Unveiling the Adsorptive Potential of Natural Biopolymers for Olive Mill Wastewater Treatment: A Synergistic Approach Using RSM-BBD, Mixture Design, Kinetics, and Mechanistic Analysis
Abstract
1. Introduction
2. Results and Discussions
2.1. OMW Characterization
2.2. Characterization of the Adsorbents
2.2.1. Proximate Analysis
2.2.2. Boehm Titration
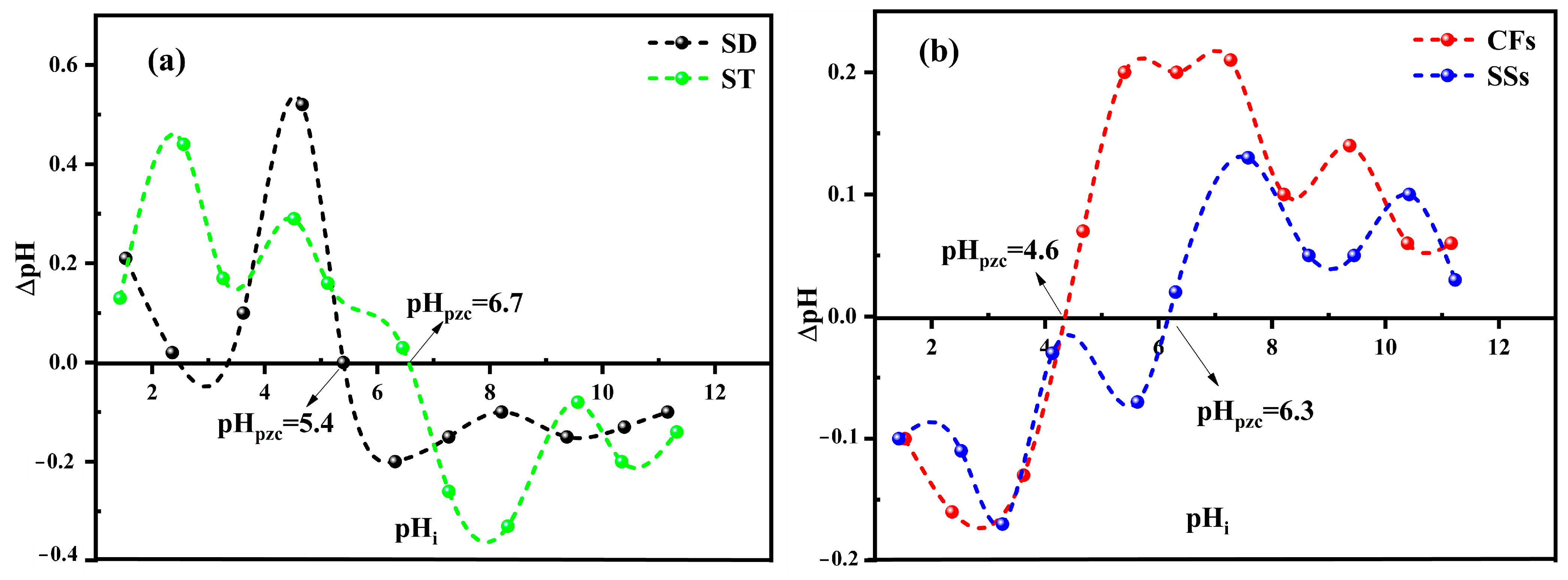
2.2.3. Isoelectric pH
2.2.4. FTIR and XRD
2.2.5. BET
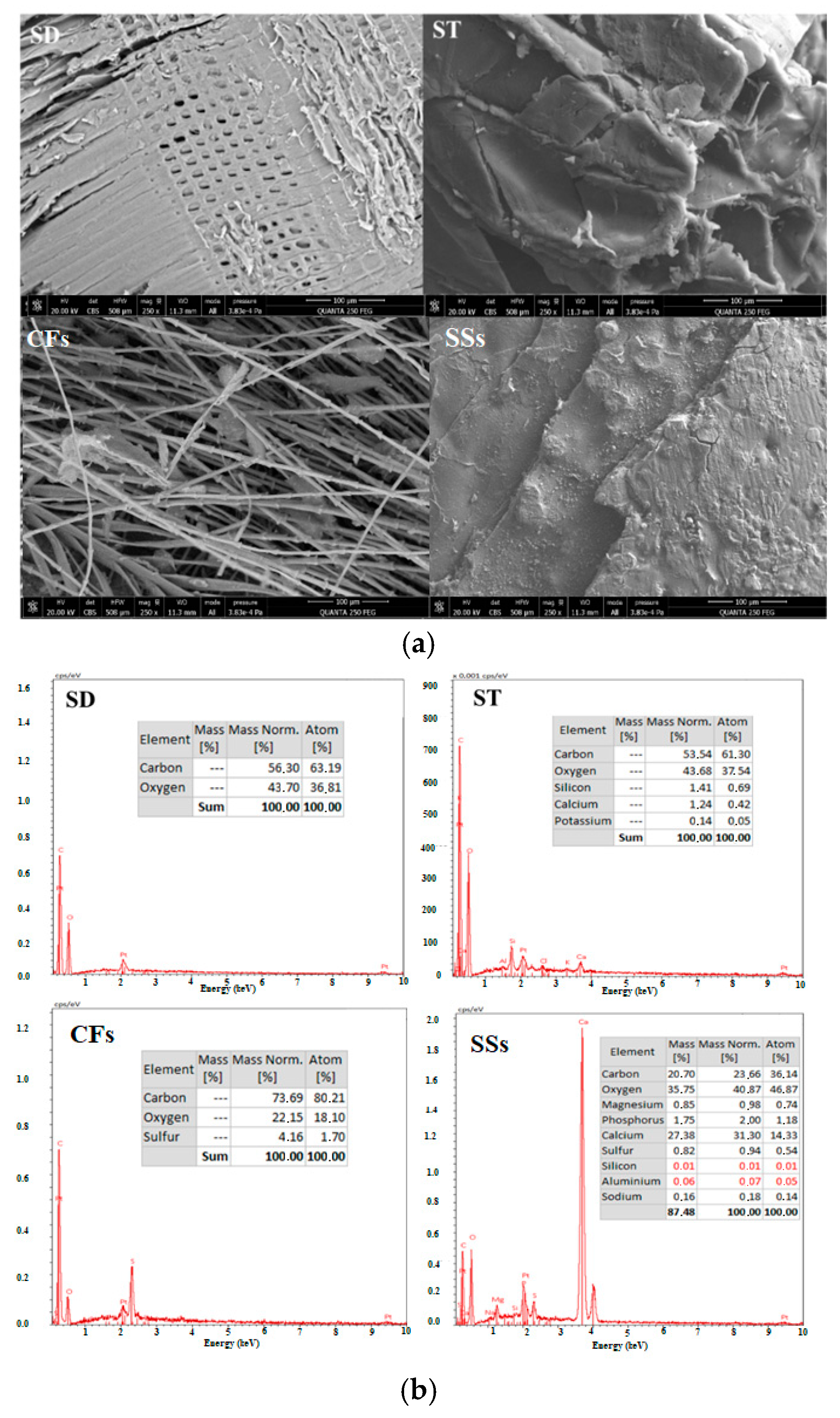
2.2.6. SEM and EDX
2.3. COD Removal
2.3.1. BBD Experimental Results
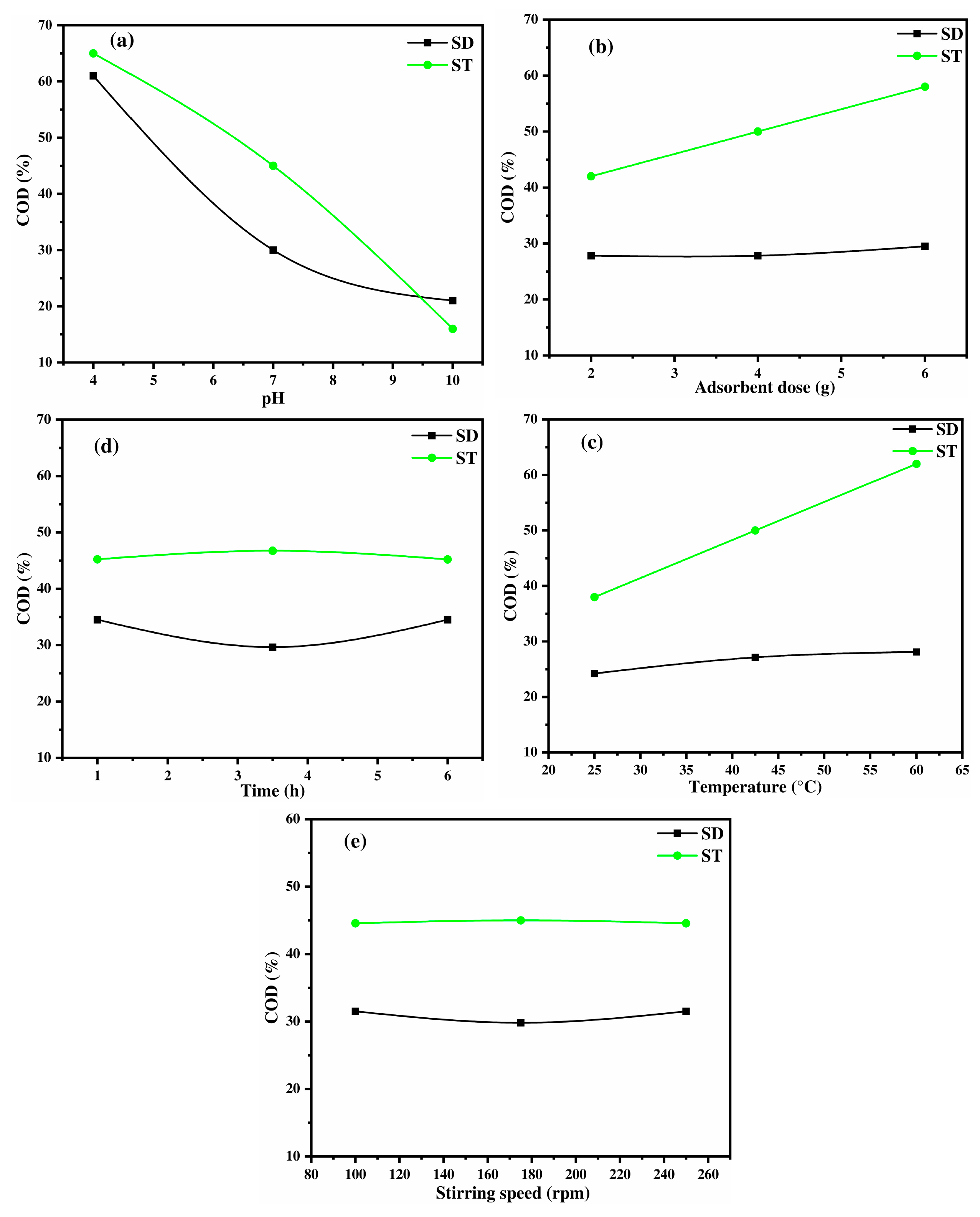
2.3.2. Lignocellulose Materials: Sawdust and Straw
2.3.3. Keratin-Based Material: Chicken Feathers
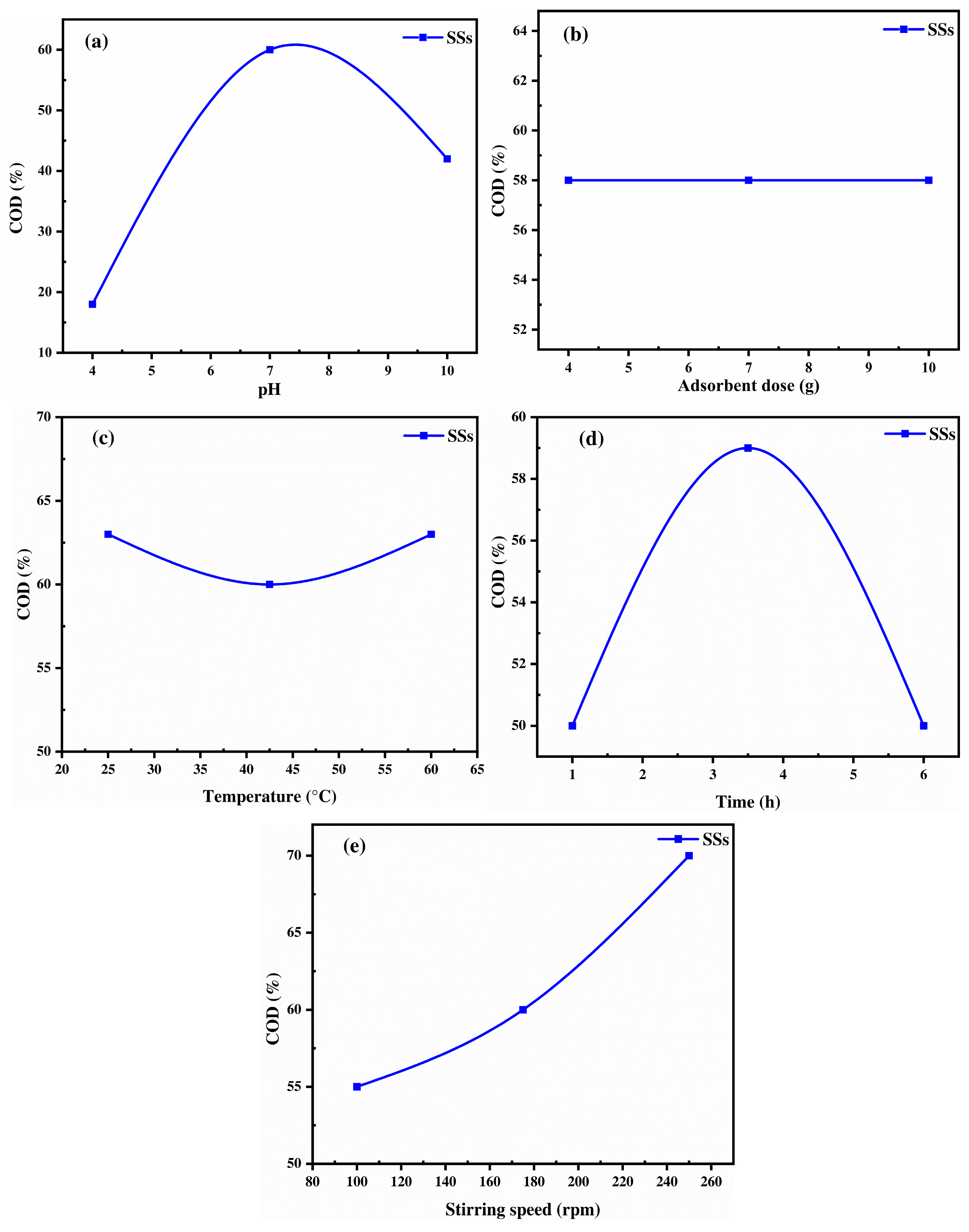
2.3.4. Chitin-Biobased Polymer: Shrimp Shells
2.4. COD Removal Optimization by RSM
2.5. Synergetic Effect of the Adsorbents for COD Removal
2.5.1. Statistical Analysis
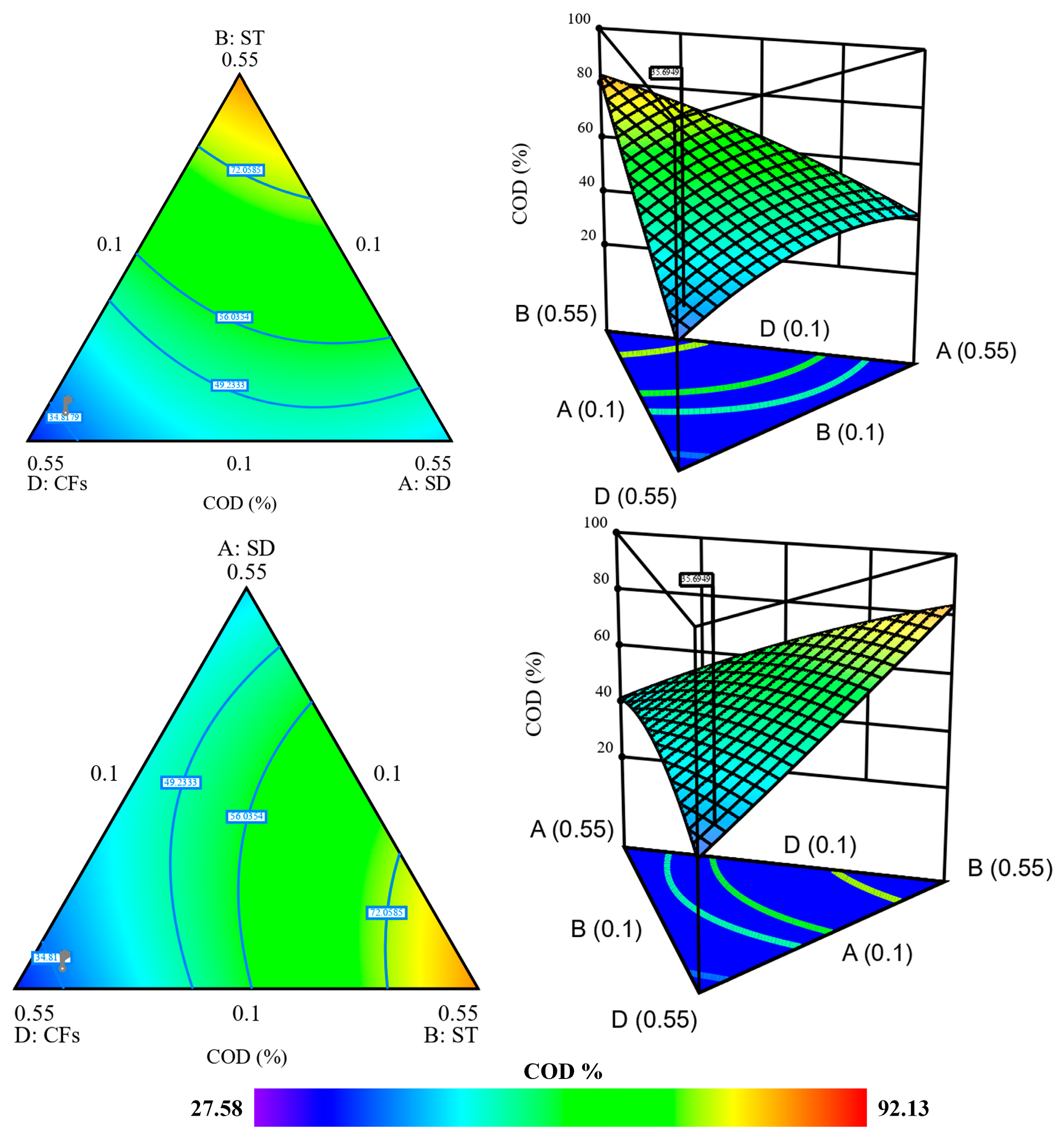
2.5.2. Contour Plot and 3D Response Surface Analysis of COD Removal by Mixture Design
2.5.3. Optimization of the Mixture Design for the Synergetic Effect of the Adsorbents
2.6. Isotherm and Kinetics for COD Removal
2.7. Thermodynamic Study of COD and Polyphenols
2.8. Adsorption Mechanisms
2.9. Desorption Analyis
2.10. Process Scalability and Cost
3. Materials and Methods
3.1. Olive Mill Wastewater (OMW) Sample Collection and Characterization
3.2. Collection and Preparation of Raw Adsorbents
3.3. Adsorbent Characterization
3.4. Adsorption Experiments
3.4.1. Batch Adsorption
3.4.2. Optimization and Modeling
3.4.3. Adsorption Isotherms, Kinetics, and Thermodynamics
3.5. Desorption
3.6. Statistical and Data Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, T.; Ren, X.; Soundari, P.G.; Chen, H.; Awasthi, S.K.; Varjani, S.; Pandey, A.; Zhang, Z.; Awasthi, M.K. Waste Biorefinery Development Toward Circular Bioeconomy with a Focus on Life-Cycle Assessment. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 199–230. ISBN 978-0-12-821878-5. [Google Scholar]
- Rout, P.R.; Goel, M.; Pandey, D.S.; Briggs, C.; Sundramurthy, V.P.; Halder, N.; Mohanty, A.; Mukherjee, S.; Varjani, S. Technological Advancements in Valorisation of Industrial Effluents Employing Hydrothermal Liquefaction of Biomass: Strategic Innovations, Barriers and Perspectives. Environ. Pollut. 2023, 316, 120667. [Google Scholar] [CrossRef]
- Shabir, S.; Ilyas, N.; Saeed, M.; Bibi, F.; Sayyed, R.Z.; Almalki, W.H. Treatment Technologies for Olive Mill Wastewater with Impacts on Plants. Environ. Res. 2023, 216. [Google Scholar] [CrossRef]
- Moussaid, D.; Moumnani, F.T.; Tanji, K.; Tayibi, S.; Benabdallah, A.C.; Kherbeche, A.; Barakat, A.; Beniazza, R. Efficient Polyphenols Compounds Photodegradation from Olive Mill Wastewater Using Solar-Light-Driven β-Cu2V2O7 and Cu3V2O8 Nanoparticles. J. Water Process Eng. 2025, 71, 107326. [Google Scholar] [CrossRef]
- Fernandes, M.J.; Gomes, J.; Carvalho, P.; Martins, R.C.; Domingues, E. Removal and Recovery of Phenolic Compounds from OMW by a Cationic Resin. Chem. Eng. Sci. 2024, 291, 119925. [Google Scholar] [CrossRef]
- El Haimer, Y.; Rich, A.; Gagnière, E.; Cogné, C.; Tahiri, S.; Mangin, D.; Siniti, M.; Yañez, E.H. Cryoconcentration of Olive Mill Wastewater for Application in Leather Tanning Process: An Alternative End to Protect the Environment and Save Raw Materials Consumption. Environ. Sci. Pollut. Res. 2025, 32, 6532–6549. [Google Scholar] [CrossRef] [PubMed]
- Al Bawab, A.F.; Abu-Dalo, M.A.; Kanaan, H.; Al-Rawashdeh, N.; Odeh, F. Removal of Phenolic Compounds from Olive Mill Wastewater (OMW) by Tailoring the Surface of Activated Carbon under Acidic and Basic Conditions. Water Sci. Technol. 2025, 91, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, M.D.; Sabatino, R.; Borgomaneiro, G.; Cesare, A.D.; Rizzo, L. Bacterial Community Dynamics in a Biofilm-Based Process after Electro-Assisted Fenton Pre-Treatment of Real Olive Mill Wastewater. Bioresour. Technol. 2025, 419, 132095. [Google Scholar] [CrossRef] [PubMed]
- Faggiano, A.; De Carluccio, M.; Fiorentino, A.; Ricciardi, M.; Cucciniello, R.; Proto, A.; Rizzo, L. Photo-Fenton like Process as Polishing Step of Biologically Co-Treated Olive Mill Wastewater for Phenols Removal. Sep. Purif. Technol. 2023, 305, 122525. [Google Scholar] [CrossRef]
- Garcia-Castello, E.; Cassano, A.; Criscuoli, A.; Conidi, C.; Drioli, E. Recovery and Concentration of Polyphenols from Olive Mill Wastewaters by Integrated Membrane System. Water Res. 2010, 44, 3883–3892. [Google Scholar] [CrossRef]
- Ben Hamou, A.; Enneiymy, M.; Farsad, S.; Amjlef, A.; Chaoui, A.; Nouj, N.; Majdoub, A.; Jada, A.; Ez-zahery, M.; El Alem, N. Novel Chemically Reduced Cobalt-Doped g-C3 N4 (CoCN- x ) as a Highly Heterogeneous Catalyst for the Super-Degradation of Organic Dyes via Peroxymonosulfate Activation. Mater. Adv. 2024, 5, 1960–1976. [Google Scholar] [CrossRef]
- Nassar, N.N.; Arar, L.A.; Marei, N.N.; Abu Ghanim, M.M.; Dwekat, M.S.; Sawalha, S.H. Treatment of Olive Mill Based Wastewater by Means of Magnetic Nanoparticles: Decolourization, Dephenolization and COD Removal. Environ. Nanotechnol. Monit. Manag. 2014, 1–2, 14–23. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Vavouraki, A.I.; Kornaros, M.E.; Paraskeva, C.A. Purification of Olive Mill Wastewater Phenols through Membrane Filtration and Resin Adsorption/Desorption. J. Hazard. Mater. 2015, 285, 69–76. [Google Scholar] [CrossRef]
- Rabichi, I.; Yaacoubi, F.E.; Sekkouri, C.; Ezzahi, K.; Ennaciri, K.; Fels, L.E.; Mohamed, H.; Baçaoui, A.; Yaacoubi, A. Optimizing Biochar Preparation for Eco-Friendly Adsorption of Polyphenols and Organic Compounds in Pilot-Scale: An Application of Doehlert Designs. Biomass Convers. Biorefinery 2024. [Google Scholar] [CrossRef]
- Akaniro, I.R.; Wang, G.; Wang, P.; Zhang, R.; Xue, W.; Ye, J.; Wong, J.W.C.; Zhao, J. pH-Tuneable Simultaneous and Selective Dye Wastewater Remediation with Digestate-Derived Biochar: Adsorption Behaviour, Mechanistic Insights and Potential Application. Green Chem. Eng. 2025, 6, 344–356. [Google Scholar] [CrossRef]
- Lua, A.C. A Comparative Study of the Pore Characteristics and Phenol Adsorption Performance of Activated Carbons Prepared from Oil-Palm Shell Wastes by Steam and Combined Steam-Chemical Activation. Green Chem. Eng. 2024, 5, 85–96. [Google Scholar] [CrossRef]
- Achak, M.; Hafidi, A.; Ouazzani, N.; Sayadi, S.; Mandi, L. Low Cost Biosorbent “Banana Peel” for the Removal of Phenolic Compounds from Olive Mill Wastewater: Kinetic and Equilibrium Studies. J. Hazard. Mater. 2009, 166, 117–125. [Google Scholar] [CrossRef]
- Achak, M.; Hafidi, A.; Mandi, L.; Ouazzani, N. Removal of Phenolic Compounds from Olive Mill Wastewater by Adsorption onto Wheat Bran. Desalination Water Treat. 2014, 52, 2875–2885. [Google Scholar] [CrossRef]
- Elayadi, F.; Achak, M.; Boumya, W.; Barka, N.; Lamy, E.; El Adlouni, C. Olive Mill Wastewater Treatment Using Natural Adsorbents: Phytotoxicity on Durum Wheat (Triticum turgidum L. var. durum) and White Bean (Phaseolus vulgaris L.) Seed Germination. Environ. Sci. Pollut. Res. 2023, 30, 109481–109499. [Google Scholar] [CrossRef]
- El Abbadi, S.; El Moustansiri, H.; Douma, M.; Bouazizi, A.; Arfoy, B.; Calvo, J.I.; Tijani, N. Enhancing the Performance of Alumina-Pillared Clay for Phenol Removal from Water Solutions and Polyphenol Removal from Olive Mill Wastewater: Characterization, Kinetics, Adsorption Performance, and Mechanism. J. Water Process Eng. 2024, 63, 105432. [Google Scholar] [CrossRef]
- Banat, F.A.; Al-Asheh, S. Biosorption of Phenol by Chicken Feathers. Environ. Eng. Policy 1999, 2, 85–90. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Bansal, M.; Garima; Wilson, K.; Gupta, S.; Dhanawat, M. Lignocellulose Biosorbents: Unlocking the Potential for Sustainable Environmental Cleanup. Int. J. Biol. Macromol. 2025, 294, 139497. [Google Scholar] [CrossRef]
- Fotodimas, I.; Ioannou, Z.; Kanlis, G.; Sarris, D.; Athanasekou, C. Sustainable Management of Shrimp Waste to Produce High-Added Value Carbonaceous Adsorbents. Sustainability 2024, 16, 10305. [Google Scholar] [CrossRef]
- Zhang, R.; Somasundaran, P. Advances in Adsorption of Surfactants and Their Mixtures at Solid/Solution Interfaces. Adv. Colloid Interface Sci. 2006, 123–126, 213–229. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive Mill Wastes: Biochemical Characterizations and Valorization Strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Azbar, N.; Bayram, A.; Filibeli, A.; Muezzinoglu, A.; Sengul, F.; Ozer, A. A Review of Waste Management Options in Olive Oil Production. Crit. Rev. Environ. Sci. Technol. 2004, 34, 209–247. [Google Scholar] [CrossRef]
- El-Gohary, F.A.; Badawy, M.I.; El-Khateeb, M.A.; El-Kalliny, A.S. Integrated Treatment of Olive Mill Wastewater (OMW) by the Combination of Fenton’s Reaction and Anaerobic Treatment. J. Hazard. Mater. 2009, 162, 1536–1541. [Google Scholar] [CrossRef]
- 28. El Herradi, E.; Boujaber, G.; Naman, M.; Laamyem, A.; El Adlouni, C.; Naman, F. Treatment of Oil Mill Wastewaters by Infiltration-Percolation on Two Types of Filters Based on Soil, Sand and Fly Ash. J. Mater. Environ. Sci. 2016, 7, 820–827. [Google Scholar]
- Mekki, H.; Anderson, M.; Benzina, M.; Ammar, E. Valorization of Olive Mill Wastewater by Its Incorporation in Building Bricks. J. Hazard. Mater. 2008, 158, 308–315. [Google Scholar] [CrossRef]
- Di Mauro, M.D.; Giardina, R.C.; Fava, G.; Mirabella, E.F.; Acquaviva, R.; Renis, M.; D’Antona, N. Polyphenolic Profile and Antioxidant Activity of Olive Mill Wastewater from Two Sicilian Olive Cultivars: Cerasuola and Nocellara Etnea. Eur. Food Res. Technol. 2017, 243, 1895–1903. [Google Scholar] [CrossRef]
- Aly, A.A.; Hasan, Y.N.Y.; Al-Farraj, A.S. Olive Mill Wastewater Treatment Using a Simple Zeolite-Based Low-Cost Method. J. Environ. Manage. 2014, 145, 341–348. [Google Scholar] [CrossRef]
- Niaounakis, M.; Halvadakis, C.P. Olive Processing Waste Management: Literature Review and Patent Survey, 2nd ed.; Niaounakis, M., Halvadakis, C.P., Eds.; Waste Management Series; Elsevier: Amsterdam, The Netherlands; London, UK, 2006; ISBN 978-0-08-044851-0. [Google Scholar]
- Venieri, D.; Rouvalis, A.; Iliopoulou-Georgudaki, J. Microbial and Toxic Evaluation of Raw and Treated Olive Oil Mill Wastewaters. J. Chem. Technol. Biotechnol. 2010, 85, 1380–1388. [Google Scholar] [CrossRef]
- Akkam, Y.; Zaitoun, M.; Aljarrah, I.; Jaradat, A.; Hmedat, A.; Alhmoud, H.; Rababah, T.; Almajwal, A.; Al-Rayyan, N. Effective Detoxification of Olive Mill Wastewater Using Multi-Step Surfactant-Based Treatment: Assessment of Environmental and Health Impact. Molecules 2024, 29, 4284. [Google Scholar] [CrossRef]
- Malvis, A.; Hodaifa, G.; Halioui, M.; Seyedsalehi, M.; Sánchez, S. Integrated Process for Olive Oil Mill Wastewater Treatment and Its Revalorization through the Generation of High Added Value Algal Biomass. Water Res. 2019, 151, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An Overview on Olive Mill Wastes and Their Valorisation Methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef]
- Vaz, T.; Quina, M.M.J.; Martins, R.C.; Gomes, J. Olive Mill Wastewater Treatment Strategies to Obtain Quality Water for Irrigation: A Review. Sci. Total Environ. 2024, 931, 172676. [Google Scholar] [CrossRef]
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Physicochemical Characterization of Chitin and Chitosan from Crab Shells. Carbohydr. Polym. 2009, 75, 15–21. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jain, S.S.; Jadhav, A.J.; Pinjari, D.V. Valorization of Keratin Based Waste. Process Saf. Environ. Prot. 2018, 115, 85–98. [Google Scholar] [CrossRef]
- Stjepanović, M.; Velić, N.; Lončarić, A.; Gašo-Sokač, D.; Bušić, V.; Habuda-Stanić, M. Adsorptive Removal of Nitrate from Wastewater Using Modified Lignocellulosic Waste Material: FTIR Interpretation Ref1. J. Mol. Liq. 2019, 285, 535–544. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Nguyen, T.V.; Chao, H.-P. Insight into the Adsorption Mechanism of Cationic Dye onto Biosorbents Derived from Agricultural Wastes: FTIR AC. Chem. Eng. Commun. 2017, 204, 1020–1036. [Google Scholar] [CrossRef]
- Moussaoui, F.; El Ouadrhiri, F.; Saleh, E.-A.M.; El Bourachdi, S.; Althomali, R.H.; Kassem, A.F.; Adachi, A.; Husain, K.; Hassan, I.; Lahkimi, A. Enhancing Hydrochar Production and Proprieties from Biogenic Waste: Merging Response Surface Methodology and Machine Learning for Organic Pollutant Remediation. J. Saudi Chem. Soc. 2024, 28, 101920. [Google Scholar] [CrossRef]
- Martins, A.F.; Cardoso, A.D.L.; Stahl, J.A.; Diniz, J. Low Temperature Conversion of Rice Husks, Eucalyptus Sawdust and Peach Stones for the Production of Carbon-like Adsorbent. Bioresour. Technol. 2007, 98, 1095–1100. [Google Scholar] [CrossRef]
- Pimentel, C.H.; Díaz-Fernández, L.; Gómez-Díaz, D.; Freire, M.S.; González-Álvarez, J. Separation of CO2 Using Biochar and KOH and ZnCl2 Activated Carbons Derived from Pine Sawdust. J. Environ. Chem. Eng. 2023, 11, 111378. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97. [Google Scholar] [CrossRef]
- Misran, E.; Pratama, W.; Napitupulu, K.I.K.; Supardan, M.D.; Iryani, D.A.; Pramananda, V. Ultrasonic-Assisted Adsorption of Methylene Blue Using Shrimp Shells as a Low-Cost Adsorbent: Evaluation on the Adsorption Isotherm, Kinetics, and Thermodynamics. South Afr. J. Chem. Eng. 2025, 52, 111–126. [Google Scholar] [CrossRef]
- Gbenebor, O.P.; Adeosun, S.O.; Lawal, G.I.; Jun, S. Role of CaCO3 in the Physicochemical Properties of Crustacean-Sourced Structural Polysaccharides. Mater. Chem. Phys. 2016, 184, 203–209. [Google Scholar] [CrossRef]
- Goudarzi, G.; Dadashian, F.; Vatanara, A.; Sepehrizadeh, Z. Optimization of Keratin Sponge Preparation Conditions for Hemostatic Application Using Response Surface Methodology (RSM). J. Polym. Environ. 2024, 32, 1135–1149. [Google Scholar] [CrossRef]
- Giraldo, L.; Moreno-Piraján, J.C. Exploring the Use of Rachis of Chicken Feathers for Hydrogen Storage. J. Anal. Appl. Pyrolysis 2013, 104, 243–248. [Google Scholar] [CrossRef]
- Mittal, A. Use of Hen Feathers as Potential Adsorbent for the Removal of a Hazardous Dye, Brilliant Blue FCF, from Wastewater. J. Hazard. Mater. 2006, 128, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Lgaz, H.; Tiwari, P.; Chung, I.-M.; Ji, G.; Prakash, R. Experimental and Theoretical Investigation of Aqueous and Methanolic Extracts of Prunus Dulcis Peels as Green Corrosion Inhibitors of Mild Steel in Aggressive Chloride Media. J. Mol. Liq. 2019, 276, 347–361. [Google Scholar] [CrossRef]
- Chen, X.; Xu, R.; Xu, Y.; Hu, H.; Pan, S.; Pan, H. Natural Adsorbent Based on Sawdust for Removing Impurities in Waste Lubricants. J. Hazard. Mater. 2018, 350, 38–45. [Google Scholar] [CrossRef]
- Phitsuwan, P.; Permsriburasuk, C.; Baramee, S.; Teeravivattanakit, T.; Ratanakhanokchai, K. Structural Analysis of Alkaline Pretreated Rice Straw for Ethanol Production. Int. J. Polym. Sci. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Wani, A.K.; Akhtar, N.; Mir, T.U.G.; Rahayu, F.; Suhara, C.; Anjli, A.; Chopra, C.; Singh, R.; Prakash, A.; El Messaoudi, N.; et al. Eco-Friendly and Safe Alternatives for the Valorization of Shrimp Farming Waste. Environ. Sci. Pollut. Res. 2023, 31, 38960–38989. [Google Scholar] [CrossRef]
- Al Bsoul, A.; Hailat, M.; Abdelhay, A.; Tawalbeh, M.; Jum’h, I.; Bani-Melhem, K. Treatment of Olive Mill Effluent by Adsorption on Titanium Oxide Nanoparticles. Sci. Total Environ. 2019, 688, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Bargaoui, M.; Jellali, S.; Azzaz, A.A.; Jeguirim, M.; Akrout, H. Optimization of Hybrid Treatment of Olive Mill Wastewaters through Impregnation onto Raw Cypress Sawdust and Electrocoagulation. Environ. Sci. Pollut. Res. 2021, 28, 24470–24485. [Google Scholar] [CrossRef]
- Elayadi, F.; Boumya, W.; Achak, M.; Chhiti, Y.; Alaoui, F.E.M.; Barka, N.; Adlouni, C.E. Experimental and Modeling Studies of the Removal of Phenolic Compounds from Olive Mill Wastewater by Adsorption on Sugarcane Bagasse. Environ. Chall. 2021, 4, 100184. [Google Scholar] [CrossRef]
- Papaoikonomou, L.; Labanaris, K.; Kaderides, K.; Goula, A.M. Adsorption–Desorption of Phenolic Compounds from Olive Mill Wastewater Using a Novel Low-Cost Biosorbent. Environ. Sci. Pollut. Res. 2021, 28, 24230–24244. [Google Scholar] [CrossRef]
- Djeziri, S.; Taleb, Z.; Djellouli, H. Kinetic Study of Adsorption of Phenolic Compounds from Olive Oil Mill Wastewater on Activated Carbon. Acta Period. Technol. 2023, 197–208. [Google Scholar] [CrossRef]
- Elayadi, F.; Achak, M.; Boumya, W.; Elamraoui, S.; Barka, N.; Lamy, E.; Beniich, N.; El Adlouni, C. Factorial Design Statistical Analysis and Optimization of the Adsorptive Removal of COD from Olive Mill Wastewater Using Sugarcane Bagasse as a Low-Cost Adsorbent. Water 2023, 15, 1630. [Google Scholar] [CrossRef]
- Boumediene, F.; Abdallah, O.; Bensebia, B.; Slovák, V. Valorization of Oak and Casuarina Fruit Shells to Reduce the Rate of Copper and Methylene Blue. Int. J. Environ. Sci. Technol. 2022, 19, 7141–7150. [Google Scholar] [CrossRef]
- Song, C.; Gao, C.; Fatehi, P.; Wang, S.; Jiang, C.; Kong, F. Influence of Structure and Functional Group of Modified Kraft Lignin on Adsorption Behavior of Dye. Int. J. Biol. Macromol. 2023, 240, 124368. [Google Scholar] [CrossRef]
- Yao, L.; Yang, H.; Yoo, C.G.; Chen, C.; Meng, X.; Dai, J.; Yang, C.; Yu, J.; Ragauskas, A.J.; Chen, X. A Mechanistic Study of Cellulase Adsorption onto Lignin. Green Chem. 2021, 23, 333–339. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Wang, J.; Xu, H.; Liu, Y.; Zhang, Z.; Li, Y.; Zhang, Y. Adsorption of Cadmium and Lead Ions by Phosphoric Acid-Modified Biochar Generated from Chicken Feather: Selective Adsorption and Influence of Dissolved Organic Matter. Bioresour. Technol. 2019, 292, 121948. [Google Scholar] [CrossRef]
- Cheong, C.W.; Lee, Y.S.; Ahmad, S.A.; Ooi, P.T.; Phang, L.Y. Chicken Feather Valorization by Thermal Alkaline Pretreatment Followed by Enzymatic Hydrolysis for Protein-Rich Hydrolysate Production. Waste Manag. 2018, 79, 658–666. [Google Scholar] [CrossRef]
- Korniłłowicz-Kowalska, T.; Bohacz, J. Biodegradation of Keratin Waste: Theory and Practical Aspects. Waste Manag. 2011, 31, 1689–1701. [Google Scholar] [CrossRef]
- Tissera, N.D.; Wijesena, R.N.; Yasasri, H.; De Silva, K.M.N.; De Silva, R.M. Fibrous Keratin Protein Bio Micro Structure for Efficient Removal of Hazardous Dye Waste from Water: Surface Charge Mediated Interfaces for Multiple Adsorption Desorption Cycles. Mater. Chem. Phys. 2020, 246, 122790. [Google Scholar] [CrossRef]
- Chao, S.-J.; Chung, K.-H.; Lai, Y.-F.; Lai, Y.-K.; Chang, S.-H. Keratin Particles Generated from Rapid Hydrolysis of Waste Feathers with Green DES/KOH: Efficient Adsorption of Fluoroquinolone Antibiotic and Its Reuse. Int. J. Biol. Macromol. 2021, 173, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Stasinakis, A.S.; Elia, I.; Petalas, A.V.; Halvadakis, C.P. Removal of Total Phenols from Olive-Mill Wastewater Using an Agricultural by-Product, Olive Pomace. J. Hazard. Mater. 2008, 160, 408–413. [Google Scholar] [CrossRef]
- Vievard, J.; Alem, A.; Pantet, A.; Ahfir, N.-D.; Arellano-Sánchez, M.G.; Devouge-Boyer, C.; Mignot, M. Bio-Based Adsorption as Ecofriendly Method for Wastewater Decontamination: A Review. Toxics 2023, 11, 404. [Google Scholar] [CrossRef]
- Caovilla, M.; Oro, C.E.D.; Mores, R.; Venquiaruto, L.D.; Mignoni, M.L.; Di Luccio, M.; Treichel, H.; Dallago, R.M.; Tres, M.V. Exploring Chicken Feathers as a Cost-Effective Adsorbent for Aqueous Dye Removal. Separations 2025, 12, 39. [Google Scholar] [CrossRef]
- Hansen, S.; Selzer, D.; Schaefer, U.F.; Kasting, G.B. An Extended Database of Keratin Binding. J. Pharm. Sci. 2011, 100, 1712–1726. [Google Scholar] [CrossRef]
- Chen, M.; Wu, D.; Deng, X.; Li, Y.; Sun, X.; Ma, J.; Chen, C.; Liao, X.; Liu, X. Chitin-Rich Shrimp Shells-Based Nitrogen-Doped Green Fluorescence Carbon Dots: New Insights for Multi-Orbital Applications. Int. J. Biol. Macromol. 2025, 304, 141029. [Google Scholar] [CrossRef] [PubMed]
- Shahib, I.I.; Ifthikar, J.; Wang, S.; Elkhlifi, Z.; Wang, J.; Chen, Z. Nitrogen-Rich Carbon Composite Fabricated from Waste Shrimp Shells for Highly Efficient Oxo-Vanadate Adsorption-Coupled Reduction. Chemosphere 2023, 340, 139915. [Google Scholar] [CrossRef] [PubMed]
- Isibor, P.O.; Akinduti, P.; Ikhiwili, O.M.; Aanuoluwa, A.T.; Dorcas, O.Y. Water Purification Potentials of Crustacean Chitosan. In Biotechnological Approaches to Sustainable Development Goals; Isibor, P.O., Akinduti, P., Oranusi, S.U., Popoola, J.O., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 269–287. ISBN 978-3-031-33369-9. [Google Scholar]
- Geetha Devi, M.; Shinoon Al-Hashmi, Z.S.; Chandra Sekhar, G. Treatment of Vegetable Oil Mill Effluent Using Crab Shell Chitosan as Adsorbent. Int. J. Environ. Sci. Technol. 2012, 9, 713–718. [Google Scholar] [CrossRef]
- Elayadi, F.; Achak, M.; Beniich, N.; Belaqziz, M.; El Adlouni, C. Factorial Design for Optimizing and Modeling the Removal of Organic Pollutants from Olive Mill Wastewater Using a Novel Low-Cost Bioadsorbent. Water. Air. Soil Pollut. 2020, 231. [Google Scholar] [CrossRef]
- Bahadir, T.; Gök, G.; Çelebi, H.; Şimşek, İ.; Gök, O. Seafood Wastes as an Attractive Biosorbent: Chitin-Based Shrimp Shells. Water. Air. Soil Pollut. 2023, 234, 145. [Google Scholar] [CrossRef]
- Boumya, W.; Khnifira, M.; Machrouhi, A.; Abdennouri, M.; Sadiq, M.; Achak, M.; Serdaroğlu, G.; Kaya, S.; Şimşek, S.; Barka, N. Adsorption of Eriochrome Black T on the Chitin Surface: Experimental Study, DFT Calculations and Molecular Dynamics Simulation. J. Mol. Liq. 2021, 331, 115706. [Google Scholar] [CrossRef]
- Elamraoui, S.; Asdiou, N.; Boumya, W.; Billah, R.E.; Achaby, M.E.; Barka, N.; Lamy, E.; Alaoui, F.E.; Chhiti, Y.; Benhida, R.; et al. Comparative Study of Raw Pinus Sylvestris Sawdust and Its Activated Carbon for Chemical Oxygen Demand and Polyphenols Removal from Olive Mill Wastewater. Biomass Convers. Biorefinery 2025. [Google Scholar] [CrossRef]
- Santi, C.A.; Cortes, S.; D’Acqui, L.P.; Sparvoli, E.; Pushparaj, B. Reduction of Organic Pollutants in Olive Mill Wastewater by Using Different Mineral Substrates as Adsorbents. Bioresour. Technol. 2008, 99, 1945–1951. [Google Scholar] [CrossRef]
- Odeh, F.; Abu-Dalo, M.; Albiss, B.; Ghannam, N.; Khalaf, A.; Amayreh, H.H.; Al Bawab, A. Coupling Magnetite and Goethite Nanoparticles with Sorbent Materials for Olive Mill Wastewater Remediation. Emergent Mater. 2022, 5, 77–88. [Google Scholar] [CrossRef]
- Yangui, A.; Abderrabba, M. Towards a High Yield Recovery of Polyphenols from Olive Mill Wastewater on Activated Carbon Coated with Milk Proteins: Experimental Design and Antioxidant Activity. Food Chem. 2018, 262, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Xiaoli, C.; Youcai, Z. Adsorption of Phenolic Compound by Aged-Refuse. J. Hazard. Mater. 2006, 137, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Al-Essa, K.; Al-Essa, E.M. Effective Approach of Activated Jordanian Bentonite by Sodium Ions for Total Phenolic Compounds Removal from Olive Mill Wastewater. J. Chem. 2021, 2021, 1–16. [Google Scholar] [CrossRef]
- Pareja-Sánchez, E.; García-Moreno, A.J.; Martínez-García, M.; Pérez-Colodrero, L.; García-Zapata, L.; García-Ruiz, R. Bio-Based Solvents for Polyphenol Recovery: Transforming Olive Mill Wastewater into High-Value Resources. J. Water Process Eng. 2025, 71, 107238. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of Methylene Blue on Low-Cost Adsorbents: A Review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Investigation of Australian Olive Mill Waste for Recovery of Biophenols. J. Agric. Food Chem. 2005, 53, 9911–9920. [Google Scholar] [CrossRef]
- Jean Rodier, B.L.; Merlet, N.; Rodier, J. L’analyse de l’eau: Eaux Naturelles, Eaux Résiduaires, Eau de Mer, 7th ed.; DUNOD, BORDAS: Paris, France, 1984; p. 1365. [Google Scholar]
- APHA; APHA. Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Public Health Association (APHA), American Water Works Association (AWWA) and Water Pollution Control Federation (WPCF): Washington, DC, USA, 1992. [Google Scholar]
- Macheix, J.J.; Fleuriet, A.; Billot JMacheix, J.J.; Fleuriet, A.; Billot, J. Fruit Phenolics; CRC Press: Boca Raton, FL, USA, 1990; p. 378. [Google Scholar]
- AFNOR. Recueil de Norme Franc ¸aise: Eau, Méthodes d’essai, 2nd ed.; AFNO: Paris, Franch, 1983; p. 621. [Google Scholar]
- Li, S.; Xu, S.; Liu, S.; Yang, C.; Lu, Q. Fast Pyrolysis of Biomass in Free-Fall Reactor for Hydrogen-Rich Gas. Fuel Process. Technol. 2004, 85, 1201–1211. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Liao, F.; Wang, Y.; Li, M.; Meng, L.; Jiang, J. Removal of Methylene Blue from Aqueous Solution with Magnetite Loaded Multi-Wall Carbon Nanotube: Kinetic, Isotherm and Mechanism Analysis. J. Hazard. Mater. 2011, 198, 282–290. [Google Scholar] [CrossRef]
- Boehm, H.-P. Surface Chemical Characterization of Carbons from Adsorption Studies. In Adsorption by Carbons; Elsevier: Amsterdam, The Netherlands, 2008; pp. 301–327. ISBN 978-0-08-044464-2. [Google Scholar]
- Naboulsi, A.; Himri, M.E.; Gharibi, E.K.; Haddad, M.E. Study of Adsorption Mechanism of Malachite Green (MG) and Basic Yellow 28 (BY28) onto Smectite Rich Natural Clays (Ghassoul) Using DFT/B3LYP and DOE/FFD. Surf. Interfaces 2022, 33, 102227. [Google Scholar] [CrossRef]
- Franco, C.A.; Cortés, F.B.; Nassar, N.N. Adsorptive Removal of Oil Spill from Oil-in-Fresh Water Emulsions by Hydrophobic Alumina Nanoparticles Functionalized with Petroleum Vacuum Residue. J. Colloid Interface Sci. 2014, 425, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; MacDonald, L.; Raj, P.; Karamalidis, A.K. Thiol-Functionalized Cellulose Adsorbents for Highly Selective Separation of Palladium over Platinum in Acidic Aqueous Solutions. Chem. Eng. J. 2024, 494, 152948. [Google Scholar] [CrossRef]
- Şahin, S.; Ciğeroğlu, Z.; Özdemir, O.K.; Bilgin, M.; Elhussein, E.; Gülmez, Ö. Recovery of Hydroxytyrosol onto Graphene Oxide Nanosheets: Equilibrium and Kinetic Models. J. Mol. Liq. 2019, 285, 213–222. [Google Scholar] [CrossRef]



| Parameter | Concentration |
|---|---|
| pH | 5.2 ± 0.5 |
| Conductivity (mS/cm) | 15.79 ± 0.06 |
| Total dissolved solids (TDSs) (g/L) | 7.51 ± 0.02 |
| Total suspended solids (TSSs) (g/L) | 3.25 ± 1.33 |
| BOD5 (g/L) | 28.50 + 1.63 |
| Total solids (TSs) (g/L) | 55.45 ± 0.03 |
| Fixed solids (FSs) (g/L) | 33.18 ± 0.03 |
| Chloride—Cl− (mg/L) | 0.00215 ± 0.004 |
| Potassium—K+ (mg/L) | 0.00329 ± 0.003 |
| Phosphate—PO43− (g/L) | 2.33 ± 0.05 |
| Ca2+ (mg/L) | 0.055 ± 0.004 |
| Mg2+ (mg/L) | 0.056 ± 0.003 |
| TKN (g/L) | 1.15 ± 0.03 |
| Nitrite—NO2− (mg/L) | 0.00083 ± 0.0005 |
| Nitrate—NO3− (mg/L) | 0.01218 ± 0.004 |
| Sulfate—SO42− (g/L) | 2.72 ± 0.02 |
| Total COD (g/L) | 223 ± 03.65 |
| Dissolved COD (g/L) | 152 ± 0.05 |
| Polyphenols (g/L) | 3.11 ± 0.06 |
| Proximate Analysis (wt.%) | ST | SD | SSs | CFs |
|---|---|---|---|---|
| Moisture | 3.24 | 4.5 | 6 | 3 |
| Ash | 2.96 | 0.5 | 3 | 4.5 |
| Volatile matter | 70.23 | 73.65 | 70.98 | 76.5 |
| Fixed carbon | 23.57 | 21.35 | 20.02 | 16 |
| Component analysis (wt.%) | ||||
| Lignin | 39.45 | 20.32 | 0 | 0 |
| Cellulose | 35.79 | 60.47 | 0 | 0 |
| Hemicellulose | 20.47 | 15.63 | 0 | 0 |
| Extractives | 4.29 | 3.58 | 0 | 0 |
| Sample | Carboxylic Acids (mmol/g) | Phenolic Acids (mmol/g) | Lactonic Acids (mmol/g) | Total Acid Groups (mmol/g) | Basic Groups (mmol/g) |
|---|---|---|---|---|---|
| ST | 4.75 | 1.98 | 0.65 | 7.38 | 0.75 |
| SD | 3.25 | 1.12 | 0.45 | 5.42 | 1.25 |
| CFs | 2.37 | 0.95 | 0.23 | 3.55 | 2.12 |
| SSs | 3.12 | 1.35 | 1.14 | 5.61 | 3.17 |
| Sample Type | SD | ST | CFs | SSs |
|---|---|---|---|---|
| BET Surface area (m2/g) | 0.2810 | 5.3215 | 0.5648 | 1.6740 |
| BJH Pore Volume (cm3/g) | 0.0583 | 0.0813 | 0.0305 | 0.0455 |
| BJH Pore Size (nm) | 3.688 | 3.822 | 6.757 | 5.895 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elamraoui, S.; Asdiou, N.; El kaim Billah, R.; El Achaby, M.; Kounbach, S.; Benhida, R.; Achak, M. Unveiling the Adsorptive Potential of Natural Biopolymers for Olive Mill Wastewater Treatment: A Synergistic Approach Using RSM-BBD, Mixture Design, Kinetics, and Mechanistic Analysis. Int. J. Mol. Sci. 2025, 26, 7738. https://doi.org/10.3390/ijms26167738
Elamraoui S, Asdiou N, El kaim Billah R, El Achaby M, Kounbach S, Benhida R, Achak M. Unveiling the Adsorptive Potential of Natural Biopolymers for Olive Mill Wastewater Treatment: A Synergistic Approach Using RSM-BBD, Mixture Design, Kinetics, and Mechanistic Analysis. International Journal of Molecular Sciences. 2025; 26(16):7738. https://doi.org/10.3390/ijms26167738
Chicago/Turabian StyleElamraoui, Sabah, Nouhaila Asdiou, Rachid El kaim Billah, Mounir El Achaby, Said Kounbach, Rachid Benhida, and Mounia Achak. 2025. "Unveiling the Adsorptive Potential of Natural Biopolymers for Olive Mill Wastewater Treatment: A Synergistic Approach Using RSM-BBD, Mixture Design, Kinetics, and Mechanistic Analysis" International Journal of Molecular Sciences 26, no. 16: 7738. https://doi.org/10.3390/ijms26167738
APA StyleElamraoui, S., Asdiou, N., El kaim Billah, R., El Achaby, M., Kounbach, S., Benhida, R., & Achak, M. (2025). Unveiling the Adsorptive Potential of Natural Biopolymers for Olive Mill Wastewater Treatment: A Synergistic Approach Using RSM-BBD, Mixture Design, Kinetics, and Mechanistic Analysis. International Journal of Molecular Sciences, 26(16), 7738. https://doi.org/10.3390/ijms26167738







