MicroRNAs and Their Inhibition in Modulating SLC5A8 Expression in the Context of Papillary Thyroid Carcinoma
Abstract
1. Introduction
2. Results
2.1. The Expression of SLC5A8 Is Lowered in PTC
2.2. In Silico Analysis Reveals Candidate MicroRNAs Regulating the Expression of SLC5A8
2.3. Luciferase Assay Confirms Binding of miR-181a-5p, miR-182-5p, and miR-494-3p to the 3′UTR of SLC5A8
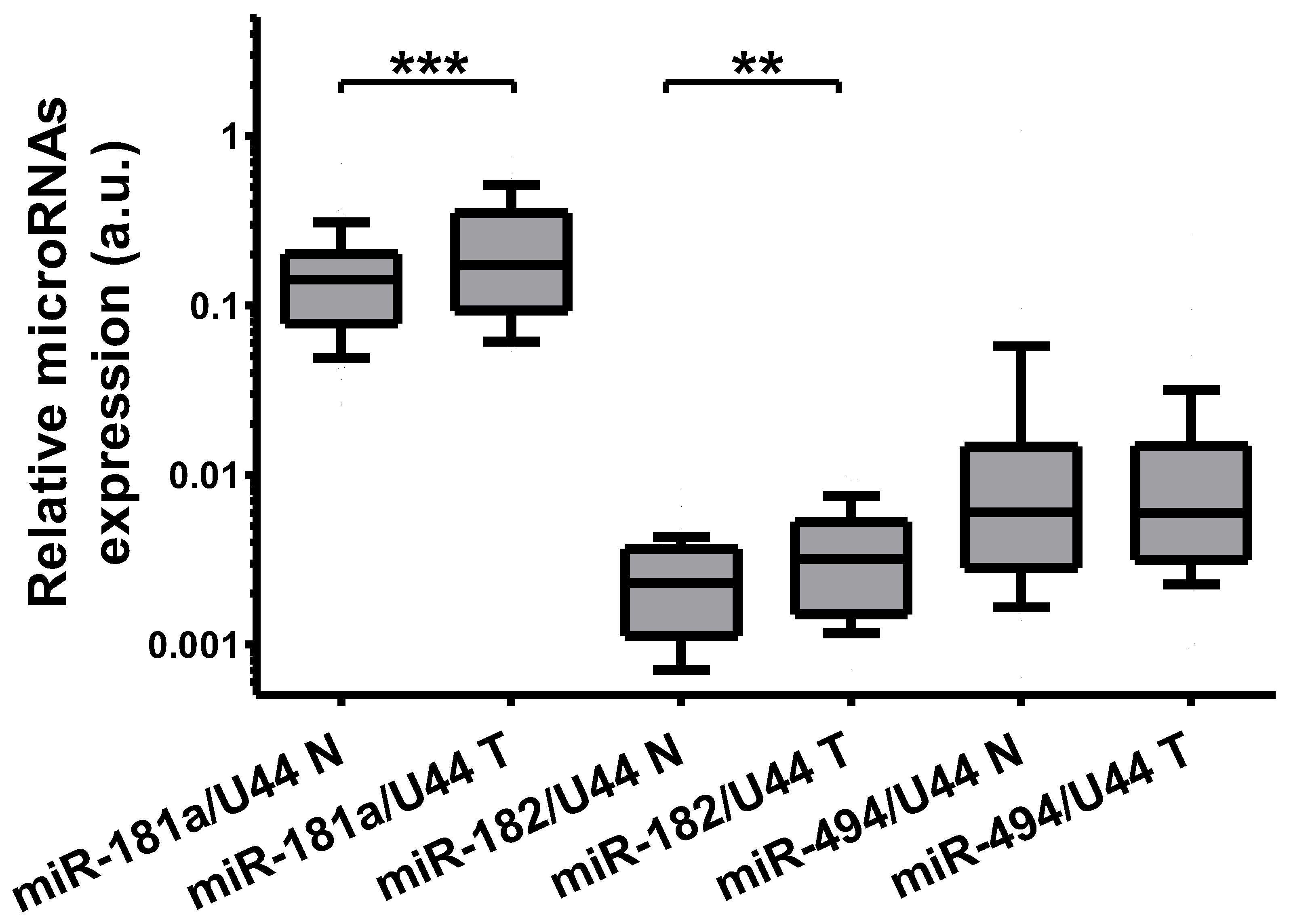
2.4. The Expression of Identified MicroRNAs Is Deregulated in Tumor Tissue
2.5. Plasmid Functionality Verification
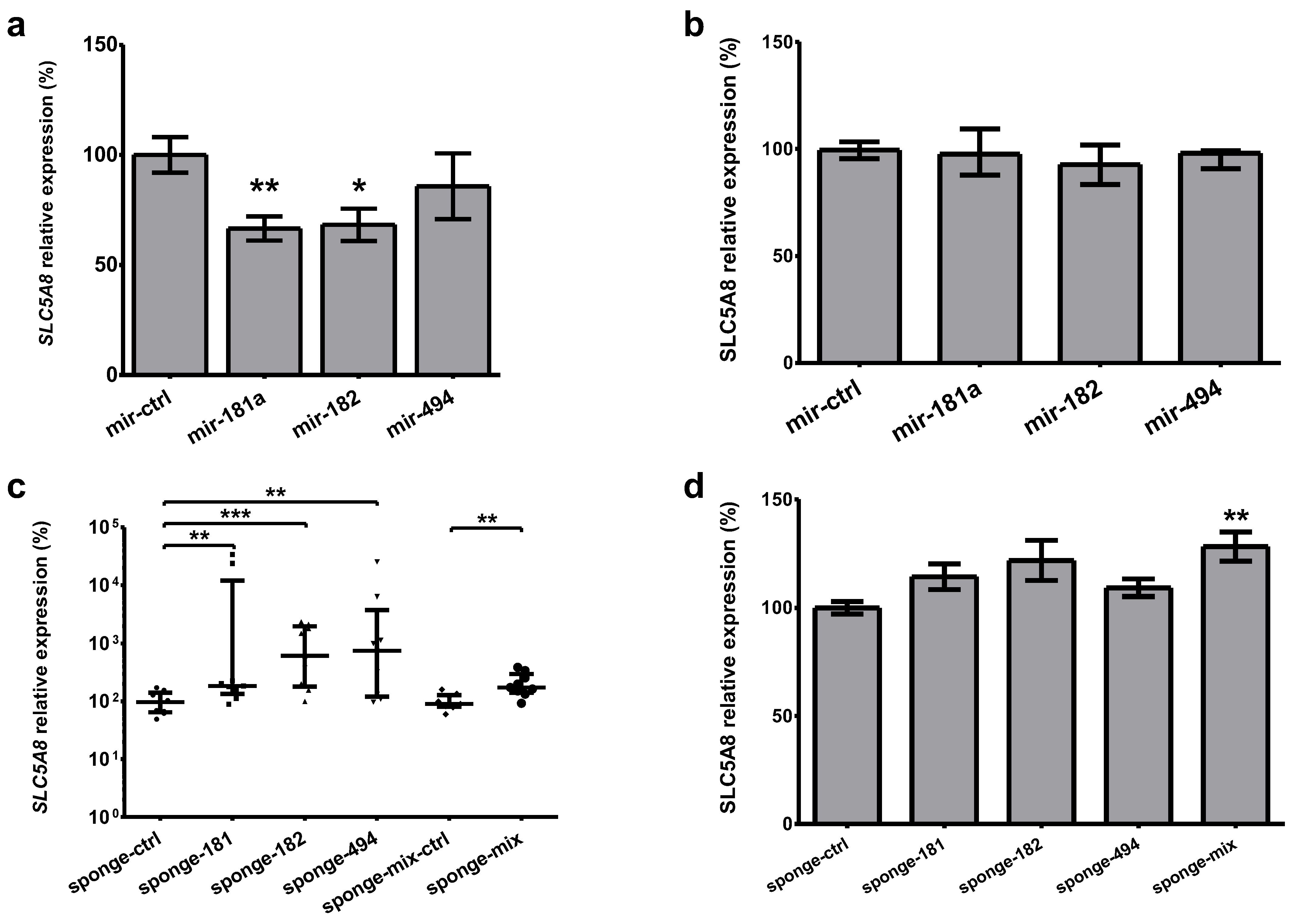
2.6. Modulation of Selected MicroRNAs Affects SLC5A8 Expression
3. Discussion
4. Materials and Methods
4.1. Tissue Samples
4.2. In Silico Identification of MicroRNAs Targeting SLC5A8 Transcripts
4.3. MicroRNA Cloning and MicroRNA-Sponges Preparation
4.4. Analysis of MicroRNA-Mediated Regulation of SLC5A8
4.5. Real-Time PCR
4.6. Protein Quantification
4.7. Transcriptome Sequencing
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganapathy, V.; Gopal, E.; Miyauchi, S.; Prasad, P.D. Biological functions of SLC5A8, a candidate tumour suppressor. Biochem. Soc. Trans. 2005, 33, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Perron, B.; Lacroix, L.; Caillou, B.; Leblanc, G.; Schlumberger, M.; Bidart, J.; Pourcher, T. Identification and characterization of a putative human iodide transporter located at the apical membrane of thyrocytes. J. Clin. Endocrinol. Metab. 2002, 87, 3500–3503. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Myeroff, L.; Smiraglia, D.; Romero, M.; Pretlow, T.; Kasturi, L.; Lutterbaugh, J.; Rerko, R.; Casey, G.; Issa, J.; et al. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc. Natl. Acad. Sci. USA 2003, 100, 8412–8417. [Google Scholar] [CrossRef]
- Hong, C.; Maunakea, A.; Jun, P.; Bollen, A.W.; Hodgson, J.G.; Goldenberg, D.D.; Weiss, W.A.; Costello, J.F. Shared epigenetic mechanisms in human and mouse gliomas inactivate expression of the growth suppressor SLC5A8. Cancer Res. 2005, 65, 3617–3623. [Google Scholar] [CrossRef]
- Park, J.Y.; Helm, J.F.; Zheng, W.; Ly, Q.P.; Hodul, P.J.; Centeno, B.A.; Malafa, M.P. Silencing of the candidate tumor suppressor gene solute carrier family 5 member 8 (SLC5A8) in human pancreatic cancer. Pancreas 2008, 36, e32–e39. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.; Karpenko, M.; Lin, M.; Claus, R.; Arab, K.; Dyckhoff, G.; Plinkert, P.; Herpel, E.; Smiraglia, D.; Plass, C. Frequently methylated tumor suppressor genes in head and neck squamous cell carcinoma. Cancer Res. 2008, 68, 4494–4499. [Google Scholar] [CrossRef]
- Elangovan, S.; Pathania, R.; Ramachandran, S.; Ananth, S.; Padia, R.N.; Srinivas, S.R.; Babu, E.; Hawthorn, L.; Schoenlein, P.V.; Boettger, T.; et al. Molecular mechanism of SLC5A8 inactivation in breast cancer. Mol. Cell. Biol. 2013, 33, 3920–3935. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yan, F.; Xu, J.; Bao, Y.; Zhu, J.; Wang, X.; Wu, J.; Li, Y.; Pu, W.; Liu, Y.; et al. Identification and validation of the methylation biomarkers of non-small cell lung cancer (NSCLC). Clin. Epigenet. 2015, 7, 3. [Google Scholar] [CrossRef]
- Park, J.Y.; Zheng, W.; Kim, D.; Cheng, J.Q.; Kumar, N.; Ahmad, N.; Pow-Sang, J. Candidate tumor suppressor gene SLC5A8 is frequently down-regulated by promoter hypermethylation in prostate tumor. Cancer Detect. Prev. 2007, 31, 359–365. [Google Scholar] [CrossRef]
- Yang, Y.; Liao, C.; Yang, Q.; Li, Y.; Tang, Y.; Xu, B. Role of hypermethylated SLC5A8 in follicular thyroid cancer diagnosis and prognosis prediction. World J. Surg. Oncol. 2023, 21, 367. [Google Scholar] [CrossRef]
- Lacroix, L.; Pourcher, T.; Magnon, C.; Bellon, N.; Talbot, M.; Intaraphairot, T.; Caillou, B.; Schlumberger, M.; Bidart, J.M. Expression of the apical iodide transporter in human thyroid tissues: A comparison study with other iodide transporters. J. Clin. Endocrinol. Metab. 2004, 89, 1423–1428. [Google Scholar] [CrossRef][Green Version]
- Porra, V.; Ferraro-Peyret, C.; Durand, C.; Selmi-Ruby, S.; Giroud, H.; Berger-Dutrieux, N.; Decaussin, M.; Peix, J.L.; Bournaud, C.; Orgiazzi, J.; et al. Silencing of the tumor suppressor gene SLC5A8 is associated with BRAF mutations in classical papillary thyroid carcinomas. J. Clin. Endocrinol. Metab. 2005, 90, 3028–3035. [Google Scholar] [CrossRef][Green Version]
- Hu, S.; Liu, D.; Tufano, R.P.; Carson, K.A.; Rosenbaum, E.; Cohen, Y.; Holt, E.H.; Kiseljak-Vassiliades, K.; Rhoden, K.J.; Tolaney, S.; et al. Association of aberrant methylation of tumor suppressor genes with tumor aggressiveness and BRAF mutation in papillary thyroid cancer. Int. J. Cancer 2006, 119, 2322–2329. [Google Scholar] [CrossRef] [PubMed]
- Nikitski, A.V.; Condello, V.; Divakaran, S.S.; Nikiforov, Y.E. Inhibition of ALK-Signaling Overcomes STRN-ALK-Induced Downregulation of the Sodium Iodine Symporter and Restores Radioiodine Uptake in Thyroid Cells. Thyroid 2023, 33, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Paroder, V.; Spencer, S.R.; Paroder, M.; Arango, D.; Schwartz, S., Jr.; Mariadason, J.M.; Augenlicht, L.H.; Eskandari, S.; Carrasco, N. Na+/monocarboxylate transport (SMCT) protein expression correlates with survival in colon cancer: Molecular characterization of SMCT. Proc. Natl. Acad. Sci. USA 2006, 103, 7270–7275. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, S.; Gopal, E.; Fei, Y.J.; Ganapathy, V. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na+-coupled transporter for short-chain fatty acids. J. Biol. Chem. 2004, 279, 13293–13296. [Google Scholar] [CrossRef]
- Coothankandaswamy, V.; Elangovan, S.; Singh, N.; Prasad, P.D.; Thangaraju, M.; Ganapathy, V. The plasma membrane transporter SLC5A8 suppresses tumour progression through depletion of survivin without involving its transport function. Biochem. J. 2013, 450, 169–178. [Google Scholar] [CrossRef]
- Coady, M.J.; Chang, M.H.; Charron, F.M.; Plata, C.; Wallendorff, B.; Sah, J.F.; Markowitz, S.D.; Romero, M.F.; Lapointe, J.Y. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J. Physiol. 2004, 557, 719–731. [Google Scholar] [CrossRef]
- Coady, M.; Wallendorff, B.; Bourgeois, F.; Charron, F.; Lapointe, J.Y. Establishing a definitive stoichiometry for the Na+/monocarboxylate cotransporter SMCT1. Biophys. J. 2007, 93, 2325–2331. [Google Scholar] [CrossRef]
- Coady, M.J.; Wallendorff, B.; Bourgeois, F.; Lapointe, J.Y. Anionic leak currents through the Na+/monocarboxylate cotransporter SMCT1. Am. J. Physiol. Cell Physiol. 2010, 298, C124–C131. [Google Scholar] [CrossRef]
- Vergara-Jaque, A.; Fong, P.; Comer, J. Iodide Binding in Sodium-Coupled Cotransporters. J. Chem. Inf. Model. 2017, 57, 3043–3055. [Google Scholar] [CrossRef]
- Thangaraju, M.; Karunakaran, S.K.; Itagaki, S.; Gopal, E.; Elangovan, S.; Prasad, P.D.; Ganapathy, V. Transport by SLC5A8 with subsequent inhibition of histone deacetylase 1 (HDAC1) and HDAC3 underlies the antitumor activity of 3-bromopyruvate. Cancer 2009, 115, 4655–4666. [Google Scholar] [CrossRef]
- Babu, E.; Ramachandran, S.; CoothanKandaswamy, V.; Elangovan, S.; Prasad, P.D.; Ganapathy, V.; Thangaraju, M. Role of SLC5A8, a plasma membrane transporter and a tumor suppressor, in the antitumor activity of dichloroacetate. Oncogene 2011, 30, 4026–4037. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, Y.L.; Wu, Y.; Yu, C.L.; Sun, Y.; Li, Y.X. Identification and characterization of the human SLC5A8 gene promoter. Cancer Genet. Cytogenet. 2010, 196, 124–132. [Google Scholar] [CrossRef]
- Thangaraju, M.; Ananth, S.; Martin, P.M.; Roon, P.; Smith, S.B.; Sterneck, E.; Prasad, P.D.; Ganapathy, V. c/ebpδ Null mouse as a model for the double knock-out of slc5a8 and slc5a12 in kidney. J. Biol. Chem. 2006, 281, 26769–26773. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Swierniak, M.; Wojcicka, A.; Czetwertynska, M.; Stachlewska, E.; Maciag, M.; Wiechno, W.; Gornicka, B.; Bogdanska, M.; Koperski, L.; de la Chapelle, A.; et al. In-depth characterization of the microRNA transcriptome in normal thyroid and papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2013, 8, E1401–E1409. [Google Scholar] [CrossRef] [PubMed]
- Wojcicka, A.; Kolanowska, M.; Jazdzewski, K. MECHANISMS IN ENDOCRINOLOGY: MicroRNA in diagnostics and therapy of thyroid cancer. Eur. J. Endocrinol. 2016, 174, R89–R98. [Google Scholar] [CrossRef] [PubMed]
- Lindow, M.; Kauppinen, S. Discovering the first microRNA-targeted drug. J. Cell Biol. 2012, 199, 407–412. [Google Scholar] [CrossRef]
- Wojcicka, A.; Gierlikowski, W.; Kotlarek, M.; Koperski, L.; Jazdzewski, K. Apical Iodide Transporter (AIT) and Its microRNA—Induced Silencing in Thyroid Malignancies. Endocr. Rev. 2014, 35, P1125. [Google Scholar]
- Gierlikowski, W.; Broniarek, K.; Cheda, Ł.; Rogulski, Z.; Kotlarek-Łysakowska, M. MiR-181a-5p Regulates NIS Expression in Papillary Thyroid Carcinoma. Int. J. Mol. Sci. 2021, 22, 6067. [Google Scholar] [CrossRef]
- Zhang, X.M.; Meng, Q.H.; Kong, F.F.; Wang, K.; Du, L.J. SLC5A8 regulates the biological behaviors of cervical cancer cells through mediating the Wnt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4679–4686. [Google Scholar] [CrossRef]
- Vargas-Sierra, O.; Hernández-Juárez, J.; Uc-Uc, P.Y.; Herrera, L.A.; Domínguez-Gómez, G.; Gariglio, P.; Díaz-Chávez, J. Role of SLC5A8 as a Tumor Suppressor in Cervical Cancer. Front. Biosci. 2024, 29, 16. [Google Scholar] [CrossRef]
- Whitman, S.; Hackanson, B.; Liyanarachchi, S.; Liu, S.; Rush, L.; Maharry, K.; Margeson, D.; Davuluri, R.; Wen, J.; Witte, T.; et al. DNA hypermethylation and epigenetic silencing of the tumor suppressor gene, SLC5A8, in acute myeloid leukemia with the MLL partial tandem duplication. Blood 2008, 112, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.X.; Wu, Y.Q.; Liu, J.; Pan, H.; Deng, W.; Sun, W.; Xie, C.; Huang, X.F. Proteome-wide analysis reveals potential therapeutic targets for Colorectal cancer: A two-sample mendelian randomization study. BMC Cancer 2023, 23, 1188. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.M.; Lee, E.J.; Jeon, E.S.; Park, C.K.; Kim, K.M. Progressive methylation during the serrated neoplasia pathway of the colorectum. Mod. Pathol. 2005, 18, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Zane, M.; Agostini, M.; Enzo, M.V.; Ide, E.C.; Del Bianco, P.; Torresan, F.; Boschin, I.M.; Pennelli, G.; Saccani, A.; Rubello, D.; et al. Circulating cell-free DNA, SLC5A8 and SLC26A4 hypermethylation, BRAFV600E: A non-invasive tool panel for early detection of thyroid cancer. Biomed. Pharmacother. 2013, 67, 723–730. [Google Scholar] [CrossRef]
- Khatami, F.; Larijani, B.; Heshmat, R.; Nasiri, S.; Haddadi-Aghdam, M.; Teimoori-Toolabi, L.; Tavangar, S.M. Hypermethylated RASSF1 and SLC5A8 promoters alongside BRAF(V600E) mutation as biomarkers for papillary thyroid carcinoma. J. Cell. Physiol. 2020, 235, 6954–6968. [Google Scholar] [CrossRef]
- Durante, C.; Puxeddu, E.; Ferretti, E.; Morisi, R.; Moretti, S.; Bruno, R.; Barbi, F.; Avenia, N.; Scipioni, A.; Verrienti, A.; et al. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J. Clin. Endocrinol. Metab. 2007, 92, 2840–2843. [Google Scholar] [CrossRef]
- Anbazhagan, A.N.; Priyamvada, S.; Kumar, A.; Jayawardena, D.; Borthakur, A.; Saksena, S.; Gill, R.K.; Alrefai, W.A.; Dudeja, P.K. miR-29a, b, and c regulate SLC5A8 expression in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G223–G231. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, S.; Liu, Y.; Wang, F.; Liu, Y.; Xiao, H. Expression of miRNAs in Papillary Thyroid Carcinomas is Associated with BRAF Mutation and Clinicopathological Features in Chinese Patients. Int. J. Endocrinol. 2013, 5, 675–681. [Google Scholar]
- The Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Eichhorn, S.W.; Guo, H.; McGeary, S.E.; Rodriguez-Mias, R.A.; Shin, C.; Baek, D.; Hsu, S.H.; Ghoshal, K.; Villén, J.; Bartel, D.P. mRNA destabilization is the dominant effect of mammalian microRNAs by the time substantial repression ensues. Mol. Cell 2014, 56, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Jie, J.; Liu, D.; Wang, Y.; Wu, Q.; Wu, T.; Fang, R. Generation of MiRNA sponge constructs targeting multiple MiRNAs. J. Clin. Lab. Anal. 2022, 36, e24527. [Google Scholar] [CrossRef]
- Thangaraju, M.; Gopal, E.; Martin, P.M.; Ananth, S.; Smith, S.B.; Prasad, P.D.; Sterneck, E.; Ganapathy, V. SLC5A8 triggers tumor cell apoptosis through pyruvate-dependent inhibition of histone deacetylases. Cancer Res. 2006, 66, 11560–11564. [Google Scholar] [CrossRef]
- Everaert, C.; Luypaert, M.; Maag, J.L.V.; Cheng, Q.X.; Dinger, M.E.; Hellemans, J.; Mestdagh, P. Benchmarking of RNA-sequencing analysis workflows using whole-transcriptome RT-qPCR expression data. Sci. Rep. 2017, 7, 1559. [Google Scholar] [CrossRef]
- Gopal, E.; Fei, Y.J.; Sugawara, M.; Miyauchi, S.; Zhuang, L.; Martin, P.; Smith, S.B.; Prasad, P.D.; Ganapathy, V. Expression of slc5a8 in kidney and its role in Na+-coupled transport of lactate. J. Biol. Chem. 2004, 279, 44522–44532. [Google Scholar] [CrossRef]
- John, B.; Enright, A.J.; Aravin, A.; Tuschl, T.; Sander, C.; Marks, D.S. Human MicroRNA targets. PLoS Biol. 2004, 2, e363. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Shomron, N.; Sandberg, R.; Hornstein, E.; Kitzman, J.; Burge, C.B. Determinants of targeting by endogenous and exogenous microRNAs and siRNAs. RNA 2007, 13, 1894–1910. [Google Scholar] [CrossRef]
- Tastsoglou, S.; Alexiou, A.; Karagkouni, D.; Skoufos, G.; Zacharopoulou, E.; Hatzigeorgiou, A.G. DIANA-microT 2023: Including predicted targets of virally encoded miRNAs. Nucleic Acids Res. 2023, 51, W148–W153. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Steffen, P.; Voß, B.; Rehmsmeier, M.; Reeder, J.; Giegerich, R. RNAshapes: An integrated RNA analysis package based on abstract shapes. Bioinformatics 2005, 22, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Jazdzewski, K.; Murray, E.L.; Franssila, K.; Jarzab, B.; Schoenberg, D.R.; de la Chapelle, A. Common SNP in pre-miR-146a decreases mature miR expression and predisposes to papillary thyroid carcinoma. Proc. Natl. Acad. Sci. USA 2008, 105, 7269–7274. [Google Scholar] [CrossRef]
- Ebert, M.S.; Neilson, J.R.; Sharp, P.A. MicroRNA sponges: Competitive inhibitors of small RNAs in mammalian cells. Nat. Methods 2007, 4, 721–726. [Google Scholar] [CrossRef]
- Wong, N.; Wang, X. miRDB: An online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2014, 43, D146–D152. [Google Scholar] [CrossRef]




| Feature | n | (%) | |
|---|---|---|---|
| Sex | Female | 44 | 90% |
| Male | 5 | 10% | |
| Histopathological subtype | PTC cf | 43 | 88% |
| PTC fv | 6 | 12% | |
| No. of foci | Single | 39 | 80% |
| Multiple | 10 | 20% | |
| Tumor diameter | Average | 12 | mm |
| Range | 1–73 | mm | |
| pT feature | pT1a | 21 | 43% |
| pT1b | 12 | 24% | |
| pT2 | 6 | 12% | |
| pT3 | 10 | 20% | |
| pT4 | 0 | 0% | |
| pN feature | N0 | 39 | 80% |
| N1a | 5 | 10% | |
| N1b | 5 | 10% | |
| cM feature | M0 | 48 | 98% |
| M1 | 1 | 2% | |
| Vascular invasion | No | 44 | 90% |
| Yes | 5 | 10% | |
| Local invasion | No | 32 | 65% |
| Capsule only | 10 | 20% | |
| Extrathyroid | 7 | 14% | |
| Stage | I | 40 | 82% |
| II | 1 | 2% | |
| III | 5 | 10% | |
| IVA | 2 | 4% | |
| IVB | 0 | 0% | |
| IVC | 1 | 2% | |
| BRAF status * | T (wild-type) | 23 | 55% |
| T/A (mutated) | 19 | 45% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gierlikowski, W.; Grzędzicka, J.; Konieczek, K.; Kotlarek-Łysakowska, M. MicroRNAs and Their Inhibition in Modulating SLC5A8 Expression in the Context of Papillary Thyroid Carcinoma. Int. J. Mol. Sci. 2025, 26, 7889. https://doi.org/10.3390/ijms26167889
Gierlikowski W, Grzędzicka J, Konieczek K, Kotlarek-Łysakowska M. MicroRNAs and Their Inhibition in Modulating SLC5A8 Expression in the Context of Papillary Thyroid Carcinoma. International Journal of Molecular Sciences. 2025; 26(16):7889. https://doi.org/10.3390/ijms26167889
Chicago/Turabian StyleGierlikowski, Wojciech, Jowita Grzędzicka, Katarzyna Konieczek, and Marta Kotlarek-Łysakowska. 2025. "MicroRNAs and Their Inhibition in Modulating SLC5A8 Expression in the Context of Papillary Thyroid Carcinoma" International Journal of Molecular Sciences 26, no. 16: 7889. https://doi.org/10.3390/ijms26167889
APA StyleGierlikowski, W., Grzędzicka, J., Konieczek, K., & Kotlarek-Łysakowska, M. (2025). MicroRNAs and Their Inhibition in Modulating SLC5A8 Expression in the Context of Papillary Thyroid Carcinoma. International Journal of Molecular Sciences, 26(16), 7889. https://doi.org/10.3390/ijms26167889







