. Elevated Serum Level of Anti-Mullerian Hormone in Patients with Polycystic Ovary Syndrome: Relationship to the Ovarian Follicle Excess and to the Follicular Arrest. J. Clin. Endocrinol. Metab. 2003, 88, 5957–5962. [Google Scholar] [CrossRef]
- Laven, J.S.E.; Mulders, A.G.M.G.J.; Visser, J.A.; Themmen, A.P.; de Jong, F.H.; Fauser, B.C.J.M. Anti-Müllerian Hormone Serum Concentrations in Normoovulatory and Anovulatory Women of Reproductive Age. J. Clin. Endocrinol. Metab. 2004, 89, 318–323. [Google Scholar] [CrossRef]
- Maliqueo, M.; Lara, H.E.; Sánchez, F.; Echiburú, B.; Crisosto, N.; Sir-Petermann, T. Placental Steroidogenesis in Pregnant Women with Polycystic Ovary Syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 166, 151–155. [Google Scholar] [CrossRef]
- Hodgins, M.B. Binding of Androgens in 5α-Reductase-Deficient Human Genital Skin Fibroblasts: Inhibition by Progesterone and Its Metabolites. J. Endocrinol. 1982, 94, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.L. Diverse Roles for Sex Hormone-Binding Globulin in Reproduction. Biol. Reprod. 2011, 85, 431–441. [Google Scholar] [CrossRef]
- Crisosto, N.; Echiburú, B.; Maliqueo, M.; Pérez, V.; Ladrón de Guevara, A.; Preisler, J.; Sánchez, F.; Sir-Petermann, T. Improvement of Hyperandrogenism and Hyperinsulinemia during Pregnancy in Women with Polycystic Ovary Syndrome: Possible Effect in the Ovarian Follicular Mass of Their Daughters. Fertil. Steril. 2012, 97, 218–224. [Google Scholar] [CrossRef] [PubMed]
- DeUgarte, C.M.; Bartolucci, A.A.; Azziz, R. Prevalence of Insulin Resistance in the Polycystic Ovary Syndrome Using the Homeostasis Model Assessment. Fertil. Steril. 2005, 83, 1454–1460. [Google Scholar] [CrossRef]
- Lim, S.S.; Norman, R.J.; Davies, M.J.; Moran, L.J. The Effect of Obesity on Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Obes. Rev. 2013, 14, 95–109. [Google Scholar] [CrossRef]
- Quinkler, M.; Sinha, B.; Tomlinson, J.W.; Bujalska, I.J.; Stewart, P.M.; Arlt, W. Androgen Generation in Adipose Tissue in Women with Simple Obesity—A Site-Specific Role for 17β-Hydroxysteroid Dehydrogenase Type 5. J. Endocrinol. 2004, 183, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Balen, A.H. Hypersecretion of Luteinizing Hormone and the Polycystic Ovary Syndrome. Hum. Reprod. 1993, 8 (Suppl. S2), 123–128. [Google Scholar] [CrossRef]
- Ford, E.S.; Giles, W.H.; Mokdad, A.H. Increasing Prevalence of the Metabolic Syndrome among US Adults. Diabetes Care 2004, 27, 2444–2449. [Google Scholar] [CrossRef] [PubMed]
- Joham, A.E.; Ranasinha, S.; Zoungas, S.; Moran, L.; Teede, H.J. Gestational Diabetes and Type 2 Diabetes in Reproductive-Aged Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2014, 99, E447–E452. [Google Scholar] [CrossRef] [PubMed]
- Helvaci, N.; Yildiz, B.O. Polycystic Ovary Syndrome as a Metabolic Disease. Nat. Rev. Endocrinol. 2025, 21, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Wild, R.A. Polycystic Ovary Syndrome: A Risk for Coronary Artery Disease? Am. J. Obstet. Gynecol. 2002, 186, 35–43. [Google Scholar] [CrossRef]
- Barry, J.A.; Azizia, M.M.; Hardiman, P.J. Risk of Endometrial, Ovarian and Breast Cancer in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2014, 20, 748–758. [Google Scholar] [CrossRef]
- Gottschau, M.; Kjaer, S.K.; Jensen, A.; Munk, C.; Mellemkjaer, L. Risk of Cancer among Women with Polycystic Ovary Syndrome: A Danish Cohort Study. Gynecol. Oncol. 2015, 136, 99–103. [Google Scholar] [CrossRef]
- Qin, J.Z.; Pang, L.H.; Li, M.J.; Fan, X.J.; Huang, R.D.; Chen, H.Y. Obstetric Complications in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Reprod. Biol. Endocrinol. 2013, 11, 56. [Google Scholar] [CrossRef]
- Glueck, C.J.; Wang, P.; Goldenberg, N.; Sieve-Smith, L. Pregnancy Outcomes among Women with Polycystic Ovary Syndrome Treated with Metformin. Hum. Reprod. 2002, 17, 2858–2864. [Google Scholar] [CrossRef]
- Hall-Craggs, M.A.; Kirkham, A.; Creighton, S.M. Renal and Urological Abnormalities Occurring with Mullerian Anomalies. J. Pediatr. Urol. 2013, 9, 27–32. [Google Scholar] [CrossRef]
- Acién, P.; Acién, M. Renal Agenesis, Associated Genital Malformations, and Responsible Genes. Fertil. Steril. 2021, 116, 1370–1371. [Google Scholar] [CrossRef]
- Mooren, E.R.M.; Cleypool, C.G.J.; de Kort, L.M.O.; Goverde, A.J.; Dik, P. A Retrospective Analysis of Female Müllerian Duct Anomalies in Association With Congenital Renal Abnormalities. J. Pediatr. Adolesc. Gynecol. 2021, 34, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Aslan, K.; Albayrak, O.; Bilgic, K.O.; Kasapoglu, I.; Avcı, B.; Uncu, G. Amh Levels May Predict for Mullerian Anomalies and Pregnancy Outcomes Patients with Pcos. Fertil. Steril. 2020, 114, e407. [Google Scholar] [CrossRef]
- Habiba, M.; Heyn, R.; Bianchi, P.; Brosens, I.; Benagiano, G. The Development of the Human Uterus: Morphogenesis to Menarche. Hum. Reprod. Update 2021, 27, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.R.; Robboy, S.J.; Kurita, T.; Isaacson, D.; Shen, J.; Cao, M.; Baskin, L.S. Development of the Human Female Reproductive Tract. Differentiation 2018, 103, 46–65. [Google Scholar] [CrossRef]
- Acién, P.; Acién, M.I. The History of Female Genital Tract Malformation Classifications and Proposal of an Updated System. Hum. Reprod. Update 2011, 17, 693–705. [Google Scholar] [CrossRef]
- Lin, P.C.; Bhatnagar, K.P.; Nettleton, G.S.; Nakajima, S.T. Female Genital Anomalies Affecting Reproduction. Fertil. Steril. 2002, 78, 899–915. [Google Scholar] [CrossRef]
- Kaufman, R.H.; Binder, G.L.; Gray, P.M.; Adam, E. Upper Genital Tract Changes Associated with Exposure in Utero to Diethylstilbestrol. Am. J. Obstet. Gynecol. 1977, 128, 51–59. [Google Scholar] [CrossRef]
- Aslan, K.; Albayrak, O.; Orhaner, A.; Kasapoglu, I.; Uncu, G. Incidence of Congenital Uterine Abnormalities in Polycystic Ovarian Syndrome (CONUTA Study). Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 271, 183–188. [Google Scholar] [CrossRef]
- Fujii, S.; Oguchi, T. Shapes of the Uterine Cavity Are Different in Women with Polycystic Ovary Syndrome. Reprod. Med. Biol. 2023, 22, e12508. [Google Scholar] [CrossRef]
- de Zegher, F.; Francois, I.; Boehmer, A.L.; Saggese, G.; Müller, J.; Hiort, O.; Sultan, C.; Clayton, P.; Brauner, R.; Cacciari, E.; et al. Androgens and Fetal Growth. Horm. Res. 1998, 50, 243–244. [Google Scholar] [CrossRef]
- Wallach, E.E.; Golan, A.; Langer, R.; Bukovsky, I.; Caspi, E. Congenital Anomalies of the Müllerian System. Fertil. Steril. 1989, 51, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Tokhunts, K.; Adamyan, M.; Chopikyan, A.; Kayfajyan, K.; Khudaverdyan, A.; Tumanyan, A. Is I-Shaped Uterus More Common in Patients with Hyperandrogenism? Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 272, 116–122. [Google Scholar] [CrossRef]
- Ege, S.; Peker, N.; Bademkıran, M.H. The Prevalence of Uterine Anomalies in Infertile Patients with Polycystic Ovary Syndrome: A Retrospective Study in a Tertiary Center in Southeastern Turkey. Turk. J. Obstet. Gynecol. 2020, 16, 224–227. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, F.; Wu, K.; Yu, D.; Zhang, Y.; Liao, Y.; Xu, G.; Wang, Y. Müllerian Duct Anomalies and Anti-Müllerian Hormone Levels in Women With Polycystic Ovary Syndrome. Cureus 2023, 15, e43848. [Google Scholar] [CrossRef] [PubMed]
- Moramezi, F.; Barati, M.; Shahbazian, N.; Golbabaei, M.; Hemadi, M. Sonographic Evaluation of Mullerian Anomalies in Women with Polycystic Ovaries. Health 2013, 05, 1313–1317. [Google Scholar] [CrossRef]
- Zaborowska, L.; Blok, J.; Ludwin, I.; Jasek, J.; Lindheim, S.R.; Martins, W.P.; Ludwin, A. Uterine Morphology and Anomalies in Women with and without Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Bermejo, C.; Martínez Ten, P.; Cantarero, R.; Diaz, D.; Pérez Pedregosa, J.; Barrón, E.; Labrador, E.; Ruiz López, L. Three-Dimensional Ultrasound in the Diagnosis of Müllerian Duct Anomalies and Concordance with Magnetic Resonance Imaging. Ultrasound Obstet. Gynecol. 2010, 35, 593–601. [Google Scholar] [CrossRef]
- Jurkovic, D.; Geipel, A.; Gruboeck, K.; Jauniaux, E.; Natucci, M.; Campbell, S. Three-Dimensional Ultrasound for the Assessment of Uterine Anatomy and Detection of Congenital Anomalies: A Comparison with Hysterosalpingography and Two-Dimensional Sonography. Ultrasound Obstet. Gynecol. 1995, 5, 233–237. [Google Scholar] [CrossRef]
- Ludwin, A.; Pityński, K.; Ludwin, I.; Banas, T.; Knafel, A. Two- and Three-Dimensional Ultrasonography and Sonohysterography versus Hysteroscopy With Laparoscopy in the Differential Diagnosis of Septate, Bicornuate, and Arcuate Uteri. J. Minim. Invasive Gynecol. 2013, 20, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Ludwin, A.; Ludwin, I. Reliability of Hysteroscopy-Based Diagnosis of Septate, Arcuate and Normal Uterus: Estimate or Guestimate? Hum. Reprod. 2016, 31, 1376–1377. [Google Scholar] [CrossRef]
- Smit, J.G.; Torrance, H.L.; Eijkemans, M.J.C.; Broekmans, F.J.M. Reply: Reliability of Hysteroscopy-Based Diagnosis of Septate, Arcuate and Normal Uterus: Estimate or Guestimate? Hum. Reprod. 2016, 31, 1377–1378. [Google Scholar] [CrossRef]
- Smit, J.G.; Kasius, J.C.; Eijkemans, M.J.C.; Veersema, S.; Fatemi, H.M.; van, E.J.S.; Campo, R.; Broekmans, F.J.M. The International Agreement Study on the Diagnosis of the Septate Uterus at Office Hysteroscopy in Infertile Patients. Fertil. Steril. 2013, 99, 2108–2113.e2. [Google Scholar] [CrossRef] [PubMed]
- Smit, J.G.; Overdijkink, S.; Mol, B.W.; Kasius, J.C.; Torrance, H.L.; Eijkemans, M.J.C.; Bongers, M.; Emanuel, M.H.; Vleugels, M.; Broekmans, F.J.M. The Impact of Diagnostic Criteria on the Reproducibility of the Hysteroscopic Diagnosis of the Septate Uterus: A Randomized Controlled Trial. Hum. Reprod. 2015, 30, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L. Variation in the Incidence of Uterine Leiomyoma Among Premenopausal Women by Age and Race. Obstet. Gynecol. 1997, 90, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.M.; Spiegelman, D.; Manson, J.E.; Goldman, M.B.; Barbieri, R.L.; Stampfer, M.J.; Willett, W.C.; Hunter, D.J. Risk of Uterine Leiomyomata among Premenopausal Women in Relation to Body Size and Cigarette Smoking. Epidemiology 1998, 9, 511–517. [Google Scholar] [CrossRef]
- Faerstein, E.; Szklo, M.; Rosenshein, N. Risk Factors for Uterine Leiomyoma: A Practice-Based Case-Control Study. I. African-American Heritage, Reproductive History, Body Size, and Smoking. Am. J. Epidemiol. 2001, 153, 1–10. [Google Scholar] [CrossRef]
- Brandon, D.D.; Erickson, T.E.; Keenan, E.J.; Strawn, E.Y.; Novy, M.J.; Burry, K.A.; Warner, C.; Clinton, G.M. Estrogen Receptor Gene Expression in Human Uterine Leiomyomata. J. Clin. Endocrinol. Metab. 1995, 80, 1876–1881. [Google Scholar] [CrossRef]
- Brandon, D.D.; Bethea, C.L.; Strawn, E.Y.; Novy, M.J.; Burry, K.A.; Harrington, M.S.; Erickson, T.E.; Warner, C.; Keenan, E.J.; Clinton, G.M. Progesterone Receptor Messenger Ribonucleic Acid and Protein Are Overexpressed in Human Uterine Leiomyomas. Am. J. Obstet. Gynecol. 1993, 169, 78–85. [Google Scholar] [CrossRef]
- Ishikawa, H.; Reierstad, S.; Demura, M.; Rademaker, A.W.; Kasai, T.; Inoue, M.; Usui, H.; Shozu, M.; Bulun, S.E. High Aromatase Expression in Uterine Leiomyoma Tissues of African-American Women. J. Clin. Endocrinol. Metab. 2009, 94, 1752–1756. [Google Scholar] [CrossRef]
- Bulun, S.E.; Simpson, E.R.; Word, R.A. Expression of the CYP19 Gene and Its Product Aromatase Cytochrome P450 in Human Uterine Leiomyoma Tissues and Cells in Culture. J. Clin. Endocrinol. Metab. 1994, 78, 736–743. [Google Scholar] [CrossRef]
- Huang, H.; Kuang, H.; Sun, F.; Diamond, M.P.; Legro, R.S.; Coutifaris, C.; Alvero, R.; Robinson, R.D.; Casson, P.R.; Christman, G.M.; et al. Lower Prevalence of Non–Cavity-Distorting Uterine Fibroids in Patients with Polycystic Ovary Syndrome than in Those with Unexplained Infertility. Fertil. Steril. 2019, 111, 1011–1019.e1. [Google Scholar] [CrossRef]
- Abdel-Gadir, A.; Oyawoye, O.O.; Chander, B.P. Coexistence of Polycystic Ovaries and Uterine Fibroids and Their Combined Effect on the Uterine Artery Blood Flow in Relation to Age and Parity. J. Reprod. Med. 2009, 54, 347–352. [Google Scholar]
- Suturina, L.; Lizneva, D.; Lazareva, L.; Darzhaev, Z. Negative Association between PCOS and Risk of Uterine Leiyomyomas in Caucasian Infertile Women. Reprod. Sci. 2016, 23, 51A–344A. [Google Scholar] [CrossRef]
- Wise, L.A.; Palmer, J.R.; Stewart, E.A.; Rosenberg, L. Polycystic Ovary Syndrome and Risk of Uterine Leiomyomata. Fertil. Steril. 2007, 87, 1108–1115. [Google Scholar] [CrossRef]
- Shen, C.-C.; Yang, A.C.; Hung, J.-H.; Hu, L.-Y.; Tsai, S.-J. A Nationwide Population-Based Retrospective Cohort Study of the Risk of Uterine, Ovarian and Breast Cancer in Women With Polycystic Ovary Syndrome. Oncologist 2015, 20, 45–49. [Google Scholar] [CrossRef]
- Panidis, D.; Tziomalos, K.; Papadakis, E.; Vosnakis, C.; Betsas, G.; Tsourdi, E.; Katsikis, I. Uterine Volume and Endometrial Thickness in the Early Follicular Phase in Patients with Polycystic Ovary Syndrome. Endocr. Pract. 2014, 20, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.; Johnson, I.; Raine-Fenning, N. Endometrial Blood Flow Is Impaired in Women with Polycystic Ovarian Syndrome Who Are Clinically Hyperandrogenic. Ultrasound Obstet. Gynecol. 2009, 34, 326–334. [Google Scholar] [CrossRef]
- Palomba, S.; Russo, T.; Orio, F.; Falbo, A.; Manguso, F.; Cascella, T.; Tolino, A.; Carmina, E.; Colao, A.; Zullo, F. Uterine Effects of Metformin Administration in Anovulatory Women with Polycystic Ovary Syndrome. Hum. Reprod. 2006, 21, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, H.; Gull, B.; Kishimoto, K.; Kataoka, M.; Nilsson, L.; Janson, P.O.; Stener-Victorin, E.; Hellström, M. Uterine Morphology and Peristalsis in Women with Polycystic Ovary Syndrome. Acta Radiol. 2012, 53, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Iatrakis, G.; Tsionis, C.; Adonakis, G.; Stoikidou, M.; Anthouli-Anagnostopoulou, F.; Parava, M.; Vouxinou, A.; Georgopoulos, N.A.; Kourounis, G. Polycystic Ovarian Syndrome, Insulin Resistance and Thickness of the Endometrium. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 127, 218–221. [Google Scholar] [CrossRef]
- Venturoli, S.; Paradisi, R.; Saviotti, E.; Porcu, E.; Fabbri, R.; Orsini, L.F.; Bovicelli, L.; Flamigni, C. Ultrasound Study of Ovarian and Uterine Morphology in Women with Polycystic Ovary Syndrome before, during and after Treatment with Cyproterone Acetate and Ethinyloestradiol. Arch. Gynecol. 1985, 237, 1–10. [Google Scholar] [CrossRef]
- Pinkas, H.; Mashiach, R.; Rabinerson, D.; Avrech, O.M.; Royburt, M.; Rufas, O.; Meizner, I.; Ben-Rafael, Z.; Fisch, B. Doppler Parameters of Uterine and Ovarian Stromal Blood Flow in Women with Polycystic Ovary Syndrome and Normally Ovulating Women Undergoing Controlled Ovarian Stimulation. Ultrasound Obstet. Gynecol. 1998, 12, 197–200. [Google Scholar] [CrossRef]
- Younesi, L.; Safarpour Lima, Z.; Akbari Sene, A.; Hosseini Jebelli, Z.; Amjad, G. Comparison of Uterine and Ovarian Stromal Blood Flow in Patients with Polycystic Ovarian Syndrome. Endocr. Connect. 2019, 8, 50–56. [Google Scholar] [CrossRef]
- Ajossa, S.; Guerriero, S.; Paoletti, A.M.; Orrù, M.; Floris, S.; Mannias, M.; Melis, G.B. Uterine Perfusion and Hormonal Pattern in Patients with Polycystic Ovary Syndrome. J. Assist. Reprod. Genet. 2001, 18, 436–440. [Google Scholar] [CrossRef]
- Mala, Y.M.; Ghosh, S.B.; Tripathi, R. Three-Dimensional Power Doppler Imaging in the Diagnosis of Polycystic Ovary Syndrome. Int. J. Gynaecol. Obstet. 2009, 105, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Vrtačnik-Bokal, E.; Meden-Vrtovec, H.; Verdenik, I. Uterine Arterial Blood Flow and the Substances of Ovarian Renin–Angiotensin System in Women with Polycystic Ovaries Undergoing in Vitro Fertilization. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 124, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Adali, E.; Kolusari, A.; Adali, F.; Yildizhan, R.; Kurdoglu, M.; Sahin, H.G. Doppler Analysis of Uterine Perfusion and Ovarian Stromal Blood Flow in Polycystic Ovary Syndrome. Int. J. Gynaecol. Obstet. 2009, 105, 154–157. [Google Scholar] [CrossRef]
- Bracho, G.S.; Altamirano, G.A.; Kass, L.; Luque, E.H.; Bosquiazzo, V.L. Hyperandrogenism Induces Histo-Architectural Changes in the Rat Uterus. Reprod. Sci. 2019, 26, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.R.; Goyeneche, A.A.; Heber, M.F.; Abruzzese, G.A.; Telleria, C.M.; Motta, A.B. Prenatally Androgenized Female Rats Develop Uterine Hyperplasia When Adult. Mol. Cell. Endocrinol. 2020, 499, 110610. [Google Scholar] [CrossRef]
- Li, S.-Y.; Song, Z.; Song, M.-J.; Qin, J.-W.; Zhao, M.-L.; Yang, Z.-M. Impaired Receptivity and Decidualization in DHEA-Induced PCOS Mice. Sci. Rep. 2016, 6, 38134. [Google Scholar] [CrossRef]
- Palmerini, M.G.; Macchiarelli, G.; Cocciolone, D.; Mascitti, I.A.; Placidi, M.; Vergara, T.; Di Emidio, G.; Tatone, C. Modulating Morphological and Redox/Glycative Alterations in the PCOS Uterus: Effects of Carnitines in PCOS Mice. Biomedicines 2023, 11, 374. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, Y.; Guo, X.; Jia, W.; Liu, G.; Zhang, J.; Li, J.; Cui, P.; Sferruzzi-Perri, A.N.; Han, Y.; et al. Hyperandrogenism and Insulin Resistance Induce Gravid Uterine Defects in Association with Mitochondrial Dysfunction and Aberrant Reactive Oxygen Species Production. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E794–E809. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and Function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, Biology and Role in Disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Jia, W.; Liu, G.; Zhang, J.; Wang, B.; Li, J.; Cui, P.; Li, X.; Lager, S.; et al. Hyperandrogenism and Insulin Resistance Modulate Gravid Uterine and Placental Ferroptosis in PCOS-like Rats. J. Endocrinol. 2020, 246, 247–263. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Xu, H.; Hu, M.; Guo, X.; Jia, W.; Liu, G.; Li, J.; Cui, P.; Lager, S.; et al. Hyperandrogenism and Insulin Resistance-induced Fetal Loss: Evidence for Placental Mitochondrial Abnormalities and Elevated Reactive Oxygen Species Production in Pregnant Rats That Mimic the Clinical Features of Polycystic Ovary Syndrome. J. Physiol. 2019, 597, 3927–3950. [Google Scholar] [CrossRef]
- Lin, S.; Jin, X.; Gu, H.; Bi, F. Relationships of Ferroptosis-Related Genes with the Pathogenesis in Polycystic Ovary Syndrome. Front. Med. 2023, 10, 1120693. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Y.; Wei, F.; Kuang, H. Regulatory Mechanism and Research Progress of Ferroptosis in Obstetrical and Gynecological Diseases. Front. Cell Dev. Biol. 2023, 11, 1146971. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, L.; Qi, C.; Hu, G.; Wang, L.; Sun, Z.; Ni, X. Cryptotanshinone alleviates polycystic ovary syndrome in rats by regulating the HMGB1/TLR4/NF-κB signaling pathway. Mol. Med. Rep. 2020, 22, 3851–3861. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, F.; Li, D.; Yan, Y.; Wang, H. Transferrin Receptor-Mediated Reactive Oxygen Species Promotes Ferroptosis of KGN Cells via Regulating NADPH Oxidase 1/PTEN Induced Kinase 1/Acyl-CoA Synthetase Long Chain Family Member 4 Signaling. Bioengineered 2021, 12, 4983–4994. [Google Scholar] [CrossRef]
- Wu, P.; Li, C.; Ye, D.M.; Yu, K.; Li, Y.; Tang, H.; Xu, G.; Yi, S.; Zhang, Z. Circular RNA CircEPSTI1 Accelerates Cervical Cancer Progression via MiR-375/409-3P/515-5p-SLC7A11 Axis. Aging 2021, 13, 4663–4673. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef]
- Palomba, S.; de Wilde, M.A.; Falbo, A.; Koster, M.P.H.; La Sala, G.B.; Fauser, B.C.J.M. Pregnancy Complications in Women with Polycystic Ovary Syndrome. Hum. Reprod. Update 2015, 21, 575–592. [Google Scholar] [CrossRef]
- Boomsma, C.; Fauser, B.; Macklon, N. Pregnancy Complications in Women with Polycystic Ovary Syndrome. Semin. Reprod. Med. 2008, 26, 072–084. [Google Scholar] [CrossRef]
- Bammann, B.I.; Coulam, C.B.; Jiang, N.-S. Total and Free Testosterone during Pregnancy. Am. J. Obstet. Gynecol. 1980, 137, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Rosato, E.; Sciarra, F.; Anastasiadou, E.; Lenzi, A.; Venneri, M.A. Revisiting the Physiological Role of Androgens in Women. Expert Rev. Endocrinol. Metab. 2022, 17, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Makieva, S.; Saunders, P.T.K.; Norman, J.E. Androgens in Pregnancy: Roles in Parturition. Hum. Reprod. Update 2014, 20, 542–559. [Google Scholar] [CrossRef]
- Perusquia, M.; Navarrete, E.; Jasso-Kamel, J.; Montano, L.M. Androgens Induce Relaxation of Contractile Activity in Pregnant Human Myometrium at Term: A Nongenomic Action on L-Type Calcium Channels1. Biol. Reprod. 2005, 73, 214–221. [Google Scholar] [CrossRef]
- Perusquía, M. Nongenomic Action of Steroids in Myometrial Contractility. Endocrine 2001, 15, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, M.; Herr, F.; Münstedt, K.; Lang, U.; Liang, O.D. Angiogenesis and Vasculogenesis in Pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, S10–S18. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Mishra, J.S.; Chinnathambi, V.; Vincent, K.L.; Patrikeev, I.; Motamedi, M.; Saade, G.R.; Hankins, G.D.; Sathishkumar, K. Elevated Testosterone Reduces Uterine Blood Flow, Spiral Artery Elongation, and Placental Oxygenation in Pregnant Rats. Hypertension 2016, 67, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Wu, W.; Xu, J.; Yu, D.; Qiao, B.; Liu, H.; Yang, B.; Li, Y.; Ling, Y.; Kuang, H. Flutamide Ameliorates Uterine Decidualization and Angiogenesis in the Mouse Hyperandrogenemia Model during Mid-Pregnancy. PLoS ONE 2019, 14, e0217095. [Google Scholar] [CrossRef]
- Palomba, S.; Piltonen, T.T.; Giudice, L.C. Endometrial Function in Women with Polycystic Ovary Syndrome: A Comprehensive Review. Hum. Reprod. Update 2021, 27, 584–618. [Google Scholar] [CrossRef]
- Bellver, J.; Pellicer, A.; García-Velasco, J.A.; Ballesteros, A.; Remohí, J.; Meseguer, M. Obesity Reduces Uterine Receptivity: Clinical Experience from 9,587 First Cycles of Ovum Donation with Normal Weight Donors. Fertil. Steril. 2013, 100, 1050–1058. [Google Scholar] [CrossRef]
- Rhee, J.S.; Saben, J.L.; Mayer, A.L.; Schulte, M.B.; Asghar, Z.; Stephens, C.; Chi, M.M.-Y.; Moley, K.H. Diet-Induced Obesity Impairs Endometrial Stromal Cell Decidualization: A Potential Role for Impaired Autophagy. Hum. Reprod. 2016, 31, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Oróstica, L.; Poblete, C.; Romero, C.; Vega, M. Pro-Inflammatory Markers Negatively Regulate IRS1 in Endometrial Cells and Endometrium from Women with Obesity and PCOS. Reprod. Sci. 2020, 27, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Apparao, K.B.C.; Lovely, L.P.; Gui, Y.; Lininger, R.A.; Lessey, B.A. Elevated Endometrial Androgen Receptor Expression in Women with Polycystic Ovarian Syndrome1. Biol. Reprod. 2002, 66, 297–304. [Google Scholar] [CrossRef]
- Maliqueo, M.; Clementi, M.; Gabler, F.; Johnson, M.C.; Palomino, A.; Sir-Petermann, T.; Vega, M. Expression of Steroid Receptors and Proteins Related to Apoptosis in Endometria of Women with Polycystic Ovary Syndrome. Fertil. Steril. 2003, 80, 812–819. [Google Scholar] [CrossRef]
- Quezada, S.; Avellaira, C.; Johnson, M.C.; Gabler, F.; Fuentes, A.; Vega, M. Evaluation of Steroid Receptors, Coregulators, and Molecules Associated with Uterine Receptivity in Secretory Endometria from Untreated Women with Polycystic Ovary Syndrome. Fertil. Steril. 2006, 85, 1017–1026. [Google Scholar] [CrossRef]
- Li, X.; Pishdari, B.; Cui, P.; Hu, M.; Yang, H.-P.; Guo, Y.-R.; Jiang, H.-Y.; Feng, Y.; Billig, H.; Shao, R. Regulation of Androgen Receptor Expression Alters AMPK Phosphorylation in the Endometrium: In Vivo and In Vitro Studies in Women with Polycystic Ovary Syndrome. Int. J. Biol. Sci. 2015, 11, 1376–1389. [Google Scholar] [CrossRef] [PubMed]
- Young, S.L. Androgens and Endometrium: New Lessons from the Corpus Luteum via the Adrenal Cortex? Fertil. Steril. 2018, 109, 623–624. [Google Scholar] [CrossRef]
- Feigenbaum, S.L.; Crites, Y.; Hararah, M.K.; Yamamoto, M.P.; Yang, J.; Lo, J.C. Prevalence of Cervical Insufficiency in Polycystic Ovarian Syndrome. Hum. Reprod. 2012, 27, 2837–2842. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, M.; Liang, X.; Yang, X. The Prevalence of Cervical Insufficiency in Chinese Women with Polycystic Ovary Syndrome Undergone ART Treatment Accompanied with Negative Prognosis: A Retrospective Study. J. Obstet. Gynaecol. 2021, 41, 888–892. [Google Scholar] [CrossRef]
- Shetelig Løvvik, T.; Stridsklev, S.; Carlsen, S.M.; Salvesen, Ø.; Vanky, E. Cervical Length and Androgens in Pregnant Women With Polycystic Ovary Syndrome: Has Metformin Any Effect? J. Clin. Endocrinol. Metab. 2016, 101, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Stridsklev, S.; Salvesen, Ø.; Salvesen, K.Å.; Carlsen, S.M.; Vanky, E. Uterine Artery Doppler in Pregnancy: Women with PCOS Compared to Healthy Controls. Int. J. Endocrinol. 2018, 2018, 2604064. [Google Scholar] [CrossRef]
- Palomba, S.; Falbo, A.; Russo, T.; Battista, L.; Tolino, A.; Orio, F.; Zullo, F. Uterine Blood Flow in Pregnant Patients with Polycystic Ovary Syndrome: Relationships with Clinical Outcomes. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 711–721. [Google Scholar] [CrossRef]
- Nouh, A.A.; Shalaby, S.M. The Predictive Value of Uterine Blood Flow in Detecting the Risk of Adverse Pregnancy Outcome in Patients with Polycystic Ovary Syndrome. Middle East Fertil. Soc. J. 2011, 16, 284–290. [Google Scholar] [CrossRef]
- Mirghani, H.M.; Salem, M.; Weerasinghe, S.D. Effect of Maternal Fasting on Uterine Arterial Blood Flow. J. Obstet. Gynaecol. Res. 2007, 33, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, S.; Chang, Y.; Fang, C.; Liu, H.; Zhang, X.; Wang, Y. Effect of Metformin Treatment during Pregnancy on Women with PCOS: A Systematic Review and Meta-Analysis. Clin. Investig. Med. 2016, 39, E120–E131. [Google Scholar] [CrossRef] [PubMed]
- Salvesen, K.Å.; Vanky, E.; Carlsen, S.M. Metformin Treatment in Pregnant Women with Polycystic Ovary Syndrome—Is Reduced Complication Rate Mediated by Changes in the Uteroplacental Circulation? Ultrasound Obstet. Gynecol. 2007, 29, 433–437. [Google Scholar] [CrossRef]
- Jamal, A.; Milani, F.; Al-Yasin, A. Evaluation of the Effect of Metformin and Aspirin on Utero Placental Circulation of Pregnant Women with PCOS. Iran. J. Reprod. Med. 2012, 10, 265–270. [Google Scholar] [PubMed]
- Stridsklev, S.; Carlsen, S.M.; Salvesen, Ø.; Clemens, I.; Vanky, E. Midpregnancy Doppler Ultrasound of the Uterine Artery in Metformin- Versus Placebo-Treated PCOS Women: A Randomized Trial. J. Clin. Endocrinol. Metab. 2014, 99, 972–977. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Conclusions | Study Type a | Population | Values in PCOS Patients vs. in Non-PCOS Patients | p Value | Author |
|---|---|---|---|---|---|---|
| Endometrial and subendometrial blood flow | Higher in PCOS patients | Prospective case–control | 67 patients (37 PCOS vs. 30 healthy controls) | Endometrial pulsatility index (PI)—mean ± SD Follicular phase: 0.96 ± 0.13 vs. 0.72 ± 0.18 Peri-ovulatory phase: 0.97 ± 0.17 vs. 0.83 ± 0.13 Luteal phase: 1.00 ± 0.15 vs. 0.80 ± 0.13 Endometrial resistance index (RI)—mean ± SD Follicular phase: 0.57 ± 0.11 vs. 0.48 ± 0.10 Peri-ovulatory phase: 0.62 ± 0.12 vs. 0.55 ± 0.11 Luteal phase: 0.636 ± 0.10 vs. 0.54 ± 0.12 Subendometrial pulsatility index (PI)—mean ± SD Follicular phase: 0.90 ± 0.13 vs. 0.65 ± 0.16 Peri-ovulatory phase: 0.91 ± 0.11 vs. 0.76 ± 0.12 Luteal phase: 0.90 ± 0.14 vs. 0.77 ± 0.12 Subendometrial resistance index (RI)—mean ± SD Follicular phase: 0.54 ± 0.10 vs. 0.47 ± 0.10 Peri-ovulatory phase: 0.56 ± 0.10 vs. 0.54 ± 0.11 Luteal phase: 0.58 ± 0.08 vs. 0.53 ± 0.12 | p < 0.05 p < 0.05 p < 0.05 p < 0.05 p < 0.05 p < 0.05 p < 0.05 p < 0.05 p < 0.05 p < 0.05 p < 0.05 p < 0.05 | Palomba et al., 2006 [94] |
| No difference between PCOS and control groups b | Prospective observational | 72 patients (36 PCOS vs. 36 controls from the assisted conception unit and the infertility clinic) | Endometrial parameters—median (range) Follicular phase: VI% 0.60 (0.06–1.45) vs. 0.86 (0.23–1.76) FI 31.57 (23.54–50.76) vs. 32.14 (24.21–69.41) VFI 0.21 (0.02–0.56) vs. 0.28 (0.07–0.65) Subendometrial parameters—median (range) Follicular phase: VI% 1.92 (0.16–5.17) vs. 2.47 (0.85–5.29) FI 39.98 (30.72–56.17) vs. 39.09 (34.66–67.08) VFI 0.76 (0.07–2.01) vs. 0.96 (0.35–2.48) | p ≥ 0.05 p ≥ 0.05 p ≥ 0.05 p ≥ 0.05 p ≥ 0.05 p ≥ 0.05 | Lam et al., 2009 [93] | |
| Uterine artery pulsatility index (PI) | Higher in PCOS patients | Prospective | 103 patients (88 PCOS vs. 15 patients with normal menstrual cycle) | Mean ± SD 2.97 ± 0.9 vs. 1.89 ± 0.2 | p < 0.05 | Ajossa et al., 2001 [100] |
| Prospective case–control | 67 patients (37 PCOS vs. 30 healthy controls) | Mean ± SD Follicular phase: 3.41 ± 0.97 vs. 2.37 ± 0.54 Peri-ovulatory phase: 3.30 ± 1.02 vs. 2.76 ± 0.41 Luteal phase: 3.32 ± 0.96 vs. 2.81 ± 0.54 | p < 0.05 p < 0.05 p < 0.05 | Palomba et al., 2006 [94] | ||
| Prospective controlled | Not reported | p < 0.05 | Vrtačnik-Bokal et al., 2006 [102] | |||
| Prospective | 97 patients (55 PCOS vs. 42 healthy controls) | Mean ± SD 4.88 ± 0.96 vs. 4.11 ± 0.82 | p < 0.01 | Adali et al., 2009 [103] | ||
| Prospective case–control | 50 patients (25 PCOS vs. 25 healthy controls) | Mean ± SD 3.74 ± 1.01 vs. 2.43 ± 0.36 | p < 0.001 | Mala et al., 2009 [101] | ||
| No difference between PCOS and control groups | Prospective case–control | 22 patients (10 PCOS vs. 12 controls) | Mean ± SD 2.9 ± 1.2 vs. 3.1 ± 0.8 | p > 0.05 | Pinkas et al., 1998 [98] | |
| Prospective observational | 72 patients (36 PCOS vs. 36 controls from the assisted conception unit and the infertility clinic) | Median (range) 2.48 (1.29–4.60) vs. 2.52 (1.24–7.04) | p ≥ 0.05 | Lam et al., 2009 [93] | ||
| Uterine artery resistance index (RI) | Higher in PCOS patients | Prospective case–control | 67 patients (37 PCOS vs. 30 healthy controls) | Mean ± SD Follicular phase: 0.96 ± 0.12 vs. 0.67 ± 0.10 Peri-ovulatory phase: 0.96 ± 0.14 vs. 0.84 ± 0.10 Luteal phase: 0.98 ± 0.14 vs. 0.84 ± 0.12 | p < 0.05 | Palomba et al., 2006 [94] |
| Prospective controlled | 49 patients (18 PCOS and 31 women with normal menstrual cycle) | Mean ± S.E. 0.90 ± 0.05 vs. 0.86 ± 0.06 | p < 0.05 | Vrtačnik-Bokal et al., 2006 [102] | ||
| Prospective case–control | 50 patients (25 PCOS vs. 25 healthy controls) | Mean ± SD 0.87 ± 0.04 vs. 0.80 ± 0.06 | p < 0.001 | Mala et al., 2009 [101] | ||
| No difference between PCOS and control group | Prospective case–control | 22 patients (10 PCOS vs. 12 controls) | Mean ± SD 0.92 ± 0.10 vs. 0.91 ± 0.07 | p > 0.05 | Pinkas et al., 1998 [98] | |
| Prospective observational | 72 patients (36 PCOS vs. 36 controls from the assisted conception unit and the infertility clinic) | Median (range) 0.86 (0.68–0.99) vs. 0.87 (0.70–0.99) | p ≥ 0.05 | Lam et al., 2009 [93] | ||
| Case–control | 77 patients (45 PCOS vs. 32 healthy controls) | Mean ± SD 0.96 ± 0.42 vs. 0.87 ± 0.13 | p ≥ 0.05 | Younesi et al., 2019 [99] | ||
| Parameter | Conclusions | Study Type a | Population | Values in PCOS Patients vs. in Non-PCOS Patients | p Value | Author |
|---|---|---|---|---|---|---|
| Uterine artery pulsatility index (PI) | Higher rate of abnormal PI | Prospective case–control | 139 patients (70 PCOS vs. 69 healthy controls) | n (%) Baseline: 42 (60.0) vs. 29 (42.0) 8 hbd: 39 (55.7) vs. 27 (39.1) 10 hbd: 38 (34.3) vs. 26 (37.7) 12 hbd: 36 (51.4) vs. 24 (34.8) 20 hbd: 36 (51.4) vs. 23 (33.3) | p < 0.05 p < 0.05 p < 0.05 p < 0.05 p < 0.05 | Palomba et al., 2010 [142] |
| Higher PI in PCOS patients | Prospective case–control | 80 patients (40 ovulatory PCOS vs. 40 healthy controls) b | Mean ± SD 8 hbd: 2.93 ± 1.02 vs. 2.4 ± 0.95 12 hbd: 2.71 ± 1.03 vs. 1.9 ± 0.89 26 hbd: 1.9 ± 1.01 vs. 1.4 ± 0.93 | p = 0.014 p = 0.001 p = 0.024 | Nouh and Shalaby, 2011 [143] | |
| No difference between PCOS/healthy patients | Prospective observational | 142 patients (24 PCOS vs. 118 healthy controls) c | Mean (CI) 1.80 (1.58–2.03) vs. 1.79 (1.70–1.87) | p = 0.18 | Stridsklev et al., 2018 [141] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaborowska, L.; Blok, J.M.; Piotrkowicz, E.; Lindheim, S.R.; Ludwin, A. Functional and Structural Uterine Changes in PCOS. Int. J. Mol. Sci. 2025, 26, 7921. https://doi.org/10.3390/ijms26167921
Zaborowska L, Blok JM, Piotrkowicz E, Lindheim SR, Ludwin A. Functional and Structural Uterine Changes in PCOS. International Journal of Molecular Sciences. 2025; 26(16):7921. https://doi.org/10.3390/ijms26167921
Chicago/Turabian StyleZaborowska, Lucja, Joanna Maria Blok, Emilia Piotrkowicz, Steven R. Lindheim, and Artur Ludwin. 2025. "Functional and Structural Uterine Changes in PCOS" International Journal of Molecular Sciences 26, no. 16: 7921. https://doi.org/10.3390/ijms26167921
APA StyleZaborowska, L., Blok, J. M., Piotrkowicz, E., Lindheim, S. R., & Ludwin, A. (2025). Functional and Structural Uterine Changes in PCOS. International Journal of Molecular Sciences, 26(16), 7921. https://doi.org/10.3390/ijms26167921





