The G-allele of rs10830963 in MTNR1B Exerts Stage-Specific Effects Across the Trajectory of Type 2 Diabetes: A Multi-State Analysis
Abstract
1. Introduction
2. Results
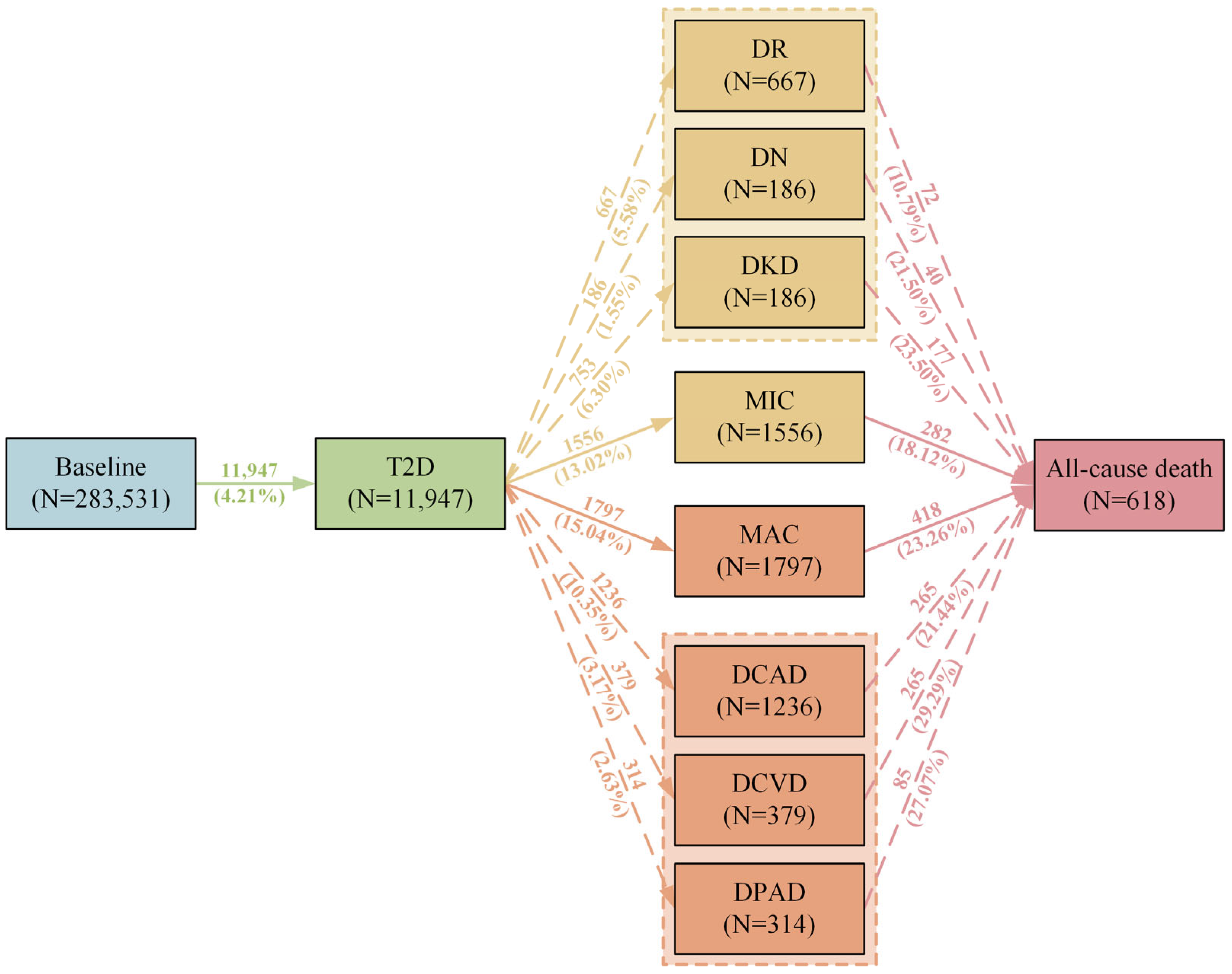
2.1. Descriptive Analysis
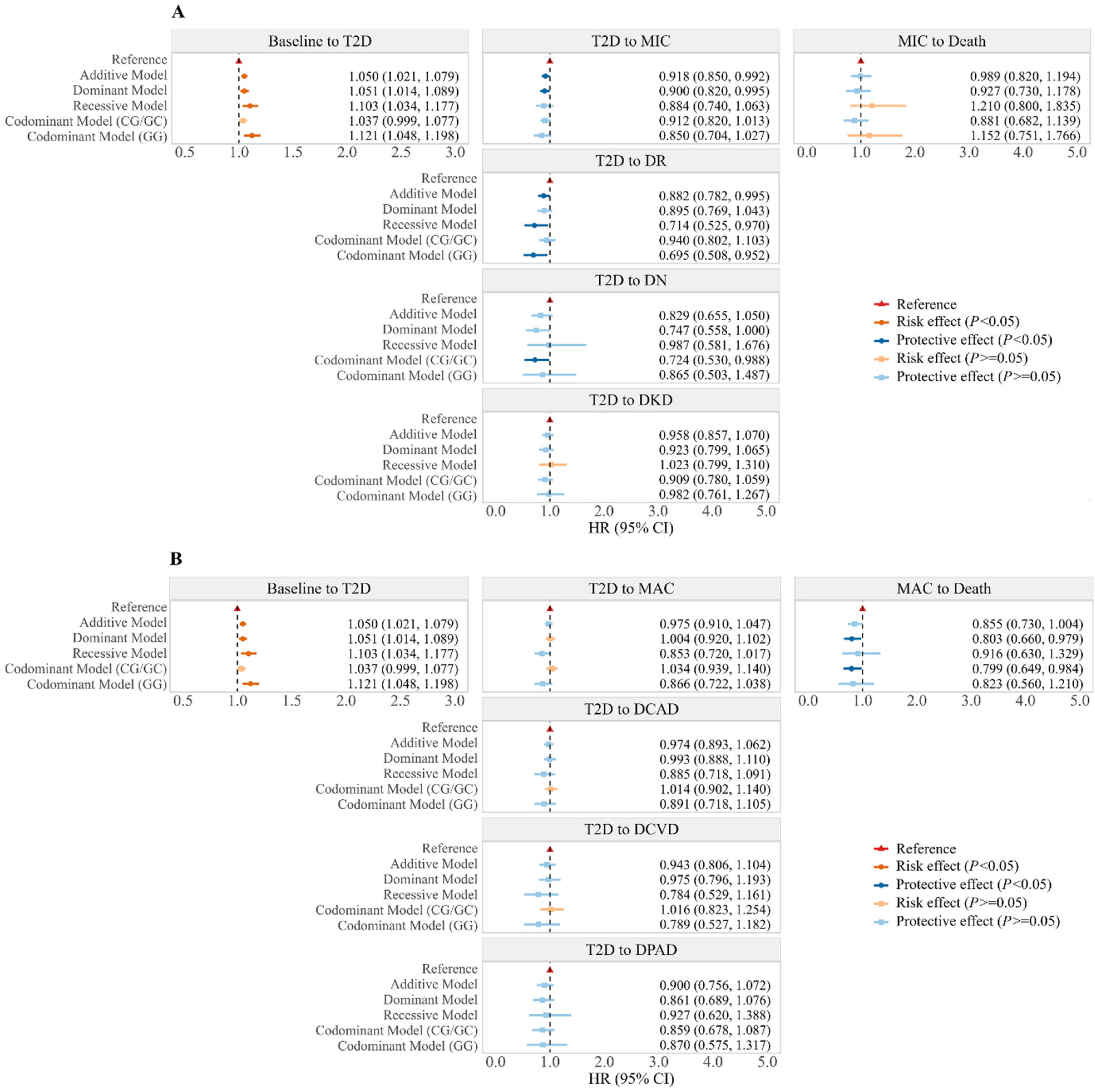
2.2. Associations Between rs10830963 and Trajectory of T2D
2.3. Associations Between rs10830963 and Onset of T2D and T2D Comorbidities
2.4. Associations Between rs10830963 and Blood Biochemical Parameters in T2D and Non-T2D Participants
2.5. Subgroup and Sensitivity Analyses
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. MTNR1B rs10830963 Genotype
4.3. Blood Biochemical Parameter Assessment
4.4. Ascertainment of Outcomes
4.5. Covariates
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef]
- Bouatia-Naji, N.; Bonnefond, A.; Cavalcanti-Proença, C.; Sparsø, T.; Holmkvist, J.; Marchand, M.; Delplanque, J.; Lobbens, S.; Rocheleau, G.; Durand, E.; et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat. Genet. 2009, 41, 89–94. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wang, H.; Zhang, Y.Y. Melatonin receptor 1B gene rs10830963 C/G polymorphism associated with type 2 diabetes mellitus: An updated meta-analysis of 13,752 participants. Heliyon 2022, 8, e11786. [Google Scholar] [CrossRef] [PubMed]
- Lyssenko, V.; Nagorny, C.L.; Erdos, M.R.; Wierup, N.; Jonsson, A.; Spégel, P.; Bugliani, M.; Saxena, R.; Fex, M.; Pulizzi, N.; et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat. Genet. 2009, 41, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wang, Y.; Chen, J.; Zhang, J.; Yu, P.; Zhang, R.; Li, S.; Tao, B.; Wang, Y.; Qiu, Y.; et al. Activation of melatonin receptor 2 but not melatonin receptor 1 mediates melatonin-conferred cardioprotection against myocardial ischemia/reperfusion injury. J. Pineal Res. 2019, 67, e12571. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, Y.; Gao, Y.; Wang, Z.; Ma, J. Melatonin Attenuates Anoxia/Reoxygenation Injury by Inhibiting Excessive Mitophagy Through the MT2/SIRT3/FoxO3a Signaling Pathway in H9c2 Cells. Drug Des. Dev. Ther. 2020, 14, 2047–2060. [Google Scholar] [CrossRef]
- Klosen, P.; Lapmanee, S.; Schuster, C.; Guardiola, B.; Hicks, D.; Pevet, P.; Felder-Schmittbuhl, M.P. MT1 and MT2 melatonin receptors are expressed in nonoverlapping neuronal populations. J. Pineal Res. 2019, 67, e12575. [Google Scholar] [CrossRef]
- Amin, M.; Rafla, B.; Wu, R.; Postolache, T.T.; Gragnoli, C. The role of melatonin receptor 1B gene (MTNR1B) in the susceptibility to depression and type 2 diabetes comorbidity. Genes Dis. 2024, 11, 101067. [Google Scholar] [CrossRef]
- Ocak, Ö.; Silan, F.; Şahin, E.M. Melatonin receptor gene polymorphisms as a risk factor in patients with diabetic peripheral neuropathy. Diabetes/Metab. Res. Rev. 2022, 38, e3573. [Google Scholar] [CrossRef]
- Tan, X.; Benedict, C. Increased Risk of Myocardial Infarction Among Patients With Type 2 Diabetes Who Carry the Common rs10830963 Variant in the MTNR1B Gene. Diabetes Care 2020, 43, 2289–2292. [Google Scholar] [CrossRef]
- Xue, P.; Tan, X.; Wu, J.; Tang, X.; Benedict, C. No association between a common type 2 diabetes risk gene variant in the melatonin receptor gene (MTNR1B) and mortality among type 2 diabetes patients. J. Pineal Res. 2022, 72, e12785. [Google Scholar] [CrossRef]
- Guo, H.; Peng, H.; Wang, S.; Hou, T.; Li, Y.; Zhang, H.; Jiang, J.; Ma, B.; Wang, M.; Wu, Y.; et al. Healthy Lifestyles Modify the Association of Melatonin Receptor 1B Gene and Ischemic Stroke: A Family-Based Cohort Study in Northern China. J. Pineal Res. 2024, 76, e13000. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, L.; Liang, Y.Y.; He, Z.; Ai, Q.H.; Chen, W.; Xue, H.; Zhou, M.; Wang, Y.; Ma, H.; et al. Interaction of night shift work with polymorphism in melatonin receptor 1B gene on incident stroke. Scand. J. Work Environ. Health 2022, 48, 372–379. [Google Scholar] [CrossRef]
- Meira-Machado, L.; de Uña-Alvarez, J.; Cadarso-Suárez, C.; Andersen, P.K. Multi-state models for the analysis of time-to-event data. Stat. Methods Med. Res. 2009, 18, 195–222. [Google Scholar] [CrossRef]
- Tan, X.; Ciuculete, D.M.; Schiöth, H.B.; Benedict, C. Associations between chronotype, MTNR1B genotype and risk of type 2 diabetes in UK Biobank. J. Intern. Med. 2020, 287, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Arikoglu, H.; Erkoc-Kaya, D.; Ipekci, S.H.; Gokturk, F.; Iscioglu, F.; Korez, M.K.; Baldane, S.; Gonen, M.S. Type 2 diabetes is associated with the MTNR1B gene, a genetic bridge between circadian rhythm and glucose metabolism, in a Turkish population. Mol. Biol. Rep. 2021, 48, 4181–4189. [Google Scholar] [CrossRef]
- Sparsø, T.; Bonnefond, A.; Andersson, E.; Bouatia-Naji, N.; Holmkvist, J.; Wegner, L.; Grarup, N.; Gjesing, A.P.; Banasik, K.; Cavalcanti-Proença, C.; et al. G-allele of Intronic rs10830963 in MTNR1B Confers Increased Risk of Impaired Fasting Glycemia and Type 2 Diabetes Through an Impaired Glucose-Stimulated Insulin Release: Studies Involving 19,605 Europeans. Diabetes 2009, 58, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Prokopenko, I.; Langenberg, C.; Florez, J.C.; Saxena, R.; Soranzo, N.; Thorleifsson, G.; Loos, R.J.; Manning, A.K.; Jackson, A.U.; Aulchenko, Y.; et al. Variants in MTNR1B influence fasting glucose levels. Nat. Genet. 2009, 41, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, J.; Luo, X.; Li, M.; Yue, Y.; Laudon, M.; Jia, Z.; Zhang, R. Neu-P11, a novel MT1/MT2 agonist, reverses diabetes by suppressing the hypothalamic-pituitary-adrenal axis in rats. Eur. J. Pharmacol. 2017, 812, 225–233. [Google Scholar] [CrossRef]
- Vejrazkova, D.; Vankova, M.; Vcelak, J.; Krejci, H.; Anderlova, K.; Tura, A.; Pacini, G.; Sumova, A.; Sladek, M.; Bendlova, B. The rs10830963 Polymorphism of the MTNR1B Gene: Association With Abnormal Glucose, Insulin and C-peptide Kinetics. Front. Endocrinol. 2022, 13, 868364. [Google Scholar] [CrossRef]
- Müssig, K.; Staiger, H.; Machicao, F.; Häring, H.U.; Fritsche, A. Genetic variants in MTNR1B affecting insulin secretion. Ann. Med. 2010, 42, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Qian, J.; Florez, J.C.; Arendt, J.; Saxena, R.; Scheer, F. Melatonin Effects on Glucose Metabolism: Time To Unlock the Controversy. Trends Endocrinol. Metab. TEM 2020, 31, 192–204. [Google Scholar] [CrossRef]
- Persaud, S.J.; Jones, P.M. A Wake-up Call for Type 2 Diabetes? N. Engl. J. Med. 2016, 375, 1090–1092. [Google Scholar] [CrossRef] [PubMed]
- Gaulton, K.J.; Ferreira, T.; Lee, Y.; Raimondo, A.; Mägi, R.; Reschen, M.E.; Mahajan, A.; Locke, A.; Rayner, N.W.; Robertson, N.; et al. Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nat. Genet. 2015, 47, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Tuomi, T.; Nagorny, C.L.F.; Singh, P.; Bennet, H.; Yu, Q.; Alenkvist, I.; Isomaa, B.; Östman, B.; Söderström, J.; Pesonen, A.K.; et al. Increased Melatonin Signaling Is a Risk Factor for Type 2 Diabetes. Cell Metab. 2016, 23, 1067–1077. [Google Scholar] [CrossRef]
- Lopez-Minguez, J.; Saxena, R.; Bandín, C.; Scheer, F.A.; Garaulet, M. Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: A randomized, cross-over study. Clin. Nutr. 2018, 37, 1133–1140. [Google Scholar] [CrossRef]
- Garaulet, M.; Lopez-Minguez, J.; Dashti, H.S.; Vetter, C.; Hernández-Martínez, A.M.; Pérez-Ayala, M.; Baraza, J.C.; Wang, W.; Florez, J.C.; Scheer, F.; et al. Interplay of Dinner Timing and MTNR1B Type 2 Diabetes Risk Variant on Glucose Tolerance and Insulin Secretion: A Randomized Crossover Trial. Diabetes Care 2022, 45, 512–519. [Google Scholar] [CrossRef]
- Yeung, H.M.; Hung, M.W.; Lau, C.F.; Fung, M.L. Cardioprotective effects of melatonin against myocardial injuries induced by chronic intermittent hypoxia in rats. J. Pineal Res. 2015, 58, 12–25. [Google Scholar] [CrossRef]
- Ge, X.; Wang, C.; Yang, G.; Maimaiti, D.; Hou, M.; Liu, H.; Yang, H.; Chen, X.; Xu, Y.; He, F. Enhancement of mitochondrial energy metabolism by melatonin promotes vascularized skeletal muscle regeneration in a volumetric muscle loss model. Free Radic. Biol. Med. 2024, 210, 146–157. [Google Scholar] [CrossRef]
- Kim, R.; Kim, M.; Jeong, S.; Kim, S.; Moon, H.; Kim, H.; Lee, M.Y.; Kim, J.; Kim, H.S.; Choi, M.; et al. Melatonin alleviates myocardial dysfunction through inhibition of endothelial-to-mesenchymal transition via the NF-κB pathway. J. Pineal Res. 2024, 76, e12958. [Google Scholar] [CrossRef]
- Reppert, S.M.; Godson, C.; Mahle, C.D.; Weaver, D.R.; Slaugenhaupt, S.A.; Gusella, J.F. Molecular characterization of a second melatonin receptor expressed in human retina and brain: The Mel1b melatonin receptor. Proc. Natl. Acad. Sci. USA 1995, 92, 8734–8738. [Google Scholar] [CrossRef]
- Ning, J.; Pan, M.; Yang, H.; Wang, Z.; Wang, X.; Guo, K.; Feng, Y.; Xie, T.; Chen, Y.; Chen, C.; et al. Melatonin Attenuates Diabetic Retinopathy by Regulating EndMT of Retinal Vascular Endothelial Cells via Inhibiting the HDAC7/FOXO1/ZEB1 Axis. J. Pineal Res. 2024, 76, e13008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, S.; Huang, Y.; Ma, M.; Li, B.; Li, C.; Zhu, X.; Xu, X.; Chen, H.; Zhang, Y.; et al. Diabetes-Related Macrovascular Complications Are Associated With an Increased Risk of Diabetic Microvascular Complications: A Prospective Study of 1518 Patients With Type 1 Diabetes and 20 802 Patients With Type 2 Diabetes in the UK Biobank. J. Am. Heart Assoc. 2024, 13, e032626. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Gao, B.; Wang, J.; Yang, C.; Zhao, M.H.; Zhang, L. The Difference Between Cystatin C- and Creatinine-Based Estimated Glomerular Filtration Rate and Risk of Diabetic Microvascular Complications Among Adults With Diabetes: A Population-Based Cohort Study. Diabetes Care 2024, 47, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Peakman, T.C.; on behalf of UK Biobank. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int. J. Epidemiol. 2008, 37, 234–244. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Fadnes, L.T.; Celis-Morales, C.; Økland, J.M.; Parra-Soto, S.; Livingstone, K.M.; Ho, F.K.; Pell, J.P.; Balakrishna, R.; Javadi Arjmand, E.; Johansson, K.A.; et al. Life expectancy can increase by up to 10 years following sustained shifts towards healthier diets in the United Kingdom. Nat. Food 2023, 4, 961–965. [Google Scholar] [CrossRef]
- Putter, H.; Fiocco, M.; Geskus, R.B. Tutorial in biostatistics: Competing risks and multi-state models. Stat. Med. 2007, 26, 2389–2430. [Google Scholar] [CrossRef]
- de Wreede, L.C.; Fiocco, M.; Putter, H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput. Methods Programs Biomed. 2010, 99, 261–274. [Google Scholar] [CrossRef]
- Wei, L.J. The accelerated failure time model: A useful alternative to the Cox regression model in survival analysis. Stat. Med. 1992, 11, 1871–1879. [Google Scholar] [CrossRef]
| Characteristics | Total (N = 283,531) | rs10830963 Genotype | P | ||
|---|---|---|---|---|---|
| CC (N = 149,392) | CG/GC (N = 112,581) | GG (N = 21,558) | |||
| Mean follow-up, year (SD *) | 13.37 (1.82) | 13.37 (1.82) | 13.37 (1.81) | 13.38 (1.83) | 0.630 |
| Age, mean (SD) | 56.06 (8.05) | 56.09 (8.05) | 56.04 (8.05) | 55.96 (8.06) | 0.037 |
| Sex, n (%) | 0.253 | ||||
| Male | 131,985 (46.55) | 69,557 (46.56) | 52,284 (46.44) | 10,144 (47.05) | |
| Female | 151,546 (53.45) | 79,835 (53.44) | 60,297 (53.56) | 11,414 (52.95) | |
| Education, n (%) | 0.672 | ||||
| Any school degree | 113,642 (40.08) | 59,892 (40.09) | 45,177 (40.12) | 8573 (39.77) | |
| Vocational qualification | 18,404 (6.49) | 9804 (6.56) | 7217 (6.41) | 1383 (6.42) | |
| College education | 98,830 (34.86) | 51,992 (34.80) | 39,252 (34.87) | 7586 (35.18) | |
| Other | 52,655 (18.57) | 27,704 (18.54) | 20,935 (18.60) | 4016 (18.63) | |
| TDI ‡, mean (SD) | −1.65 (2.87) | −1.65 (2.87) | −1.65 (2.87) | −1.66 (2.87) | 0.792 |
| Income, n (%) | 0.390 | ||||
| <GBP § 18,000 | 48,081 (16.96) | 25,223 (16.88) | 19,170 (17.03) | 3688 (17.10) | |
| GBP 18,000 to GBP 30,999 | 63,420 (22.37) | 33,510 (22.43) | 25,074 (22.27) | 4836 (22.43) | |
| GBP 31,000 to GBP 51,999 | 70,661 (24.92) | 37,278 (24.95) | 27,919 (24.80) | 5464 (25.35) | |
| GBP 52,000 to GBP 100,000 | 58,000 (20.45) | 30,530 (20.44) | 23,090 (20.51) | 4380 (20.32) | |
| ≥GBP 100,000 | 15,105 (5.33) | 7911 (5.30) | 6051 (5.37) | 1143 (5.30) | |
| Unknown | 28,264 (9.97) | 14,940 (10.00) | 11,277 (10.02) | 2047 (9.50) | |
| BMI †, n (%) | 0.129 | ||||
| Normal | 97,389 (34.35) | 51,634 (34.56) | 38,468 (34.17) | 7287 (33.80) | |
| Underweight | 1352 (0.48) | 707 (0.48) | 532 (0.47) | 113 (0.52) | |
| Overweight | 124,245 (43.82) | 65,289 (43.70) | 49,501 (43.97) | 9455 (43.86) | |
| Obese | 60,545 (21.35) | 31,762 (21.26) | 24,080 (21.39) | 4703 (21.82) | |
| Alcohol intake frequency, n (%) | 0.012 | ||||
| Never | 15,285 (5.39) | 8163 (5.47) | 6049 (5.37) | 1073 (4.98) | |
| Occasional | 56,964 (20.09) | 29,760 (19.92) | 22,829 (20.28) | 4375 (20.29) | |
| Moderate | 147,664 (52.08) | 78,059 (52.25) | 58,406 (51.88) | 11,199 (51.95) | |
| Heavy | 63,618 (22.44) | 33,410 (22.36) | 25,297 (22.47) | 4911 (22.78) | |
| Smoking status, n (%) | 0.582 | ||||
| Never | 157,382 (55.51) | 82,931 (55.51) | 62,593 (55.60) | 11,858 (55.01) | |
| Previous | 97,887 (34.52) | 51,567 (34.52) | 38,812 (34.47) | 7508 (34.83) | |
| Current | 28,262 (9.97) | 14,894 (9.97) | 11,176 (9.93) | 2192 (10.16) | |
| Sedentary time, n (%) | 0.447 | ||||
| ≥4 h | 76,136 (26.85) | 40,081 (26.83) | 30,334 (26.94) | 5721 (26.54) | |
| <4 h | 207,395 (73.15) | 109,311 (73.17) | 82,247 (73.06) | 15,837 (73.46) | |
| Sleep duration, n (%) | 0.665 | ||||
| <7 h or >9 h | 68,971 (24.33) | 36,292 (24.29) | 27,473 (24.40) | 5206 (24.15) | |
| 7~9 h | 214,560 (75.67) | 113,100 (75.71) | 85,108 (75.60) | 16,352 (75.85) | |
| Healthy diet, n (%) | 0.661 | ||||
| Yes | 125,199 (44.16) | 65,938 (44.14) | 49,678 (44.13) | 9583 (44.45) | |
| No | 158,332 (55.84) | 83,454 (55.86) | 62,903 (55.87) | 11,975 (55.55) | |
| Physical activity, n (%) | 0.281 | ||||
| Low | 50,611 (17.85) | 26,747 (17.91) | 19,975 (17.74) | 3889 (18.04) | |
| Moderate | 116,144 (40.96) | 61,328 (41.05) | 45,963 (40.83) | 8853 (41.07) | |
| High | 116,776 (41.19) | 61,317 (41.04) | 46,643 (41.43) | 8816 (40.89) | |
| Hypertension, n (%) | 0.741 | ||||
| Yes | 172,082 (60.69) | 58,801 (39.36) | 44,155 (39.22) | 8493 (39.40) | |
| No | 111,449 (39.31) | 90,591 (60.64) | 68,426 (60.78) | 13,065 (60.60) | |
| Transitions | Additive Model (Continuous) | Dominant Model (GC/CG + GG vs. CC) | Recessive Model (GG vs. CC + GC/CG) | Codominant Model | |
|---|---|---|---|---|---|
| GC/CG vs. CC | GG vs. CC | ||||
| Baseline to T2D | 0.966 (0.947, 0.986) | 0.965 (0.941, 0.990) | 0.932 (0.890, 0.977) | 0.974 (0.948, 1.001) | 0.922 (0.878, 0.967) |
| T2D to MIC | 1.067 (1.030, 1.105) | 1.084 (1.037, 1.133) | 1.087 (1.000, 1.182) | 1.076 (1.027, 1.127) | 1.122 (1.030, 1.222) |
| T2D to DR | 1.062 (1.016, 1.110) | 1.055 (0.997, 1.115) | 1.176 (1.047, 1.320) | 1.031 (0.973, 1.093) | 1.191 (1.059, 1.341) |
| T2D to DN | 1.072 (0.974, 1.180) | 1.124 (0.997, 1.267) | 0.982 (0.793, 1.216) | 1.143 (1.006, 1.299) | 1.038 (0.833, 1.292) |
| T2D to DKD | 1.069 (1.015, 1.125) | 1.099 (1.030, 1.173) | 1.045 (0.928, 1.177) | 1.102 (1.029, 1.180) | 1.088 (0.964, 1.230) |
| MIC to Death | 0.984 (0.949, 1.020) | 0.993 (0.948, 1.041) | 0.939 (0.866, 1.017) | 1.005 (0.956, 1.057) | 0.941 (0.866, 1.022) |
| T2D to MAC | 1.030 (0.991, 1.071) | 1.027 (0.978, 1.079) | 1.078 (0.983, 1.183) | 1.016 (0.965, 1.070) | 1.086 (0.987, 1.195) |
| T2D to DCAD | 1.021 (0.974, 1.071) | 1.017 (0.957, 1.080) | 1.064 (0.950, 1.192) | 1.007 (0.945, 1.073) | 1.067 (0.950, 1.200) |
| T2D to DCVD | 1.064 (0.986, 1.147) | 1.061 (0.965, 1.168) | 1.156 (0.959, 1.394) | 1.040 (0.941, 1.150) | 1.176 (0.971, 1.425) |
| T2D to DPAD | 1.044 (0.980, 1.112) | 1.088 (1.004, 1.178) | 0.958 (0.833, 1.100) | 1.109 (1.018, 1.208) | 0.999 (0.866, 1.153) |
| MAC to Death | 1.009 (0.976, 1.042) | 1.010 (0.970, 1.052) | 1.013 (0.937, 1.096) | 1.009 (0.966, 1.053) | 1.017 (0.939, 1.102) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Dou, X.; He, M.; Su, Y.; Lin, H.; Yang, Y. The G-allele of rs10830963 in MTNR1B Exerts Stage-Specific Effects Across the Trajectory of Type 2 Diabetes: A Multi-State Analysis. Int. J. Mol. Sci. 2025, 26, 7855. https://doi.org/10.3390/ijms26167855
Huang Y, Dou X, He M, Su Y, Lin H, Yang Y. The G-allele of rs10830963 in MTNR1B Exerts Stage-Specific Effects Across the Trajectory of Type 2 Diabetes: A Multi-State Analysis. International Journal of Molecular Sciences. 2025; 26(16):7855. https://doi.org/10.3390/ijms26167855
Chicago/Turabian StyleHuang, Yao, Xiuping Dou, Man He, Yang Su, Hualiang Lin, and Yin Yang. 2025. "The G-allele of rs10830963 in MTNR1B Exerts Stage-Specific Effects Across the Trajectory of Type 2 Diabetes: A Multi-State Analysis" International Journal of Molecular Sciences 26, no. 16: 7855. https://doi.org/10.3390/ijms26167855
APA StyleHuang, Y., Dou, X., He, M., Su, Y., Lin, H., & Yang, Y. (2025). The G-allele of rs10830963 in MTNR1B Exerts Stage-Specific Effects Across the Trajectory of Type 2 Diabetes: A Multi-State Analysis. International Journal of Molecular Sciences, 26(16), 7855. https://doi.org/10.3390/ijms26167855






