Transcriptomic Response to Neuromuscular Electrical Stimulation in Muscle, Brain, and Plasma EVs in WT and Klotho-Deficient Mice
Abstract
1. Introduction
2. Results
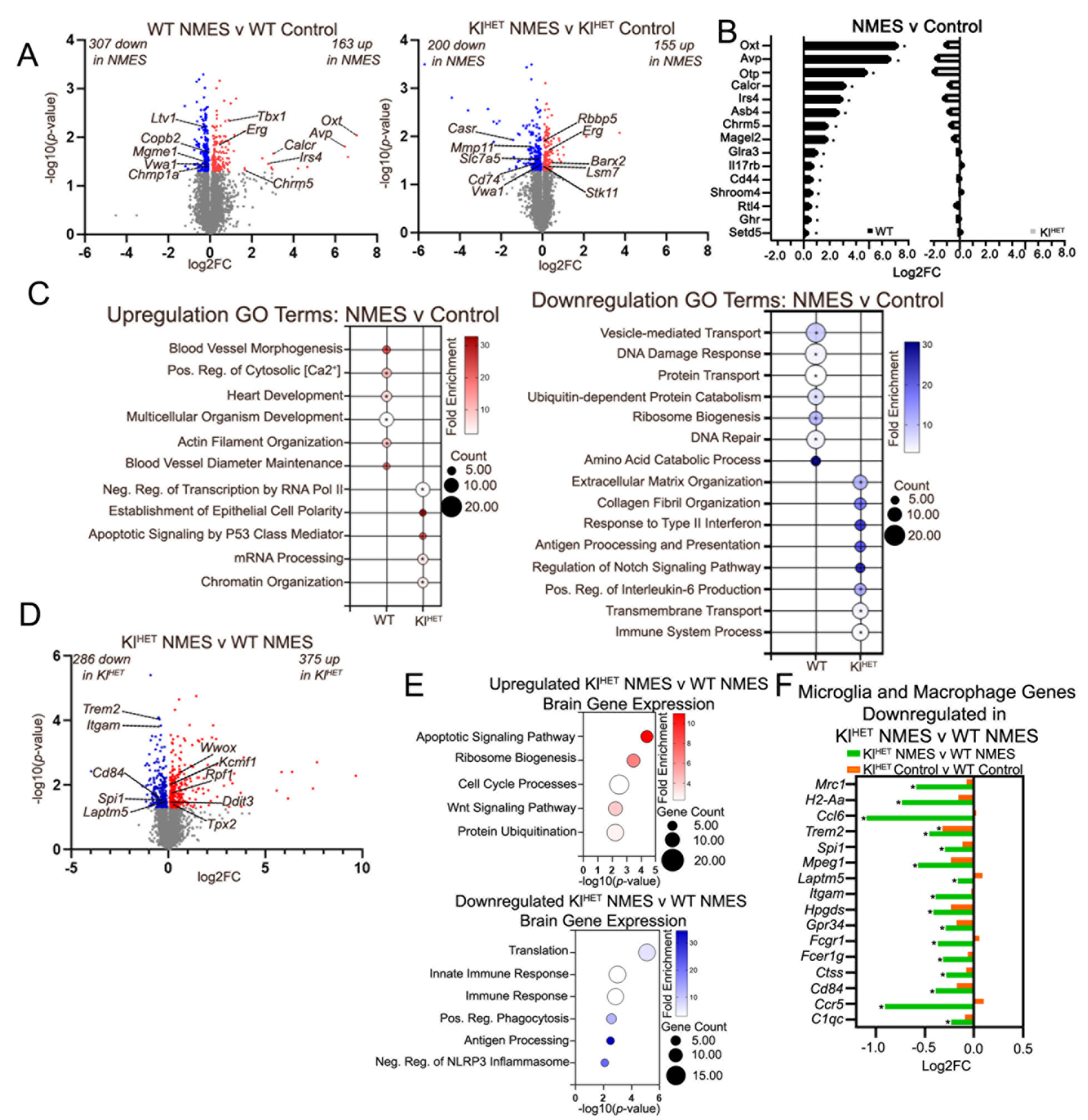
2.1. NMES Treatment Activates Metabolic Response in Muscle Tissue Similarly in WT and KlHET Mice
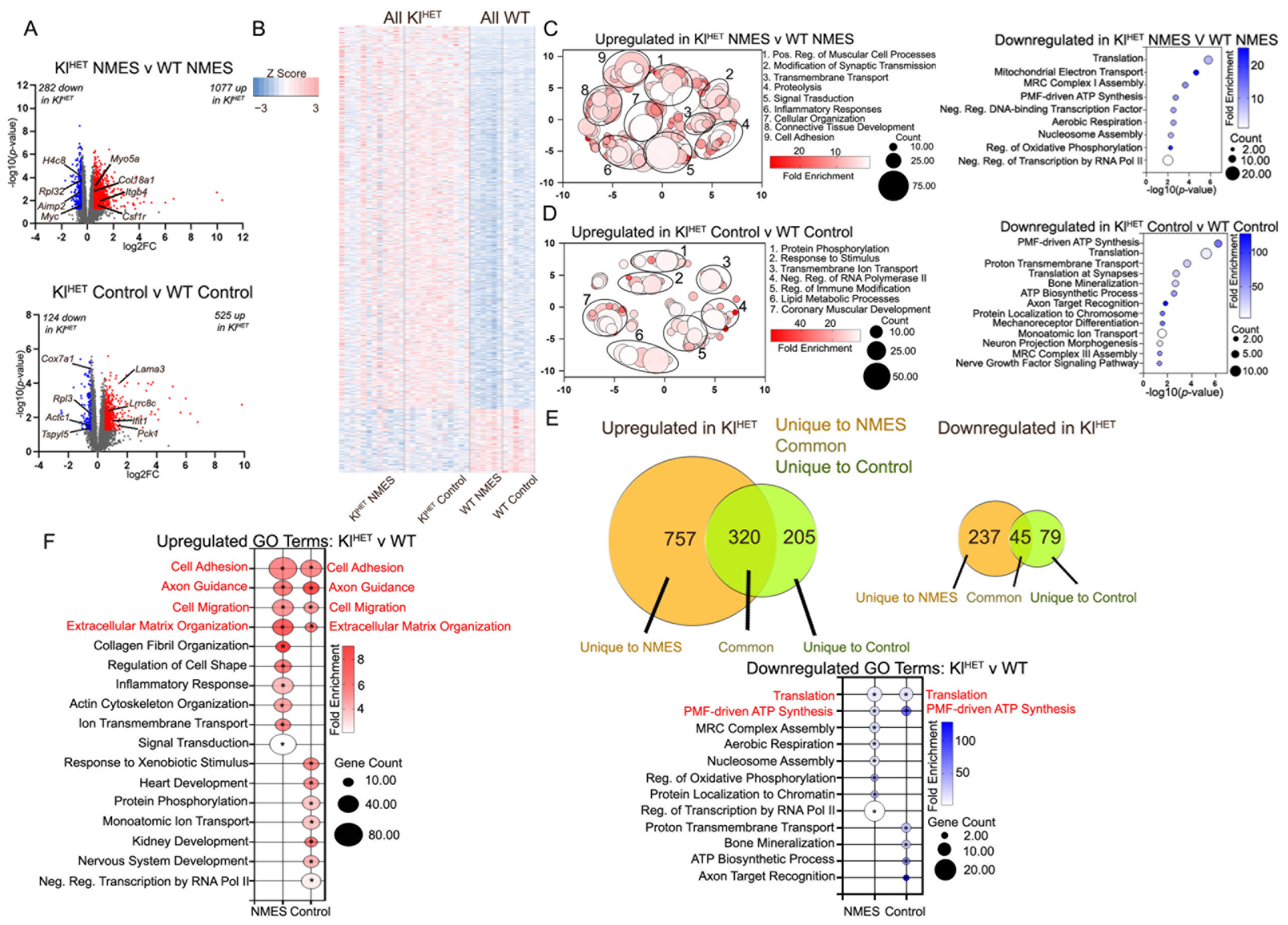
2.2. KLOTHO Deficiency Significantly Affects Muscle Transcriptome
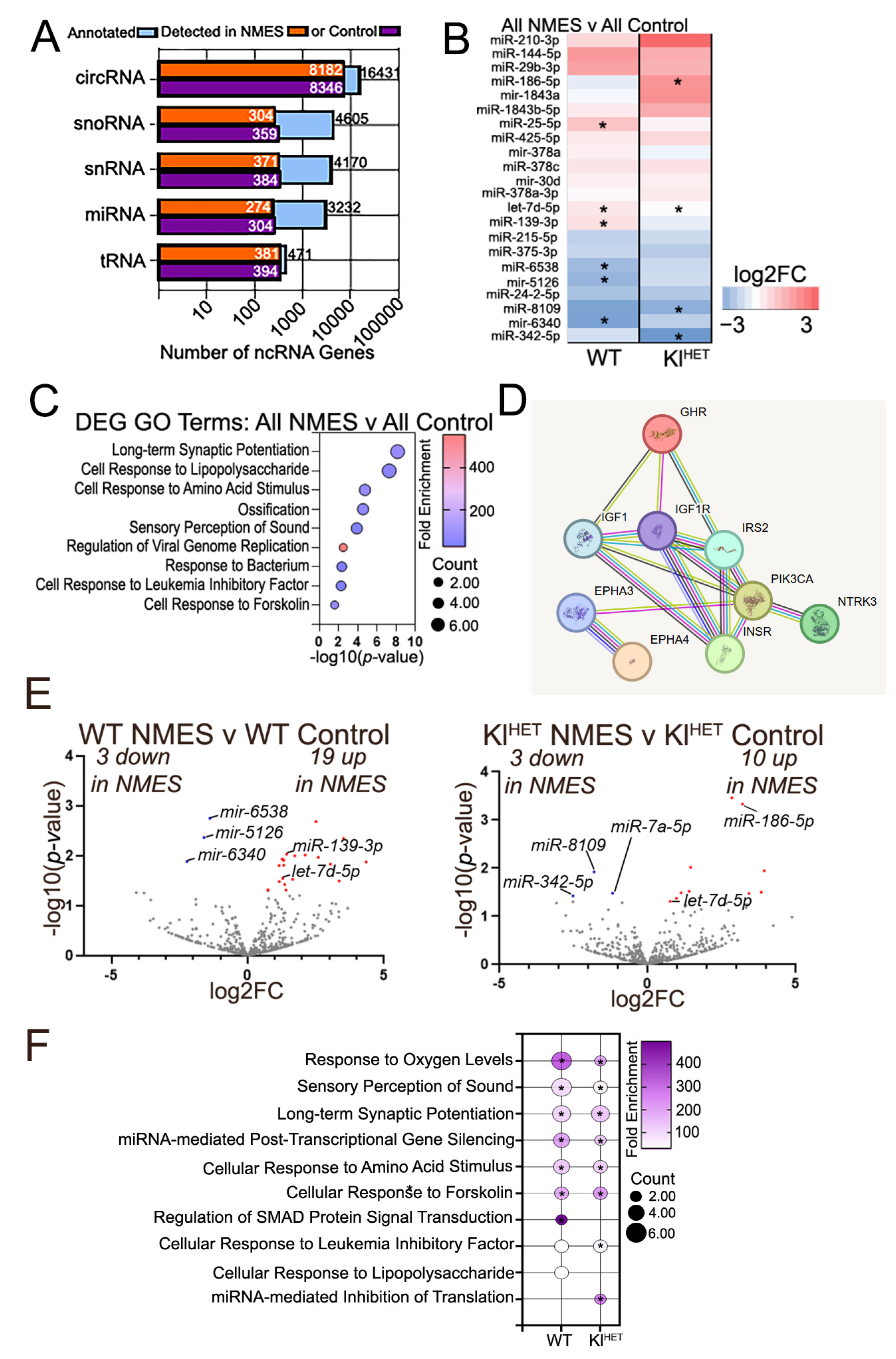
2.3. Functional Patterns Emerge in Small Non-Coding RNA Signatures of Plasma EVs Associated with NMES in WT and KlHET Mice
2.4. Effect of NMES on Brain Transcriptome Indicates Little Overlap in WT Compared to KlHET
3. Discussion
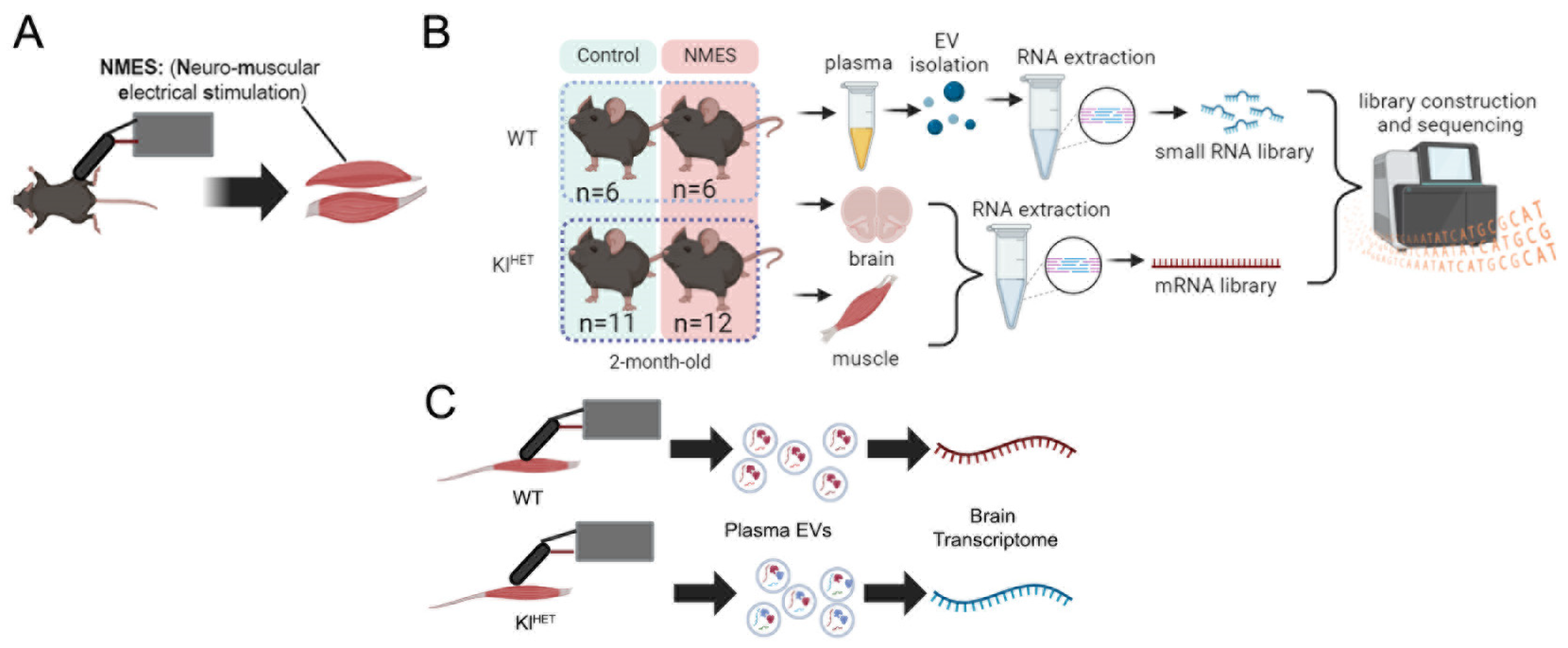
4. Materials and Methods
4.1. RNA Alignment and Differential Enrichment
4.2. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bean, A.C.; Sahu, A.; Piechocki, C.; Gualerzi, A.; Picciolini, S.; Bedoni, M.; Ambrosio, F. Neuromuscular electrical stimulation enhances the ability of serum extracellular vesicles to regenerate aged skeletal muscle after injury. Exp. Gerontol. 2023, 177, 112179. [Google Scholar] [CrossRef]
- Kristensen, M.G.H.; Busk, H.; Wienecke, T. Neuromuscular Electrical Stimulation Improves Activities of Daily Living Post Stroke: A Systematic Review and Meta-analysis. Arch. Rehabil. Res. Clin. Transl. 2022, 4, 100167. [Google Scholar] [CrossRef]
- Sanchez, M.J.; Mossayebi, A.; Sigaroodi, S.; Apaflo, J.N.; Galvan, M.J.; Min, K.; Agullo, F.J.; Wagler, A.; Bajpeyi, S. Effects of neuromuscular electrical stimulation on glycemic control: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1222532. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Masuda, T.; Kamiya, K.; Hamazaki, N.; Akiyama, A.; Kamada, Y.; Maekawa, E.; Noda, C.; Yamaoka-Tojo, M.; Ako, J. A single Session of Neuromuscular Electrical Stimulation Enhances Vascular Endothelial Function and Peripheral Blood Circulation in Patients with Acute Myocardial Infarction. Int. Heart J. 2016, 57, 676–681. [Google Scholar] [CrossRef]
- Distefano, G.; Ferrari, R.J.; Weiss, C.; Deasy, B.M.; Boninger, M.L.; Fitzgerald, G.K.; Huard, J.; Ambrosio, F.; Musaro, A. Neuromuscular electrical stimulation as a method to maximize the beneficial effects of muscle stem cells transplanted into dystrophic skeletal muscle. PLoS ONE 2013, 8, e54922. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Burtscher, J.; Millet, G.P.; Place, N.; Kayser, B.; Zanou, N. The Muscle-Brain Axis and Neurodegenerative Diseases: The Key Role of Mitochondria in Exercise-Induced Neuroprotection. Int. J. Mol. Sci. 2021, 22, 6479. [Google Scholar] [CrossRef]
- Flodin, J.; Reitzner, S.M.; Emanuelsson, E.B.; Sundberg, C.J.; Ackermann, P. The effect of neuromuscular electrical stimulation on the human skeletal muscle transcriptome. Acta Physiol. 2024, 240, e14129. [Google Scholar] [CrossRef]
- Ransom, L.S.; Liu, C.S.; Dunsmore, E.; Palmer, C.R.; Nicodemus, J.; Ziomek, D.; Williams, N.; Chun, J. Human brain small extracellular vesicles contain selectively packaged, full-length mRNA. Cell Rep. 2024, 43, 114061. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, I.A.; Mehler, M.F. Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease. Nat. Rev. Neurosci. 2012, 13, 528–541. [Google Scholar] [CrossRef]
- Jimenez-Ramirez, I.A.; Castano, E. Non-coding RNAs in the pathogenesis of Alzheimer’s disease: Beta-amyloid aggregation, Tau phosphorylation and neuroinflammation. Mol. Biol. Rep. 2025, 52, 183. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Fitz, N.F.; Sahu, A.; Lu, Y.; Ambrosio, F.; Lefterov, I.; Koldamova, R. Extracellular Vesicles in Young Serum Contribute to the Restoration of Age-Related Brain Transcriptomes and Cognition in Old Mice. Int. J. Mol. Sci. 2023, 24, 12550. [Google Scholar] [CrossRef]
- Sahu, A.; Clemens, Z.J.; Shinde, S.N.; Sivakumar, S.; Pius, A.; Bhatia, A.; Picciolini, S.; Carlomagno, C.; Gualerzi, A.; Bedoni, M.; et al. Regulation of aged skeletal muscle regeneration by circulating extracellular vesicles. Nat. Aging 2021, 1, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Nelson, V.K.; Jha, N.K.; Nuli, M.V.; Gupta, S.; Kanna, S.; Gahtani, R.M.; Hani, U.; Singh, A.K.; Abomughaid, M.M.; Abomughayedh, A.M.; et al. Unveiling the impact of aging on BBB and Alzheimer’s disease: Factors and therapeutic implications. Ageing Res. Rev. 2024, 98, 102224. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Stewart, T.; Banks, W.A.; Zhang, J. The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Curr. Pharm. Des. 2017, 23, 6206–6214. [Google Scholar] [CrossRef]
- Abdelsalam, M.; Ahmed, M.; Osaid, Z.; Hamoudi, R.; Harati, R. Insights into Exosome Transport through the Blood-Brain Barrier and the Potential Therapeutical Applications in Brain Diseases. Pharmaceuticals 2023, 16, 571. [Google Scholar] [CrossRef]
- Corrêa, H.d.L.; Raab, A.T.O.; Araújo, T.M.; Deus, L.A.; Reis, A.L.; Honorato, F.S.; Rodrigues-Silva, P.L.; Neves, R.V.P.; Brunetta, H.S.; da Silva Mori, M.A.; et al. A systematic review and meta-analysis demonstrating Klotho as an emerging exerkine. Sci. Rep. 2022, 12, 17587. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.Y.; Iyaswamy, A.; Sreenivasmurthy, S.G.; Krishnamoorthi, S.; Guan, X.-J.; Zhu, Z.; Su, C.-F.; Liu, J.; Kan, Y.; Zhang, Y.; et al. Klotho an Autophagy Stimulator as a Potential Therapeutic Target for Alzheimer’s Disease: A Review. Biomedicines 2022, 10, 705. [Google Scholar] [CrossRef]
- Sahu, A.; Mamiya, H.; Shinde, S.N.; Cheikhi, A.; Winter, L.L.; Vo, N.V.; Stolz, D.; Roginskaya, V.; Tang, W.-Y.; Croix, C.S.; et al. Age-related declines in alpha-Klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat. Commun. 2018, 9, 4859. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, M. Emerging role of alpha-Klotho in energy metabolism and cardiometabolic diseases. Diabetes Metab. Syndr. 2023, 17, 102854. [Google Scholar] [CrossRef]
- Re, V.L.; Russelli, G.; Gerfo, E.L.; Alduino, R.; Bulati, M.; Iannolo, G.; Terzo, D.; Martucci, G.; Anzani, S.; Panarello, G.; et al. Cognitive outcomes in patients treated with neuromuscular electrical stimulation after coronary artery bypass grafting. Front. Neurol. 2023, 14, 1209905. [Google Scholar] [CrossRef] [PubMed]
- Dubnov, S.; Bennett, E.R.; Yayon, N.; Yakov, O.; Bennett, D.A.; Seshadri, S.; Mufson, E.; Tzur, Y.; Greenberg, D.; Kuro-o, M.; et al. Knockout of the longevity gene Klotho perturbs aging and Alzheimer’s disease-linked brain microRNAs and tRNA fragments. Commun. Biol. 2024, 7, 720. [Google Scholar] [CrossRef]
- Ambrosio, F.; Fitzgerald, G.K.; Ferrari, R.; Distefano, G.; Carvell, G. A murine model of muscle training by neuromuscular electrical stimulation. J. Vis. Exp. 2012, 63, e3914. [Google Scholar] [CrossRef]
- Bettahi, I.; Krishnankutty, R.; Jaganjac, M.; Suleiman, N.N.M.; Ramanjaneya, M.; Jerobin, J.; Hassoun, S.; Alkasem, M.; Abdelhakam, I.; Iskandarani, A.; et al. Differences in protein expression, at the basal state and at 2 h of insulin infusion, in muscle biopsies from healthy Arab men with high or low insulin sensitivity measured by hyperinsulinemic euglycemic clamp. Front. Endocrinol. 2023, 13, 1024832. [Google Scholar] [CrossRef]
- Yao, J.; Saraf, F.; Rathore, V.S.; Darkazanli, K.; Liu, Y.; Korivi, M.; Bhaskar, L.V.K.S. Importance of selected genetic determinants on endurance performance and physical strength: A narrative review. Front. Physiol. 2025, 16, 1568334. [Google Scholar] [CrossRef]
- Carey, K.A.; Farnfield, M.M.; Tarquinio, S.D.; Cameron-Smith, D. Impaired expression of Notch signaling genes in aged human skeletal muscle. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Singh, C.; Lee, C.; DiMagno, K.; O’DOnnell, M.; Kaczmarek, M.; Ahmed, A.; Salvo-Schaich, J.; Perez, A.; Letsou, W.; et al. Mustn1 ablation in skeletal muscle results in increased glucose tolerance concomitant with upregulated GLUT expression in male mice. Physiol. Rep. 2023, 11, e15674. [Google Scholar] [CrossRef] [PubMed]
- Dziembowska, I.; Wójcik, M.; Bukowski, J.; Żekanowska, E. Physical Training Increases Erythroferrone Levels in Men. Biology 2021, 10, 1215. [Google Scholar] [CrossRef]
- Lausch, E.; Keppler, R.; Hilbert, K.; Cormier-Daire, V.; Nikkel, S.; Nishimura, G.; Unger, S.; Spranger, J.; Superti-Furga, A.; Zabel, B. Mutations in MMP9 and MMP13 determine the mode of inheritance and the clinical spectrum of metaphyseal anadysplasia. Am. J. Hum. Genet. 2009, 85, 168–178. [Google Scholar] [CrossRef]
- Colman, M.; Castori, M.; Micale, L.; Ritelli, M.; Colombi, M.; Ghali, N.; Van Dijk, F.; Marsili, L.; Weeks, A.; Vandersteen, A.; et al. Atypical variants in COL1A1 and COL3A1 associated with classical and vascular Ehlers-Danlos syndrome overlap phenotypes: Expanding the clinical phenotype based on additional case reports. Clin. Exp. Rheumatol. 2022, 40 (Suppl. S134), 46–62. [Google Scholar] [CrossRef] [PubMed]
- Hangelbroek, R.W.J.; Fazelzadeh, P.; Tieland, M.; Boekschoten, M.V.; Hooiveld, G.J.E.J.; van Duynhoven, J.P.M.; Timmons, J.A.; Verdijk, L.B.; de Groot, L.C.P.G.M.; van Loon, L.J.C.; et al. Expression of protocadherin gamma in skeletal muscle tissue is associated with age and muscle weakness. J. Cachexia Sarcopenia Muscle 2016, 7, 604–614. [Google Scholar] [CrossRef]
- Lee, M.H.; Kang, S.; Um, K.-H.; Lee, S.W.; Hwang, H.; Baek, K.; Choi, J.W. Brain-targeted delivery of neuroprotective survival gene minimizing hematopoietic cell contamination: Implications for Parkinson’s disease treatment. J. Transl. Med. 2024, 22, 53. [Google Scholar] [CrossRef] [PubMed]
- Marotta, M.; Ruiz-Roig, C.; Sarria, Y.; Peiro, J.L.; Nuñez, F.; Ceron, J.; Munell, F.; Roig-Quilis, M. Muscle genome-wide expression profiling during disease evolution in mdx mice. Physiol. Genom. 2009, 37, 119–132. [Google Scholar] [CrossRef]
- Ramanathan, G.; Zhao, Y.; Gupta, R.; Langmo, S.; Bhetraratana, M.; Yin, F.; Driscoll, W.; Ricks, J.; Louie, A.; Stewart, J.A.; et al. Integrated hepatic transcriptomics and metabolomics identify Pck1 as a key factor in the broad dysregulation induced by vehicle pollutants. Part. Fibre Toxicol. 2024, 21, 55. [Google Scholar] [CrossRef]
- Krick, S.; Baumlin, N.; Aller, S.P.; Aguiar, C.; Grabner, A.; Sailland, J.; Mendes, E.; Schmid, A.; Qi, L.; David, N.V.; et al. Klotho Inhibits Interleukin-8 Secretion from Cystic Fibrosis Airway Epithelia. Sci. Rep. 2017, 7, 14388. [Google Scholar] [CrossRef]
- Langston, P.K.; Mathis, D. Immunological regulation of skeletal muscle adaptation to exercise. Cell Metab. 2024, 36, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J. Insulin-like growth factor-I regulation of immune function: A potential therapeutic target in autoimmune diseases? Pharmacol. Rev. 2010, 62, 199–236. [Google Scholar] [CrossRef]
- de Gonzalo-Calvo, D.; Dávalos, A.; Montero, A.; García-González, A.; Tyshkovska, I.; González-Medina, A.; Soares, S.M.A.; Martínez-Camblor, P.; Casas-Agustench, P.; Rabadán, M.; et al. Circulating inflammatory miRNA signature in response to different doses of aerobic exercise. J. Appl. Physiol. 2015, 119, 124–134. [Google Scholar] [CrossRef]
- Abramova, O.; Zorkina, Y.; Ushakova, V.; Zubkov, E.; Morozova, A.; Chekhonin, V. The role of oxytocin and vasopressin dysfunction in cognitive impairment and mental disorders. Neuropeptides 2020, 83, 102079. [Google Scholar] [CrossRef] [PubMed]
- Bymaster, F.P.; McKinzie, D.L.; Felder, C.C.; Wess, J. Use of M1-M5 muscarinic receptor knockout mice as novel tools to delineate the physiological roles of the muscarinic cholinergic system. Neurochem. Res. 2003, 28, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Agodi, A.; Ojeda-Granados, C.; Maugeri, A.; Barchitta, M.; Coco, O.; Pezzino, S.; Magro, G.; La Greca, G.; Latteri, F.S.; Castorina, S.; et al. Changes in Gut Microbial Composition and DNA Methylation in Obese Patients with NAFLD After Bariatric Surgery. Int. J. Mol. Sci. 2024, 25, 11510. [Google Scholar] [CrossRef]
- Birdsey, G.M.; Shah, A.V.; Dufton, N.; Reynolds, L.E.; Almagro, L.O.; Yang, Y.; Aspalter, I.M.; Khan, S.T.; Mason, J.C.; Dejana, E.; et al. The endothelial transcription factor ERG promotes vascular stability and growth through Wnt/β-catenin signaling. Dev. Cell 2015, 32, 82–96. [Google Scholar] [CrossRef]
- Arribat, Y. Genetic alterations of VWA1: A new link between extracellular matrix and neuromuscular diseases. Brain 2021, 144, 362–365. [Google Scholar] [CrossRef]
- Deschauer, M.; Hengel, H.; Rupprich, K.; Kreiß, M.; Schlotter-Weigel, B.; Grimmel, M.; Admard, J.; Schneider, I.; Alhaddad, B.; Gazou, A.; et al. Bi-allelic truncating mutations in VWA1 cause neuromyopathy. Brain 2021, 144, 574–583. [Google Scholar] [CrossRef]
- Fitz, N.F.; Nam, K.N.; Wolfe, C.M.; Letronne, F.; Playso, B.E.; Iordanova, B.E.; Kozai, T.D.Y.; Biedrzycki, R.J.; Kagan, V.E.; Tyurina, Y.Y.; et al. Phospholipids of APOE lipoproteins activate microglia in an isoform-specific manner in preclinical models of Alzheimer’s disease. Nat. Commun. 2021, 12, 3416. [Google Scholar] [CrossRef]
- Lu, Y.; Saibro-Girardi, C.; Fitz, N.F.; McGuire, M.R.; Ostach, M.A.; Mamun-Or-Rashid, A.; Lefterov, I.; Koldamova, R. Multi-transcriptomics reveals brain cellular responses to peripheral infection in Alzheimer’s disease model mice. Cell Rep. 2023, 42, 112785. [Google Scholar] [CrossRef]
- Fitz, N.F.; Wolfe, C.M.; Playso, B.E.; Biedrzycki, R.J.; Lu, Y.; Nam, K.N.; Lefterov, I.; Koldamova, R. Trem2 deficiency differentially affects phenotype and transcriptome of human APOE3 and APOE4 mice. Mol. Neurodegener. 2020, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.; Liu, Y.; Santagiuliana, G.; Barandun, G.; Junior, P.T.; Güder, F.; Bastiaansen, C.W.; Baxendale, M.; Fenwick, O.; Papageorgiou, D.G.; et al. Self-powered ultrasensitive and highly stretchable temperature-strain sensing composite yarns. Mater. Horiz. 2021, 8, 2513–2519. [Google Scholar] [CrossRef]
- Colleluori, G.; Viola, V.; Bathina, S.; Armamento-Villareal, R.; Qualls, C.; Giordano, A.; Villareal, D.T. Effect of aerobic or resistance exercise, or both on insulin secretion, ciliary neurotrophic factor, and insulin-like growth factor-1 in dieting older adults with obesity. Clin. Nutr. 2025, 51, 50–62. [Google Scholar] [CrossRef]
- Vilarino-Garcia, T.; Polonio-Gonzalez, M.L.; Perez-Perez, A.; Ribalta, J.; Arrieta, F.; Aguilar, M.; Obaya, J.C.; Gimeno-Orna, J.A.; Iglesias, P.; Navarro, J.; et al. Role of Leptin in Obesity, Cardiovascular Disease, and Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 2338. [Google Scholar] [CrossRef]
- McGuire, M.J.; Ishii, M. Leptin Dysfunction and Alzheimer’s Disease: Evidence from Cellular, Animal, and Human Studies. Cell Mol. Neurobiol. 2016, 36, 203–217. [Google Scholar] [CrossRef]
- Nyomba, B.; Johnson, M.; Berard, L.; Murphy, L.J. Relationship between serum leptin and the insulin-like growth factor-I system in humans. Metabolism 1999, 48, 840–844. [Google Scholar] [CrossRef]
- Raber, J.; Olsen, R.H.; Su, W.; Foster, S.; Xing, R.; Acevedo, S.F.; Sherman, L.S. CD44 is required for spatial memory retention and sensorimotor functions. Behav. Brain Res. 2014, 275, 146–149. [Google Scholar] [CrossRef]
- Vagena, E.; Crneta, J.; Engström, P.; He, L.; Yulyaningsih, E.; Korpel, N.L.; Cheang, R.T.; Bachor, T.P.; Huang, A.; Michel, G.; et al. ASB4 modulates central melanocortinergic neurons and calcitonin signaling to control satiety and glucose homeostasis. Sci. Signal. 2022, 15, eabj8204. [Google Scholar] [CrossRef] [PubMed]
- Elabd, S.; Sabry, I. Two Birds with One Stone: Possible Dual-Role of Oxytocin in the Treatment of Diabetes and Osteoporosis. Front. Endocrinol. 2015, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Yang, C.; Chen, T.H.; Al-Azawi, N.; Hsu, W.H. Effect of AVP and oxytocin on insulin release: Involvement of V1b receptors. Am. J. Physiol. 1995, 269 Pt 1, E1095–E1100. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Maxwell-Warburton, A.; Hasib, A.; Ma, L.; Kang, L. The Membrane Receptor CD44: Novel Insights into Metabolism. Trends Endocrinol. Metab. 2022, 33, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fang, X.; Liu, Y.; Lu, X.; Liu, Y.; Zhao, Z.; Liu, B.; Yang, R. Lipid Regulatory Element Interact with CD44 on Mitochondrial Bioenergetics in Bovine Adipocyte Differentiation and Lipometabolism. J. Agric. Food Chem. 2024, 72, 17481–17498. [Google Scholar] [CrossRef]
- Lotri-Koffi, A.; Pauly, M.; Lemarié, E.; Godin-Ribuot, D.; Tamisier, R.; Pépin, J.-L.; Vivodtzev, I. Chronic neuromuscular electrical stimulation improves muscle mass and insulin sensitivity in a mouse model. Sci. Rep. 2019, 9, 7252. [Google Scholar] [CrossRef]
- Zheng, H.; Tian, F.; Sun, W.; Zheng, L.; Xiao, W. Neuromuscular Electrical Stimulation Improves Frontal Ankle Motor Control in Individuals with Chronic Ankle Instability During Drop Landing. Am. J. Phys. Med. Rehabil. 2024, 103, 890–896. [Google Scholar] [CrossRef]
- Deczkowska, A.; Keren-Shaul, H.; Weiner, A.; Colonna, M.; Schwartz, M.; Amit, I. Disease-Associated Microglia: A Universal Immune Sensor of Neurodegeneration. Cell 2018, 173, 1073–1081. [Google Scholar] [CrossRef]
- Gleeson, M. Immune function in sport and exercise. J. Appl. Physiol. 2007, 103, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, R.; Demontis, F.; Perrimon, N.; Goldberg, A.L. Mechanisms of muscle growth and atrophy in mammals and Drosophila. Dev. Dyn. 2014, 243, 201–215. [Google Scholar] [CrossRef]
- Parker, F.; Peckham, M. Disease mutations in striated muscle myosins. Biophys. Rev. 2020, 12, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Uçar, N.; Öner, H.; Kuş, M.A.; Karaca, H.; Fırat, T. The effect of neuromuscular electrical stimulation applied at different muscle lengths on muscle architecture and sarcomere morphology in rats. Anat. Rec. 2024, 307, 356–371. [Google Scholar] [CrossRef]
- Kishlyansky, M.; Vojnovic, J.; Roudier, E.; Gineste, C.; Decary, S.; Forn, P.; Bergeron, R.; Desplanches, D.; Birot, O. Striated muscle angio-adaptation requires changes in Vasohibin-1 expression pattern. Biochem. Biophys. Res. Commun. 2010, 399, 359–364. [Google Scholar] [CrossRef]
- Rivard, A.; Silver, M.; Chen, D.; Kearney, M.; Magner, M.; Annex, B.; Peters, K.; Isner, J.M. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am. J. Pathol. 1999, 154, 355–363. [Google Scholar] [CrossRef]
- Perosa, V.; Priester, A.; Ziegler, G.; Cardenas-Blanco, A.; Dobisch, L.; Spallazzi, M.; Assmann, A.; Maass, A.; Speck, O.; Oltmer, J.; et al. Hippocampal vascular reserve associated with cognitive performance and hippocampal volume. Brain 2020, 143, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Rego, B.V.; Weiss, D.; Humphrey, J.D. A fast, robust method for quantitative assessment of collagen fibril architecture from transmission electron micrographs. Microsc. Microanal. 2023, 29, 2099–2107. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujimori, T.; Nabeshima, Y. Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1alpha-hydroxylase gene. Endocrinology 2002, 143, 683–689. [Google Scholar] [CrossRef]
- Mencke, R.; Hillebrands, J.-L. The role of the anti-ageing protein Klotho in vascular physiology and pathophysiology. Ageing Res. Rev. 2017, 35, 124–146. [Google Scholar] [CrossRef]
- Kalna, V.; Yang, Y.; Peghaire, C.R.; Frudd, K.; Hannah, R.; Shah, A.V.; Osuna Almagro, L.; Boyle, J.J.; Gottgens, B.; Ferrer, J.; et al. The Transcription Factor ERG Regulates Super-Enhancers Associated with an Endothelial-Specific Gene Expression Program. Circ. Res. 2019, 124, 1337–1349. [Google Scholar] [CrossRef]
- Roush, S.; Slack, F.J. The let-7 family of microRNAs. Trends Cell Biol. 2008, 18, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Aroca-Esteban, J.; Souza-Neto, F.V.; Aguilar-Latorre, C.; Tribaldo-Torralbo, A.; González-López, P.; Ruiz-Simón, R.; Álvarez-Villareal, M.; Ballesteros, S.; de Ceniga, M.V.; Landete, P.; et al. Potential protective role of let-7d-5p in atherosclerosis progression reducing the inflammatory pathway regulated by NF-kappaB and vascular smooth muscle cells proliferation. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167327. [Google Scholar] [CrossRef] [PubMed]
- Buonfiglioli, A.; Efe, I.E.; Guneykaya, D.; Ivanov, A.; Huang, Y.; Orlowski, E.; Krüger, C.; Deisz, R.A.; Markovic, D.; Flüh, C.; et al. let-7 MicroRNAs Regulate Microglial Function and Suppress Glioma Growth through Toll-like Receptor 7. Cell Rep. 2019, 29, 3460–3471.E7. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.; Salmond, N.; Halvaei, S.; Johnson, A.; Williams, K.C. Separation and isolation of CD9-positive extracellular vesicles from plasma using flow cytometry. Nanoscale Adv. 2023, 5, 4435–4446. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’DRiscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Collins, D.F. Central contributions to contractions evoked by tetanic neuromuscular electrical stimulation. Exerc. Sport Sci. Rev. 2007, 35, 102–109. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat. Rev. Endocrinol. 2012, 8, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.-F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavanaugh, C.A.; Moore, A.E.; Fitz, N.F.; Lefterov, I.; Koldamova, R. Transcriptomic Response to Neuromuscular Electrical Stimulation in Muscle, Brain, and Plasma EVs in WT and Klotho-Deficient Mice. Int. J. Mol. Sci. 2025, 26, 7849. https://doi.org/10.3390/ijms26167849
Cavanaugh CA, Moore AE, Fitz NF, Lefterov I, Koldamova R. Transcriptomic Response to Neuromuscular Electrical Stimulation in Muscle, Brain, and Plasma EVs in WT and Klotho-Deficient Mice. International Journal of Molecular Sciences. 2025; 26(16):7849. https://doi.org/10.3390/ijms26167849
Chicago/Turabian StyleCavanaugh, Catherine Anne, Amanda E. Moore, Nicholas Francis Fitz, Iliya Lefterov, and Radosveta Koldamova. 2025. "Transcriptomic Response to Neuromuscular Electrical Stimulation in Muscle, Brain, and Plasma EVs in WT and Klotho-Deficient Mice" International Journal of Molecular Sciences 26, no. 16: 7849. https://doi.org/10.3390/ijms26167849
APA StyleCavanaugh, C. A., Moore, A. E., Fitz, N. F., Lefterov, I., & Koldamova, R. (2025). Transcriptomic Response to Neuromuscular Electrical Stimulation in Muscle, Brain, and Plasma EVs in WT and Klotho-Deficient Mice. International Journal of Molecular Sciences, 26(16), 7849. https://doi.org/10.3390/ijms26167849





