Study of the Effectiveness of Skin Restoration Using a Biopolymer Hydrogel Scaffold with Encapsulated Mesenchymal Stem Cells
Abstract
1. Introduction
2. Results and Discussion
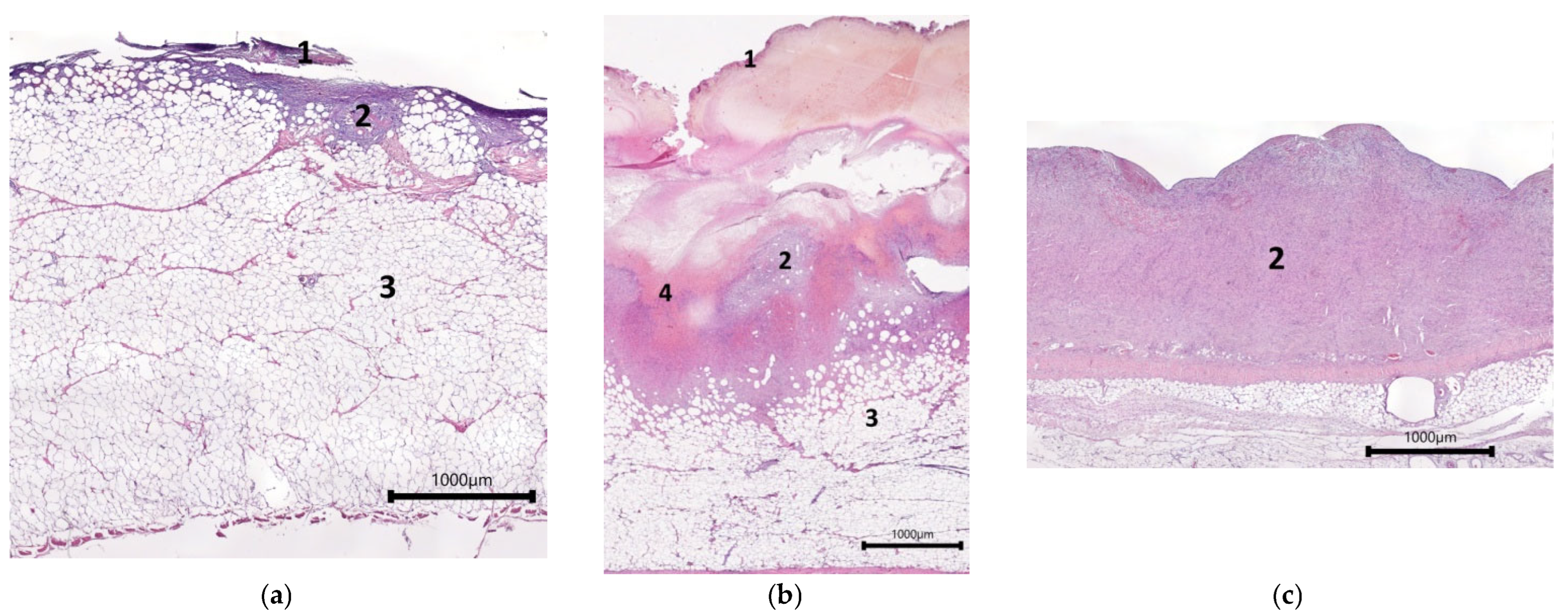
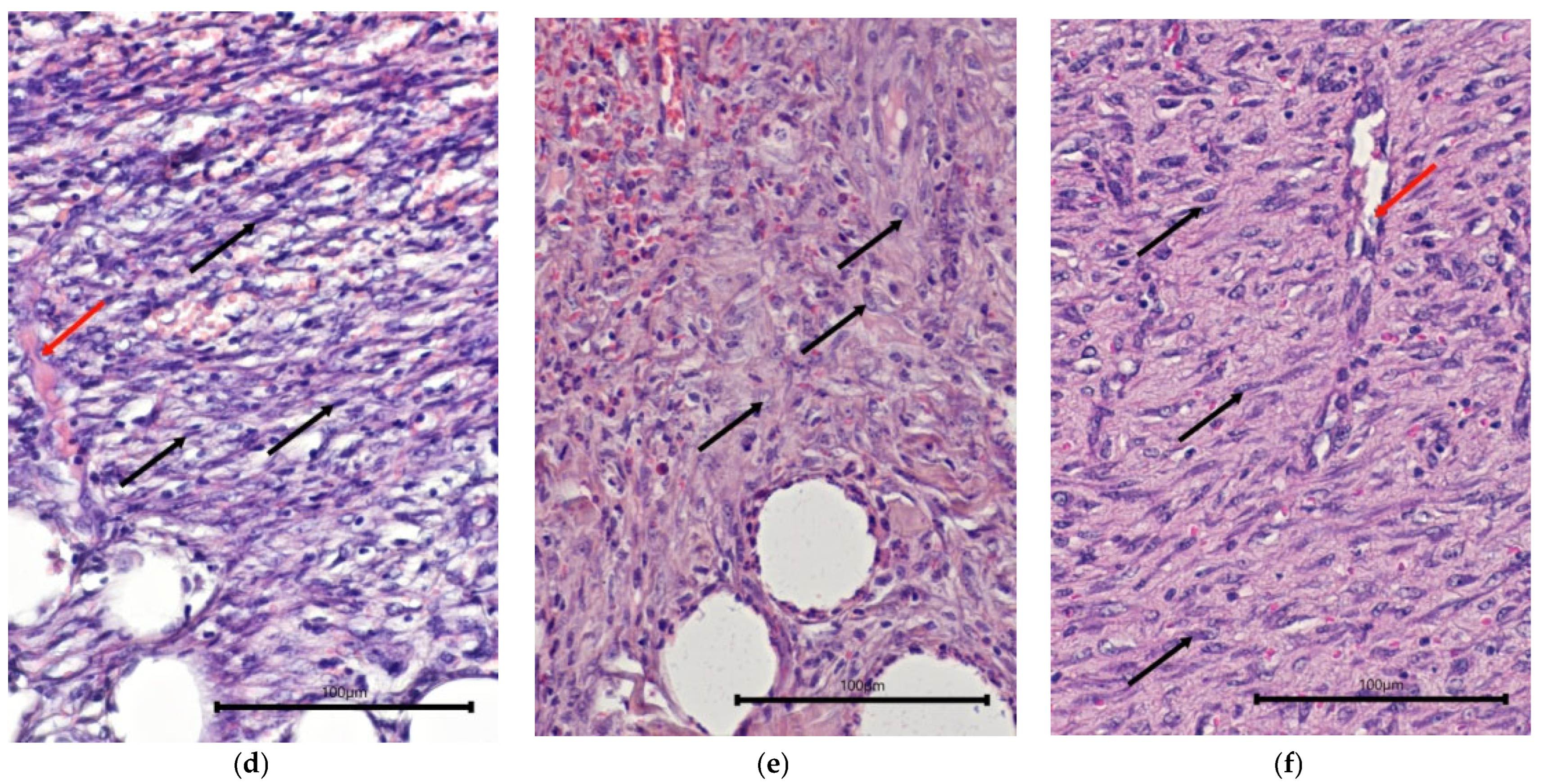
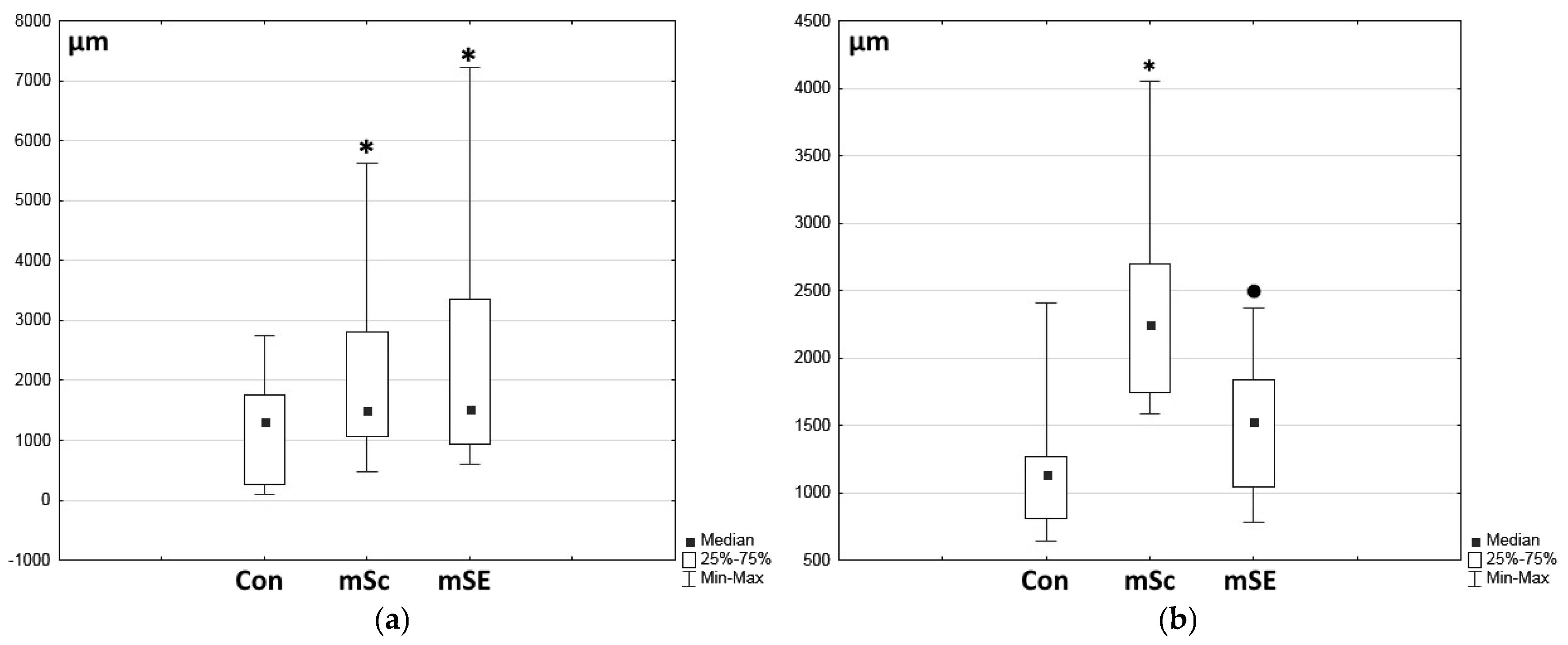
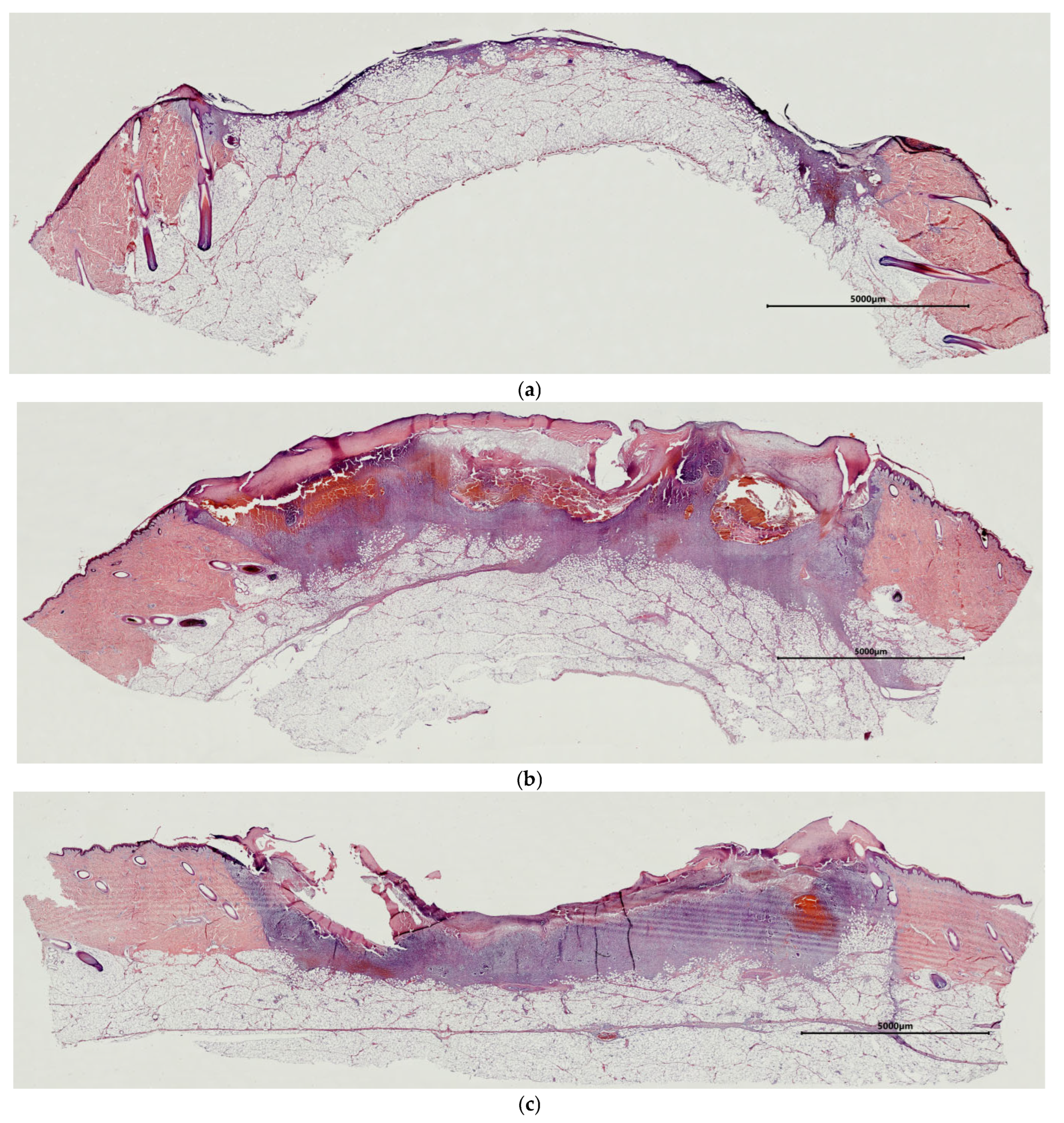
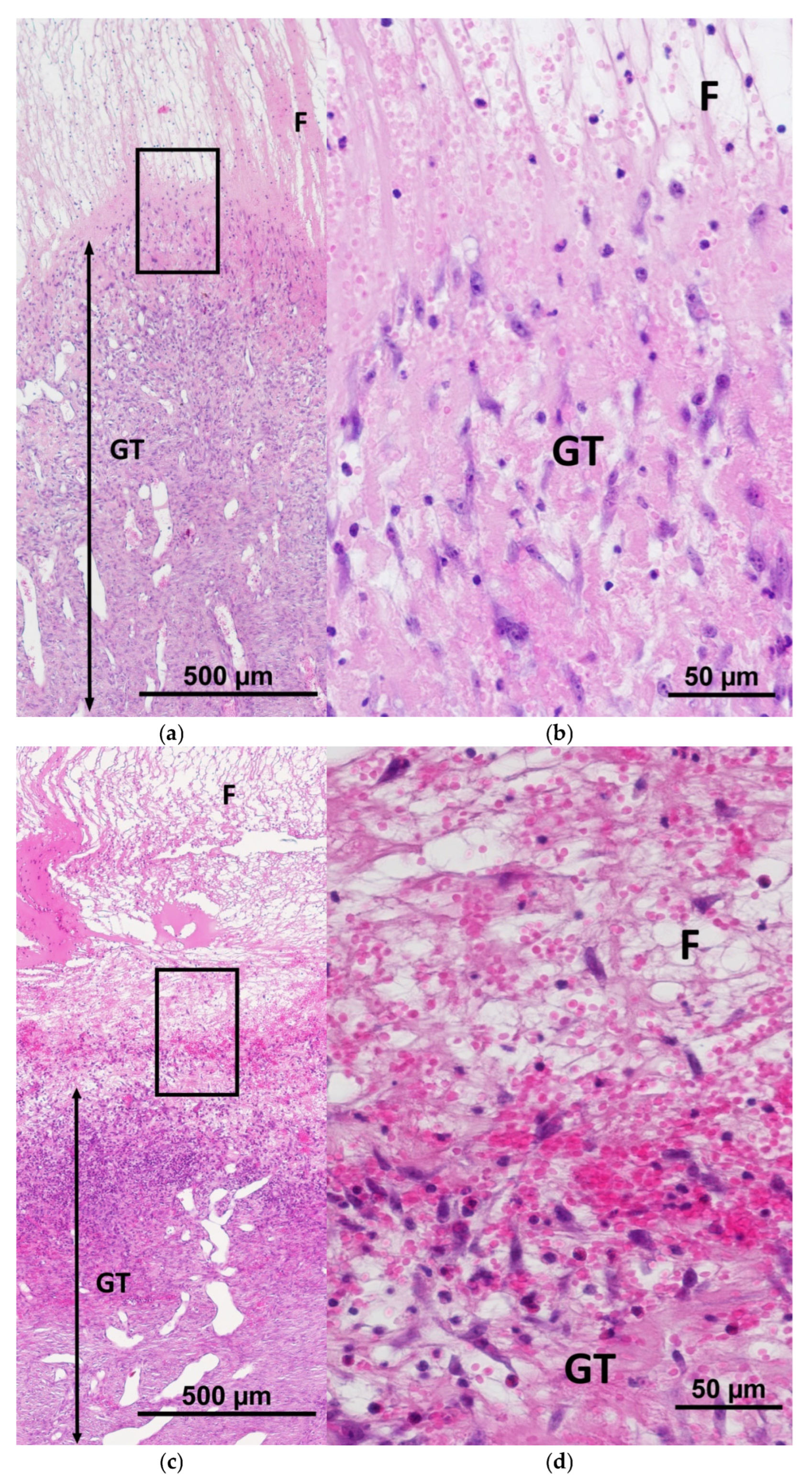
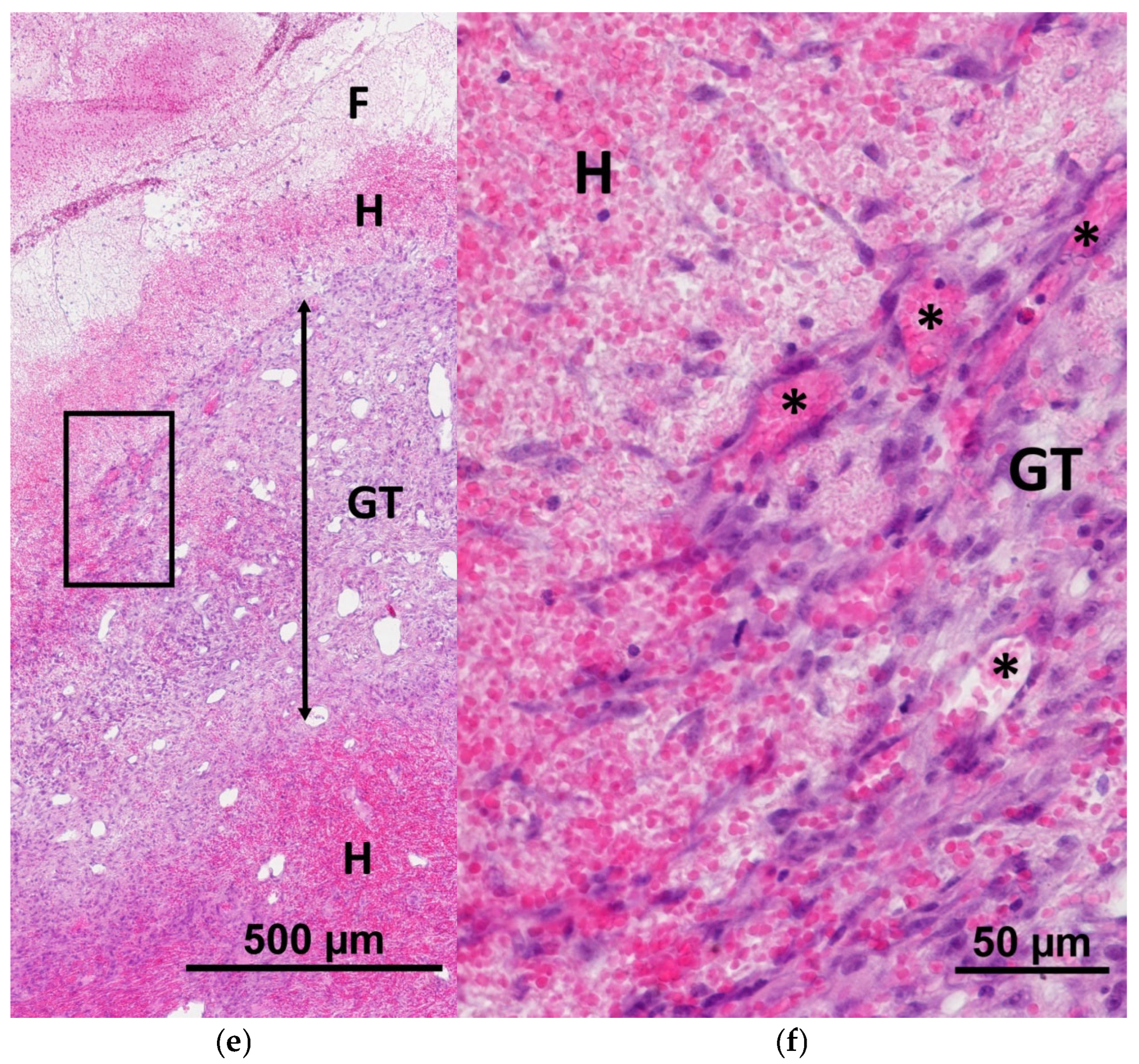
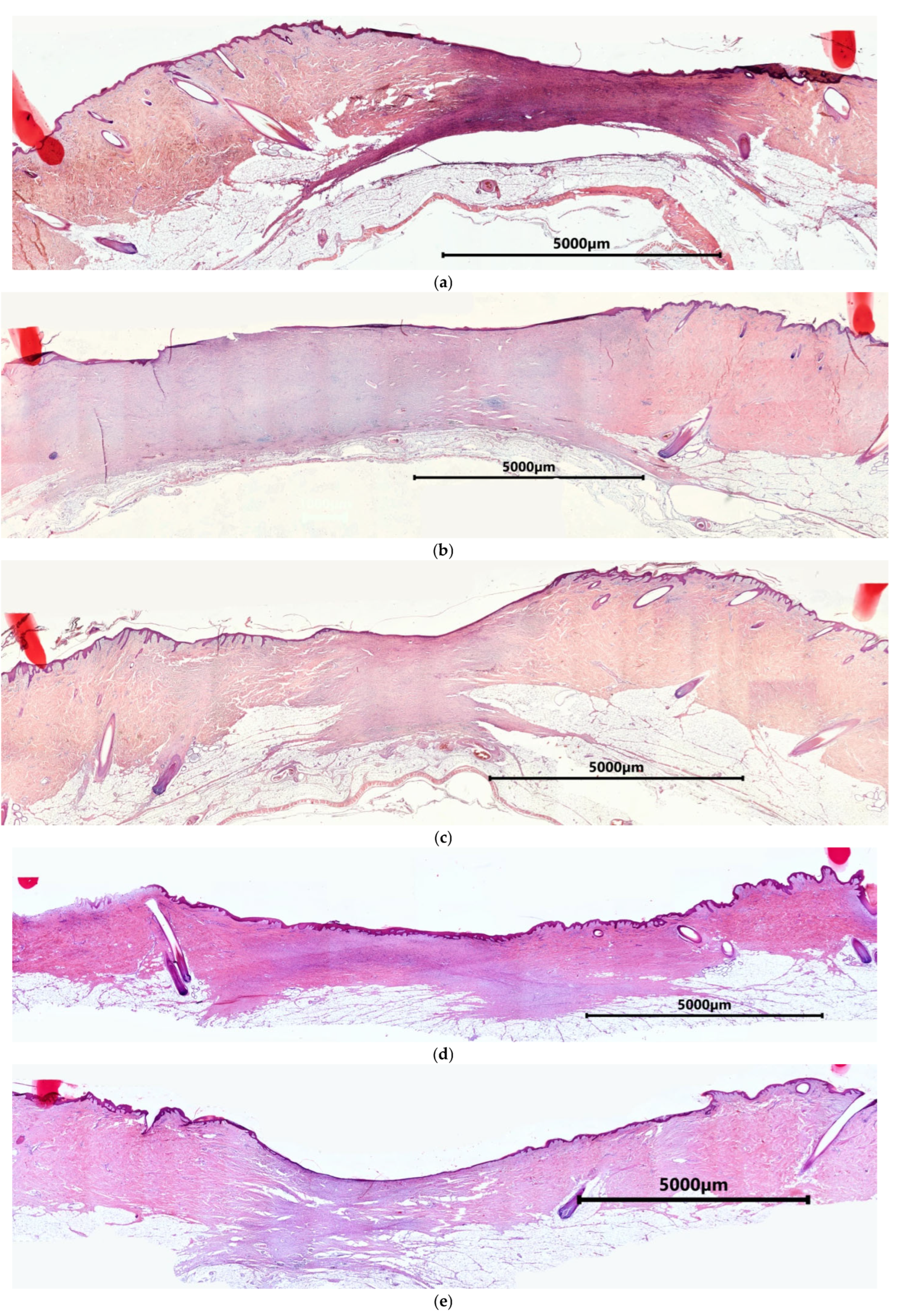
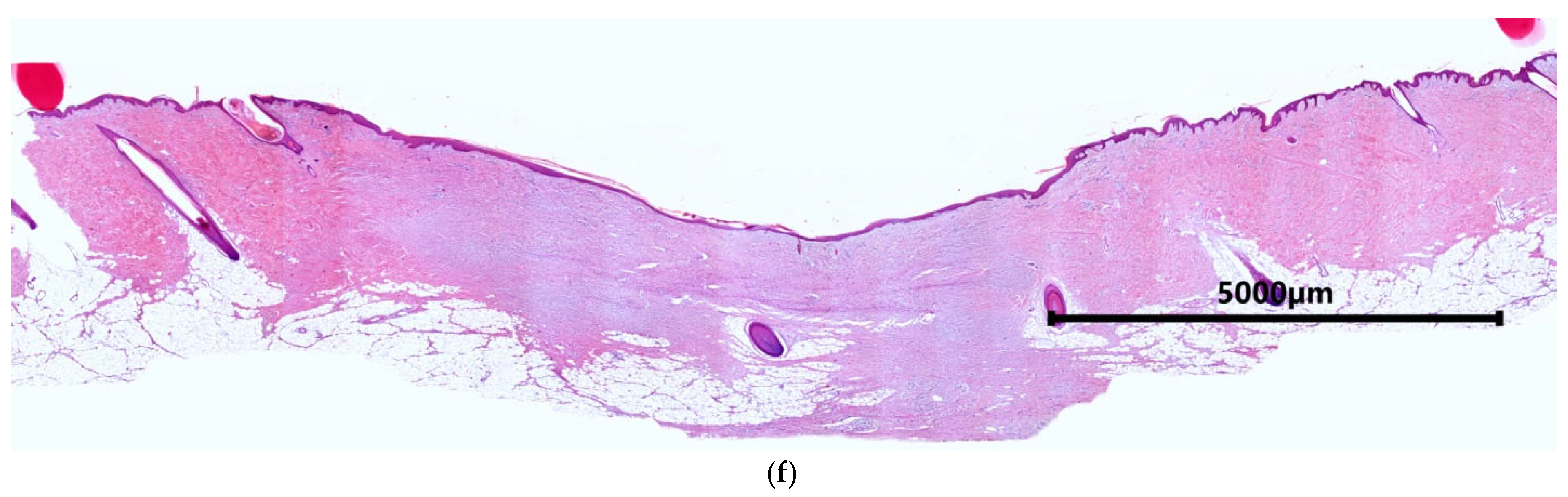
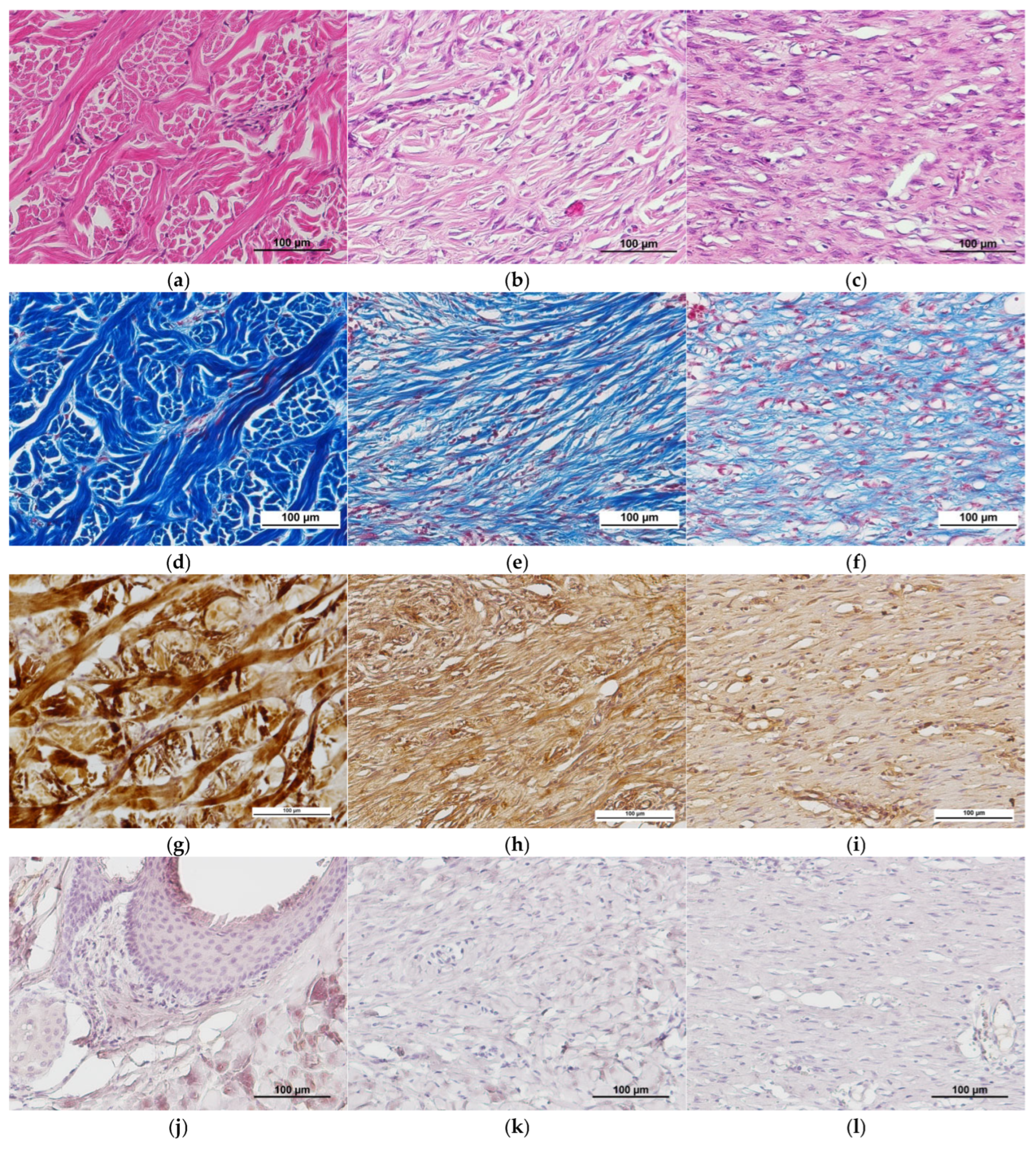
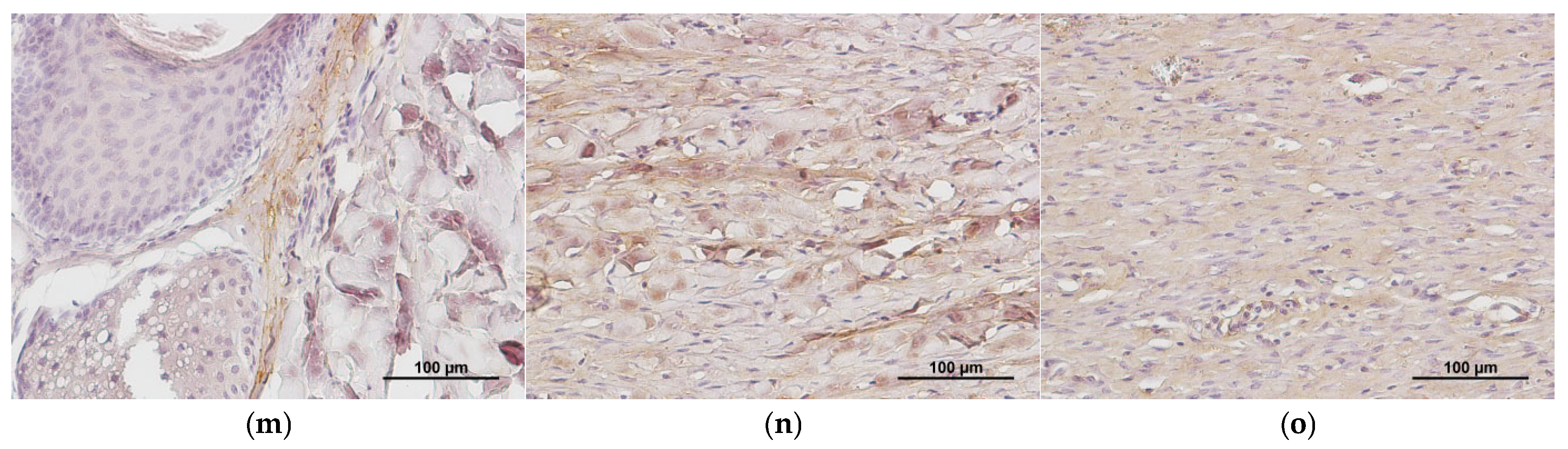
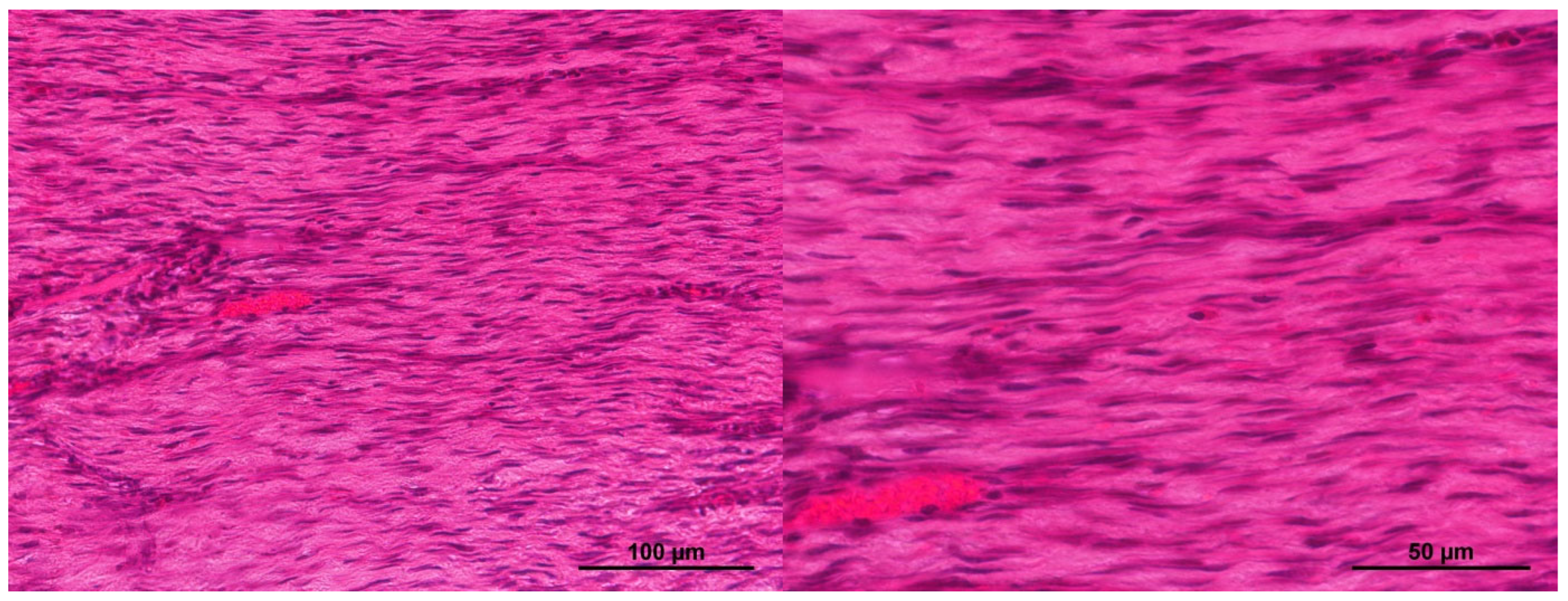
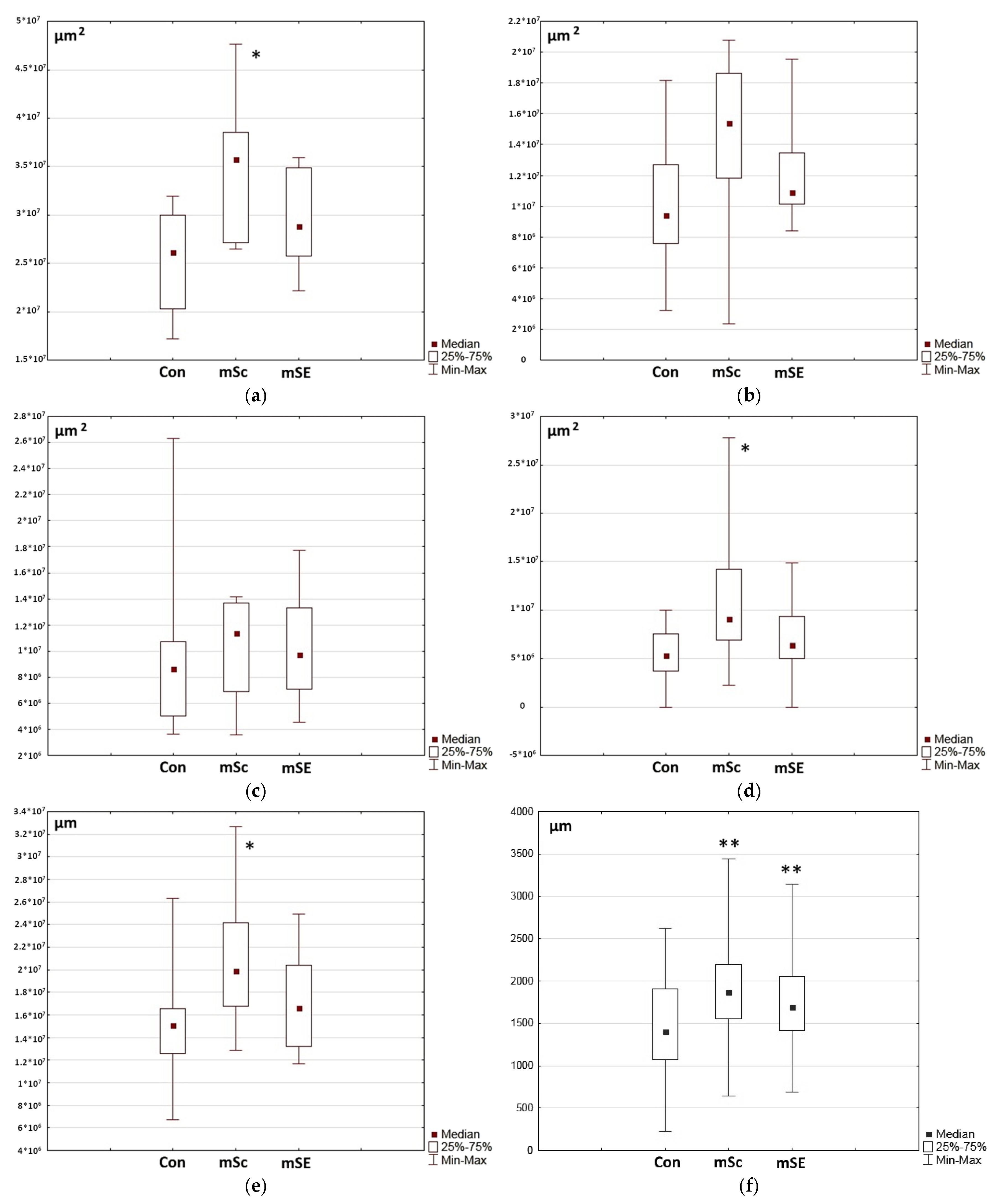
2.1. Characteristics of the Condition of the Wounds on Day 7 of the Study
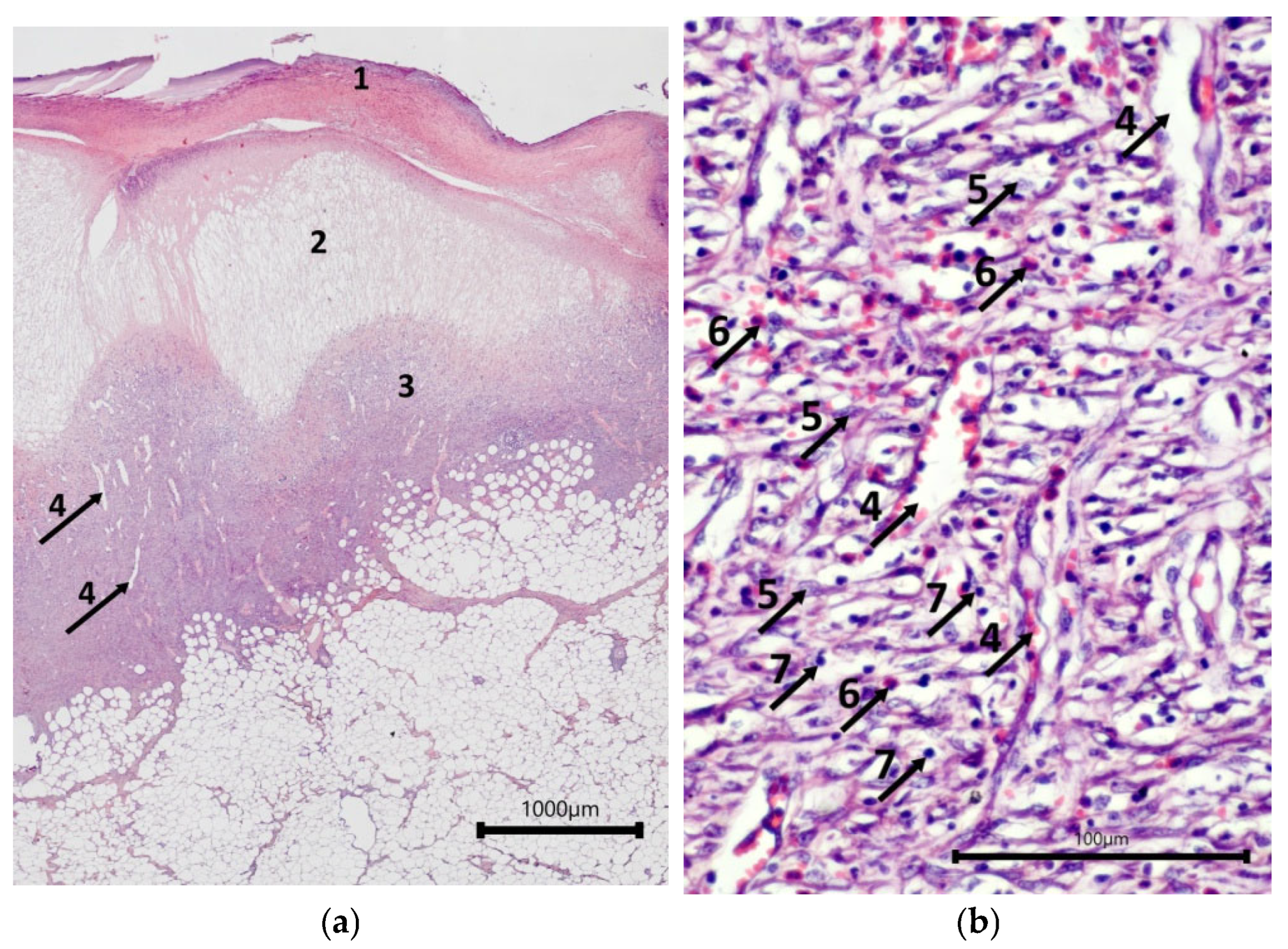
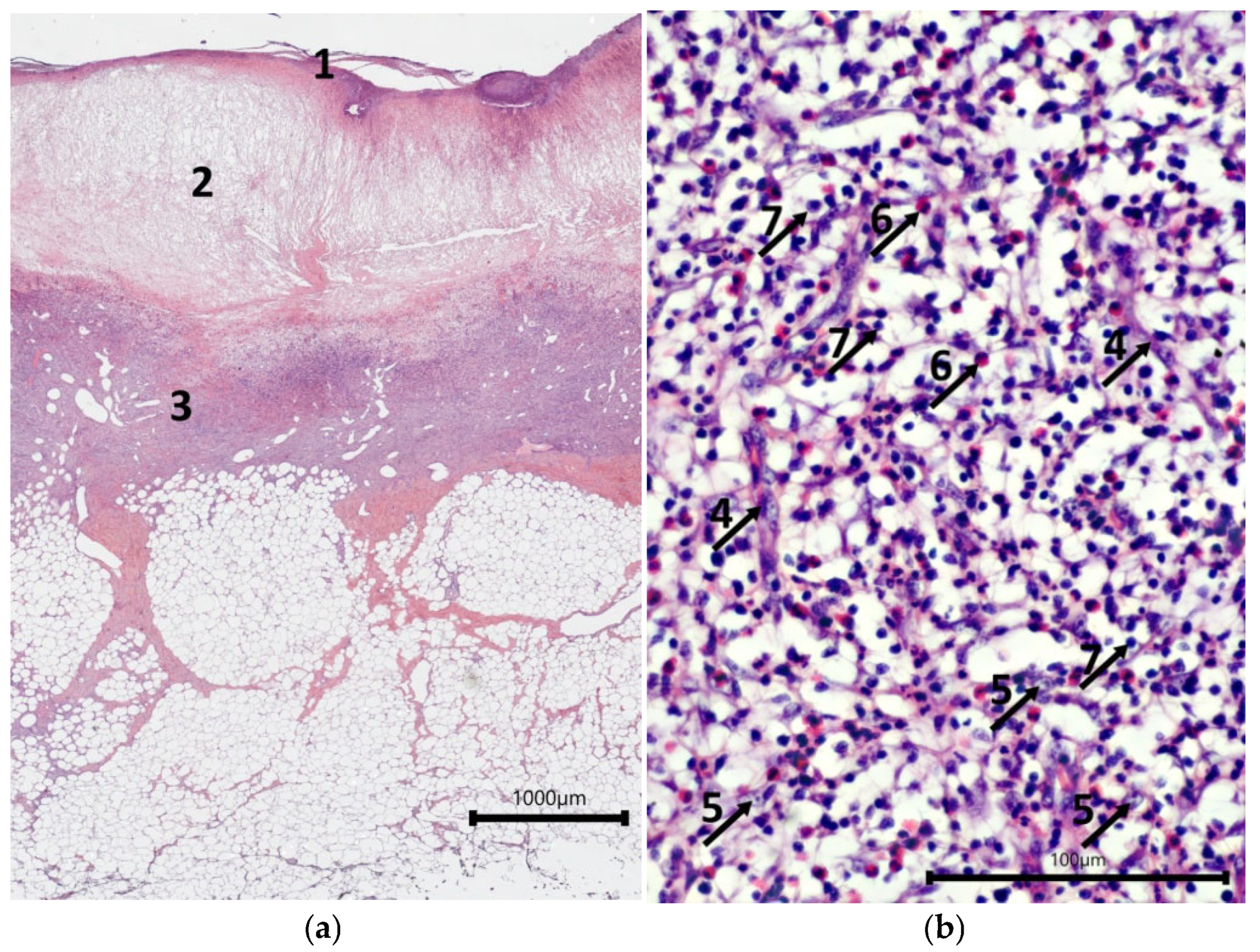
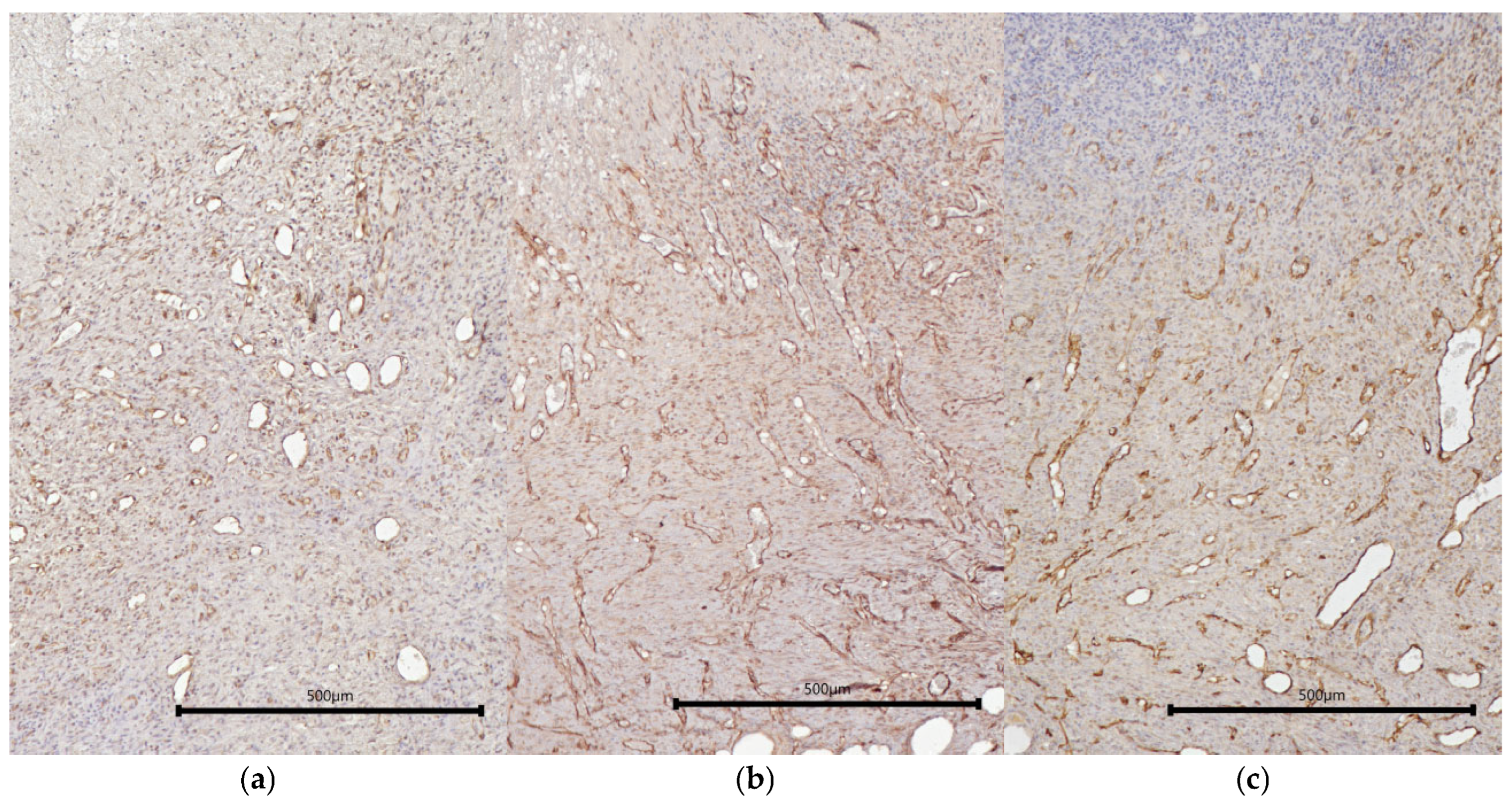
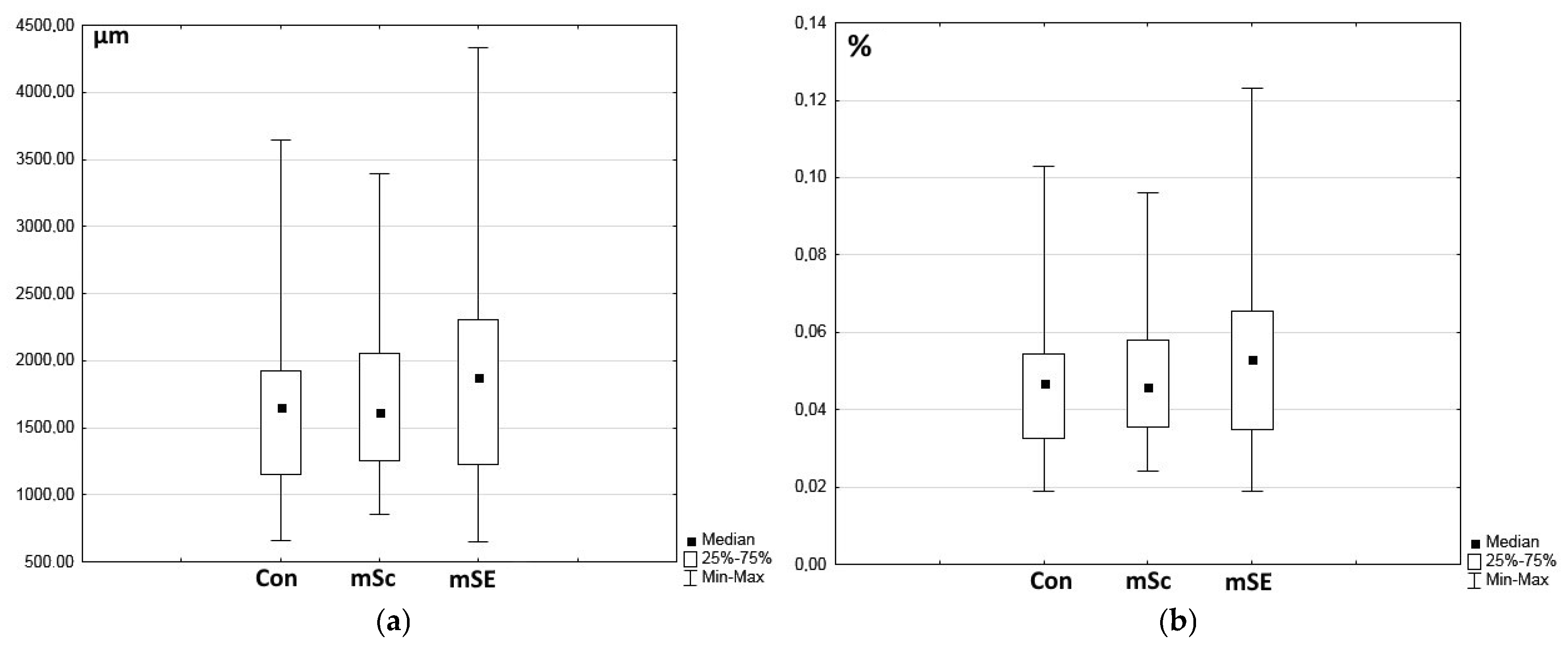
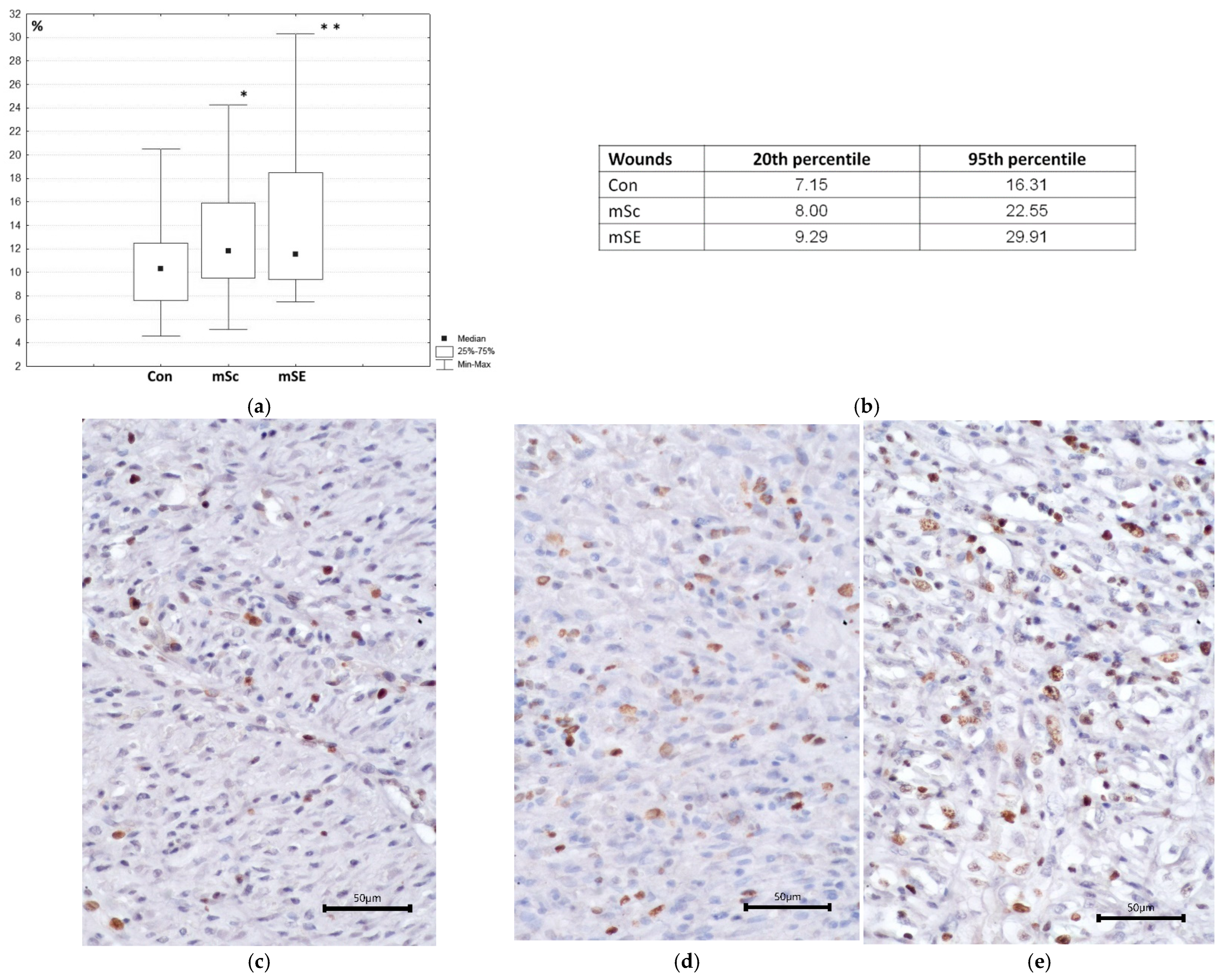
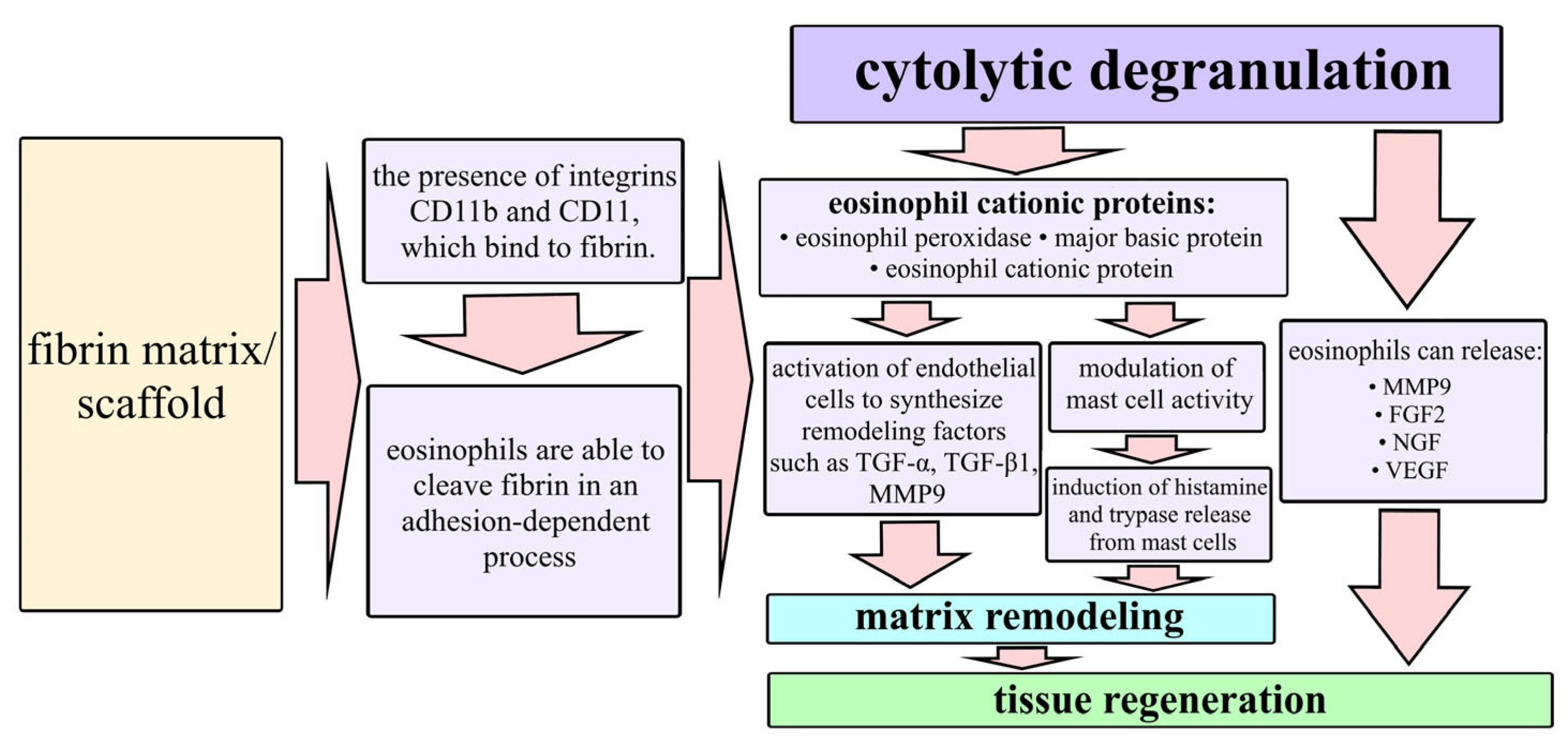
2.2. Characteristics of the Wound Defect Condition on Day 42 of the Study
3. Materials and Methods
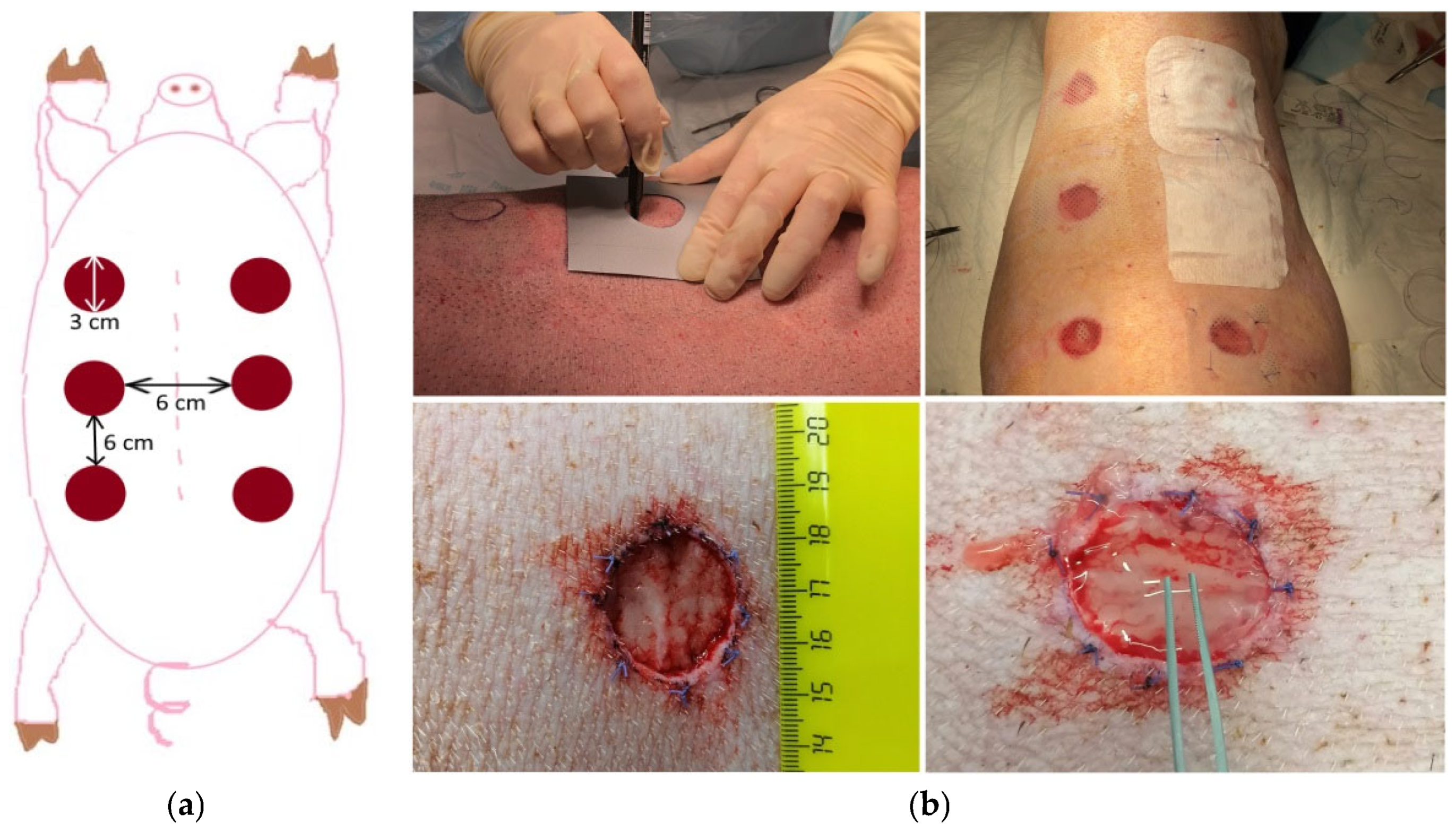
3.1. Animals
3.2. Animal Sedation and Anesthetization
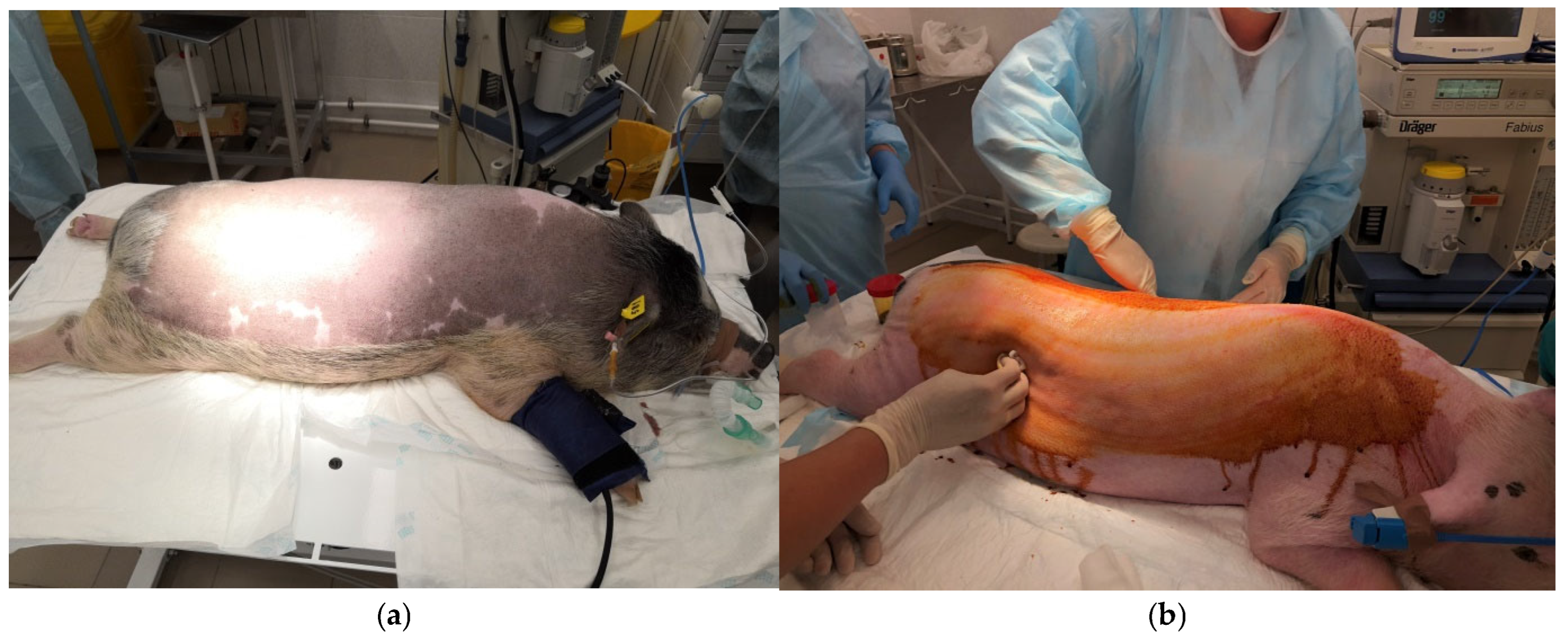
3.3. Preparatory Procedures
3.4. Monitoring of Animals During Surgery
3.5. Extraction of Biomaterial to Obtain Pig Mesenchymal Stem Cells and Pig Blood Plasma
3.6. Obtaining an ASC Culture
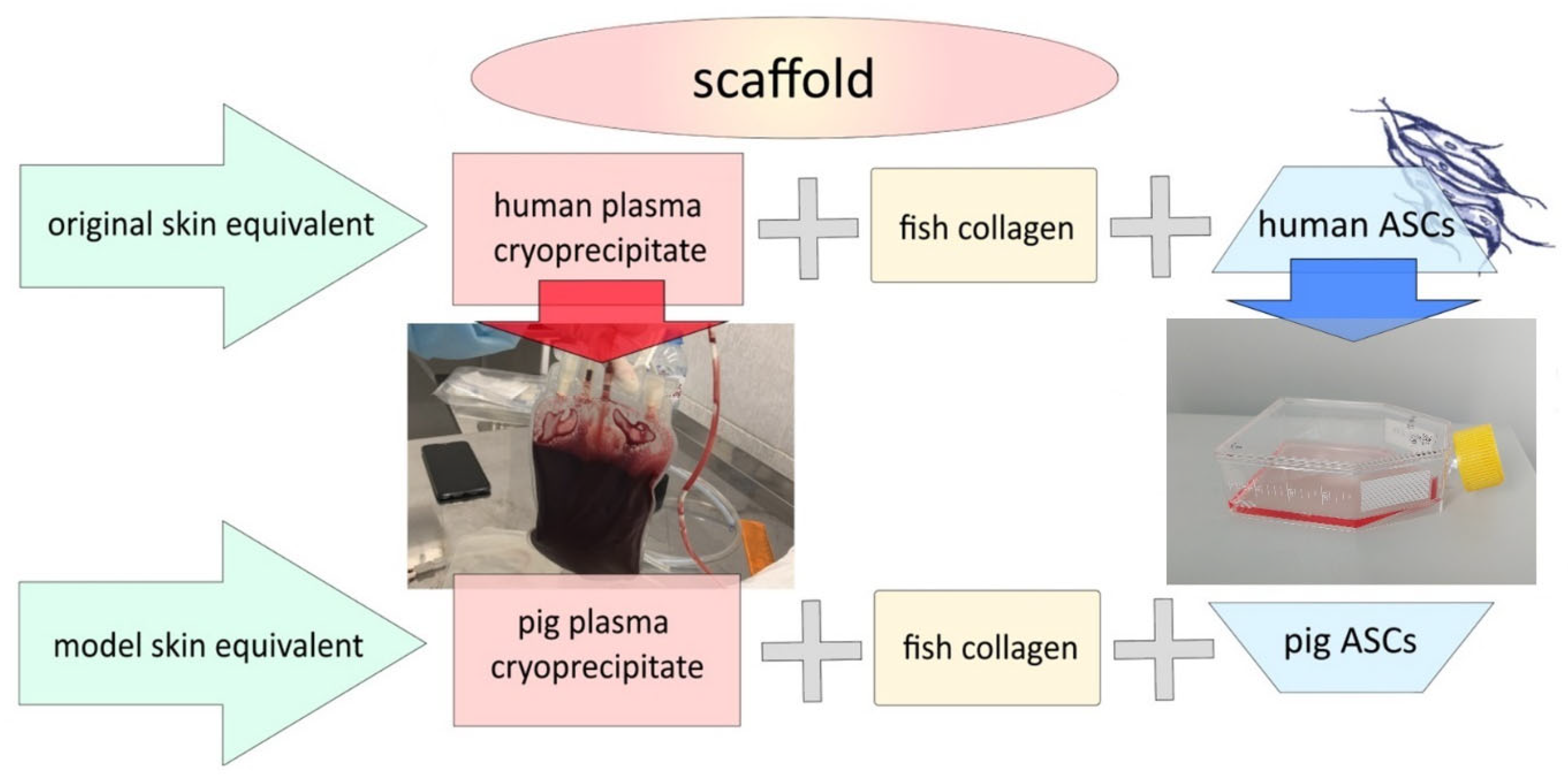
3.7. Formation of the Model Scaffold Carrier and Skin Equivalents
3.8. Wound Formation
3.9. Post-Surgery Period
3.10. Animal Euthanasia
3.11. Morphological Studies of Biomaterial
3.11.1. Light Microscopy
3.11.2. Immunohistochemistry
3.11.3. Morphometric Examinations
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freeman, A.E.; Igel, H.J.; Herrman, B.J.; Kleinfeld, K.L. Growth and Characterization of Human Skin Epithelial Cell Cultures. In Vitro 1976, 12, 352–362. [Google Scholar] [CrossRef]
- Netzlaff, F.; Lehr, C.M.; Wertz, P.W.; Schaefer, U.F. The Human Epidermis Models EpiSkin®, SkinEthic® and EpiDerm®: An Evaluation of Morphology and Their Suitability for Testing Phototoxicity, Irritancy, Corrosivity, and Substance Transport. Eur. J. Pharm. Biopharm. 2005, 60, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.A.; Helder, R.W.J.; El Ghalbzouri, A. Human Skin Equivalents: Impaired Barrier Function in Relation to the Lipid and Protein Properties of the Stratum Corneum. Adv. Drug Deliv. Rev. 2021, 175, 113802. [Google Scholar] [CrossRef] [PubMed]
- Suhail, S.; Sardashti, N.; Jaiswal, D.; Rudraiah, S.; Misra, M.; Kumbar, S.G. Engineered Skin Tissue Equivalents for Product Evaluation and Therapeutic Applications. Biotechnol. J. 2019, 14, 1900022. [Google Scholar] [CrossRef] [PubMed]
- Integra Dermal Regeneration Template. Available online: https://www.integralife.com/ (accessed on 3 June 2025).
- Choudhury, S.; Das, A. Advances in Generation of Three-Dimensional Skin Equivalents: Pre-Clinical Studies to Clinical Therapies. Cytotherapy 2021, 23, 1–9. [Google Scholar] [CrossRef]
- Apligraf. Available online: https://www.apligraf.com/pdf/Apligraf-FactSheet.pdf (accessed on 3 June 2025).
- Epicel. Available online: https://www.epicel.com/patients/ (accessed on 3 June 2025).
- TransCyte(Dermagraft-Tc). Available online: https://profiles.biocentury.com/products/transcyte_(dermagraft-tc) (accessed on 3 June 2025).
- Riabinin, A.; Pankratova, M.; Rogovaya, O.; Vorotelyak, E.; Terskikh, V.; Vasiliev, A. Ideal Living Skin Equivalents, from Old Technologies and Models to Advanced Ones: The Prospects for an Integrated Approach. Biomed. Res. Int. 2024, 2024, 9947692. [Google Scholar] [CrossRef]
- OrCel Wound Sealant. Available online: https://ichgcp.net/ru/clinical-trials-registry/NCT00270972?ysclid=mbggc2dgwq811373821 (accessed on 3 June 2025).
- AlloDerm. Available online: https://hcp.alloderm.com/performance (accessed on 3 June 2025).
- Vecin, N.M.; Kirsner, R.S. Skin Substitutes as Treatment for Chronic Wounds: Current and Future Directions. Front. Med. 2023, 10, 1154567. [Google Scholar] [CrossRef]
- Liu, T.; Qiu, C.; Ben, C.; Li, H.; Zhu, S. One-Step Approach for Full-Thickness Skin Defect Reconstruction in Rats Using Minced Split-Thickness Skin Grafts with Pelnac Overlay. Burn. Trauma 2019, 7, 19. [Google Scholar] [CrossRef]
- Dearman, B.L.; Boyce, S.T.; Greenwood, J.E. Comparison of Biopolymer Scaffolds for the Fabrication of Skin Substitutes in a Porcine Wound Model. Wound Repair Regen. 2023, 31, 87–98. [Google Scholar] [CrossRef]
- Kavanagh, F.; Singhal, S.; Rozen, W.M. Split Thickness Skin Graft Compression: A Scoping Review. Gland Surg. 2023, 12, 297. [Google Scholar] [CrossRef]
- Stephenson, A.J.; Griffiths, R.W.; Hausse-Brown, T.P.L. Patterns of Contraction in Human Full Thickness Skin Grafts. Br. J. Plast. Surg. 2000, 53, 397–402. [Google Scholar] [CrossRef]
- Matthew, E.; Braza, M.P.F. 1 Split-Thickness Skin Grafts; StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Singh, M.; Nuutila, K.; Kruse, C.; Robson, M.C.; Caterson, E.; Eriksson, E. Challenging the Conventional Therapy: Emerging Skin Graft Techniques for Wound Healing. Plast. Reconstr. Surg. 2015, 136, 524e–530e. [Google Scholar] [CrossRef] [PubMed]
- Wood, F.M.; Stoner, M.L.; Fowler, B.V.; Fear, M.W. The Use of a Non-Cultured Autologous Cell Suspension and Integra® Dermal Regeneration Template to Repair Full-Thickness Skin Wounds in a Porcine Model: A One-Step Process. Burns 2007, 33, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Damaraju, S.M.; Mintz, B.R.; Park, J.G.; Gandhi, A.; Saini, S.; Molnar, J.A. Skin Substitutes with Noncultured Autologous Skin Cell Suspension Heal Porcine Full-Thickness Wounds in a One-Stage Procedure. Int. Wound J. 2022, 19, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Foubert, P.; Barillas, S.; Gonzalez, A.D.; Alfonso, Z.; Zhao, S.; Hakim, I.; Meschter, C.; Tenenhaus, M.; Fraser, J.K. Uncultured Adipose-Derived Regenerative Cells (ADRCs) Seeded in Collagen Scaffold Improves Dermal Regeneration, Enhancing Early Vascularization and Structural Organization Following Thermal Burns. Burns 2015, 41, 1504–1516. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, J.; Wang, G.; Wen, H. Vascularization of Lando® Dermal Scaffold in an Acute Full-Thickness Skin-Defect Porcine Model. J. Plast. Surg. Hand Surg. 2018, 52, 204–209. [Google Scholar] [CrossRef]
- Egorikhina, M.N.; Rubtsova, Y.P.; Charykova, I.N.; Bugrova, M.L.; Bronnikova, I.I.; Mukhina, P.A.; Sosnina, L.N.; Aleynik, D.Y. Biopolymer Hydrogel Scaffold as an Artificial Cell Niche for Mesenchymal Stem Cells. Polymers 2020, 12, 2550. [Google Scholar] [CrossRef]
- Egorikhina, M.N.; Timofeeva, L.B.; Linkova, D.D.; Rubtsova, Y.P.; Bugrova, M.L.; Charykova, I.N.; Ryabkov, M.G.; Kobyakova, I.I.; Farafontova, E.A.; Aleynik, D.Y. Biocompatibility study of hydrogel biopolymer scaffold with encapsulated mesenchymal stem cells. Polymers 2023, 15, 1337. [Google Scholar] [CrossRef]
- Valent, P.; Degenfeld-Schonburg, L.; Sadovnik, I.; Horny, H.P.; Arock, M.; Simon, H.U.; Reiter, A.; Bochner, B.S. Eosinophils and Eosinophil-Associated Disorders: Immunological, Clinical, and Molecular Complexity. Semin. Immunopathol. 2021, 43, 423–438. [Google Scholar] [CrossRef]
- Weller, P.F.; Spencer, L.A. Functions of Tissue-Resident Eosinophils. Nat. Rev. Immunol. 2017, 17, 746–760. [Google Scholar] [CrossRef]
- Abdala Valencia, H.; Loffredo, L.F.; Misharin, A.V.; Berdnikovs, S. Phenotypic Plasticity and Targeting of Siglec-FhighCD11clow Eosinophils to the Airway in a Murine Model of Asthma. Allergy Eur. J. Allergy Clin. Immunol. 2016, 71, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Barthel, S.R.; Jarjour, N.N.; Mosher, D.F.; Johansson, M.W. Dissection of the Hyperadhesive Phenotype of Airway Eosinophils in Asthma. Am. J. Respir. Cell Mol. Biol. 2006, 35, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Spoelstra, F.M.; Hovenga, H.; Noordhoek, J.A.; Postma, D.S.; Kauffman, H.F. Changes in CD11b and L-Selectin Expression on Eosinophils Are Mediated by Human Lung Fibroblasts in Vitro. Am. J. Respir. Crit. Care Med. 1998, 158, 769–777. [Google Scholar] [CrossRef]
- Yoon, J.; Ponikau, J.U.; Lawrence, C.B.; Kita, H. Innate Antifungal Immunity of Human Eosinophils Mediated by a Β2 Integrin, CD11b. J. Immunol. 2008, 181, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Coden, M.E.; Loffredo, L.F.; Walker, M.T.; Jeong, B.M.; Nam, K.; Bochner, B.S.; Abdala-Valencia, H.; Berdnikovs, S. Fibrinogen Is a Specific Trigger for Cytolytic Eosinophil Degranulation. J. Immunol. 2020, 204, 438–448. [Google Scholar] [CrossRef]
- Zagai, U.; Lundahl, J.; Klominek, J.; Venge, P.; Sköld, C.M. Eosinophil Cationic Protein Stimulates Migration of Human Lung Fibroblasts in Vitro. Scand. J. Immunol. 2009, 69, 381–386. [Google Scholar] [CrossRef]
- Venge, P.; Byström, J. Molecules in Focus Eosinophil Cationic Protein (ECP). Int. J. Biochem. Cell Biol. 1998, 30, 433–437. [Google Scholar] [CrossRef]
- Topic, R.Z.; Dodig, S. Eosinophil Cationic Protein–Current Concepts and Controversies. Biochem. Medica 2011, 21, 111–121. [Google Scholar] [CrossRef]
- Venge, P.; Byström, J.; Carlson, M.; Håkansson, L.; Karawacjzyk, M.; Peterson, C.; Sevéus, L.; Trulson, A. Eosinophil Cationic Protein (ECP): Molecular and Biological Properties and the Use of ECP as a Marker of Eosinophil Activation in Disease. Clin. Exp. Allergy 1999, 29, 1172–1186. [Google Scholar] [CrossRef]
- Ogasawara, H.; Furuno, M.; Edamura, K.; Noguchi, M. Peptides of Major Basic Protein and Eosinophil Cationic Protein Activate Human Mast Cells. Biochem. Biophys. Rep. 2020, 21, 100719. [Google Scholar] [CrossRef]
- Coden, M.E.; Berdnikovs, S. Eosinophils in Wound Healing and Epithelial Remodeling: Is Coagulation a Missing Link? J. Leukoc. Biol. 2020, 108, 93–103. [Google Scholar] [CrossRef]
- Aibibu, D.; Hild, M.; Wöltje, M.; Cherif, C. Textile Cell-Free Scaffolds for in Situ Tissue Engineering Applications. J. Mater. Sci. Mater. Med. 2016, 27, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Muylaert, D.E.P.; Fledderus, J.O.; Bouten, C.V.C.; Dankers, P.Y.W.; Verhaar, M.C. Combining Tissue Repair and Tissue Engineering; Bioactivating Implantable Cell-Free Vascular Scaffolds. Heart 2014, 100, 1825–1830. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. The Biomaterials Conundrum in Tissue Engineering. Tissue Eng.-Part A 2014, 20, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wang, W.; Li, L.; Meng, H.Y.; Feng, C.Z.; Dong, Y.Y.; Fang, X.C.; Dong, Q.Q.; Jiang, W.; Xin, H.L.; et al. Recombinant Human Epidermal Growth Factor Combined with Vacuum Sealing Drainage for Wound Healing in Bama Pigs. Mil. Med. Res. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Zhao, B.; Gao, J.; Xia, L.; Xing, F.; Kong, Y.; Li, Y.; Zhang, G. Detection of Type I and III Collagen in Porcine Acellular Matrix Using HPLC-MS. Regen. Biomater. 2021, 7, 577–582. [Google Scholar] [CrossRef]
- Kuo, T.Y.; Huang, C.C.; Shieh, S.J.; Wang, Y.B.; Lin, M.J.; Wu, M.C.; Huang, L.L.H. Skin Wound Healing Assessment via an Optimized Wound Array Model in Miniature Pigs. Sci. Rep. 2022, 12, 445. [Google Scholar] [CrossRef]
- Grant, S.A.; Deeken, C.R.; Hamilton, S.R.; Grant, D.A.; Bachman, S.L.; Ramshaw, B.J. A Comparative Study of the Remodeling and Integration of a Novel AuNP-Tissue Scaffold and Commercial Tissue Scaffolds in a Porcine Model. J. Biomed. Mater. Res.-Part A 2013, 101, 2778–2787. [Google Scholar] [CrossRef]
- Aleynik, D.Y.; Charykova, I.N.; Rubtsova, Y.P.; Linkova, D.D.; Farafontova, E.A.; Egorikhina, M.N. Specific Features of the Functional Activity of Human Adipose Stromal Cells in the Structure of a Partial Skin-Equivalent. Int. J. Mol. Sci. 2024, 25, 6290. [Google Scholar] [CrossRef]
- Egorikhina, M.N.; Charykova, I.N.; Aleynik, D.Y.; Rubtsova, Y.P. Method for Determining the Viability of Cells in Biomedical Cell Products in the Process of Regeneration. Patent 2 777 257, 1 August 2022. [Google Scholar]
- Egorikhina, M.N.; Aleynik, D.Y.; Rubtsova, Y.P.; Levin, G.Y.; Charykova, I.N.; Semenycheva, L.L.; Bugrova, M.L.; Zakharychev, E.A. Hydrogel Scaffolds Based on Blood Plasma Cryoprecipitate and Collagen Derived from Various Sources: Structural, Mechanical and Biological Characteristics. Bioact. Mater. 2019, 4, 334–345. [Google Scholar] [CrossRef]
- Semenycheva, L.L.; Astanina, M.V.; Kuznetsova, J.L.; Valetova, N.B.; Geras’kina, E.V.; Tarankova, O.A. Method for Production of Acetic Dispersion of High Molecular Fish Collagen. Patent 2567171, 10 November 2015. [Google Scholar]
- Egorikhina, M.N.; Aleinik, D.Y.; Rubtsova, Y.P.; Charykova, I.N.; Struchcov, A.A.; Ezhevskaya, A.A.; Zagrekov, V.I.; Sosnina, L.N.; Zagaynova, E.V. Biomedical Cell Product Model for Preclinical Studies Carried out on a Large Laboratory Animal. Vestn. Transplantologii I Iskusstv. Organov 2020, 22, 142–156. [Google Scholar] [CrossRef]
| Brand | Manufacturer | Product Description |
|---|---|---|
| Integra® DRT (Dermal Regeneration Template) [5,6] | Integra LifeSciences (Princeton, NJ, USA) | Thin, cell-free silicone film covering a porous matrix made of bovine collagen and glycosaminoglycans |
| Apligraf®/Graftskin® [6,7] | Organogenisis (Canton, MA, USA) | Fibroblasts and collagen combined in a dermal matrix seeded with keratinocytes to form an epidermal-like layer |
| Epicel® [8] | Genzyme (Cambridge, MA, USA) | Autologous keratinocytes grown ex vivo in the presence of a mouse fibroblast feeder layer |
| TransCyte®/Dermagraft® Advanced Tissue [6,9] | Shire Regenerative Medicine (Lexington, MA, USA) | Cryopreserved skin substitute: human fibroblasts seeded onto a polymer mesh and cultured ex vivo |
| TransCyte® [6,10] | Shire Regenerative Medicine (Lexington, MA, USA) | Human allogeneic fibroblasts derived from newborn foreskin seeded onto a silicone-coated degradable nylon mesh sponge and cultured ex vivo for 4–6 weeks; these secrete extracellular matrix components and growth factors |
| OrCel® [6,11] | FortiCell Bioscience (New York, NY, USA) | Human epidermal keratinocytes and dermal fibroblasts cultured in separate layers with type I bovine collagen |
| Alloderm® [12] | Strattice® LifeCell Co.® (Branchburg, NJ, USA) | Cell-free matrix of cadaver skin |
| Laserskin® [10] | Fidia Advanced Biopolymers (Turin, Italy) | Autologous keratinocytes and fibroblasts from skin biopsy samples, cultured on a laser-microperforated biodegradable matrix of benzyl-esterified hyaluronic acid |
| PermaDerm® [10] | Regenicin, Inc. (New York, NY, USA) | Autologous keratinocytes and fibroblasts cultured on a bovine collagen support |
| StrataGraft® [6,10] | The Luminis Group, Ltd. for Stratatech Corp (Madison, WI, USA) | Patented, immortalized keratinocytes of the NIKS® (Normal Immortal Keratinocytes) line, together with dermal fibroblasts on a collagen support |
| GraftJacket® [10] | Wright Medical Technology (Arlington, TN, USA) | Micronized decellularized human dermis with dermal matrix and preserved basement membrane for ingrowth of blood vessels |
| Biobrane [6] | Smith & Nephew (London, UK) | Biosynthetic adhesive wound dressing constructed with an outer silicone membrane and an inner nylon mesh with added type I pig collagen |
| EpiDex® [13] | Anika Therapeutics (Bedford, MA, USA) | An epidermal equivalent derived from isolated hair follicle keratinocytes cultured on a matrix |
| BioSeed® [13] | BioTissue Technologies AG (Freiburg im Breisgau, Germany) | An epidermal equivalent derived from autologous keratinocytes and a fibrin sealant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorikhina, M.N.; Timofeeva, L.B.; Rubtsova, Y.P.; Farafontova, E.A.; Linkova, D.D.; Charykova, I.N.; Ryabkov, M.G.; Ezhevskaya, A.A.; Levicheva, E.A.; Aleynik, D.Y. Study of the Effectiveness of Skin Restoration Using a Biopolymer Hydrogel Scaffold with Encapsulated Mesenchymal Stem Cells. Int. J. Mol. Sci. 2025, 26, 7840. https://doi.org/10.3390/ijms26167840
Egorikhina MN, Timofeeva LB, Rubtsova YP, Farafontova EA, Linkova DD, Charykova IN, Ryabkov MG, Ezhevskaya AA, Levicheva EA, Aleynik DY. Study of the Effectiveness of Skin Restoration Using a Biopolymer Hydrogel Scaffold with Encapsulated Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2025; 26(16):7840. https://doi.org/10.3390/ijms26167840
Chicago/Turabian StyleEgorikhina, Marfa N., Lidia B. Timofeeva, Yulia P. Rubtsova, Ekaterina A. Farafontova, Dariya D. Linkova, Irina N. Charykova, Maksim G. Ryabkov, Anna A. Ezhevskaya, Ekaterina A. Levicheva, and Diana Ya. Aleynik. 2025. "Study of the Effectiveness of Skin Restoration Using a Biopolymer Hydrogel Scaffold with Encapsulated Mesenchymal Stem Cells" International Journal of Molecular Sciences 26, no. 16: 7840. https://doi.org/10.3390/ijms26167840
APA StyleEgorikhina, M. N., Timofeeva, L. B., Rubtsova, Y. P., Farafontova, E. A., Linkova, D. D., Charykova, I. N., Ryabkov, M. G., Ezhevskaya, A. A., Levicheva, E. A., & Aleynik, D. Y. (2025). Study of the Effectiveness of Skin Restoration Using a Biopolymer Hydrogel Scaffold with Encapsulated Mesenchymal Stem Cells. International Journal of Molecular Sciences, 26(16), 7840. https://doi.org/10.3390/ijms26167840










