Investigation of Adsorption Kinetics and Isotherms of Synthetic Dyes on Biochar Derived from Post-Coagulation Sludge
Abstract
1. Introduction
2. Results and Discussion
2.1. Activated Biochar Characteristics
2.1.1. FTIR Measurements
2.1.2. Specific Surface Area Measurement
2.1.3. Microscopic Images
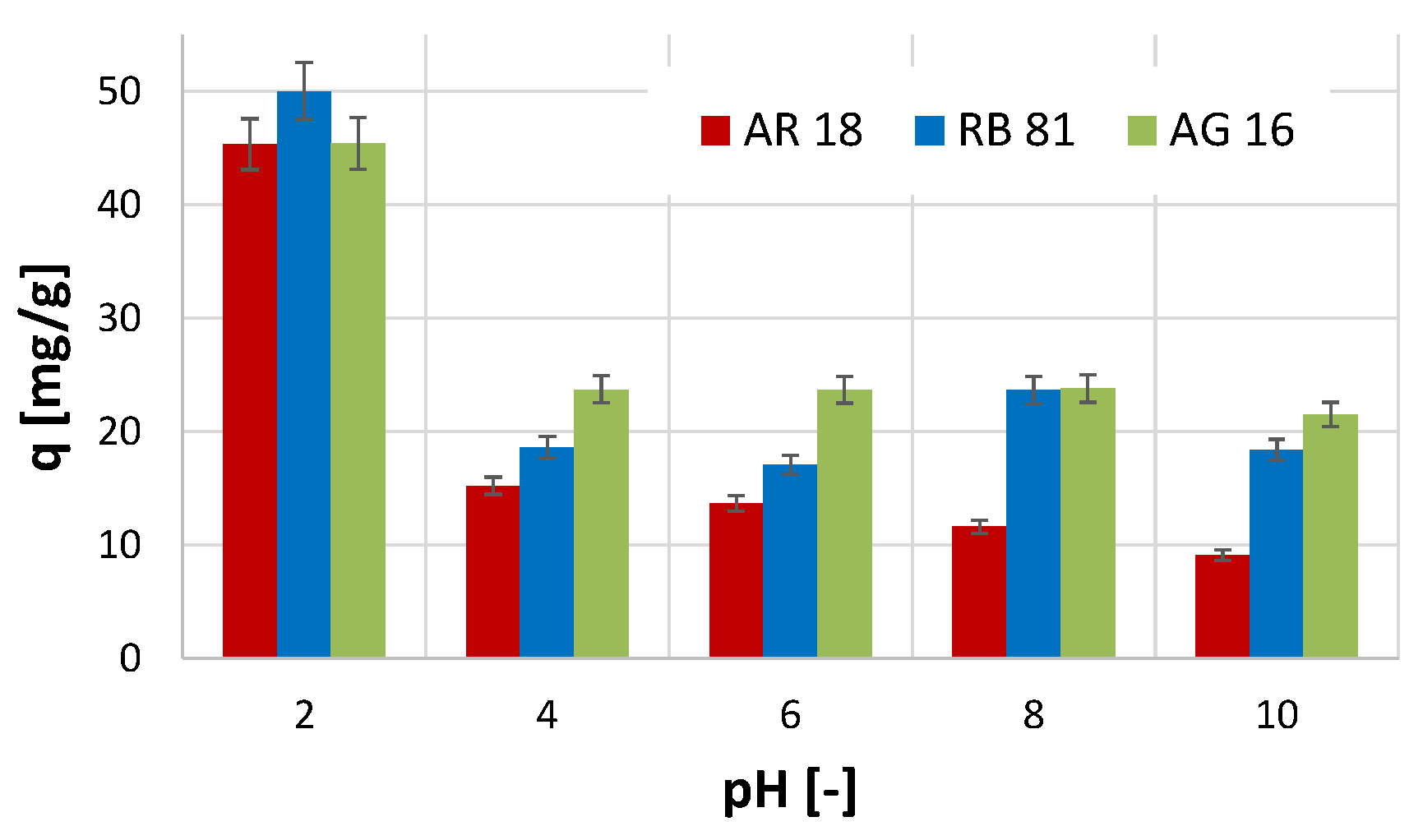
2.2. The Effect of pH
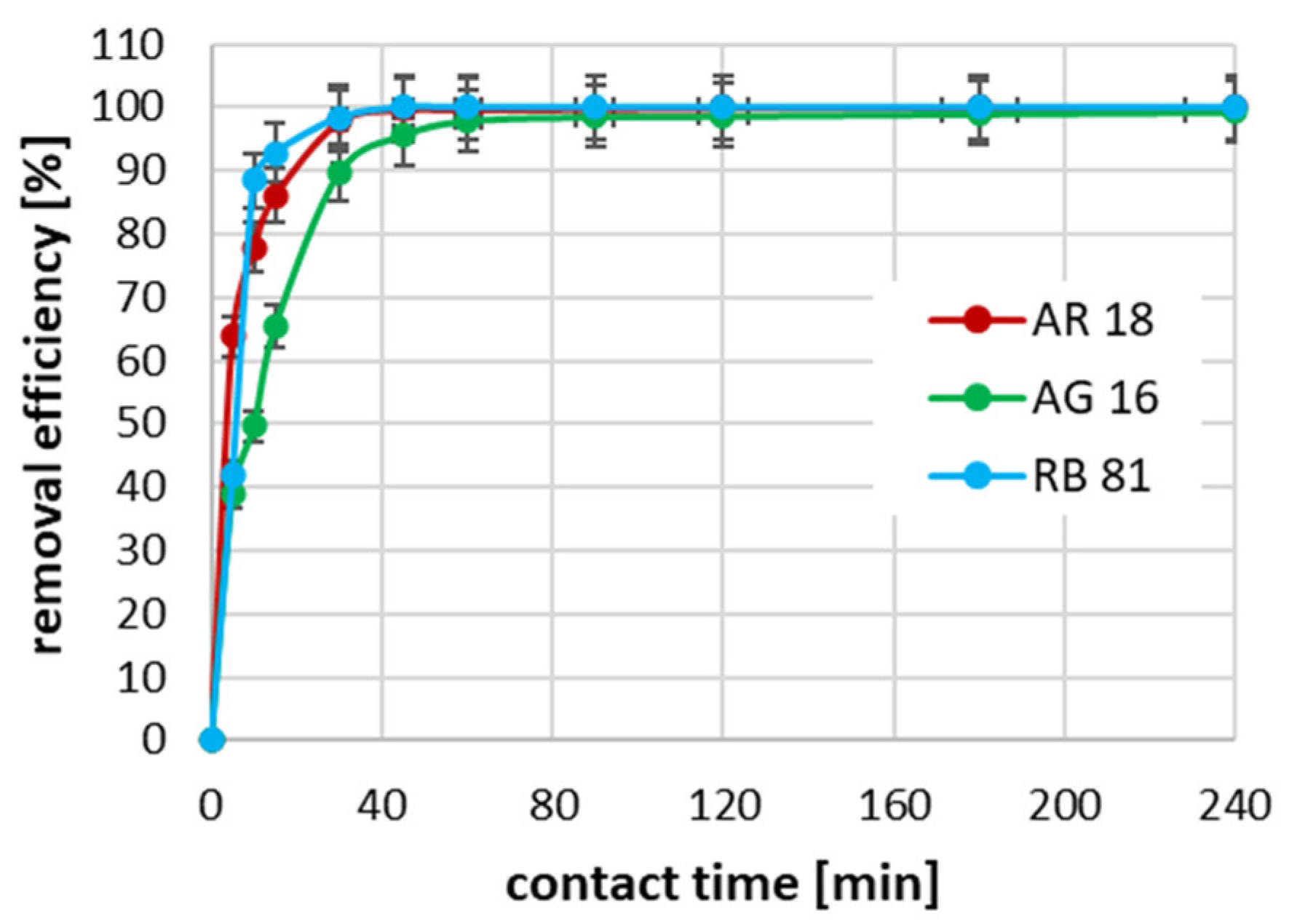
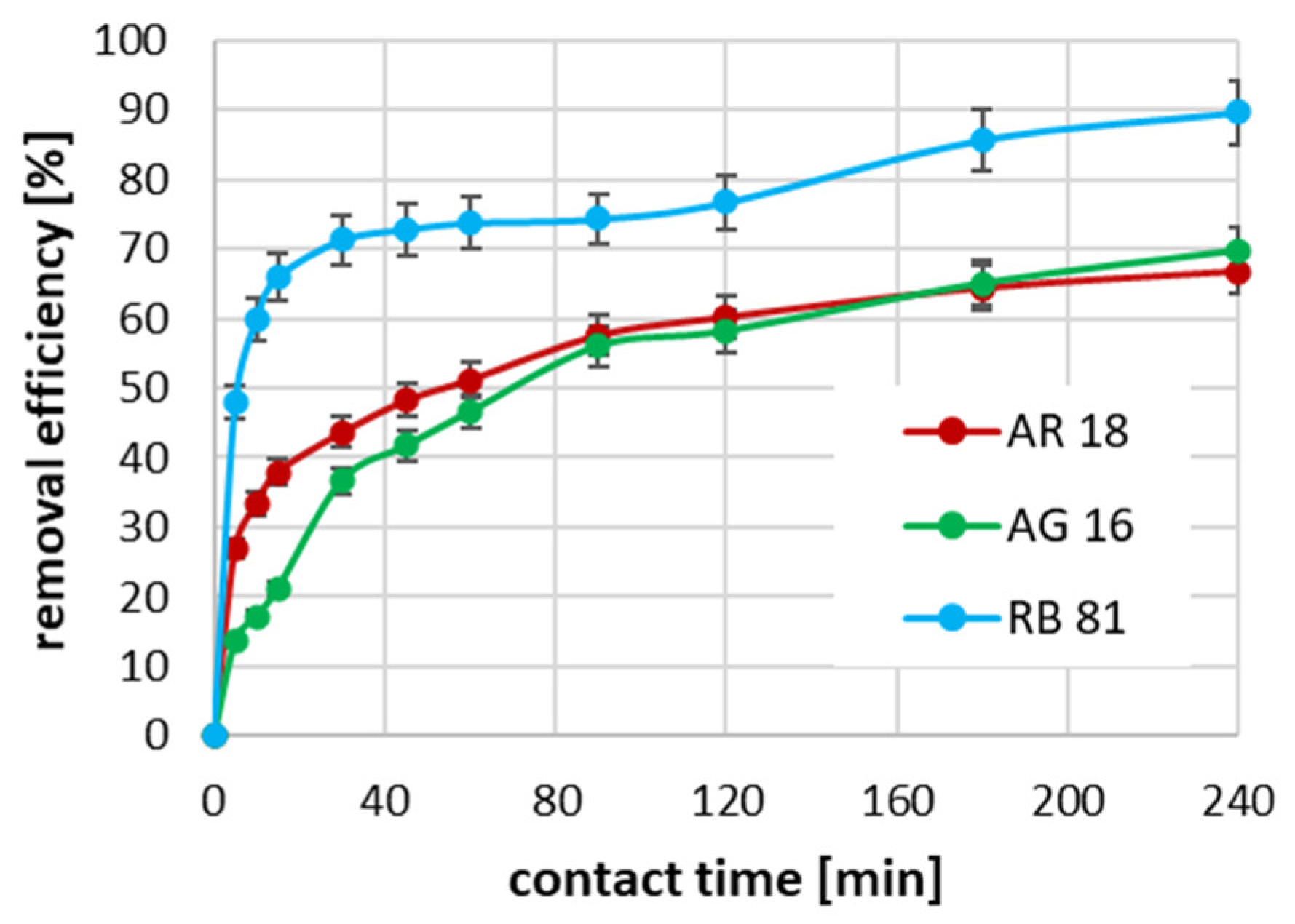
2.3. The Effect of Contact Time on Adsorption Efficiency
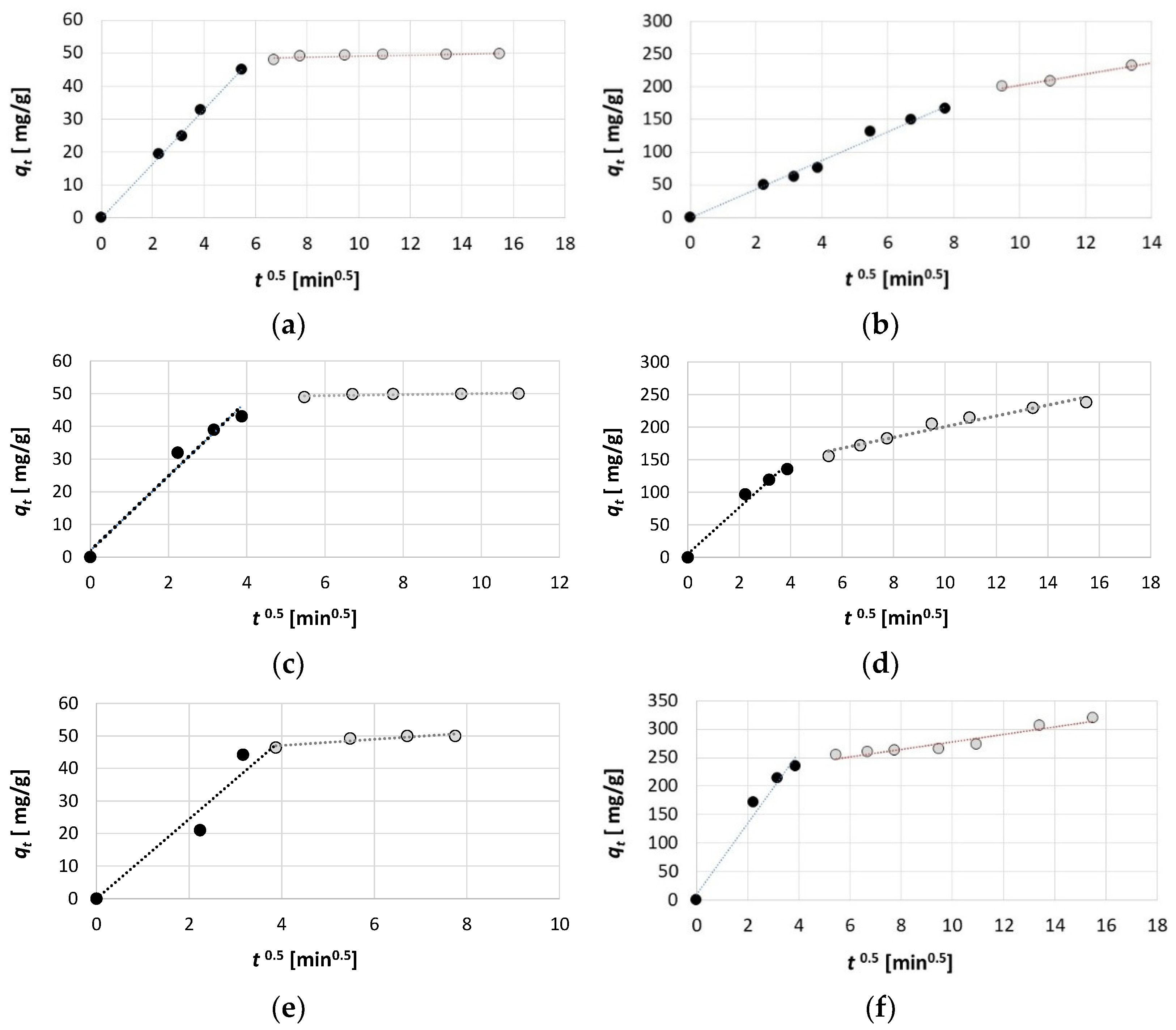
2.4. The Results of Adsorption Kinetics
2.5. The Results of Adsorption Isotherm
3. Materials and Methods
3.1. Activated Biochar Preparation
3.2. Dye Characteristics
3.3. Batch Adsorption Process
3.3.1. Effect of pH
3.3.2. Effect of Contact Time
3.3.3. Adsorption Kinetics
3.3.4. Adsorption Isotherm
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tony, M.A. Valorization of Undervalued Aluminum-Based Waterworks Sludge Waste for the Science of “The 5 Rs’ Criteria”. Appl. Water Sci. 2022, 12, 20. [Google Scholar] [CrossRef]
- Castro-Jiménez, C.C.; Saldarriaga-Molina, J.C.; García, E.F. Physical-Chemical Characterisation of an Alum-Based Water Treatment Sludge in Different Raw Water Turbidity Scenarios. Heliyon 2024, 10, e37579. [Google Scholar] [CrossRef] [PubMed]
- Ihaddaden, S.; Aberkane, D.; Boukerroui, A.; Robert, D. Removal of Methylene Blue (Basic Dye) by Coagulation-Flocculation with Biomaterials (Bentonite and Opuntia Ficus Indica). J. Water Process Eng. 2022, 49, 102952. [Google Scholar] [CrossRef]
- Shawal, N.B.M.; Razali, N.A.; Hairom, N.H.H.; Yatim, N.I.I.; Rasit, N.; Harun, M.H.C.; Kasan, N.; Hamzah, S. Parametric Study of Coagulant Recovery from Water Treatment Sludge towards Water Circular Economy. Water Sci. Technol. 2023, 88, 3142–3150. [Google Scholar] [CrossRef]
- Nayeri, D.; Mousavi, S.A. A Comprehensive Review on the Coagulant Recovery and Reuse from Drinking Water Treatment Sludge. J. Environ. Manag. 2022, 319, 115649. [Google Scholar] [CrossRef]
- Yu, B.; Li, X.; Yan, H.; Zhang, M.; Ma, J.; Lian, K. Recycling of Sludge Residue as a Coagulant for Phosphorus Removal from Aqueous Solutions. Environ. Monit. Assess. 2024, 196, 576. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho Gomes, S.; Zhou, J.L.; Li, W.; Long, G. Progress in Manufacture and Properties of Construction Materials Incorporating Water Treatment Sludge: A Review. Resour. Conserv. Recycl. 2019, 145, 148–159. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, K.; Ahad, A.; Alam, M. Characterization of Water Treatment Sludge and Its Reuse as Coagulant. J. Environ. Manag. 2016, 182, 606–611. [Google Scholar] [CrossRef]
- Zeng, H.; Yu, Y.; Wang, F.; Zhang, J.; Li, D. Arsenic(V) Removal by Granular Adsorbents Made from Water Treatment Residuals Materials and Chitosan. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124036. [Google Scholar] [CrossRef]
- Ren, B.; Lyczko, N.; Zhao, Y.; Nzihou, A. Alum Sludge as an Efficient Sorbent for Hydrogen Sulfide Removal: Experimental, Mechanisms and Modeling Studies. Chemosphere 2020, 248, 126010. [Google Scholar] [CrossRef] [PubMed]
- Belzile, N.; Chen, Y.-W. Re-Utilization of Drinking Water Treatment Residuals (DWTR): A Review Focused on the Adsorption of Inorganic and Organic Contaminants in Wastewater and Soil. Environ. Sci. Water Res. Technol. 2024, 10, 1019–1033. [Google Scholar] [CrossRef]
- Shamaki, M.; Adu-Amankwah, S.; Black, L. Reuse of UK Alum Water Treatment Sludge in Cement-Based Materials. Constr. Build. Mater. 2021, 275, 122047. [Google Scholar] [CrossRef]
- Mañosa, J.; Cerezo-Piñas, M.; Maldonado-Alameda, A.; Formosa, J.; Giro-Paloma, J.; Rosell, J.R.; Chimenos, J.M. Water Treatment Sludge as Precursor in Non-Dehydroxylated Kaolin-Based Alkali-Activated Cements. Appl. Clay Sci. 2021, 204, 106032. [Google Scholar] [CrossRef]
- Reactive Blue 81-Dye. Available online: https://www.worlddyevariety.com/reactive-dyes/reactive-blue-81.html (accessed on 28 March 2025).
- Acid Red 18-Dye. Available online: http://www.worlddyevariety.com/acid-dyes/acid-red-18.html (accessed on 28 March 2025).
- Acid Green 16-Dye. Available online: http://www.worlddyevariety.com/acid-dyes/acid-green-16.html (accessed on 28 March 2025).
- Oplatowska, M.; Donnelly, R.F.; Majithiya, R.J.; Glenn Kennedy, D.; Elliott, C.T. The Potential for Human Exposure, Direct and Indirect, to the Suspected Carcinogenic Triphenylmethane Dye Brilliant Green from Green Paper Towels. Food Chem. Toxicol. 2011, 49, 1870–1876. [Google Scholar] [CrossRef]
- Wrońska-Nofer, T.; Wiśniewska-Knypl, J.; Wyszyńska, K.; Dziubałtowska, E. Genotoxicity of Industrial Dyes under the Inductive Effect of Ethanol on Monooxygenase System in Mice. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 1997, 392, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Wrzesińska, K.; Wawrzkiewicz, M.; Szymczyk, K. Physicochemical Interactions in C.I. Acid Green 16–Lewatit S 6368 A Systems–Kinetic, Equilibrium, Auxiliaries Addition and Thermodynamic Aspects. J. Mol. Liq. 2021, 331, 115748. [Google Scholar] [CrossRef]
- Poopal, R.-K.; Ashwini, R.; Ramesh, M.; Li, B.; Ren, Z. Triphenylmethane Dye (C52H54N4O12) Is Potentially a Hazardous Substance in Edible Freshwater Fish at Trace Level: Toxicity, Hematology, Biochemistry, Antioxidants, and Molecular Docking Evaluation Study. Environ. Sci. Pollut. Res. Int. 2023, 30, 28759–28779. [Google Scholar] [CrossRef] [PubMed]
- Ardila-Leal, L.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Quevedo-Hidalgo, B.E. A Brief History of Colour, the Environmental Impact of Synthetic Dyes and Removal by Using Laccases. Molecules 2021, 26, 3813. [Google Scholar] [CrossRef]
- Lucová, M.; Hojerová, J.; Pažoureková, S.; Klimová, Z. Absorption of Triphenylmethane Dyes Brilliant Blue and Patent Blue through Intact Skin, Shaven Skin and Lingual Mucosa from Daily Life Products. Food Chem. Toxicol. 2013, 52, 19–27. [Google Scholar] [CrossRef]
- Feng, J.; Cerniglia, C.E.; Chen, H. Toxicological Significance of Azo Dye Metabolism by Human Intestinal Microbiota. Front. Biosci. (Elite Ed) 2012, 4, 568–586. [Google Scholar] [CrossRef]
- Jiang, L.-L.; Li, K.; Yan, D.-L.; Yang, M.-F.; Ma, L.; Xie, L.-Z. Toxicity Assessment of 4 Azo Dyes in Zebrafish Embryos. Int. J. Toxicol. 2020, 39, 115–123. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Luqman, M.; Muhammad, S.; Hanif, U.; Sardar, A.A.; Ali, S.; Hasnain, A.; Tufail, M.; Khan, Z.I.; Hussain, M.I.; et al. Genotoxicity of Synthetic Food Colors on Nitrogen-Fixing Bacteria in Agricultural Lands Irrigated with Wastewater of Corresponding Industries. Sustainability 2023, 15, 2897. [Google Scholar] [CrossRef]
- Golka, K.; Kopps, S.; Myslak, Z.W. Carcinogenicity of Azo Colorants: Influence of Solubility and Bioavailability. Toxicol. Lett. 2004, 151, 203–210. [Google Scholar] [CrossRef] [PubMed]
- El-Abhar, H.S.; Ali-Tammam, M.; Zahran, S.A.; Ali, A.E.; Mansour, S.M. Coloring Outside the Lines: Exploring the Dysbiotic Impact of Azo Dyes on Gut Health and Inflammation. Cell Signal. 2024, 2, 104–107. [Google Scholar] [CrossRef]
- Guo, G.; Qian, X.; Li, T.; Gao, S.; Zhang, B.; Wang, L.; Liu, K.; Gu, C.; Chen, D. A Smartphone-Integrated Nanosensor Based on N, P Co-Doped Graphene Quantum Dots for Fluorescence Detection of Acid Red 18 in Food. Curr. Appl. Phys. 2023, 56, 92–99. [Google Scholar] [CrossRef]
- Hu, H.; Xing, H.; Wang, L.; Zhang, T.; Guo, G.; Li, T.; Wang, X.; Chen, D. Smartphone-Assisted Fluorimetric and Colorimetric Dual-Recognition Sensing Platform Based on Nitrogen-Doped Carbon Dots for Visual Monitoring of Acid Red 18 in Food. Dye. Pigment. 2024, 225, 112098. [Google Scholar] [CrossRef]
- Garcia, V.S.G.; de Freitas Tallarico, L.; Rosa, J.M.; Suzuki, C.F.; Roubicek, D.A.; Nakano, E.; Borrely, S.I. Multiple Adverse Effects of Textile Effluents and Reactive Red 239 Dye to Aquatic Organisms. Environ. Sci. Pollut. Res. 2021, 28, 63202–63214. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, G.A.R.; de Lapuente, J.; Teixidó, E.; Porredón, C.; Borràs, M.; de Oliveira, D.P. Textile Dyes Induce Toxicity on Zebrafish Early Life Stages. Environ. Toxicol. Chem. 2016, 35, 429–434. [Google Scholar] [CrossRef]
- Leme, D.M.; de Oliveira, G.A.R.; Meireles, G.; Brito, L.B.; Rodrigues, L.d.B.; Palma de Oliveira, D. Eco- and Genotoxicological Assessments of Two Reactive Textile Dyes. J. Toxicol. Environ. Health A 2015, 78, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Leme, D.M.; Primo, F.L.; Gobo, G.G.; da Costa, C.R.V.; Tedesco, A.C.; de Oliveira, D.P. Genotoxicity Assessment of Reactive and Disperse Textile Dyes Using Human Dermal Equivalent (3D Cell Culture System). J. Toxicol. Environ. Health A 2015, 78, 466–480. [Google Scholar] [CrossRef]
- Muñoz, X.; Clofent, D.; Cruz, M.-J. Occupational Respiratory Allergy to Reactive Dyes. Curr. Opin. Allergy Clin. Immunol. 2023, 23, 70–75. [Google Scholar] [CrossRef]
- Alam, M.Z.; Anwar, A.H.M.F. Nutrients Adsorption onto Biochar and Alum Sludge for Treating Stormwater. J. Water Environ. Technol. 2020, 18, 132–146. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Khan, M.T.; Zubair, M.; Bilal, M.; Sajid, M. Removal of Pharmaceuticals from Water Using Sewage Sludge-Derived Biochar: A Review. Chemosphere 2022, 289, 133196. [Google Scholar] [CrossRef] [PubMed]
- Pająk, M. Alum Sludge as an Adsorbent for Inorganic and Organic Pollutants Removal from Aqueous Solutions: A Review. Int. J. Environ. Sci. Technol. 2023, 20, 10953–10972. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Silverstein-Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Fernandes, G.B.; de Oliveira Alves, R.; Marconsini, L.T.; de Oliveira, M.P.; Passos, R.R.; Profeti, D.; Profeti, L.P.R. Macadamia Nut Residue-Derived Biochar: An Eco-Friendly Solution for β-Naphthol and Reactive Black-5 Removal. Catal. Today 2025, 445, 115050. [Google Scholar] [CrossRef]
- Tran, T.K.C.; Truong, T.T.T.; Le, A.L.; Do, D.A.M.; Nguyen, T.G.; Tran, T.D.; Pham, T.D. Synthesis, Characterization of Novel Protein-Modified Rice Husk Biochar and Their Applications for Highly Adsorptive Removal Azo Dye in Water. Environ. Technol. Innov. 2025, 37, 104037. [Google Scholar] [CrossRef]
- Kavitha, G.; Subhapriya, P.; Dhanapal, V.; Dineshkumar, G.; Venkateswaran, V. Dye Removal Kinetics and Adsorption Studies of Activated Carbon Derived from the Stems of Phyllanthus reticulatus. Mater. Today Proc. 2021, 45, 7934–7938. [Google Scholar] [CrossRef]
- Bazarin, G.; Módenes, A.N.; Espinoza-Quiñones, F.R.; Borba, C.E.; Trigueros, D.E.G.; Dall’Oglio, I.C. High Removal Performance of Reactive Blue 5G Dye from Industrial Dyeing Wastewater Using Biochar in a Fixed-Bed Adsorption System: Approaches and Insights Based on Modeling, Isotherms, and Thermodynamics Study. J. Environ. Chem. Eng. 2024, 12, 111761. [Google Scholar] [CrossRef]
- Grimm, A.; Conrad, S.; Gentili, F.G.; Mikkola, J.P.; Hu, T.; Lassi, U.; Silva, L.F.O.; Lima, E.C.; dos Reis, G.S. Highly Efficient Boron/Sulfur-Modified Activated Biochar for Removal of Reactive Dyes from Water: Kinetics, Isotherms, Thermodynamics, and Regeneration Studies. Colloids Surf. A Physicochem. Eng. Asp. 2025, 713, 136486. [Google Scholar] [CrossRef]
- Rápó, E.; Tonk, S. Factors Affecting Synthetic Dye Adsorption; Desorption Studies: A Review of Results from the Last Five Years (2017–2021). Molecules 2021, 26, 5419. [Google Scholar] [CrossRef]
- Khasri, A.; Jamir, M.R.M.; Ahmad, A.A.; Ahmad, M.A. Adsorption of Remazol Brilliant Violet 5r Dye from Aqueous Solution onto Melunak and Rubberwood Sawdust Based Activated Carbon: Interaction Mechanism, Isotherm, Kinetic and Thermodynamic Properties. Desalin. Water Treat. 2021, 216, 401–411. [Google Scholar] [CrossRef]
- Alhujaily, A.; Yu, H.; Zhang, X.; Ma, F. Adsorptive Removal of Anionic Dyes from Aqueous Solutions Using Spent Mushroom Waste. Appl. Water Sci. 2020, 10, 183. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie Der Sogenannten Adsorption Geloster Stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Low, M.J.D. Kinetics of Chemisorption of Gases on Solids. Chem. Rev. 1960, 60, 267–312. [Google Scholar] [CrossRef]
- Ngulube, T.; Gumbo, J.R.; Masindi, V.; Maity, A. Calcined Magnesite as an Adsorbent for Cationic and Anionic Dyes: Characterization, Adsorption Parameters, Isotherms and Kinetics Study. Heliyon 2018, 4, e00838. [Google Scholar] [CrossRef] [PubMed]
- Keskinkan, O.; Goksu, M.Z.L.; Basibuyuk, M.; Forster, C.F. Heavy Metal Adsorption Properties of a Submerged Aquatic Plant (Ceratophyllum demersum). Bioresour. Technol. 2004, 92, 197–200. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Saxena, M.; Sharma, N.; Saxena, R. Highly Efficient and Rapid Removal of a Toxic Dye: Adsorption Kinetics, Isotherm, and Mechanism Studies on Functionalized Multiwalled Carbon Nanotubes. Surf. Interfaces 2020, 21, 100639. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, L.; Zhang, G.; Yan, T.; Yan, L.; Wei, Q.; Du, B. Removal of Pb(II) and Methylene Blue from Aqueous Solution by Magnetic Hydroxyapatite-Immobilized Oxidized Multi-Walled Carbon Nanotubes. J. Colloid Interface Sci. 2017, 494, 380–388. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, K.; Wang, F.; Sun, S.; Li, D.; Zhang, J. Preparation of Adsorbent Based on Water Treatment Residuals and Chitosan by Homogeneous Method with Freeze-Drying and Its As(V) Removal Performance. Int. J. Biol. Macromol. 2021, 184, 313–324. [Google Scholar] [CrossRef] [PubMed]
- De Marques Neto, J.O.; Bellato, C.R.; Milagres, J.L.; Pessoa, K.D.; De Alvarenga, E.S. Preparation and Evaluation of Chitosan Beads Immobilized with Iron(III) for the Removal of As(III) and As(V) from Water. J. Braz. Chem. Soc. 2013, 24, 121–132. [Google Scholar] [CrossRef]
- Gamoudi, S.; Srasra, E. Adsorption of Organic Dyes by HDPy+-Modified Clay: Effect of Molecular Structure on the Adsorption. J. Mol. Struct. 2019, 1193, 522–531. [Google Scholar] [CrossRef]
- Yan, L.; Qin, L.; Yu, H.; Li, S.; Shan, R.; Du, B. Adsorption of Acid Dyes from Aqueous Solution by CTMAB Modified Bentonite: Kinetic and Isotherm Modeling. J. Mol. Liq. 2015, 211, 1074–1081. [Google Scholar] [CrossRef]
- Khan, T.A.; Nouman, M.; Dua, D.; Khan, S.A.; Alharthi, S.S. Adsorptive Scavenging of Cationic Dyes from Aquatic Phase by H3PO4 Activated Indian Jujube (Ziziphus mauritiana) Seeds Based Activated Carbon: Isotherm, Kinetics, and Thermodynamic Study. J. Saudi Chem. Soc. 2022, 26, 101417. [Google Scholar] [CrossRef]
- Gao, Q.; Zhu, H.; Luo, W.J.; Wang, S.; Zhou, C.G. Preparation, Characterization, and Adsorption Evaluation of Chitosan-Functionalized Mesoporous Composites. Microporous Mesoporous Mater. 2014, 193, 15–26. [Google Scholar] [CrossRef]
- Dragan, E.S.; Apopei Loghin, D.F. Enhanced Sorption of Methylene Blue from Aqueous Solutions by Semi-IPN Composite Cryogels with Anionically Modified Potato Starch Entrapped in PAAm Matrix. Chem. Eng. J. 2013, 234, 211–222. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, T.; Liu, X.; Zhang, Y.; Jin, H. Adsorption Behavior of Multi-Walled Carbon Nanotubes for the Removal of Olaquindox from Aqueous Solutions. J. Hazard. Mater. 2011, 197, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Song, Z.; He, J. Diffusion-Controlled Protein Adsorption in Mesoporous Silica. J. Phys. Chem. B 2011, 115, 7744–7750. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.E.; Hu, Y.Y.; Xie, L.; Peng, K. Biosorption Behavior of Azo Dye by Inactive CMC Immobilized Aspergillus Fumigatus Beads. Bioresour. Technol. 2008, 99, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Özer, A.; Akkaya, G.; Turabik, M. The Biosorption of Acid Red 337 and Acid Blue 324 on Enteromorpha Prolifera: The Application of Nonlinear Regression Analysis to Dye Biosorption. Chem. Eng. J. 2005, 112, 181–190. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, C.; Hou, B.; Wang, Y.; Hao, C.; Wu, J. Carbon Composite Lignin-Based Adsorbents for the Adsorption of Dyes. Chemosphere 2018, 206, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem 1906, 57, 385–470. [Google Scholar]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Peers, A.M. Elovich Adsorption Kinetics and the Heterogeneous Surface. J. Catal. 1965, 4, 499–503. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. The Equation of the Characteristic Curve of Activated Charcoal. Proc. Acad. Sci. Phys. Chem. Sect. 1947, 55, 331–337. [Google Scholar]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Temkin, M.I.; Pyzhev, V. Kinetics of Ammonia Synthesis on Promoted Iron Catalyst. Acta Phys. Chim. USSR 1940, 12, 327–356. [Google Scholar]
- De Castro, M.L.F.A.; Abad, M.L.B.; Sumalinog, D.A.G.; Abarca, R.R.M.; Paoprasert, P.; de Luna, M.D.G. Adsorption of Methylene Blue Dye and Cu(II) Ions on EDTA-Modified Bentonite: Isotherm, Kinetic and Thermodynamic Studies. Sustain. Environ. Res. 2018, 28, 197–205. [Google Scholar] [CrossRef]
- Araújo, C.S.T.; Almeida, I.L.S.; Rezende, H.C.; Marcionilio, S.M.L.O.; Léon, J.J.L.; de Matos, T.N. Elucidation of Mechanism Involved in Adsorption of Pb(II) onto Lobeira Fruit (Solanum lycocarpum) Using Langmuir, Freundlich and Temkin Isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- Can, N.; Ömür, B.C.; Altındal, A. Modeling of Heavy Metal Ion Adsorption Isotherms onto Metallophthalocyanine Film. Sens. Actuators B Chem. 2016, 237, 953–961. [Google Scholar] [CrossRef]
- Quiñones, I.; Guiochon, G. Extension of a Jovanovic–Freundlich Isotherm Model to Multicomponent Adsorption on Heterogeneous Surfaces. J. Chromatogr. A 1998, 796, 15–40. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Ordonez, D.; Valencia, A.; Elhakiem, H.; Chang, N.B.; Wanielista, M.P. Adsorption Thermodynamics and Kinetics of Advanced Green Environmental Media (AGEM) for Nutrient Removal and Recovery in Agricultural Discharge and Stormwater Runoff. Environ. Pollut. 2020, 266, 115172. [Google Scholar] [CrossRef] [PubMed]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; DADA, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar] [CrossRef]
- Mortazavi, S.; Aghaei, H. Make Proper Surfaces for Immobilization of Enzymes: Immobilization of Lipase and α-Amylase on Modified Na-Sepiolite. Int. J. Biol. Macromol. 2020, 164, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhong, L.; Wang, H.; Li, J.; Cheng, H.; Ma, Q. Process Optimization of Polyphenol Oxidase Immobilization: Isotherm, Kinetic, Thermodynamic and Removal of Phenolic Compounds. Int. J. Biol. Macromol. 2021, 185, 792–803. [Google Scholar] [CrossRef]
- Khan, T.A.; Khan, E.A. Shahjahan Adsorptive Uptake of Basic Dyes from Aqueous Solution by Novel Brown Linseed Deoiled Cake Activated Carbon: Equilibrium Isotherms and Dynamics. J. Environ. Chem. Eng. 2016, 4, 3084–3095. [Google Scholar] [CrossRef]
- Ullah, S.; Bustam, M.A.; Al-Sehemi, A.G.; Assiri, M.A.; Abdul Kareem, F.A.; Mukhtar, A.; Ayoub, M.; Gonfa, G. Influence of Post-Synthetic Graphene Oxide (GO) Functionalization on the Selective CO2/CH4 Adsorption Behavior of MOF-200 at Different Temperatures; an Experimental and Adsorption Isotherms Study. Microporous Mesoporous Mater. 2020, 296, 110002. [Google Scholar] [CrossRef]
- Mihaly-Cozmuta, L.; Mihaly-Cozmuta, A.; Peter, A.; Nicula, C.; Tutu, H.; Silipas, D.; Indrea, E. Adsorption of Heavy Metal Cations by Na-Clinoptilolite: Equilibrium and Selectivity Studies. J. Environ. Manag. 2014, 137, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.N.; Alsulami, R.A.; Alhakami, M.H.; Baata, M.; Alotaibi, M.F.; Alshehri, S.; Alzahrani, S.M.; Alwafi, A.M.; Aljufareen, M.A.; Albarqi, M.M.; et al. Investigation of Adsorption Isotherms and Thermodynamic Models of Uranium Biosorption from Aqueous Solutions by Rumex Acetosella. J. Radioanal. Nucl. Chem. 2025, 334, 2251–2270. [Google Scholar] [CrossRef]
- Pieczykolan, B.; Krzyżowska, P. Removal of Helaktyn Blue F-2R via Adsorption onto Modified Post-Coagulation Sludge. Desalin. Water Treat. 2022, 275, 103–115. [Google Scholar] [CrossRef]
- Pieczykolan, B.; Solecka, B. Kinetics and Adsorption Isotherm Studies of Methylene Blue and Direct Red 81 onto Post-Coagulation Sludge. Desalin. Water Treat. 2023, 305, 201–216. [Google Scholar] [CrossRef]
- RB 81 PubChem-Topological Polar Surface Area. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/101586671#section=Computed-Properties (accessed on 20 May 2025).
- AR 18 PubChem-Topological Polar Surface Area. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/C.I.-Acid-Red-18#section=Computed-Properties (accessed on 20 May 2025).
- AG 16 PubChem-Topological Polar Surface Area. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/160685#section=Computed-Properties (accessed on 20 May 2025).
- Prasad, A.L.; Santhi, T.; Manonmani, S. Recent Developments in Preparation of Activated Carbons by Microwave: Study of Residual Errors. Arab. J. Chem. 2015, 8, 343–354. [Google Scholar] [CrossRef]


| Kinetics Model | Formula | Eq. | Ref. | Parameters |
|---|---|---|---|---|
| Pseudo-first-order | (1) | [47] | qt—the dye adsorbed per unit mass of activated biochar at each contact time [mg/g]; qe—the dye adsorbed per unit mass of activated biochar at the equilibrium state [mg/g]; k1—the constant rate of the pseudo-first-order model [1/min]; t—contact time [min]; k2—the constant rate of the pseudo-second-order model [g/(mg·min)]; a—regarded as the initial sorption rate [mg/(g·min)]; b—constant related to the extent of surface coverage; KIPD—the intraparticle diffusion rate constant [mg/(g·min0.5)]; C—a constant depicting the boundary-layer effects [mg/g]; | |
| Pseudo-second-order | (2) | [48] | ||
| Elovich | (3) | [49,50] | ||
| Intraparticle diffusion | (4) | [51,52] |
| Kinetics Model | Initial Dye Concentration | Param. | Unit | Dye | ||
|---|---|---|---|---|---|---|
| AR 18 | AG 16 | RB 81 | ||||
| Pseudo-first-order | 100 mg/dm3 | k1 | 1/min | 0.17673 | 0.07703 | 0.15205 |
| qe | mg/g | 49.3 | 49.4 | 50.1 | ||
| R2 | 0.940 | 0.988 | 0.922 | |||
| 700 mg/dm3 | k1 | 1/min | 0.07319 | 0.02434 | 0.16076 | |
| qe | mg/g | 207.3 | 232.7 | 276.4 | ||
| R2 | 0.802 | 0.979 | 0.742 | |||
| Pseudo-second-order | 100 mg/dm3 | k2 | g/(mg·min) | 0.00734 | 0.00246 | 0.00389 |
| qe | mg/g | 50.6 | 51.6 | 55.2 | ||
| R2 | 0.984 | 0.963 | 0.843 | |||
| 700 mg/dm3 | k2 | g/(mg·min) | 0.00042 | 0.00010 | 0.00087 | |
| qe | mg/g | 231.0 | 279.4 | 294.2 | ||
| R2 | 0.928 | 0.992 | 0.878 | |||
| Elovich | 100 mg/dm3 | a | mg/(g·min) | 562.47 | 19.74 | 25.55 |
| b | g/mg | 0.177 | 0.107 | 0.085 | ||
| R2 | 0.863 | 0.870 | 0.720 | |||
| 700 mg/dm3 | a | mg/(g·min) | 81.05 | 11.53 | 2034.78 | |
| b | g/mg | 0.026 | 0.014 | 0.031 | ||
| R2 | 0.996 | 0.990 | 0.933 | |||
| RB 81 | AG 16 | AR 18 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 mg/dm3 | 700 mg/dm3 | 100 mg/dm3 | 700 mg/dm3 | 100 mg/dm3 | 700 mg/dm3 | |||||||
| 1st Stage | 2nd Stage | 1st Stage | 2nd Stage | 1st Stage | 2nd Stage | 1st Stage | 2nd Stage | 1st Stage | 2nd Stage | 1st Stage | 2nd Stage | |
| KIPD | 12.217 | 0.9392 | 62.612 | 6.6381 | 8.1955 | 0.1611 | 21.849 | 8.4398 | 11.419 | 0.1566 | 35.668 | 8.2545 |
| C | 0 | 43.315 | 9.9839 | 211.75 | 0.2068 | 47.423 | 0 | 118.04 | 2.0082 | 48.448 | 5.2061 | 118.13 |
| R2 | 0.9843 | 0.846 | 0.9746 | 0.9184 | 0.9971 | 0.6659 | 0.9873 | 0.9923 | 0.9700 | 0.5707 | 0.9793 | 0.9576 |
| Isotherm Model | Formula | Eq. | Ref. |
|---|---|---|---|
| Freundlich | (5) | [67] | |
| Langmuir | (6) | [68,69] | |
| Jovanovic | (7) | [70] | |
| Dubinin–Radushkevich (D–R) | (8) | [71] | |
| (9) | |||
| (10) | |||
| SIPS | (11) | [72] | |
| Temkin | (12) | [73] | |
| (13) |
| Isotherm Model | Parameter | AR 18 | AG 16 | RB 81 |
|---|---|---|---|---|
| Freundlich | 1/n | 0.18115 | 0.31021 | 0.23449 |
| KF | 65.001 | 23.004 | 52.970 | |
| R2 | 0.970 | 0.860 | 0.941 | |
| RMSE | 10.39 | 18.83 | 22.21 | |
| Langmuir | qm | 202.07 | 204.49 | 253.54 |
| KL | 0.05928 | 0.00845 | 0.01946 | |
| R2 | 0.848 | 0.911 | 0.935 | |
| RMSE | 29.71 | 47.46 | 26.66 | |
| Jovanovic | qmax | 197.00 | 173.86 | 233.00 |
| KJ | 0.02948 | 0.00726 | 0.01173 | |
| R2 | 0.913 | 0.994 | 0.924 | |
| RMSE | 37.02 | 6.80 | 32.72 | |
| Dubinin–Radushkevich | QS | 197.31 | 171.09 | 223.01 |
| KDR | 212.006 | 1130.504 | 320.750 | |
| E | 0.05 | 0.02 | 0.04 | |
| R2 | 0.815 | 0.956 | 0.739 | |
| RMSE | 43.95 | 14.35 | 44.75 | |
| SIPS | qmS | 1594.95 | 204.49 | 402.83 |
| KS | 0.04151 | 0.00845 | 0.08526 | |
| SP | 0.200 | 1 | 0.442 | |
| R2 | 0.971 | 0.967 | 0.952 | |
| RMSE | 10.34 | 9.14 | 21.25 | |
| Temkin | KT | 17.036 | 1.941 | 240.798 |
| bT | 112.24 | 84.71 | 136.11 | |
| BT | 21.70 | 28.76 | 17.90 | |
| R2 | 0.931 | 0.889 | 0.760 | |
| RMSE | 15.89 | 21.70 | 35.90 |
| Dye | Type of Dye | Adsorbent | Adsorption Capacity [mg/g] | Ref. |
|---|---|---|---|---|
| Reactive Black 5 | Azo dye | macadamia-nut-residue-derived biochar | 2.8–3.1 | [39] |
| Reactive Orange 16 | Azo dye | activated carbon derived from the stems of Phyllanthus reticulatus | 67.93–100.5 | [41] |
| New Coccine | Azo dye | protein-modified rice husk biochar | 16–32 | [40] |
| Reactive Black 5 | Azo dye | pine tree logging residues | 1260–1363 | [43] |
| Remazol Brilliant Violet 5R | Azo dye | melunak-based activated carbon | 238.33 | [45] |
| Remazol Brilliant Violet 5R | Azo dye | rubberwood-sawdust-based activated carbon | 204.08 | [45] |
| AR 18 | AG 16 | RB 81 | |
|---|---|---|---|
| Molecular structure |  |  |  |
| Molecular weight | 604.48 | 560.62 | 808.49 |
| Molecular formula | C20H11N2Na3O10S3 | C27H25N2NaO6S2 | C25H14Cl2N7Na3O10S3 |
| C.I. number | 16255 | 44025 | 18245 |
| Colour | Red powder (red) | Green (variegated dark green powder) | Blue powder (red light blue) |
| Dye class | Single azo class | triarylmethane class | Single azo class |
| Topological polar Surface area | 242 Å2 | 137 Å2 | 304 Å2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieczykolan, B. Investigation of Adsorption Kinetics and Isotherms of Synthetic Dyes on Biochar Derived from Post-Coagulation Sludge. Int. J. Mol. Sci. 2025, 26, 7912. https://doi.org/10.3390/ijms26167912
Pieczykolan B. Investigation of Adsorption Kinetics and Isotherms of Synthetic Dyes on Biochar Derived from Post-Coagulation Sludge. International Journal of Molecular Sciences. 2025; 26(16):7912. https://doi.org/10.3390/ijms26167912
Chicago/Turabian StylePieczykolan, Barbara. 2025. "Investigation of Adsorption Kinetics and Isotherms of Synthetic Dyes on Biochar Derived from Post-Coagulation Sludge" International Journal of Molecular Sciences 26, no. 16: 7912. https://doi.org/10.3390/ijms26167912
APA StylePieczykolan, B. (2025). Investigation of Adsorption Kinetics and Isotherms of Synthetic Dyes on Biochar Derived from Post-Coagulation Sludge. International Journal of Molecular Sciences, 26(16), 7912. https://doi.org/10.3390/ijms26167912





