Fibroblast Activation Protein (FAP) as a Serum Biomarker for Fibrotic Ovarian Aging: A Clinical Validation Study Based on Translational Transcriptomic Targets
Abstract
1. Introduction
2. Results
2.1. ML Identification of Possible Candidates with Divergent Age-Related Expression in Ovarian Tissue
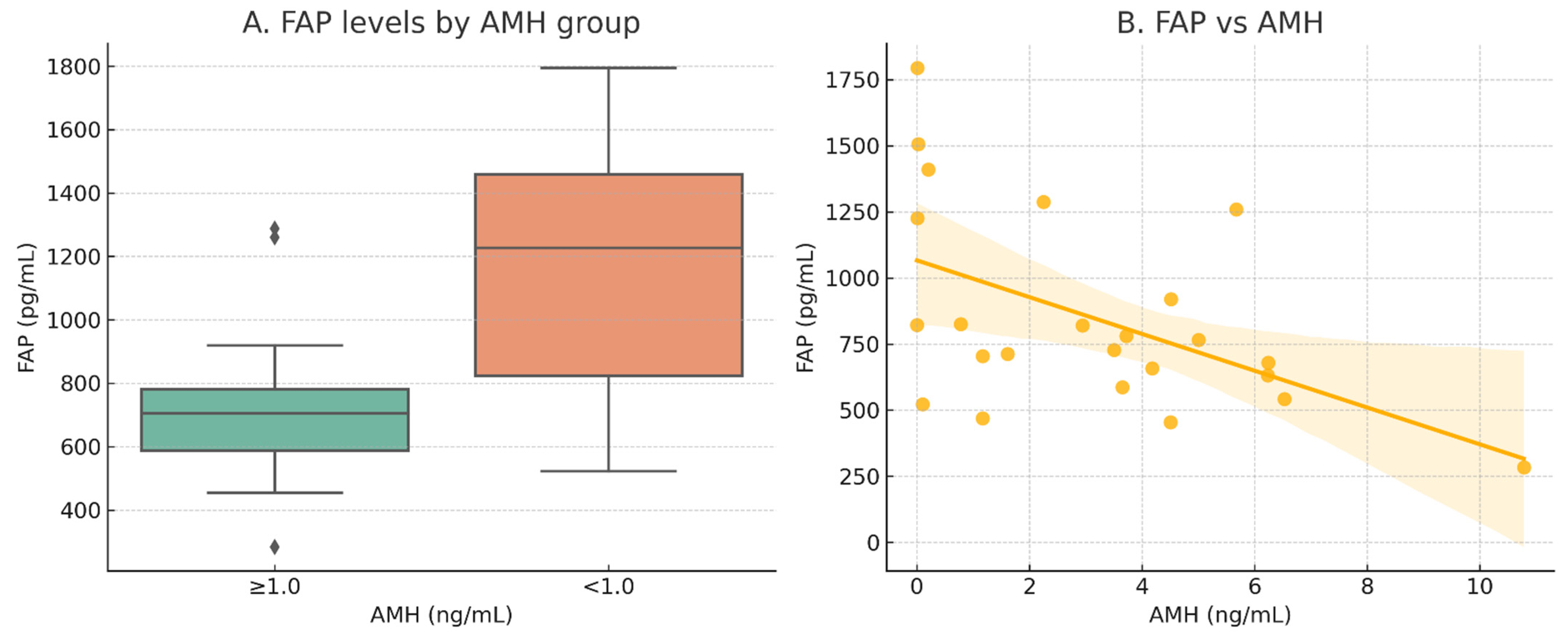
2.2. Circulating FAP Specifically Reflects Ovarian Reserve in Real-World Samples
3. Discussion
4. Materials and Methods
4.1. Bioinformatic Identification of Ovarian Aging Biomarker Candidates
4.1.1. Data Acquisition and Preprocessing
4.1.2. Supervised Machine Learning (ML) and Feature Selection
4.1.3. Pathway Enrichment Analysis
4.2. Clinical Validation Study Design
Cohort Recruitment
4.3. Laboratory Measurements
4.3.1. Serum Biomarker Quantification
4.3.2. Hormonal Assays
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chon, S.J.; Umair, Z.; Yoon, M.S. Premature Ovarian Insufficiency: Past, Present, and Future. Front. Cell Dev. Biol. 2021, 9, 672890. [Google Scholar] [CrossRef]
- Jiao, X.; Meng, T.; Zhai, Y.; Zhao, L.; Luo, W.; Liu, P.; Qin, Y. Ovarian Reserve Markers in Premature Ovarian Insufficiency: Within Different Clinical Stages and Different Etiologies. Front. Endocrinol. 2021, 12, 601752. [Google Scholar] [CrossRef]
- Cavalcante, M.B.; Sampaio, O.G.M.; Câmara, F.E.A.; Schneider, A.; de Ávila, B.M.; Prosczek, J.; Masternak, M.M.; Campos, A.R. Ovarian aging in humans: Potential strategies for extending reproductive lifespan. GeroScience 2023, 45, 2121–2133. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, C.; Yang, C.; Shan, X.; Meng, X.Q.; Zhang, M. Unveiling the role of chronic inflammation in ovarian aging: Insights into mechanisms and clinical implications. Hum. Reprod. 2024, 39, 1599–1607. [Google Scholar] [CrossRef]
- Jamil, Z.; Fatima, S.S.; Ahmed, K.; Malik, R. Anti-Mullerian Hormone: Above and Beyond Conventional Ovarian Reserve Markers. Dis. Markers 2016, 2016, 5246217. [Google Scholar] [CrossRef]
- Karaviti, E.; Karaviti, D.; Kani, E.R.; Chatziandreou, E.; Paschou, S.A.; Psaltopoulou, T.; Kalantaridou, S.; Lambrinoudaki, I. The role of anti-Müllerian hormone: Insights into ovarian reserve, primary ovarian insufficiency, and menopause prediction. Endocrine 2025, 89, 338–355. [Google Scholar] [CrossRef]
- Jeong, H.G.; Kim, S.K.; Lee, J.R.; Jee, B.C. Correlation of oocyte number with serum anti-Müllerian hormone levels measured by either Access or Elecsys in fresh in vitro fertilization cycles. Clin. Exp. Reprod. Med. 2022, 49, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, H.O.; Lee, S.Y.; Park, E.A.; Choi, K.H.; Kang, K.; Yu, E.J.; Koong, M.K.; Lee, K.A. Serum miR-329-3p as a potential biomarker for poor ovarian response in an in vitro fertilization. Clin. Exp. Reprod. Med. 2025, 52, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Isola, J.V.V.; Ocañas, S.R.; Hubbart, C.R.; Ko, S.; Mondal, S.A.; Hense, J.D.; Carter, H.N.C.; Schneider, A.; Kovats, S.; Alberola-Ila, J.; et al. A single-cell atlas of the aging mouse ovary. Nat. Aging 2024, 4, 145–162. [Google Scholar] [CrossRef]
- Umehara, T.; Winstanley, Y.E.; Andreas, E.; Morimoto, A.; Williams, E.J.; Smith, K.M.; Carroll, J.; Febbraio, M.A.; Shimada, M.; Russell, D.L.; et al. Female reproductive life span is extended by targeted removal of fibrotic collagen from the mouse ovary. Sci. Adv. 2022, 8, eabn4564. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Wang, Y.; Yu, Y. Ovarian fibrosis: Molecular mechanisms and potential therapeutic targets. J. Ovarian Res. 2024, 17, 139. [Google Scholar] [CrossRef]
- Bae, S.J.; Jo, Y.; Cho, M.K.; Jin, J.S.; Kim, J.Y.; Shim, J.; Kim, Y.H.; Park, J.-K.; Ryu, D.; Lee, H.J.; et al. Identification and analysis of novel endometriosis biomarkers via integrative bioinformatics. Front. Endocrinol. 2022, 13, 942368. [Google Scholar] [CrossRef]
- Wu, W.; Liu, C.; Farrar, C.A.; Ma, L.; Dong, X.; Sacks, S.H.; Li, K.; Zhou, W. Collectin-11 Promotes the Development of Renal Tubulointerstitial Fibrosis. J. Am. Soc. Nephrol. 2018, 29, 168–181. [Google Scholar] [CrossRef]
- Davis, S.R.; Pinkerton, J.; Santoro, N.; Simoncini, T. Menopause—Biology, consequences, supportive care, and therapeutic options. Cell 2023, 186, 4038–4058. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Wang, X.; Yang, J.; Kim, S.; Hudgins, A.D.; Gamliel, A.; Pei, M.; Contreras, D.; Devos, M.; Guo, Q.; et al. Molecular and genetic insights into human ovarian aging from single-nuclei multi-omics analyses. Nat. Aging 2025, 5, 275–290. [Google Scholar] [CrossRef]
- Steiner, A.Z.; Jukic, A.M.Z. Impact of female age and nulligravidity on fecundity in an older reproductive age cohort. Fertil. Steril. 2016, 105, 1584–1588.e1. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, L.; Yang, D.; Luo, J.; Gao, J.; Wang, J.; Guo, H.; Li, J.; Wang, F.; Wu, J.; et al. Chronic stress causes ovarian fibrosis to impair female fertility in mice. Cell. Signal. 2024, 122, 111334. [Google Scholar] [CrossRef] [PubMed]
- Camaioni, A.; Ucci, M.A.; Campagnolo, L.; De Felici, M.; Klinger, F.G. The process of ovarian aging: It is not just about oocytes and granulosa cells. J. Assist. Reprod. Genet. 2022, 39, 783–792. [Google Scholar] [CrossRef]
- Shen, L.; Liu, J.; Luo, A.; Wang, S. The stromal microenvironment and ovarian aging: Mechanisms and therapeutic opportunities. J. Ovarian Res. 2023, 16, 237. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- García-Domínguez, M. Pathological and Inflammatory Consequences of Aging. Biomolecules 2025, 15, 404. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Basalova, N.; Alexandrushkina, N.; Grigorieva, O.; Kulebyakina, M.; Efimenko, A. Fibroblast Activation Protein Alpha (FAPα) in Fibrosis: Beyond a Perspective Marker for Activated Stromal Cells? Biomolecules 2023, 13, 1718. [Google Scholar] [CrossRef]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef] [PubMed]
- Amargant, F.; Magalhaes, C.; Pritchard, M.T.; Duncan, F.E. Systemic low-dose anti-fibrotic treatment attenuates ovarian aging in the mouse. GeroScience 2025, 47, 3475–3495. [Google Scholar] [CrossRef]
- Ouni, E.; Peaucelle, A.; Haas, K.T.; Van Kerk, O.; Dolmans, M.-M.; Tuuri, T.; Otala, M.; Amorim, C.A. A blueprint of the topology and mechanics of the human ovary for next-generation bioengineering and diagnosis. Nat. Commun. 2021, 12, 5603. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.T.; Kim, Y.O.; Yan, X.Z.; Abe, H.; Aslam, M.; Park, K.S.; Zhao, X.Y.; Jia, J.D.; Klein, T.; You, H.; et al. Fibroblast Activation Protein Activates Macrophages and Promotes Parenchymal Liver Inflammation and Fibrosis. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 841–867. [Google Scholar] [CrossRef]
- Lavis, P.; Garabet, A.; Cardozo, A.K.; Bondue, B. The fibroblast activation protein alpha as a biomarker of pulmonary fibrosis. Front. Med. 2024, 11, 1393778. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.; Farrar, C.A.; Sacks, S.H. Structural and functional diversity of collectins and ficolins and their relationship to disease. Semin. Immunopathol. 2018, 40, 75–85. [Google Scholar] [CrossRef]
- Hao, J.; Liu, L.; Chang, B.; Zhao, Y.; Lai, Y.; Tian, C.; Xu, H.; Wang, H.; Ji, L.; Yang, J. Blood-detected mitochondrial biomarker NSUN4: A potential indicator of ovarian aging. Exp. Gerontol. 2025, 208, 112825. [Google Scholar] [CrossRef]
- Salemi, F.; Jambarsang, S.; Kheirkhah, A.; Salehi-Abargouei, A.; Ahmadnia, Z.; Hosseini, H.A.; Lotfi, M.; Amer, S. The best ovarian reserve marker to predict ovarian response following controlled ovarian hyperstimulation: A systematic review and meta-analysis. Syst. Rev. 2024, 13, 303. [Google Scholar] [CrossRef]
- Baker, K.M.; FernandezCriado, R.; Eaton, J.L.; Mensah, V.A. The Clinical Utility of Measures of Ovarian Reserve. Obstet. Gynecol. Surv. 2025, 80, 121–133. [Google Scholar] [CrossRef]
- Lee, H.J.; Noh, H.K.; Joo, J.K. Comparison of ART outcome in patients with poor ovarian response according to POSEIDON criteria. Sci. Rep. 2022, 12, 17723. [Google Scholar] [CrossRef] [PubMed]
- Peigné, M.; Bernard, V.; Dijols, L.; Creux, H.; Robin, G.; Hocké, C.; Grynberg, M.; Dewailly, D.; Sonigo, C. Using serum anti-Müllerian hormone levels to predict the chance of live birth after spontaneous or assisted conception: A systematic review and meta-analysis. Hum. Reprod. 2023, 38, 1789–1806. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.H.; Lee, H.J.; Joo, J.K.; Na, Y.J. Which Ovarian Reserve Marker is More Reliable in IVF Patients with AMH and AFC Discordance? Clin. Exp. Obstet. Gynecol. 2024, 51, 49. [Google Scholar] [CrossRef]
- Wu, M.; Tang, W.; Chen, Y.; Xue, L.; Dai, J.; Li, Y.; Zhu, X.; Wu, C.; Xiong, J.; Zhang, J.; et al. Spatiotemporal transcriptomic changes of human ovarian aging and the regulatory role of FOXP1. Nat. Aging 2024, 4, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Golezar, S.; Keshavarz, Z.; Ramezani Tehrani, F.; Ebadi, A.; Zayeri, F.; Golezar, M.H. Primary ovarian insufficiency quality of life scale (POIQOLS): Development and psychometric properties. BMC Women’s Health 2022, 22, 481. [Google Scholar] [CrossRef]
- Golezar, S.; Ramezani Tehrani, F.; Khazaei, S.; Ebadi, A.; Keshavarz, Z. The global prevalence of primary ovarian insufficiency and early menopause: A meta-analysis. Climacteric 2019, 22, 403–411. [Google Scholar] [CrossRef]
- Cooper, O.O.; Quint, E.H.; Smith, Y.R.; Dendrinos, M.L. FSH receptor variant: An unusual cause of secondary amenorrhea. J. Pediatr. Adolesc. Gynecol. 2025, in press. [CrossRef]
- Iwase, A.; Osuka, S.; Goto, M.; Murase, T.; Nakamura, T.; Takikawa, S.; Manabe, S.; Kikkawa, F. Anti-Müllerian hormone for screening, diagnosis, evaluation and prediction of functional ovarian reserve: A clinical perspective. J. Obstet. Gynaecol. Res. 2023, 49, 5150–5167. [Google Scholar] [CrossRef]
| Biomarker | Correlated Parameter | Cohort | Correlation Coefficient (r) | p-Value |
|---|---|---|---|---|
| FAP | Chronological Age | Overall (n = 72) | 0.089 | 0.458 |
| AMH | Subcohort (n = 24) | −0.517 | 0.01 | |
| FSH | Subcohort (n = 24) | 0.147 | 0.492 | |
| LH | Subcohort (n = 24) | −0.012 | 0.957 | |
| COLEC11 | Chronological Age | Overall (n = 72) | 0.415 | <0.001 |
| AMH | Subcohort (n = 24) | −0.258 | 0.223 |
| A | ||||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n = 72) | <45 years (n = 46) | ≥45 years (n = 26) | ||||||
| Variables | Mean | SD | Mean | SD | Mean | SD | t | p-Value |
| COLEC11 | 197.26 | 51.80 | 183.25 | 38.48 | 222.06 | 62.87 | −2.860 | 0.007 |
| FAP | 859.20 | 353.16 | 798.12 | 269.29 | 967.27 | 452.40 | −1.740 | 0.091 |
| B | ||||||||
| Overall (n = 24) | <45 years (n = 18) | ≥45 years (n = 6) | ||||||
| Variables | Mean | SD | Mean | SD | Mean | SD | t | p-Value |
| COLEC11 | 169.06 | 35.66 | 163.31 | 30.64 | 186.29 | 46.71 | −1.127 | 0.300 |
| FAP | 849.63 | 374.26 | 760.72 | 297.38 | 1116.36 | 479.40 | −1.711 | 0.135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.J.; Jo, Y.; Wei, S.; Yu, E.H.; Lee, S.; Ryu, D.; Joo, J.K. Fibroblast Activation Protein (FAP) as a Serum Biomarker for Fibrotic Ovarian Aging: A Clinical Validation Study Based on Translational Transcriptomic Targets. Int. J. Mol. Sci. 2025, 26, 7807. https://doi.org/10.3390/ijms26167807
Lee HJ, Jo Y, Wei S, Yu EH, Lee S, Ryu D, Joo JK. Fibroblast Activation Protein (FAP) as a Serum Biomarker for Fibrotic Ovarian Aging: A Clinical Validation Study Based on Translational Transcriptomic Targets. International Journal of Molecular Sciences. 2025; 26(16):7807. https://doi.org/10.3390/ijms26167807
Chicago/Turabian StyleLee, Hyun Joo, Yunju Jo, Shibo Wei, Eun Hee Yu, Sul Lee, Dongryeol Ryu, and Jong Kil Joo. 2025. "Fibroblast Activation Protein (FAP) as a Serum Biomarker for Fibrotic Ovarian Aging: A Clinical Validation Study Based on Translational Transcriptomic Targets" International Journal of Molecular Sciences 26, no. 16: 7807. https://doi.org/10.3390/ijms26167807
APA StyleLee, H. J., Jo, Y., Wei, S., Yu, E. H., Lee, S., Ryu, D., & Joo, J. K. (2025). Fibroblast Activation Protein (FAP) as a Serum Biomarker for Fibrotic Ovarian Aging: A Clinical Validation Study Based on Translational Transcriptomic Targets. International Journal of Molecular Sciences, 26(16), 7807. https://doi.org/10.3390/ijms26167807






