Vagal Oxytocin Receptors as Molecular Targets in Gut–Brain Signaling: Implications for Appetite, Satiety, Obesity, and Esophageal Motility—A Narrative Review
Abstract
1. Introduction
2. Peripheral Oxytocin in Metabolic and Gastrointestinal Regulation
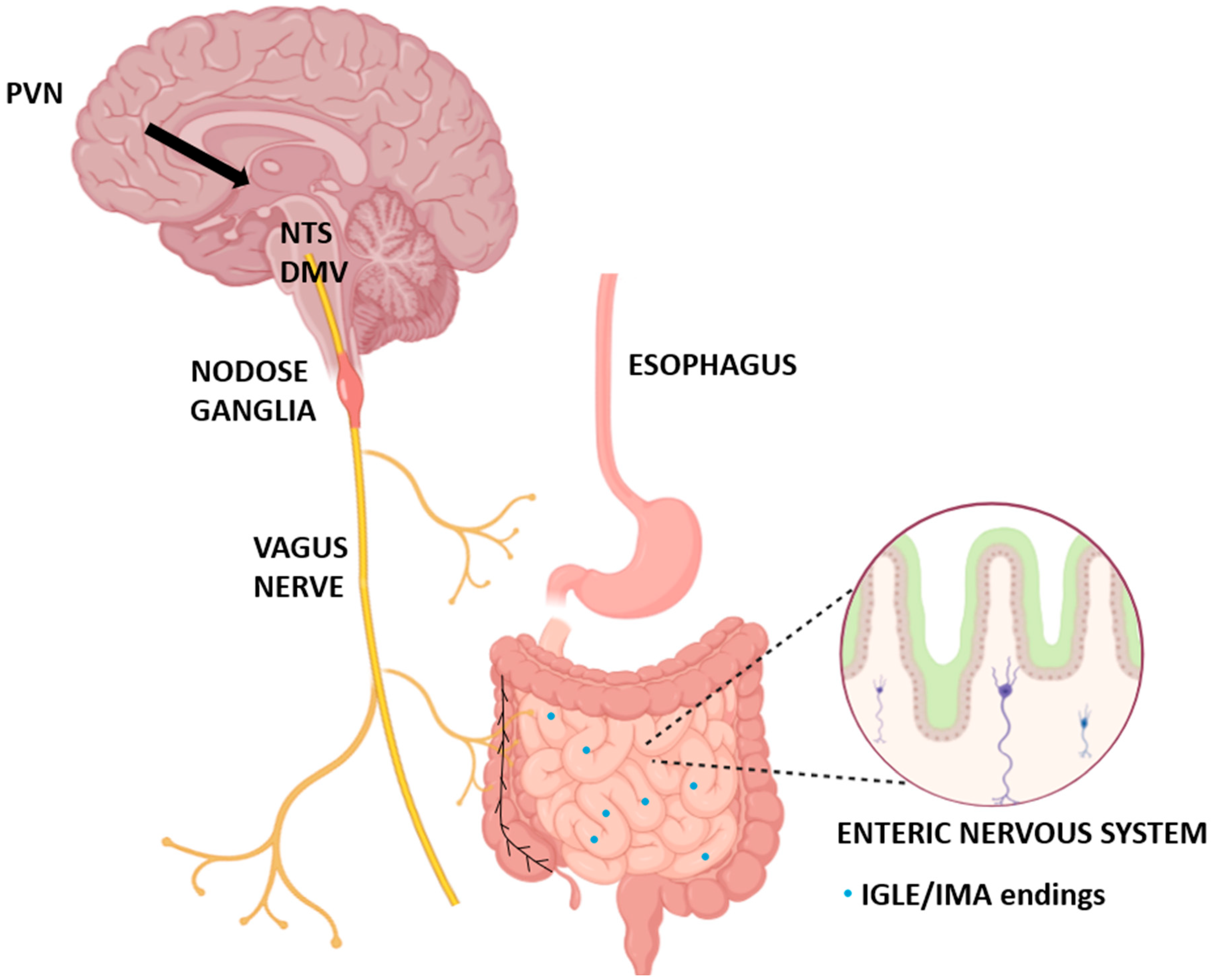
3. The Vagus Nerve as a Central Gut–Brain Interface for Oxytocin Signaling
4. Vagal Oxytocin Receptors: Localization, Specialization, and Plasticity
4.1. Distribution and Neuronal Diversity of Vagal Oxytocin Receptors
4.2. Regional and Functional Specialization
4.3. Developmental and Contextual Plasticity of Vagal Oxytocin Signaling
4.4. Functional Mapping and Connectivity
4.4.1. Integration with Brainstem Nuclei
4.4.2. Peripheral Connectivity to Gastrointestinal Organs
5. Intracellular Signaling and Neurochemical Interactions of Vagal Oxytocin Receptors
5.1. Receptor Pharmacology and Kinetics
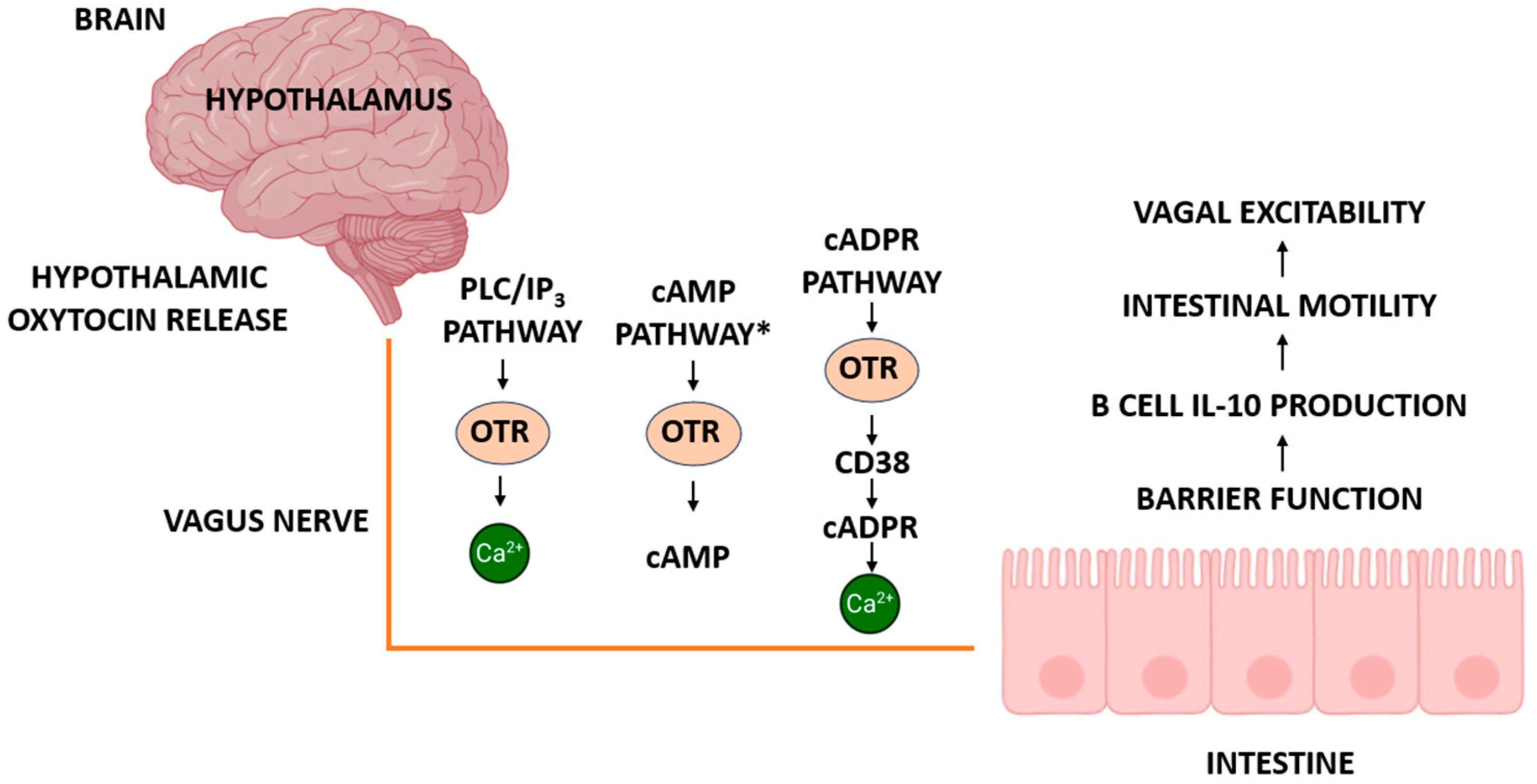
5.2. Downstream Signaling Pathways
5.2.1. Canonical GPCR Signaling
5.2.2. MAPK and NO Signaling: Alternative Intracellular Pathways
5.2.3. Network-Level Crosstalk with Neuromodulators
5.2.4. ADP-Ribosyl Cyclases and cADPR Signaling in Vagal and Intestinal Function
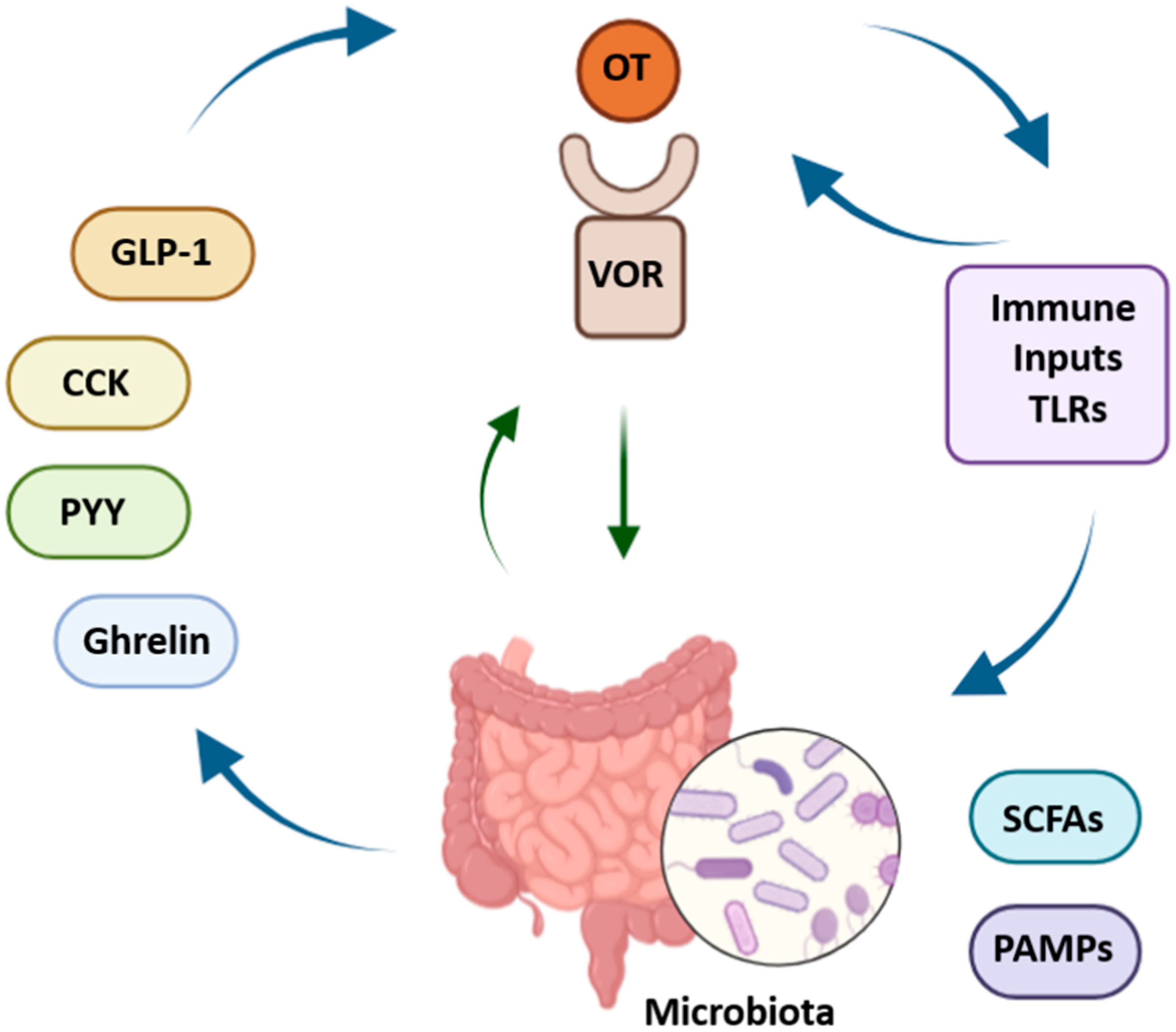
5.3. Interactions with Gut Peptides and Hormones
5.3.1. Integration of Vagal Oxytocin Receptors with Gut-Derived Peptides
5.3.2. Modulation of Vagal Neurotransmission by Oxytocin
6. Role of Vagal Oxytocin Receptors in Esophageal Motility
6.1. Esophageal Peristalsis and Swallowing
6.2. Regulation of Lower Esophageal Sphincter Function
6.3. Sex-Specific Effects and Pathophysiology
7. Vagal Oxytocin Receptors in Appetite and Gastrointestinal Control
7.1. Peripheral Oxytocin and Satiety Signaling
7.2. Meal Size, Macronutrient Selection, and Patterns of Food Intake
7.3. Thermogenesis and Metabolic Regulation
7.4. Sex Differences and Behavioral Outcomes
8. The Gut–Brain–Vagus Axis: A Systems Biology Perspective
8.1. Integration of Gut, Brain, and Vagus Nerve
8.2. Influence of Microbiota on Vagal Oxytocin Signaling
8.3. Inflammation and Vagal Function
8.4. Metabolic Stress and Plasticity of Vagal Circuits
9. Translational and Therapeutic Implications
9.1. Targeting Vagal Oxytocin Receptors in Gastrointestinal and Metabolic Disorders
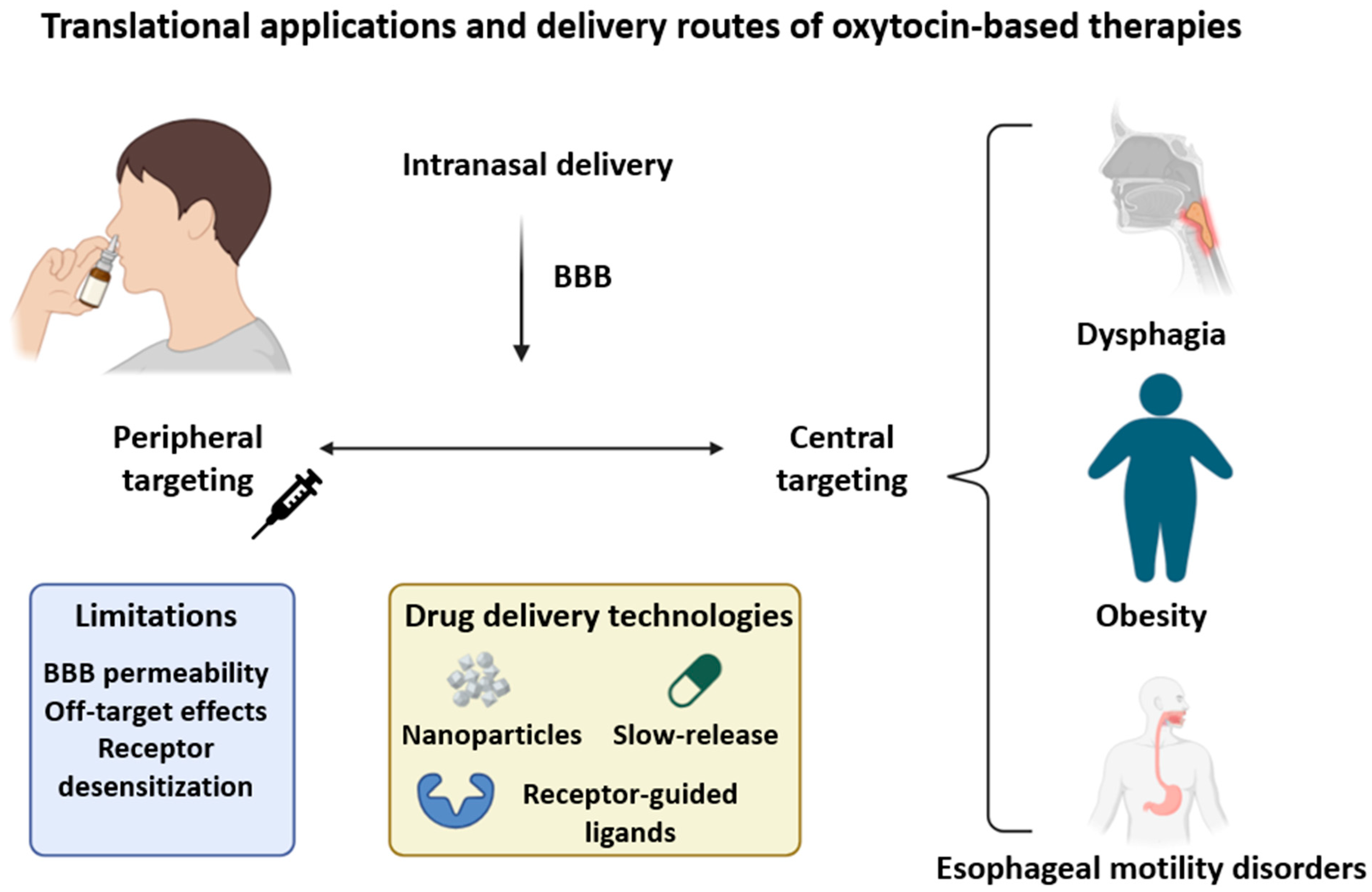
9.2. Drug Delivery and Ligand Design: Challenges and Opportunities
9.3. Peripheral Versus Central Targeting Strategies
10. Future Directions and Open Questions
10.1. Technological Innovations for Vagal Oxytocin Research
10.1.1. Spatial Transcriptomics and Single-Nucleus RNA Sequencing
10.1.2. Optogenetic and Chemogenetic Approaches
10.1.3. Advanced Imaging and Functional Mapping
10.2. Key Unanswered Questions
10.3. Translational Potential and Pathways to Clinical Application
10.4. Mechanistic and Translational Gaps in VOR-Mediated Appetite Control
11. Summary of Key Studies on Vagal Oxytocin Signaling
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takayanagi, Y.; Onaka, T. Roles of Oxytocin in Stress Responses, Allostasis and Resilience. Int. J. Mol. Sci. 2021, 23, 150. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.C.; Liu, X.; Jia, S.; Wang, X.; Li, T.; Yu, J.; Parpura, V.; Wang, Y. Neural Functions of Hypothalamic Oxytocin and Its Regulation. ASN Neuro 2022, 14, 17590914221100706. [Google Scholar] [CrossRef] [PubMed]
- Everett, N.A.; Turner, A.; Costa, P.A.; Baracz, S.J.; Cornish, J.L. The Vagus Nerve Mediates the Suppressing Effects of Peripherally Administered Oxytocin on Methamphetamine Self-Administration and Seeking in Rats. Neuropsychopharmacology 2020, 46, 297–304. [Google Scholar] [CrossRef]
- Liu, H.; Yang, G.; Wang, H. Oxytocin/Oxytocin Receptor Signalling in the Gastrointestinal System: Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2024, 25, 10935. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.G.; Tamir, H.; Gross, K.J.; Chen, J.; Anwar, M.; Gershon, M.D. Expression and Developmental Regulation of Oxytocin (OT) and Oxytocin Receptors (OTR) in the Enteric Nervous System (ENS) and Intestinal Epithelium. J. Comp. Neurol. 2008, 512, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Asker, M.; Krieger, J.; Marić, I.; Bedel, E.; van der Steen, J.T.; Börchers, S.; Wen, Y.; Longo, F.; Aronsson, P.; Winder, M.; et al. Vagal Oxytocin Receptors Are Necessary for Esophageal Motility and Function. JCI Insight 2025, 10, e190108. [Google Scholar] [CrossRef]
- Jiang, Y.; Travagli, R.A. Hypothalamic–Vagal Oxytocinergic Neurocircuitry Modulates Gastric Emptying and Motility Following Stress. J. Physiol. 2020, 598, 4941–4955. [Google Scholar] [CrossRef]
- Perelló, M.; Cornejo, M.P.; Francesco, P.N.D.; Fernández, G.; Gautron, L.; Valdivia, L.S. The Controversial Role of the Vagus Nerve in Mediating Ghrelin’s Actions: Gut Feelings and Beyond. IBRO Neurosci. Rep. 2022, 12, 228–239. [Google Scholar] [CrossRef]
- Ottaviani, M.M.; Macefield, V.G. Structure and Functions of the Vagus Nerve in Mammals. Compr. Physiol. 2022, 12, 3989–4037. [Google Scholar] [CrossRef]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Chen, L.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J. Inflamm. Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef]
- Neuhuber, W.; Berthoud, H. Functional Anatomy of the Vagus System—Emphasis on the Somato-Visceral Interface. Auton. Neurosci. 2021, 236, 102887. [Google Scholar] [CrossRef]
- Li, H.; Page, A.J. Altered Vagal Signaling and Its Pathophysiological Roles in Functional Dyspepsia. Front. Neurosci. 2022, 16, 858612. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, C.; Barrett, L.F.; Quigley, K.S. Signal Processing in the Vagus Nerve: Hypotheses Based on New Genetic and Anatomical Evidence. Biol. Psychol. 2023, 182, 108626. [Google Scholar] [CrossRef] [PubMed]
- Jameson, H.; Bateman, R.; Byrne, P.; Dyavanapalli, J.; Wang, X.; Jain, V.; Mendelowitz, D. Oxytocin Neuron Activation Prevents Hypertension That Occurs with Chronic Intermittent Hypoxia/Hypercapnia in Rats. AJP Heart Circ. Physiol. 2016, 310, H1549–H1557. [Google Scholar] [CrossRef] [PubMed]
- Szafoni, S.; Piegza, M. Progress in Personalized Psychiatric Therapy with the Example of Using Intranasal Oxytocin in PTSD Treatment. J. Pers. Med. 2022, 12, 1067. [Google Scholar] [CrossRef]
- Kremsmayr, T.; Schober, G.; Kaltenböck, M.; Hoare, B.L.; Brierley, S.M.; Muttenthaler, M. Oxytocin Analogues for the Oral Treatment of Abdominal Pain. Angew. Chem. Int. Ed. 2024, 63, e202415333. [Google Scholar] [CrossRef]
- Camerino, C. Oxytocin Involvement in Body Composition Unveils the True Identity of Oxytocin. Int. J. Mol. Sci. 2021, 22, 6383. [Google Scholar] [CrossRef]
- Assinder, S.J. The Importance of Experimental Investigation of the Peripheral Oxytocin System. Methods Mol. Biol. 2021, 2384, 1–17. [Google Scholar] [CrossRef]
- Cuesta-Marti, C.; Uhlig, F.; Muguerza, B.; Hyland, N.P.; Clarke, G.; Schellekens, H. Microbes, Oxytocin and Stress: Converging Players Regulating Eating Behavior. J. Neuroendocrinol. 2023, 35, e13243. [Google Scholar] [CrossRef]
- Wang, R.; Han, M.; Lv, X.; Yu, Y.; Chai, S.; Qu, C.; Liu, C.Y. Inhibitory Action of Oxytocin on Spontaneous Contraction of Rat Distal Colon by Nitrergic Mechanism: Involvement of Cyclic GMP and Apamin-sensitive K+ Channels. Acta Physiol. 2017, 221, 182–192. [Google Scholar] [CrossRef]
- Xi, T.-F.; Li, D.-N.; Li, Y.-Y.; Qin, Y.; Wang, H.-H.; Song, N.; Zhang, Q.; Ding, Y.; Shi, X.-Z.; Xie, D.-P. Central 5-Hydroxytryptamine (5-HT) Mediates Colonic Motility by Hypothalamus Oxytocin-Colonic Oxytocin Receptor Pathway. Biochem. Biophys. Res. Commun. 2018, 508, 959–964. [Google Scholar] [CrossRef]
- Morton, G.J.; Thatcher, B.S.; Reidelberger, R.D.; Ogimoto, K.; Wolden-Hanson, T.; Baskin, D.G.; Schwartz, M.W.; Blevins, J.E. Peripheral Oxytocin Suppresses Food Intake and Causes Weight Loss in Diet-Induced Obese Rats. Am. J. Physiol.-Endocrinol. Metab. 2011, 302, E134–E144. [Google Scholar] [CrossRef]
- Lawson, E.A.; Marengi, D.A.; DeSanti, R.; Holmes, T.M.; Schoenfeld, D.; Tolley, C. Oxytocin Reduces Caloric Intake in Men. Obesity 2015, 23, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Ong, Z.Y.; Bongiorno, D.M.; Hernando, M.A.; Grill, H.J. Effects of Endogenous Oxytocin Receptor Signaling in Nucleus Tractus Solitarius on Satiation-Mediated Feeding and Thermogenic Control in Male Rats. Endocrinology 2017, 158, 2826–2836. [Google Scholar] [CrossRef] [PubMed]
- Burmester, V.; Gibson, E.L.; Butler, G.K.L.; Bailey, A.; Terry, P. Oxytocin Reduces Post-Stress Sweet Snack Intake in Women without Attenuating Salivary Cortisol. Physiol. Behav. 2019, 212, 112704. [Google Scholar] [CrossRef]
- Kerem, L.; Lawson, E.A. The Effects of Oxytocin on Appetite Regulation, Food Intake and Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 7737. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, R.; Wu, R.; Gu, Y.; Lu, Y. The Effects of Oxytocin to Rectify Metabolic Dysfunction in Obese Mice Are Associated with Increased Thermogenesis. Mol. Cell. Endocrinol. 2020, 514, 110903. [Google Scholar] [CrossRef]
- Rochman, M.; Kellerman, K.; Jankowski, M.P.; Rothenberg, M.E. The Oesophagus as an Immune Organ. Nat. Rev. Gastroenterol. Hepatol. 2025. [Google Scholar] [CrossRef]
- Olszewski, P.K.; Klockars, A.; Schiöth, H.B.; Levine, A.S. Oxytocin as Feeding Inhibitor: Maintaining Homeostasis in Consummatory Behavior. Pharmacol. Biochem. Behav. 2010, 97, 47–54. [Google Scholar] [CrossRef]
- Welch, M.G.; Margolis, K.G.; Li, Z.; Gershon, M.D. Oxytocin Regulates Gastrointestinal Motility, Inflammation, Macromolecular Permeability, and Mucosal Maintenance in Mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 307, G848–G862. [Google Scholar] [CrossRef]
- Yeomans, D.C.; Hanson, L.R.; Carson, D.S.; Tunstall, B.J.; Lee, M.R.; Tzabazis, A.; Jacobs, D.L.; Frey, W.H. Nasal Oxytocin for the Treatment of Psychiatric Disorders and Pain: Achieving Meaningful Brain Concentrations. Transl. Psychiatry 2021, 11, 388. [Google Scholar] [CrossRef]
- Petrut, S.; Bragaru, A.M.; Munteanu, A.E.; Moldovan, A.G.; Moldovan, C.; Rusu, E. Gut over Mind: Exploring the Powerful Gut–Brain Axis. Nutrients 2025, 17, 842. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.-R. Vagal and Hormonal Gut–Brain Communication: From Satiation to Satisfaction. Neurogastroenterol. Motil. 2008, 20, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Travagli, R.A.; Anselmi, L. Vagal Neurocircuitry and Its Influence on Gastric Motility. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Maejima, Y.; Yokota, S.; Nishimori, K.; Shimomura, K. The Anorexigenic Neural Pathways of Oxytocin and Their Clinical Implication. Neuroendocrinology 2018, 107, 91–104. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Parmila, K.; Wang, L.; Hidema, S.; Nishimori, K.; Yada, T. Relay of Peripheral Oxytocin to Central Oxytocin Neurons via Vagal Afferents for Regulating Feeding. Biochem. Biophys. Res. Commun. 2019, 519, 553–558. [Google Scholar] [CrossRef]
- Biddinger, J.E.; Elson, A.E.T.; Fathi, P.A.; Sweet, S.R.; Nishimori, K.; Ayala, J.E.; Simerly, R.B.; Sternson, S.M. AgRP Neurons Mediate Activity- Dependent Development of Oxytocin Connectivity and Autonomic Regulation. In Proceedings of the National Academy of Sciences, Washington, DC, USA, 9–12 September 2024. [Google Scholar]
- Komisaruk, B.R.; Frangos, E. Vagus Nerve Afferent Stimulation: Projection into the Brain, Reflexive Physiological, Perceptual, and Behavioral Responses, and Clinical Relevance. Auton. Neurosci. 2021, 237, 102908. [Google Scholar] [CrossRef]
- Prescott, S.L.; Liberles, S.D. Internal Senses of the Vagus Nerve. Neuron 2022, 110, 579–599. [Google Scholar] [CrossRef]
- Mörkl, S.; Butler, M.I.; Wagner-Skacel, J. Gut-Brain-Crosstalk- the Vagus Nerve and the Microbiota-Gut-Brain Axis in Depression. A Narrat. Review. J. Affect. Disord. Rep. 2023, 13, 100607. [Google Scholar]
- Wang, X.; Ribeiro, C.; Nilsson, A.C.; Escobar, J.B.; Alber, B.R.; Bethea, J.R.; Polotsky, V.Y.; Kay, M.W.; Schunke, K.J.; Mendelowitz, D. Oxytocin Receptor Expression and Activation in Parasympathetic Brainstem Cardiac Vagal Neurons. bioRxiv 2025. [Google Scholar] [CrossRef]
- Wang, Y.B.; de Lartigue, G.; Page, A.J. Dissecting the Role of Subtypes of Gastrointestinal Vagal Afferents. Front. Physiol. 2020, 11, 643. [Google Scholar] [CrossRef]
- Biddinger, J.E.; Lazarenko, R.M.; Scott, M.M.; Simerly, R.B. Leptin Suppresses Development of GLP-1 Inputs to the Paraventricular Nucleus of the Hypothalamus. eLife 2020, 9, e59857. [Google Scholar] [CrossRef] [PubMed]
- Clyburn, C.; Browning, K.N. Glutamatergic Plasticity within Neurocircuits of the Dorsal Vagal Complex and the Regulation of Gastric Functions. Am. J. Physiol.-Gastrointest. Liver Physiol. 2021, 320, G880–G887. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Lin, Y.-T.; Tseng, W.-D.; Tseng, P.; Hua, G.-L.; Chao, Y.; Wu, Y. Neuroimmunomodulation of Vagus Nerve Stimulation and the Therapeutic Implications. Front. Aging Neurosci. 2023, 15, 1173987. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Guo, L.; Suo, M.; Sun, Y.; Wu, J.; Zhang, X.Y.; Liu, C.Y. Role of the Nitrergic Pathway in Motor Effects of Oxytocin in Rat Proximal Colon. Neurogastroenterol. Motil. 2016, 28, 1815–1823. [Google Scholar] [CrossRef]
- Browning, K.N.; Carson, K.E. Central Neurocircuits Regulating Food Intake in Response to Gut Inputs—Preclinical Evidence. Nutrients 2021, 13, 908. [Google Scholar] [CrossRef]
- Matsunaga, M.; Konagaya, T.; Nogimori, T.; Yoneda, M.; Kasugai, K.; Ohira, H.; Kaneko, H. Inhibitory Effect of Oxytocin on Accelerated Colonic Motility Induced by Water-avoidance Stress in Rats. Neurogastroenterol. Motil. 2009, 21, 856-e59. [Google Scholar] [CrossRef]
- Qin, J.; Feng, M.; Wang, C.; Ye, Y.; Wang, P.S.; Liu, C. Oxytocin Receptor Expressed on the Smooth Muscle Mediates the Excitatory Effect of Oxytocin on Gastric Motility in Rats. Neurogastroenterol. Motil. 2009, 21, 430–438. [Google Scholar] [CrossRef]
- Mano-Otagiri, A.; Ohata, H.; Iwasaki-Sekino, A.; Nemoto, T.; Shibasaki, T. Ghrelin Suppresses Noradrenaline Release in the Brown Adipose Tissue of Rats. J. Endocrinol. 2009, 201, 341–349. [Google Scholar] [CrossRef]
- Krieger, J.; da Conceição, E.P.S.; Sanchez-Watts, G.; Arnold, M.; Pettersen, K.G.; Mohammed, M.; Modica, S.; Lossel, P.; Morrison, S.F.; Madden, C.J.; et al. Glucagon-like Peptide-1 Regulates Brown Adipose Tissue Thermogenesis via the Gut-Brain Axis in Rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2018, 315, R708–R720. [Google Scholar] [CrossRef]
- Székely, M. The Vagus Nerve in Thermoregulation and Energy Metabolism. Auton. Neurosci. 2000, 85, 26–38. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Maejima, Y.; Suyama, S.; Yoshida, M.; Arai, T.; Katsurada, K.; Parmila, K.; Nakabayashi, H.; Kakei, M.; Yada, T. Peripheral Oxytocin Activates Vagal Afferent Neurons to Suppress Feeding in Normal and Leptin-Resistant Mice: A Route for Ameliorating Hyperphagia and Obesity. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2014, 308, R360–R369. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Mesgarzadeh, S.; Ramesh, K.; Huey, E.L.; Liu, Y.; Gray, L.; Aitken, T.J.; Chen, Y.; Beutler, L.R.; Ahn, J.S.; et al. Genetic Identification of Vagal Sensory Neurons That Control Feeding. Cell 2019, 179, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Melo, V.; Saldanha, R.R.M.; Santos, C.R.d.; Cruz, J.d.C.; Lira, V.A.; Santana-Filho, V.J.; Michelini, L.C. Ovarian Hormone Deprivation Reduces Oxytocin Expression in Paraventricular Nucleus Preautonomic Neurons and Correlates with Baroreflex Impairment in Rats. Front. Physiol. 2016, 7, 461. [Google Scholar] [CrossRef]
- Dantzler, H.A.; Kline, D.D. Exaggerated Potassium Current Reduction by Oxytocin in Visceral Sensory Neurons Following Chronic Intermittent Hypoxia. Auton. Neurosci. 2020, 229, 102735. [Google Scholar] [CrossRef]
- Alqudah, M.; Razzaq, R.A.; Alfaqih, M.A.; Al-Shboul, O.; Al-Dwairi, A.; Taha, S. Mechanism of Oxytocin-Induced Contraction in Rat Gastric Circular Smooth Muscle. Int. J. Mol. Sci. 2022, 24, 441. [Google Scholar] [CrossRef]
- Kobashi, M.; Shimatani, Y.; Fujita, M. Oxytocin Increased Intragastric Pressure in the Forestomach of Rats via the Dorsal Vagal Complex. Physiol. Behav. 2023, 261, 114087. [Google Scholar] [CrossRef]
- Holmes, G.M.; Browning, K.N.; Babic, T.; Fortna, S.R.; Coleman, F.H.; Travagli, R.A. Vagal Afferent Fibres Determine the Oxytocin-induced Modulation of Gastric Tone. J. Physiol. 2013, 591, 3081–3100. [Google Scholar] [CrossRef]
- Ong, Z.Y.; Alhadeff, A.L.; Grill, H.J. Medial Nucleus Tractus Solitarius Oxytocin Receptor Signaling and Food Intake Control: The Role of Gastrointestinal Satiation Signal Processing. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 308, R800–R806. [Google Scholar] [CrossRef]
- Bellusci, L.; DuBar, S.G.; Kuah, M.; Castellano, D.; Muralidaran, V.; Jones, E.A.; Rozeboom, A.M.; Gillis, R.A.; Vicini, S.; Sahibzada, N. Interactions between Brainstem Neurons That Regulate the Motility to the Stomach. J. Neurosci. 2022, 42, 5212–5228. [Google Scholar] [CrossRef]
- Brierley, D.I.; Holt, M.K.; Singh, A.; de Araujo, A.; McDougle, M.; Vergara, M.; Afaghani, M.H.; Lee, S.J.; Scott, K.A.; Maske, C.B.; et al. Central and Peripheral GLP-1 Systems Independently Suppress Eating. Nat. Metab. 2021, 3, 258–273. [Google Scholar] [CrossRef]
- Yao, S.; Kendrick, K.M. Effects of Intranasal Administration of Oxytocin and Vasopressin on Social Cognition and Potential Routes and Mechanisms of Action. Pharmaceutics 2022, 14, 323. [Google Scholar] [CrossRef] [PubMed]
- Talpo, F.; Spaiardi, P.; Castagno, A.N.; Maniezzi, C.; Raffin, F.; Terribile, G.; Sancini, G.; Pisani, A.; Biella, G. Neuromodulatory Functions Exerted by Oxytocin on Different Populations of Hippocampal Neurons in Rodents. Front. Cell. Neurosci. 2023, 17, 1082010. [Google Scholar] [CrossRef] [PubMed]
- Carson, K.E.; Alvarez, J.; Mackley, J.; Browning, K.N. Perinatal High Fat Diet Exposure Alters Oxytocin and Corticotropin Releasing Factor Inputs onto Vagal Neurocircuits Controlling Gastric Motility. J. Physiol. 2023, 601, 2853–2875. [Google Scholar] [CrossRef]
- Feng, M.; Qin, J.; Wang, C.; Ye, Y.; Wang, S.; Xie, D.-P.; Wang, P.S.; Liu, C. Estradiol Upregulates the Expression of Oxytocin Receptor in Colon in Rats. Am. J. Physiol.-Endocrinol. Metab. 2009, 296, E1059–E1066. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.M.; Blevins, J.E. Coming Full Circle: Contributions of Central and Peripheral Oxytocin Actions to Energy Balance. Endocrinology 2012, 154, 589–596. [Google Scholar] [CrossRef]
- Baribeau, D.; Anagnostou, E. Oxytocin and Vasopressin: Linking Pituitary Neuropeptides and Their Receptors to Social Neurocircuits. Front. Neurosci. 2015, 9, 335. [Google Scholar] [CrossRef]
- Dumais, K.M.; Veenema, A.H. Vasopressin and Oxytocin Receptor Systems in the Brain: Sex Differences and Sex-Specific Regulation of Social Behavior. Front. Neuroendocrinol. 2015, 40, 1–23. [Google Scholar] [CrossRef]
- Zhang, V.R.; Ng, Q.X.; Ren, Y.P.; Tang, A.S.P.; Sulaimi, F.; Yaow, C.Y.L.; Siah, K.T.H. Diagnostic Criteria and Symptom Profiles in Adult Idiopathic Gastroparesis: A Systematic Review. J. Neurogastroenterol. Motil. 2025, 31, 296–303. [Google Scholar] [CrossRef]
- Ohlsson, B.; Ringström, G.; Abrahamsson, H.; Simrén, M.; Björnsson, E.S. Oxytocin Stimulates Colonic Motor Activity in Healthy Women. Neurogastroenterol. Motil. 2004, 16, 233–240. [Google Scholar] [CrossRef]
- Thienel, M.; Fritsche, A.; Heinrichs, M.; Peter, A.; Ewers, M.; Lehnert, H.; Born, J.; Hallschmid, M. Oxytocin’s Inhibitory Effect on Food Intake Is Stronger in Obese than Normal-Weight Men. Int. J. Obes. 2016, 40, 1707–1714. [Google Scholar] [CrossRef]
- Scott, K.A.; Tan, Y.; Johnson, D.N.; Elsaafien, K.; Baumer-Harrison, C.; Méndez-Hernández, R.; Kirchner, M.K.; Eikenberry, S.A.; Jessica, M.; Stern, J.E.; et al. Mechanosensation of the Heart and Gut Elicits Hypometabolism and Vigilance in Mice. Nat. Metab. 2025, 7, 263–275. [Google Scholar] [CrossRef]
- Grabauskas, G.; Owyang, C. Plasticity of Vagal Afferent Signaling in the Gut. Medicina 2017, 53, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, M.; Xuan, S.-Y.; Fujita, M.; Mitoh, Y.; Matsuo, R. Central Ghrelin Inhibits Reflex Swallowing Elicited by Activation of the Superior Laryngeal Nerve in the Rat. Regul. Pept. 2010, 160, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Yosten, G.L.C.; Samson, W.K. The Anorexigenic and Hypertensive Effects of Nesfatin-1 Are Reversed by Pretreatment with an Oxytocin Receptor Antagonist. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010, 298, R1642–R1647. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Luo, X.; Gao, S.; Sun, X.; Li, Z.; Gong, Y. The Effects of Nesfatin-1 in the Paraventricular Nucleus on Gastric Motility and Its Potential Regulation by the Lateral Hypothalamic Area in Rats. J. Neurochem. 2014, 132, 266–275. [Google Scholar] [CrossRef]
- Sun, X.; Liu, B.; Yuan, Y.; Ying, R.; Pang, R.; Li, Q. Neural and Hormonal Mechanisms of Appetite Regulation during Eating. Front. Nutr. 2025, 12, 1484827. [Google Scholar] [CrossRef]
- Hume, C.; Leng, G. Oxytocin Neurons: Integrators of Hypothalamic and Brainstem Circuits in the Regulation of Macronutrient-Specific Satiety. Curr. Opin. Physiol. 2019, 12, 65–71. [Google Scholar] [CrossRef]
- Liu, C.M.; Spaulding, M.O.; Rea, J.J.; Noble, E.E.; Kanoski, S.E. Oxytocin and Food Intake Control: Neural, Behavioral, and Signaling Mechanisms. Int. J. Mol. Sci. 2021, 22, 10859. [Google Scholar] [CrossRef]
- Guillebaud, F.; Roussel, G.; Félix, B.; Troadec, J.; Dallaporta, M.; Abysique, A. Interaction between Nesfatin-1 and Oxytocin in the Modulation of the Swallowing Reflex. Brain Res. 2019, 1711, 173–182. [Google Scholar] [CrossRef]
- Wang, T.; Tufenkjian, A.; Ajijola, O.A.; Oka, Y. Molecular and Functional Diversity of the Autonomic Nervous System. Nat. reviews. Neurosci. 2025. [Google Scholar] [CrossRef]
- Holt, M.K. The Ins and Outs of the Caudal Nucleus of the Solitary Tract: An Overview of Cellular Populations and Anatomical Connections. J. Neuroendocrinol. 2022, 34, e13132. [Google Scholar] [CrossRef] [PubMed]
- Dyavanapalli, J. Novel Approaches to Restore Parasympathetic Activity to the Heart in Cardiorespiratory Diseases. Am. J. Physiol.-Heart Circ. Physiol. 2020, 319, H1153–H1161. [Google Scholar] [CrossRef] [PubMed]
- Gillis, R.A.; Dezfuli, G.; Bellusci, L.; Vicini, S.; Niaz, A. Brainstem Neuronal Circuitries Controlling Gastric Tonic and Phasic Contractions: A Review. Cell. Mol. Neurobiol. 2021, 42, 333–360. [Google Scholar] [CrossRef]
- Mansuy-Aubert, V.; Ravussin, Y. Short Chain Fatty Acids: The Messengers from down Below. Front. Neurosci. 2023, 17, 1197759. [Google Scholar] [CrossRef]
- Bonaz, B. Enteric Neuropathy and the Vagus Nerve: Therapeutic Implications. Neurogastroenterol. Motil. 2024, 37, e14842. [Google Scholar] [CrossRef]
- Williams, E.K.; Chang, R.B.; Strochlic, D.E.; Umans, B.D.; Lowell, B.B.; Liberles, S.D. Sensory Neurons That Detect Stretch and Nutrients in the Digestive System. Cell 2016, 166, 209. [Google Scholar] [CrossRef]
- Bülbül, M.; Babygirija, R.; Ludwig, K.; Takahashi, T. Central Oxytocin Attenuates Augmented Gastric Postprandial Motility Induced by Restraint Stress in Rats. Neurosci. Lett. 2010, 479, 302–306. [Google Scholar] [CrossRef]
- Monstein, H.; Grahn, N.; Truedsson, M.; Ohlsson, B. Oxytocin and Oxytocin-Receptor mRNA Expression in the Human Gastrointestinal Tract: A Polymerase Chain Reaction Study. Regul. Pept. 2004, 119, 39–44. [Google Scholar] [CrossRef]
- Che, T.; Sun, H.; Li, J.; Yu, X.; Zhu, D.; Xue, B.; Liu, K.; Zhang, M.; Kunze, W.; Liu, C. Oxytocin Hyperpolarizes Cultured Duodenum Myenteric Intrinsic Primary Afferent Neurons by Opening BKCa Channels through IP3 Pathway. J. Neurochem. 2012, 121, 516–525. [Google Scholar] [CrossRef]
- Llewellyn-Smith, I.J.; Kellett, D.O.; Jordan, D.L.; Browning, K.N.; Travagli, R.A. Oxytocin-immunoreactive Innervation of Identified Neurons in the Rat Dorsal Vagal Complex. Neurogastroenterol. Motil. 2011, 24, e136–e146. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, H.; Xie, W.; Zhang, Z.; Smrcka, A.V.; Wu, D. Roles of PLC-Β2 and -Β3 and PI3Kγ in Chemoattractant-Mediated Signal Transduction. Science 2000, 287, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Badshah, M.; Ibrahim, J.; Su, N.; Whiley, P.A.F.; Middendorff, R.; Whittaker, M.R.; Exintaris, B. Age-Dependent Effects of Oxytocin and Oxytocin Receptor Antagonists on Bladder Contractions: Implications for the Treatment of Overactive Bladder Syndrome. Biomedicines 2024, 12, 674. [Google Scholar] [CrossRef] [PubMed]
- Caruso, E.A.; Nunzio, V.D.; Tutino, V.; Notarnicola, M. The Endocannabinoid System: Implications in Gastrointestinal Physiology and Pathology. Int. J. Mol. Sci. 2025, 26, 1306. [Google Scholar] [CrossRef]
- Zhou, L.; Ghee, S.M.; See, R.E.; Reichel, C.M. Oxytocin Differentially Affects Sucrose Taking and Seeking in Male and Female Rats. Behav. Brain Res. 2015, 283, 184–190. [Google Scholar] [CrossRef]
- Hornby, P.J.; Abrahams, T. Central Control of Lower Esophageal Sphincter Relaxation. Am. J. Med. 2000, 108, 90–98. [Google Scholar] [CrossRef]
- Parsons, S.P.; Huizinga, J.D. Nitric Oxide Is Essential for Generating the Minute Rhythm Contraction Pattern in the Small Intestine, Likely via ICC-DMP. Front. Neurosci. 2021, 14, 592664. [Google Scholar] [CrossRef]
- Idrizaj, E.; Traini, C.; Vannucchi, M.G.; Baccari, M.C. Nitric Oxide: From Gastric Motility to Gastric Dysmotility. Int. J. Mol. Sci. 2021, 22, 9990. [Google Scholar] [CrossRef]
- Van den Houte, K.; Scarpellini, E.; Verbeure, W.; Mori, H.; Schol, J.; Masuy, I.; Carbone, F.; Tack, J. The Role of GI Peptides in Functional Dyspepsia and Gastroparesis: A Systematic Review. Front. Psychiatry 2020, 11, 172. [Google Scholar] [CrossRef]
- Cawthon, C.R.; de La Serre, C.B. The Critical Role of CCK in the Regulation of Food Intake and Diet-Induced Obesity. Peptides 2021, 138, 170492. [Google Scholar] [CrossRef]
- Pirník, Z.; Kořínková, L.; Osacká, J.; Železná, B.; Kuneš, J.; Maletínská, L. Cholecystokinin System Is Involved in the Anorexigenic Effect of Peripherally Applied Palmitoylated Prolactin-Releasing Peptide in Fasted Mice. Physiol. Res. 2021, 70, 579–590. [Google Scholar] [CrossRef]
- Schalla, M.A.; Taché, Y.; Stengel, A. Neuroendocrine Peptides of the Gut and Their Role in the Regulation of Food Intake. Compr. Physiol. 2021, 11, 1679–1730. [Google Scholar] [CrossRef]
- Tack, J.; Verbeure, W.; Mori, H.; Schol, J.; Van den Houte, K.; Huang, I.; Balsiger, L.M.; Broeders, B.; Colomier, E.; Scarpellini, E.; et al. The Gastrointestinal Tract in Hunger and Satiety Signalling. United Eur. Gastroenterol. J. 2021, 9, 727–734. [Google Scholar] [CrossRef]
- Roh, E.; Choi, K.M. Hormonal Gut–Brain Signaling for the Treatment of Obesity. Int. J. Mol. Sci. 2023, 24, 3384. [Google Scholar] [CrossRef]
- Chang, R.B. Body Thermal Responses and the Vagus Nerve. Neurosci. Lett. 2019, 698, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Burmester, V.; Higgs, S.; Terry, P. Rapid-Onset Anorectic Effects of Intranasal Oxytocin in Young Men. Appetite 2018, 130, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Masule, M.V.; Rathod, S.; Agrawal, Y.O.; Patil, C.R.; Nakhate, K.T.; Ojha, S.; Goyal, S.N.; Mahajan, U.B. Ghrelin Mediated Regulation of Neurosynaptic Transmitters in Depressive Disorders. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100113. [Google Scholar] [CrossRef]
- Li, S.; Liu, M.; Cao, S.; Liu, B.; Li, D.; Wang, Z.; Sun, H.; Cui, Y.; Shi, Y. The Mechanism of the Gut-Brain Axis in Regulating Food Intake. Nutrients 2023, 15, 3728. [Google Scholar] [CrossRef]
- Barakat, G.M.; Ramadan, W.; Assi, G.; Khoury, N.B.E. Satiety: A Gut–Brain–Relationship. J. Physiol. Sci. 2024, 74, 11. [Google Scholar] [CrossRef]
- Yahagi, A.; Iseki, M.; Yaku, K.; Nakagawa, T.; Itoh, M.; Mukai, T.; Ishihara, K. Aberrant humoral immune responses and intestinal homeostasis in Cd38 Bst1 double knockout mice. Immunohorizons 2025, 9, vlaf029. [Google Scholar] [CrossRef]
- Kim, H.S.; Jung, C.H. Oral Semaglutide, the First Ingestible Glucagon-Like Peptide-1 Receptor Agonist: Could It Be a Magic Bullet for Type 2 Diabetes? Int. J. Mol. Sci. 2021, 22, 9936. [Google Scholar] [CrossRef]
- Hoffman, S.; Adeli, K. Glucagon-like Peptide (GLP)-1 Regulation of Lipid and Lipoprotein Metabolism. Med. Rev. 2024, 4, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.K. Mechanisms of Action and Therapeutic Applications of GLP-1 and Dual GIP/GLP-1 Receptor Agonists. Front. Endocrinol. 2024, 15, 1431292. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yan, Q.; Wang, P.; Guo, W.; Yu, L. Therapeutic Potential of Ghrelin/GOAT/GHSR System in Gastrointestinal Disorders. Front. Nutr. 2024, 11, 1422431. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Young, W.S.; Song, J. Characterization of Oxytocin Receptor Expression Within Various Neuronal Populations of the Mouse Dorsal Hippocampus. Front. Mol. Neurosci. 2020, 13, 40. [Google Scholar] [CrossRef]
- Chukwunyere, U.; Mercan, M.; Şehirli, A.Ö.; Abacıoğlu, N. Possible Cytoprotective Mechanisms of Oxytocin against 5-Fluorouracil-Induced Gastrointestinal Mucositis. Mol. Biol. Rep. 2022, 49, 4055–4059. [Google Scholar] [CrossRef]
- Gould, F.; Lammers, A.R.; Mayerl, C.J.; German, R.Z. Specific Vagus Nerve Lesion Have Distinctive Physiologic Mechanisms of Dysphagia. Front. Neurol. 2019, 10, 1301. [Google Scholar] [CrossRef]
- Chen, J. Ineffective Esophageal Motility and the Vagus: Current Challenges and Future Prospects. Clin. Exp. Gastroenterol. 2016, 9, 291–299. [Google Scholar] [CrossRef]
- Tack, J.; Pandolfino, J.E. Pathophysiology of Gastroesophageal Reflux Disease. Gastroenterology 2017, 154, 277. [Google Scholar] [CrossRef]
- Lin, S.; Li, H.; Fang, X. Esophageal Motor Dysfunctions in Gastroesophageal Reflux Disease and Therapeutic Perspectives. J. Neurogastroenterol. Motil. 2019, 25, 499–507. [Google Scholar] [CrossRef]
- Vogt, C.; Panoskaltsis-Mortari, A. Tissue Engineering of the Gastroesophageal Junction. J. Tissue Eng. Regen. Med. 2020, 14, 855–868. [Google Scholar] [CrossRef]
- Rosen, R.D.; Winters, R. Physiology, Lower Esophageal Sphincter; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Noble, E.E.; Billington, C.J.; Kotz, C.M.; Wang, C. Oxytocin in the Ventromedial Hypothalamic Nucleus Reduces Feeding and Acutely Increases Energy Expenditure. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2014, 307, R737–R745. [Google Scholar] [CrossRef]
- Olson, B.R.; Drutarosky, M.D.; CHOW, M.-S.; Hruby, V.J.; Stricker, E.M.; Verbalis, J.G. Oxytocin and an Oxytocin Agonist Administered Centrally Decrease Food Intake in Rats. Peptides 1991, 12, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.; Arnold, M.; Pettersen, K.G.; Lossel, P.; Langhans, W.; Lee, S.J. Knockdown of GLP-1 Receptors in Vagal Afferents Affects Normal Food Intake and Glycemia. Diabetes 2015, 65, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Seelke, A.M.H.; Rhine, M.A.; Khun, K.; Shweyk, A.N.; Scott, A.M.; Bond, J.; Graham, J.L.; Havel, P.J.; Wolden-Hanson, T.; Bales, K.L.; et al. Intranasal Oxytocin Reduces Weight Gain in Diet-Induced Obese Prairie Voles. Physiol. Behav. 2018, 196, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Powley, T.L.; Baronowsky, E.A.; Gilbert, J.M.; Hudson, C.N.; Martin, F.N.; Mason, J.K.; McAdams, J.L.; Phillips, R.J. Vagal Afferent Innervation of the Lower Esophageal Sphincter. Auton. Neurosci. 2013, 177, 129–142. [Google Scholar] [CrossRef]
- Ng, Q.X.; Yaow, C.Y.L.; Moo, J.R.; Koo, S.W.K.; Loo, E.X.L.; Siah, K.T.H. A systematic review of the association between environmental risk factors and the development of irritable bowel syndrome. J. Gastroenterol. Hepatol. 2024, 39, 1780–1787. [Google Scholar] [CrossRef]
- Barbara, G.; Regazzi, M.G.; Barbaro, M.R.; Dothel, G.; Tarricone, I. The Brain-Gut Axis and the Gender. In Health and Gender; Springer: Cham, Switzerland, 2019; p. 245. [Google Scholar]
- Harsányiová, J.; Ru, F.; Žatko, T.; Kollárik, M.; Hennel, M. Vagus Nerves Provide a Robust Afferent Innervation of the Mucosa Throughout the Body of the Esophagus in the Mouse. Dysphagia 2019, 35, 471–478. [Google Scholar] [CrossRef]
- Roberts, Z.S.; Wolden-Hanson, T.; Matsen, M.E.; Ryu, V.; Vaughan, C.H.; Graham, J.L.; Havel, P.J.; Chukri, D.W.; Schwartz, M.W.; Morton, G.J.; et al. Chronic Hindbrain Administration of Oxytocin Is Sufficient to Elicit Weight Loss in Diet-Induced Obese Rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2017, 313, R357–R371. [Google Scholar] [CrossRef]
- Ohlsson, B.; Truedsson, M.; Djerf, P.; Sundler, F. Oxytocin Is Expressed throughout the Human Gastrointestinal Tract. Regul. Pept. 2006, 135, 7–11. [Google Scholar] [CrossRef]
- Arletti, R.; Benelli, A.; Bertolini, A. Oxytocin Inhibits Food and Fluid Intake in Rats. Physiol. Behav. 1990, 48, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Arletti, R.; Benelli, A.; Bertolini, A. Influence of Oxytocin on Feeding Behavior in the Rat. Peptides 1989, 10, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Gartner, S.N.; Klockars, A.; Prosser, C.G.; Carpenter, E.; Levine, A.S.; Olszewski, P.K. Identification of Central Mechanisms Underlying Anorexigenic Effects of Intraperitoneal L-Tryptophan. Neuroreport 2018, 29, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Dyavanapalli, J.; Rodriguez, J.; Santos, C.R.d.; Escobar, J.B.; Dwyer, M.; Schloen, J.; Lee, K.-M.; Wolaver, W.; Wang, X.; Dergacheva, O.; et al. Activation of Oxytocin Neurons Improves Cardiac Function in a Pressure-Overload Model of Heart Failure. JACC Basic Transl. Sci. 2020, 5, 484–497. [Google Scholar] [CrossRef]
- Ding, C.; Leow, M.K.; Magkos, F. Oxytocin in Metabolic Homeostasis: Implications for Obesity and Diabetes Management. Obes. Rev. 2018, 20, 22–40. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Z.; Ma, B.; Fan, L.; Yi, N.; Lü, B.; Wang, Q.; Liu, R. GLP-1 Improves Adipocyte Insulin Sensitivity Following Induction of Endoplasmic Reticulum Stress. Front. Pharmacol. 2018, 9, 1168. [Google Scholar] [CrossRef]
- Labyb, M.; Chrétien, C.; Caillon, A.; Rohner-Jeanrenaud, F.; Altirriba, J. Oxytocin Administration Alleviates Acute but Not Chronic Leptin Resistance of Diet-Induced Obese Mice. Int. J. Mol. Sci. 2018, 20, 88. [Google Scholar] [CrossRef]
- McCormack, S.E.; Blevins, J.E.; Lawson, E.A. Metabolic Effects of Oxytocin. Endocr. Rev. 2019, 41, 121–145. [Google Scholar] [CrossRef]
- Tanday, N.; English, A.; Lafferty, R.A.; Flatt, P.R.; Irwin, N. Benefits of Sustained Upregulated Unimolecular GLP-1 and CCK Receptor Signalling in Obesity-Diabetes. Front. Endocrinol. 2021, 12, 674704. [Google Scholar] [CrossRef]
- Head, M.A.; Levine, A.S.; Christian, D.; Klockars, A.; Olszewski, P.K. Effect of Combination of Peripheral Oxytocin and Naltrexone at Subthreshold Doses on Food Intake, Body Weight and Feeding-Related Brain Gene Expression in Male Rats. Physiol. Behav. 2021, 238, 113464. [Google Scholar] [CrossRef]
- Dong, Y.; Carty, J.R.E.; Goldstein, N.; He, Z.; Hwang, E.; Chau, D.; Wallace, B.; Kabahizi, A.; Lieu, L.; Peng, Y.; et al. Time and Metabolic State-Dependent Effects of GLP-1R Agonists on NPY/AgRP and POMC Neuronal Activity in Vivo. Mol. Metab. 2021, 54, 101352. [Google Scholar] [CrossRef] [PubMed]
- Austin, G.O.; Tomás, A. Variation in Responses to Incretin Therapy: Modifiable and Non-Modifiable Factors. Front. Mol. Biosci. 2023, 10, 1170181. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.-C.; Zhou, L.; Ghee, S.M.; See, R.E.; Reichel, C.M. Oxytocin Decreases Cocaine Taking, Cocaine Seeking, and Locomotor Activity in Female Rats. Exp. Clin. Psychopharmacol. 2015, 24, 55–64. [Google Scholar] [CrossRef]
- Rogers, R.C.; Hermann, G.E. Hypothalamic Paraventricular Nucleus Stimulation-Induced Gastric Acid Secretion and Bradycardia Suppressed by Oxytocin Antagonist. Peptides 1986, 7, 695–700. [Google Scholar] [CrossRef]
- Lefèvre, A.; Benusiglio, D.; Tang, Y.; Krabichler, Q.; Charlet, A.; Grinevich, V. Oxytocinergic Feedback Circuitries: An Anatomical Basis for Neuromodulation of Social Behaviors. Front. Neural Circuits 2021, 15, 688234. [Google Scholar] [CrossRef]
- van der Klaauw, A.A.; Ziauddeen, H.; Keogh, J.M.; Henning, E.; Dachi, S.V.; Fletcher, P.C.; Farooqi, I.S. Oxytocin Administration Suppresses Hypothalamic Activation in Response to Visual Food Cues. Sci. Rep. 2017, 7, 4266. [Google Scholar] [CrossRef]
- Quintana, D.; Smerud, K.T.; Andreassen, O.A.; Djupesland, P.G. Evidence for Intranasal Oxytocin Delivery to the Brain: Recent Advances and Future Perspectives. Ther. Deliv. 2018, 9, 515–525. [Google Scholar] [CrossRef]
- Lee, M.R.; Jayant, R.D. Penetration of the Blood–brain barrier by Peripheral Neuropeptides: New Approaches to Enhancing Transport and Endogenous Expression. Cell Tissue Res. 2018, 375, 287–293. [Google Scholar] [CrossRef]
- Smith, A.S.; Korgan, A.C.; Young, W.S. Oxytocin Delivered Nasally or Intraperitoneally Reaches the Brain and Plasma of Normal and Oxytocin Knockout Mice. Pharmacol. Res. 2019, 146, 104324. [Google Scholar] [CrossRef]
- Maejima, Y.; Iwasaki, Y.; Yamahara, Y.; Kodaira, M.; Sedbazar, U.; Yada, T. Peripheral Oxytocin Treatment Ameliorates Obesity by Reducing Food Intake and Visceral Fat Mass. Aging 2011, 3, 1169–1177. [Google Scholar] [CrossRef]
- Augustine, M.E.; Leerkes, E.M.; Smolen, A.; Calkins, S.D. Relations between Early Maternal Sensitivity and Toddler Self-Regulation: Exploring Variation by Oxytocin and Dopamine D2 Receptor Genes. Dev. Psychobiol. 2018, 60, 789–804. [Google Scholar] [CrossRef]
- Takayanagi, Y.; Kasahara, Y.; Onaka, T.; Takahashi, N.; Kawada, T.; Nishimori, K. Oxytocin Receptor-De¢cient Mice Developed Late-Onset Obesity. Neuroreport 2008, 19, 951–955. [Google Scholar] [CrossRef]

| Domain | Mechanisms and Effects | Key Findings |
|---|---|---|
| Satiety signaling |
|
|
| Meal patterns |
|
|
| Metabolic regulation |
|
|
| Sex differences |
|
|
| Study | Model | Study Type | Key Characteristics | Main Findings | Quality/Limitations |
|---|---|---|---|---|---|
| [3] | Rat | Experimental | Methamphetamine self-administration; peripheral OT administration | Vagus nerve mediates OT’s suppressing effects on drug seeking behavior | High quality; specific behavioral paradigm |
| [5] | Mouse/Rat | Histological/Developmental | Expression of OT/OTR in enteric nervous system across development | OT and OTR widely expressed in ENS and intestinal epithelium | High quality; comprehensive developmental analysis |
| [6] | Mouse | Experimental/Genetic | Vagal OTR mouse model; esophageal motility assessment | Vagal OTRs necessary for normal esophageal motility and function | High quality; genetic approach with functional outcomes |
| [7] | Rat | Electrophysiological | Stress-induced gastric motility; hypothalamic–vagal circuitry | OT modulates gastric emptying via hypothalamic–vagal pathways following stress | High quality; mechanistic approach |
| [14] | Rat | Physiological | Chronic intermittent hypoxia model; cardiovascular outcomes | OT neuron activation prevents hypertension in sleep apnea model | Good quality; clinically relevant model |
| [20] | Rat | Pharmacological | Distal colon motility; nitrergic mechanisms | OT inhibits colonic contractions via NO-cGMP-K+ channel pathway | High quality; detailed mechanistic analysis |
| [21] | Mouse | Pharmacological/Behavioral | Central 5-HT mediation; colonic motility assessment | Central 5-HT mediates colonic motility through hypothalamic OT–colonic OTR pathway | Good quality; dual central–peripheral approach |
| [22] | Rat | Metabolic | Diet-induced obesity model; peripheral OT administration | Peripheral OT suppresses food intake and causes weight loss in obese rats | High quality; clinically relevant obesity model |
| [23] | Human | Clinical trial | Single-dose intranasal OT in healthy men | OT reduces caloric intake in men | Moderate quality; small sample, single-dose design |
| [24] | Rat | Neurophysiological | NTS OTR signaling; feeding behavior | Endogenous OTR signaling in NTS controls satiation and thermogenesis | High quality; specific brain region focus |
| [25] | Human | Clinical trial | Post-stress eating in women; intranasal OT | OT reduces sweet snack intake without affecting cortisol | Good quality; gender-specific, stress paradigm |
| [27] | Mouse | Metabolic | Obese mouse model; thermogenesis assessment | OT improves metabolic dysfunction via increased thermogenesis | Good quality; mechanistic metabolic focus |
| [30] | Mouse | Pharmacological | GI motility, inflammation, permeability assessment | OT regulates multiple GI functions including motility and inflammation | High quality; comprehensive GI analysis |
| [36] | Mouse | Neuroanatomical | Peripheral-to-central OT relay via vagal afferents | Peripheral OT activates central OT neurons via vagal pathway for feeding control | High quality; novel relay mechanism identified |
| [53] | Mouse | Pharmacological | Vagal afferent activation; feeding behavior in normal and leptin-resistant mice | Peripheral OT activates vagal afferents to suppress feeding in both normal and leptin-resistant states | High quality; clinically relevant leptin resistance model |
| [59] | Rat | Electrophysiological | Vagal afferent fiber role in OT-induced gastric modulation | Vagal afferents determine OT-induced gastric tone modulation | High quality; direct neural recording approach |
| [60] | Rat | Pharmacological | NTS OTR signaling; satiation signal processing | NTS OTR signaling processes GI satiation signals for food intake control | High quality; specific satiation mechanism focus |
| [48] | Rat | Stress model | Water-avoidance stress; colonic motility | OT inhibits stress-induced accelerated colonic motility | Good quality; stress-specific GI effects |
| [49] | Rat | Pharmacological | Gastric smooth muscle; motility assessment | OTRs on gastric smooth muscle mediate excitatory effects on motility | Good quality; direct tissue-level analysis |
| [54] | Mouse | Genetic/Optogenetic | Vagal sensory neuron identification and manipulation | Genetic identification of specific vagal neurons controlling feeding | High quality; cutting-edge genetic tools |
| [58] | Rat | Physiological | Forestomach pressure; dorsal vagal complex involvement | OT increases intragastric pressure via dorsal vagal complex | Good quality; specific gastric region focus |
| [71] | Human | Clinical | Healthy women; colonic motor activity assessment | OT stimulates colonic motor activity in healthy women | Good quality; gender-specific clinical data |
| [72] | Human | Clinical trial | Obese vs. normal-weight men; food intake assessment | OT’s anorexic effects stronger in obese than normal-weight men | Good quality; BMI-stratified analysis |
| [128] | Prairie vole | Metabolic | Diet-induced obesity model; intranasal OT treatment | Intranasal OT reduces weight gain in diet-induced obese prairie voles | Moderate quality; non-traditional rodent model |
| [133] | Rat | Pharmacological | Chronic hindbrain OT administration; weight loss | Chronic hindbrain OT sufficient to elicit weight loss in obese rats | High quality; chronic treatment paradigm |
| [154] | Mouse | Metabolic | Peripheral OT treatment; obesity and food intake | Peripheral OT ameliorates obesity by reducing food intake and visceral fat | Good quality; comprehensive metabolic assessment |
| [144] | Rat | Pharmacological | Combined OT and naltrexone treatment; feeding behavior | Subthreshold OT–naltrexone combination affects feeding and brain gene expression | Good quality; novel combination therapy approach |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacka, A.; Śniegocki, M.; Ziółkowska, E.A. Vagal Oxytocin Receptors as Molecular Targets in Gut–Brain Signaling: Implications for Appetite, Satiety, Obesity, and Esophageal Motility—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 7812. https://doi.org/10.3390/ijms26167812
Nowacka A, Śniegocki M, Ziółkowska EA. Vagal Oxytocin Receptors as Molecular Targets in Gut–Brain Signaling: Implications for Appetite, Satiety, Obesity, and Esophageal Motility—A Narrative Review. International Journal of Molecular Sciences. 2025; 26(16):7812. https://doi.org/10.3390/ijms26167812
Chicago/Turabian StyleNowacka, Agnieszka, Maciej Śniegocki, and Ewa A. Ziółkowska. 2025. "Vagal Oxytocin Receptors as Molecular Targets in Gut–Brain Signaling: Implications for Appetite, Satiety, Obesity, and Esophageal Motility—A Narrative Review" International Journal of Molecular Sciences 26, no. 16: 7812. https://doi.org/10.3390/ijms26167812
APA StyleNowacka, A., Śniegocki, M., & Ziółkowska, E. A. (2025). Vagal Oxytocin Receptors as Molecular Targets in Gut–Brain Signaling: Implications for Appetite, Satiety, Obesity, and Esophageal Motility—A Narrative Review. International Journal of Molecular Sciences, 26(16), 7812. https://doi.org/10.3390/ijms26167812








