Ajuba as a Potential Nutrition-Responsive Biomarker for the Prevention of Age-Related Sarcopenia
Abstract
1. Introduction
2. Results
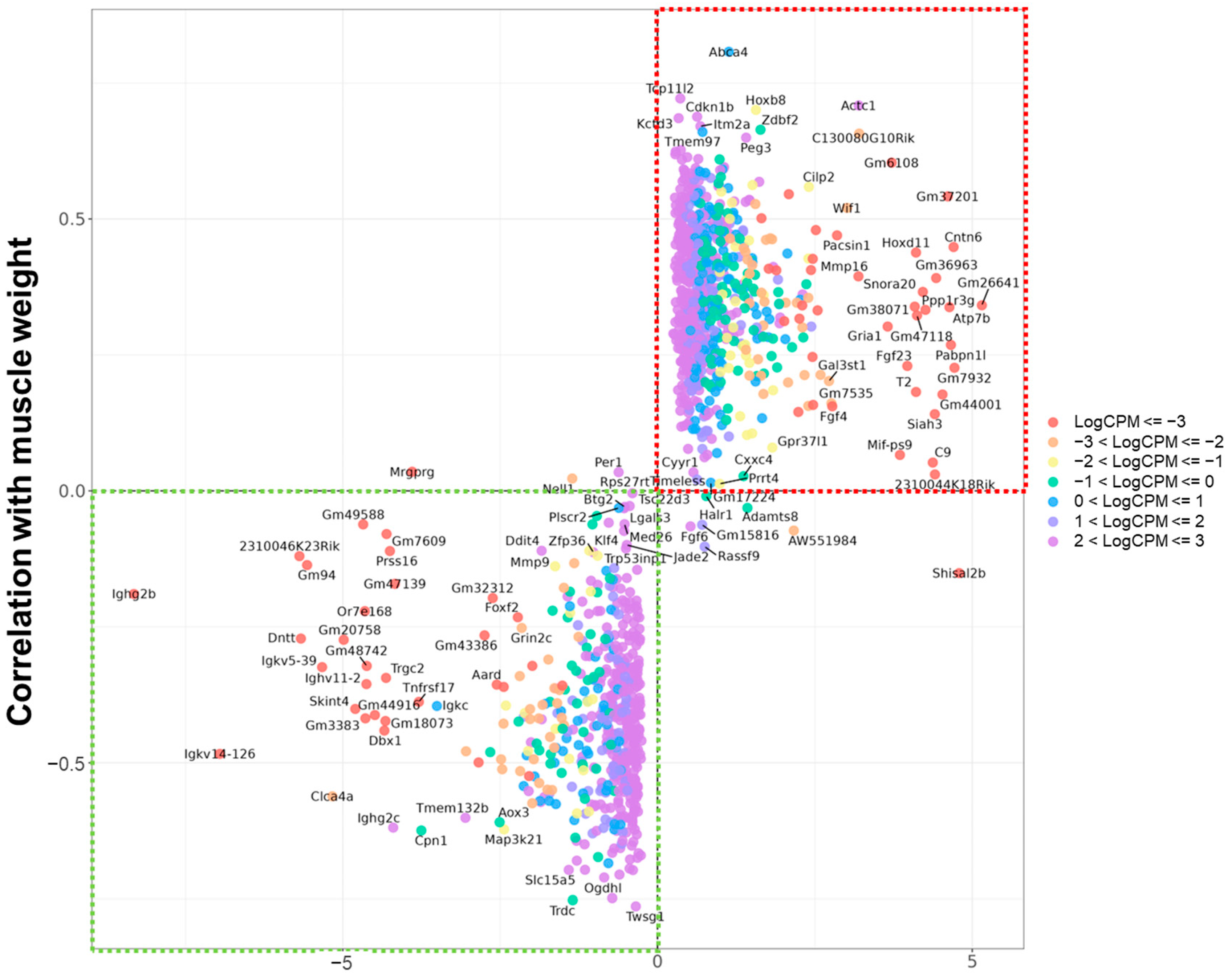
2.1. Identification of Muscle-Related Candidate Genes Among Aging-Associated DEGs
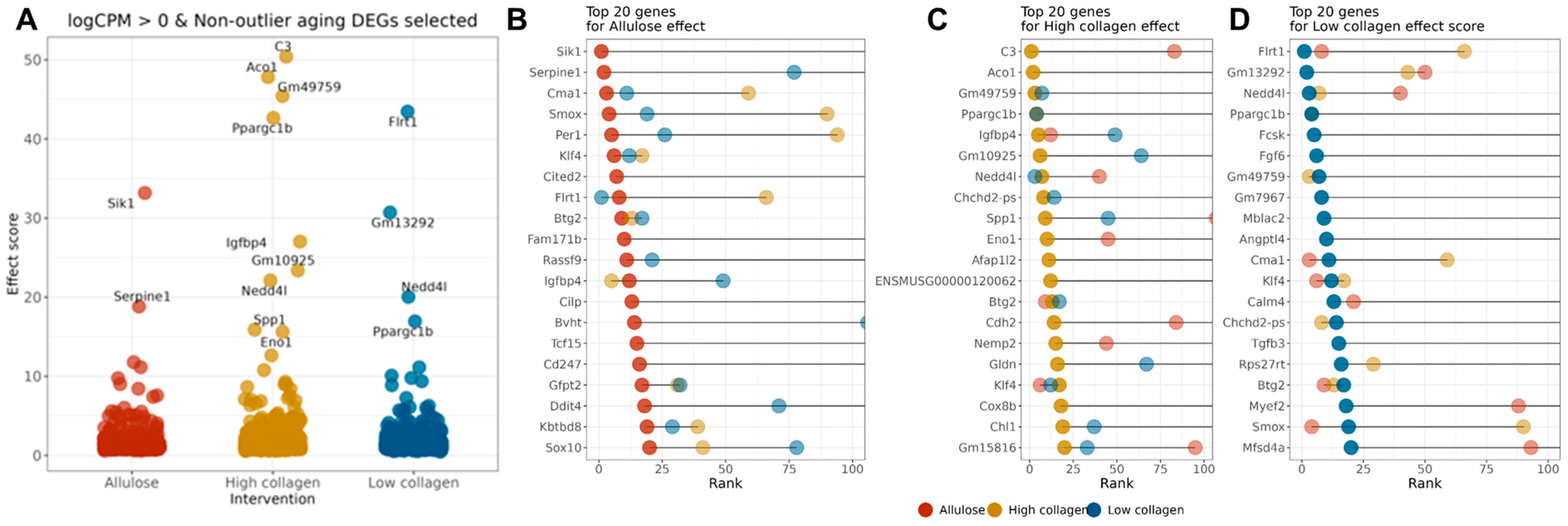
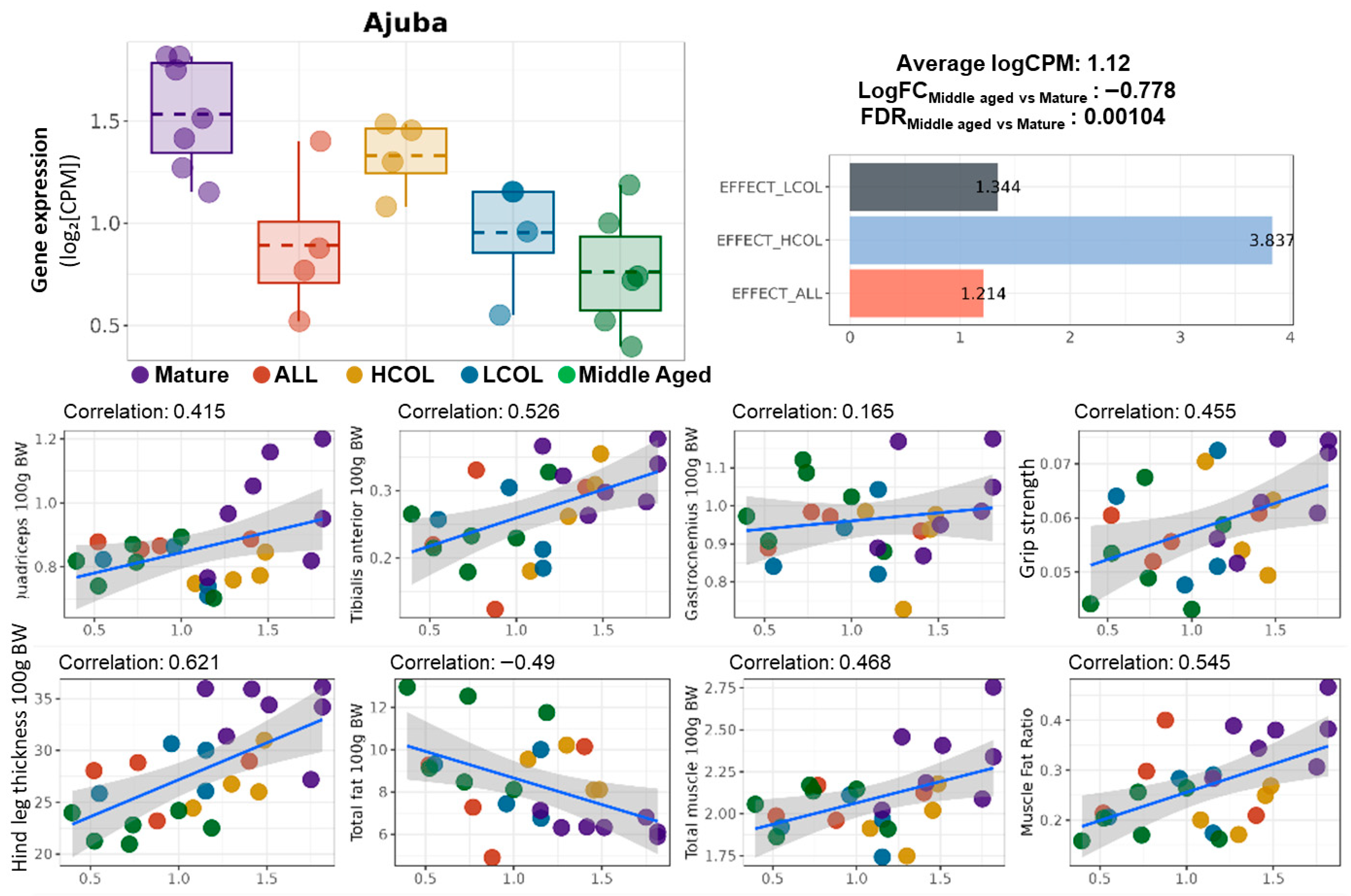
2.2. Effect-Score-Based Prioritization of Allulose- and Collagen Hydrolysate-Responsive Aging Genes
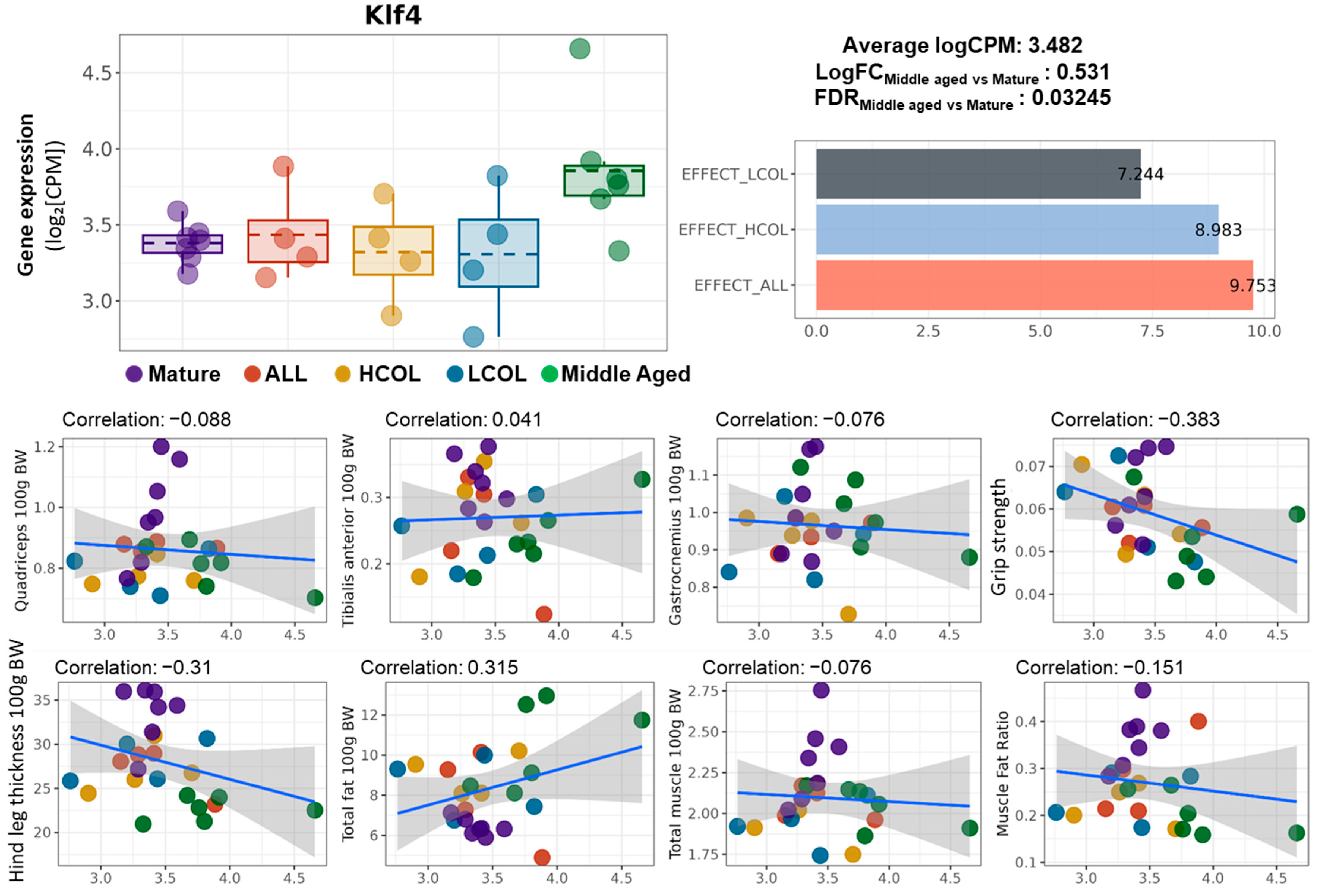
2.3. Ajuba Identified as a Nutrition-Responsive Biomarker Downstream of Klf4
3. Discussion
4. Materials and Methods
4.1. Animals and Diet
4.2. mRNA-Seq and Analysis
4.3. Effect Score Calculation
4.4. WGCNA and Integrated Analysis
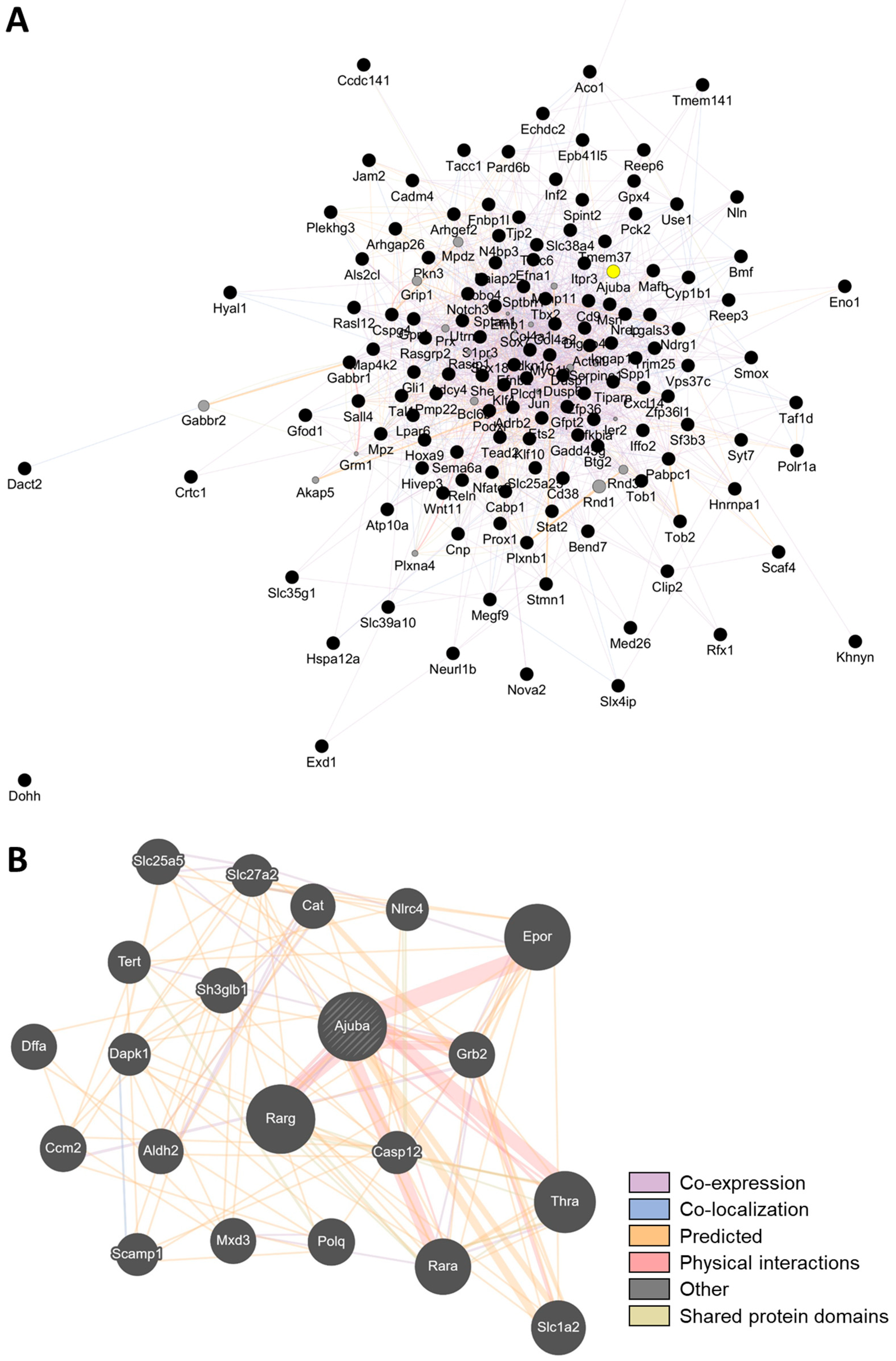
4.5. Gene Interaction Network Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gustafsson, T.; Ulfhake, B. Aging skeletal muscles: What are the mechanisms of age-related loss of strength and muscle mass, and can we impede its development and progression? Int. J. Mol. Sci. 2024, 25, 10932. [Google Scholar] [CrossRef]
- Rodríguez-Mañas, L.; Alonso-Bouzón, C.; Blackman, M.R. Relationships among frailty, sarcopenia and the endocrine-metabolic changes of advanced age: Pathophysiology, prevention, diagnosis, and treatment. In Endocrinology of Aging; Elsevier: Amsterdam, The Netherlands, 2021; pp. 523–545. [Google Scholar]
- Hida, T.; Harada, A.; Imagama, S.; Ishiguro, N. Managing Sarcopenia and Its Related-Fractures to Improve Quality of Life in Geriatric Populations. Aging Dis. 2014, 5, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kim, D.H.; Pack, S.P. Marine-Derived Bioactive Ingredients in Functional Foods for Aging: Nutritional and Therapeutic Perspectives. Mar. Drugs 2024, 22, 496. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.-Y.; Kim, C.Y. A comprehensive review of pathological mechanisms and natural dietary ingredients for the management and prevention of sarcopenia. Nutrients 2023, 15, 2625. [Google Scholar] [CrossRef]
- Lee, C.H.; Kwon, Y.; Park, S.; Kim, T.; Kim, M.S.; Kim, E.J.; Jung, J.I.; Min, S.; Park, K.-H.; Jeong, J.H.; et al. The Impact of Ulmus macrocarpa extracts on a model of sarcopenia-induced C57BL/6 mice. Int. J. Mol. Sci. 2024, 25, 6197. [Google Scholar] [CrossRef]
- Kwon, E.Y.; Kim, J.W.; Choi, J.Y.; Hwang, K.; Han, Y. Setaria viridis Ethanol Extract Attenuates Muscle Loss and Body Fat Reduction in Sarcopenic Obesity by Regulating AMPK in High-Fat Diet-Induced Obese Mice. Food Sci. Nutr. 2025, 13, e70655. [Google Scholar] [CrossRef]
- Picó, C.; Serra, F.; Rodríguez, A.M.; Keijer, J.; Palou, A. Biomarkers of Nutrition and Health: New Tools for New Approaches. Nutrients 2019, 11, 1092. [Google Scholar] [CrossRef]
- Chaudhary, D.; Guleria, D.; Aggarwal, H.; Mishra, V.; Chauhan, A.; Dufossé, L.; Joshi, N.C. Nutrigenomics and Personalized Diets-Tailoring Nutrition for Optimal Health. Appl. Food Res. 2025, 5, 100980. [Google Scholar] [CrossRef]
- Lagoumintzis, G.; Patrinos, G.P. Triangulating nutrigenomics, metabolomics and microbiomics toward personalized nutrition and healthy living. Hum. Genom. 2023, 17, 109. [Google Scholar] [CrossRef]
- Heianza, Y.; Qi, L. Gene-Diet Interaction and Precision Nutrition in Obesity. Int. J. Mol. Sci. 2017, 18, 787. [Google Scholar] [CrossRef]
- Nieman, D.C. Multiomics approach to precision sports nutrition: Limits, challenges, and possibilities. Front. Nutr. 2021, 8, 796360. [Google Scholar] [CrossRef]
- Kim, J.-E.; Kwon, E.-Y.; Han, Y. A collagen hydrolysate containing tripeptides ameliorates sarcopenia in middle-aged mice. Molecules 2022, 27, 2718. [Google Scholar] [CrossRef]
- Kim, J.E.; Kwon, E.Y.; Han, Y. Allulose attenuated age-associated sarcopenia via regulating IGF-1 and myostatin in aged mice. Mol. Nutr. Food Res. 2022, 66, 2100549. [Google Scholar] [CrossRef] [PubMed]
- Diamant, I.; Clarke, D.J.; Evangelista, J.E.; Lingam, N.; Ma’ayan, A. Harmonizome 3.0: Integrated knowledge about genes and proteins from diverse multi-omics resources. Nucleic Acids Res. 2025, 53, D1016–D1028. [Google Scholar] [CrossRef] [PubMed]
- Schimizzi, G.V.; Longmore, G.D. Ajuba proteins. Curr. Biol. 2015, 25, R445–R446. [Google Scholar] [CrossRef] [PubMed]
- Gropper, S.S. The role of nutrition in chronic disease. Nutrients 2023, 15, 664. [Google Scholar] [CrossRef]
- Cristina, N.M.; Lucia, d.A. Nutrition and healthy aging: Prevention and treatment of gastrointestinal diseases. Nutrients 2021, 13, 4337. [Google Scholar] [CrossRef]
- Ispoglou, T.; Wilson, O.; McCullough, D.; Aldrich, L.; Ferentinos, P.; Lyall, G.; Stavropoulos-Kalinoglou, A.; Duckworth, L.; Brown, M.A.; Sutton, L. A narrative review of non-pharmacological strategies for managing sarcopenia in older adults with cardiovascular and metabolic diseases. Biology 2023, 12, 892. [Google Scholar] [CrossRef]
- Nakahara, S.; Takasaki, M.; Abe, S.; Kakitani, C.; Nishioka, S.; Wakabayashi, H.; Maeda, K. Aggressive nutrition therapy in malnutrition and sarcopenia. Nutrition 2021, 84, 111109. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Li, S. Advances in nutritional supplementation for sarcopenia management. Front. Nutr. 2023, 10, 1189522. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, J.; Dai, T.; Li, X.; Chen, L.; He, Z.; Guo, M.; Zhao, J.; Xu, L. A review of KLF4 and inflammatory disease: Current status and future perspective. Pharmacol. Res. 2024, 207, 107345. [Google Scholar] [CrossRef]
- Huang, T.; Yin, J.; Ren, S.e.; Zhang, X. Protective effects of KLF4 on blood–brain barrier and oxidative stress after cerebral ischemia–reperfusion in rats through the Nrf2/Trx1 pathway. Cytokine 2023, 169, 156288. [Google Scholar] [CrossRef]
- Wang, L.; Shen, F.; Stroehlein, J.R.; Wei, D. Context-dependent functions of KLF4 in cancers: Could alternative splicing isoforms be the key? Cancer Lett. 2018, 438, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.-D.; Zhao, F. KLF4 suppresses the proliferation and metastasis of NSCLC cells via inhibition of MSI2 and regulation of the JAK/STAT3 signaling pathway. Transl. Oncol. 2022, 22, 101396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.; Lu, C.; Zhang, H. KLF4 suppresses the proliferation of perihilar cholangiocarcinoma by negatively regulating GDF15 and phosphorylating AKT. Oncol. Rep. 2023, 50, 222. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Wang, X.; Xu, R.; Liang, Z.; Zhu, Q.; Chen, M.; Lin, Z.; Li, C.; Duo, T.; Tong, X. KLF4 regulates skeletal muscle development and regeneration by directly targeting P57 and Myomixer. Cell Death Dis. 2023, 14, 612. [Google Scholar] [CrossRef]
- Thakur, M.D.; Feng, Y.; Jagannathan, R.; Seppa, M.J.; Skeath, J.B.; Longmore, G.D. Ajuba LIM proteins are negative regulators of the Hippo signaling pathway. Curr. Biol. 2010, 20, 657–662. [Google Scholar] [CrossRef]
- Jagannathan, R.; Schimizzi, G.V.; Zhang, K.; Loza, A.J.; Yabuta, N.; Nojima, H.; Longmore, G.D. AJUBA LIM proteins limit hippo activity in proliferating cells by sequestering the hippo core kinase complex in the cytosol. Mol. Cell. Biol. 2016, 36, 2526–2542. [Google Scholar] [CrossRef]
- Razzell, W.; Bustillo, M.E.; Zallen, J.A. The force-sensitive protein Ajuba regulates cell adhesion during epithelial morphogenesis. J. Cell Biol. 2018, 217, 3715–3730. [Google Scholar] [CrossRef]
- McCormack, J.; Bruche, S.; Ouadda, A.; Ishii, H.; Lu, H.; Garcia-Cattaneo, A.; Chávez-Olórtegui, C.; Lamarche-Vane, N.; Braga, V. The scaffold protein Ajuba suppresses CdGAP activity in epithelia to maintain stable cell-cell contacts. Sci. Rep. 2017, 7, 9249. [Google Scholar] [CrossRef]
- Nola, S.; Daigaku, R.; Smolarczyk, K.; Carstens, M.; Martin-Martin, B.; Longmore, G.; Bailly, M.; Braga, V.M. Ajuba is required for Rac activation and maintenance of E-cadherin adhesion. J. Cell Biol. 2011, 195, 855–871. [Google Scholar] [CrossRef] [PubMed]
- Pratt, S.J.; Epple, H.; Ward, M.; Feng, Y.; Braga, V.M.; Longmore, G.D. The LIM protein Ajuba influences p130Cas localization and Rac1 activity during cell migration. J. Cell Biol. 2005, 168, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. In Babraham Bioinformatics; Babraham Institute: Cambridge, UK, 2010; Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 12 August 2025).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Cunningham, F.; Allen, J.E.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Bennett, R.; et al. Ensembl 2022. Nucleic Acids Res. 2021, 50, D988–D995. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38 (Suppl. S2), W214–W220. [Google Scholar] [CrossRef]

| Ingredient (g) | AIN-93G | AIN-93G + 3% ALL | AIN-93G + 0.2% LCOL | AIN-93G + 0.2% HCOL |
|---|---|---|---|---|
| Casein | 200 | 200 | 200 | 200 |
| Corn starch | 397.5 | 397.5 | 397.5 | 397.5 |
| Sucrose | 100 | 100 | 100 | 100 |
| Maltodextrin | 132 | 132 | 132 | 132 |
| Cellulose | 50 | 20 | 48 | 48 |
| Soybean oil | 70 | 70 | 70 | 70 |
| Mineral mix * | 35 | 35 | 35 | 35 |
| Vitamin mix † | 10 | 10 | 10 | 10 |
| TBHQ, antioxidant | 0.014 | 0.014 | 0.014 | 0.014 |
| L-Cystine | 3 | 3 | 3 | 3 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 |
| Allulose | 30 | |||
| Low-molecular-weight collagen hydrolysate | - | 2 | - | |
| High-molecular-weight collagen hydrolysate | - | - | 2 | |
| Total (g) | 1000 | 1000 | 1000 | 1000 |
| Total protein contents (g) | 200 | 200 | 202 | 202 |
| Calories (cal/g) | 3948 | 3948 | 3956 | 3956 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Pack, S.P. Ajuba as a Potential Nutrition-Responsive Biomarker for the Prevention of Age-Related Sarcopenia. Int. J. Mol. Sci. 2025, 26, 7869. https://doi.org/10.3390/ijms26167869
Han Y, Pack SP. Ajuba as a Potential Nutrition-Responsive Biomarker for the Prevention of Age-Related Sarcopenia. International Journal of Molecular Sciences. 2025; 26(16):7869. https://doi.org/10.3390/ijms26167869
Chicago/Turabian StyleHan, Youngji, and Seung Pil Pack. 2025. "Ajuba as a Potential Nutrition-Responsive Biomarker for the Prevention of Age-Related Sarcopenia" International Journal of Molecular Sciences 26, no. 16: 7869. https://doi.org/10.3390/ijms26167869
APA StyleHan, Y., & Pack, S. P. (2025). Ajuba as a Potential Nutrition-Responsive Biomarker for the Prevention of Age-Related Sarcopenia. International Journal of Molecular Sciences, 26(16), 7869. https://doi.org/10.3390/ijms26167869






