Immunological Landscape and Molecular Therapeutic Targets of the Tumor Microenvironment in Hepatocellular Carcinoma
Abstract
1. Introduction
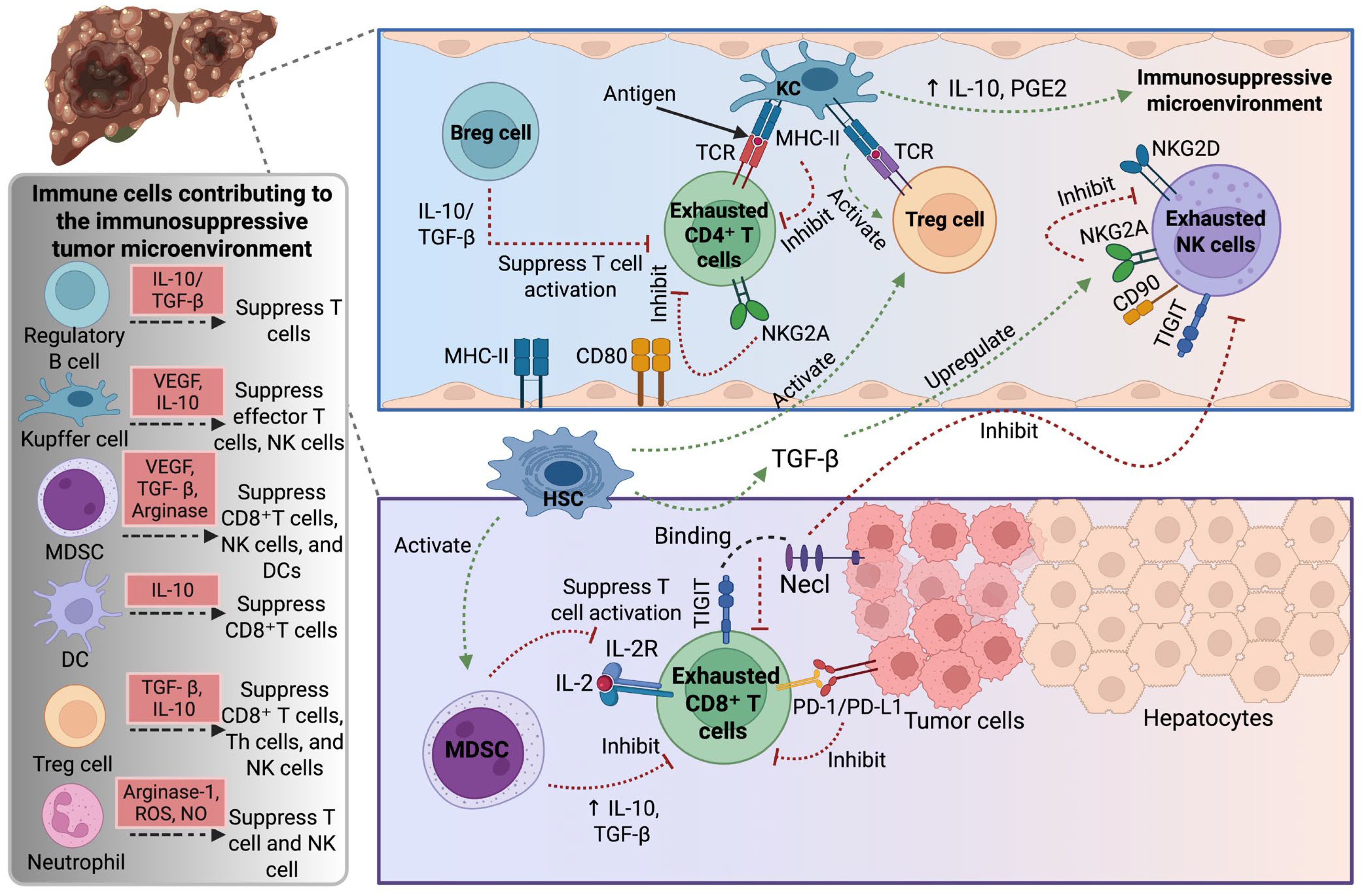
2. Tumor Microenvironment in Hepatocellular Carcinoma
3. Immune Cells in Hepatocellular Carcinoma
3.1. T Cells
3.1.1. Regulatory T Cells
3.1.2. CD8+ T Cells
3.2. Natural Killer Cells
3.3. Myeloid Cells
3.4. Cancer-Associated Fibroblasts
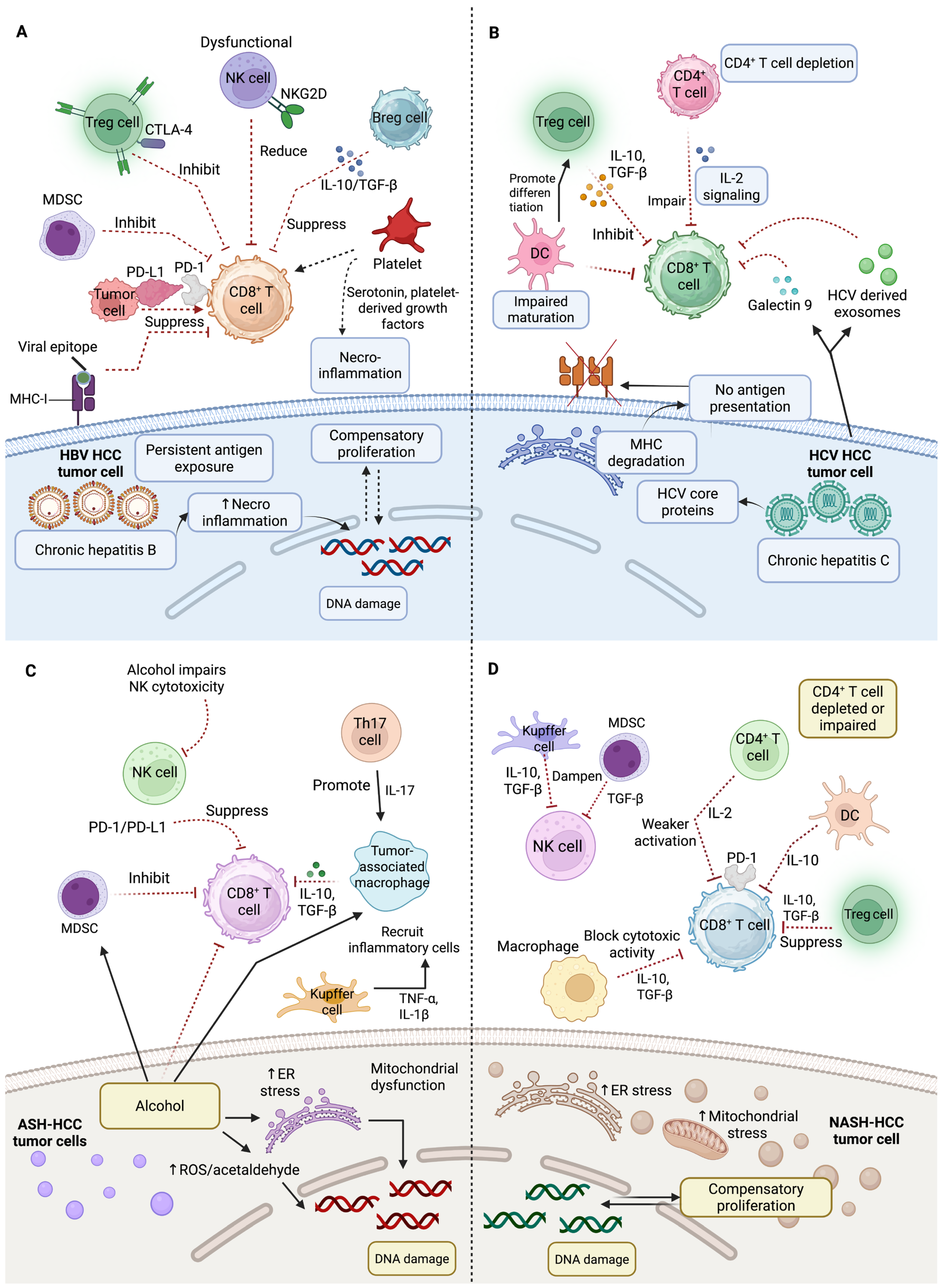
4. Etiological Influences of Tumor Microenvironment
4.1. Immune Landscape in Virus-Related HCC
4.2. Immunological Landscape of Non-Viral HCC
5. Pathways and Signaling Cascades Mediating Immune Evasion
5.1. Role of Wnt/β-Catenin Signaling in HCC Tumorigenesis and Immune Evasion
5.2. TGF-β Signaling in HCC Progression and Immune Suppression
5.3. Immune Checkpoint Expression and Immune Evasion in HCC
6. Conventional and Advanced Therapies for Hepatocellular Carcinoma Management
6.1. Curative and Localized Treatment Approaches
6.2. Systemic and Immunotherapeutic Approaches
6.3. Barriers and Advancements for Treatment
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Donne, R.; Lujambio, A. The liver cancer immune microenvironment: Therapeutic implications for hepatocellular carcinoma. Hepatology 2023, 77, 1773–1796. [Google Scholar] [CrossRef] [PubMed]
- Zarlashat, Y.; Mushtaq, H.; Pham, L.; Abbas, W.; Sato, K. Advancements in immunotherapeutic treatments for hepatocellular carcinoma: Potential of combination therapies. Int. J. Mol. Sci. 2024, 25, 6830. [Google Scholar] [CrossRef] [PubMed]
- Zarlashat, Y.; Ambreen, A.; Zafar, M.; Mushtaq, H.; Munir, B.; Mujahid, M.; Ghaffar, A. Effect of doxorubicin and paclitaxel on the selective oncogenes expression level of hepatocellular carcinoma RAS/RAF/MEK/ERK pathway in Huh-7 cell line. Agrobiol. Rec. 2024, 18, 1–11. [Google Scholar] [CrossRef]
- Mushtaq, H.; Zarlashat, Y.; Ambreen, A.; Mujahid, M.; Kausar, S.; Shafqat, D. Reviewing advances in understanding and targeting the MAPK signaling pathway in hepatocellular carcinoma progression and therapeutics. Agrobiol. Rec. 2024, 15, 103–116. [Google Scholar] [CrossRef]
- Shlyakhtina, Y.; Moran, K.L.; Portal, M.M. Genetic and non-genetic mechanisms underlying cancer evolution. Cancers 2021, 13, 1380. [Google Scholar] [CrossRef]
- Safri, F.; Nguyen, R.; Zerehpooshnesfchi, S.; George, J.; Qiao, L. Heterogeneity of hepatocellular carcinoma: From mechanisms to clinical implications. Cancer Gene Ther. 2024, 31, 1105–1112. [Google Scholar] [CrossRef]
- Zarlashat, Y.; Tayyeba, A.; Hussain, S. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in hepatocellular carcinoma: From inflammation to clinical applications. Cancer Plus 2024, 6, 5758. [Google Scholar] [CrossRef]
- Kotsari, M.; Dimopoulou, V.; Koskinas, J.; Armakolas, A. Immune system and hepatocellular carcinoma (HCC): New insights into HCC progression. Int. J. Mol. Sci. 2023, 24, 11471. [Google Scholar] [CrossRef]
- Ringelhan, M.; Pfister, D.; O’Connor, T.; Pikarsky, E.; Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 2018, 19, 222–232. [Google Scholar] [CrossRef]
- Kudo, M. Immuno-oncology therapy for hepatocellular carcinoma: Current status and ongoing trials. Liver Cancer 2019, 8, 221–238. [Google Scholar] [CrossRef]
- Ju, C.; Tacke, F. Hepatic macrophages in homeostasis and liver diseases: From pathogenesis to novel therapeutic strategies. Cell. Mol. Immunol. 2016, 13, 316–327. [Google Scholar] [CrossRef]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef]
- Kurebayashi, Y.; Ojima, H.; Tsujikawa, H.; Kubota, N.; Maehara, J.; Abe, Y.; Kitago, M.; Shinoda, M.; Kitagawa, Y.; Sakamoto, M. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology 2018, 68, 1025–1041. [Google Scholar] [CrossRef]
- Ding, W.; Xu, X.; Qian, Y.; Xue, W.; Wang, Y.; Du, J.; Jin, L.; Tan, Y. Prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma: A meta-analysis. Medicine 2018, 97, 13301. [Google Scholar] [CrossRef] [PubMed]
- Heymann, F.; Peusquens, J.; Ludwig-Portugall, I.; Kohlhepp, M.; Ergen, C.; Niemietz, P.; Martin, C.; van Rooijen, N.; Ochando, J.C.; Randolph, G.J.; et al. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology 2015, 62, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.; Nunez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, H.; Sun, B.; Karin, M. The immunobiology of hepatocellular carcinoma in humans and mice: Basic concepts and therapeutic implications. J. Hepatol. 2020, 72, 167–182. [Google Scholar] [CrossRef]
- Liu, D.; Heij, L.R.; Czigany, Z.; Dahl, E.; Lang, S.A.; Ulmer, T.F.; Luedde, T.; Neumann, U.P.; Bednarsch, J. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 127. [Google Scholar] [CrossRef]
- Wang, X.; Gao, X.; Liu, Y.; Fan, S.; Ma, Q. Progress of research on the regulatory pathway of the plant shade-avoidance syndrome. Front. Plant Sci. 2020, 11, 439. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, Y.; Li, B. CD8+ T cell exhaustion and cancer immunotherapy. Cancer Lett. 2023, 559, 216043. [Google Scholar] [CrossRef]
- Barsch, M.; Salié, H.; Schlaak, A.E.; Zhang, Z.; Hess, M.; Mayer, L.S.; Tauber, C.; Otto-Mora, P.; Ohtani, T.; Nilsson, T.; et al. T-cell exhaustion and residency dynamics inform clinical outcomes in hepatocellular carcinoma. J. Hepatol. 2022, 77, 397–409. [Google Scholar] [CrossRef]
- Magen, A.; Hamon, P.; Fiaschi, N.; Soong, B.Y.; Park, M.D.; Mattiuz, R.; Humblin, E.; Troncoso, L.; D’souza, D.; Dawson, T.; et al. Intratumoral dendritic cell–CD4+ T helper cell niches enable CD8+ T cell differentiation following PD-1 blockade in hepatocellular carcinoma. Nat. Med. 2023, 29, 1389–1399. [Google Scholar] [CrossRef]
- Patsoukis, N.; Brown, J.; Petkova, V.; Liu, F.; Li, L.; Boussiotis, V.A. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci. Signal. 2012, 5, ra46. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zheng, B.; Goswami, S.; Meng, L.; Zhang, D.; Cao, C.; Li, T.; Zhu, F.; Ma, L.; Zhang, Z.; et al. PD1Hi CD8+ T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J. Immunother. Cancer 2019, 7, 331. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D. PD-L1, TMB, and other potential predictors of response to immunotherapy for hepatocellular carcinoma: How can they assist drug clinical trials? Expert Opin. Investig. Drug. 2022, 31, 415–423. [Google Scholar] [CrossRef]
- Jenkins, E.; Whitehead, T.; Fellermeyer, M.; Davis, S.J.; Sharma, S. The current state and future of T-cell exhaustion research. Oxf. Open Immunol. 2023, 4, iqad006. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chatterjee, M.; Ghosh, P.; Ganguly, K.K.; Basu, M.; Ghosh, M.K. Targeting PD-1/PD-L1 in cancer immunotherapy: An effective strategy for treatment of triple-negative breast cancer (TNBC) patients. Genes Dis. 2023, 10, 1318–1350. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.-Y.; Breder, V.V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Results of KEYNOTE-240: Phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J. Clin. Oncol. 2019, 37, 4004. [Google Scholar] [CrossRef]
- Galasso, L.; Cerrito, L.; Maccauro, V.; Termite, F.; Ainora, M.E.; Gasbarrini, A.; Zocco, M.A. Hepatocellular Carcinoma and the Multifaceted Relationship with Its Microenvironment: Attacking the Hepatocellular Carcinoma Defensive Fortress. Cancers 2024, 16, 1837. [Google Scholar] [CrossRef]
- Sangro, B.; Gomez-Martin, C.; de la Mata, M.; Iñarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.I.; Larrea, E.; Alfaro, C.; Sarobe, P.; et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013, 59, 81–88. [Google Scholar] [CrossRef]
- Beyzaei, Z.; Shojazadeh, A.; Geramizadeh, B. The role of regulatory T cells in liver transplantation. Transpl. Immunol. 2022, 70, 101512. [Google Scholar] [CrossRef]
- Xydia, M.; Rahbari, R.; Ruggiero, E.; Macaulay, I.; Tarabichi, M.; Lohmayer, R.; Wilkening, S.; Michels, T.; Brown, D.; Vanuytven, S.; et al. Common clonal origin of conventional T cells and induced regulatory T cells in breast cancer patients. Nat. Commun. 2021, 12, 1119. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Nishikawa, H. Roles of regulatory T cells in cancer immunity. Int. Immunol. 2016, 28, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; You, M.; Fu, J.; Tian, M.; Zhong, X.; Du, C.; Hong, Z.; Zhu, Z.; Liu, J.; Markowitz, G.J.; et al. Intratumoral stem-like CCR4+ regulatory T cells orchestrate the immunosuppressive microenvironment in HCC associated with hepatitis B. J. Hepatol. 2022, 76, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Speiser, D.E.; Chijioke, O.; Schaeuble, K.; Münz, C. CD4+ T cells in cancer. Nat. Cancer 2023, 4, 317–329. [Google Scholar] [CrossRef]
- Prieto, J.; Melero, I.; Sangro, B.J. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 681–700. [Google Scholar] [CrossRef]
- Budhu, A.; Forgues, M.; Ye, Q.-H.; Jia, H.-L.; He, P.; Zanetti, K.A.; Kammula, U.S.; Chen, Y.; Qin, L.-X.; Tang, Z.-Y.; et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell 2006, 10, 99–111. [Google Scholar] [CrossRef]
- Pocino, K.; Stefanile, A.; Basile, V.; Napodano, C.; D’Ambrosio, F.; Di Santo, R.; Callà, C.A.M.; Gulli, F.; Saporito, R.; Ciasca, G.; et al. Cytokines and hepatocellular carcinoma: Biomarkers of a deadly embrace. J. Pers. Med. 2022, 13, 5. [Google Scholar] [CrossRef]
- Rico Montanari, N.; Anugwom, C.M.; Boonstra, A.; Debes, J.D. The role of cytokines in the different stages of hepatocellular carcinoma. Cancers 2021, 13, 4876. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Yan, J.; Xu, J.; Pang, X.-H.; Chen, M.-S.; Li, L.; Wu, C.; Li, S.-P.; Zheng, L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J. Hepatol. 2009, 50, 980–989. [Google Scholar] [CrossRef]
- Wilke, C.M.; Kryczek, I.; Wei, S.; Zhao, E.; Wu, K.; Wang, G.; Zou, W. Th17 cells in cancer: Help or hindrance? Carcinogenesis 2011, 32, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.R.; Nelson, M.H.; Himes, R.A.; Li, Z.; Mehrotra, S.; Paulos, C.M. Th17 cells in cancer: The ultimate identity crisis. Front. Immunol. 2014, 5, 276. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.-T.; Fan, X.-P.; Fan, Y.-C.; Zhao, J.; Wang, K. Change in the Treg/Th17 cell imbalance in hepatocellular carcinoma patients and its clinical value. Medicine 2017, 96, e7704. [Google Scholar] [CrossRef] [PubMed]
- Pallett, L.J.; Burton, A.R.; Amin, O.E.; Rodriguez-Tajes, S.; Patel, A.A.; Zakeri, N.; Jeffery-Smith, A.; Swadling, L.; Schmidt, N.M.; Baiges, A.; et al. Longevity and replenishment of human liver-resident memory T cells and mononuclear phagocytes. J. Exp. Med. 2020, 217, e20200050. [Google Scholar] [CrossRef]
- Zhang, Q.; He, Y.; Luo, N.; Patel, S.J.; Han, Y.; Gao, R.; Modak, M.; Carotta, S.; Haslinger, C.; Kind, D.; et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell 2019, 179, 829–845.e20. [Google Scholar] [CrossRef]
- Payne, K.K.; Mine, J.A.; Biswas, S.; Chaurio, R.A.; Perales-Puchalt, A.; Anadon, C.M.; Costich, T.L.; Harro, C.M.; Walrath, J.; Ming, Q. BTN3A1 governs antitumor responses by coordinating αβ and γδ T cells. Science 2020, 369, 942–949. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, L.; Zhong, Y.; Zhou, K.; Hou, Y.; Wang, Z.; Zhang, Z.; Xie, J.; Wang, C.; Chen, D.; et al. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell 2021, 184, 404–421.e16. [Google Scholar] [CrossRef]
- Song, G.; Shi, Y.; Zhang, M.; Goswami, S.; Afridi, S.; Meng, L.; Ma, J.; Chen, Y.; Lin, Y.; Zhang, J. Global immune characterization of HBV/HCV-related hepatocellular carcinoma identifies macrophage and T-cell subsets associated with disease progression. Cell Discov. 2020, 6, 90. [Google Scholar] [CrossRef]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef]
- Yan, F.; Zhu, B.; Shi, K.; Zhang, Y.; Zeng, X.; Zhang, Q.; Yang, Z.; Wang, X. Prognostic and therapeutic potential of imbalance between PD-1+ CD8 and ICOS+ Treg cells in advanced HBV-HCC. Cancer Sci. 2024, 115, 2553–2564. [Google Scholar] [CrossRef]
- Kaseb, A.O.; Vence, L.; Blando, J.; Yadav, S.S.; Ikoma, N.; Pestana, R.C.; Vauthey, J.N.; Allison, J.P.; Sharma, P. Immunologic correlates of pathologic complete response to preoperative immunotherapy in hepatocellular carcinoma. Cancer Immunol. Res. 2019, 7, 1390–1395. [Google Scholar] [CrossRef]
- Yang, M.; Vanderwert, E.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Li, G. The Important Roles of Natural Killer Cells in Liver Fibrosis. Biomedicines 2023, 11, 1391. [Google Scholar] [CrossRef] [PubMed]
- Hudspeth, K.; Donadon, M.; Cimino, M.; Pontarini, E.; Tentorio, P.; Preti, M.; Hong, M.; Bertoletti, A.; Bicciato, S.; Invernizzi, P.; et al. Human liver-resident CD56bright/CD16neg NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J. Autoimmun. 2016, 66, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Guerra, N.; Fessas, P.; Murphy, R.; Mineo, T.; Mauri, F.A.; Mukherjee, S.K.; Thursz, M.; Wong, C.N.; Sharma, R.; et al. Immune-based therapies for hepatocellular carcinoma. Oncogene 2020, 39, 3620–3637. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.-D.; Ljunggren, H.-G.; La Cava, A.; Van Kaer, L. Organ-specific features of natural killer cells. Nat. Rev. Immunol. 2011, 11, 658–671. [Google Scholar] [CrossRef]
- Kiaei, S.Z.F.; Nouralishahi, A.; Ghasemirad, M.; Barkhordar, M.; Ghaffari, S.; Kheradjoo, H.; Saleh, M.; Mohammadzadehsaliani, S.; Molaeipour, Z. Advances in natural killer cell therapies for breast cancer. Immunol. Cell Biol. 2023, 101, 705–726. [Google Scholar] [CrossRef]
- Poggi, A.; Zocchi, M.R. Natural killer cells and immune-checkpoint inhibitor therapy: Current knowledge and new challenges. Mol. Ther. Oncolytics 2022, 24, 26–42. [Google Scholar] [CrossRef]
- Boneva, E.; Shivarov, V.; Ivanova, M. A Concise Review of the Role of the NKG2D Receptor and Its Ligands in Cancer. Immuno 2025, 5, 9. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.-D.; Sun, L. Recent advances in molecular mechanisms of the NKG2D pathway in hepatocellular carcinoma. Biomolecules 2020, 10, 301. [Google Scholar] [CrossRef]
- Sanchez-Correa, B.; Valhondo, I.; Hassouneh, F.; Lopez-Sejas, N.; Pera, A.; Bergua, J.M.; Arcos, M.J.; Bañas, H.; Casas-Avilés, I.; Durán, E.; et al. DNAM-1 and the TIGIT/PVRIG/TACTILE axis: Novel immune checkpoints for natural killer cell-based cancer immunotherapy. Cancers 2019, 11, 877. [Google Scholar] [CrossRef]
- Klekowski, J.; Zielińska, D.; Hofman, A.; Zajdel, N.; Gajdzis, P.; Chabowski, M. Clinical Significance of Nectins in HCC and Other Solid Malignant Tumors: Implications for Prognosis and New Treatment Opportunities—A Systematic Review. Cancers 2023, 15, 3983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, Y. Targeting NK cell checkpoint receptors or molecules for cancer immunotherapy. Front. Immunol. 2020, 11, 1295. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.K.; Lee, B.C.; Kim, H.-J.; Lee, J.-J.; Chung, I.-J.; Cho, S.B.; Koh, Y.S. A phase I study of locoregional high-dose autologous natural killer cell therapy with hepatic arterial infusion chemotherapy in patients with locally advanced hepatocellular carcinoma. Front. Immunol. 2022, 13, 879452. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef]
- Zhu, Y.-H.; Zheng, J.-H.; Jia, Q.-Y.; Duan, Z.-H.; Yao, H.-F.; Yang, J.; Sun, Y.-W.; Jiang, S.-H.; Liu, D.-J.; Huo, Y.-M. Immunosuppression, immune escape, and immunotherapy in pancreatic cancer: Focused on the tumor microenvironment. Cell. Oncol. 2023, 46, 17–48. [Google Scholar] [CrossRef]
- Groth, C.; Hu, X.; Weber, R.; Fleming, V.; Altevogt, P.; Utikal, J.; Umansky, V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br. J. Cancer 2019, 120, 16–25. [Google Scholar] [CrossRef]
- Bruni, D.; Angell, H.K.; Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef]
- Lee, W.-C.; Wang, Y.-C.; Cheng, C.-H.; Wu, T.-H.; Lee, C.-F.; Wu, T.-J.; Chou, H.-S.; Chan, K.-M. Myeloid-derived suppressor cells in the patients with liver resection for hepatitis B virus-related hepatocellular carcinoma. Sci. Rep. 2019, 9, 2269. [Google Scholar] [CrossRef]
- Vonderlin, J.; Chavakis, T.; Sieweke, M.; Tacke, F. The multifaceted roles of macrophages in NAFLD pathogenesis. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 1311–1324. [Google Scholar] [CrossRef]
- Hashimoto, A.; Sarker, D.; Reebye, V.; Jarvis, S.; Sodergren, M.H.; Kossenkov, A.; Sanseviero, E.; Raulf, N.; Vasara, J.; Andrikakou, P.; et al. Upregulation of C/EBPα inhibits suppressive activity of myeloid cells and potentiates antitumor response in mice and patients with cancer. Clin. Cancer Res. 2021, 27, 5961–5978. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Greten, T.F.; Schwabe, R.; Bardeesy, N.; Ma, L.; Goyal, L.; Kelley, R.K.; Wang, X.W. Immunology and immunotherapy of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, C.M.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The role of tumor microenvironment in cancer metastasis: Molecular mechanisms and therapeutic opportunities. Cancers 2021, 13, 2053. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Bucher, S.; Rosenberg, K.; Tsui, M.; Burhan, D.; Hoffman, D.; Cho, S.-J.; Rangaswami, A.; Breese, M.; Leung, S.; et al. Single-cell analysis of hepatoblastoma identifies tumor signatures that predict chemotherapy susceptibility using patient-specific tumor spheroids. Nat. Commun. 2022, 13, 4878. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, S.; Xu, C.; Liu, B.; Shen, J. A phase Ib/II study of BLU-554, a fibroblast growth factor receptor 4 inhibitor in combination with CS1001, an anti-PD-L1, in patients with locally advanced or metastatic hepatocellular carcinoma. Investig. New Drugs 2023, 41, 162–167. [Google Scholar] [CrossRef]
- Lee, T.K.-W.; Guan, X.-Y.; Ma, S. Cancer stem cells in hepatocellular carcinoma—From origin to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 26–44. [Google Scholar] [CrossRef]
- Lavie, D.; Ben-Shmuel, A.; Erez, N.; Scherz-Shouval, R. Cancer-associated fibroblasts in the single-cell era. Nat. Cancer 2022, 3, 793–807. [Google Scholar] [CrossRef]
- Boissonnas, A.; Combadière, C. Modulating the tumor-associated macrophage landscape. Nat. Immunol. 2022, 23, 481–482. [Google Scholar] [CrossRef]
- Nault, J.-C. Pathogenesis of hepatocellular carcinoma according to aetiology. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 937–947. [Google Scholar] [CrossRef]
- Borgia, M.; Dal Bo, M.; Toffoli, G. Role of virus-related chronic inflammation and mechanisms of cancer immune-suppression in pathogenesis and progression of hepatocellular carcinoma. Cancers 2021, 13, 4387. [Google Scholar] [CrossRef]
- Zarlashat, Y.; Abbas, S.; Ghaffar, A. Hepatocellular carcinoma: Beyond the border of advanced stage therapy. Cancers 2024, 16, 2034. [Google Scholar] [CrossRef]
- D’souza, S.; Lau, K.C.; Coffin, C.S.; Patel, T.R. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 5759. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.G.; Boyd, A.; Combe, E.; Testoni, B.; Zoulim, F. Covalently closed circular DNA: The ultimate therapeutic target for curing HBV infections. J. Hepatol. 2021, 75, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Sitia, G.; Aiolfi, R.; Di Lucia, P.; Mainetti, M.; Fiocchi, A.; Mingozzi, F.; Esposito, A.; Ruggeri, Z.M.; Chisari, F.V.; Iannacone, M.; et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc. Natl. Acad. Sci. USA 2012, 109, E2165–E2172. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-C.; Yeh, C.-M.; Hu, Y.-W.; Chen, C.-C.; Liu, C.-J.; Su, C.-W.; Huo, T.-I.; Huang, Y.-H.; Chao, Y.; Chen, T.-J.; et al. Antiplatelet therapy is associated with a better prognosis for patients with hepatitis B virus-related hepatocellular carcinoma after liver resection. Ann. Surg. Oncol. 2016, 23, 874–883. [Google Scholar] [CrossRef]
- Tan, A.T.; Yang, N.; Krishnamoorthy, T.L.; Oei, V.; Chua, A.; Zhao, X.; Tan, H.S.; Chia, A.; Le Bert, N.; Low, D.; et al. Use of expression profiles of HBV-DNA integrated into genomes of hepatocellular carcinoma cells to select T cells for immunotherapy. Gastroenterology 2019, 156, 1862–1876.e9. [Google Scholar] [CrossRef]
- Fisicaro, P.; Barili, V.; Rossi, M.; Montali, I.; Vecchi, A.; Acerbi, G.; Laccabue, D.; Zecca, A.; Penna, A.; Missale, G.; et al. Pathogenetic mechanisms of T cell dysfunction in chronic HBV infection and related therapeutic approaches. Front. Immunol. 2020, 11, 849. [Google Scholar] [CrossRef]
- Dumolard, L.; Hilleret, M.-N.; Costentin, C.; Mercey-Ressejac, M.; Sturm, N.; Gerster, T.; Decaens, T.; Jouvin-Marche, E.; Marche, P.N.; Macek Jilkova, Z. Differences in the intrahepatic expression of immune checkpoint molecules on T cells and natural killer cells in chronic HBV patients. Front. Immunol. 2025, 15, 1489770. [Google Scholar] [CrossRef]
- Lim, C.J.; Lee, Y.H.; Pan, L.; Lai, L.; Chua, C.; Wasser, M.; Lim, T.K.H.; Yeong, J.; Toh, H.C.; Lee, S.Y.; et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut 2019, 68, 916–927. [Google Scholar] [CrossRef]
- Goto, K.; Roca Suarez, A.A.; Wrensch, F.; Baumert, T.F.; Lupberger, J. Hepatitis C virus and hepatocellular carcinoma: When the host loses its grip. Int. J. Mol. Sci. 2020, 21, 3057. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.; Finn, R.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Han, K.-H.; Harding, J.; Merle, P.; et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann. Oncol. 2019, 30, v874–v875. [Google Scholar] [CrossRef]
- Heim, M.H.; Thimme, R. Innate and adaptive immune responses in HCV infections. J. Hepatol. 2014, 61, S14–S25. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, R.; Tu, Z.; Xu, Y.; Firpi, R.J.; Rosen, H.R.; Liu, C.; Nelson, D.R. An immunomodulatory role for CD4+ CD25+ regulatory T lymphocytes in hepatitis C virus infection. Hepatology 2004, 40, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Abel, M.; Sène, D.; Pol, S.; Bourlière, M.; Poynard, T.; Charlotte, F.; Cacoub, P.; Caillat-Zucman, S. Intrahepatic virus-specific IL-10-producing CD8 T cells prevent liver damage during chronic hepatitis C virus infection. Hepatology 2006, 44, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Hirano, J.; Yoshio, S.; Sakai, Y.; Songling, L.; Suzuki, T.; Itoh, Y.; Zhang, H.; Chen, D.V.; Haga, S.; Oomori, H.; et al. Hepatitis C virus modulates signal peptide peptidase to alter host protein processing. Proc. Natl. Acad. Sci. USA 2021, 118, e2026184118. [Google Scholar] [CrossRef]
- Pallett, L.J.; Gill, U.S.; Quaglia, A.; Sinclair, L.V.; Jover-Cobos, M.; Schurich, A.; Singh, K.P.; Thomas, N.; Das, A.; Chen, A.; et al. Metabolic regulation of hepatitis B immunopathology by myeloid-derived suppressor cells. Nat. Med. 2015, 21, 591–600. [Google Scholar] [CrossRef]
- Cao, D.; Liu, H. Dysregulated cholesterol regulatory genes in hepatocellular carcinoma. Eur. J. Med. Res. 2023, 28, 580. [Google Scholar] [CrossRef]
- Gutierrez-Cuevas, J.; Lucano-Landeros, S.; Lopez-Cifuentes, D.; Santos, A.; Armendariz-Borunda, J. Epidemiologic, genetic, pathogenic, metabolic, epigenetic aspects involved in NASH-HCC: Current therapeutic strategies. Cancers 2022, 15, 23. [Google Scholar] [CrossRef]
- Schmidt, N.M.; Wing, P.A.; Diniz, M.O.; Pallett, L.J.; Swadling, L.; Harris, J.M.; Burton, A.R.; Jeffery-Smith, A.; Zakeri, N.; Amin, O.E.; et al. Targeting human Acyl-CoA: Cholesterol acyltransferase as a dual viral and T cell metabolic checkpoint. Nat. Commun. 2021, 12, 2814. [Google Scholar] [CrossRef]
- Li, X.; Ramadori, P.; Pfister, D.; Seehawer, M.; Zender, L.; Heikenwalder, M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat. Rev. Cancer 2021, 21, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.; Liu, Y.; Lu, L.; Zhang, G.; Zhou, W.; Wu, T.; Wang, L.; Xu, H.; Ji, G. The pathogenesis of HCC driven by NASH and the preventive and therapeutic effects of natural products. Front. Pharmacol. 2022, 13, 944088. [Google Scholar] [CrossRef] [PubMed]
- Beyaz Coşkun, A.; Sağdiçoğlu Celep, A.G. Therapeutic modulation methods of gut microbiota and gut-liver axis. Crit. Rev. Food Sci. Nutr. 2022, 62, 6505–6515. [Google Scholar] [CrossRef]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef]
- Zhu, Q.; Ma, Y.; Liang, J.; Wei, Z.; Li, M.; Zhang, Y.; Liu, M.; He, H.; Qu, C.; Cai, J.; et al. AHR mediates the aflatoxin B1 toxicity associated with hepatocellular carcinoma. Signal Transduct. Target. Ther. 2021, 6, 299. [Google Scholar] [CrossRef]
- Liu, K.; Wang, F.-S.; Xu, R. Neutrophils in liver diseases: Pathogenesis and therapeutic targets. Cell. Mol. Immunol. 2021, 18, 38–44. [Google Scholar] [CrossRef]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
- Geh, D.; Leslie, J.; Rumney, R.; Reeves, H.L.; Bird, T.G.; Mann, D.A. Neutrophils as potential therapeutic targets in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 257–273. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef]
- Malehmir, M.; Pfister, D.; Gallage, S.; Szydlowska, M.; Inverso, D.; Kotsiliti, E.; Leone, V.; Peiseler, M.; Surewaard, B.G.; Rath, D.; et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat. Med. 2019, 25, 641–655. [Google Scholar] [CrossRef]
- Hayashi, T.; Shibata, M.; Oe, S.; Miyagawa, K.; Honma, Y.; Harada, M. Antiplatelet therapy improves the prognosis of patients with hepatocellular carcinoma. Cancers 2020, 12, 3215. [Google Scholar] [CrossRef]
- Sutti, S.; Albano, E. Adaptive immunity: An emerging player in the progression of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 81–92. [Google Scholar] [CrossRef]
- Wolf, M.J.; Adili, A.; Piotrowitz, K.; Abdullah, Z.; Boege, Y.; Stemmer, K.; Ringelhan, M.; Simonavicius, N.; Egger, M.; Wohlleber, D.; et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 2014, 26, 549–564. [Google Scholar] [CrossRef]
- Foerster, F.; Gairing, S.J.; Müller, L.; Galle, P.R. NAFLD-driven HCC: Safety and efficacy of current and emerging treatment options. J. Hepatol. 2022, 76, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Marinović, S.; Lenartić, M.; Mladenić, K.; Šestan, M.; Kavazović, I.; Benić, A.; Krapić, M.; Rindlisbacher, L.; Cokarić Brdovčak, M.; Sparano, C.; et al. NKG2D-mediated detection of metabolically stressed hepatocytes by innate-like T cells is essential for initiation of NASH and fibrosis. Sci. Immunol. 2023, 8, eadd1599. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Galarreta, M.; Bresnahan, E.; Molina-Sánchez, P.; Lindblad, K.E.; Maier, B.; Sia, D.; Puigvehi, M.; Miguela, V.; Casanova-Acebes, M.; Dhainaut, M.; et al. β-catenin activation promotes immune escape and resistance to anti–PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019, 9, 1124–1141. [Google Scholar] [CrossRef] [PubMed]
- Cadoux, M.; Caruso, S.; Pham, S.; Gougelet, A.; Pophillat, C.; Riou, R.; Loesch, R.; Colnot, S.; Nguyen, C.T.; Calderaro, J.; et al. Expression of NKG2D ligands is downregulated by β-catenin signalling and associates with HCC aggressiveness. J. Hepatol. 2021, 74, 1386–1397. [Google Scholar] [CrossRef]
- Moeini, A.; Torrecilla, S.; Tovar, V.; Montironi, C.; Andreu-Oller, C.; Peix, J.; Higuera, M.; Pfister, D.; Ramadori, P.; Pinyol, R.; et al. An immune gene expression signature associated with development of human hepatocellular carcinoma identifies mice that respond to chemopreventive agents. Gastroenterology 2019, 157, 1383–1397.e11. [Google Scholar] [CrossRef]
- Montironi, C.; Castet, F.; Haber, P.K.; Pinyol, R.; Torres-Martin, M.; Torrens, L.; Mesropian, A.; Wang, H.; Puigvehi, M.; Maeda, M.; et al. Inflamed and non-inflamed classes of HCC: A revised immunogenomic classification. Gut 2023, 72, 129–140. [Google Scholar] [CrossRef]
- Deldar Abad Paskeh, M.; Mirzaei, S.; Ashrafizadeh, M.; Zarrabi, A.; Sethi, G. Wnt/β-catenin signaling as a driver of hepatocellular carcinoma progression: An emphasis on molecular pathways. J. Hepatocell. Carcinoma 2021, 8, 1415–1444. [Google Scholar] [CrossRef]
- Xu, C.; Xu, Z.; Zhang, Y.; Evert, M.; Calvisi, D.F.; Chen, X. β-Catenin signaling in hepatocellular carcinoma. J. Clin. Investig. 2022, 132, e154515. [Google Scholar] [CrossRef]
- Strait, A.A.; Wang, X.J. The role of transforming growth factor-beta in immune suppression and chronic inflammation of squamous cell carcinomas. Mol. Carcinog. 2020, 59, 745–753. [Google Scholar] [CrossRef]
- Chan, M.K.-K.; Chan, E.L.-Y.; Ji, Z.Z.; Chan, A.S.-W.; Li, C.; Leung, K.-T.; To, K.-F.; Tang, P.M.-K. Transforming growth factor-β signaling: From tumor microenvironment to anticancer therapy. Explor. Target. Anti-Tumor Ther. 2023, 4, 316. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sanchez, E.; Vaquero, J.; Férnandez-Barrena, M.G.; Lasarte, J.J.; Avila, M.A.; Sarobe, P.; Reig, M.; Calvo, M.; Fabregat, I. The TGF-β pathway: A pharmacological target in hepatocellular carcinoma? Cancers 2021, 13, 3248. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Li, S.; Deng, J.; Li, N.; Yu, F.; Jiang, Z.; Zhang, J.; Shi, X.; Hu, X. The current status and future of PD-L1 in liver cancer. Front. Immunol. 2023, 14, 1323581. [Google Scholar] [CrossRef]
- Wen, W.; Zhang, Y.; Zhang, H.; Chen, Y. Clinical outcomes of PD-1/PD-L1 inhibitors in patients with advanced hepatocellular carcinoma: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2023, 149, 969–978. [Google Scholar] [CrossRef]
- Joerg, V.; Scheiner, B.; Fulgenzi, C.A.; Schönlein, M.; Kocheise, L.; Lohse, A.W.; Huber, S.; Wege, H.; Kaseb, A.; Wang, Y.; et al. Efficacy and safety of atezolizumab/bevacizumab in patients with HCC after prior systemic therapy: A global, observational study. Hepatol. Commun. 2023, 7, e0302. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Kelley, R.K.; Rimassa, L.; Cheng, A.-L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef]
- Lee, H.A.; Lee, S.; Lee, H.L.; Song, J.E.; Lee, D.H.; Han, S.; Shim, J.H.; Kim, B.H.; Choi, J.Y.; Rhim, H.; et al. The efficacy of treatment for hepatocellular carcinoma in elderly patients. J. Liver Cancer 2023, 23, 362–376. [Google Scholar] [CrossRef]
- Yegin, E.G.; Oymaci, E.; Karatay, E.; Coker, A. Progress in surgical and nonsurgical approaches for hepatocellular carcinoma treatment. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 234–256. [Google Scholar] [CrossRef]
- Zhang, L.; Hong, W.; Wang, Z.; Zheng, C.; Liang, B.; Shi, H. Safety and Effectiveness of Transarterial Chemoembolization in Hepatocellular Carcinoma Patients Aged Greater versus Less Than 80 Years. Clin. Interv. Aging 2023, 18, 1883–1892. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, L.; Li, M.; Jiang, P.; Wang, J.; Li, C. Advances in radiotherapy and immunity in hepatocellular carcinoma. J. Transl. Med. 2023, 21, 526. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Gerolami, R.; Caparello, C.; et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J. Hepatol. 2018, 69, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Chan, S.; Minami, T.; Chishina, H.; Aoki, T.; Takita, M.; Hagiwara, S.; Minami, Y.; Ida, H.; et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and Child–Pugh a liver function: A proof-of-concept study. Cancers 2019, 11, 1084. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wang, S.; Xia, L.; Sun, Z.; Chan, K.M.; Bernards, R.; Qin, W.; Chen, J.; Xia, Q.; Jin, H. Hepatocellular carcinoma: Signaling pathways and therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 35. [Google Scholar] [CrossRef]
- Girardi, D.M.; Sousa, L.P.; Miranda, T.A.; Haum, F.N.; Pereira, G.C.; Pereira, A.A. Systemic therapy for advanced hepatocellular carcinoma: Current stand and perspectives. Cancers 2023, 15, 1680. [Google Scholar] [CrossRef]
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970. [Google Scholar] [CrossRef]
- Yau, T.; Zagonel, V.; Santoro, A.; Acosta-Rivera, M.; Choo, S.P.; Matilla, A.; He, A.R.; Cubillo Gracian, A.; El-Khoueiry, A.B.; Sangro, B.; et al. Nivolumab plus cabozantinib with or without ipilimumab for advanced hepatocellular carcinoma: Results from cohort 6 of the CheckMate 040 trial. J. Clin. Oncol. 2023, 41, 1747–1757. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Hsu, C.-H.; Li, D.; Burgoyne, A.M.; Cotter, C.; Badhrinarayanan, S.; Wang, Y.; Yin, A.; Edubilli, T.R.; et al. Tiragolumab in combination with atezolizumab and bevacizumab in patients with unresectable, locally advanced or metastatic hepatocellular carcinoma (MORPHEUS-Liver): A randomised, open-label, phase 1b–2, study. Lancet Oncol. 2025, 26, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Ryoo, B.-Y.; Hsu, C.-H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.P.; Spahn, J.; Liu, B.; Abdullah, H.; et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020, 21, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Erstad, D.J.; Tanabe, K.K. Hepatocellular carcinoma: Early-stage management challenges. J. Hepatocell. Carcinoma 2017, 4, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Schlachterman, A.; Craft, W.W., Jr.; Hilgenfeldt, E.; Mitra, A.; Cabrera, R. Current and future treatments for hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 8478. [Google Scholar] [CrossRef]
- Dufour, J.-F. Current Challenges in Treating Unresectable Hepatocellular Carcinoma. Healthbook TIMES Oncol. Hematol. 2023, 18, 24–27. [Google Scholar]
- Zhang, H.; Zhang, W.; Jiang, L.; Chen, Y. Recent advances in systemic therapy for hepatocellular carcinoma. Biomark. Res. 2022, 10, 3. [Google Scholar] [CrossRef]
- Addissouky, T.A.; Sayed, I.E.T.E.; Ali, M.M.; Wang, Y.; Baz, A.E.; Khalil, A.A.; Elarabany, N. Latest advances in hepatocellular carcinoma management and prevention through advanced technologies. Egypt. Liver J. 2024, 14, 2. [Google Scholar] [CrossRef]

| Trial ID | Treatment | Trial Phase | N of Patients | Targets | Status |
|---|---|---|---|---|---|
| NCT05337137 | Nivolumab and Relatlimab + Bevacizumab | Phase I/II | 162 | PD-1, LAG-3, VEGF | Active |
| NCT03412773 | Tislelizumab vs. Sorafenib | Multiregional phase III | 674 | PD-1 | Completed |
| NCT04826406 | Camrelizumab plus Apatinib | Phase II | 40 | PD-1 receptor | Recruiting |
| NCT06560827 | CT011 CAR-GPC3 T cells injection | Phase I | 30 | CAR T cells | Recruiting |
| NCT06144385 | CAR-GPC3 T cells | Phase I | 20 | CAR T cells | Recruiting |
| NCT06084884 | AZD5851 | Phase I/II | 84 | CAR-T therapy directed against GPC3 | Recruiting |
| NCT05652920 | Ori-C101 Hepatic arterial infusion | Phase I/II | 105 | GPC3-directed chimeric antigen receptor-modified T cells | Recruiting |
| NCT05323201 | fhB7H3.CAR-Ts + Fludarabine + Cyclophosphamide | Phase I/II | 15 | Human B7H3 CAR-T cells | Recruiting |
| NCT05155189 | C-CAR031 + Lenvatinib + PD-1(L1) monoclonal antibody | Phase I | 44 | GPC3 armored CART cell injection (C-CAR031) | Recruiting |
| NCT06001567 | Avatrombopag | Phase II | 30 | Platelets | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarlashat, Y.; Ghaffar, A.; Guerra, F.; Picca, A. Immunological Landscape and Molecular Therapeutic Targets of the Tumor Microenvironment in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2025, 26, 7836. https://doi.org/10.3390/ijms26167836
Zarlashat Y, Ghaffar A, Guerra F, Picca A. Immunological Landscape and Molecular Therapeutic Targets of the Tumor Microenvironment in Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2025; 26(16):7836. https://doi.org/10.3390/ijms26167836
Chicago/Turabian StyleZarlashat, Yusra, Abdul Ghaffar, Flora Guerra, and Anna Picca. 2025. "Immunological Landscape and Molecular Therapeutic Targets of the Tumor Microenvironment in Hepatocellular Carcinoma" International Journal of Molecular Sciences 26, no. 16: 7836. https://doi.org/10.3390/ijms26167836
APA StyleZarlashat, Y., Ghaffar, A., Guerra, F., & Picca, A. (2025). Immunological Landscape and Molecular Therapeutic Targets of the Tumor Microenvironment in Hepatocellular Carcinoma. International Journal of Molecular Sciences, 26(16), 7836. https://doi.org/10.3390/ijms26167836







