Genome-Wide Identification and Expression Analysis of the WRKY Gene Families in Vaccinium bracteatum
Abstract
1. Introduction
2. Results
2.1. Identification of WRKY Families in V. bracteatum
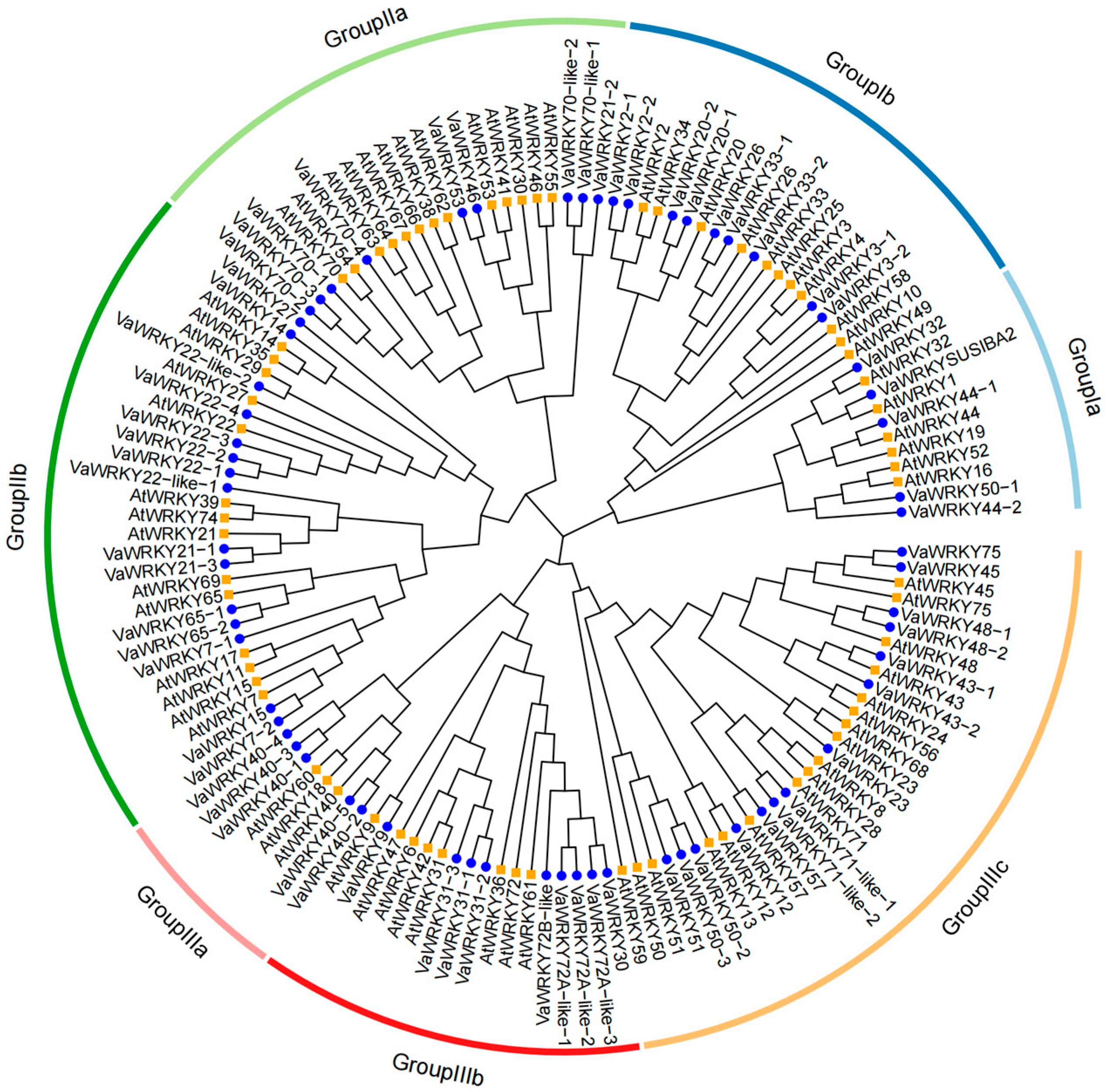
2.2. Phylogenetic Analysis of WRKY Gene Families in V. bracteatum
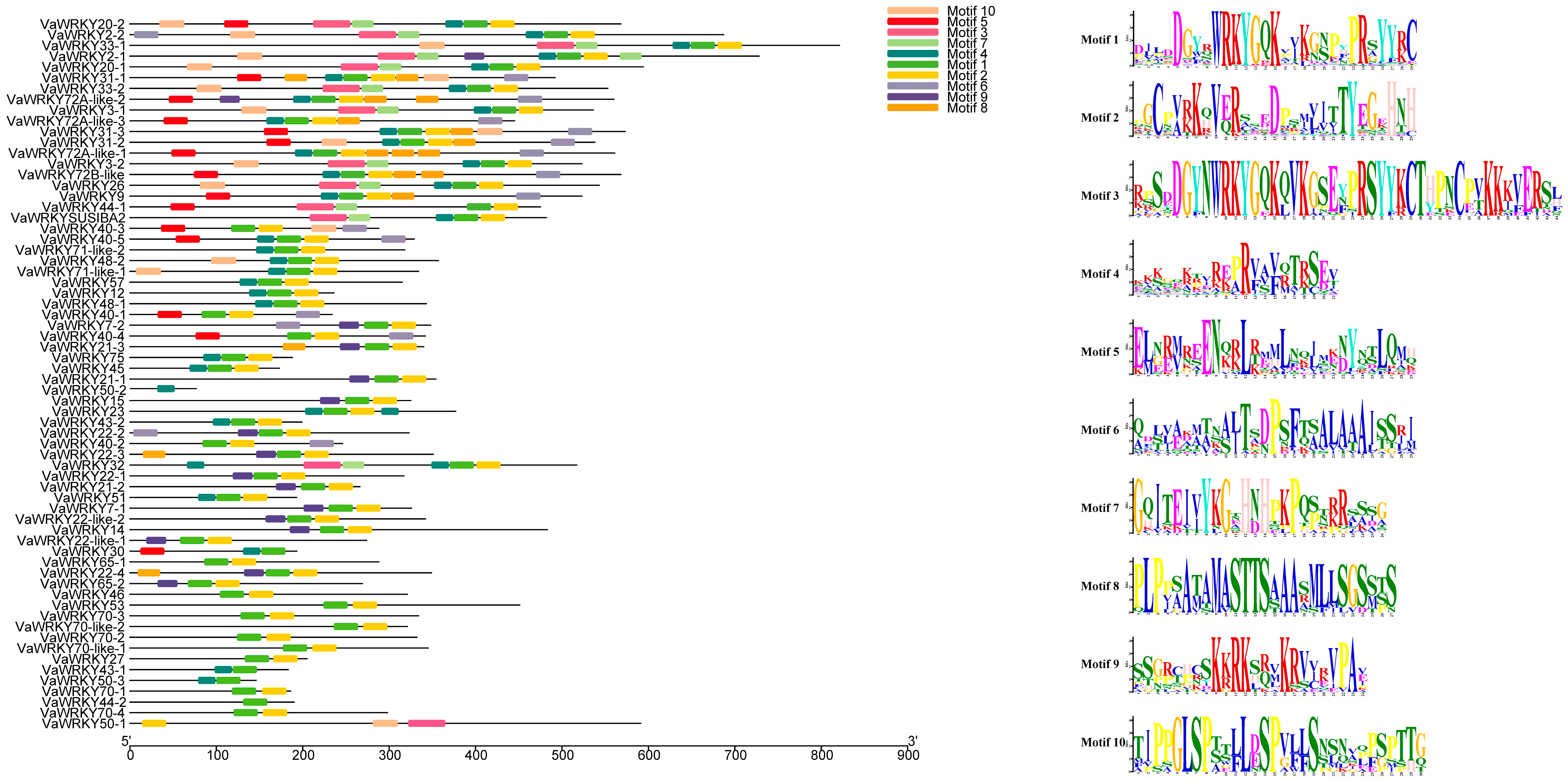
2.3. Gene Structure and Conserved Domain Analysis of WRKY Gene Families in V. bracteatum
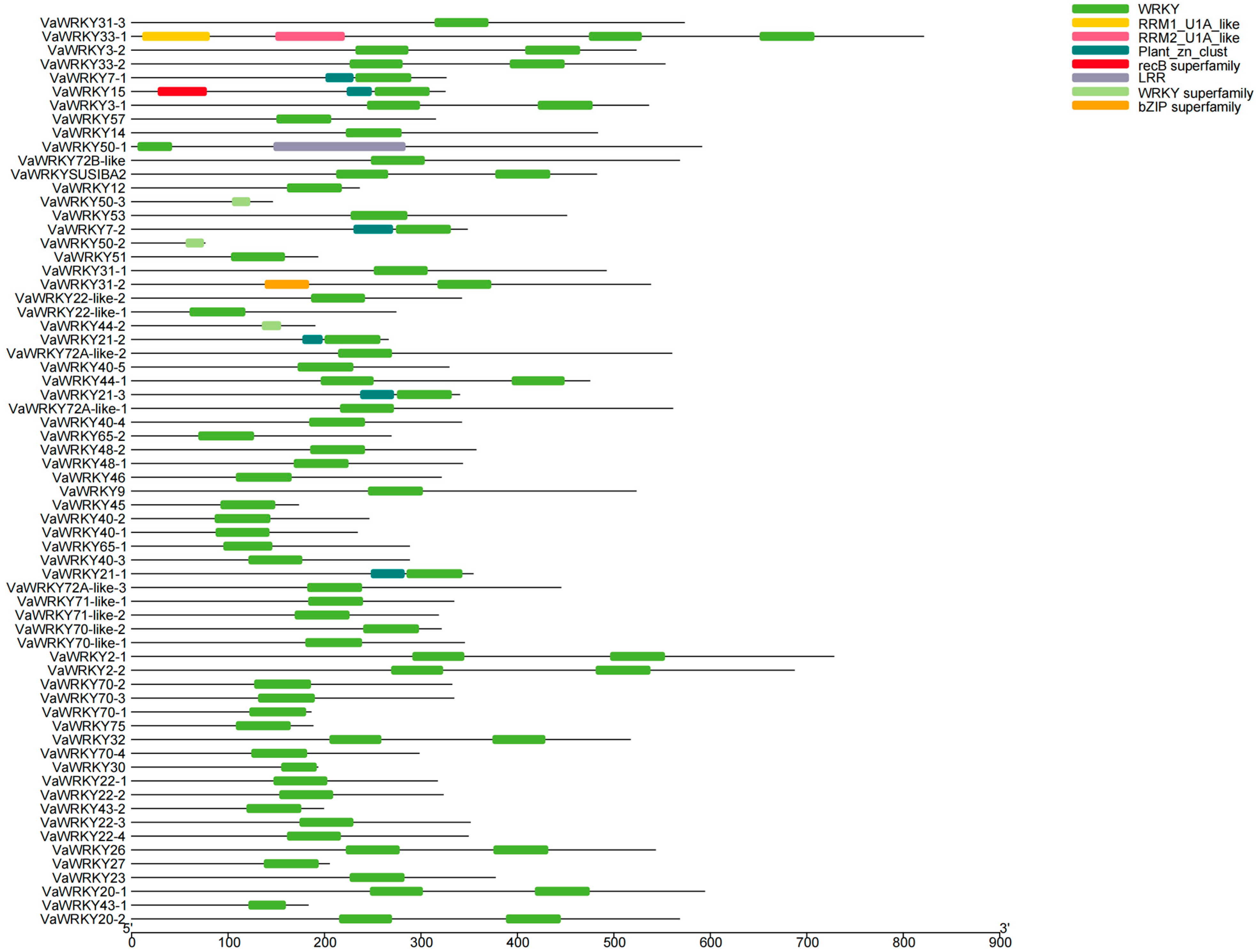
2.4. Domain Analysis of the VaWRKY Gene Families
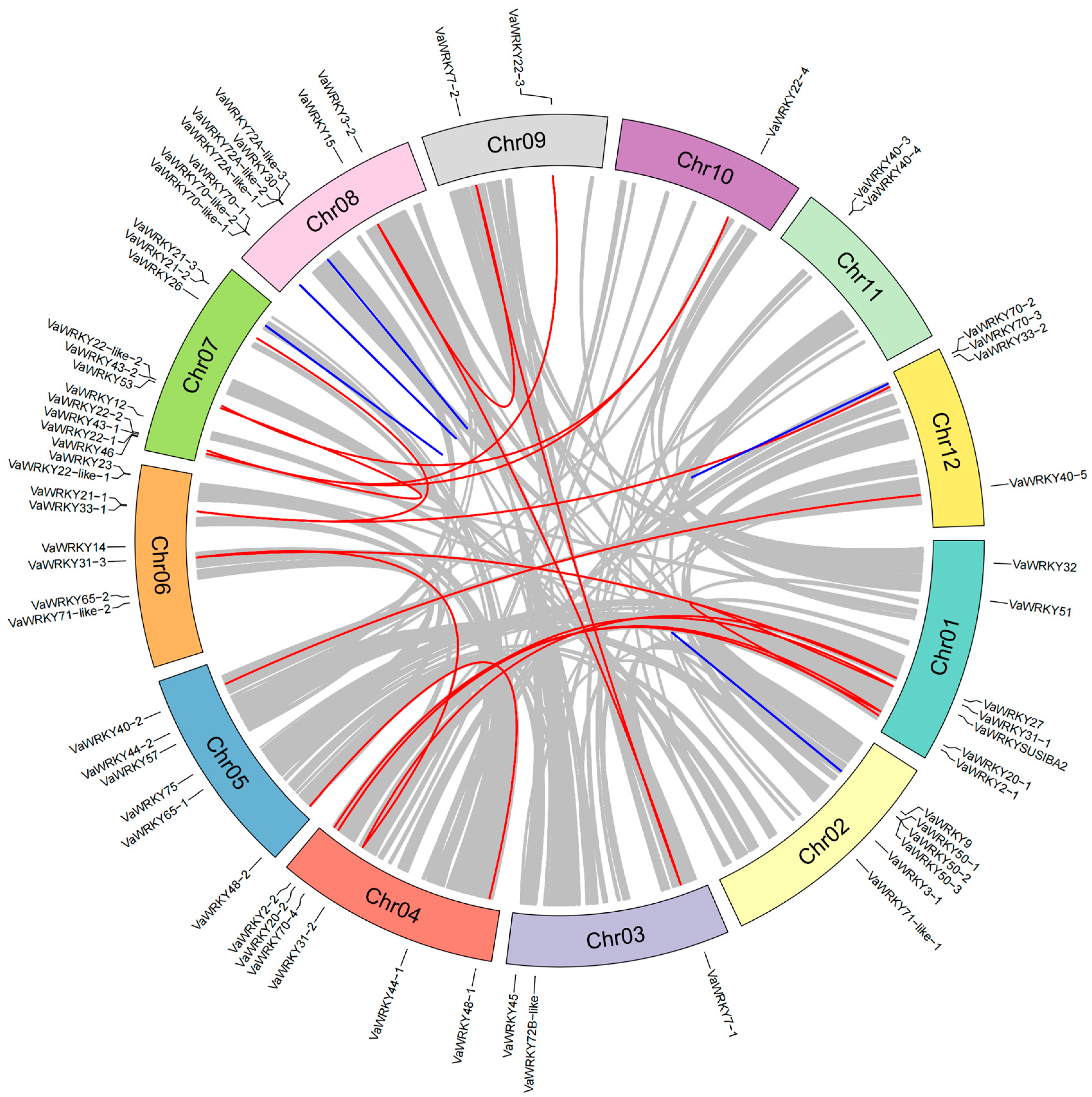
2.5. Chromosome Localization and Collinearity Analysis of the VaWRKY Gene Families
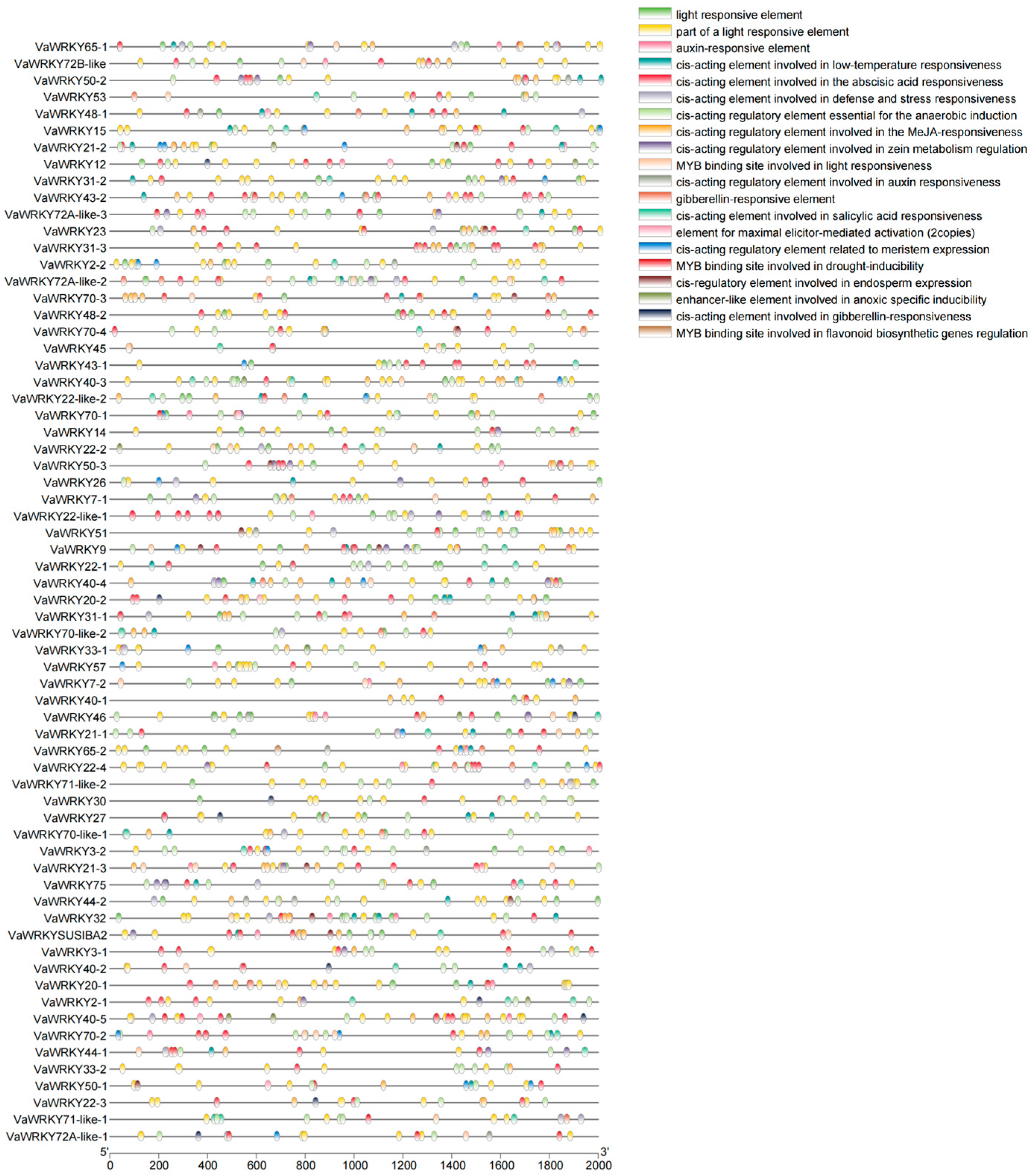
2.6. Promoter and Homology Analysis of the WRKY Gene Families in V. bracteatum
2.7. Expression Pattern Analysis of VaWRKY
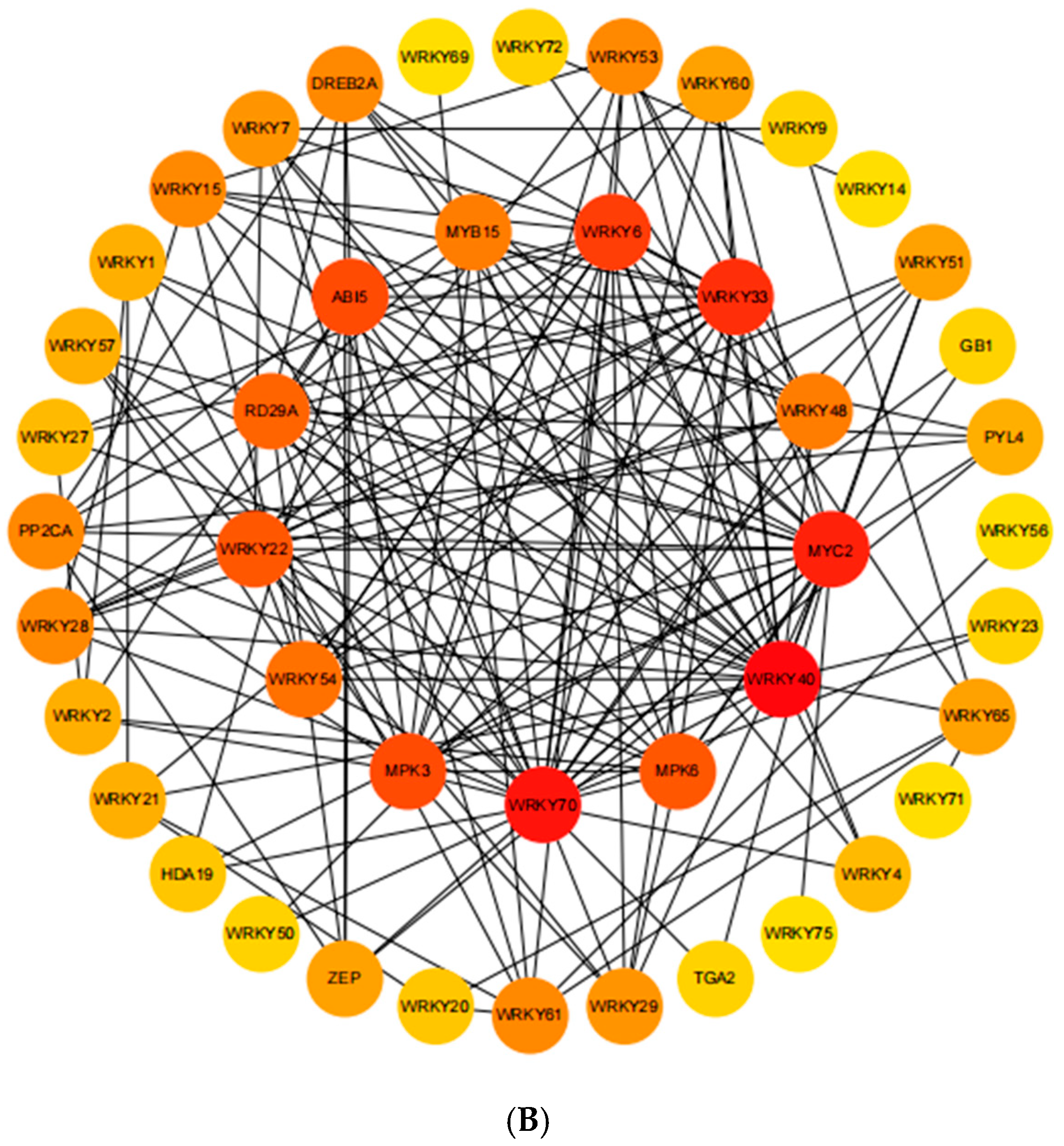
2.8. Analysis of Protein Interaction Networks
2.9. Three-Dimensional Structure Analysis of WRKY Protein in V. bracteatum
3. Discussion
3.1. Analysis of the Evolutionary Trajectory and Structural Characteristics of the VaWRKY Gene Families
3.2. Chromosomal Distribution Pattern and Gene Duplication Mechanism of the VaWRKY Gene Families
3.3. Construction of Cis—Elements in the VaWRKY Gene Promoter and Expression Regulation Network
3.4. Analysis of the VaWRKY Protein Interaction Network and Functional Prediction
4. Materials and Methods
4.1. Plant Resources
4.2. Genome-Wide Identification and Classification of WRKY Gene Families
4.3. The Establishment of the Phylogenetic Tree
4.4. Analysis of Introns, Exons, and Regulatory Sequences
4.5. Analysis of Related Structural Domains
4.6. Visualization and Collinearity Analysis of Chromosomal Positioning
4.7. Analysis of Promoter Sequence
4.8. Expression Patterns Within Different Fruit Stages
4.9. Analysis of Protein–Protein Interaction Expression
4.10. Protein 3D Structure Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NJ | neighbor-joining |
| ABA | abscisic acid |
| MeJA | methyl jasmonate |
| DRE | defective in RNA polymerase II transcription factor D |
| MAPK | mitogen-activated protein kinase |
| MAP | microtubule-associated proteins |
References
- Wang, H.; Chen, W.; Xu, Z.; Chen, M.; Yu, D. Functions of WRKYs in plant growth and development. Trends Plant Sci. 2023, 28, 630–645. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, N.; Zhao, S. Functional characterization of a WRKY family gene involved in somatic embryogenesis in Panax ginseng. Protoplasma 2020, 257, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Puccio, G.; Crucitti, A.; Tiberini, A.; Mauceri, A.; Taglienti, A.; Palumbo Piccionello, A.; Carimi, F.; van Kaauwen, M.; Scholten, O.; Sunseri, F.; et al. WRKY Gene Family Drives Dormancy Release in Onion Bulbs. Cells 2022, 11, 1100. [Google Scholar] [CrossRef]
- Zhu, R.; Gao, N.; Luo, J.; Shi, W. Genome and Transcriptome Analysis of the Torreya grandis WRKY Gene Family during Seed Development. Genes 2024, 15, 267. [Google Scholar] [CrossRef]
- Zhao, N.; He, M.; Li, L.; Cui, S.; Hou, M.; Wang, L.; Mu, G.; Liu, L.; Yang, X. Identification and expression analysis of WRKY gene family under drought stress in peanut (Arachis hypogaea L.). PLoS ONE 2020, 15, e0231396. [Google Scholar] [CrossRef]
- Liu, X.; Li, D.; Zhang, S.; Xu, Y.; Zhang, Z. Genome-wide characterization of the rose (Rosa chinensis) WRKY family and role of RcWRKY41 in gray mold resistance. BMC Plant Biol. 2019, 19, 522. [Google Scholar] [CrossRef]
- Wani, S.H.; Anand, S.; Singh, B.; Bohra, A.; Joshi, R. WRKY transcription factors and plant defense responses: Latest discoveries and future prospects. Plant Cell Rep. 2021, 40, 1071–1085. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, D.; Zeng, B.; Liu, X.; Yang, J.; Gao, W.; Ma, X. Characterization of the WRKY gene family reveals its contribution to the adaptability of almond (Prunus dulcis). PeerJ 2022, 10, e13491. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Krishnamoorthi, S.; Goh, H.; Leong, R.; Sanson, A.C.; Urano, D. Crosstalk between heterotrimeric G protein-coupled signaling pathways and WRKY transcription factors modulating plant responses to suboptimal micronutrient conditions. J. Exp. Bot. 2020, 71, 3227–3239. [Google Scholar] [CrossRef]
- Chen, H.; Shi, Y.; An, L.; Yang, X.; Liu, J.; Dai, Z.; Zhang, Y.; Li, T.; Ahammed, G.J. Overexpression of SlWRKY6 enhances drought tolerance by strengthening antioxidant defense and stomatal closure via ABA signaling in Solanum lycopersicum L. Plant Physiol. Biochem. 2024, 213, 108855. [Google Scholar] [CrossRef]
- Mao, C.; Zhang, J.; Zhang, Y.; Wang, B.; Li, W.; Wang, X.; Huang, L. Genome-wide analysis of the WRKY gene family and their response to low-temperature stress in elephant grass. BMC Genom. 2024, 25, 947. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Z.; Wang, S.; Feng, S.; Wang, R.; Zhu, C.; Zhong, L.; Cheng, Y.; Bao, M.; Zhang, F. DcWRKY33 promotes petal senescence in carnation (Dianthus caryophyllus L.) by activating genes involved in the biosynthesis of ethylene and abscisic acid and accumulation of reactive oxygen species. Plant J. 2023, 113, 698–715. [Google Scholar] [CrossRef]
- Ahmad, Z.; Ramakrishnan, M.; Wang, C.; Rehman, S.; Shahzad, A.; Wei, Q. Unravelling the role of WRKY transcription factors in leaf senescence: Genetic and molecular insights. J. Adv. Res. 2024, 74, 191–206. [Google Scholar] [CrossRef]
- Liu, F.; Dou, T.; Hu, C.; Zhong, Q.; Sheng, O.; Yang, Q.; Deng, G.; He, W.; Gao, H.; Li, C.; et al. WRKY transcription factor MaWRKY49 positively regulates pectate lyase genes during fruit ripening of Musa acuminata. Plant Physiol. Biochem. 2023, 194, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, R.; Su, W.; Sun, T.; Qi, M.; Zhang, X.; Wei, F.; Yu, Z.; Xiao, F.; Yan, L.; et al. A comprehensive analysis of the WRKY family in soybean and functional analysis of GmWRKY164-GmGSL7c in resistance to soybean mosaic virus. BMC Genom. 2024, 25, 620. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Niu, Y.; Zhang, Y.; Wen, G.; Zhao, C.; Jiang, M. Evolution of the WRKY66 Gene Family and Its Mutations Generated by the CRISPR/Cas9 System Increase the Sensitivity to Salt Stress in Arabidopsis. Int. J. Mol. Sci. 2023, 24, 3071. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, J.; Chang, X.; Dong, N.; Chen, B.; Wang, J.; Zha, L.; Gui, S. Genome-wide identification and expression profiling of the WRKY gene family reveals abiotic stress response mechanisms in Platycodon grandiflorus. Int. J. Biol. Macromol. 2024, 257, 128617. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Zhang, N.; Zou, Y.; Hao, Y.; Pan, J.; Liu, Y.; Zhang, W.; Li, B. Genome-wide identification and expression analysis of WRKY gene family members in red clover (Trifolium pratense L.). Front. Plant Sci. 2023, 14, 1289507. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Xu, M.; Wang, Y.; Cheng, S.; Liebrecht, A.; Qian, H.; Zhang, H.; Qi, X. Anti-diabetic activity of Vaccinium bracteatum Thunb. leaves’ polysaccharide in STZ-induced diabetic mice. Int. J. Biol. Macromol. 2013, 61, 317–321. [Google Scholar] [CrossRef]
- Fan, M.; Fan, Y.; Rao, Z.; Li, Y.; Qian, H.; Zhang, H.; Wu, G.; Qi, X.; Wang, L. Comparative investigation on metabolite changes in ‘wu mi’ production by Vaccinium bracteatum Thunb. leaves based on multivariate data analysis using UPLC–QToF–MS. Food Chem. 2019, 286, 146–153. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Chen, J.; Hou, S.; Zhang, Q.; Meng, J.; Zhang, Y.; Du, J.; Wang, C.; Liang, D.; Guo, Y. Genome-Wide Identification and Analysis of the WRKY Gene Family in Asparagus officinalis. Genes 2023, 14, 1704. [Google Scholar] [CrossRef]
- Xiong, R.; Peng, Z.; Zhou, H.; Xue, G.; He, A.; Yao, X.; Weng, W.; Wu, W.; Ma, C.; Bai, Q.; et al. Genome-wide identification, structural characterization and gene expression analysis of the WRKY transcription factor family in pea (Pisum sativum L.). BMC Plant Biol. 2024, 24, 113. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Zhou, X.; Liu, S.; Zhuang, Y. Identification of WRKY gene family and characterization of cold stress-responsive WRKY genes in eggplant. PeerJ 2020, 8, e8777. [Google Scholar] [CrossRef]
- Song, H.; Wang, P.; Lin, J.Y.; Zhao, C.; Bi, Y.; Wang, X. Genome-Wide Identification and Characterization of WRKY Gene Family in Peanut. Front. Plant Sci. 2016, 7, 534. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Chen, C.; Li, C.; Liu, J.; Liu, C.; He, Y. Genome-wide investigation of WRKY gene family in pineapple: Evolution and expression profiles during development and stress. BMC Genom. 2018, 19, 490. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Cushman, S.A.; Wang, S.-C.; Wang, F.; Li, Q.; Liu, H.-L.; Li, Y. Genome-wide investigation of the WRKY transcription factor gene family in weeping forsythia: Expression profile and cold and drought stress responses. Genetica 2023, 151, 153–165. [Google Scholar] [CrossRef]
- Yang, X.; Shuang, Z.; Wendong, G.; Tenghui, W.; Zhenyu, F.; Wang, Y. Genome-wide identification and expression analysis of the WRKY gene family in cabbage (Brassica oleracea var. capitata L.). Biotechnol. Biotechnol. Equip. 2022, 36, 759–772. [Google Scholar] [CrossRef]
- Dou, L.; Zhang, X.; Pang, C.; Song, M.; Wei, H.; Fan, S.; Yu, S. Genome-wide analysis of the WRKY gene family in cotton. Mol. Genet. Genom. 2014, 289, 1103–1121. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Chang, X.; Zhi, Y.; Wang, L.; Xing, G.; Song, W.; Nie, X. Evolution and Identification of the WRKY Gene Family in Quinoa (Chenopodium quinoa). Genes 2019, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Xue, C.; Lin, Y.; Yan, Q.; Chen, J.; Wu, R.; Chen, X.; Yuan, X. Identification and Functional Characterization of WRKY, PHD and MYB Three Salt Stress Responsive Gene Families in Mungbean (Vigna radiata L.). Genes 2023, 14, 463. [Google Scholar] [CrossRef]
- Wei, Y.; Shi, H.; Xia, Z.; Tie, W.; Ding, Z.; Yan, Y.; Wang, W.; Hu, W.; Li, K. Genome-Wide Identification and Expression Analysis of the WRKY Gene Family in Cassava. Front. Plant Sci. 2016, 7, 25. [Google Scholar] [CrossRef]
- van Verk, M.C.; Pappaioannou, D.; Neeleman, L.; Bol, J.F.; Linthorst, H.J. A Novel WRKY transcription factor is required for induction of PR-1a gene expression by salicylic acid and bacterial elicitors. Plant Physiol. 2008, 146, 1983–1995. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Tian, A.G.; Zou, H.F.; Xie, Z.M.; Lei, G.; Huang, J.; Wang, C.M.; Wang, H.W.; Zhang, J.S.; Chen, S.Y. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol. J. 2008, 6, 486–503. [Google Scholar] [CrossRef]
- He, H.; Dong, Q.; Shao, Y.; Jiang, H.; Zhu, S.; Cheng, B.; Xiang, Y. Genome-wide survey and characterization of the WRKY gene family in Populus trichocarpa. Plant Cell Rep. 2012, 31, 1199–1217. [Google Scholar] [CrossRef]
- Wei, K.F.; Chen, J.; Chen, Y.F.; Wu, L.J.; Xie, D.X. Molecular phylogenetic and expression analysis of the complete WRKY transcription factor family in maize. DNA Res. 2012, 19, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Mackay, J.P.; Crossley, M. Zinc fingers are sticking together. Trends Biochem. Sci. 1998, 23, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vision, T.J.; Brown, D.G.; Tanksley, S.D. The origins of genomic duplications in Arabidopsis. Science 2000, 290, 2114–2117. [Google Scholar] [CrossRef]
- Mao, H.; Yu, L.; Li, Z.; Liu, H.; Han, R. Molecular evolution and gene expression differences within the HD-Zip transcription factor family of Zea mays L. Genetica 2016, 144, 243–257. [Google Scholar] [CrossRef]
- Gao, X.; Liu, X.; Zhang, H.; Cheng, L.; Wang, X.; Zhen, C.; Du, H.; Chen, Y.; Yu, H.; Zhu, B.; et al. Genome-Wide Identification, Expression, and Interaction Analysis of the Auxin Response Factor and AUX/IAA Gene Families in Vaccinium bracteatum. Int. J. Mol. Sci. 2024, 25, 8385. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Park, Y.H.; Kwon, S.I.; Yun, C.; Ahn, I.; Park, S.R.; Hwang, D.J. Identification of AtWRKY75 as a transcriptional regulator in the defense response to Pcc through the screening of Arabidopsis activation-tagged lines. PLANT Biotechnol. Rep. 2014, 8, 183–192. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Yang, Y.; Wang, Y.; Mo, Y.; Zhang, R.; Zhang, Y.; Ma, J.; Wei, C.; Zhang, X. Identification and expression analyses of WRKY genes reveal their involvement in growth and abiotic stress response in watermelon (Citrullus lanatus). PLoS ONE 2018, 13, e0191308. [Google Scholar] [CrossRef]
- Asif, M.H.; Lakhwani, D.; Pathak, S.; Gupta, P.; Bag, S.K.; Nath, P.; Trivedi, P.K. Transcriptome analysis of ripe and unripe fruit tissue of banana identifies major metabolic networks involved in fruit ripening process. BMC Plant Biol. 2014, 14, 316. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.; Zheng, Z.; Fan, B.; Yu, J.Q.; Chen, Z. Characterization of the promoter and extended C-terminal domain of Arabidopsis WRKY33 and functional analysis of tomato WRKY33 homologues in plant stress responses. J. Exp. Bot. 2015, 66, 4567–4583. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, M.; Shen, M.; Bu, S.; Zhu, B.; He, F.; Zhang, X.; Gao, X.; Xiao, J. Chromosome-level genome assembly and annotation of the native Chinese wild blueberry Vaccinium bracteatum. Fruit Res. 2022, 2, 8. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Zhou, J.; Liang, X.; Chen, Y.; Liu, X.; Zhen, C.; Zhang, H.; Xiao, J.; Gao, X. Genome-Wide Identification and Expression Analysis of the WRKY Gene Families in Vaccinium bracteatum. Int. J. Mol. Sci. 2025, 26, 7835. https://doi.org/10.3390/ijms26167835
Du H, Zhou J, Liang X, Chen Y, Liu X, Zhen C, Zhang H, Xiao J, Gao X. Genome-Wide Identification and Expression Analysis of the WRKY Gene Families in Vaccinium bracteatum. International Journal of Molecular Sciences. 2025; 26(16):7835. https://doi.org/10.3390/ijms26167835
Chicago/Turabian StyleDu, Haijing, Jianqiang Zhou, Xiaoran Liang, Yufei Chen, Xiaohui Liu, Cheng Zhen, Hong Zhang, Jiaxin Xiao, and Xuan Gao. 2025. "Genome-Wide Identification and Expression Analysis of the WRKY Gene Families in Vaccinium bracteatum" International Journal of Molecular Sciences 26, no. 16: 7835. https://doi.org/10.3390/ijms26167835
APA StyleDu, H., Zhou, J., Liang, X., Chen, Y., Liu, X., Zhen, C., Zhang, H., Xiao, J., & Gao, X. (2025). Genome-Wide Identification and Expression Analysis of the WRKY Gene Families in Vaccinium bracteatum. International Journal of Molecular Sciences, 26(16), 7835. https://doi.org/10.3390/ijms26167835





