Decoding Pancreatic Neuroendocrine Tumors: Molecular Profiles, Biomarkers, and Pathways to Personalized Therapy
Abstract
1. Introduction
2. Molecular Landscape and Evolving Insights into Pancreatic Neuroendocrine Tumors
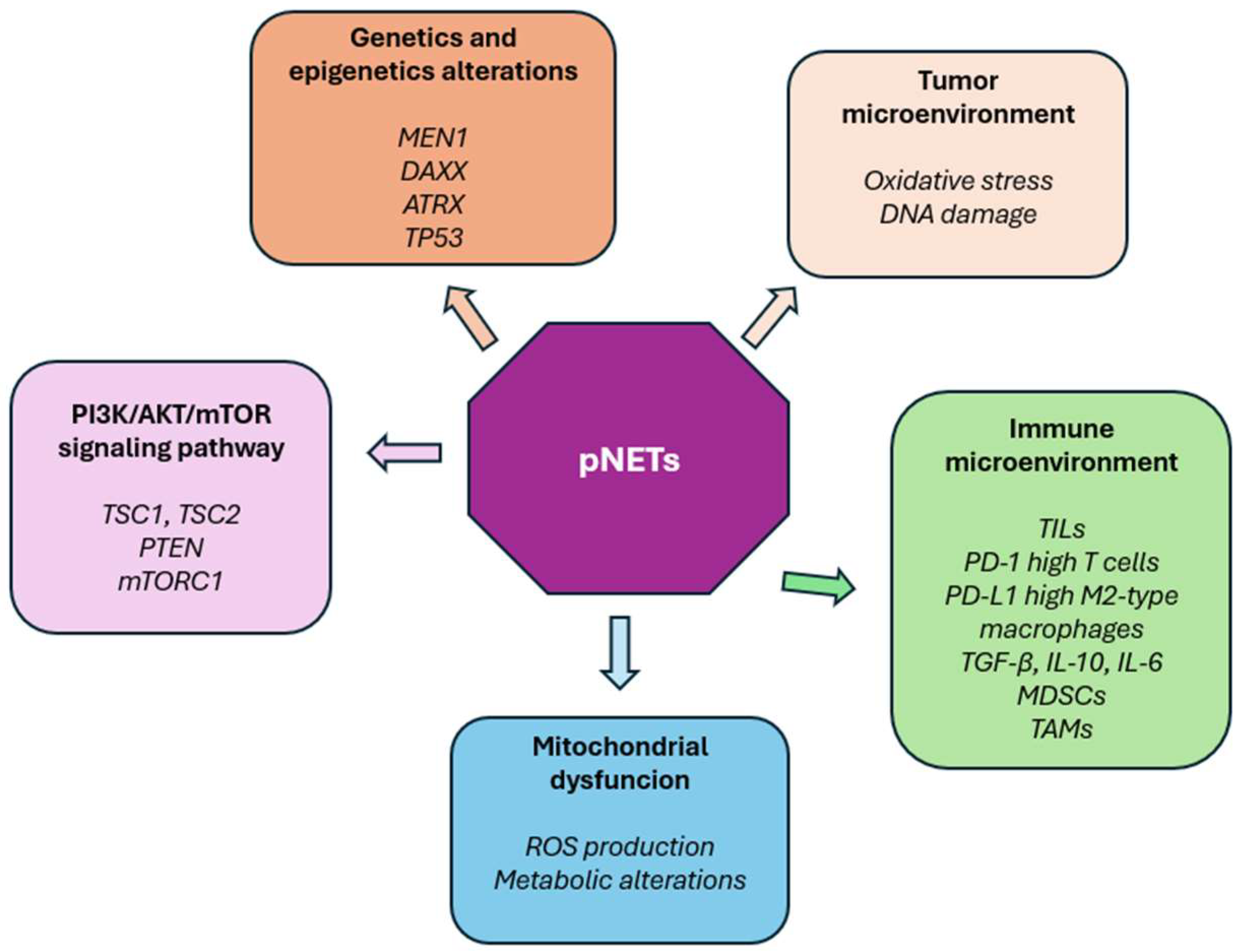
2.1. Genomic and Epigenetic Alterations: Key Drivers of pNETs Pathogenesis
2.2. From Genomics to Multi-Omics: Unraveling Complexity and Therapeutic Opportunities in pNETs
2.3. The Tumor Microenvironment in pNETs: Driver of Progression, Immune Evasion and Therapeutic Resistance
3. Old and New Biomarkers in pNETs: An Integrated Diagnostic Approach
3.1. Established Markers: Chromogranin A, Ki-67 Index
3.1.1. Chromogranin A (CgA) in pNETs: Clinical Utility and Diagnostic Limitations
3.1.2. Ki-67 in pNETs: A Valuable but Imperfect Grading Tool
3.2. Emerging Molecular Biomarkers: Circulating Tumor DNA, microRNAs
3.2.1. Circulating Tumor DNA in pNETs: Emerging Insights and Challenges for Clinical Implementation
3.2.2. MiRNAs in pNETs: Promising Biomarkers with Emerging but Limited Clinical Validation
3.3. Somatostatin Receptor 2 (SSTR2) in pNETs: Established Clinical Biomarker with Recognized Strengths and Ongoing Challenges
4. Therapeutic Implications and Personalized Approaches of pNETs
4.1. Somatostatin Analogs (SSAs): Established Foundations and Limitations
4.2. Peptide Receptor Radionuclide Therapy (PRRT): A Paradigm Shift with Strategic Challanges
4.3. Targeted Agents and Molecular Pathways: Opportunities and Obstacles
4.4. Immunotherapy: Emerging Concepts and Clinical Challenges
5. The Microbiome as a Gateway to Precision Medicine in Neuroendocrine Tumors
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Halfdanarson, T.R.; Rabe, K.G. Pancreatic neuroendocrine tumors (PNETs): Incidence, prognosis and recent trend toward improved survival. Ann. Oncol. 2008, 19, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Kos-Kudła, B.; Castaño, J. European Neuroendocrine Tumour Society (ENETS) 2023 guidance paper for nonfunctioning pancreatic neuroendocrine tumours. J. Neuroendocrinol. 2023, 35, e13343. [Google Scholar] [CrossRef]
- Leoncini, E.; Carioli, G. Risk factors for neuroendocrine neoplasms: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Luchini, C.; Pantanowitz, L. Ki-67 assessment of pancreatic neuroendocrine neoplasms: Systematic review and meta-analysis of manual vs. digital pathology scoring. Mod. Pathol. 2022, 35, 712–720. [Google Scholar] [CrossRef]
- Zhao, C.L.; Dabiri, B. Improving fine needle aspiration to predict the tumor biological aggressiveness in pancreatic neuroendocrine tumors using Ki-67 proliferation index, phosphorylated histone H3 (PHH3), and BCL-2. Ann. Diagn. Pathol. 2023, 65, 152149. [Google Scholar] [CrossRef]
- Cortegoso Valdivia, P.; Rizza, S. Ki-67 assessment in pancreatic neuroendocrine tumors: Is EUS-FNA still a valid ally? Pancreatology 2021, 21, 496–497. [Google Scholar] [CrossRef]
- Scarpa, A.; Chang, D.K. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature 2017, 543, 65–71, Erratum in Nature 2017, 550, 548. [Google Scholar] [CrossRef] [PubMed]
- Uccella, S. Molecular Classification of Gastrointestinal and Pancreatic Neuroendocrine Neoplasms: Are We Ready for That? Endocr. Pathol. 2024, 35, 91–106. [Google Scholar] [CrossRef]
- Maluchenko, A.; Maksimov, D. Molecular Basis of Pancreatic Neuroendocrine Tumors. Int. J. Mol. Sci. 2024, 25, 11017. [Google Scholar] [CrossRef]
- Hofland, J.; Falconi, M. European Neuroendocrine Tumor Society 2023 guidance paper for functioning pancreatic neuroendocrine tumour syndromes. J. Neuroendocrinol. 2023, 35, e13318. [Google Scholar] [CrossRef]
- Shen, X.; Wang, X. Molecular biology of pancreatic neuroendocrine tumors: From mechanism to translation. Front. Oncol. 2022, 12, 967071. [Google Scholar] [CrossRef]
- Majer, A.D.; Hua, X. Menin in Cancer. Genes 2024, 15, 1231. [Google Scholar] [CrossRef]
- Amorim, J.P.; Santos, G. The Role of ATRX in the Alternative Lengthening of Telomeres (ALT) Phenotype. Genes 2016, 7, 66. [Google Scholar] [CrossRef]
- Marinoni, I. Prognostic value of DAXX/ATRX loss of expression and ALT activation in PanNETs: Is it time for clinical implementation? Gut 2022, 71, 847–848. [Google Scholar] [CrossRef]
- Lakis, V.; Lawlor, R.T. DNA methylation patterns identify subgroups of pancreatic neuroendocrine tumors with clinical association. Commun. Biol. 2021, 4, 155. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Pelle’, E. The Tumor Microenvironment in Neuroendocrine Tumors: Biology and Therapeutic Implications. Neuroendocrinology 2019, 109, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Saleh, Z.; Moccia, M.C. Pancreatic Neuroendocrine Tumors: Signaling Pathways and Epigenetic Regulation. Int. J. Mol. Sci. 2024, 25, 1331. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.; Blenkiron, C. Recurrent loss of heterozygosity correlates with clinical outcome in pancreatic neuroendocrine cancer. NPJ Genom. Med. 2018, 3, 18. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.J. Malignant potential of neuroendocrine microtumor of the pancreas harboring high-grade transformation: Lesson learned from a patient with von Hippel-Lindau syndrome. J. Pathol. Transl. Med. 2024, 58, 91–97. [Google Scholar] [CrossRef]
- Zhou, L.; Baba, Y. KRAS, BRAF, and PIK3CA mutations, and patient prognosis in 126 pancreatic cancers: Pyrosequencing technology and literature review. Med. Oncol. 2016, 33, 32. [Google Scholar] [CrossRef]
- Joseph, N.M.; Umetsu, S.E. Progression of Low-Grade Neuroendocrine Tumors (NET) to High-Grade Neoplasms Harboring the NEC-Like Co-alteration of RB1 and TP53. Endocr. Pathol. 2024, 35, 325–337. [Google Scholar] [CrossRef]
- Missiaglia, E.; Dalai, I. Pancreatic endocrine tumors: Expression profiling evidences a role for AKT-mTOR pathway. J. Clin. Oncol. 2010, 28, 245–255. [Google Scholar] [CrossRef]
- Ito, T.; Okusaka, T. Everolimus for advanced pancreatic neuroendocrine tumours: A subgroup analysis evaluating Japanese patients in the RADIANT-3 trial. Jpn. J. Clin. Oncol. 2012, 42, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Martins Peçanha, F.L.; Jaafar, R. The Transcription Factor YY1 Is Essential for Normal DNA Repair and Cell Cycle in Human and Mouse β-Cells. Diabetes 2022, 71, 1694–1705. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.S.; Laddha, S.V. ATRX, DAXX or MEN1 mutant pancreatic neuroendocrine tumors are a distinct alpha-cell signature subgroup. Nat. Commun. 2018, 9, 4158. [Google Scholar] [CrossRef]
- Sadanandam, A.; Wullschleger, S. A Cross-Species Analysis in Pancreatic Neuroendocrine Tumors Reveals Molecular Subtypes with Distinctive Clinical, Metastatic, Developmental, and Metabolic Characteristics. Cancer Discov. 2015, 5, 1296–1313. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Kalloger, S.E. Proteotranscriptomic classification and characterization of pancreatic neuroendocrine neoplasms. Cell Rep. 2021, 37, 109817. [Google Scholar] [CrossRef]
- Cejas, P.; Drier, Y. Enhancer signatures stratify and predict outcomes of non-functional pancreatic neuroendocrine tumors. Nat. Med. 2019, 25, 1260–1265, Erratum in Nat. Med. 2019, 25, 1627. [Google Scholar] [CrossRef]
- Boons, G.; Vandamme, T. PDX1 DNA Methylation Distinguishes Two Subtypes of Pancreatic Neuroendocrine Neoplasms with a Different Prognosis. Cancers 2020, 12, 1461. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, A.; Pipinikas, C.P. Epigenetic landscape of pancreatic neuroendocrine tumours reveals distinct cells of origin and means of tumour progression. Commun. Biol. 2020, 3, 740. [Google Scholar] [CrossRef]
- Cros, J.; Hentic, O. MGMT expression predicts response to temozolomide in pancreatic neuroendocrine tumors. Endocr. Relat. Cancer 2016, 23, 625–633. [Google Scholar] [CrossRef]
- Melone, V.; Salvati, A. Identification of functional pathways and molecular signatures in neuroendocrine neoplasms by multi-omics analysis. J. Transl. Med. 2022, 20, 306. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S. Single-cell RNA sequencing reveals spatiotemporal heterogeneity and malignant progression in pancreatic neuroendocrine tumor. Int. J. Biol. Sci. 2021, 17, 3760–3775. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, S.E.; Dowrey, T.W. Intertumoral lineage diversity and immunosuppressive transcriptional programs in well-differentiated gastroenteropancreatic neuroendocrine tumors. Sci. Adv. 2023, 9, eadd9668. [Google Scholar] [CrossRef]
- Cui, Y.; Yuan, Q. Determination and characterization of molecular heterogeneity and precision medicine strategies of patients with pancreatic cancer and pancreatic neuroendocrine tumor based on oxidative stress and mitochondrial dysfunction-related genes. Front. Endocrinol. 2023, 14, 1127441. [Google Scholar] [CrossRef]
- Li, K.; Deng, Z. The Role of Oxidative Stress in Tumorigenesis and Progression. Cells 2024, 13, 441. [Google Scholar] [CrossRef]
- Pipinikas, C.P.; Berner, A.M. The evolving (epi)genetic landscape of pancreatic neuroendocrine tumours. Endocr. Relat. Cancer 2019, 26, R519–R544. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz López, K.G.; Toledo Guzmán, M.E. mTORC1 as a Regulator of Mitochondrial Functions and a Therapeutic Target in Cancer. Front. Oncol. 2019, 9, 1373. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Singh, A. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef]
- Jannin, A.; Dessein, A.F. Metabolism of pancreatic neuroendocrine tumors: What can omics tell us? Front. Endocrinol. 2023, 14, 1248575. [Google Scholar] [CrossRef]
- Kuo, C.L.; Lin, Y.C. The mitochondrial stress signaling tunes immunity from a view of systemic tumor microenvironment and ecosystem. iScience 2024, 27, 110710. [Google Scholar] [CrossRef]
- Lyu, Y.; Wang, T. Mitochondrial Damage-Associated Molecular Patterns and Metabolism in the Regulation of Innate Immunity. J. Innate Immun. 2023, 15, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Guo, N. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm. Sin. B 2020, 10, 2156–2170. [Google Scholar] [CrossRef]
- Yellapragada, S.V.; Forsythe, S.D. The Role of the Tumor Microenvironment in Gastroenteropancreatic Neuroendocrine Tumors. Int. J. Mol. Sci. 2025, 26, 5635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Wang, W.Q. The tumor immune microenvironment in gastroenteropancreatic neuroendocrine neoplasms. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 188311. [Google Scholar] [CrossRef] [PubMed]
- Egal, E.S.A.; Jacenik, D. Translational challenges in pancreatic neuroendocrine tumor immunotherapy. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188640. [Google Scholar] [CrossRef] [PubMed]
- Neves, F.R.; Martins, A.L. Characterization of the Pancreatic Neuroendocrine Neoplasm Immune Microenvironment. Cancer Med. 2025, 14, e70798. [Google Scholar] [CrossRef]
- Jacenik, D.; Lebish, E.J. MK2 Promotes the Development and Progression of Pancreatic Neuroendocrine Tumors Mediated by Macrophages and Metabolomic Factors. Int. J. Mol. Sci. 2022, 23, 13561. [Google Scholar] [CrossRef]
- Takahashi, D.; Kojima, M. Profiling the Tumour Immune Microenvironment in Pancreatic Neuroendocrine Neoplasms with Multispectral Imaging Indicates Distinct Subpopulation Characteristics Concordant with WHO 2017 Classification. Sci. Rep. 2018, 8, 13166. [Google Scholar] [CrossRef]
- Greenberg, J.; Limberg, J. Metastatic pancreatic neuroendocrine tumors feature elevated T cell infiltration. JCI Insight 2022, 7, e160130. [Google Scholar] [CrossRef]
- Meng, D.; Zhao, L. Identification of the Immune Subtypes for the Prediction of Metastasis in Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2023, 113, 719–735. [Google Scholar] [CrossRef]
- Deftos, L.J. Chromogranin A: Its role in endocrine function and as an endocrine and neuroendocrine tumor marker. Endocr. Rev. 1991, 12, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Helle, K.B.; Metz-Boutigue, M.H. Chromogranins: From discovery to current times. Pflug. Arch. 2018, 470, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Helle, K.B.; Corti, A. The endocrine role for chromogranin A: A prohormone for peptides with regulatory properties. Cell Mol. Life Sci. 2007, 64, 2863–2886. [Google Scholar] [CrossRef] [PubMed]
- Oberg, K.; Modlin, I.M. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015, 16, e435–e446. [Google Scholar] [CrossRef]
- Tseng, C.M.; Cheng, T.Y. Low accuracy of chromogranin A for diagnosing early-stage pancreatic neuroendocrine tumors. Oncol. Lett. 2018, 15, 8951–8958. [Google Scholar] [CrossRef]
- Modlin, I.M.; Drozdov, I. A multianalyte PCR blood test outperforms single analyte ELISAs (chromogranin A, pancreastatin, neurokinin A) for neuroendocrine tumor detection. Endocr. Relat. Cancer 2014, 21, 615–628. [Google Scholar] [CrossRef]
- Pregun, I.; Herszényi, L. Effect of proton-pump inhibitor therapy on serum chromogranin a level. Digestion 2011, 84, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Baudin, E.; Bidart, J.M. Impact of chromogranin A measurement in the work-up of neuroendocrine tumors. Ann. Oncol. 2001, 12 (Suppl. 2), S79–S82. [Google Scholar] [CrossRef]
- van Balveren, J.A.; Erdem-Eraslan, L. Awareness of drug laboratory test interactions is important for prevention of unnecessary additional diagnostics: An example. Clin. Chim. Acta 2022, 530, 99–103. [Google Scholar] [CrossRef]
- Massironi, S.; Rossi, R.E. Chromogranin A in diagnosing and monitoring patients with gastroenteropancreatic neuroendocrine neoplasms: A large series from a single institution. Neuroendocrinology 2014, 100, 240–249. [Google Scholar] [CrossRef]
- Tsai, H.J.; Hsiao, C.F. The Prognostic and Predictive Role of Chromogranin A in Gastroenteropancreatic Neuroendocrine Tumors—A Single-Center Experience. Front. Oncol. 2021, 11, 741096. [Google Scholar] [CrossRef]
- Rogowski, W.; Wachuła, E. Baseline chromogranin A and its dynamics are prognostic markers in gastroenteropancreatic neuroendocrine tumors. Future Oncol. 2017, 13, 1069–1079. [Google Scholar] [CrossRef]
- Yao, J.C.; Pavel, M. Chromogranin A and neuron-specific enolase as prognostic markers in patients with advanced pNET treated with everolimus. J. Clin. Endocrinol. Metab. 2011, 96, 3741–3749. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M. CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Huizing, D.M.V.; Aalbersberg, E.A. Early response assessment and prediction of overall survival after peptide receptor radionuclide therapy. Cancer Imaging 2020, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Welin, S. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann. Oncol. 2013, 24, 152–160. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, D.; Grossman, A. Consensus Conference participants; European Neuroendocrine Tumor Society. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Biochemical markers. Neuroendocrinology 2009, 90, 194–202. [Google Scholar] [CrossRef]
- Rindi, G.; Klöppel, G. Frascati Consensus Conference participants; European Neuroendocrine Tumor Society (ENETS). TNM staging of foregut (neuro)endocrine tumors: A consensus proposal including a grading system. Virchows Arch. 2006, 449, 395–401. [Google Scholar] [CrossRef]
- Rindi, G.; Klimstra, D.S. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Basturk, O.; Yang, Z. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am. J. Surg. Pathol. 2015, 39, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Kroneman, T.N.; Voss, J.S. Comparison of Three Ki-67 Index Quantification Methods and Clinical Significance in Pancreatic Neuroendocrine Tumors. Endocr. Pathol. 2015, 26, 255–262. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Rinke, A. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms. Systemic Therapy 2, Chemotherapy. Neuroendocrinology 2017, 105, 281–294. [Google Scholar] [CrossRef]
- Partelli, S.; Muffatti, F. The size of well differentiated pancreatic neuroendocrine tumors correlates with Ki67 proliferative index and is not associated with age. Dig. Liver Dis. 2019, 51, 735–740. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Fine, R.L. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011, 117, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; O’Toole, D. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef]
- Klöppel, G.; La Rosa, S. Ki67 labeling index: Assessment and prognostic role in gastroenteropancreatic neuroendocrine neoplasms. Virchows Arch. 2018, 472, 341–349, Erratum in Virchows Arch. 2018, 472, 515. [Google Scholar] [CrossRef]
- Lea, D.; Gudlaugsson, E.G. Digital Image Analysis of the Proliferation Markers Ki67 and Phosphohistone H3 in Gastroenteropancreatic Neuroendocrine Neoplasms: Accuracy of Grading Compared With Routine Manual Hot Spot Evaluation of the Ki67 Index. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 499–505. [Google Scholar] [CrossRef]
- Vélayoudom-Céphise, F.L.; Duvillard, P.; Foucan, L. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr. Relat. Cancer 2013, 20, 649–657. [Google Scholar] [CrossRef]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Mouliere, F.; Chandrananda, D. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci. Transl. Med. 2018, 10, eaat4921. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Shi, C. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011, 331, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Cowzer, D.; Shah, R.H. Clinical utility of plasma cell-free DNA in pancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer 2024, 31, e230292. [Google Scholar] [CrossRef]
- Lamarca, A.; Frizziero, M. Molecular Profiling of Well-Differentiated Neuroendocrine Tumours: The Role of ctDNA in Real-World Practice. Cancers 2022, 14, 1017. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, I.; Campolo, F. Comparative Targeted Genome Profiling between Solid and Liquid Biopsies in Gastroenteropancreatic Neuroendocrine Neoplasms: A Proof-of-Concept Pilot Study. Neuroendocrinology 2024, 115, 422–433. [Google Scholar] [CrossRef]
- Dang, D.K.; Park, B.H. Circulating tumor DNA: Current challenges for clinical utility. J. Clin. Investig. 2022, 132, e154941. [Google Scholar] [CrossRef]
- Roldo, C.; Missiaglia, E. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J. Clin. Oncol. 2006, 24, 4677–4684. [Google Scholar] [CrossRef]
- Matyasovska, N.; Valkova, N. Deep sequencing reveals distinct microRNA-mRNA signatures that differentiate pancreatic neuroendocrine tumor from non-diseased pancreas tissue. BMC Cancer 2025, 25, 669. [Google Scholar] [CrossRef] [PubMed]
- Nyirő, G.; Szeredás, B.K. miRNA Expression Profiling in G1 and G2 Pancreatic Neuroendocrine Tumors. Cancers 2024, 16, 2528. [Google Scholar] [CrossRef]
- Xiong, M.; Wang, P. The diagnostic and prognostic values of microRNA-196a in cancer. Biosci. Rep. 2021, 41, BSR20203559. [Google Scholar] [CrossRef]
- Kövesdi, A.; Kurucz, P.A. Circulating miRNA Increases the Diagnostic Accuracy of Chromogranin A in Metastatic Pancreatic Neuroendocrine Tumors. Cancers 2020, 12, 2488. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B. Concomitant expression of several peptide receptors in neuroendocrine tumours: Molecular basis for in vivo multireceptor tumour targeting. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 781–793. [Google Scholar] [CrossRef]
- Virgolini, I.; Ambrosini, V. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2004–2010. [Google Scholar] [CrossRef]
- Ginj, M.; Zhang, H. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc. Natl. Acad. Sci. USA 2006, 103, 16436–16441. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur. J. Nucl. Med. 2001, 28, 836–846, Erratum in Eur. J. Nucl. Med. 2001, 28, 1433. [Google Scholar] [CrossRef] [PubMed]
- Guenter, R.; Aweda, T. Overexpression of somatostatin receptor type 2 in neuroendocrine tumors for improved Ga68-DOTATATE imaging and treatment. Surgery 2020, 167, 189–196, Erratum in Surgery 2020, 170, 351. [Google Scholar] [CrossRef] [PubMed]
- Deppen, S.A.; Liu, E. Safety and Efficacy of 68Ga-DOTATATE PET/CT for Diagnosis, Staging, and Treatment Management of Neuroendocrine Tumors. J. Nucl. Med. 2016, 57, 708–714. [Google Scholar] [CrossRef]
- Johnbeck, C.B.; Knigge, U. Head-to-Head Comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: A Prospective Study of 59 Patients with Neuroendocrine Tumors. J. Nucl. Med. 2017, 58, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Lau, W.F. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: Clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics 2015, 35, 500–516. [Google Scholar] [CrossRef]
- Ambrosini, V.; Campana, D. 68Ga-DOTANOC PET/CT clinical impact in patients with neuroendocrine tumors. J. Nucl. Med. 2010, 51, 669–673. [Google Scholar] [CrossRef]
- Pauwels, E.; Cleeren, F. Somatostatin receptor PET ligands—The next generation for clinical practice. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 311–331. [Google Scholar]
- Haug, A.R.; Cindea-Drimus, R. The role of 68Ga-DOTATATE PET/CT in suspected neuroendocrine tumors. J. Nucl. Med. 2012, 53, 1686–1692. [Google Scholar] [CrossRef]
- Sadowski, S.M.; Neychev, V. Prospective Study of 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography for Detecting Gastro-Entero-Pancreatic Neuroendocrine Tumors and Unknown Primary Sites. J. Clin. Oncol. 2016, 34, 588–596. [Google Scholar] [CrossRef]
- Gabriel, M.; Decristoforo, C. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J. Nucl. Med. 2007, 48, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Faggiano, A. Long-acting somatostatin analogs and well differentiated neuroendocrine tumors: A 20-year-old story. J. Endocrinol. Investig. 2024, 47, 35–46. [Google Scholar] [CrossRef]
- Geisler, L.; Mohr, R. The Role of miRNA in the Pathophysiology of Neuroendocrine Tumors. Int. J. Mol. Sci. 2021, 22, 8569. [Google Scholar] [CrossRef]
- Maratta, M.G.; Chiloiro, S. Somatostatin Analogs Versus Active Surveillance in Small Pancreatic Neuroendocrine Tumors. Pancreas 2025, 54, e524–e529. [Google Scholar] [CrossRef]
- Rinke, A.; Wittenberg, M. PROMID Study Group. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology 2017, 104, 26–32. [Google Scholar] [CrossRef]
- Lauricella, E.; Vilisova, S. The current status of somatostatin analogs in the treatment of neuroendocrine tumors and future perspectives. Expert. Rev. Neurother. 2025, 25, 245–258. [Google Scholar] [CrossRef]
- La Salvia, A.; Sesti, F. Somatostatin Analogue Therapy in MEN1-Related Pancreatic Neuroendocrine Tumors from Evidence to Clinical Practice: A Systematic Review. Pharmaceuticals 2021, 14, 1039. [Google Scholar] [CrossRef]
- Bodei, L.; Mueller-Brand, J. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816, Erratum in Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 584. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G. NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dahan, L. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513, Erratum in Engl. J. Med. 2011, 364, 1082. [Google Scholar] [CrossRef]
- Theiler, D.; Cattaneo, M. Safety and Efficacy of Peptide-Receptor Radionuclide Therapy in Elderly Neuroendocrine Tumor Patients. Cancers 2021, 13, 6290. [Google Scholar] [CrossRef]
- van der Zwan, W.A.; Bodei, L. GEPNETs update: Radionuclide therapy in neuroendocrine tumors. Eur. J. Endocrinol. 2015, 172, R1–R8. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Bogdani, C. Emerging innovations in theranostics for pancreatic neuroendocrine tumors. NPJ Precis. Oncol. 2025, 9, 146. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: First clinical experience on the efficacy and safety. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 934–946. [Google Scholar] [CrossRef]
- Fazio, N.; Carnaghi, C. Relationship between metabolic toxicity and efficacy of everolimus in patients with neuroendocrine tumors: A pooled analysis from the randomized, phase 3 RADIANT-3 and RADIANT-4 trials. Cancer 2021, 127, 2674–2682. [Google Scholar] [CrossRef] [PubMed]
- Salazar, R.; Garcia-Carbonero, R. Phase II Study of BEZ235 versus Everolimus in Patients with Mammalian Target of Rapamycin Inhibitor-Naïve Advanced Pancreatic Neuroendocrine Tumors. Oncologist 2018, 23, 766-e90. [Google Scholar] [CrossRef]
- Tijeras-Raballand, A.; Neuzillet, C. Resistance to targeted therapies in pancreatic neuroendocrine tumors (PNETs): Molecular basis, preclinical data, and counteracting strategies. Target. Oncol. 2012, 7, 173–181. [Google Scholar] [CrossRef]
- Allen, E.; Walters, I.B. Brivanib, a dual FGF/VEGF inhibitor, is active both first and second line against mouse pancreatic neuroendocrine tumors developing adaptive/evasive resistance to VEGF inhibition. Clin. Cancer Res. 2011, 17, 5299–5310. [Google Scholar] [CrossRef]
- Sennino, B.; Ishiguro-Oonuma, T. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. 2012, 2, 270–287. [Google Scholar] [CrossRef]
- Blumenthal, G.M.; Cortazar, P. FDA approval summary: Sunitinib for the treatment of progressive well-differentiated locally advanced or metastatic pancreatic neuroendocrine tumors. Oncologist 2012, 17, 1108–1113. [Google Scholar] [CrossRef]
- Xu, J.; Shen, L. Surufatinib in advanced pancreatic neuroendocrine tumours (SANET-p): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 1489–1499. [Google Scholar] [CrossRef]
- Chan, J.A.; Geyer, S. Phase 3 Trial of Cabozantinib to Treat Advanced Neuroendocrine Tumors. N. Engl. J. Med. 2025, 392, 653–665. [Google Scholar] [CrossRef]
- Ye, X.; Yu, Y. Clinical immunotherapy in pancreatic cancer. Cancer Immunol. Immunother. 2024, 73, 64. [Google Scholar] [CrossRef]
- Xu, J.X.; Wu, D.H. Immunotherapies for well-differentiated grade 3 gastroenteropancreatic neuroendocrine tumors: A new category in the World Health Organization classification. World J. Gastroenterol. 2021, 27, 8123–8137. [Google Scholar] [CrossRef]
- Strosberg, J.; Mizuno, N. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study. Clin. Cancer Res. 2020, 26, 2124–2130. [Google Scholar] [CrossRef]
- Pellegrino, C.; Favalli, N. Peptide-guided adaptor-CAR T-Cell therapy for the treatment of SSTR2-expressing neuroendocrine tumors. Oncoimmunology 2024, 13, 2412371. [Google Scholar] [CrossRef]
- Jancewicz, I.; Śmiech, M. New CEACAM-targeting 2A3 single-domain antibody-based chimeric antigen receptor T-cells produce anticancer effects in vitro and in vivo. Cancer Immunol. Immunother. 2024, 73, 30, Erratum in Cancer Immunol. Immunother. 2024, 73, 184. [Google Scholar] [CrossRef]
- Wong-Rolle, A.; Wei, H.K. Unexpected guests in the tumor microenvironment: Microbiome in cancer. Protein Cell 2021, 12, 426–435. [Google Scholar] [CrossRef]
- Cao, Z.; Huang, J. Causal Relationships between Gut Microbiotas, Blood Metabolites, and Neuroendocrine Tumors: A Mediated Mendelian Randomization Study. Neuroendocrinology 2024, 114, 981–992. [Google Scholar] [CrossRef]
- Massironi, S.; Facciotti, F. Intratumor Microbiome in Neuroendocrine Neoplasms: A New Partner of Tumor Microenvironment? A Pilot Study. Cells 2022, 11, 692. [Google Scholar] [CrossRef]
- Mohamed, A.; Asa, S.L. The Role of the Microbiome in Gastroentero-Pancreatic Neuroendocrine Neoplasms (GEP-NENs). Curr. Issues Mol. Biol. 2022, 44, 2015–2028. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Jiang, S.J. Investigating the causal relationship between gut microbiota and gastroenteropancreatic neuroendocrine neoplasms: A bidirectional Mendelian randomization study. Front. Microbiol. 2024, 15, 1420167. [Google Scholar] [CrossRef]
| Biomarker | Advantages | Challenges | Clinical Readiness |
|---|---|---|---|
| Chromogranin A (CgA) | - Widely used for diagnosis, prognosis, and monitoring [57,58,59,64,65,66,67,68,70]. - Reflects tumor burden and secretory activity. | - Assay variability and lack of standardization. - False positives from benign conditions and PPI use. - Limited sensitivity in small or early tumors [63,64,65,66]. | Established clinical biomarker; best used in combination with imaging and other tests [70]. |
| Ki-67 | - Standard for grading and prognostication [9,71,72,73,74,75,76,77,78,79,80,81,82]. - Guides treatment decisions. - Correlates with survival. | - Intratumoral heterogeneity. - Interobserver variability. - Manual counting affects reproducibility [79,80]. | Gold standard histopathological marker; requires standardized assessment protocols [80,81]. |
| SSTR2 | - Central to imaging (68Ga-DOTATATE PET/CT) and targeted therapies [95,96,97,98,99,100,101,102,103,104,105,106,107,108]. - High sensitivity and specificity. | - Reduced sensitivity for lesions < 5 mm. - False negatives in SSTR-negative tumors. - Technical challenges [108]. | Well-established for clinical use; crucial for staging and therapy planning. |
| Biomarker | Advantages | Challenges | Clinical Readiness |
|---|---|---|---|
| ctDNA | - Non-invasive, dynamic tumor genotyping and monitoring [83,84,85,86]. - Captures genetic and epigenetic info. - Potential early detection of progression. | - Low levels in early/low-grade tumors. - Methodological variability and lack of standardization [89]. - Limited large-scale validation. | Promising but experimental; requires further validation and standardization [88,89]. |
| miRNAs | - Stable in biofluids; non-invasive testing [90,109]. - Diagnostic and prognostic potential. - Can improve accuracy combined with CgA [91,92,93,94]. | - Lack of methodological standardization. - Limited large-scale prospective validation. - Heterogeneous data [90,91,92,93,94,109]. | Early clinical research stage; promising but needs robust validation and harmonization. |
| Therapy | Advantages | Challenges |
|---|---|---|
| SSAs | Good tolerability, proven PFS benefit [67,108] | Limited curative potential, mainly disease control |
| PRRT | High response rates, personalized via imaging [114,116] | Toxicity risk, limited in low SSTR expression tumors [118] |
| mTOR inhibitors | Improved PFS, target key pathway [122] | Resistance and side effects limit efficacy [123] |
| Anti-angiogenic TKIs | Target tumor microenvironment, immunomodulatory [127,128,129,130,131] | Side effects, resistance |
| ICIs | Potential synergy with other treatments [133,134] | Low monotherapy efficacy, experimental combinations [130,131,132] |
| Therapy | Biomarkers/Selection Criteria | Personalization Potential | Notes |
|---|---|---|---|
| SSAs | SSTR expression; hormone secretion profile [108,110] | Selection by receptor expression; hormone status | Standard first-line therapy |
| PRRT | High uptake on ^68Ga-DOTATATE PET/CT [116,120,121] | Theranostic approach enables patient-specific use | Requires functional imaging and renal function |
| mTOR inhibitors | Mutations in TSC2, PTEN, PIK3CA [19] | Potential for genomic-driven combinations | Resistance limits long-term efficacy |
| Anti-angiogenic TKIs | VEGF/VEGFR expression and angiogenic markers [125,126] | Emerging biomarkers to optimize therapy | Immunomodulatory effects under evaluation |
| ICIs | PD-L1 expression, tumor mutational burden [130,131,132] | Low predictive biomarkers; combos to enhance effect | Early-phase trials ongoing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galasso, L.; Vitale, F.; Giansanti, G.; Esposto, G.; Borriello, R.; Mignini, I.; Nicoletti, A.; Zileri Dal Verme, L.; Gasbarrini, A.; Ainora, M.E.; et al. Decoding Pancreatic Neuroendocrine Tumors: Molecular Profiles, Biomarkers, and Pathways to Personalized Therapy. Int. J. Mol. Sci. 2025, 26, 7814. https://doi.org/10.3390/ijms26167814
Galasso L, Vitale F, Giansanti G, Esposto G, Borriello R, Mignini I, Nicoletti A, Zileri Dal Verme L, Gasbarrini A, Ainora ME, et al. Decoding Pancreatic Neuroendocrine Tumors: Molecular Profiles, Biomarkers, and Pathways to Personalized Therapy. International Journal of Molecular Sciences. 2025; 26(16):7814. https://doi.org/10.3390/ijms26167814
Chicago/Turabian StyleGalasso, Linda, Federica Vitale, Gabriele Giansanti, Giorgio Esposto, Raffaele Borriello, Irene Mignini, Alberto Nicoletti, Lorenzo Zileri Dal Verme, Antonio Gasbarrini, Maria Elena Ainora, and et al. 2025. "Decoding Pancreatic Neuroendocrine Tumors: Molecular Profiles, Biomarkers, and Pathways to Personalized Therapy" International Journal of Molecular Sciences 26, no. 16: 7814. https://doi.org/10.3390/ijms26167814
APA StyleGalasso, L., Vitale, F., Giansanti, G., Esposto, G., Borriello, R., Mignini, I., Nicoletti, A., Zileri Dal Verme, L., Gasbarrini, A., Ainora, M. E., & Zocco, M. A. (2025). Decoding Pancreatic Neuroendocrine Tumors: Molecular Profiles, Biomarkers, and Pathways to Personalized Therapy. International Journal of Molecular Sciences, 26(16), 7814. https://doi.org/10.3390/ijms26167814







