Fusobacterium nucleatum and Gastric Cancer: An Emerging Connection
Abstract
1. Introduction
2. Materials and Methods
3. Biological and Pathogenic Features of F. nucleatum
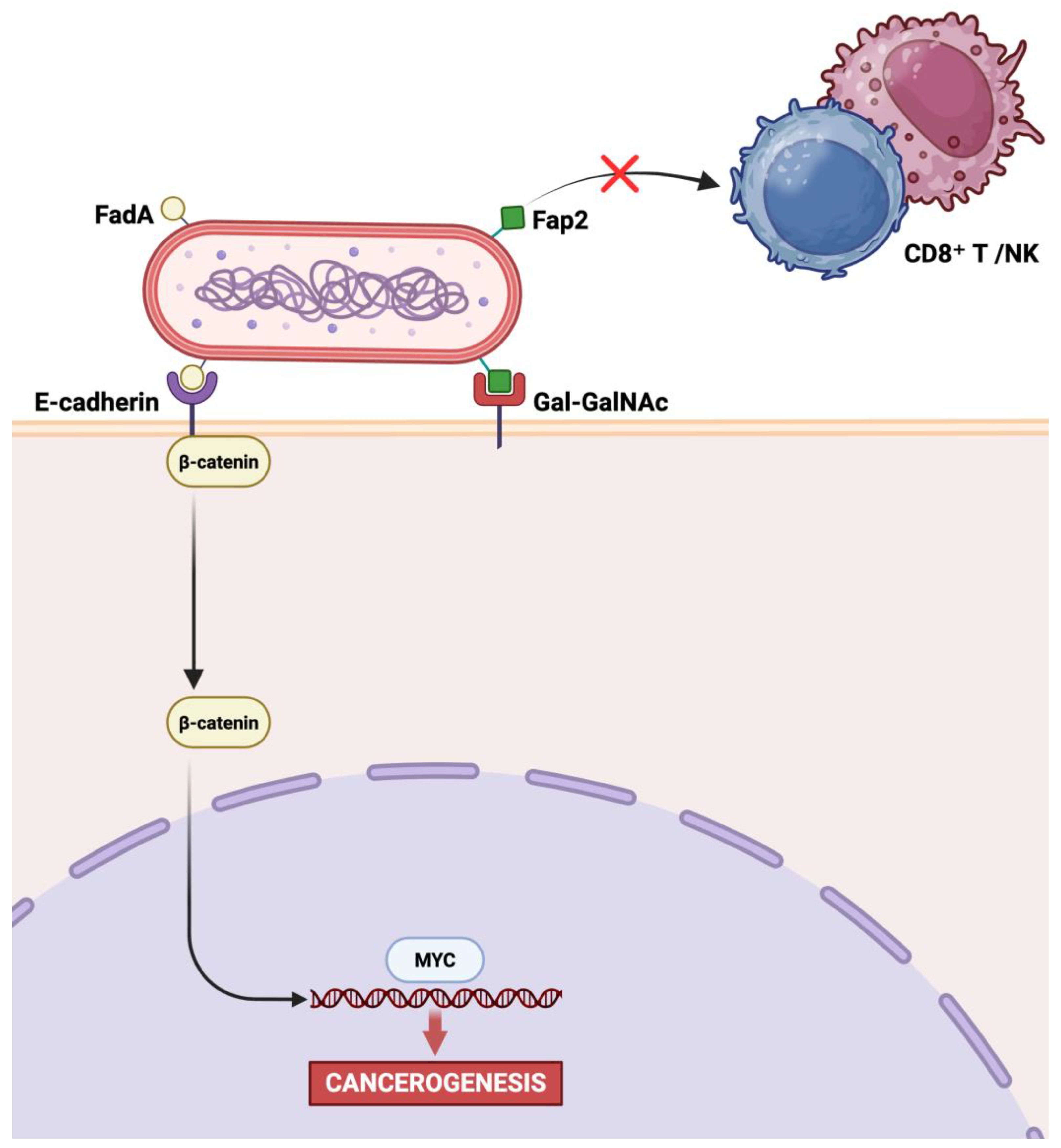
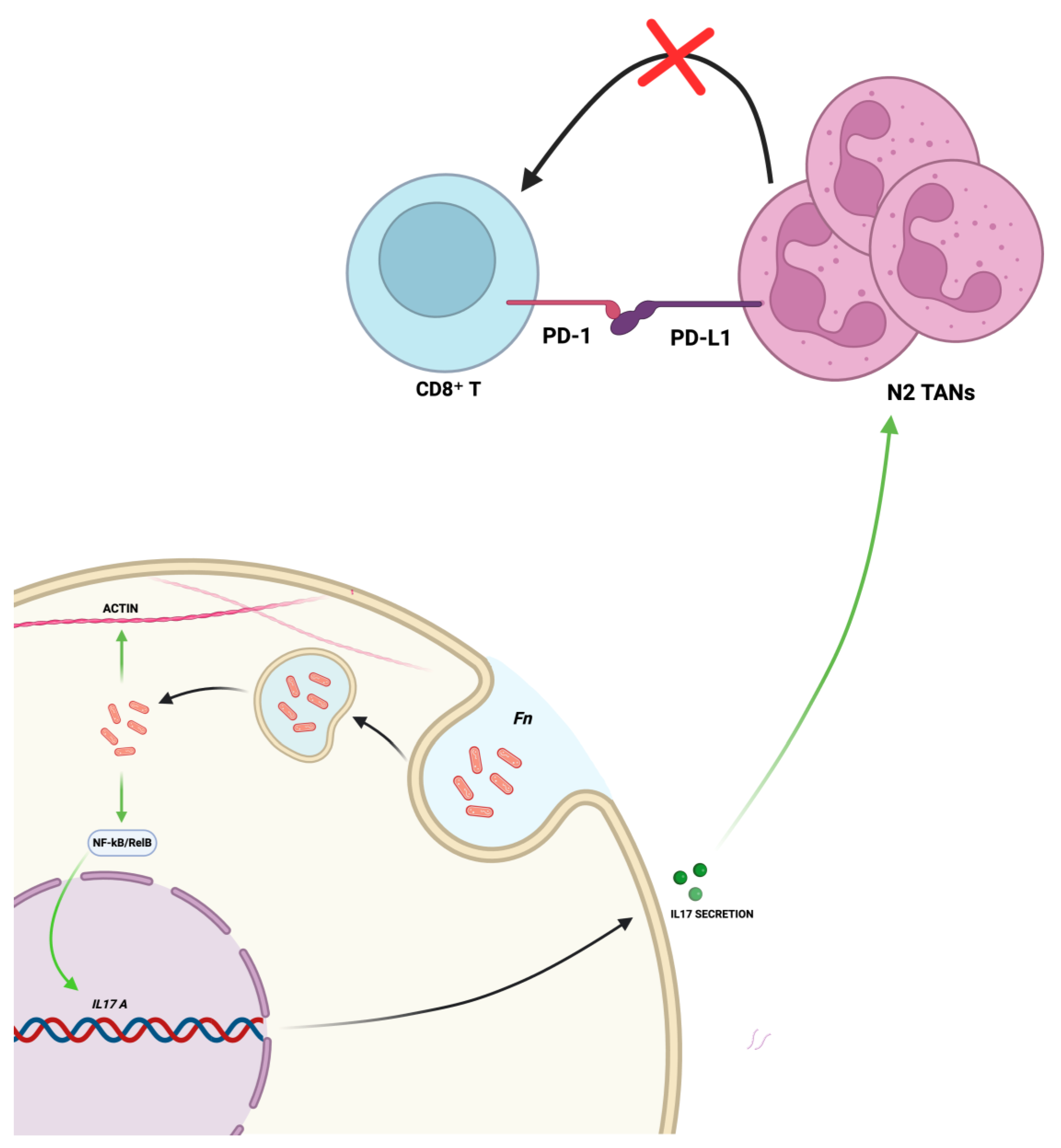
4. Dysbiosis-Driven Carcinogenesis: Evidence for F. nucleatum Involvement in GC
5. Methods for the Detection of F. nucleatum in the Stomach: Where We Stand
6. Future Directions
6.1. Direct Antimicrobial Strategies
6.2. Targeting Bacterial Virulence Factors
6.3. Modulating the Host Immune Response and Combination Therapies
6.4. Microbiota-Based Interventions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Robles Alonso, V.; Guarner, F. Linking the Gut Microbiota to Human Health. Br. J. Nutr. 2013, 109, S21–S26. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as Vitamin Suppliers to Their Host: A Gut Microbiota Perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.A.; Kleerebezem, M.; Brummer, R.-J.; Cani, P.D.; Mercenier, A.; MacDonald, T.T.; Garcia-Ródenas, C.L.; Wells, J.M. Can Probiotics Modulate Human Disease by Impacting Intestinal Barrier Function? Br. J. Nutr. 2017, 117, 93–107. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018, 6, 116. [Google Scholar] [CrossRef]
- Biedermann, L.; Rogler, G. Environmental Factors and Their Impact on the Intestinal Microbiota: A Role for Human Disease? Dig. Dis. 2012, 30, 20–27. [Google Scholar] [CrossRef]
- Wegierska, A.E.; Charitos, I.A.; Topi, S.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med. 2022, 52, 2355–2369. [Google Scholar] [CrossRef]
- Cusumano, G.; Flores, G.A.; Venanzoni, R.; Angelini, P. The Impact of Antibiotic Therapy on Intestinal Microbiota: Dysbiosis, Antibiotic Resistance, and Restoration Strategies. Antibiotics 2025, 14, 371. [Google Scholar] [CrossRef]
- Aviles-Jimenez, F.; Vazquez-Jimenez, F.; Medrano-Guzman, R.; Mantilla, A.; Torres, J. Stomach Microbiota Composition Varies between Patients with Non-Atrophic Gastritis and Patients with Intestinal Type of Gastric Cancer. Sci. Rep. 2014, 4, 4202. [Google Scholar] [CrossRef]
- Castaño-Rodríguez, N.; Goh, K.-L.; Fock, K.M.; Mitchell, H.M.; Kaakoush, N.O. Dysbiosis of the Microbiome in Gastric Carcinogenesis. Sci. Rep. 2017, 7, 15957. [Google Scholar] [CrossRef]
- Iwu, C.D.; Iwu-Jaja, C.J. Gastric Cancer Epidemiology: Current Trend and Future Direction. Hygiene 2023, 3, 256–268. [Google Scholar] [CrossRef]
- Warren, J.R.; Marshall, B. Unidentified Curved Bacilli on Gastric Epithelium in Active Chronic Gastritis. Lancet 1983, 1, 1273–1275. [Google Scholar] [CrossRef]
- Plottel, C.S.; Blaser, M.J. Microbiome and Malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef]
- Zeng, R.; Gou, H.; Lau, H.C.H.; Yu, J. Stomach Microbiota in Gastric Cancer Development and Clinical Implications. Gut 2024, 73, 2062–2073. [Google Scholar] [CrossRef]
- Kamali, N.; Talebi Bezmin Abadi, A.; Rahimi, F.; Forootan, M. Fusobacterium Nucleatum Confirmed in Gastric Biopsies of Patients without Helicobacter Pylori. BMC Res. Notes 2025, 18, 109. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-Y.; Tung, S.-Y.; Pan, H.-Y.; Yen, C.-W.; Xu, H.-W.; Lin, Y.-J.; Deng, Y.-F.; Hsu, W.-T.; Wu, C.-S.; Li, C. Increased Abundance of Clostridium and Fusobacterium in Gastric Microbiota of Patients with Gastric Cancer in Taiwan. Sci. Rep. 2018, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Y.; Zhai, E.; Zhao, R.; Qian, Y.; Huang, Z.; Liu, Y.; Zhao, Z.; Xu, X.; Liu, J.; et al. Intratumoral Fusobacterium Nucleatum Recruits Tumor-Associated Neutrophils to Promote Gastric Cancer Progression and Immune Evasion. Cancer Res. 2025, 85, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Li, X.; Zhang, M.; Shang, Z.; Luo, Z.; Wang, Y.; Gui, X.; Liu, Q.; Li, T.; Zeng, S.; et al. Fusobacterium nucleatum-Induced Exosomal HOTTIP Promotes Gastric Cancer Progression Through the microRNA-885-3p/EphB2 Axis. Cancer Sci. 2023, 114, 2360–2374. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Chen, J.; Liu, Y.; Huang, Z.; Xu, X.; Qian, Y.; Zhao, R.; Liu, J.; Zhao, Z.; et al. IDDF2023-ABS-0058 Fusobacterium nucleatum Intracellular Parasitism Activates the NF-KB/IL-17 Signaling Pathway Induce Neutrophil Recruitment and Promote Gastric Cancer Progression. Gut 2023, 72, A70–A72. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Fusobacterium Nucleatum—Symbiont, Opportunist and Oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J.; Periasamy, S.; Jakubovics, N.S. Oral Multispecies Biofilm Development and the Key Role of Cell–Cell Distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef]
- Guo, L.; Shokeen, B.; He, X.; Shi, W.; Lux, R. Streptococcus mutans SpaP Binds to RadD of Fusobacterium nucleatum ssp. Polymorphum. Mol. Oral Microbiol. 2017, 32, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, C.W.; Lux, R.; Haake, S.K.; Shi, W. The Fusobacterium nucleatum Outer Membrane Protein RadD Is an Arginine-inhibitable Adhesin Required for Inter-species Adherence and the Structured Architecture of Multispecies Biofilm. Mol. Microbiol. 2009, 71, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Krisanaprakornkit, S.; Kimball, J.R.; Weinberg, A.; Darveau, R.P.; Bainbridge, B.W.; Dale, B.A. Inducible Expression of Human β-Defensin 2 by Fusobacterium nucleatum in Oral Epithelial Cells: Multiple Signaling Pathways and Role of Commensal Bacteria in Innate Immunity and the Epithelial Barrier. Infect. Immun. 2000, 68, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.-H.; Chun, S.; Park, C.; Lee, J.-H.; Lee, S.-W.; Lee, T.-H. Transcriptome Profiling Analysis of Senescent Gingival Fibroblasts in Response to Fusobacterium nucleatum Infection. PLoS ONE 2017, 12, e0188755. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Ghosh, S.K.; Shokeen, B.; Eapan, B.; Lux, R.; Kiselar, J.; Nithianantham, S.; Young, A.; Pandiyan, P.; McCormick, T.S.; et al. FAD-I, a Fusobacterium nucleatum Cell Wall-Associated Diacylated Lipoprotein That Mediates Human Beta Defensin 2 Induction through Toll-Like Receptor-1/2 (TLR-1/2) and TLR-2/6. Infect. Immun. 2016, 84, 1446–1456. [Google Scholar] [CrossRef]
- Taxman, D.J.; Swanson, K.V.; Broglie, P.M.; Wen, H.; Holley-Guthrie, E.; Huang, M.T.-H.; Callaway, J.B.; Eitas, T.K.; Duncan, J.A.; Ting, J.P.Y. Porphyromonas gingivalis Mediates Inflammasome Repression in Polymicrobial Cultures through a Novel Mechanism Involving Reduced Endocytosis. J. Biol. Chem. 2012, 287, 32791–32799. [Google Scholar] [CrossRef]
- Saito, A.; Kokubu, E.; Inagaki, S.; Imamura, K.; Kita, D.; Lamont, R.J.; Ishihara, K. Porphyromonas gingivalis Entry into Gingival Epithelial Cells Modulated by Fusobacterium nucleatum Is Dependent on Lipid Rafts. Microb. Pathog. 2012, 53, 234–242. [Google Scholar] [CrossRef]
- Metzger, Z.; Lin, Y.-Y.; Dimeo, F.; Ambrose, W.W.; Trope, M.; Arnold, R.R. Synergistic Pathogenicity of Porphyromonas gingivalis and Fusobacterium nucleatum in the Mouse Subcutaneous Chamber Model. J. Endod. 2009, 35, 86–94. [Google Scholar] [CrossRef]
- Xu, M.; Yamada, M.; Li, M.; Liu, H.; Chen, S.G.; Han, Y.W. FadA from Fusobacterium nucleatum Utilizes Both Secreted and Nonsecreted Forms for Functional Oligomerization for Attachment and Invasion of Host Cells. J. Biol. Chem. 2007, 282, 25000–25009. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum Promotes Colorectal Carcinogenesis by Modulating E-Cadherin/β-Catenin Signaling via Its FadA Adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef]
- Fardini, Y.; Wang, X.; Témoin, S.; Nithianantham, S.; Lee, D.; Shoham, M.; Han, Y.W. Fusobacterium nucleatum Adhesin FadA Binds Vascular Endothelial Cadherin and Alters Endothelial Integrity. Mol. Microbiol. 2011, 82, 1468–1480. [Google Scholar] [CrossRef]
- Zheng, Z.; Jin, W.; Guo, W.; Jin, Z.; Zuo, Y. Oral Fusobacterium Nucleatum Exacerbates Ulcerative Colitis via the Oral-Gut Axis: Mechanisms and Therapeutic Implications. Front. Cell. Infect. Microbiol. 2025, 15, 1564169. [Google Scholar] [CrossRef]
- Tortora, S.C.; Agurto, M.G.; Martello, L.A. The Oral-Gut-Circulatory Axis: From Homeostasis to Colon Cancer. Front. Cell. Infect. Microbiol. 2023, 13, 1289452. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, S.; Sheng, H.; Zhen, Y.; Wu, B.; Li, Z.; Chen, D.; Zhou, H. Oral Fusobacterium nucleatum Resists the Acidic PH of the Stomach Due to Membrane Erucic Acid Synthesized via Enoyl-CoA Hydratase-Related Protein FnFabM. J. Oral Microbiol. 2025, 17, 2453964. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Brennan, M.T.; Sasser, H.C.; Fox, P.C.; Paster, B.J.; Bahrani-Mougeot, F.K. Bacteremia Associated with Toothbrushing and Dental Extraction. Circulation 2008, 117, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- Gethings-Behncke, C.; Coleman, H.G.; Jordao, H.W.T.; Longley, D.B.; Crawford, N.; Murray, L.J.; Kunzmann, A.T. Fusobacterium nucleatum in the Colorectum and Its Association with Cancer Risk and Survival: A Systematic Review and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2020, 29, 539–548. [Google Scholar] [CrossRef]
- Yamamura, K.; Baba, Y.; Miyake, K.; Nakamura, K.; Shigaki, H.; Mima, K.; Kurashige, J.; Ishimoto, T.; Iwatsuki, M.; Sakamoto, Y.; et al. Fusobacterium nucleatum in gastroenterological cancer: Evaluation of measurement methods using quantitative polymerase chain reaction and a literature review. Oncol Lett. 2017, 14, 6373–6378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, J.; Li, Q.; Fu, X. Fusobacterium nucleatum Contributes to the Carcinogenesis of Colorectal Cancer by Inducing Inflammation and Suppressing Host Immunity. Transl. Oncol. 2019, 12, 846–851. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic Analysis Identifies Association of Fusobacterium with Colorectal Carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum Infection Is Prevalent in Human Colorectal Carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef]
- McCoy, A.N.; Araújo-Pérez, F.; Azcárate-Peril, A.; Yeh, J.J.; Sandler, R.S.; Keku, T.O. Fusobacterium Is Associated with Colorectal Adenomas. PLoS ONE 2013, 8, e53653. [Google Scholar] [CrossRef]
- Bullman, S.; Pedamallu, C.S.; Sicinska, E.; Clancy, T.E.; Zhang, X.; Cai, D.; Neuberg, D.; Huang, K.; Guevara, F.; Nelson, T.; et al. Analysis of Fusobacterium Persistence and Antibiotic Response in Colorectal Cancer. Science (1979) 2017, 358, 1443–1448. [Google Scholar] [CrossRef]
- Petkevicius, V.; Lehr, K.; Kupcinskas, J.; Link, A. Fusobacterium nucleatum: Unraveling Its Potential Role in Gastric Carcinogenesis. World J. Gastroenterol. 2024, 30, 3972–3984. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Ge, Q.-X.; Cao, J.; Zhou, Y.-J.; Du, Y.-L.; Shen, B.; Wan, Y.-J.Y.; Nie, Y.-Q. Association of Fusobacterium nucleatum Infection with Colorectal Cancer in Chinese Patients. World J. Gastroenterol. 2016, 22, 3227. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, L.; Schmid, J.; Ebert, M.; Soucek, P.; Kunicka, T.; Liska, V.; Bruha, J.; Neary, P.; Dezeeuw, N.; Tommasino, M.; et al. Fusobacterium nucleatum Associates with Stages of Colorectal Neoplasia Development, Colorectal Cancer and Disease Outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium nucleatum in Colorectal Carcinoma Tissue and Patient Prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef]
- Guo, P.; Tian, Z.; Kong, X.; Yang, L.; Shan, X.; Dong, B.; Ding, X.; Jing, X.; Jiang, C.; Jiang, N.; et al. FadA Promotes DNA Damage and Progression of Fusobacterium nucleatum-Induced Colorectal Cancer through up-Regulation of Chk2. J. Exp. Clin. Cancer Res. 2020, 39, 202. [Google Scholar] [CrossRef]
- Yang, Y.; Weng, W.; Peng, J.; Hong, L.; Yang, L.; Toiyama, Y.; Gao, R.; Liu, M.; Yin, M.; Pan, C.; et al. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor−κB, and Up-Regulating Expression of MicroRNA-21. Gastroenterology 2017, 152, 851–866.e24. [Google Scholar] [CrossRef]
- Quah, S.Y.; Bergenholtz, G.; Tan, K.S. Fusobacterium nucleatum Induces Cytokine Production through Toll-like-receptor-independent Mechanism. Int. Endod. J. 2014, 47, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, A.; Cocomazzi, A.; Basso, M.; Cenci, T.; Ricci, R.; Pierconti, F.; Cassano, A.; Fiorentino, V.; Barone, C.; Bria, E.; et al. C-MYC Expression Is a Possible Keystone in the Colorectal Cancer Resistance to EGFR Inhibitors. Cancers 2020, 12, 638. [Google Scholar] [CrossRef] [PubMed]
- Abed, J.; Emgård, J.E.M.; Zamir, G.; Faroja, M.; Almogy, G.; Grenov, A.; Sol, A.; Naor, R.; Pikarsky, E.; Atlan, K.A.; et al. Fap2 Mediates Fusobacterium Nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe 2016, 20, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Abed, J.; Maalouf, N.; Parhi, L.; Chaushu, S.; Mandelboim, O.; Bachrach, G. Tumor Targeting by Fusobacterium nucleatum: A Pilot Study and Future Perspectives. Front. Cell. Infect. Microbiol. 2017, 7, 295. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 Protein of Fusobacterium nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Huang, S.-T.; Chen, J.; Lian, L.-Y.; Cai, H.-H.; Zeng, H.-S.; Zheng, M.; Liu, M.-B. Intratumoral Levels and Prognostic Significance of Fusobacterium nucleatum in Cervical Carcinoma. Aging 2020, 12, 23337–23350. [Google Scholar] [CrossRef]
- Audirac-Chalifour, A.; Torres-Poveda, K.; Bahena-Román, M.; Téllez-Sosa, J.; Martínez-Barnetche, J.; Cortina-Ceballos, B.; López-Estrada, G.; Delgado-Romero, K.; Burguete-García, A.I.; Cantú, D.; et al. Cervical Microbiome and Cytokine Profile at Various Stages of Cervical Cancer: A Pilot Study. PLoS ONE 2016, 11, e0153274. [Google Scholar] [CrossRef]
- Spor, A.; Koren, O.; Ley, R. Unravelling the Effects of the Environment and Host Genotype on the Gut Microbiome. Nat. Rev. Microbiol. 2011, 9, 279–290. [Google Scholar] [CrossRef]
- Mancabelli, L.; Milani, C.; Lugli, G.A.; Turroni, F.; Mangifesta, M.; Viappiani, A.; Ticinesi, A.; Nouvenne, A.; Meschi, T.; van Sinderen, D.; et al. Unveiling the Gut Microbiota Composition and Functionality Associated with Constipation through Metagenomic Analyses. Sci. Rep. 2017, 7, 9879. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The Gut Microbiome in Atherosclerotic Cardiovascular Disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Marieb, E.N.; Hoehn, K. Human Anatomy and Physiology; Pearson Education: London, UK, 2019. [Google Scholar]
- Mantzourani, I.; Chondrou, P.; Bontsidis, C.; Karolidou, K.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Galanis, A.; Plessas, S. Assessment of the Probiotic Potential of Lactic Acid Bacteria Isolated from Kefir Grains: Evaluation of Adhesion and Antiproliferative Properties in in Vitro Experimental Systems. Ann. Microbiol. 2019, 69, 751–763. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Sjövall, H.; Hansson, G.C. The Gastrointestinal Mucus System in Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sun, C.; Li, M.; Hu, G.; Zhao, X.-M.; Chen, W.-H. Compared to Histamine-2 Receptor Antagonist, Proton Pump Inhibitor Induces Stronger Oral-to-Gut Microbial Transmission and Gut Microbiome Alterations: A Randomised Controlled Trial. Gut 2024, 73, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Piazuelo, M.B. The Gastric Precancerous Cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Cryer, B.; McArthur, K.; Huet, B.; Lee, E. Effects of Aging and Gastritis on Gastric Acid and Pepsin Secretion in Humans: A Prospective Study. Gastroenterology 1996, 110, 1043–1052. [Google Scholar] [CrossRef]
- Coker, O.O.; Dai, Z.; Nie, Y.; Zhao, G.; Cao, L.; Nakatsu, G.; Wu, W.K.; Wong, S.H.; Chen, Z.; Sung, J.J.Y.; et al. Mucosal Microbiome Dysbiosis in Gastric Carcinogenesis. Gut 2018, 67, 1024–1032. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Pereira-Marques, J.; Pinto-Ribeiro, I.; Costa, J.L.; Carneiro, F.; Machado, J.C.; Figueiredo, C. Gastric Microbial Community Profiling Reveals a Dysbiotic Cancer-Associated Microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, R.; Chen, S.; Sun, B.; Zhang, K. Analysis of Gastric Microbiome Reveals Three Distinctive Microbial Communities Associated with the Occurrence of Gastric Cancer. BMC Microbiol. 2022, 22, 184. [Google Scholar] [CrossRef]
- Yu, D.; Yang, J.; Jin, M.; Zhou, B.; Shi, L.; Zhao, L.; Zhang, J.; Lin, Z.; Ren, J.; Liu, L.; et al. Fecal Streptococcus Alteration Is Associated with Gastric Cancer Occurrence and Liver Metastasis. mBio 2021, 12, e0299421. [Google Scholar] [CrossRef]
- Dai, D.; Yang, Y.; Yu, J.; Dang, T.; Qin, W.; Teng, L.; Ye, J.; Jiang, H. Interactions between Gastric Microbiota and Metabolites in Gastric Cancer. Cell Death Dis. 2021, 12, 1104. [Google Scholar] [CrossRef]
- Chen, X.-H.; Wang, A.; Chu, A.-N.; Gong, Y.-H.; Yuan, Y. Mucosa-Associated Microbiota in Gastric Cancer Tissues Compared With Non-Cancer Tissues. Front. Microbiol. 2019, 10, 1261. [Google Scholar] [CrossRef]
- Shao, D.; Vogtmann, E.; Liu, A.; Qin, J.; Chen, W.; Abnet, C.C.; Wei, W. Microbial Characterization of Esophageal Squamous Cell Carcinoma and Gastric Cardia Adenocarcinoma from a High-risk Region of China. Cancer 2019, 125, 3993–4002. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, X.; Zeng, R.; Wu, Q.; Sun, H.; Wu, W.; Zhang, X.; Sun, G.; Yan, B.; Wu, L.; et al. Changes of the Gastric Mucosal Microbiome Associated With Histological Stages of Gastric Carcinogenesis. Front. Microbiol. 2020, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Gantuya, B.; El-Serag, H.B.; Matsumoto, T.; Ajami, N.J.; Oyuntsetseg, K.; Azzaya, D.; Uchida, T.; Yamaoka, Y. Gastric Microbiota in Helicobacter Pylori-Negative and -Positive Gastritis Among High Incidence of Gastric Cancer Area. Cancers 2019, 11, 504. [Google Scholar] [CrossRef] [PubMed]
- Boehm, E.T.; Thon, C.; Kupcinskas, J.; Steponaitiene, R.; Skieceviciene, J.; Canbay, A.; Malfertheiner, P.; Link, A. Fusobacterium nucleatum Is Associated with Worse Prognosis in Lauren’s Diffuse Type Gastric Cancer Patients. Sci. Rep. 2020, 10, 16240. [Google Scholar] [CrossRef] [PubMed]
- Nascimento Araujo, C.d.; Amorim, A.T.; Barbosa, M.S.; Alexandre, J.C.P.L.; Campos, G.B.; Macedo, C.L.; Marques, L.M.; Timenetsky, J. Evaluating the Presence of Mycoplasma Hyorhinis, Fusobacterium Nucleatum, and Helicobacter Pylori in Biopsies of Patients with Gastric Cancer. Infect. Agent Cancer 2021, 16, 70. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Z.; Tang, X.; Zhao, F.; He, M.; Liu, C.; Zhou, D.; Wang, L.; Gu, B.; Yuan, Y.; et al. Colonization of Fusobacterium nucleatum Is an Independent Predictor of Poor Prognosis in Gastric Cancer Patients with Venous Thromboembolism: A Retrospective Cohort Study. Thromb. J. 2023, 21, 2. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Ożarowski, M.; Stasiewicz, M. Carcinogenic microbiota and its role in colorectal cancer development. Semin. Cancer Biol. 2022, 86 Pt 3, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Baba, Y.; Ishimoto, T.; Gu, X.; Zhang, J.; Nomoto, D.; Okadome, K.; Baba, H.; Qiu, P. Gut Microbiome in Gastrointestinal Cancer: A Friend or Foe? Int. J. Biol. Sci. 2022, 18, 4101–4117. [Google Scholar] [CrossRef]
- Brennan, C.A.; Clay, S.L.; Lavoie, S.L.; Bae, S.; Lang, J.K.; Fonseca-Pereira, D.; Rosinski, K.G.; Ou, N.; Glickman, J.N.; Garrett, W.S. Fusobacterium nucleatum drives a pro-inflammatory intestinal microenvironment through metabolite receptor-dependent modulation of IL-17 expression. Gut Microbes. 2021, 13, 1987780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yong, X.; Mu, D.; Ni, H.; Wang, X.; Zhang, T.; Chang, X.; He, S.; Zhou, D. Regulation of the CD8⁺ T cell and PDL1/PD1 axis in gastric cancer: Unraveling the molecular landscape. Crit. Rev. Oncol. Hematol. 2025, 212, 104750. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Sun, S.; Zhao, M.; Zhang, B.; Xiong, H. The mechanisms and clinical significance of CD8+ T cell exhaustion in anti-tumor immunity. Cancer Biol. Med. 2025, 22, 460–480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, E.M.; Gong, J.; Klempner, S.J.; Chao, J. Advances in immuno-oncology biomarkers for gastroesophageal cancer: Programmed death ligand 1, microsatellite instability, and beyond. World J. Gastroenterol. 2018, 24, 2686–2697. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, Y.; Ahn, S.; Kim, K.-M. PD-L1 as a Biomarker in Gastric Cancer Immunotherapy. J. Gastric Cancer 2025, 25, 177. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, J.; Qian, M.; Han, W.; Tian, M.; Li, Z.; Wang, Z.; He, S.; Wu, K. PD-L1 Expression and the Prognostic Significance in Gastric Cancer: A Retrospective Comparison of Three PD-L1 Antibody Clones (SP142, 28–8 and E1L3N). Diagn. Pathol. 2018, 13, 91. [Google Scholar] [CrossRef]
- Högner, A.; Moehler, M. Immunotherapy in Gastric Cancer. Curr. Oncol. 2022, 29, 1559–1574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, T.; Meng, X.; Wang, D.; Fu, W.; Li, X. Neutrophil Transcriptional Deregulation by the Periodontal Pathogen Fusobacterium Nucleatum in Gastric Cancer: A Bioinformatic Study. Dis. Markers 2022, 2022, 9584507. [Google Scholar] [CrossRef]
- Hsieh, Y.-Y.; Tung, S.-Y.; Pan, H.-Y.; Chang, T.-S.; Wei, K.-L.; Chen, W.-M.; Deng, Y.-F.; Lu, C.-K.; Lai, Y.-H.; Wu, C.-S.; et al. Fusobacterium nucleatum Colonization Is Associated with Decreased Survival of Helicobacter pylori -Positive Gastric Cancer Patients. World J. Gastroenterol. 2021, 27, 7311–7323. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Meng, X.; Ma, G.; Zhang, X.; Yin, H.; Miao, Y.; He, F. Extracellular Vesicles from Fusobacterium nucleatum: Roles in the Malignant Phenotypes of Gastric Cancer. Cell Cycle 2024, 23, 294–307. [Google Scholar] [CrossRef]
- Galasso, L.; Termite, F.; Mignini, I.; Esposto, G.; Borriello, R.; Vitale, F.; Nicoletti, A.; Paratore, M.; Ainora, M.E.; Gasbarrini, A.; et al. Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights. Cancers 2025, 17, 368. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Jia, Z.; Tang, D.; Zhang, Z.; Gao, H.; He, K.; Feng, Q. Fusobacterium nucleatum Facilitates Apoptosis, ROS Generation, and Inflammatory Cytokine Production by Activating AKT/MAPK and NF-ΚB Signaling Pathways in Human Gingival Fibroblasts. Oxid Med. Cell. Longev. 2019, 2019, 1681972. [Google Scholar] [CrossRef] [PubMed]
- Okita, Y.; Koi, M.; Takeda, K.; Ross, R.; Mukherjee, B.; Koeppe, E.; Stoffel, E.M.; Galanko, J.A.; McCoy, A.N.; Keku, T.O.; et al. Fusobacterium nucleatum infection correlates with two types of microsatellite alterations in colorectal cancer and triggers DNA damage. Gut Pathog. 2020, 12, 46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsieh, Y.-Y.; Kuo, W.-L.; Hsu, W.-T.; Tung, S.-Y.; Li, C. Fusobacterium nucleatum-Induced Tumor Mutation Burden Predicts Poor Survival of Gastric Cancer Patients. Cancers 2022, 15, 269. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, Y.; Dong, A.; Elsabahy, M.; Yang, Y.-W.; Gao, H. Emerging Strategies for Combating Fusobacterium nucleatum in Colorectal Cancer Treatment: Systematic Review, Improvements and Future Challenges. Exploration 2024, 4, 20230092. [Google Scholar] [CrossRef]
- Ohsawa, M.; Nishi, H.; Emi, M.; Yoshikawa, T.; Hamai, Y.; Ibuki, Y.; Kurokawa, T.; Hirohata, R.; Kitasaki, N.; Kawada-Matsuo, M.; et al. Impact of Fusobacterium nucleatum in the Treatment of Cancer, Including Radiotherapy and Its Future Potential in Esophageal Cancer. J. Radiat. Res. 2024, 65, i126–i134. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.; Li, T.; Jiang, Z.; Yuan, Y.; Chen, X. Fusobacterium nucleatum Promotes Megakaryocyte Maturation in Patients with Gastric Cancer via Inducing the Production of Extracellular Vesicles Containing 14-3-3ε. Infect. Immun. 2023, 91, e0010223. [Google Scholar] [CrossRef]
- Chen, W.-D.; Zhang, X.; Zhang, M.-J.; Zhang, Y.-P.; Shang, Z.-Q.; Xin, Y.-W.; Zhang, Y. Salivary Fusobacterium nucleatum Serves as a Potential Diagnostic Biomarker for Gastric Cancer. World J. Gastroenterol. 2022, 28, 4120–4132. [Google Scholar] [CrossRef]
- Aziz, S.; Rasheed, F.; Akhter, T.S.; Zahra, R.; König, S. Microbial Proteins in Stomach Biopsies Associated with Gastritis, Ulcer, and Gastric Cancer. Molecules 2022, 27, 5410. [Google Scholar] [CrossRef]
- Lu, J.; Wei, W.; Zheng, D. Fusobacterium nucleatum in Colorectal Cancer: Ally Mechanism and Targeted Therapy Strategies. Research 2025, 8, 0640. [Google Scholar] [CrossRef]
- Li, Y.; Xing, S.; Chen, F.; Li, Q.; Dou, S.; Huang, Y.; An, J.; Liu, W.; Zhang, G. Intracellular Fusobacterium nucleatum Infection Attenuates Antitumor Immunity in Esophageal Squamous Cell Carcinoma. Nat. Commun. 2023, 14, 5788. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, Y.; Liang, W.; Wong, C.C.; Qin, H.; Gao, Y.; Liang, M.; Song, L.; Zhang, Y.; Fan, M.; et al. Fusobacterium nucleatum Facilitates Anti-PD-1 Therapy in Microsatellite Stable Colorectal Cancer. Cancer Cell 2024, 42, 1729–1746.e8. [Google Scholar] [CrossRef]
- Luo, M.; Li, Q.; Gu, Q.; Zhang, C. Fusobacterium nucleatum: A Novel Regulator of Antitumor Immune Checkpoint Blockade Therapy in Colorectal Cancer. Am. J. Cancer Res. 2024, 14, 3962–3975. [Google Scholar] [CrossRef]
- Van der Merwe, M.; Van Niekerk, G.; Botha, A.; Engelbrecht, A.-M. The Onco-Immunological Implications of Fusobacterium nucleatum in Breast Cancer. Immunol. Lett. 2021, 232, 60–66. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Y.; Huang, Y.; Liu, L.; Li, S.; Huang, B.; Chen, W. Causal Relationship between Infection and Gastrointestinal Cancers: A Multivariable Mendelian Randomization Study. Chin. Clin. Oncol. 2025, 14, 31. [Google Scholar] [CrossRef]
- Nakamura, S.; Hojo, M. Diagnosis and Treatment for Gastric Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma. J. Clin. Med. 2022, 12, 120. [Google Scholar] [CrossRef]
- Khan, J.; Ullah, A.; Waheed, A.; Karki, N.R.; Adhikari, N.; Vemavarapu, L.; Belakhlef, S.; Bendjemil, S.M.; Mehdizadeh Seraj, S.; Sidhwa, F.; et al. Gastrointestinal Stromal Tumors (GIST): A Population-Based Study Using the SEER Database, Including Management and Recent Advances in Targeted Therapy. Cancers 2022, 14, 3689. [Google Scholar] [CrossRef]
- Castri, F.; Ravegnini, G.; Lodoli, C.; Fiorentino, V.; Abatini, C.; Giustiniani, M.C.; Angelini, S.; Ricci, R. Gastroblastoma in Old Age. Histopathology 2019, 75, 778–782. [Google Scholar] [CrossRef] [PubMed]
| Method | Target Gene(s)/Marker(s) | Sample Type(s) | Key Strengths |
|---|---|---|---|
| qPCR/qRT-PCR [16,17,39,77,91,98] | 16S rRNA, nusG | Gastric biopsy (fresh or FFPE) | Sensitive, quantitative, high throughput |
| Nested PCR [89,95] | nusG | Gastric biopsy (FFPE) | Enhanced specificity and sensitivity; ideal for low biomass or degraded samples |
| ddPCR [99] | nusG | Saliva | Ultra-sensitive and highly specific; absolute quantification without standard curve |
| FISH [19,78] | Fn-specific DNA probes (5′-CGCAATACAGAGTTGAGCCCTGC−3′) (5′-CTTGTAGTTCCGC(C/T)TACCTC−3′) | Gastric biopsy (FFPE) | Enables spatial localization and visualization of microbial–host interactions |
| High-definition mass spectrometry | Fn-specific proteins (atpD, FN0857, FN1974, FN0813, clpB, FN1649, FN1546) | Gastric biopsy (FFPE) | Confirms viability and metabolic activity of bacteria; proteomic-level specificity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorino, J.; Della Mura, M.; Ingravallo, G.; Cazzato, G.; Pizzimenti, C.; Zuccalà, V.; Pepe, L.; Germanà, E.; Martini, M.; Ieni, A.; et al. Fusobacterium nucleatum and Gastric Cancer: An Emerging Connection. Int. J. Mol. Sci. 2025, 26, 7915. https://doi.org/10.3390/ijms26167915
Sorino J, Della Mura M, Ingravallo G, Cazzato G, Pizzimenti C, Zuccalà V, Pepe L, Germanà E, Martini M, Ieni A, et al. Fusobacterium nucleatum and Gastric Cancer: An Emerging Connection. International Journal of Molecular Sciences. 2025; 26(16):7915. https://doi.org/10.3390/ijms26167915
Chicago/Turabian StyleSorino, Joana, Mario Della Mura, Giuseppe Ingravallo, Gerardo Cazzato, Cristina Pizzimenti, Valeria Zuccalà, Ludovica Pepe, Emanuela Germanà, Maurizio Martini, Antonio Ieni, and et al. 2025. "Fusobacterium nucleatum and Gastric Cancer: An Emerging Connection" International Journal of Molecular Sciences 26, no. 16: 7915. https://doi.org/10.3390/ijms26167915
APA StyleSorino, J., Della Mura, M., Ingravallo, G., Cazzato, G., Pizzimenti, C., Zuccalà, V., Pepe, L., Germanà, E., Martini, M., Ieni, A., & Fiorentino, V. (2025). Fusobacterium nucleatum and Gastric Cancer: An Emerging Connection. International Journal of Molecular Sciences, 26(16), 7915. https://doi.org/10.3390/ijms26167915










