Systems and Molecular Biology of Longevity and Preventive Medicine: Brain-Energy–Microbiome–Exposome Synergies in Blue Zones and the Cilento Case
Abstract
1. Introduction
- Clarify the biological and systemic pathways through which positive environmental and social conditions promote physiological resilience and healthy aging. internal regulatory states.
- Identify key integrative nodes–such as the CNS-ANS-ENS axis, immune-bioelectric interfaces, and the gut–brain-microbiota loop–that mediate adaptive responses across systems.
- Propose a scientifically grounded, systems-based model for aging as a modifiable process, with implications for geroscience, preventive medicine, and context-aware public health.
- Offer translational insights derived from real-world models of exceptional longevity, especially those observed in Cilento and other BZ, to guide the development of effective, evidence-based healthspan-promoting strategies.
2. Methods
2.1. Literature Search Strategy
2.2. Study Selection Criteria
- Original research, systematic reviews, or meta-analytical involving human or animal models directly addressing mechanisms of aging, resilience, and multisystemic integration.
- Longitudinal studies, Randomized controlled trials (RCTs), and observational research providing mechanistic insights into interactions among neurophysiological, epigenetic, immunological, microbial, and environmental factors.
- Studies examining emotional regulation, stress reactivity, empathy, or contemplative practices as mediators of neuroaffective and autonomic coherence.
- Comparative research on sociobiological determinants of longevity in BZ and Mediterranean populations.
- Studies lacking methodological rigor or transparency.
- Reports focused exclusively on pathological conditions without reference to healthy aging or resilience mechanisms.
- Publications not available in English.
2.3. Data Extraction and Thematic Organization
- Study design, setting, and sample characteristics.
- Primary biological or environmental variables examined.
- Main findings and reported limitations.
- Potential biases or confounding factors noted by the authors.
- Brain, nutriregulation, and energy metabolism.
- ANS and energy homeostasis.
- Gut microbiome.
- Epigenetic and genetic regulation.
- Telomere biology.
- Exposome.
- Insights from BZ (Cilento model).
- Physical activity and quality of life (QoL).
2.4. Integration of Original Fieldwork
2.5. Theoretical Frameworks
2.6. Bias Mitigation
2.7. Data Synthesis
3. The Brain, Nutriregulation, and Energy Metabolism in Lifestyle-Mediated Longevity
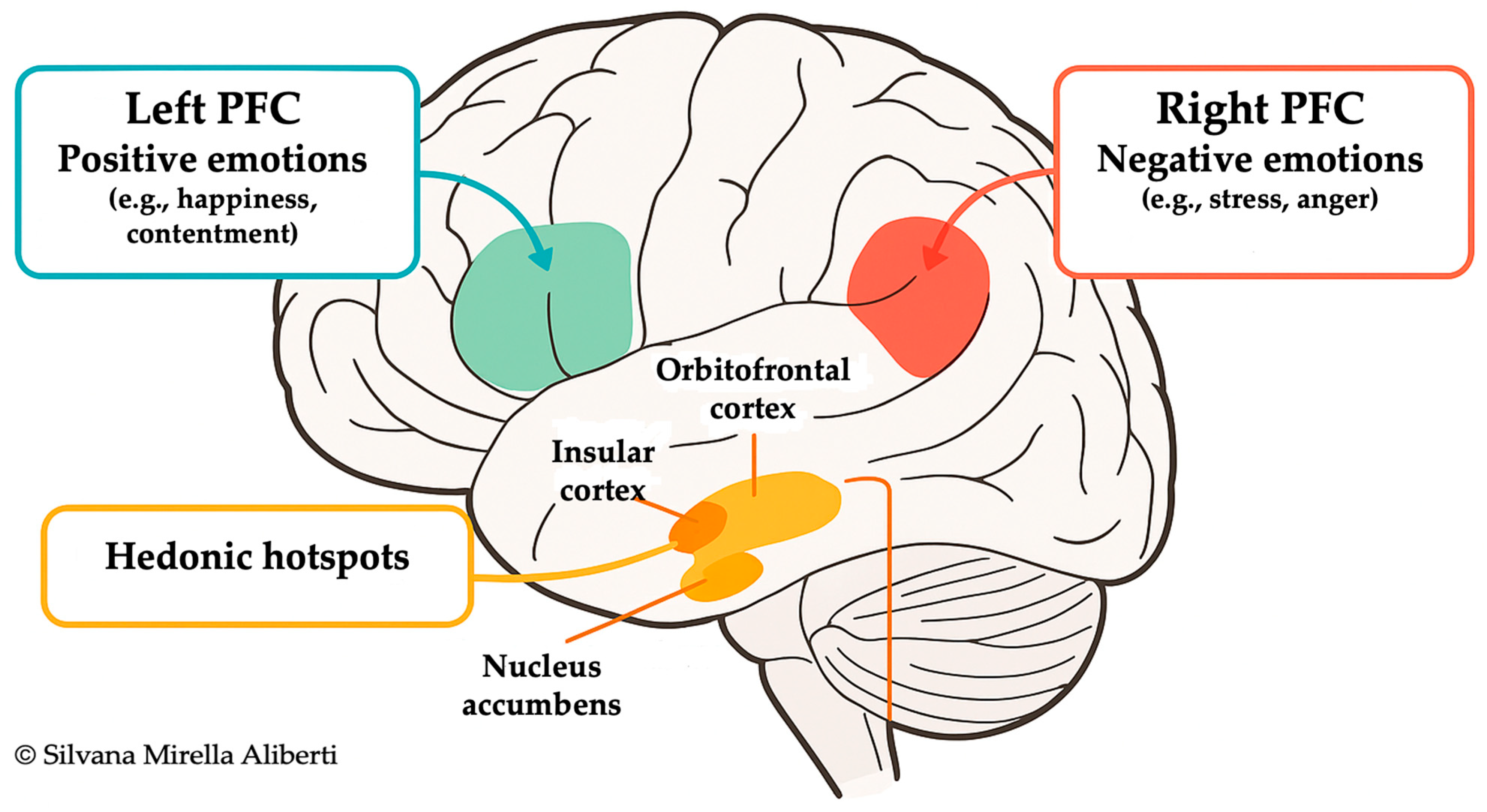
3.1. Prefrontal Cortex and Lifestyle Regulation
3.2. Emotional Processing and Autonomic Modulation
3.3. Stress, Sociocultural Context, and Neuroendocrine Aging
4. The Autonomic Nervous System: Energy Homeostasis and Neurophysiological Mediation of Aging
4.1. Sympathetic-Parasympathetic Balance and Aging Mechanisms
4.2. Bioelectrical Signaling and the Neuroenergetic Interface
4.3. The Gut–Brain-Immune Axis
4.4. Vagal Tone, Positive Emotion, and Longevity
5. The Gut Microbiome as a Central Regulator of Energy Metabolism and Longevity
6. Epigenetic and Genetic Regulation
7. Telomeres, Energy Metabolism, and Longevity
8. The Exposome and Its Role in Healthy Aging and Energy Metabolism
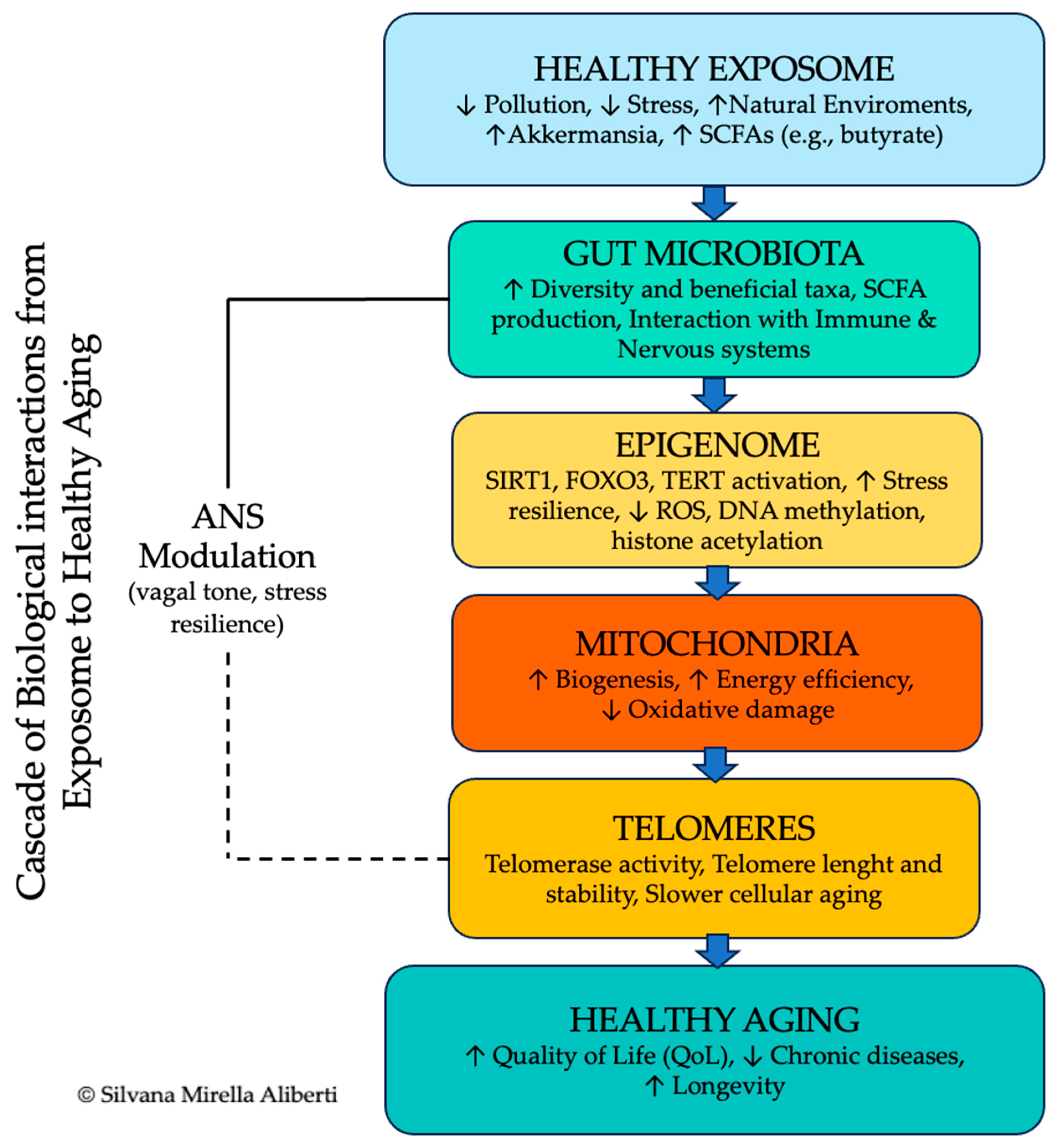
| Factor | Role in Longevity | Mechanisms Involved | Examples/Zones | Preventive/Clinical Implications |
|---|---|---|---|---|
| Exposome | Influences biological aging via environmental quality and pollutant load | Environmental toxins, microbiome-mitochondria axis, endocrine disruptors, noise, light | Cilento, Nicoya, Ikaria, Martinique, Loma Linda | Air and water quality monitoring; low-pollution housing design; urban green planning; reduction in endocrine disruptor exposure in diet and household |
| Microbiome | Modulates inflammation, improves metabolism, enhances immune and cognitive resilience | SCFA production (butyrate), NAD+, AMPK, sirtuins, autophagy | Mediterranean, fermented diets, Martinique | Targeted probiotic/prebiotic supplementation; SCFA-enhancing diets (fiber-rich, polyphenols); microbiome profiling for personalized nutrition |
| Epigenetics | Tunes gene expression in response to lifestyle and environment | DNA methylation, histone modification, microRNA, HDAC inhibition via SCFAs | Plant-based, polyphenol-rich diets | Polyphenol supplementation (resveratrol, EGCG); lifestyle interventions tracking epigenetic biomarkers; nutrigenomic counseling |
| Energy Metabolism | Enhances mitochondrial efficiency, buffers stress, supports cellular homeostasis | HRV, HPA axis, vagal tone, PGC-1α, mitochondrial biogenesis | Cilento, yoga traditions, Loma Linda | Vagal nerve stimulation (non-invasive devices); regular aerobic exercise; yoga/pranayama; Mediterranean-style diet rich in mitochondrial cofactors (CoQ10, polyphenols) |
| Telomeres | Maintains genomic stability, delays cellular senescence | Telomerase activation, anti-inflammatory signaling, oxidative stress reduction | Cilento, Nicoya, Okinawa | Stress-reduction programs; antioxidant-rich diets (vitamins C, E, carotenoids); structured exercise; omega-3 fatty acids for telomere protection |
| Cerebral Cortex | Governs conscious life choices, stress resilience, and behavior regulation | Top-down control, neuroplasticity, executive function, neuroimaging | All BZ, Loma Linda | Structured cognitive training programs; MBSR; community education on health literacy; promotion of lifelong learning |
9. Insights from Blue Zones: The Cilento Model
- Low levels of environmental pollutants.
- Predominantly plant-based, polyphenol-rich Mediterranean diet.
- High levels of daily physical activity.
- Tight-knit social structures and intergenerational bonding.
10. Physical Activity, Energy Metabolism, and Quality of Life
11. Discussion
Implication for Preventive Medicine
- Lifestyle Interventions
- Environmental Policies
- Microbiome-Based Strategies
- Precision Diagnostics
12. Conclusions
13. Recommendations and Policy Proposals
- Integrate Mitochondrial Health into Public Health and Aging PoliciesTo advance energy-efficient aging as a public health priority, it is essential to:
- Incorporate validated metrics of mitochondrial function, such as VO2 max, lactate threshold, and circulating mitochondrial-derived peptides, into chronic disease screening and healthy aging assessments.
- Expand access to community-based physical activity programs, especially in underserved or rural areas, to enhance mitochondrial biogenesis, maintain telomere integrity, and reduce inflammation across the lifespan.
- Ensure Equitable Access to Biological and Environmental DiagnosticsDisparities in access to aging-related biomarkers hinder the implementation of precision prevention. Therefore:
- Public health system should subsidize diagnostic platforms for telomere length, mitochondrial function, gut microbiota composition, and cumulative exposome burden, prioritizing vulnerable populations.
- Regional Longevity and Exposome Monitoring Centers should be established–modeled on paradigms observed in BZ such as Cilento–to longitudinally monitor biological aging and environmental exposures across diverse ecological contexts.
- Institutionalize Microbiome Health in National Nutrition and Clinical GuidelinesGiven the pivotal role of gut microbiome in modulating host metabolism, immunity, and epigenetic aging:
- National dietary recommendations should explicitly support microbiome integrity, advocating high-fiber, polyphenol-rich, and minimally processed foods, along with the regular inclusion of fermented products.
- Clinical practice should integrate microbiome assessments–particularly for patients with metabolic, autoimmune, or rare diseases–and leverage personalized nutrition platforms based on individual microbiota profiles and functional signatures.
- Position Environmental Health as a Foundational Pillar of Longevity ScienceRecognizing the exposome as a primary modulator of aging biology necessitates a robust environmental health infrastructure:
- National and municipal policy should target measurable reductions in air pollutants (e.g., PM2.5, NO2), heavy metals, and endocrine-disrupting compounds.
- Urban design initiatives should prioritize the development of biophilic environments, including green spaces and urban BZ, to mitigate chronic stress and enhance physiological resilience.
- Regulatory oversight must expand to include the long-term health impacts of food additives, microplastics, and packaging-related xenobiotics.
- Embed Neuro-Metabolic-Environmental Literacy in Education and Health PromotionTo cultivate lifelong resilience, educational frameworks must address the dynamic interplay between behavior, biology, and the environment:
- Multilevel campaigns should be implemented in schools, families, and clinical settings to increase awareness of the brain-microbiome-energy metabolism axis and its implications for physical and mental health.
- Curricula should include experimental learning in mindful eating, movement literacy, sleep hygiene, and emotional regulation, alongside ecological awareness and sustainability practices (e.g., eco-nutrition).
- Promote Transdisciplinary Research and Precision PreventionThe complexity of the aging process requires convergence science approaches:
- Dedicated funding should support longitudinal cohort studies and mechanistic trials investigating how the exposome, energy metabolism, and the gut microbiome converge to shape aging trajectories.
- Integrated translational platforms should bridge clinical care, environmental science, genomics, and public health, fostering data interoperability and policy-relevant insights.
- Artificial Intelligence and machine learning applications should be developed to model personalized aging trajectories based on genome-exposome-microbiome interactions, enabling early risk detection and targeted intervention.
- Formally Recognize and Support Emerging BZRegions such as Cilento offer replicable models of sustainable longevity:
- Criteria should be developed to formally recognize emerging BZ based on convergent evidence from epidemiological, molecular, and exposomic data.
- These regions should serve as testbeds for scalable, culturally adapted aging interventions and international collaboration in geroscience and environmental health policy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BZs | Blue Zones |
| LBZs | Longevity Blue Zones |
| SCFAs | Short-chain fatty acids (e.g., butyrate, acetate, propionate) |
| QoL | Quality of Life |
| HRV | Heart-Rate Variability |
| HPA | Hypothalamic–Pituitary–Adrenal axis |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| NAD+ | Nicotinamide Adenine Dinucleotide (oxidized form) |
| AMPK | AMP-activated protein kinase |
| HDAC | Histone Deacetylase |
| CNS | Central nervous system |
| ANS | Autonomic nervous system |
| ENS | Enteric nervous system |
| EEG | Electroencephalogram |
| ECG | Electrocardiogram |
| PFC | Prefrontal cortex |
| ATP | Adenosine Triphosphate |
| ROS | Reactive oxygen species |
| HF-HRV | High-frequency heart-rate variability |
| OFC | Orbitofrontal Cortex |
| BDNF | Brain-derived neurotrophic factor |
| SNS | Sympathetic nervous system |
| PNS | parasympathetic nervous system |
| TNTs | tunneling nanotubes |
| DNA | Deoxyribonucleic Acid |
| BBB | Blood–brain barrier |
| HA | Histone acetylation |
| SNPs | Single-nucleotide polymorphisms |
| FOXO3 | Forkhead Box 03 |
| SIRT1 | Sirtuin 1 |
| mtDNA | Mitochondrial DNA |
| LTL | Leukocyte telomere length |
| CRP | C-reactive protein |
| TERT | Telomerase Reverse Transcriptase |
| CEMI | Conscious electromagnetic information field |
| EMFs | Electromagnetic fields |
| TAFA4 | Tumor necrosis factor (TNT) Alpha-induced Protein 8-Like2 |
| VNS | Vagus nerve stimulation |
| MBSR | Mindfulness-based stress reduction |
| LPS | lipopolysaccharides |
| mTOR | Mechanistic target of Rapamycin |
References
- Passarino, G.; De Rango, F.; Montesanto, A. Human longevity: Genetics or Lifestyle? It takes two to tango. Immun. Ageing 2016, 13, 12. [Google Scholar] [CrossRef]
- Wild, C.P. The exposome: From concept to utility. Int. J. Epidemiol. 2012, 41, 24–32. [Google Scholar] [CrossRef]
- Poulain, M.; Herm, A.; Pes, G. The blue zones: Areas of exceptional longevity around the world. Vienna Yearb. Popul. Res. 2013, 11, 87–108. [Google Scholar] [CrossRef]
- Buettner, D. The Blue Zones: 9 Lessons for Living Longer from the People Who’ve Lived the Longest; National Geographic Books: Washington, DC, USA, 2012; ISBN 978-1-4262-0400-5. [Google Scholar]
- Poulain, M.; Herm, A. Validation of Exceptional Longevity in Martinique. Available online: https://longevitybluezone.com/wp-content/uploads/2023/03/validation-martinique-26-mars-eng.pdf (accessed on 14 July 2025).
- Aliberti, S.M.; Donato, A.; Funk, R.H.W.; Capunzo, M. A Narrative Review Exploring the Similarities between Cilento and the Already Defined “Blue Zones” in Terms of Environment, Nutrition, and Lifestyle: Can Cilento Be Considered an Undefined “Blue Zone”? Nutrients 2024, 16, 729. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, S.M.; De Caro, F.; Funk, R.H.W.; Schiavo, L.; Gonnella, J.; Boccia, G.; Capunzo, M. Extreme Longevity: Analysis of the Direct or Indirect Influence of Environmental Factors on Old, Nonagenarians, and Centenarians in Cilento, Italy. Int. J. Environ. Res. Public Health 2022, 19, 1589. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, S.M.; Funk, R.H.W.; Schiavo, L.; Giudice, A.; Ciaglia, E.; Puca, A.A.; Gonnella, J.; Capunzo, M. Clinical Status, Nutritional Behavior, and Lifestyle, and Determinants of Community Well-Being of Patients from the Perspective of Physicians: A Cross-Sectional Study of Young Older Adults, Nonagenarians, and Centenarians in Salerno and Province, Italy. Nutrients 2022, 14, 3665. [Google Scholar] [CrossRef]
- Aliberti, S.M.; Funk, R.H.W.; Ciaglia, E.; Gonnella, J.; Giudice, A.; Vecchione, C.; Puca, A.A.; Capunzo, M. Old, Nonagenarians, and Centenarians in Cilento, Italy and the Association of Lifespan with the Level of Some Physicochemical Elements in Tap Drinking Water. Nutrients 2023, 15, 218. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, S.M.; Capunzo, M. The Power of Environment: A Comprehensive Review of the Exposome’s Role in Healthy Aging, Longevity, and Preventive Medicine—Lessons from Blue Zones and Cilento. Nutrients 2025, 17, 722. [Google Scholar] [CrossRef]
- Aliberti, S.M.; Capunzo, M.; Galimberti, D.; Accardi, G.; Aiello, A.; Calabrò, A.; Caruso, C.; Candore, G. Ageing Trajectories: Exposome-Driven Pathobiological Mechanisms and Implications for Prevention from Blue Zones and Italian Longevity Hotspots Such as Cilento and Sicilian Mountain Villages. Int. J. Mol. Sci. 2025, 26, 4796. [Google Scholar] [CrossRef]
- McKlveen, J.M.; Myers, B.; Herman, J.P. The medial prefrontal cortex: Coordinator of autonomic, neuroendocrine and behavioural responses to stress. J. Neuroendocrinol. 2015, 27, 446–456. [Google Scholar] [CrossRef]
- Duan, M.; Xu, Y.; Li, Y.; Feng, H.; Chen, Y. Targeting brain-peripheral immune responses for secondary brain injury after ischemic and hemorrhagic stroke. J. Neuroinflamm. 2024, 21, 102. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef]
- Luo, J.; Liang, S.; Jin, F. Gut microbiota and healthy longevity. Sci. China Life Sci. 2024, 67, 2590–2602. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.W.; Jones, D.P. The nature of nurture: Refining the definition of the exposome. Toxicol. Sci. 2014, 137, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Na, D.; Zhang, Z.; Meng, M.; Li, M.; Gao, J.; Kong, J.; Zhang, G.; Guo, Y. Energy Metabolism and Brain Aging: Strategies to Delay Neuronal Degeneration. Cell. Mol. Neurobiol. 2025, 45, 38. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Gianaros, P.J. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 2010, 1186, 190–222. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Funk, R.H.W. Reflections about a “Membrane” between Mind and Brain. Arch. Anat. Physiol. 2024, 9, 7–20. [Google Scholar] [CrossRef]
- Funk, R.H.W. Essay on information processes in the human brain. Arch. Anat. Physiol. 2024, 9, 1–6. [Google Scholar] [CrossRef]
- Funk, R.H.W. Minimal Units of Consciousness and Possible Evolution of Intelligence. Eur. J. Appl. Sci. 2024, 12, 151–188. [Google Scholar] [CrossRef]
- Funk, R.H.W. Biophysical mechanisms complementing “classical” cell biology. Front. Biosci. 2018, 23, 921–939. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E. The brain’s dark energy. Sci. Am. 2010, 302, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Reichert, S.; Stier, A. Does oxidative stress shorten telomeres in vivo? A review. Biol. Lett. 2017, 13, 20170463. [Google Scholar] [CrossRef]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Gutierrez-Mariscal, F.M.; Ojeda-Rodriguez, A.; Perez-Martinez, P.; Lopez-Miranda, J. The Mediterranean Diet. In Nutrition, Fitness, and Mindfulness—An Evidence-Based Guide for Clinicians, 2nd ed.; Uribarri, J., Vassalotti, J.A., Eds.; Humana Cham: Totowa, NJ, USA, 2025; pp. 19–37. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Davidson, R.J. What does the prefrontal cortex “do” in affect: Perspectives on frontal EEG asymmetry research. Biol. Psychol. 2004, 67, 219–233. [Google Scholar] [CrossRef]
- Kop, W.J.; Synowski, S.J.; Newell, M.E.; Schmidt, L.A.; Waldstein, S.R.; Fox, N.A. Autonomic nervous system reactivity to positive and negative mood induction: The role of acute psychological responses and frontal electrocortical activity. Biol. Psychol. 2011, 86, 230–238. [Google Scholar] [CrossRef]
- Wittling, W.; Block, A.; Genzel, S.; Schweiger, E. Hemisphere asymmetry in parasympathetic control of the heart. Neuropsychologia 1998, 36, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, F. Dual-Brain Psychology: A novel theory and treatment based on cerebral laterality and psychopathology. Front. Psychol. 2022, 13, 986374. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.C.; Berridge, K.C. Advances in the neurobiological bases for food ‘liking’ versus ‘wanting’. Physiol. Behav. 2014, 136, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Kringelbach, M.L.; Berridge, K.C. The Neuroscience of Happiness and Pleasure. Soc. Res. 2010, 77, 659–678. [Google Scholar] [CrossRef]
- Georgiadis, J.R.; Kringelbach, M.L.; Pfaus, J.G. Sex for fun: A synthesis of human and animal neurobiology. Nat. Rev. Urol. 2012, 9, 486–498. [Google Scholar] [CrossRef]
- Small, D.M.; Zatorre, R.J.; Dagher, A.; Evans, A.C.; Jones-Gotman, M. Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain 2001, 124, 1720–1733. [Google Scholar] [CrossRef]
- Veldhuizen, M.G.; Douglas, D.; Aschenbrenner, K.; Gitelman, D.R.; Small, D.M. The anterior insular cortex represents breaches of taste identity expectation. J. Neurosci. 2010, 31, 14735–14744. [Google Scholar] [CrossRef]
- Singer, T.; Critchley, H.D.; Preuschoff, K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn. Sci. 2009, 13, 334–340. [Google Scholar] [CrossRef]
- de Vignemont, F.; Singer, T. The empathic brain: How, when and why? Trends Cogn. Sci. 2006, 10, 435–441. [Google Scholar] [CrossRef]
- Singer, T. A neuroscience perspective on the plasticity of the social and relational brain. Ann. N. Y. Acad. Sci. 2025, 1547, 52–74. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel—Now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Chen, W.G.; Schloesser, D.; Arensdorf, A.M.; Simmons, J.M.; Cui, C.; Valentino, R.; Gnadt, J.W.; Nielsen, L.; Hillaire-Clarke, C.S.; Spruance, V.; et al. The Emerging Science of Interoception: Sensing, Integrating, Interpreting, and Regulating Signals within the Self. Trends Neurosci. 2021, 44, 3–16. [Google Scholar] [CrossRef]
- Rodríguez-Vidal, L.; Alcauter, S.; Barrios, F.A. The functional connectivity of the human claustrum, according to the Human Connectome Project database. PLoS ONE 2024, 19, e0298349. [Google Scholar] [CrossRef] [PubMed]
- Pockett, S. The electromagnetic field theory of consciousness: A testable hypothesis about the characteristics of conscious as opposed to non-conscious fields. J. Conscious. Stud. 2012, 19, 191–223. [Google Scholar]
- McFadden, J. Integrating information in the brain’s EM field: The cemi field theory of consciousness. Neurosci. Conscious. 2020, 2020, niaa016. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.W.; Hunt, T. Electromagnetic-field theories of qualia: Can they improve upon standard neuroscience? Front. Psychol. 2023, 14, 1015967. [Google Scholar] [CrossRef]
- Braitenberg, V. Thoughts on the cerebral cortex. J. Theor. Biol. 1974, 46, 421–447. [Google Scholar] [CrossRef]
- Heck, D.H.; Varga, S. “The great mixing machine”: Multisensory integration and brain-breath coupling in the cerebral cortex. Pflügers Arch. 2023, 475, 5–11. [Google Scholar] [CrossRef]
- Zhi, G.; Xiu, R. Quantum Theory of Consciousness. J. Appl. Math. Phys. 2023, 11, 2652–2670. [Google Scholar] [CrossRef]
- Hameroff, S.; Penrose, R. Consciousness in the universe. Phys. Life Rev. 2014, 11, 39–78. [Google Scholar] [CrossRef]
- Söderpalm, A.H.; Berridge, K.C. The hedonic impact and intake of food are increased by midazolam microinjection in the parabrachial nucleus. Brain Res. 2000, 877, 288–297. [Google Scholar] [CrossRef]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef]
- Shiota, M.N.; Neufeld, S.L.; Yeung, W.H.; Moser, S.E.; Perea, E.F. Feeling good: Autonomic nervous system responding in five positive emotions. Emotion 2011, 11, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Steffener, J. Education and age-related differences in cortical thickness and volume across the lifespan. Neurobiol. Aging 2021, 102, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Puhlmann, L.M.C.; Vrtička, P.; Linz, R.; Valk, S.L.; Papassotiriou, I.; Chrousos, G.P.; Engert, V.; Singer, T. Serum BDNF Increase After 9-Month Contemplative Mental Training Is Associated with Decreased Cortisol Secretion and Increased Dentate Gyrus Volume: Evidence From a Randomized Clinical Trial. Biol. Psychiatry Glob. Open Sci. 2024, 5, 100414. [Google Scholar] [CrossRef] [PubMed]
- Ask, T.F.; Sütterlin, S. Prefrontally modulated vagal neuroimmunomodulation is associated with telomere length. Front. Neurosci. 2022, 16, 1063162. [Google Scholar] [CrossRef]
- Marques-Deak, A.; Cizza, G.; Sternberg, E. Brain-immune interactions and disease susceptibility. Mol. Psychiatry 2005, 10, 239–250. [Google Scholar] [CrossRef]
- Steinman, L. Elaborate interactions between the immune and nervous systems. Nat. Immunol. 2004, 5, 575–581. [Google Scholar] [CrossRef]
- Mueller, B.; Figueroa, A.; Robinson-Papp, J. Structural and functional connections between the autonomic nervous system, hypothalamic-pituitary-adrenal axis, and the immune system: A context and time dependent stress response network. Neurol. Sci. 2022, 43, 951–960. [Google Scholar] [CrossRef]
- Mueller, S.N. Neural control of immune cell trafficking. J. Exp. Med. 2022, 219, e20211604. [Google Scholar] [CrossRef]
- Daëron, M. The immune system as a system of relations. Front. Immunol. 2022, 13, 984678. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Bioelectric signaling: Reprogrammable circuits underlying embryogenesis, regeneration, and cancer. Cell 2021, 184, 1971–1989. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Large-scale biophysics: Ion flows and regeneration. Trends Cell Biol. 2007, 17, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Funk, R.H.; Monsees, T.; Ozkucur, N. Electromagnetic effects—From cell biology to medicine. Prog. Histochem. Cytochem. 2009, 43, 177–264. [Google Scholar] [CrossRef]
- Wittig, D.; Wang, X.; Walter, C.; Gerdes, H.H.; Funk, R.H.; Roehlecke, C. Multi-level communication of human retinal pigment epithelial cells via tunneling nanotubes. PLoS ONE 2012, 7, e33195. [Google Scholar] [CrossRef]
- Scholkmann, F. Two emerging topics regarding long-range physical signaling in neurosystems: Membrane nanotubes and electromagnetic fields. J. Integr. Neurosci. 2015, 14, 135–153. [Google Scholar] [CrossRef]
- Schwab, W.; Funk, R.H.W. Innervation pattern of different cartilaginous tissues in the rat. Acta Anat. 1998, 163, 184–190. [Google Scholar] [CrossRef]
- Funk, R.H.W.; Scholkmann, F. The significance of bioelectricity on all levels of organization of an organism Part 1: From the subcellular level to cells. Prog. Biophys. Mol. Biol. 2023, 177, 185–201. [Google Scholar] [CrossRef]
- Farmer, D.G.; Dutschmann, M.; Paton, J.F.; Pickering, A.E.; McAllen, R.M. Brainstem sources of cardiac vagal tone and respiratory sinus arrhythmia. J. Physiol. 2016, 594, 7249–7265. [Google Scholar] [CrossRef]
- Jerath, R.; Beveridge, C.; Barnes, V.A. Self-Regulation of Breathing as an Adjunctive Treatment of Insomnia. Front. Psychiatry 2019, 9, 780. [Google Scholar] [CrossRef]
- Rubik, B.; Muehsam, D.; Hammerschlag, R.; Jain, S. Biofield Science and Healing: History, Terminology, and Concepts. Glob. Adv. Health Med. 2015, 4, 8–14. [Google Scholar] [CrossRef]
- Islam, K.U.S.; Meli, N.; Blaess, S. The Development of the Mesoprefrontal Dopaminergic System in Health and Disease. Front. Neural Circuits 2021, 15, 746582. [Google Scholar] [CrossRef] [PubMed]
- McFadden, J.; Al-Khalili, J. The origins of quantum biology. Proc. Math. Phys. Eng. Sci. 2018, 474, 20180674. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Hatch, A.M.; Arcidiacono, S.M.; Pearce, S.C.; Pantoja-Feliciano, I.G.; Doherty, L.A.; Soares, J.W. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Front. Microbiol. 2018, 9, 2013. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.K.; Oh, J.S. Interaction of the Vagus Nerve and Serotonin in the Gut-Brain Axis. Int. J. Mol. Sci. 2025, 26, 1160. [Google Scholar] [CrossRef]
- Kasarello, K.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Communication of gut microbiota and brain via immune and neuroendocrine signaling. Front. Microbiol. 2023, 14, 1118529. [Google Scholar] [CrossRef]
- Hadaya, J.; Ardell, J.L. Autonomic Modulation for Cardiovascular Disease. Front. Physiol. 2020, 11, 617459. [Google Scholar] [CrossRef]
- DeLalio, L.J.; Sved, A.F.; Stocker, S.D. Sympathetic Nervous System Contributions to Hypertension: Updates and Therapeutic Relevance. Can. J. Cardiol. 2020, 36, 712–720. [Google Scholar] [CrossRef]
- Carson, R.C.; Butcher, J.N.; Mineka, S.; Hooley, J.M. Abnormal Psychology and Modern Life, 13th ed.; Allyn & Bacon: Boston, MA, USA, 2006. [Google Scholar]
- Quansah, M.; David, M.A.; Martins, R.; El-Omar, E.; Aliberti, S.M.; Capunzo, M.; Jensen, S.O.; Tayebi, M. The Beneficial Effects of Lactobacillus Strains on Gut Microbiome in Alzheimer’s Disease: A Systematic Review. Healthcare 2025, 13, 74. [Google Scholar] [CrossRef]
- Danner, D.D.; Snowdon, D.A.; Friesen, W.V. Positive emotions in early life and longevity: Findings from the nun study. J. Pers. Soc. Psychol. 2001, 80, 804–813. [Google Scholar] [CrossRef]
- Fredrickson, B.L.; Levenson, R.W. Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cogn. Emot. 1998, 12, 191–220. [Google Scholar] [CrossRef] [PubMed]
- Black, D.S.; Slavich, G.M. Mindfulness meditation and the immune system: A systematic review of randomized controlled trials. Ann. N. Y. Acad. Sci. 2016, 1373, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lee, E.K.P.; Mak, E.C.W.; Ho, C.Y.; Wong, S.Y.S. Mindfulness-based interventions: An overall review. Br. Med. Bull. 2021, 138, 41–57. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Bowles, N.P.; Gray, J.D.; Hill, M.N.; Hunter, R.G.; Karatsoreos, I.N.; Nasca, C. Mechanisms of stress in the brain. Nat. Neurosci. 2015, 18, 1353–1363. [Google Scholar] [CrossRef]
- Mathur, M.B.; Epel, E.; Kind, S.; Desai, M.; Parks, C.G.; Sandler, D.P.; Khazeni, N. Perceived stress and telomere length: A systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain Behav. Immun. 2016, 54, 158–169. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Steenbergen, L.; Maraver, M.J.; Actis-Grosso, R.; Ricciardelli, P.; Colzato, L.S. Recognizing emotions in bodies: Vagus nerve stimulation enhances recognition of anger while impairing sadness. Cogn. Affect. Behav. Neurosci. 2021, 21, 1246–1261. [Google Scholar] [CrossRef]
- Wilmanski, T.; Diener, C.; Rappaport, N.; Patwardhan, S.; Wiedrick, J.; Lapidus, J.; Earls, J.C.; Zimmer, A.; Glusman, G.; Robinson, M.; et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 2021, 3, 274–286. [Google Scholar] [CrossRef]
- Aliberti, S.M.; Sacco, A.M.; Belviso, I.; Romano, V.; Di Martino, A.; Russo, E.; Collet, S.; Ciancaleoni Bartoli, I.; Tuzi, M.; Capunzo, M.; et al. Potential Impact of Physical Activity on Measures of Well-Being and Quality of Life in People with Rare Diseases: A Nationwide Cross-Sectional Study in Italy. Healthcare 2024, 12, 1822. [Google Scholar] [CrossRef]
- Mongi-Bragato, B.; Zamponi, E.; García-Keller, C.; Assis, M.A.; Virgolini, M.B.; Mascó, D.H.; Zimmer, A.; Cancela, L.M. Enkephalin is essential for the molecular and behavioral expression of cocaine sensitization. Addict. Biol. 2016, 21, 326–338. [Google Scholar] [CrossRef]
- Behnke, M.; Kreibig, S.D.; Kaczmarek, L.D.; Assink, M.; Gross, J.J. Autonomic Nervous System Activity During Positive Emotions: A Meta-Analytic Review. Emot. Rev. 2022, 14, 132–160. [Google Scholar] [CrossRef]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.C.; Scicluna, B.P.; Arts, R.J.; Gresnigt, M.S.; Lachmandas, E.; Giamarellos-Bourboulis, E.J.; Kox, M.; Manjeri, G.R.; Wagenaars, J.A.; Cremer, O.L.; et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat. Immunol. 2016, 17, 406–413. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Belkaid, Y.; Harrison, O.J. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- Fraga, M.F.; Ballestar, E.; Paz, M.F.; Ropero, S.; Setien, F.; Ballestar, M.L.; Heine-Suñer, D.; Cigudosa, J.C.; Urioste, M.; Benitez, J.; et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. USA 2005, 102, 10604–10609. [Google Scholar] [CrossRef]
- Ciaglia, E.; Montella, F.; Lopardo, V.; Basile, C.; Esposito, R.M.; Maglio, C.; Longo, R.; Maciag, A.; Puca, A.A. The Genetic and Epigenetic Arms of Human Ageing and Longevity. Biology 2025, 14, 92. [Google Scholar] [CrossRef]
- Ramzan, F.; Vickers, M.H.; Mithen, R.F. Epigenetics, microRNA and Metabolic Syndrome: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 5047. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut Microbiota and Extreme Longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef]
- Cole, S.W. Human social genomics. PLoS Genet. 2014, 10, e1004601. [Google Scholar] [CrossRef]
- Codd, V.; Nelson, C.P.; Albrecht, E.; Mangino, M.; Deelen, J.; Buxton, J.L.; Hottenga, J.J.; Fischer, K.; Esko, T.; Surakka, I.; et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat. Genet. 2013, 45, 422–427. [Google Scholar] [CrossRef]
- Willcox, B.J.; Donlon, T.A.; He, Q.; Chen, R.; Grove, J.S.; Yano, K.; Masaki, K.H.; Willcox, D.C.; Rodriguez, B.; Curb, J.D. FOXO3A genotype is strongly associated with human longevity. Proc. Natl. Acad. Sci. USA 2008, 105, 13987–13992. [Google Scholar] [CrossRef]
- Feinberg, A.P. Phenotypic plasticity and the epigenetics of human disease. Nature 2007, 447, 433–440. [Google Scholar] [CrossRef]
- Morris, B.J.; Willcox, B.J.; Donlon, T.A. Genetic and epigenetic regulation of human aging and longevity. Biochim. Biophys. Acta. Mol. Basis Dis. 2019, 1865, 1718–1744. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef]
- Polom, J.; Boccardi, V. Employing Nutrition to Delay Aging: A Plant-Based Telomere-Friendly Dietary Revolution. Nutrients 2025, 17, 2004. [Google Scholar] [CrossRef]
- Crous-Bou, M.; Fung, T.T.; Prescott, J.; Julin, B.; Du, M.; Sun, Q.; Rexrode, K.M.; Hu, F.B.; De Vivo, I. Mediterranean diet and telomere length in Nurses’ Health Study: Population based cohort study. BMJ 2014, 349, g6674. [Google Scholar] [CrossRef] [PubMed]
- Tyrka, A.R.; Parade, S.H.; Price, L.H.; Kao, H.T.; Porton, B.; Philip, N.S.; Welch, E.S.; Carpenter, L.L. Alterations of Mitochondrial DNA Copy Number and Telomere Length with Early Adversity and Psychopathology. Biol. Psychiatry 2016, 79, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. The gut microbiome as a modulator of healthy ageing. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 565–584. [Google Scholar] [CrossRef]
- Irwin, M.R.; Cole, S.W. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 2011, 11, 625–632. [Google Scholar] [CrossRef]
- Rentscher, K.E.; Carroll, J.E.; Cole, S.W.; Repetti, R.L.; Robles, T.F. Relationship closeness buffers the effects of perceived stress on transcriptomic indicators of cellular stress and biological aging marker p16INK4a. Aging 2020, 12, 16476–16490. [Google Scholar] [CrossRef]
- Crocco, P.; De Rango, F.; Dato, S.; Rose, G.; Passarino, G. Telomere length as a function of age at population level parallels human survival curves. Aging 2021, 13, 204–218. [Google Scholar] [CrossRef]
- Schellnegger, M.; Hofmann, E.; Carnieletto, M.; Kamolz, L.P. Unlocking longevity: The role of telomeres and its targeting interventions. Front. Aging 2024, 5, 1339317. [Google Scholar] [CrossRef]
- Vidacek, N.Š.; Nanic, L.; Ravlic, S.; Sopta, M.; Geric, M.; Gajski, G.; Garaj-Vrhovac, V.; Rubelj, I. Telomeres, Nutrition, and Longevity: Can We Really Navigate Our Aging? J. Gerontol. Biol. Sci. Med. Sci. 2017, 73, 39–47. [Google Scholar] [CrossRef]
- Chakravarti, D.; LaBella, K.A.; DePinho, R.A. Telomeres: History, health, and hallmarks of aging. Cell 2021, 184, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Daniela, M.; Catalina, L.; Ilie, O.; Paula, M.; Daniel-Andrei, I.; Ioana, B. Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects. Antioxidants 2022, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yu, X.; Zhang, C.; Wang, Y.; Sun, Y.; Sun, H.; Zhang, H.; Shi, Y.; He, X. Telomeres and Mitochondrial Metabolism: Implications for Cellular Senescence and Age-related Diseases. Stem Cell Rev. Rep. 2022, 18, 2315–2327. [Google Scholar] [CrossRef] [PubMed]
- Schellnegger, M.; Lin, A.C.; Hammer, N.; Kamolz, L.P. Physical Activity on Telomere Length as a Biomarker for Aging: A Systematic Review. Sports Med. Open 2022, 8, 111. [Google Scholar] [CrossRef]
- Uchino, B.N.; Trettevik, R.; Kent de Grey, R.G.; Cronan, S.; Hogan, J.; Baucom, B.R.W. Social support, social integration, and inflammatory cytokines: A meta-analysis. Health Psychol. 2018, 37, 462–471. [Google Scholar] [CrossRef]
- Picard, M.; McEwen, B.S. Psychological Stress and Mitochondria: A Systematic Review. Psychosom. Med. 2018, 80, 141–153. [Google Scholar] [CrossRef]
- Carroll, J.E.; Diez Roux, A.V.; Fitzpatrick, A.L.; Seeman, T. Low social support is associated with shorter leukocyte telomere length in late life: Multi-ethnic study of atherosclerosis. Psychosom. Med. 2013, 75, 171–177. [Google Scholar] [CrossRef]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, J.; Hu, P.; Deng, C.; Luo, Z.; Zhao, J.; Zhang, Z.; Yi, W.; Zhu, G.; Zheng, G.; et al. Key factors in epidemiological exposure and insights for environmental management: Evidence from meta-analysis. Environ. Pollut. 2024, 362, 124991. [Google Scholar] [CrossRef]
- Rook, G.A. Regulation of the immune system by biodiversity from the natural environment: An ecosystem service essential to health. Proc. Natl. Acad. Sci. USA 2013, 110, 18360–18367. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Hicks, M.R.; Pyle, A.D. The emergence of the stem cell niche. Trends Cell Biol. 2023, 33, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.; Coan, J.A. Beyond Nature Versus Nurture: The Emergence of Emotion. Affect. Sci. 2023, 4, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Godara, M.; Singer, T. Resilient Stress Reactivity Profiles Predict Mental Health Gains from Online Contemplative Training: A Randomized Clinical Trial. J. Pers. Med. 2024, 14, 493. [Google Scholar] [CrossRef]
- McDonnell, A. The Sixth Sense-Emotional Contagion; Review of Biophysical Mechanisms Influencing Information Transfer in Groups. J. Behav. Brain Sci. 2014, 4, 342–374. [Google Scholar] [CrossRef]
- Aliberti, S.M.; Donato, A.; Funk, R.H.W.; Capunzo, M. Longevity Blue Zones. Encyclopedia. Available online: https://encyclopedia.pub/entry/55867 (accessed on 18 July 2025).
- Masi, D.; Kumar, V.; Garza-Reyes, J.A.; Godsell, J. Towards a more circular economy: Exploring the awareness, practices, and barriers from a focal firm perspective. Prod. Plan. Control. 2018, 29, 539–550. [Google Scholar] [CrossRef]
- Cacciatore, S.; Mao, S.; Nuñez, M.V.; Massaro, C.; Spadafora, L.; Bernardi, M.; Perone, F.; Sabouret, P.; Biondi-Zoccai, G.; Banach, M.; et al. Urban health inequities and healthy longevity: Traditional and emerging risk factors across the cities and policy implications. Aging Clin. Exp. Res. 2025, 37, 143. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Feher, A.; Csipo, T.; Forrai, J.; Dosa, N.; Peterfi, A.; Lehoczki, A.; Tarantini, S.; et al. Nutrition Strategies Promoting Healthy Aging: From Improvement of Cardiovascular and Brain Health to Prevention of Age-Associated Diseases. Nutrients 2022, 15, 47. [Google Scholar] [CrossRef]
- Schübel, R.; Nattenmüller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.L.; von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef]
- Nutile, T.; Ruggiero, D.; Herzig, A.F.; Tirozzi, A.; Nappo, S.; Sorice, R.; Marangio, F.; Bellenguez, C.; Leutenegger, A.L.; Ciullo, M. Whole-Exome Sequencing in the Isolated Populations of Cilento from South Italy. Sci. Rep. 2019, 9, 4059. [Google Scholar] [CrossRef]
- Russo, F.; Caporaso, N.; Paduano, A.; Sacchi, R. Phenolic compounds in fresh and dried figs from Cilento (Italy), by considering breba crop and full crop, in comparison to Turkish and Greek dried figs. J. Food Sci. 2014, 79, C1278–C1284. [Google Scholar] [CrossRef]
| Domain | Recommendation/Policy Proposal | Target | Objective | Biological Mechanism/Rationale |
|---|---|---|---|---|
| Diagnostics & Monitoring | Public funding for telomere, mitochondrial, microbiome, and exposome biomarkers | Governments, regional health systems | Improve access to predictive tools for biological aging | Enables early detection of metabolic decline and biological age acceleration |
| Education & Literacy | Integrate education on nutrition, stress, environment, and microbiome in schools and communities | Ministries of Education and Health | Foster awareness and autonomy in preventive health | Shapes health behaviors and enhances brain-microbiome-energy literacy |
| Urban Planning & Environment | Promote urban greening, walkability, and air/water quality improvements | Municipalities, local governments | Recalibrate the exposome for healthier aging environments | Reduces oxidative stress, improves mitochondrial and immune function |
| Food & Nutrition Policies | Support local agriculture and Mediterranean diets in public canteens | Agricultural ministries, schools, hospitals | Encourage adoption of nutrient-dense and sustainable diets | Promotes microbial diversity, epigenetic stability, and energy efficiency |
| Accessible Physical Activity | Fund public fitness areas, walking routes, and adapted programs for older and chronically diseases | Local governments, healthcare providers | Reduce inequality in access to physical activity | Enhances mitochondrial biogenesis, telomerase activity, and vagal tone |
| Precision Preventive Medicine | Integrate microbiome, mitochondrial, telomeric, and exposome data into prevention protocols | Health systems, clinical research centers | Promote tailored interventions based on individual biology | Enables stratified interventions and personalized risk reduction |
| Territorial Health Equity | Strengthen healthcare infrastructure in rural or under-resourced areas, including emerging BZ | National and EU-level policymakers | Address health disparities and regional diagnostic gaps | Reduces geographic inequalities in healthy aging potential |
| Research & Innovation | Fund longitudinal studies on the brain-energy-environment axis and aging biomarkers | Ministries of Research, universities, EU | Validate interventions and develop scalable diagnostic tools | Supports evidence-based, cross-domain geroscience innovation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliberti, S.M.; Capunzo, M.; Funk, R.H.W. Systems and Molecular Biology of Longevity and Preventive Medicine: Brain-Energy–Microbiome–Exposome Synergies in Blue Zones and the Cilento Case. Int. J. Mol. Sci. 2025, 26, 7887. https://doi.org/10.3390/ijms26167887
Aliberti SM, Capunzo M, Funk RHW. Systems and Molecular Biology of Longevity and Preventive Medicine: Brain-Energy–Microbiome–Exposome Synergies in Blue Zones and the Cilento Case. International Journal of Molecular Sciences. 2025; 26(16):7887. https://doi.org/10.3390/ijms26167887
Chicago/Turabian StyleAliberti, Silvana Mirella, Mario Capunzo, and Richard H. W. Funk. 2025. "Systems and Molecular Biology of Longevity and Preventive Medicine: Brain-Energy–Microbiome–Exposome Synergies in Blue Zones and the Cilento Case" International Journal of Molecular Sciences 26, no. 16: 7887. https://doi.org/10.3390/ijms26167887
APA StyleAliberti, S. M., Capunzo, M., & Funk, R. H. W. (2025). Systems and Molecular Biology of Longevity and Preventive Medicine: Brain-Energy–Microbiome–Exposome Synergies in Blue Zones and the Cilento Case. International Journal of Molecular Sciences, 26(16), 7887. https://doi.org/10.3390/ijms26167887








