The Supportive Role of Plant-Based Substances in AMD Treatment and Their Potential
Abstract
1. Introduction
2. Clinical Manifestations and Disease Course
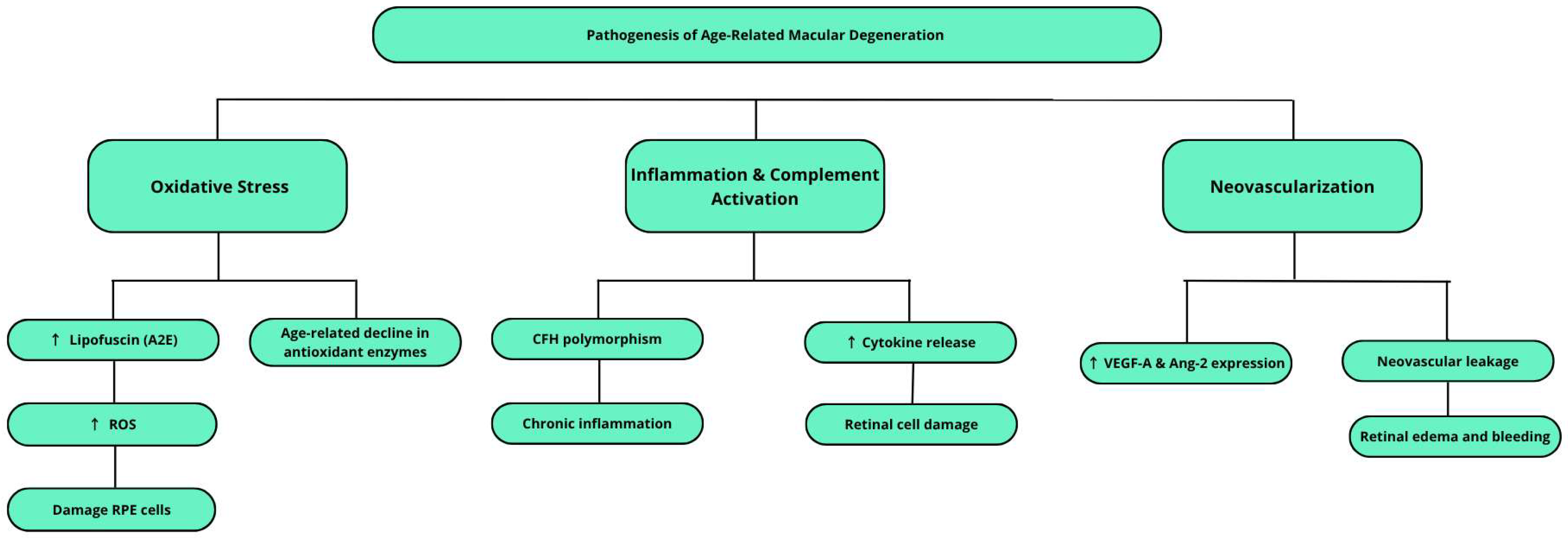
3. Etiopathogenesis of Age-Related Macular Degeneration (AMD)
3.1. Oxidative Stress
3.2. Inflammatory Processes and Complement System Activation
3.3. Neovascularization
4. Plant Substances with Potential in AMD Treatment
4.1. Plant Compounds with Newly Revealed Therapeutic Potential in the Treatment of AMD
4.1.1. Silymarin
4.1.2. Anthocyanins
4.2. Lutein and Zeaxanthin
4.3. Polyphenols
Curcumin
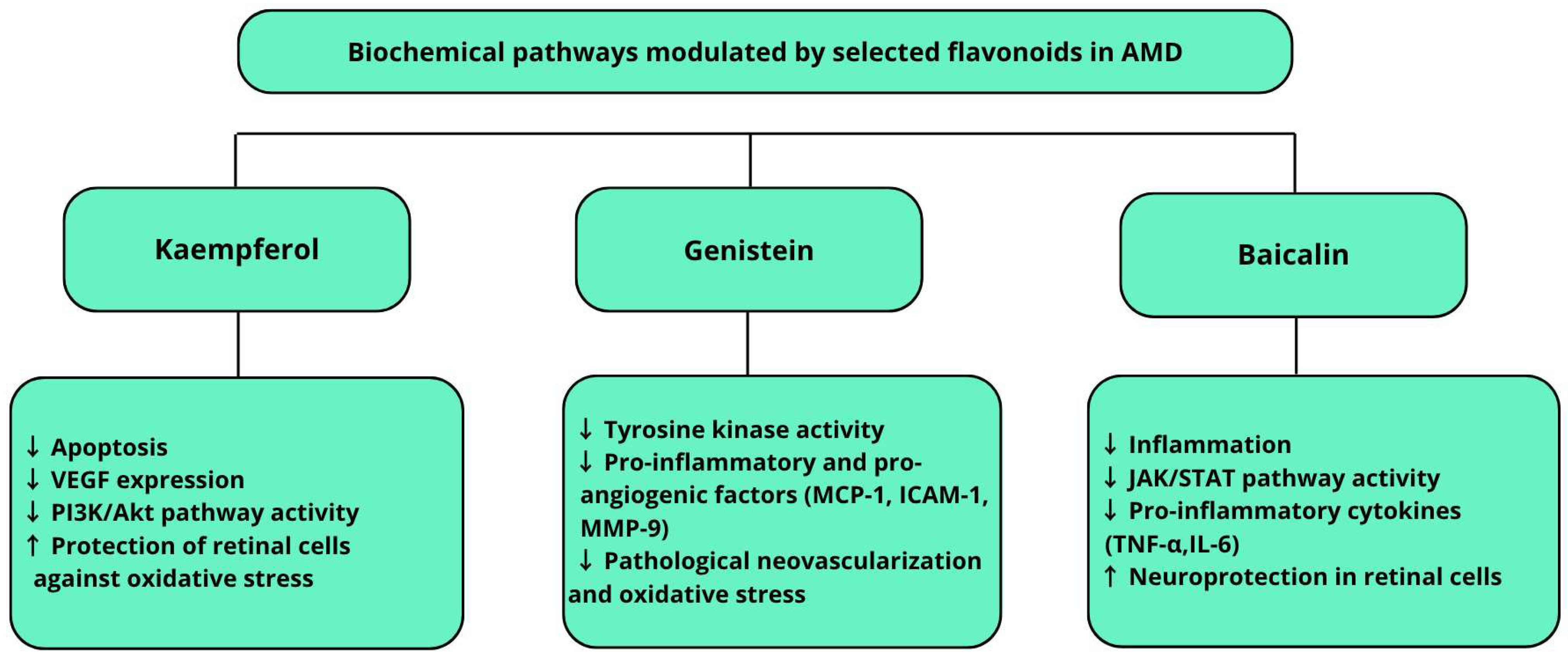
4.4. Flavonoids
| Substance Name | Source of Substances | Mode of Action | Clinical Evidence | Comments |
|---|---|---|---|---|
| Curcumin | Activation of autophagy [74] | |||
| Turmeric rhizome (Curcuma longa) | Antiangiogenic [85] | In vivo [86] In vitro [74,85] | Curcumin’s effects were observed even at a relatively low concentration of 10 μM, suggesting its high efficacy and therapeutic potential [74] | |
| Anti-inflammatory [85,86] | ||||
| Sylibinin | Milk thistle (Sylibum marianum) | Antioxidant [4] | In vivo & in vitro [4] | An interesting aspect of silybinin’s action is that it increases HIF-1α protein levels without affecting its mRNA, indicating regulation at the level of protein translation or stability, rather than transcription |
| Antiangiogenic [4] | ||||
| Anthocyane | Blueberry (Vaccinium angustifolium), Blueberry (Vaccinium myrtillus) | Anti-inflammatory [35,37] | In vivo [35] | Studies indicate that anthocyanins can penetrate the blood–retina barrier, allowing them to have a direct protective effect on retinal cells in AMD |
| Antioxidant [35,37] | ||||
| Epigallocatechin-3-gallate (EGCG) | Green tea | Antioxidant [52,54] | In vitro [54] | It also affects the regulation of the signaling pathways responsible for cell apoptosis, which may contribute to protecting the retina from degeneration |
| Resveratrol | Grape skins, red wine | Antioxidant [56,57] Anti-inflammatory [56,57] Neuroprotective [56,57] | In vivo [56,57] | |
| Chlorogenic acid (CGA) | Green coffee, Jerusalem artichoke, blueberries | Antioxidant [60] Anti-inflammatory [61,62] | In vivo [61,62] | In animal models, CGA has shown the ability to inhibit choroidal neovascularization |
| Kaempferol | Brassica vegetables, berries, tea | Antioxidant [91,92] Anti-inflammatory [91,92] Neuroprotective [94] | In vitro [91,92] In vivo [94] | There is research into new delivery systems for kaempferol, such as nanoemulsions, which improve its bioavailability and may increase its therapeutic efficacy |
| Baicalin | Root of Scutellaria baicalensis | Antioxidant [98,99,100,101] Anti-inflammatory [98,99,100,101] Neuroprotective [99,100] | In vivo [98] In vivo & in vitro [100] | A recent study developed a new form of baicalin administration, an in situ gel containing a nanoemulsion of the active ingredient |
| Genistein | Common soybean (Glycine max) | Anti-inflammatory [104,105] Antioxidant [104,105] Anti-angiogenic [106,107] | In vivo [106,107] | It is known as a selective tyrosine kinase (PTK) inhibitor |
5. Limitations
6. Conclusions
7. Materials and Methods
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3-MA | 3-Methyladenine |
| A2E | A specific bisretinoid (component of lipofuscin) |
| AKT | Protein Kinase B |
| Ang-2 | Angiopoietin-2 |
| AMD | Age-related Macular Degeneration |
| APOE | Apolipoprotein E |
| ARMS2 | Age-Related Maculopathy Susceptibility 2 |
| ARPE-19 | Human Retinal Pigment Epithelial Cell Line |
| ATP | Adenosine Triphosphate |
| BAE | Bilberry anthocyanin extract |
| Bax | Bcl-2-associated X protein (pro-apoptotic protein) |
| Bcl-2 | B-cell lymphoma 2 (anti-apoptotic protein) |
| C2 | Complement Component 2 |
| C3 | Complement Component 3 |
| CAA | Cellular Antioxidant Activity |
| cAMP | Cyclic adenosine monophosphate |
| Caspase-3 | Cysteine-aspartic acid protease 3 (key apoptosis executioner enzyme) |
| CAT | Catalase |
| CFB | Complement Factor B |
| CFH | Complement Factor H |
| CGA | Chlorogenic Acid |
| CNV | Choroidal Neovascularization |
| CTGF | Connective tissue growth factor |
| c-Myc | Cellular Myelocytomatosis oncogene |
| Cyfip2 | Cytoplasmic FMR1-interacting protein 2 |
| DHA | Docosahexaenoic Acid |
| EGCG | Epigallocatechin-3-gallate |
| eNOS | endothelial nitric oxide synthase |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ERG | Electroretinography |
| Fz | Frizzled receptor |
| GPx | Glutathione Peroxidase |
| HA-KA-NLCs | Hyaluronic Acid Modified Kaempferol Nanostructured Lipid Carriers |
| HIF-1 | Hypoxia-inducible factor 1 |
| HIF-1α | Hypoxia-Inducible Factor 1-alpha |
| HO-1 | Heme oxygenase-1 |
| HREC | Human Retinal Endothelial Cells |
| HTRA1 | High Temperature Requirement A Serine Peptidase 1 |
| IAI | Intravitreal Anti-VEGF Injections |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IL | Interleukin |
| IL-1β | Interleukin-1 beta |
| LC3 | Microtubule-associated protein 1A/1B-light chain 3 |
| LRP5/6 | Low-density lipoprotein receptor-related protein 5/6 |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MMP-9 | Matrix Metalloproteinase-9 |
| MPOD | Macular Pigment Optical Density |
| mTOR | Mammalian Target Of Rapamycin |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NOD-, LRR- and Pyrin Domain-Containing Protein 3 |
| NQO1 | NAD(P)H quinone dehydrogenase 1 |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| OCT | Optical Coherence Tomography |
| PE | Phosphatidylethanolamine |
| PHD2 | Prolyl Hydroxylase Domain-containing protein 2 |
| PI3K | Phosphoinositide 3-kinase |
| PI3K/Akt/mTOR | Phosphoinositide 3-kinase/Protein Kinase B/Mechanistic Target of Rapamycin |
| PI3K/Akt/mTOR/p70S6K | Expanded signaling pathway including p70 S6 kinase |
| PKA | Protein Kinase A |
| PTK | Protein Tyrosine Kinase |
| PUFA | Polyunsaturated Fatty Acids |
| ROS | Reactive Oxygen Species |
| RPE | Retinal Pigment Epithelium |
| SIRT1 | Sirtuin 1 |
| SOD | Superoxide Dismutase |
| TCF/LEF | T-cell factor/Lymphoid enhancer factor |
| TIMP3 | Tissue Inhibitor of Metalloproteinases 3 |
| TLR4 | Toll-Like Receptor 4 |
| TNF-α | Tumor necrosis factor alpha |
| ULK1 | Unc-51 Like Autophagy Activating Kinase 1 |
| VASP | Vasodilator-stimulated phosphoprotein |
| VEGF | Vascular endothelial growth factor |
| VEGFA | Vascular Endothelial Growth Factor A (gene) |
| VHL | Von Hippel–Lindau tumor suppressor protein |
References
- Marchesi, N.; Capierri, M.; Pascale, A.; Barbieri, A. Different Therapeutic Approaches for Dry and Wet AMD. Int. J. Mol. Sci. 2024, 25, 13053. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yu, T.; Zhao, J.; Zhu, X.; Xin, W.; Zhang, F.; Zhang, L. Role of Flavonoids in Age-Related Macular Degeneration. Biomed. Pharmacother. 2023, 159, 114259. [Google Scholar] [CrossRef]
- Kao, Y.-W.; Hsu, S.-K.; Chen, J.Y.-F.; Lin, I.-L.; Chen, K.-J.; Lee, P.-Y.; Ng, H.-S.; Chiu, C.-C.; Cheng, K.-C. Curcumin Metabolite Tetrahydrocurcumin in the Treatment of Eye Diseases. Int. J. Mol. Sci. 2020, 22, 212. [Google Scholar] [CrossRef]
- Lin, C.; Li, C.; Liao, P.; Tse, L.; Huang, W.; Cheng, H.; Cheng, Y. Silibinin Inhibits VEGF Secretion and Age-related Macular Degeneration in a Hypoxia-dependent Manner through the PI-3 Kinase/Akt/mTOR Pathway. Br. J. Pharmacol. 2013, 168, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Layana, A.; Cabrera-López, F.; García-Arumí, J.; Arias-Barquet, L.; Ruiz-Moreno, J.M. Early and Intermediate Age-Related Macular Degeneration: Update and Clinical Review. Clin. Interv. Aging 2017, 12, 1579–1587. [Google Scholar] [CrossRef]
- Nashine, S. Potential Therapeutic Candidates for Age-Related Macular Degeneration (AMD). Cells 2021, 10, 2483. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Y.; Li, J.; Wu, E.; Tang, T.; Singla, R.K.; Shen, B.; Zhang, M. Natural Products for the Treatment of Age-Related Macular Degeneration. Phytomedicine 2024, 130, 155522. [Google Scholar] [CrossRef]
- Deng, Y.; Qiao, L.; Du, M.; Qu, C.; Wan, L.; Li, J.; Huang, L. Age-Related Macular Degeneration: Epidemiology, Genetics, Pathophysiology, Diagnosis, and Targeted Therapy. Genes Dis. 2022, 9, 62–79. [Google Scholar] [CrossRef]
- Domènech, E.B.; Marfany, G. The Relevance of Oxidative Stress in the Pathogenesis and Therapy of Retinal Dystrophies. Antioxidants 2020, 9, 347. [Google Scholar] [CrossRef]
- Al-Zamil, W.; Yassin, S. Recent Developments in Age-Related Macular Degeneration: A Review. Clin. Interv. Aging 2017, 12, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Janik-Papis, K.; Ulińska, M.; Krzyzanowska, A.; Stoczyńska, E.; Borucka, A.I.; Woźniak, K.; Małgorzata, Z.; Szaflik, J.P.; Blasiak, J. Role of oxidative mechanisms in the pathogenesis of age-related macular degeneration. Klin. Oczna 2009, 111, 168–173. [Google Scholar]
- Hogg, R.E.; Woodside, J.V. Mediterranean Diet and Age-Related Macular Degeneration: Is It Time to Attempt Dietary Modification? Ophthalmology 2019, 126, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Gasparri, C.; Riva, A.; Petrangolini, G.; Barrile, G.C.; Cavioni, A.; Razza, C.; Tartara, A.; Perna, S. Diet and Ideal Food Pyramid to Prevent or Support the Treatment of Diabetic Retinopathy, Age-Related Macular Degeneration, and Cataracts. Front. Med. 2023, 10, 1168560. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research Group. A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation with Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss: AREDS Report No. 8. Arch. Ophthalmol. Chic. Ill 1960 2001, 119, 1417–1436. [Google Scholar] [CrossRef]
- Evans, J.R.; Lawrenson, J.G. Antioxidant Vitamin and Mineral Supplements for Slowing the Progression of Age-Related Macular Degeneration. Cochrane Database Syst. Rev. 2017, 2017, CD000254. [Google Scholar] [CrossRef]
- Fabre, M.; Mateo, L.; Lamaa, D.; Baillif, S.; Pagès, G.; Demange, L.; Ronco, C.; Benhida, R. Recent Advances in Age-Related Macular Degeneration Therapies. Molecules 2022, 27, 5089. [Google Scholar] [CrossRef]
- Solomon, S.D.; Lindsley, K.; Vedula, S.S.; Krzystolik, M.G.; Hawkins, B.S. Anti-Vascular Endothelial Growth Factor for Neovascular Age-Related Macular Degeneration. Cochrane Database Syst. Rev. 2019, 2019, CD005139. [Google Scholar] [CrossRef]
- Holz, F.G.; Sadda, S.R.; Staurenghi, G.; Lindner, M.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; Csaky, K.; et al. Imaging Protocols in Clinical Studies in Advanced Age-Related Macular Degeneration. Ophthalmology 2017, 124, 464–478. [Google Scholar] [CrossRef]
- Hanout, M.; Ferraz, D.; Ansari, M.; Maqsood, N.; Kherani, S.; Sepah, Y.J.; Rajagopalan, N.; Ibrahim, M.; Do, D.V.; Nguyen, Q.D. Therapies for Neovascular Age-Related Macular Degeneration: Current Approaches and Pharmacologic Agents in Development. BioMed Res. Int. 2013, 2013, 830837. [Google Scholar] [CrossRef]
- Moore, N.A.; Bracha, P.; Hussain, R.M.; Morral, N.; Ciulla, T.A. Gene Therapy for Age-Related Macular Degeneration. Expert Opin. Biol. Ther. 2017, 17, 1235–1244. [Google Scholar] [CrossRef]
- Nowak, J.Z.; Bienias, W. Age-related macular degeneration (AMD): Etiopathogenesis and therapeutic strategies. Postepy Hig. Med. Dosw. Online 2007, 61, 83–94. [Google Scholar]
- Sasaki, M.; Kawasaki, R.; Yanagi, Y. Early Stages of Age-Related Macular Degeneration: Racial/Ethnic Differences and Proposal of a New Classification Incorporating Emerging Concept of Choroidal Pathology. J. Clin. Med. 2022, 11, 6274. [Google Scholar] [CrossRef]
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.E.; Den Hollander, A.I. Risk Factors for Progression of Age-related Macular Degeneration. Ophthalmic Physiol. Opt. 2020, 40, 140–170. [Google Scholar] [CrossRef]
- Wong, J.H.C.; Ma, J.Y.W.; Jobling, A.I.; Brandli, A.; Greferath, U.; Fletcher, E.L.; Vessey, K.A. Exploring the Pathogenesis of Age-Related Macular Degeneration: A Review of the Interplay between Retinal Pigment Epithelium Dysfunction and the Innate Immune System. Front. Neurosci. 2022, 16, 1009599. [Google Scholar] [CrossRef]
- Marie, M.; Bigot, K.; Angebault, C.; Barrau, C.; Gondouin, P.; Pagan, D.; Fouquet, S.; Villette, T.; Sahel, J.-A.; Lenaers, G.; et al. Light Action Spectrum on Oxidative Stress and Mitochondrial Damage in A2E-Loaded Retinal Pigment Epithelium Cells. Cell Death Dis. 2018, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Furso, J.; Zadlo, A.; Szewczyk, G.; Sarna, T.J. Photoreactivity of Bis-Retinoid A2E Complexed with a Model Protein in Selected Model Systems. Cell Biochem. Biophys. 2020, 78, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Böhm, E.W.; Buonfiglio, F.; Voigt, A.M.; Bachmann, P.; Safi, T.; Pfeiffer, N.; Gericke, A. Oxidative Stress in the Eye and Its Role in the Pathophysiology of Ocular Diseases. Redox Biol. 2023, 68, 102967. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.-S.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary Sources of Lutein and Zeaxanthin Carotenoids and Their Role in Eye Health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef]
- Clark, S.J.; Perveen, R.; Hakobyan, S.; Morgan, B.P.; Sim, R.B.; Bishop, P.N.; Day, A.J. Impaired Binding of the Age-Related Macular Degeneration-Associated Complement Factor H 402H Allotype to Bruch’s Membrane in Human Retina. J. Biol. Chem. 2010, 285, 30192–30202. [Google Scholar] [CrossRef]
- Canonica, J.; Foxton, R.; Garrido, M.G.; Lin, C.-M.; Uhles, S.; Shanmugam, S.; Antonetti, D.A.; Abcouwer, S.F.; Westenskow, P.D. Delineating Effects of Angiopoietin-2 Inhibition on Vascular Permeability and Inflammation in Models of Retinal Neovascularization and Ischemia/Reperfusion. Front. Cell. Neurosci. 2023, 17, 1192464. [Google Scholar] [CrossRef]
- García-Ramírez, M.; Turch, M.; Simó-Servat, O.; Hernández, C.; Simó, R. Silymarin Prevents Diabetes-Induced Hyperpermeability in Human Retinal Endothelial Cells. Endocrinol. Diabetes Nutr. 2018, 65, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Rautiainen, S.; Park, S.J.; Kim, E.; Lee, I.-M.; Glynn, R.J.; Buring, J.E.; Christen, W.G. Intake of Blueberries, Anthocyanins, and Risk of Eye Disease in Women. J. Nutr. 2024, 154, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.Y.; Lee, M.-H.; Kim, H.-M.; Chung, H.-C.; Kim, D.-U.; Lee, J.-H.; Jeong, K.W. Protective Effect of Ribes Nigrum Extract against Blue Light-Induced Retinal Degeneration In Vitro and In Vivo. Antioxidants 2022, 11, 832. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Zhang, B.; Wang, S. Berries and Their Active Compounds in Prevention of Age-Related Macular Degeneration. Antioxidants 2024, 13, 1558. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Lu, F.; Yang, X.; Deng, Q.; Ji, B.; Huang, F. Retinoprotective Effects of Bilberry Anthocyanins via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Mechanisms in a Visible Light-Induced Retinal Degeneration Model in Pigmented Rabbits. Molecules 2015, 20, 22395–22410. [Google Scholar] [CrossRef]
- Bornsek, S.M.; Ziberna, L.; Polak, T.; Vanzo, A.; Ulrih, N.P.; Abram, V.; Tramer, F.; Passamonti, S. Bilberry and Blueberry Anthocyanins Act as Powerful Intracellular Antioxidants in Mammalian Cells. Food Chem. 2012, 134, 1878–1884. [Google Scholar] [CrossRef]
- Gerding, H. Primäre oder sekundäre Prophylaxe der AMD mit Anthocyanen? Klin. Monatsblätter Augenheilkd. 2009, 226, 216–219. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration-Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Tarsis, S.L. Preliminary Identification of the Human Macular Pigment. Vision Res. 1985, 25, 1531–1535. [Google Scholar] [CrossRef]
- Vishwanathan, R.; Schalch, W.; Johnson, E.J. Macular Pigment Carotenoids in the Retina and Occipital Cortex Are Related in Humans. Nutr. Neurosci. 2016, 19, 95–101. [Google Scholar] [CrossRef]
- Bhosale, P.; Li, B.; Sharifzadeh, M.; Gellermann, W.; Frederick, J.M.; Tsuchida, K.; Bernstein, P.S. Purification and Partial Characterization of a Lutein-Binding Protein from Human Retina. Biochemistry 2009, 48, 4798–4807. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; George, E.W.; Rognon, G.T.; Gorusupudi, A.; Ranganathan, A.; Chang, F.-Y.; Shi, L.; Frederick, J.M.; Bernstein, P.S. Imaging Lutein and Zeaxanthin in the Human Retina with Confocal Resonance Raman Microscopy. Proc. Natl. Acad. Sci. USA 2020, 117, 12352–12358. [Google Scholar] [CrossRef] [PubMed]
- Widomska, J.; SanGiovanni, J.P.; Subczynski, W.K. Why Is Zeaxanthin the Most Concentrated Xanthophyll in the Central Fovea? Nutrients 2020, 12, 1333. [Google Scholar] [CrossRef]
- Jang, W.; Choi, J.; Kim, H. Associations of Mediterranean Diet Score and Age-Related Macular Degeneration in Korean Elderly. BMC Public Health 2024, 24, 2846. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W. Macular Carotenoids: Lutein and Zeaxanthin. Dev. Ophthalmol. 2005, 38, 70–88. [Google Scholar] [CrossRef]
- Scripsema, N.K.; Hu, D.-N.; Rosen, R.B. Lutein, Zeaxanthin, and Meso-Zeaxanthin in the Clinical Management of Eye Disease. J. Ophthalmol. 2015, 2015, 865179. [Google Scholar] [CrossRef]
- Wu, J.; Cho, E.; Willett, W.C.; Sastry, S.M.; Schaumberg, D.A. Intakes of Lutein, Zeaxanthin, and Other Carotenoids and Age-Related Macular Degeneration During 2 Decades of Prospective Follow-Up. JAMA Ophthalmol. 2015, 133, 1415–1424. [Google Scholar] [CrossRef]
- Cho, E.; Hankinson, S.E.; Rosner, B.; Willett, W.C.; Colditz, G.A. Prospective Study of Lutein/Zeaxanthin Intake and Risk of Age-Related Macular Degeneration. Am. J. Clin. Nutr. 2008, 87, 1837–1843. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Dou, H.-L.; Huang, F.-F.; Xu, X.-R.; Zou, Z.-Y.; Lu, X.-R.; Lin, X.-M. Changes Following Supplementation with Lutein and Zeaxanthin in Retinal Function in Eyes with Early Age-Related Macular Degeneration: A Randomised, Double-Blind, Placebo-Controlled Trial. Br. J. Ophthalmol. 2015, 99, 371–375. [Google Scholar] [CrossRef]
- Sawa, M.; Shunto, T.; Nishiyama, I.; Yokoyama, A.; Shigeta, R.; Miura, S.; Kawasaki, R. Effects of Lutein Supplementation in Japanese Patients with Unilateral Age-Related Macular Degeneration: The Sakai Lutein Study. Sci. Rep. 2020, 10, 5958. [Google Scholar] [CrossRef]
- Blasiak, J.; Chojnacki, J.; Szczepanska, J.; Fila, M.; Chojnacki, C.; Kaarniranta, K.; Pawlowska, E. Epigallocatechin-3-Gallate, an Active Green Tea Component to Support Anti-VEGFA Therapy in Wet Age-Related Macular Degeneration. Nutrients 2023, 15, 3358. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (‒)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2022, 24, 340. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Quon, M.J.; Kim, J. New Insights into the Mechanisms of Polyphenols beyond Antioxidant Properties; Lessons from the Green Tea Polyphenol, Epigallocatechin 3-Gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Li, S.; Su, D.; Hu, S.; Hu, Q.; Sun, D. Epigallocatechin Gallate Ameliorates Retinal Pigment Epithelial Cell Damage via the CYFIP2 /AKT Pathway. Toxicol. Appl. Pharmacol. 2025, 495, 117124. [Google Scholar] [CrossRef] [PubMed]
- Holczer, M.; Besze, B.; Zámbó, V.; Csala, M.; Bánhegyi, G.; Kapuy, O. Epigallocatechin-3-Gallate (EGCG) Promotes Autophagy-Dependent Survival via Influencing the Balance of mTOR-AMPK Pathways upon Endoplasmic Reticulum Stress. Oxid. Med. Cell. Longev. 2018, 2018, 6721530. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, L.; Wang, F.; Zhang, J. Resveratrol Improved Mitochondrial Biogenesis by Activating SIRT1/PGC-1α Signal Pathway in SAP. Sci. Rep. 2024, 14, 26216. [Google Scholar] [CrossRef]
- Lee, C.S.; Choi, E.Y.; Lee, S.C.; Koh, H.J.; Lee, J.H.; Chung, J.H. Resveratrol Inhibits Hypoxia-Induced Vascular Endothelial Growth Factor Expression and Pathological Neovascularization. Yonsei Med. J. 2015, 56, 1678. [Google Scholar] [CrossRef]
- Kotronis, C.; Datseris, I.; Tzanidaki, M.-E.; Rouvas, A.; Gouliopoulos, N. One-Year Outcomes of Resveratrol Supplement with Aflibercept versus Aflibercept Monotherapy in Wet Age-Related Macular Degeneration. Int. J. Ophthalmol. 2023, 16, 1496–1502. [Google Scholar] [CrossRef]
- Datseris, I.; Rouvas, A.; Tzanidaki, M.-E.; Kardara, M.; Geros, V.; Gouliopoulos, N. Resveratrol Supplementation in Wet AMD: Association with Fewer Intravitreal Injections and Reduced Macular Fibrosis. Clin. Ophthalmol. 2025, 19, 217–225. [Google Scholar] [CrossRef]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic Acid: A Review on Its Mechanisms of Anti-Inflammation, Disease Treatment, and Related Delivery Systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef]
- Park, J.J.; Hwang, S.J.; Park, J.-H.; Lee, H.-J. Chlorogenic Acid Inhibits Hypoxia-Induced Angiogenesis via down-Regulation of the HIF-1α/AKT Pathway. Cell. Oncol. Dordr. Neth. 2015, 38, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.M.; Jung, W.; Park, S.-B.; Kim, C.-S.; Kim, J.S. Aster Koraiensis Extract and Chlorogenic Acid Inhibit Retinal Angiogenesis in a Mouse Model of Oxygen-Induced Retinopathy. Evid.-Based Complement. Altern. Med. ECAM 2018, 2018, 6402650. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Yu, H.G.; Sohn, J. The Anti-Angiogenic Effect of Chlorogenic Acid on Choroidal Neovascularization. Korean J. Ophthalmol. 2010, 24, 163. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Amini, M.A.; Karbasi, A.; Vahabirad, M.; Khanaghaei, M.; Alizamir, A. Mechanistic Insight into Age-Related Macular Degeneration (AMD): Anatomy, Epidemiology, Genetics, Pathogenesis, Prevention, Implications, and Treatment Strategies to Pace AMD Management. Chonnam Med. J. 2023, 59, 143–159. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Blasiak, J.; Liton, P.; Boulton, M.; Klionsky, D.J.; Sinha, D. Autophagy in Age-Related Macular Degeneration. Autophagy 2023, 19, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Hyttinen, J.M.T.; Koskela, A.; Blasiak, J.; Kaarniranta, K. Autophagy in Drusen Biogenesis Secondary to Age-Related Macular Degeneration. Acta Ophthalmol. 2024, 102, 759–772. [Google Scholar] [CrossRef]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Du, N.; Tso, M.O.; Neufeld, A.H. Autophagy and Exosomes in the Aged Retinal Pigment Epithelium: Possible Relevance to Drusen Formation and Age-Related Macular Degeneration. PLoS ONE 2009, 4, e4160. [Google Scholar] [CrossRef]
- Saadat, K.A.S.M.; Murakami, Y.; Tan, X.; Nomura, Y.; Yasukawa, T.; Okada, E.; Ikeda, Y.; Yanagi, Y. Inhibition of Autophagy Induces Retinal Pigment Epithelial Cell Damage by the Lipofuscin Fluorophore A2E. FEBS Open Bio 2014, 4, 1007–1014. [Google Scholar] [CrossRef]
- Peddada, K.V.; Brown, A.; Verma, V.; Nebbioso, M. Therapeutic Potential of Curcumin in Major Retinal Pathologies. Int. Ophthalmol. 2019, 39, 725–734. [Google Scholar] [CrossRef]
- Ammon, H.P.; Wahl, M.A. Pharmacology of Curcuma Longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef]
- Kuzminska, J.; Szyk, P.; Mlynarczyk, D.T.; Bakun, P.; Muszalska-Kolos, I.; Dettlaff, K.; Sobczak, A.; Goslinski, T.; Jelinska, A. Curcumin Derivatives in Medicinal Chemistry: Potential Applications in Cancer Treatment. Molecules 2024, 29, 5321. [Google Scholar] [CrossRef]
- Chandrasekaran, P.R.; Madanagopalan, V.G. Role of Curcumin in Retinal Diseases-A Review. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2022, 260, 1457–1473. [Google Scholar] [CrossRef]
- Pinelli, R.; Ferrucci, M.; Biagioni, F.; Bumah, V.; Scaffidi, E.; Puglisi-Allegra, S.; Fornai, F. Curcumin as a Perspective Protection for Retinal Pigment Epithelium during Autophagy Inhibition in the Course of Retinal Degeneration. Curr. Neuropharmacol. 2023, 21, 2227–2232. [Google Scholar] [CrossRef]
- Bo, Q.; Shen, M.; Xiao, M.; Liang, J.; Zhai, Y.; Zhu, H.; Jiang, M.; Wang, F.; Luo, X.; Sun, X. 3-Methyladenine Alleviates Experimental Subretinal Fibrosis by Inhibiting Macrophages and M2 Polarization Through the PI3K/Akt Pathway. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2020, 36, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.-W.; Yu, W.; Wang, Y.-N.; Zhang, X.-Y.; Song, S.-Q.; Gong, S.-Y.; Meng, L.-Y.; Gan, C.; Liu, B.-J.; Gong, Q. Effects of Autophagy Inhibitor 3-Methyladenine on a Diabetic Mice Model. Int. J. Ophthalmol. 2023, 16, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Hu, Y.; Chen, Y.; Zhou, K.K.; Zhang, B.; Gao, G.; Ma, J. The Pathogenic Role of the Canonical Wnt Pathway in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4371–4379. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, Y.; Lin, M.; Lee, K.; Mott, R.A.; Ma, J. Pathogenic Role of the Wnt Signaling Pathway Activation in Laser-Induced Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2013, 54, 141–154. [Google Scholar] [CrossRef]
- Tuo, J.; Wang, Y.; Cheng, R.; Li, Y.; Chen, M.; Qiu, F.; Qian, H.; Shen, D.; Penalva, R.; Xu, H.; et al. Wnt Signaling in Age-Related Macular Degeneration: Human Macular Tissue and Mouse Model. J. Transl. Med. 2015, 13, 330. [Google Scholar] [CrossRef]
- Vallée, A. Curcumin and Wnt/Βcatenin Signaling in Exudative Agerelated Macular Degeneration (Review). Int. J. Mol. Med. 2022, 49, 79. [Google Scholar] [CrossRef]
- Tamai, K.; Zeng, X.; Liu, C.; Zhang, X.; Harada, Y.; Chang, Z.; He, X. A Mechanism for Wnt Coreceptor Activation. Mol. Cell 2004, 13, 149–156. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Vallée, J.-N. Curcumin: A Therapeutic Strategy in Cancers by Inhibiting the Canonical WNT/β-Catenin Pathway. J. Exp. Clin. Cancer Res. CR 2019, 38, 323. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-X.; Dun, Y.; Wu, W.; Shen, J.; Zhang, F.; Zhang, L. Curcumin Suppresses the Wnt/β-Catenin Signaling Pathway by Inhibiting NKD2 Methylation to Ameliorate Intestinal Ischemia/Reperfusion Injury. Kaohsiung J. Med. Sci. 2024, 40, 175–187. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, K.; Liu, J.; Yang, J.; Tian, Y.; Yang, C.; Li, Y.; Shao, M.; Su, W.; Song, N. Curcumin Regulates Cancer Progression: Focus on ncRNAs and Molecular Signaling Pathways. Front. Oncol. 2021, 11, 660712. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, Y.; Cui, G. Effects of TNF-Alpha and Curcumin on the Expression of VEGF in Raji and U937 Cells and on Angiogenesis in ECV304 Cells. Chin. Med. J. 2005, 118, 2052–2057. [Google Scholar]
- Zhang, Z.-B.; Luo, D.-D.; Xie, J.-H.; Xian, Y.-F.; Lai, Z.-Q.; Liu, Y.-H.; Liu, W.-H.; Chen, J.-N.; Lai, X.-P.; Lin, Z.-X.; et al. Curcumin’s Metabolites, Tetrahydrocurcumin and Octahydrocurcumin, Possess Superior Anti-Inflammatory Effects in Vivo Through Suppression of TAK1-NF-κB Pathway. Front. Pharmacol. 2018, 9, 1181. [Google Scholar] [CrossRef]
- Muangnoi, C.; Sharif, U.; Ratnatilaka Na Bhuket, P.; Rojsitthisak, P.; Paraoan, L. Protective Effects of Curcumin Ester Prodrug, Curcumin Diethyl Disuccinate against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells: Potential Therapeutic Avenues for Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 3367. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Shen, R.; Tao, J.; Huang, L.; Shi, H.; Chen, H.; Wang, Y.; Wang, T. Curcumin Suppresses the Colon Cancer Proliferation by Inhibiting Wnt/β-Catenin Pathways via miR-130a. Front. Pharmacol. 2017, 8, 877. [Google Scholar] [CrossRef]
- Xu, X.; Han, N.; Zhao, F.; Fan, R.; Guo, Q.; Han, X.; Liu, Y.; Luo, G. Inefficacy of Anti-VEGF Therapy Reflected in VEGF-Mediated Photoreceptor Degeneration. Mol. Ther. Nucleic Acids 2024, 35, 102176. [Google Scholar] [CrossRef]
- Utpal, B.K.; Sutradhar, B.; Zehravi, M.; Sweilam, S.H.; Durgawale, T.P.; Arjun, U.V.N.V.; Shanmugarajan, T.S.; Kannan, S.P.; Prasad, P.D.; Usman, M.R.M.; et al. Cellular Stress Response and Neuroprotection of Flavonoids in Neurodegenerative Diseases: Clinical Insights into Targeted Therapy and Molecular Signaling Pathways. Brain Res. 2025, 1847, 149310. [Google Scholar] [CrossRef]
- Wu, J.-S.; Chu, P.-Y.; Hsu, W.-Y.; Chuang, T.-H.; Yu, Y.-C.; Pan, Y.-C.; Lin, Y.-T.; Tang, C.-H.; Lee, C.-L.; Wu, Y.-C. Fractionation and Identification of Ocular Protective Compounds from Kochiae Fructus against Oxidative Damage in Retinal Pigment Epithelium Cells. J. Ethnopharmacol. 2025, 341, 119328. [Google Scholar] [CrossRef] [PubMed]
- Al Sabaani, N. Kaempferol Protects Against Hydrogen Peroxide-Induced Retinal Pigment Epithelium Cell Inflammation and Apoptosis by Activation of SIRT1 and Inhibition of PARP1. J. Ocul. Pharmacol. Ther. 2020, 36, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; An, Y.; He, X.; Zhang, D.; He, W. Protection of Kaempferol on Oxidative Stress-Induced Retinal Pigment Epithelial Cell Damage. Oxid. Med. Cell. Longev. 2018, 2018, 1610751. [Google Scholar] [CrossRef]
- Noguchi, J.L.; Seu, M.Y.; Qiao, J.B.; Tan, I.R.; Swaminathan, S.R.; McDonnell, J.F.; Tan, Z.; Bu, P. Kaempferol Protects Against Retinal Photoreceptor Degeneration in a Mouse Model of Light-Induced Retinal Injury. J. Ocul. Pharmacol. Ther. 2023, 39, 80–85. [Google Scholar] [CrossRef]
- Colombo, M.; Melchiades, G.D.L.; Figueiró, F.; Battastini, A.M.O.; Teixeira, H.F.; Koester, L.S. Validation of an HPLC-UV Method for Analysis of Kaempferol-Loaded Nanoemulsion and Its Application to in Vitro and in Vivo Tests. J. Pharm. Biomed. Anal. 2017, 145, 831–837. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, J.; Cui, X.; Hou, J.; Yu, F.; Wang, J.; Wang, X.; Chen, C.; Tong, L. Hyaluronic Acid Modified Nanostructured Lipid Carrier for Targeting Delivery of Kaempferol to NSCLC: Preparation, Optimization, Characterization, and Performance Evaluation In Vitro. Molecules 2022, 27, 4553. [Google Scholar] [CrossRef]
- Ghosh, A.; Khanam, N.; Nath, D. Solid Lipid Nanoparticle: A Potent Vehicle of the Kaempferol for Brain Delivery through the Blood-Brain Barrier in the Focal Cerebral Ischemic Rat. Chem. Biol. Interact. 2024, 397, 111084. [Google Scholar] [CrossRef]
- Sun, H.-J.; Jin, X.-M.; Xu, J.; Xiao, Q. Baicalin Alleviates Age-Related Macular Degeneration via miR-223/NLRP3-Regulated Pyroptosis. Pharmacology 2020, 105, 28–38. [Google Scholar] [CrossRef]
- Xiao, J.-R.; Do, C.-W.; To, C.-H. Potential Therapeutic Effects of Baicalein, Baicalin, and Wogonin in Ocular Disorders. J. Ocul. Pharmacol. Ther. 2014, 30, 605–614. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, D.; Wang, W.; Wang, Q.; Li, M.; Ma, X. Protective Effect of Baicalin on Oxidative Stress Injury in Retinal Ganglion Cells through the JAK/STAT Signaling Pathway in Vitro and in Vivo. Front. Pharmacol. 2024, 15, 1443472. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wang, Y.; Zhao, C.; Zhao, B.; Wang, J. The Pharmacological Efficacy of Baicalin in Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 9317. [Google Scholar] [CrossRef]
- Anderson, D.H.; Talaga, K.C.; Rivest, A.J.; Barron, E.; Hageman, G.S.; Johnson, L.V. Characterization of β Amyloid Assemblies in Drusen: The Deposits Associated with Aging and Age-Related Macular Degeneration. Exp. Eye Res. 2004, 78, 243–256. [Google Scholar] [CrossRef]
- Pan, W.; Sun, R.; Yu, Y.; Liu, Y.; Mu, Y.; Gong, H.; Fan, H.; Zhang, Y.; He, L.; He, H.; et al. Baicalein Nanoemulsion in Situ GEL for Dry Age-Related Macular Degeneration. Int. J. Pharm. 2025, 675, 125494. [Google Scholar] [CrossRef]
- Rasheed, S.; Rehman, K.; Shahid, M.; Suhail, S.; Akash, M.S.H. Therapeutic Potentials of Genistein: New Insights and Perspectives. J. Food Biochem. 2022, 46. [Google Scholar] [CrossRef]
- Nazari-Khanamiri, F.; Ghasemnejad-Berenji, M. Cellular and Molecular Mechanisms of Genistein in Prevention and Treatment of Diseases: An Overview. J. Food Biochem. 2021, 45, e13972. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Noda, K.; Tagawa, Y.; Inafuku, S.; Dong, Y.; Fukuhara, J.; Dong, Z.; Ando, R.; Kanda, A.; Ishida, S. Genistein Attenuates Choroidal Neovascularization. J. Nutr. Biochem. 2014, 25, 1177–1182. [Google Scholar] [CrossRef]
- Wang, B.; Zou, Y.; Li, H.; Yan, H.; Pan, J.-S.; Yuan, Z.-L. Genistein Inhibited Retinal Neovascularization and Expression of Vascular Endothelial Growth Factor and Hypoxia Inducible Factor 1α in a Mouse Model of Oxygen-Induced Retinopathy. J. Ocul. Pharmacol. Ther. 2005, 21, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.X.; Jalil, J.; Lam, K.W.; Husain, K.; Premakumar, C.M. Genistein: A Review on Its Anti-Inflammatory Properties. Front. Pharmacol. 2022, 13, 820969. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, R.; Zhang, P.; Li, J.; Yue, Y.; Hu, Y.; Cheng, W.; Pan, X. Genistein Suppresses Tumor Necrosis Factor α-Induced Inflammation via Modulating Reactive Oxygen Species/Akt/Nuclear Factor κB and Adenosine Monophosphate-Activated Protein Kinase Signal Pathways in Human Synoviocyte MH7A Cells. Drug Des. Devel. Ther. 2014, 315, 315–323. [Google Scholar] [CrossRef]
- Yan, G.; Xiao, C.; He, G.; Yin, X.; Chen, N.; Cao, Y.; He, Q. Global Phosphoproteomic Effects of Natural Tyrosine Kinase Inhibitor, Genistein, on Signaling Pathways. Proteomics 2010, 10, 976–986. [Google Scholar] [CrossRef]
- Busby, M.G.; Jeffcoat, A.R.; Bloedon, L.T.; Koch, M.A.; Black, T.; Dix, K.J.; Heizer, W.D.; Thomas, B.F.; Hill, J.M.; Crowell, J.A.; et al. Clinical Characteristics and Pharmacokinetics of Purified Soy Isoflavones: Single-Dose Administration to Healthy Men. Am. J. Clin. Nutr. 2002, 75, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Bansal, K.; Singh, V.; Mishra, S.; Bajpai, M. Articulating the Pharmacological and Nanotechnological Aspects ofGenistein: Current and Future Prospectives. Curr. Pharm. Biotechnol. 2024, 25, 807–824. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.J.; Pinheiro, M.; Neves, A.R.; Pinto, M.M.M. Therapeutic Potential of Genistein: Preclinical Studies, ClinicalEvidence, and Nanotechnology Application. Curr. Med. Chem. 2023, 30, 2480–2517. [Google Scholar] [CrossRef] [PubMed]
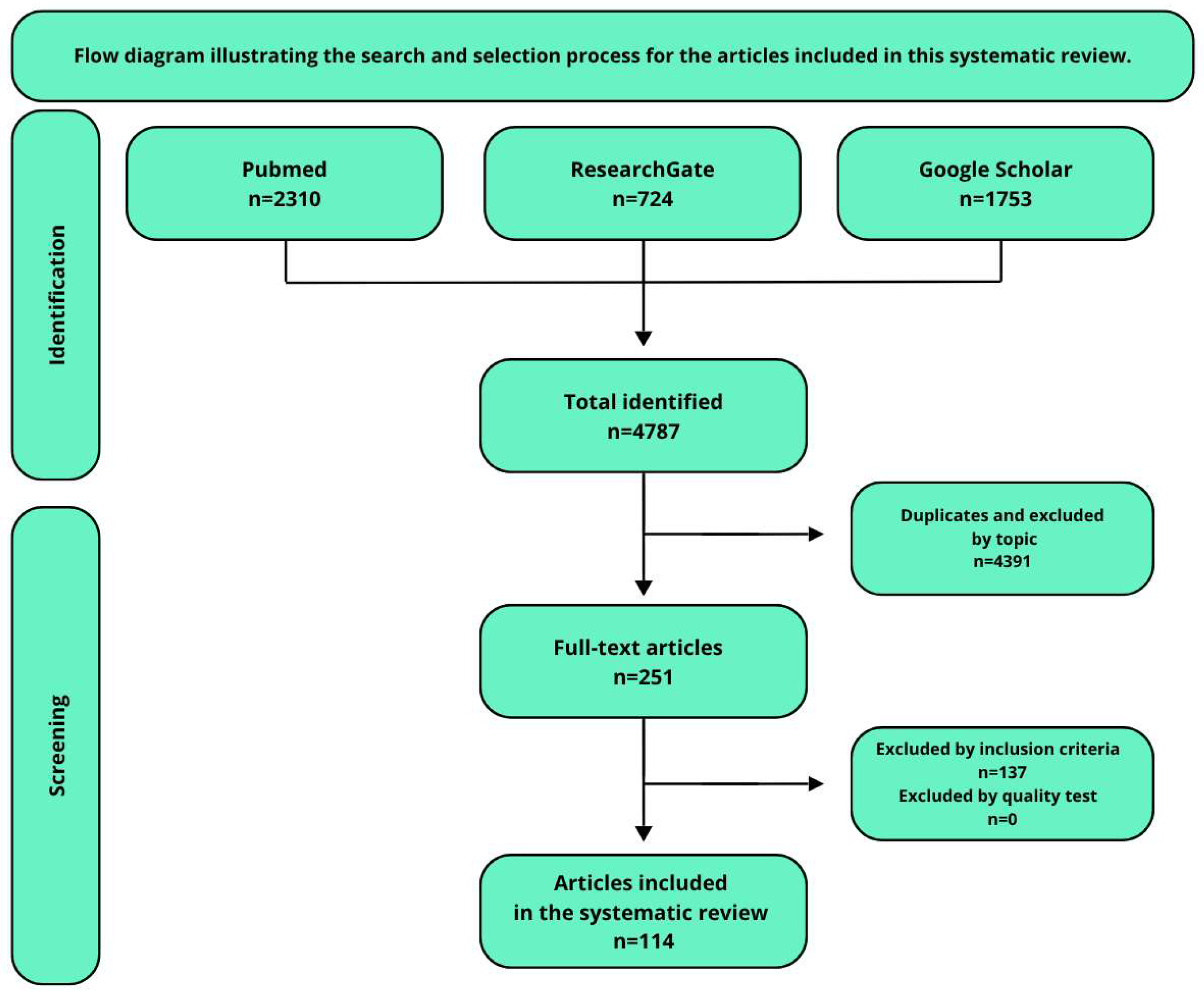
| Criteria | Details |
|---|---|
| Databases searched | PubMed, Google Scholar, ResearchGate |
| Search dates | 21 April 2009–4 December 2024 |
| Search terms | “Anthocyanins in the treatment of AMD,” “AMD pathogenesis and symptoms,” “plant-derived substances used in the treatment of AMD,” “genetic background of AMD,” “Mediterranean diet in the treatment of AMD” |
| Language restriction | English only |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Study types included | Randomized controlled trials (RCTs), in vitro and in vivo experiments, observational studies, narrative and systematic reviews |
| Peer review status | Majority of included studies were peer-reviewed |
| Reference management | Zotero (v7.0.15) used for duplicate removal and organization |
| Total articles included | 114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klusek, K.; Kijowska, M.; Kiełbus, M.; Sławińska, J.; Kuźmiuk, D.; Chorągiewicz, T.; Rejdak, R.; Dolar-Szczasny, J. The Supportive Role of Plant-Based Substances in AMD Treatment and Their Potential. Int. J. Mol. Sci. 2025, 26, 7906. https://doi.org/10.3390/ijms26167906
Klusek K, Kijowska M, Kiełbus M, Sławińska J, Kuźmiuk D, Chorągiewicz T, Rejdak R, Dolar-Szczasny J. The Supportive Role of Plant-Based Substances in AMD Treatment and Their Potential. International Journal of Molecular Sciences. 2025; 26(16):7906. https://doi.org/10.3390/ijms26167906
Chicago/Turabian StyleKlusek, Karolina, Magdalena Kijowska, Maria Kiełbus, Julia Sławińska, Dominika Kuźmiuk, Tomasz Chorągiewicz, Robert Rejdak, and Joanna Dolar-Szczasny. 2025. "The Supportive Role of Plant-Based Substances in AMD Treatment and Their Potential" International Journal of Molecular Sciences 26, no. 16: 7906. https://doi.org/10.3390/ijms26167906
APA StyleKlusek, K., Kijowska, M., Kiełbus, M., Sławińska, J., Kuźmiuk, D., Chorągiewicz, T., Rejdak, R., & Dolar-Szczasny, J. (2025). The Supportive Role of Plant-Based Substances in AMD Treatment and Their Potential. International Journal of Molecular Sciences, 26(16), 7906. https://doi.org/10.3390/ijms26167906






