Advances in Cryopreservation Strategies for 3D Biofabricated Constructs: From Hydrogels to Bioprinted Tissues
Abstract
1. Introduction
Literature Search Strategy
2. Cryoprotective Biomaterials for Three-Dimensional Constructs
2.1. Polysaccharide-Based Hydrogels
2.1.1. Hyaluronic Acid
2.1.2. Alginate
2.1.3. Chitosan
2.1.4. Dextran
2.1.5. Agarose
2.1.6. Nanocellulose-Based Bioinks
2.2. Protein-Based Biomaterials
2.2.1. Silk Fibroin
2.2.2. Sericin
2.3. Synthetic Polymers
2.3.1. Polyethylene Glycol
2.3.2. Polyvinyl Alcohol
2.3.3. Polyurethane (PU)
2.4. Comparative Insights Across Cryoprotective Biomaterials
3. Cryopreservation in 3D Constructs: From Non-Bioprinted to Bioprinted Systems
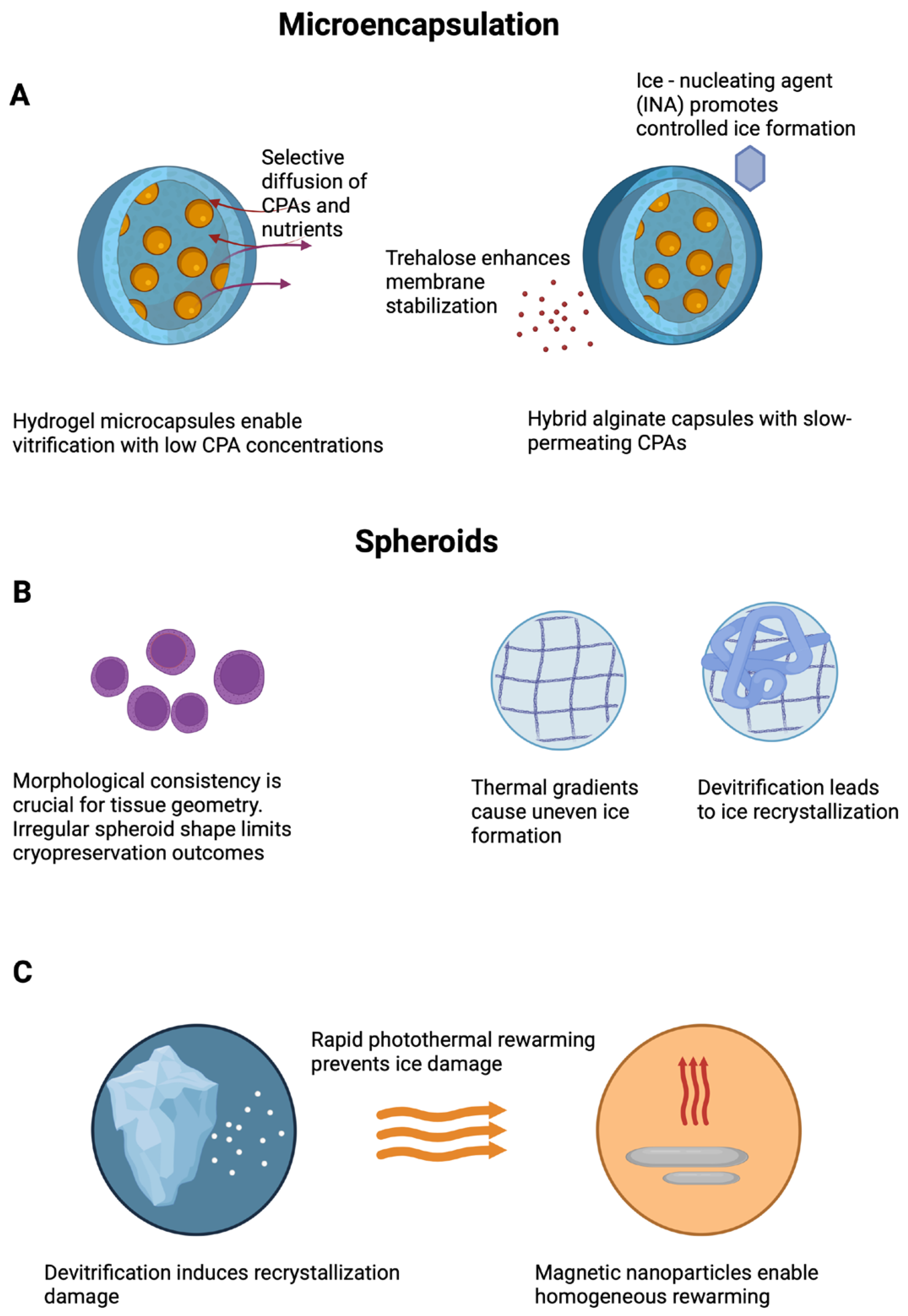
3.1. Microencapsulation and Microspheres
3.2. Macroscopic Hydrogels/Scaffolds Not Fabricated by Bioprinting
3.3. Bioprinted Constructs: Challenges and Emerging Strategies
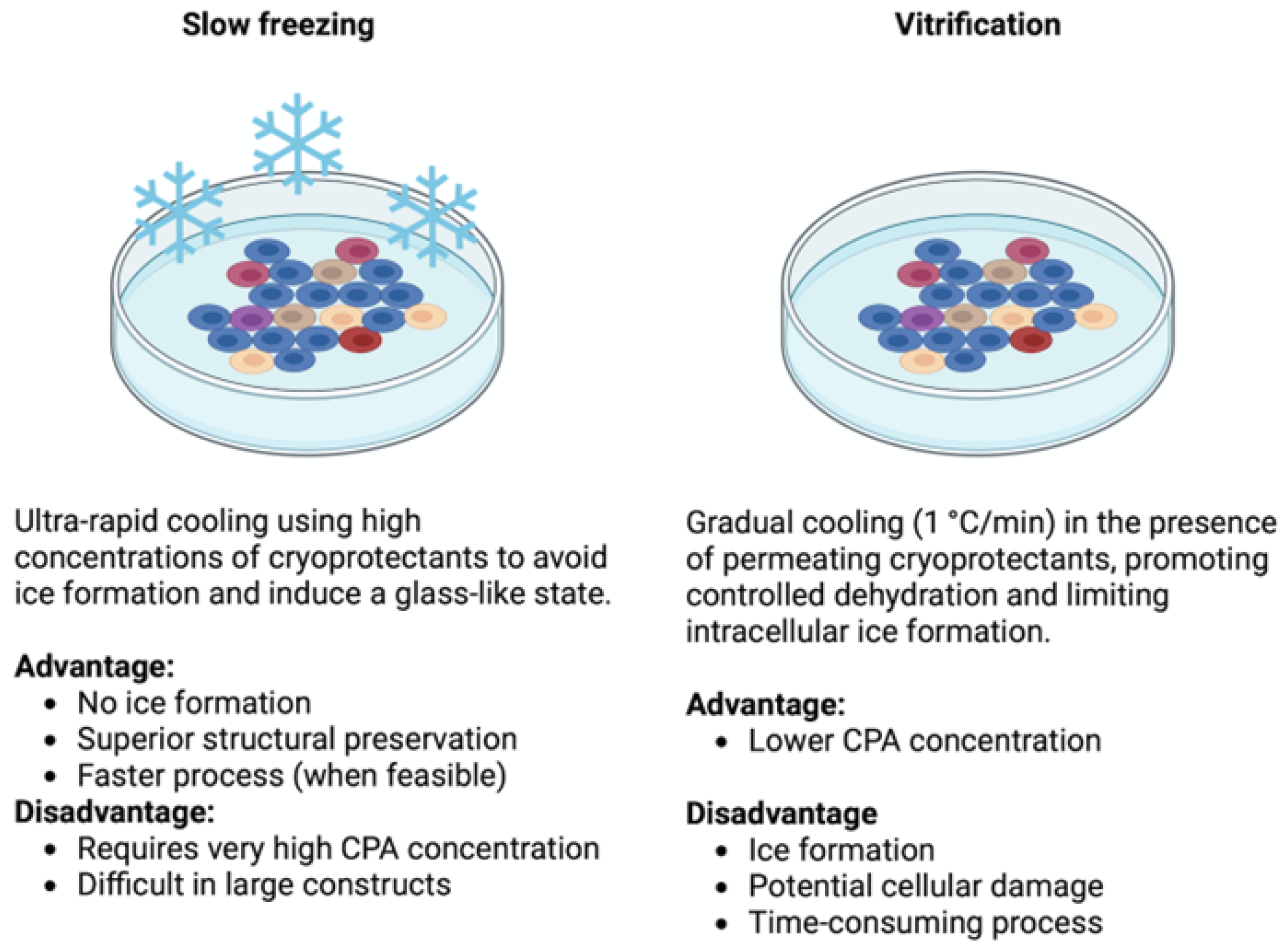
3.4. Overcoming Cryopreservation Challenges in 3D Systems: Safe Cryoprotectants and Effective Protocols
4. Future Perspectives and Regulatory Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, T.; Zhao, G. Ice Inhibition for Cryopreservation: Materials, Strategies, and Challenges. Adv. Sci. 2021, 8, 2002425. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Lee, Y.B.; Hinton, T.J.; Feinberg, A.W.; Alsberg, E. Cryopreserved Cell-Laden Alginate Microgel Bioink for 3D Bioprinting of Living Tissues. Mater. Today Chem. 2019, 12, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kudaibergen, G.; Zhunussova, M.; Mun, E.A.; Ramankulov, Y.; Ogay, V. Macroporous Cell-Laden Gelatin/Hyaluronic Acid/Chondroitin Sulfate Cryogels for Engineered Tissue Constructs. Gels 2022, 8, 590. [Google Scholar] [CrossRef] [PubMed]
- Savina, I.N.; Zoughaib, M.; Yergeshov, A.A. Design and Assessment of Biodegradable Macroporous Cryogels as Advanced Tissue Engineering and Drug Carrying Materials. Gels 2021, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Raees, S.; Ullah, F.; Javed, F.; Akil, H.M.; Jadoon Khan, M.; Safdar, M.; Din, I.U.; Alotaibi, M.A.; Alharthi, A.I.; Bakht, M.A.; et al. Classification, Processing, and Applications of Bioink and 3D Bioprinting: A Detailed Review. Int. J. Biol. Macromol. 2023, 232, 123476. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and Functional Human Liver from an IPSC-Derived Organ Bud Transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.F.; Glasscock, C.; McClanahan, D.R.; Benson, J.D.; Higgins, A.Z. Toxicity Minimized Cryoprotectant Addition and Removal Procedures for Adherent Endothelial Cells. PLoS ONE 2015, 10, e0142828. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, C.; Bailey, T.L.; Murray, K.; Gibson, M.I. Polyampholytes as Emerging Macromolecular. Cryoprotectants 2019, 21, 7–17. [Google Scholar] [CrossRef]
- Budharaju, H.; Sundaramurthi, D.; Sethuraman, S. Insights on the Role of Cryoprotectants in Enhancing the Properties of Bioinks Required for Cryobioprinting of Biological Constructs. J. Mater. Sci. Mater. Med. 2025, 36, 8. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.H.; Brockbank, K.G.M. Development of a Vitrification Preservation Process for Bioengineered Epithelial Constructs. Cells 2022, 11, 1115. [Google Scholar] [CrossRef] [PubMed]
- Malpique, R.; Osório, L.M.; Ferreira, D.S.; Ehrhart, F.; Brito, C.; Zimmermann, H.; Alves, P.M. Alginate Encapsulation as a Novel Strategy for the Cryopreservation of Neurospheres. Tissue Eng. Part C Methods 2010, 16, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Hardikar, A.A.; Risbud, M.V.; Bhonde, R.R. Improved Post-Cryopreservation Recovery Following Encapsulation of Islets in Chitosan-Alginate Microcapsules. Transplant. Proc. 2000, 32, 824–825. [Google Scholar] [CrossRef] [PubMed]
- Prát, T.; Vladimír Velebný, K.N. Hyaluronic Acid as Effective Cryoprotective Agent for HMSC Cryopreservation. Cryobiology 2022, 109, 47. [Google Scholar] [CrossRef]
- Cheng, Y.; Yu, Y.; Zhang, Y.; Zhao, G.; Zhao, Y. Cold-Responsive Nanocapsules Enable the Sole-Cryoprotectant-Trehalose Cryopreservation of β Cell–Laden Hydrogels for Diabetes Treatment. Small 2019, 15, e1904290. [Google Scholar] [CrossRef] [PubMed]
- Congdon, T.; Notman, R.; Gibson, M.I. Antifreeze (Glyco)Protein Mimetic Behavior of Poly(Vinyl Alcohol): Detailed Structure Ice Recrystallization Inhibition Activity Study. Biomacromolecules 2013, 14, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Whale, T.F.; Rosillo-Lopez, M.; Murray, B.J.; Salzmann, C.G. Ice Nucleation Properties of Oxidized Carbon Nanomaterials. J. Phys. Chem. Lett. 2015, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Huh, K.M.; Kang, S.W. Applications of Biomaterials in 3d Cell Culture and Contributions of 3d Cell Culture to Drug Development and Basic Biomedical Research. Int. J. Mol. Sci. 2021, 22, 2491. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent Advances in Biomaterials for 3D Scaffolds: A Review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D. Hydrogel Scaffolds for Tissue Engineering: The Importance of Polymer Choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Nagaraja, K.; Bhattacharyya, A.; Jung, M.; Kim, D.; Khatun, M.R.; Noh, I. 3D Bioprintable Self-Healing Hyaluronic Acid Hydrogel with Cysteamine Grafting for Tissue Engineering. Gels 2024, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Pilbauerova, N.; Schmidt, J.; Soukup, T.; Prat, T.; Nesporova, K.; Velebny, V.; Suchanek, J. Innovative Approach in the Cryogenic Freezing Medium for Mesenchymal Stem Cells. Biomolecules 2022, 12, 610. [Google Scholar] [CrossRef] [PubMed]
- Gryshkov, O.; Mutsenko, V.; Tarusin, D.; Khayyat, D.; Naujok, O.; Riabchenko, E.; Nemirovska, Y.; Danilov, A.; Petrenko, A.Y.; Glasmacher, B. Coaxial Alginate Hydrogels: From Self-Assembled 3D Cellular Constructs to Long-Term Storage. Int. J. Mol. Sci. 2021, 22, 3096. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Satish Kumar, R.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Effect of Cryopreservation and Microencapsulation of Lactic Acid Bacterium Enterococcus Faecium MC13 for Long-Term Storage. Biochem. Eng. J. 2011, 58–59, 140–147. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Zhang, L.; Wu, L.; Chen, Y.; Xie, D.; Chen, W. Molecular Sciences Hydrogel Cryopreservation System: An Effective Method for Cell Storage. Int. J. Mol. Sci. 2018, 19, 3330. [Google Scholar] [CrossRef] [PubMed]
- Ciptawati, E.; Takase, H.; Watanabe, N.M.; Okamoto, Y.; Nur, H.; Umakoshi, H. Preparation and Characterization of Biodegradable Sponge-like Cryogel Particles of Chitosan via the Inverse Leidenfrost (ILF) Effect. ACS Omega 2024, 9, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Musilová, L.; Achbergerová, E.; Vítková, L.; Kolařík, R.; Martínková, M.; Minařík, A.; Mráček, A.; Humpolíček, P.; Pecha, J. Cross-Linked Gelatine by Modified Dextran as a Potential Bioink Prepared by a Simple and Non-Toxic Process. Polymers. 2022, 14, 391. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Seo, D.; Park, K.D.; Shim, H.-E.; Park, J.H.; Ann, E.-J.; Kim, J.; Lee, J.Y.; Lee, J.; Kang, S.-W. Optimization of Alginate/Gelatin/Dextran-Aldehyde Bioink for 3D Bioprinting and Cell Engraftment. Int. J. Bioprint. Null 2025, 11, 8440. [Google Scholar] [CrossRef]
- Pescosolido, L.; Schuurman, W.; Malda, J.; Matricardi, P.; Alhaique, F.; Coviello, T.; Van Weeren, P.R.; Dhert, W.J.A.; Hennink, W.E.; Vermonden, T. Hyaluronic Acid and Dextran-Based Semi-IPN Hydrogels as Biomaterials for Bioprinting. Biomacromolecules 2011, 12, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Mandal, S.S.; Bauri, S.; Maiti, P. 3D Bioprinting and Its Innovative Approach for Biomedical Applications. MedComm 2023, 4, e194. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Peng, S.; Chen, Y.; Chu, B. Agarose Hydrogels for Bone Tissue Engineering, from Injectables to Bioprinting. Gels 2025, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Wenger, L.; Radtke, C.P.; Gerisch, E.; Kollmann, M.; Niemeyer, C.M.; Rabe, K.S.; Hubbuch, J. Systematic Evaluation of Agarose- and Agar-Based Bioinks for Extrusion-Based Bioprinting of Enzymatically Active Hydrogels. Front. Bioeng. Biotechnol. 2022, 10, 928878. [Google Scholar] [CrossRef] [PubMed]
- López-Marcial, G.R.; Zeng, A.Y.; Osuna, C.; Dennis, J.; García, J.M.; O’Connell, G.D. Agarose-Based Hydrogels as Suitable Bioprinting Materials for Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 3610–3616. [Google Scholar] [CrossRef]
- Wang, X. Advanced Polymers for Three-Dimensional (3D) Organ Bioprinting. Micromachines 2019, 10, 814. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yue, Z.; Lucarelli, E.; Wallace, G.G. Hybrid Printing Using Cellulose Nanocrystals Reinforced GelMA/HAMA Hydrogels for Improved Structural Integration. Adv. Healthc. Mater. 2020, 9, 2001410. [Google Scholar] [CrossRef] [PubMed]
- Waidi, Y.O.; Kariim, I.; Datta, S. Bioprinting of Gelatin-Based Materials for Orthopedic Application. Front. Bioeng. Biotechnol. 2024, 12, 1357460. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Vernon, B.; Jeong, B. Low-Molecular-Weight PEGs for Cryopreservation of Stem Cell Spheroids. Biomater. Res. 2024, 28, 0037. [Google Scholar] [CrossRef] [PubMed]
- Khetan, S.; Burdick, J.A. Patterning Network Structure to Spatially Control Cellular Remodeling and Stem Cell Fate within 3-Dimensional Hydrogels. Biomaterials 2010, 31, 8228–8234. [Google Scholar] [CrossRef] [PubMed]
- Khetan, S.; Corey, O. Maintenance of Stem Cell Viability and Differentiation Potential Following Cryopreservation within 3-Dimensional Hyaluronic Acid Hydrogels. Cryobiology 2019, 90, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Pangjantuk, A.; Kaokaen, P.; Kunhorm, P.; Chaicharoenaudomrung, N.; Noisa, P. 3D Culture of Alginate-Hyaluronic Acid Hydrogel Supports the Stemness of Human Mesenchymal Stem Cells. Sci. Rep. 2024, 14, 4436. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Lee, G.W.; An, S.; Seong, K.Y.; Lee, J.S.; Yang, S.Y. Enhanced Cellular Cryopreservation by Biopolymer-Associated Suppression of Rhoa/Rock Signaling Pathway. Materials 2021, 14, 6056. [Google Scholar] [CrossRef] [PubMed]
- Ziani, K.; Espona-Noguera, A.; Crisóstomo, V.; Casado, J.G.; Sanchez-Margallo, F.M.; Saenz del Burgo, L.; Ciriza, J.; Pedraz, J.L. Characterization of Encapsulated Porcine Cardiosphere-Derived Cells Embedded in 3D Alginate Matrices. Int. J. Pharm. 2021, 599, 120454. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, F.; Wang, X.; Geng, X.; Zhao, S.; Liu, H.; Dou, D.; Leng, Y.; Wang, L.; Fan, Y. Biomaterial Inks for Extrusion-Based 3D Bioprinting: Property, Classification, Modification, and Selection. Int. J. Bioprint. 2022, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Torres García, D.; Salehi, M.; Webber, M.J.; van Kasteren, S.I.; Eelkema, R. Dynamic Covalent Dextran Hydrogels as Injectable, Self-Adjuvating Peptide Vaccine Depots. ACS Chem. Biol. 2023, 18, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.T.; Jang, I.S.; Dang, T.V.; Kim, Y.H.; Lee, D.H.; Choi, H.S.; Yu, B.J.; Kim, M. Il Effective Cryopreservation of a Bioluminescent Auxotrophic Escherichia Coli-Based Amino Acid Array to Enable Long-Term Ready-to-Use Applications. Biosensors 2021, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Okoro, O.V.; Nie, L.; Petri, D.F.S.; Shavandi, A. Protein-Based 3D Biofabrication of Biomaterials. Bioengineering 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tripathy, N.; Chung, E.J. Targeting and Therapeutic Peptide-Based Strategies for Polycystic Kidney Disease. Adv. Drug Deliv. Rev. 2020, 161–162, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.M.; Gansau, J.; Buckley, C.T.; Al-Jubori, M.T.; Raad Mohammed, R.; Herrero-Gómez, A.; Azagra, M.; Marco-Rius, I. A Cryopreservation Method for Bioengineered 3D Cell Culture Models. Biomed. Mater. 2022, 17, 045023. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Jin, L.; Luo, Z.; Yu, J.; Shi, S.; Zhang, Z.; Shen, M.; Chen, H.; Li, X.; Song, Z. An Injectable Thermosensitive Polymeric Hydrogel for Sustained Release of Avastin® to Treat Posterior Segment Disease. Int. J. Pharm. 2015, 490, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Deller, R.C.; Vatish, M.; Mitchell, D.A.; Gibson, M.I. Synthetic Polymers Enable Non-Vitreous Cellular Cryopreservation by Reducing Ice Crystal Growth during Thawing. Nat. Commun. 2014 51 2014, 5, 3244. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X. Synthetic Polymers for Organ 3D Printing. Polymers 2020, 12, 1765. [Google Scholar] [CrossRef] [PubMed]
- Klbik, I.; Čechová, K.; Milovská, S.; Švajdlenková, H.; Maťko, I.; Lakota, J.; Šauša, O. Polyethylene Glycol 400 Enables Plunge-Freezing Cryopreservation of Human Keratinocytes. J. Mol. Liq. 2023, 379, 121711. [Google Scholar] [CrossRef]
- Dias, C.L.; Ala-Nissila, T.; Wong-ekkabut, J.; Vattulainen, I.; Grant, M.; Karttunen, M. The Hydrophobic Effect and Its Role in Cold Denaturation. Cryobiology 2010, 60, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.M.; Gansau, J.; Buckley, C.T. Priming and Cryopreservation of Microencapsulated Marrow Stromal Cells as a Strategy for Intervertebral Disc Regeneration. Biomed. Mater. 2018, 13, 034106. [Google Scholar] [CrossRef] [PubMed]
- Massie, I.; Selden, C.; Hodgson, H.; Fuller, B. Cryopreservation of Encapsulated Liver Spheroids for a Bioartificial Liver: Reducing Latent Cryoinjury Using an Ice Nucleating Agent. Tissue Eng. Part C Methods 2011, 17, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Awan, M.; Erro, E.; Forster-Brown, E.; Brookshaw, T.; Chandel, S.; Chalmers, S.A.; Watt, A.; Fuller, B.; Selden, C. Dimethyl Sulfoxide for Cryopreservation of Alginate Encapsulated Liver Cell Spheroids in Bioartificial Liver Support; Assessments of Cryoprotectant Toxicity Tolerance and Dilution Strategies. Cryobiology 2022, 106, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, I.N.; Olivos, D.J.; Brinker, A.; Alvarez, M.B.; Smith, L.J.; Chu, T.M.G.; Kacena, M.A.; Wagner, D.R. Scaffold-Free Bioprinting of Mesenchymal Stem Cells Using the Regenova Printer: Spheroid Characterization and Osteogenic Differentiation. Bioprinting 2019, 15, e00050. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, E.; Zhao, G. Advanced Cryopreservation Engineering Strategies: The Critical Step to Utilize Stem Cell Products. Cell Regen. 2023, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Li, Y.; Chen, T. Techniques for Fabrication and Construction of Three-Dimensional Scaffolds for Tissue Engineering. Int. J. Nanomed. 2013, 8, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Khan, I.; Chen, J.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B.; Ali, F.; Khan, I.; Chen, J.; Akhtar, K.; et al. Emerging Fabrication Strategies of Hydrogels and Its Applications. Gels 2022, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, J.; Bian, W.; Li, Z.; Li, D.; Snedeker, J.G. A Novel Silk–TCP–PEEK Construct for Anterior Cruciate Ligament Reconstruction: An off-the Shelf Alternative to a Bone–Tendon–Bone Autograft*. Biofabrication 2014, 6, 015010. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rodrigues, J.; Tomás, H. Injectable and Biodegradable Hydrogels: Gelation, Biodegradation and Biomedical Applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Fatimi, A.; Liu, Y.; Musuc, A.M.; Fajardo, A.R.; Gowda, B.H.J.; Vora, L.K.; Shavandi, A.; Okoro, O.V. Recent Advances in 3D Bioprinted Polysaccharide Hydrogels for Biomedical Applications: A Comprehensive Review. Carbohydr. Polym. 2025, 348, 122845. [Google Scholar] [CrossRef]
- Tappa, K.; Jammalamadaka, U. Novel Biomaterials Used in Medical 3D Printing Techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Warburton, L.; Rubinsky, B. Cryopreservation of 3D Bioprinted Scaffolds with Temperature-Controlled-Cryoprinting. Gels 2023, 9, 502. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Parisi, C.; Di Silvio, L.; Dini, D.; Forte, A.E. Cryogenic 3D Printing of Super Soft Hydrogels. Sci. Rep. 2017, 7, 16293. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Rubinsky, B. Temperature-Controlled 3D Cryoprinting Inks Made of Mixtures of Alginate and Agar. Gels 2023, 9, 689. [Google Scholar] [CrossRef] [PubMed]
- Hammoudeh, S.M.; Hammoudeh, A.M.; Hamoudi, R. High-Throughput Quantification of the Effect of DMSO on the Viability of Lung and Breast Cancer Cells Using an Easy-to-Use Spectrophotometric Trypan Blue-Based Assay. Histochem. Cell Biol. 2019, 152, 75. [Google Scholar] [CrossRef] [PubMed]
- Ntai, A.; La Spada, A.; De Blasio, P.; Biunno, I. Trehalose to Cryopreserve Human Pluripotent Stem Cells. Stem Cell Res. 2018, 31, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kang, K.; Yoon, S.; Kim, J.S.; Park, S.A.; Kim, W.D.; Lee, S.B.; Ryu, K.Y.; Jeong, J.; Choi, D. Prolongation of Liver-Specific Function for Primary Hepatocytes Maintenance in 3D Printed Architectures. Organogenesis 2018, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.J. Cryopreservation: Vitrification and Controlled Rate Cooling. Methods Mol. Biol. 2017, 1590, 41–77. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, T.; Takeuchi, Y.; Ishibashi, K.; Gotoh, N.; Hirata, E.; Kuroda, K. Cryopreservation of Tissues by Slow-Freezing Using an Emerging Zwitterionic Cryoprotectant. Sci. Rep. 2023, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Wowk, B. Thermodynamic Aspects of Vitrification. Cryobiology 2010, 60, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Loutradi, K.E.; Kolibianakis, E.M.; Venetis, C.A.; Papanikolaou, E.G.; Pados, G.; Bontis, I.; Tarlatzis, B.C. Cryopreservation of Human Embryos by Vitrification or Slow Freezing: A Systematic Review and Meta-Analysis. Fertil. Steril. 2008, 90, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Glujovsky, D.; Riestra, B.; Sueldo, C.; Fiszbajn, G.; Repping, S.; Nodar, F.; Papier, S.; Ciapponi, A. Vitrification versus Slow Freezing for Women Undergoing Oocyte Cryopreservation. Cochrane Database Syst. Rev. 2014, 2014, CD010047. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Rajan, R.; Ahmed, S. Bridging Polymer Chemistry and Cryobiology. Polym. J. 2023, 55, 105–115. [Google Scholar] [CrossRef]
- Aghajani, M.; Garshasbi, H.R.; Naghib, S.M.; Mozafari, M.R. 3D Printing of Hydrogel Polysaccharides for Biomedical Applications: A Review. Biomedicines 2025, 13, 731. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lee, J.M.; Yeong, W.Y. Smart Hydrogels for 3D Bioprinting. Int. J. Bioprint. 2015, 1, 3–14. [Google Scholar] [CrossRef]
- Rumon, M.M.H.; Akib, A.A.; Sarkar, S.D.; Khan, M.A.R.; Uddin, M.M.; Nasrin, D.; Roy, C.K. Polysaccharide-Based Hydrogels for Advanced Biomedical Engineering Applications. ACS Polym. Au 2024, 4, 463. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Yang, M.; Wang, L.; Li, W.; Liu, M.; Jin, Y.; Wang, Y.; Yang, R.; Wang, Y.; Zhang, K.; et al. Hydrogels for 3D Bioprinting in Tissue Engineering and Regenerative Medicine: Current Progress and Challenges. Int. J. Bioprint. 2023, 9, 207–238. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Z.; Xu, Y.; Xia, J.; Xu, Z.; Zhu, S.; Jin, M. Preparation of Chitosan/Recombinant Human Collagen-Based Photo-Responsive Bioinks for 3D Bioprinting. Gels 2022, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Mora-Boza, A.; Włodarczyk-Biegun, M.K.; Del Campo, A.; Vázquez-Lasa, B.; Román, J.S.; Mora-Boza, A. Chitosan-Based Inks: 3D Printing and Bioprinting Strategies to Improve Shape Fidelity, Mechanical Properties, and Biocompatibility of 3D Scaffolds. Biomecánica 2019, 27, 7–16. [Google Scholar] [CrossRef]
- Maturavongsadit, P.; Paravyan, G.; Shrivastava, R.; Benhabbour, S.R. Thermo-/PH-Responsive Chitosan-Cellulose Nanocrystals Based Hydrogel with Tunable Mechanical Properties for Tissue Regeneration Applications. Materialia 2020, 12, 100681. [Google Scholar] [CrossRef]
- Gaweł, J.; Milan, J.; Żebrowski, J.; Płoch, D.; Stefaniuk, I.; Kus-Liśkiewicz, M. Biomaterial Composed of Chitosan, Riboflavin, and Hydroxyapatite for Bone Tissue Regeneration. Sci. Rep. 2023, 13, 17004. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Ee, L.Y.; Yau Li, S.F. Recent Advances in 3D Printing of Nanocellulose: Structure, Preparation, and Application Prospects. Nanoscale Adv. 2021, 3, 1167–1208. [Google Scholar] [CrossRef] [PubMed]
- García-Lizarribar, A.; Fernández-Garibay, X.; Velasco-Mallorquí, F.; Castaño, A.G.; Samitier, J.; Ramon-Azcon, J. Composite Biomaterials as Long-Lasting Scaffolds for 3D Bioprinting of Highly Aligned Muscle Tissue. Macromol. Biosci. 2018, 18, 1800167. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Huda, M.; Hasan, A.; Khan, M. Biomedical Applications of Collagen: A Review. Rev. Clin. Pharmacol. Pharmacokinet. Int. Ed. 2024, 38, 73–86. [Google Scholar] [CrossRef]
- Teo, Y.C.; Abbas, A.; Park, E.J.; Barbut, C.; Guo, J.; Goh, D.; Poh, J.; Yeong, S.; Loong, W.; Mok, J.; et al. 3D Printed Bioactive PLGA Dermal Scaffold for Burn Wound Treatment. ACS Mater. Au 2023, 2023, 272. [Google Scholar] [CrossRef] [PubMed]
| Material Type | Examples | Key Properties | Cryoprotective Function | Applications | References |
|---|---|---|---|---|---|
| Hyaluronic acid (HA) | MeHA, HMW-HA | Viscoelastic, ECM-mimetic, chemically modifiable | Uniform CPA diffusion, maintains differentiation | MSCs, tumor models | [17,18,19,20,21] |
| Alginate | Ca2+-crosslinked, OMA | Fast gelation, functionalizable | Encapsulation, electrosprayed core–shell systems | Neural spheroids, biofabricated constructs | [2,11,22] |
| Chitosan | Alginate–chitosan capsules, cryogels | Polycationic, biodegradable, stabilizing | Capsule reinforcement, modulates permeability, cryogel matrix | Cell encapsulation, islet preservation, cryoscaffolds | [23,24,25] |
| Dextran | Dex-ald, Dex-HEMA | Injectable, photopolymerizable | Potential osmotic buffering, scaffold structure | Long-term cultures, DMSO-free systems | [26,27,28,29] |
| Agarose | Low melt, composites | Thermally stable, bioinert | Ice crystal barrier, structural integrity | Cartilage engineering, enzyme scaffolds | [19,30,31,32] |
| Nanocellulose | CNFs, CNCs | High stiffness, shear-thinning | Prevents freeze–thaw damage, ECM stability | Hybrid bioinks, cryoprinting | [33,34,35,36] |
| Material Type | Examples | Key Properties | Cryoprotective Function | Applications | References |
|---|---|---|---|---|---|
| Silk Fibroin (SF) | Methacrylated SF | Shear-thinning, β-sheet stability, pH/temp-responsive | Supports cell–cell/matrix interaction, reduces DMSO toxicity | Tissue engineering, hepatic modeling, cryogel interactions | [35,45,46,47] |
| Sericin | Genipin-crosslinked | Antioxidant, epithelial regenerative, bioadhesive | ROS scavenging, membrane stabilization, osmotic buffering | Epithelial scaffolds, cryopreservation without DMSO | [36,48,49] |
| Material Type | Examples | Key Properties | Cryoprotective Function | Applications | References |
|---|---|---|---|---|---|
| Polyethylene glycol (PEG) | PEG200, PEG400, PEGDA | Hydrophilic, modifiable, non-toxic, crosslinkable | Intracellular protection, osmotic buffering, ice inhibition | Cryopreservatio, hydrogel bioinks | [36,51,52,53] |
| Polyvinyl alcohol (PVA) | Freeze–thaw hydrogels | Physically crosslinked, IRI-active, non-permeating | Suppresses ice growth, enhances morphology and post thaw viability | Cryopreservation additives, tissue scaffolds | [15,50] |
| Polyurethane (PU) | Thermoresponsive PU | Elastic, biodegradable, tunable stiffness | Maintains structure/function during freeze–thaw cycles | Neural/vascular tissue printing, injectable cryogels | [15,50,51,53] |
| Biomaterial | Cryoprotective Function | Printability | Stability | Remarks/Limitations | References |
|---|---|---|---|---|---|
| Hyaluronic acid (HA) | ECM mimicry, osmotic buffering | Tunable (e.g., MeHA) | Low | Often combined with stronger polymers | [17,18,19,20,21] |
| Alginate | Osmotic buffering, water retention | Excellent (ionic crosslinking) | Moderate | Requires peptide modification | [2,11,22] |
| Chitosan | Membrane stabilization, capsule reinforcement | Limited (requires acidic pH) | Moderate | Complex gelation, used in encapsulation | [24,25,41] |
| Dextran | IRI potential, CPA diffusion | Shear-thinning, injectable | Moderate | Suitable for injectable cryogels | [26,27,28,29] |
| Agarose | Thermal stability, structural retention | Limited (thermo-gelling) | High | Supports architecture retention; lacks cell interaction | [19,30,31,32] |
| Nanocellulose (CNC, CNF) | Rheological tuning, shape fidelity | High (in blends) | High | Bioinert unless functionalized | [33,36] |
| Silk fibroin | DMSO reduction, membrane protection | Tunable (physical/chemical) | High | Good mechanical properties post thaw | [35,45,46,47] |
| Sericin | Antioxidant, membrane-protective | Blendable, injectable | Low | Bioactive additive with limited mechanical support | [36,48,49] |
| PEG/PEGDA | CPA delivery, osmotic buffering | High (photo/chemical) | Good | Synthetic, tunable stiffness and gelation | [36,51,52,53] |
| PVA | Ice recrystallization inhibition (IRI) | Blendable | High | Inert, used as non-toxic additive | [15,50] |
| PU | Elastic cryogelation, shape memory | Cryogel-based | High | Promising for scaffold integrity post thaw | [15,50,51,53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziani, K.; Saenz-del-Burgo, L.; Pedraz, J.L.; Ciriza, J. Advances in Cryopreservation Strategies for 3D Biofabricated Constructs: From Hydrogels to Bioprinted Tissues. Int. J. Mol. Sci. 2025, 26, 6908. https://doi.org/10.3390/ijms26146908
Ziani K, Saenz-del-Burgo L, Pedraz JL, Ciriza J. Advances in Cryopreservation Strategies for 3D Biofabricated Constructs: From Hydrogels to Bioprinted Tissues. International Journal of Molecular Sciences. 2025; 26(14):6908. https://doi.org/10.3390/ijms26146908
Chicago/Turabian StyleZiani, Kaoutar, Laura Saenz-del-Burgo, Jose Luis Pedraz, and Jesús Ciriza. 2025. "Advances in Cryopreservation Strategies for 3D Biofabricated Constructs: From Hydrogels to Bioprinted Tissues" International Journal of Molecular Sciences 26, no. 14: 6908. https://doi.org/10.3390/ijms26146908
APA StyleZiani, K., Saenz-del-Burgo, L., Pedraz, J. L., & Ciriza, J. (2025). Advances in Cryopreservation Strategies for 3D Biofabricated Constructs: From Hydrogels to Bioprinted Tissues. International Journal of Molecular Sciences, 26(14), 6908. https://doi.org/10.3390/ijms26146908









