CD4+ T Cell Subsets and PTPN22 as Novel Biomarkers of Immune Dysregulation in Dilated Cardiomyopathy
Abstract
1. Introduction
2. Results
2.1. Immune Alterations in Dilated Cardiomyopathy
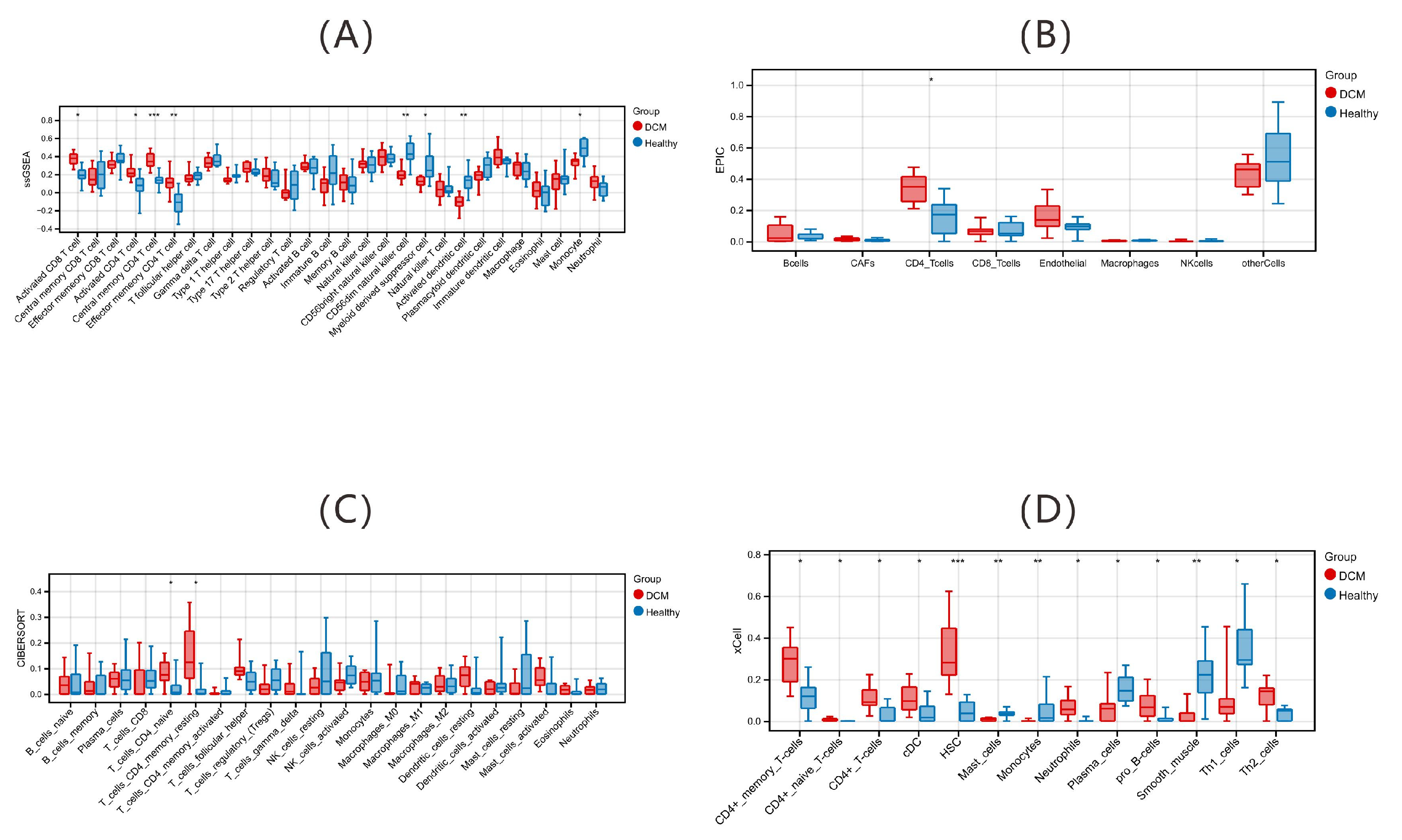
2.1.1. Immune Alterations via Deconvolution Algorithms
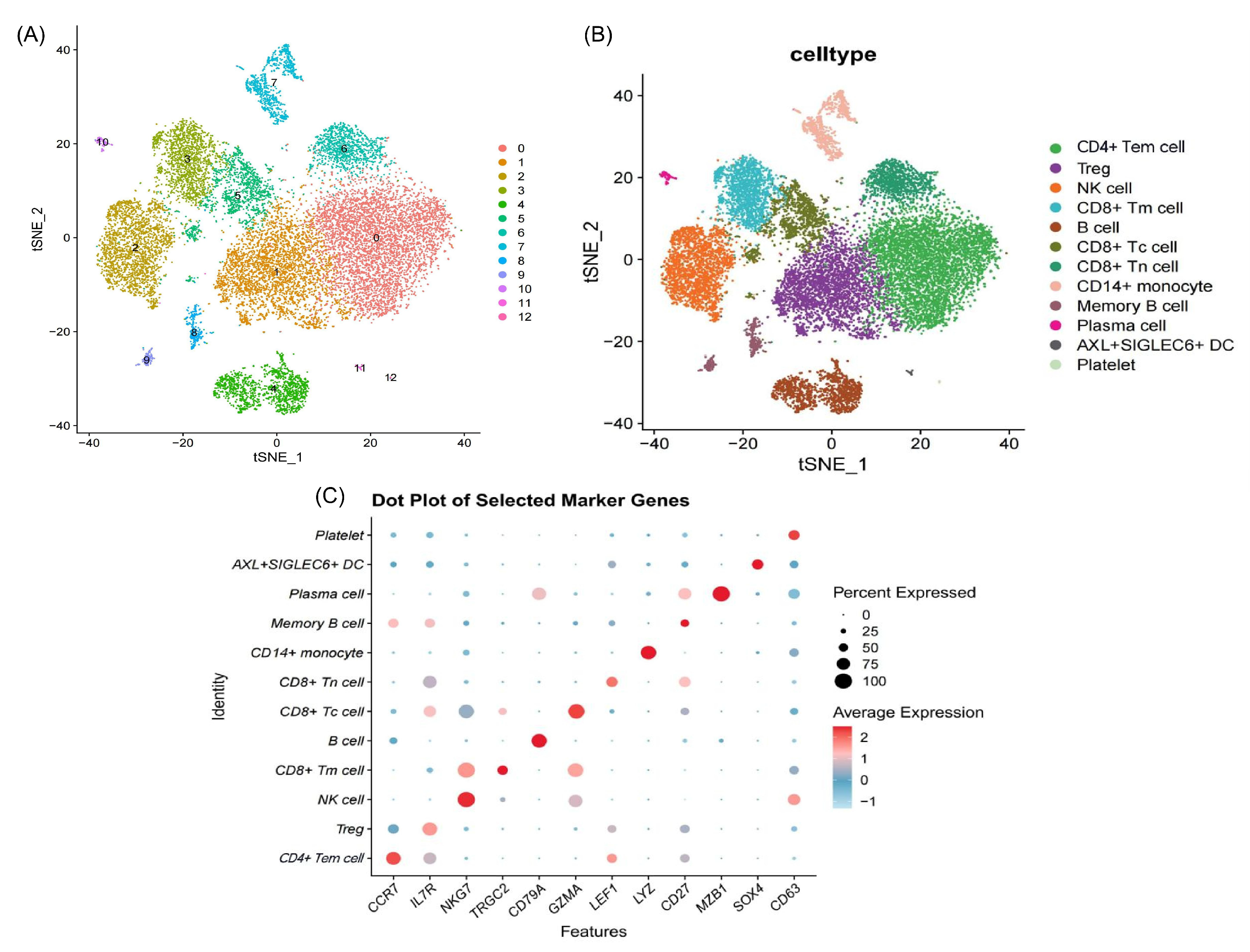
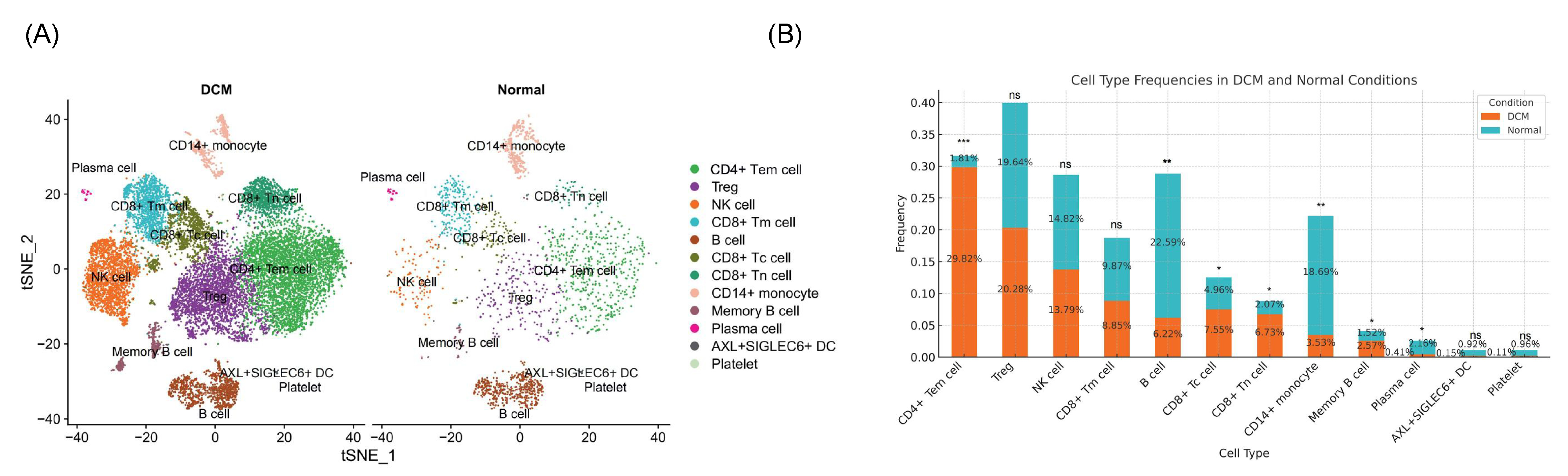
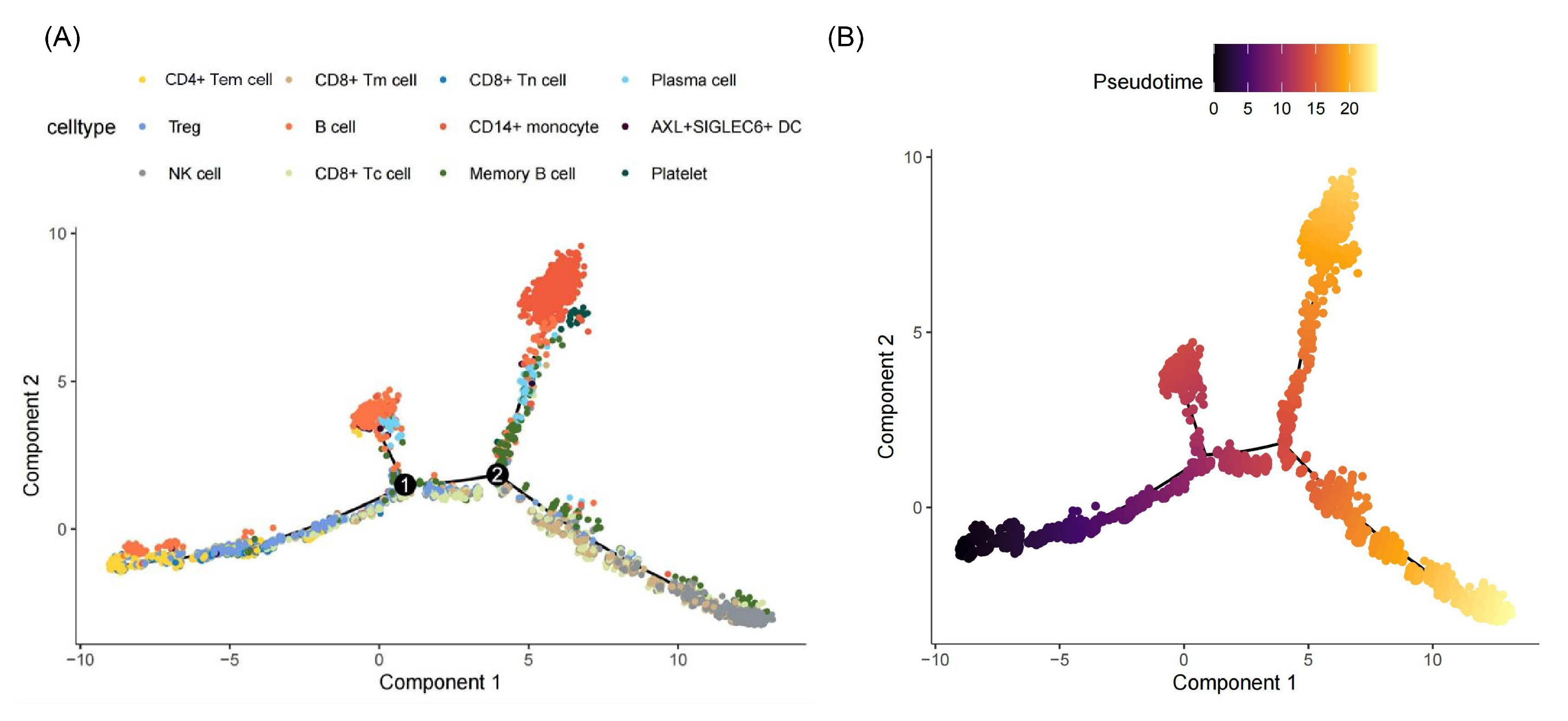
2.1.2. Immune Alterations via Single-Cell RNA Sequencing
2.2. Flow Cytometry Validates Algorithm-Predicted T Cell Shifts
2.3. Identification and Functions of Differentially Expressed Genes
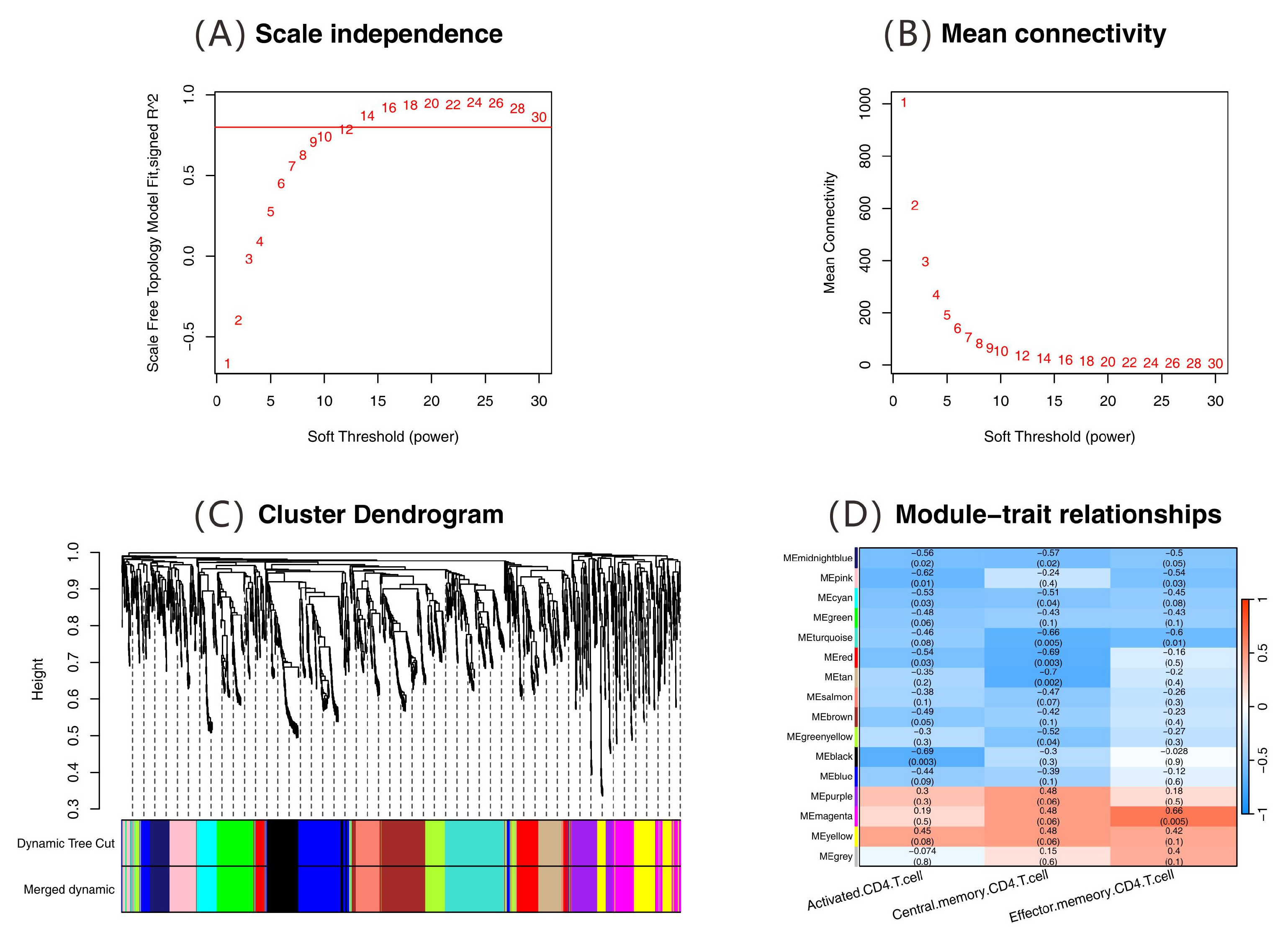
2.4. Analysis of Immune-Related Gene Networks
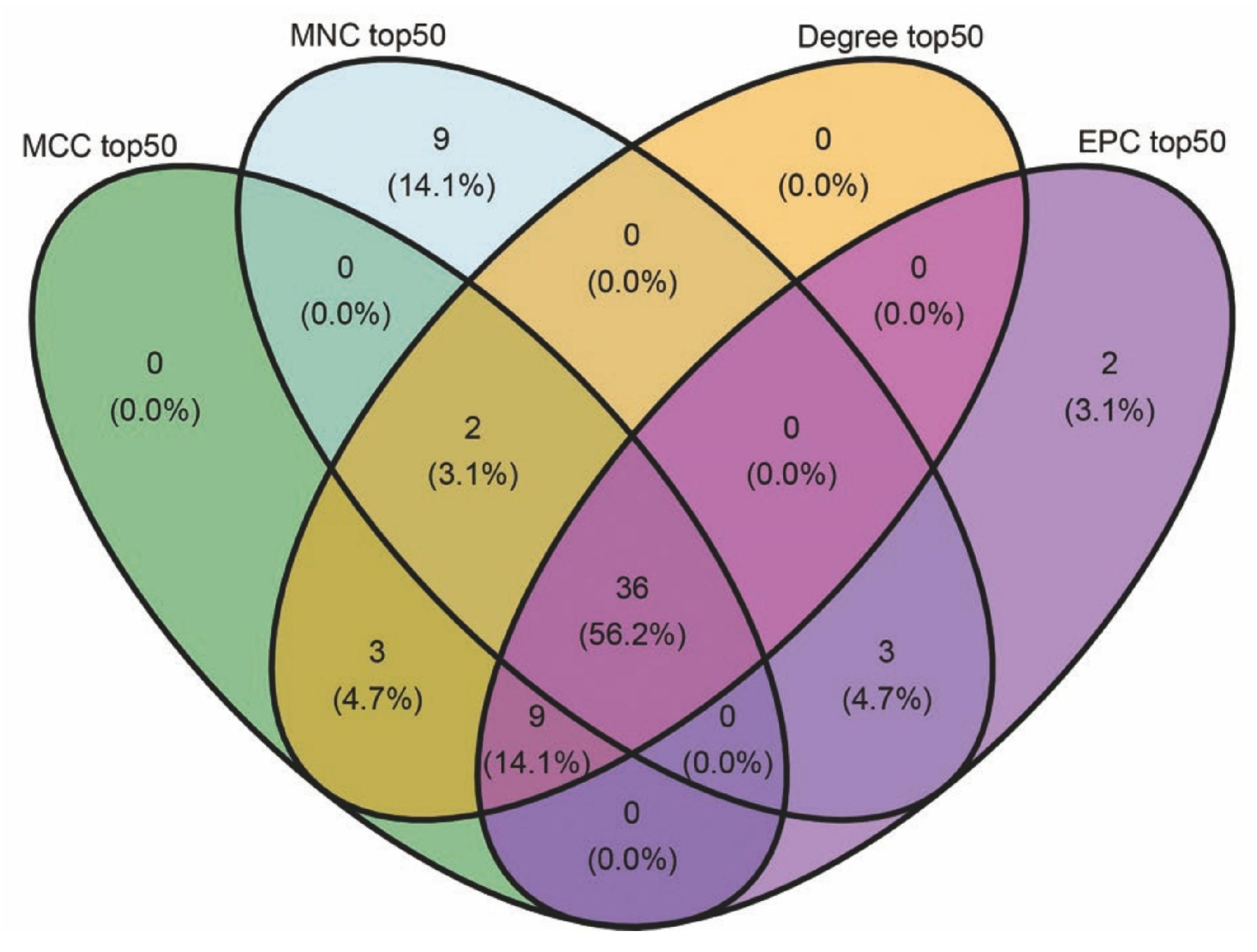
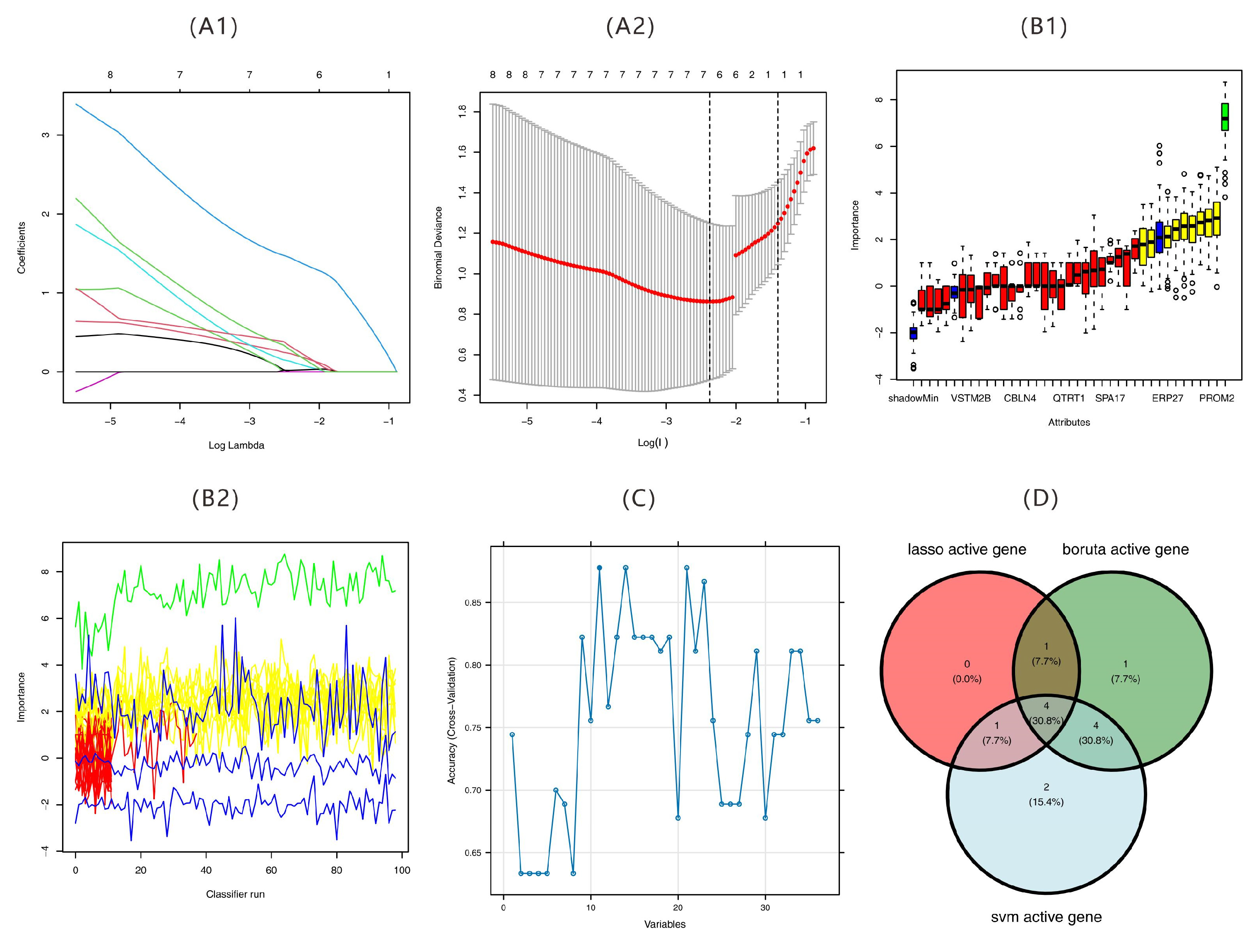
2.5. Results of Feature Gene Screening
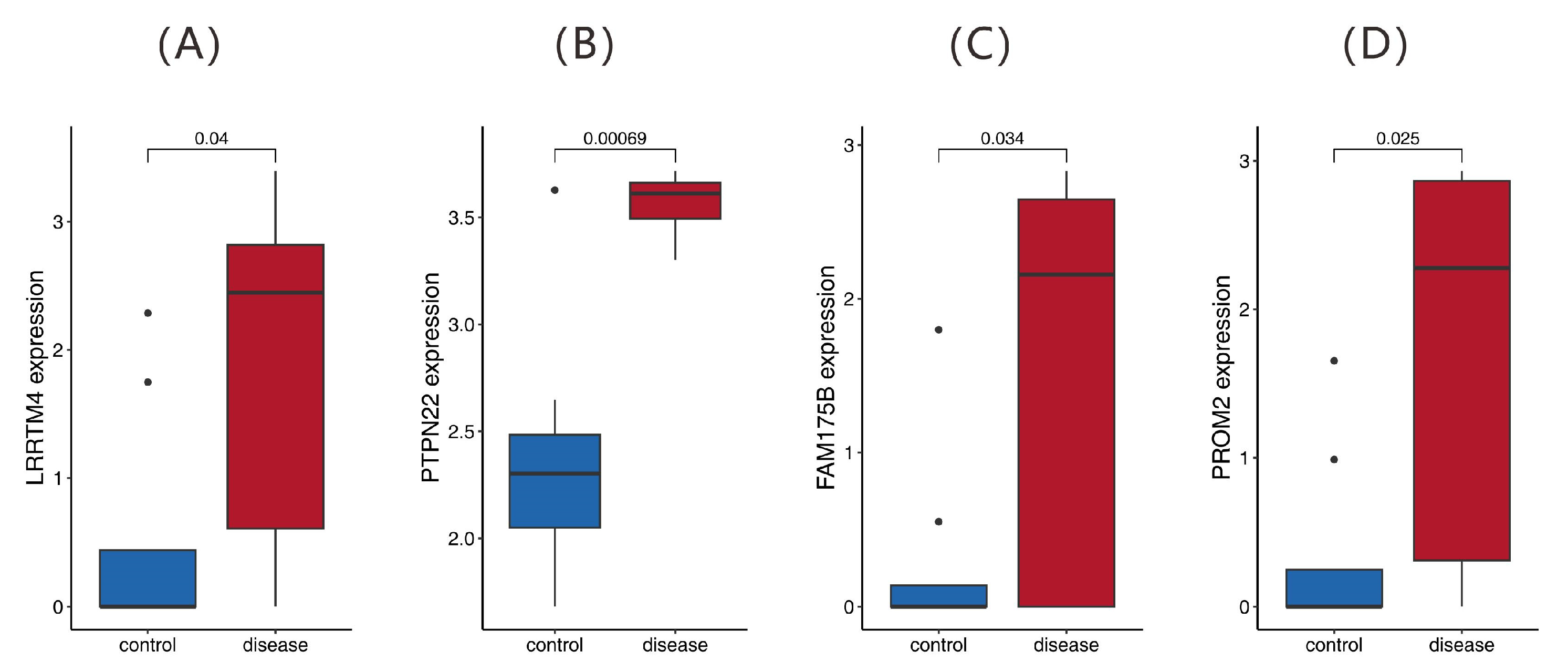
2.6. Validation of Feature Genes
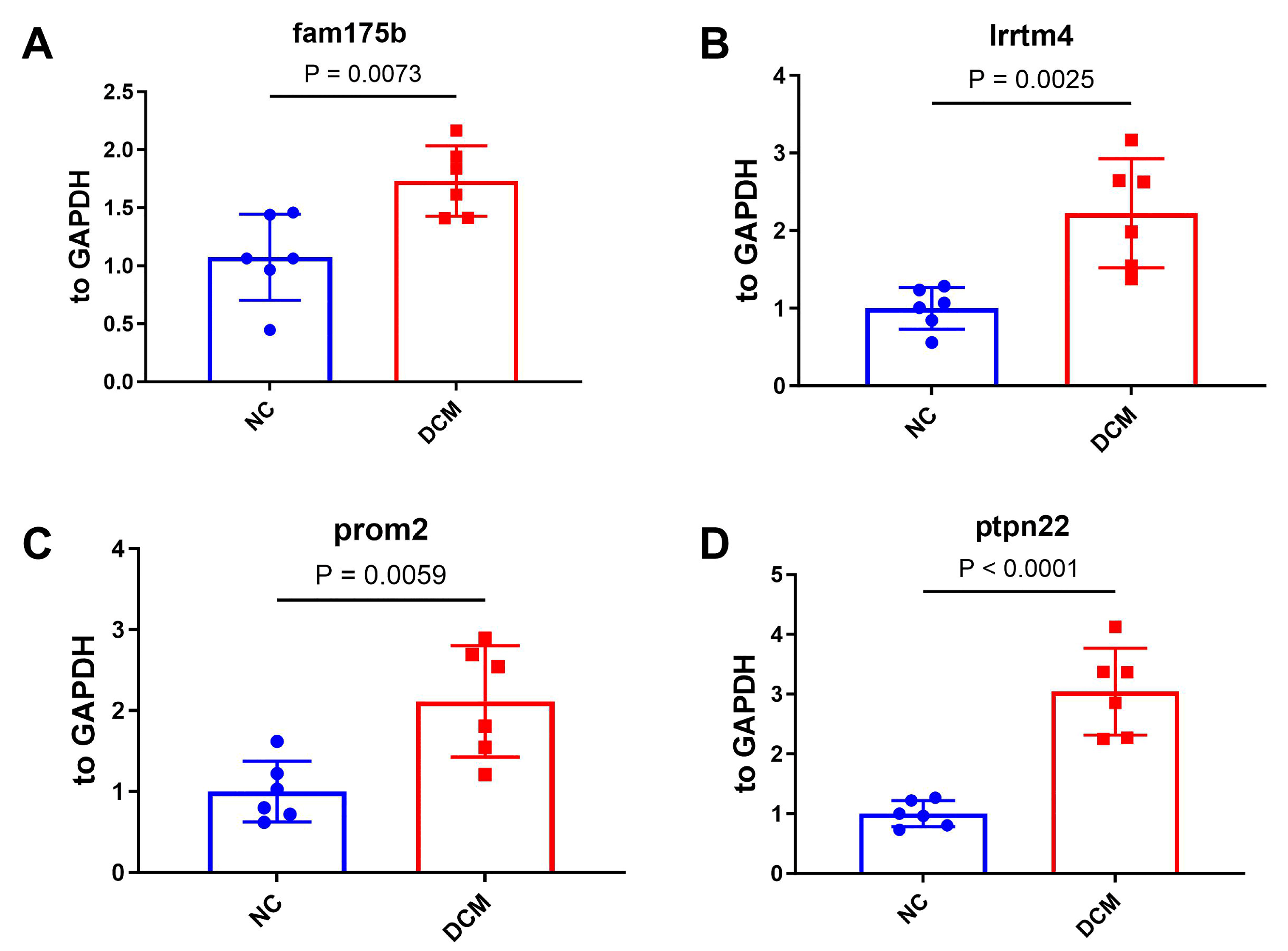
2.6.1. Validation of Feature Genes by qPCR
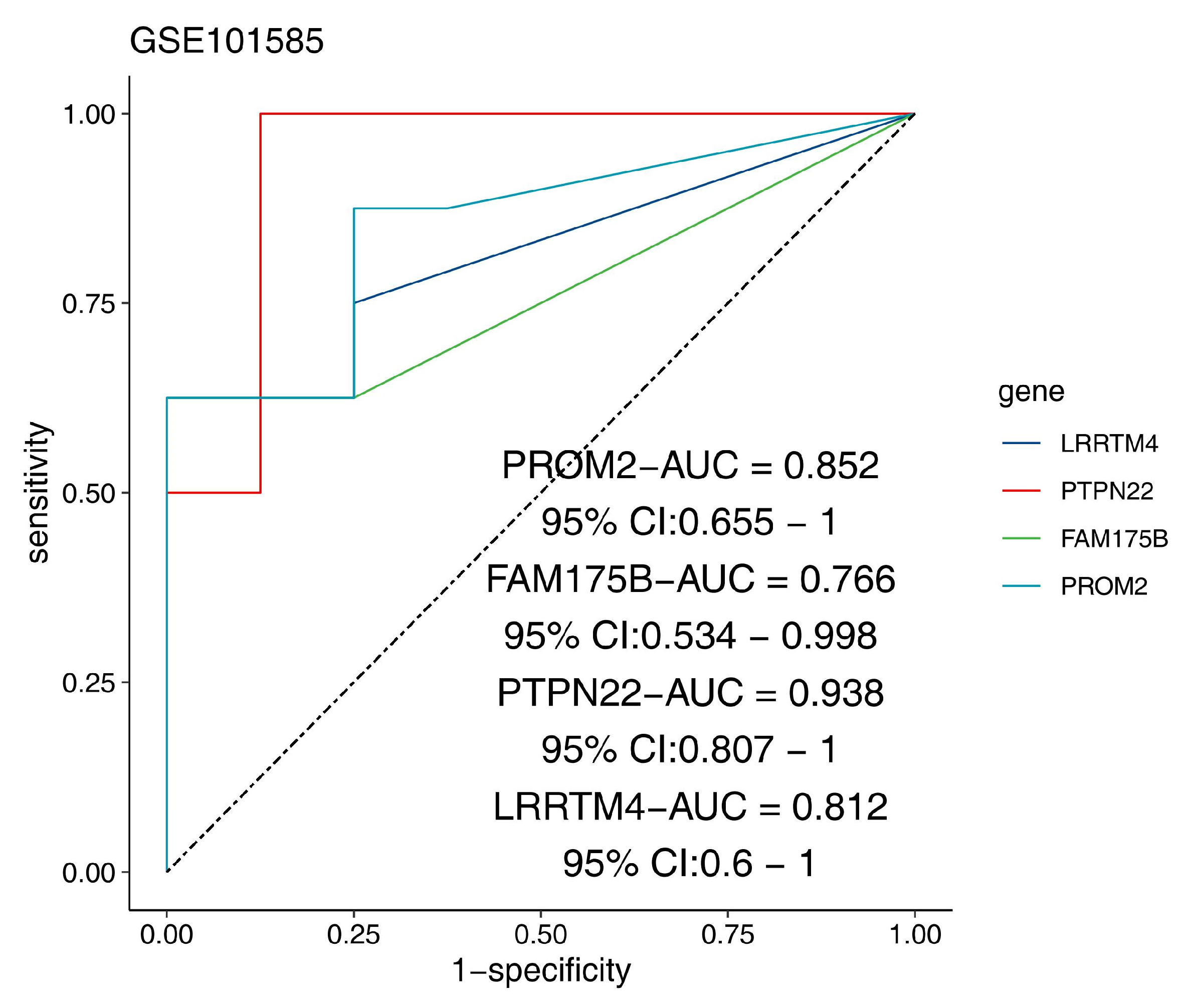
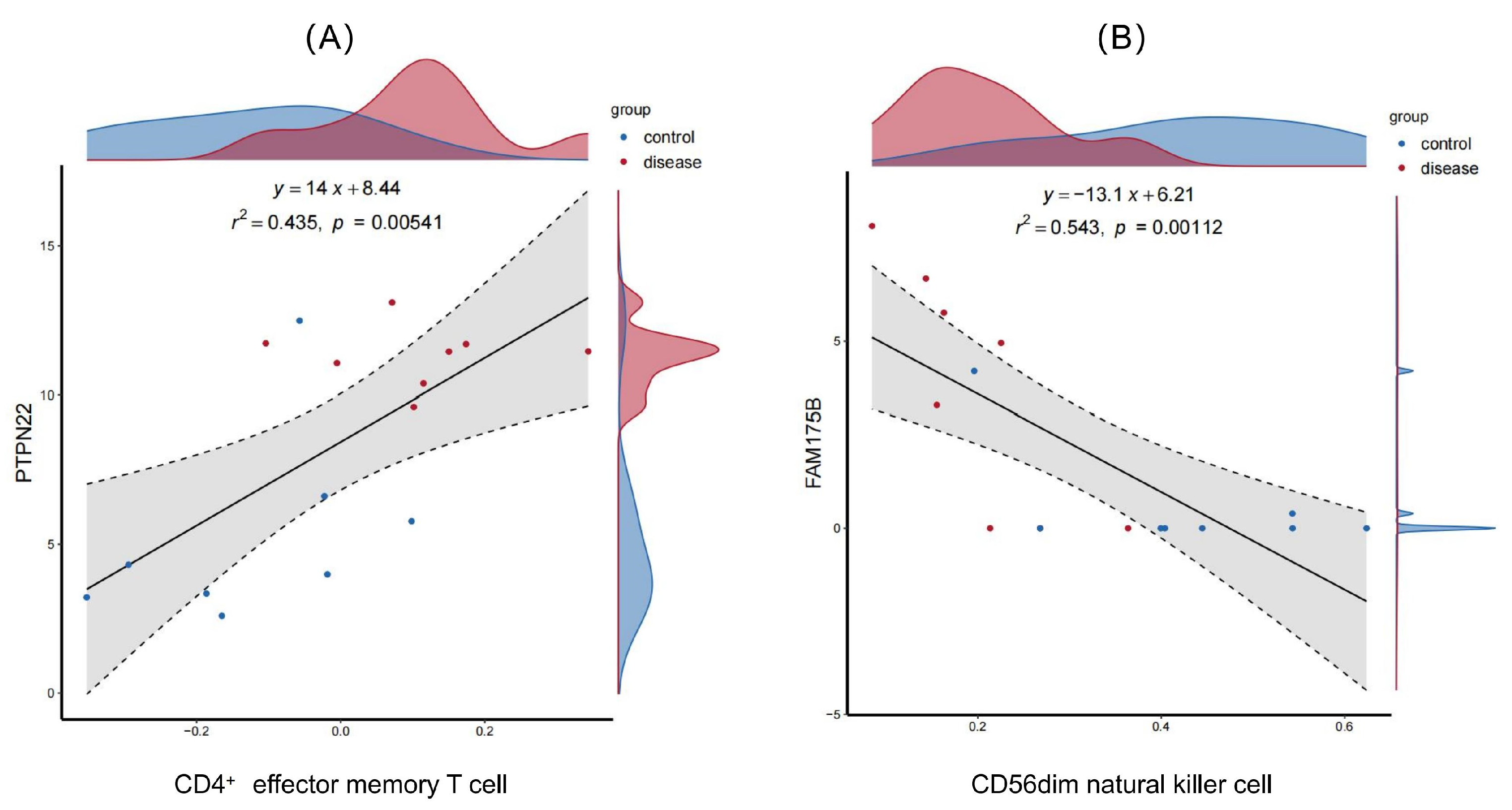
2.6.2. Validation of the Diagnostic Efficacy and Immune Correlation of the Feature Genes
2.7. Drug Target Prediction
3. Discussion
3.1. T Cell Activation and Immune Dysregulation in the Pathogenesis of DCM
3.2. The Role of Key Diagnostic Markers and Immune Pathways in the Pathogenesis and Progression of DCM
3.3. Identification of Interaction Patterns Between Diagnostic Genes and Small-Molecule Drugs
3.4. Limitations
4. Materials and Methods
4.1. Data Sources and Preprocessing
4.2. Estimation at the Immune Cell Level
4.2.1. Multi-Algorithm Deconvolution of Immune Cell Subsets
4.2.2. Single-Cell RNA Sequencing Analysis of Immune Cell Subsets
4.3. Validation at the Immune Cell Level
4.3.1. Research Subjects
4.3.2. Multicolor Flow Cytometry for the T Cell Subset
4.3.3. Flow Cytometry Data Analysis and Statistical Processing
4.4. Differentially Expressed Gene Screening
4.5. Construction of the Gene CoExpression Network
4.6. Machine Learning Feature Selection
4.7. Diagnostic Gene Validation and Immune Association
4.7.1. qPCR for mRNA
4.7.2. Diagnostic Performance and Immune Correlation
4.8. Drug–Gene Interactions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DCM | dilated cardiomyopathy |
| scRNA-seq | single-cell RNA sequencing |
| PCA | Principal Component Analysis |
| t-SNE | t-Distributed Stochastic Neighbor Embedding |
| TEM cells | effector memory T cells |
| TCM cells | central memory T cells |
| TCE cells | terminal effector T cells |
| WGCNA | weighted gene coexpression network analysis |
| PPI | protein–protein interaction |
| LRRTM4 | leucine rich repeat transmembrane neuronal 4 |
| PTPN22 | protein tyrosine phosphatase non-receptor type 22 |
| FAM175B | family with sequence similarity 175 member B |
| PROM2 | prominin 2 |
| DSigDB | drug signatures database |
| Th17 cells | type 17 helper T cells |
| Tc cells | cytotoxic T cells |
| Th1 cells | type 1 helper T cells |
| Th2 cells | type 2 helper T cells |
| FPKM | fragments per kilobase of exon per million fragments mapped |
| DEGs | differentially expressed genes |
| NCBI GEO | National Center for Biotechnology Information Gene Expression Omnibus |
| ssGSEA | single-sample gene set enrichment analysis |
| EPIC | epigenetic prediction of immune cell composition |
| xCell | xCell: digitally unlocking the immune microenvironment |
| CIBERSORT | cell-type identification by estimating relative subsets of RNA transcripts |
| IOBR | immuno-oncology biological research |
| PBMCs | peripheral blood mononuclear cells |
| PBS | phosphate-buffered saline |
| MCPcounter | microenvironment cell populations counter |
| SVM-RFE | support vector machine recursive feature elimination |
| FDR | false discovery rate |
| cDCs | conventional dendritic cells |
| HSCs | hematopoietic stem cells |
| UMAP | uniform manifold approximation and projection |
| NYHA | New York Heart Association |
| qPCR | quantitative PCR |
| HLA-DOB | human leukocyte antigen D obligate beta |
| CVB3 | coxsackievirus B3 |
| ATF4 | activating transcription factor 4 |
| NICD | notch intracellular domain |
| HFpEF | heart failure with preserved ejection fraction |
| APCs | antigen-presenting cells |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator 1 alpha |
| LV | left ventricular |
| CAD | coronary artery disease |
| LVEDD | left ventricular end-diastolic dimension |
| LVEDV | left ventricular end-diastolic volume |
| LVEF | left ventricular ejection fraction |
| log2FC | log2-fold change |
| CITE-seq | cellular indexing of transcriptomes and epitopes by sequencing |
References
- Yang, J.; Liao, Y.; Yuan, J.; Wang, C.; Cheng, X.; Zhao, D. Chinese Society of Cardiology, Chinese Myocarditis and Cardiomyopathy Cooperative Group 2018 Chinese guidelines for diagnosis and treatment of dilated cardiomyopathy. J. Clin. Cardiol. 2018, 34, 421–434. [Google Scholar] [CrossRef]
- Schultheiss, H.P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Primers 2019, 5, 32. [Google Scholar] [CrossRef]
- Gan, T.; Hu, J.; Aledan, A.K.O.; Liu, W.; Li, C.; Lu, S.; Wang, Y.; Xu, Q.; Wang, Y.; Wang, Z. Exploring the pathogenesis and immune infiltration in dilated cardiomyopathy complicated with atrial fibrillation by bioinformatics analysis. Front. Immunol. 2023, 14, 1049351. [Google Scholar] [CrossRef]
- Taylor, J.; Yeung, A.C.Y.; Ashton, A.; Faiz, A.; Guryev, V.; Fang, B.; Lal, S.; Grosser, M.; dos Remedios, C.G.; Braet, F.; et al. Transcriptomic Comparison of Human Peripartum and Dilated Cardiomyopathy Identifies Differences in Key Disease Pathways. J. Cardiovasc. Dev. Dis. 2023, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Zhou, R.; Li, T.; Hua, Y.; Zhou, K.; Li, Y.; Luo, S.; An, Q. The Molecular Role of Immune Cells in Dilated Cardiomyopathy. Medicina 2023, 59, 1246. [Google Scholar] [CrossRef]
- Kadhi, A.; Mohammed, F.; Nemer, G. The Genetic Pathways Underlying Immunotherapy in Dilated Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 613295. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. ESC Scientific Document Group. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Lv, Z.; Yu, Z.; Wang, K.; Xu, C.; Li, Y.; Wang, Y. Exploration of dilated cardiomyopathy for biomarkers and immune microenvironment: Evidence from RNA-seq. BMC Cardiovasc. Disord. 2022, 22, 320. [Google Scholar] [CrossRef]
- Sun, S.; Lu, J.; Lai, C.; Feng, Z.; Sheng, X.; Liu, X.; Wang, Y.; Huang, C.; Shen, Z.; Lv, Q.; et al. Transcriptome analysis uncovers the autophagy-mediated regulatory patterns of the immune microenvironment in dilated cardiomyopathy. J. Cell. Mol. Med. 2022, 26, 4101–4112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cheng, L.; Li, Z.; Li, H.; Liu, Y.; Zhan, H.; Xu, H.; Huang, Y.; Feng, F.; Li, Y. Immune cells and related cytokines in dilated cardiomyopathy. Biomed. Pharmacother. 2024, 171, 116159. [Google Scholar] [CrossRef]
- Hao, J.; Li, W.; Jiao, W.; Li, F.; Xie, Y. IL-17 affects the immune regulation of CD4+ T cells in dilated cardiomyopathy through JAK/STAT pathway. Cardiol. Young 2025, 35, 16–23. [Google Scholar] [CrossRef]
- Nevers, T.; Salvador, A.M.; Velazquez, F.; Ngwenyama, N.; Carrillo-Salinas, F.J.; Aronovitz, M.; Blanton, R.M.; Alcaide, P. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J. Exp. Med. 2017, 214, 3311–3329. [Google Scholar] [CrossRef]
- Fang, Y.; Ma, K.; Huang, Y.M.; Dang, Y.; Liu, Z.; Xu, Y.; Zheng, X.L.; Yang, X.; Huo, Y.; Dai, X. Fibronectin leucine-rich transmembrane protein 2 drives monocyte differentiation into macrophages via the UNC5B-Akt/mTOR axis. Front. Immunol. 2023, 14, 1162004. [Google Scholar] [CrossRef]
- Zhang, S.; Kong, X.; Yao, M.; Qi, J.; Li, Y.; Liang, H.; Zhou, Y. Met-Flow analyses of the metabolic heterogeneity associated with different stages of cord blood-derived hematopoietic cell differentiation. Front. Immunol. 2024, 15, 1425585. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, X.; Wei, Z.; Dong, J.; Lu, J.; Tang, Q.; Lu, F.; Cen, Z.; Wu, W. CD4+TEM cells drive the progression from acute myocarditis to dilated cardiomyopathy in CVB3-induced BALB/c mice. Int. Immunopharmacol. 2024, 127, 111304. [Google Scholar] [CrossRef]
- He, B.; Quan, L.P.; Cai, C.Y.; Yu, D.Y.; Yan, W.; Wei, Q.J.; Zhang, Z.; Huang, X.N.; Liu, L. Dysregulation and imbalance of innate and adaptive immunity are involved in the cardiomyopathy progression. Front. Cardiovasc. Med. 2022, 9, 973279. [Google Scholar] [CrossRef]
- Efthimiadis, I.; Skendros, P.; Sarantopoulos, A.; Boura, P. CD4+/CD25+ T-Lymphocytes and Th1/Th2 regulation in dilated cardiomyopathy. Hippokratia 2011, 15, 335–342. [Google Scholar] [PubMed]
- Lei, Z.; Cao, J.; Wu, J.; Lu, Y.; Ni, L.; Hu, X. Identification of the communal pathogenesis and immune landscape between viral myocarditis and dilated cardiomyopathy. ESC Heart Fail. 2024, 11, 282–292. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Wang, J.J.; Shen, J.; Abuduwufuer, K.; Zhang, Z.; Dong, Z.; Wen, Z.; He, J.; Chen, S.; et al. Spatiotemporal transcriptomics elucidates the pathogenesis of fulminant viral myocarditis. Signal Transduct. Target. Ther. 2025, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Hope, J.L.; Otero, D.C.; Bae, E.A.; Stairiker, C.J.; Palete, A.B.; Faso, H.A.; Lin, M.; Henriquez, M.L.; Roy, S.; Seo, H.; et al. PSGL-1 attenuates early TCR signaling to suppress CD8+ T cell progenitor differentiation and elicit terminal CD8+ T cell exhaustion. Cell Rep. 2023, 42, 112436. [Google Scholar] [CrossRef]
- Weiss, S.A.; Huang, A.Y.; Fung, M.E.; Martinez, D.; Chen, A.C.Y.; LaSalle, T.J.; Miller, B.C.; Scharer, C.D.; Hegde, M.; Nguyen, T.H.; et al. Epigenetic tuning of PD-1 expression improves exhausted T cell function and viral control. Nat. Immunol. 2024, 25, 1871–1883. [Google Scholar] [CrossRef]
- Fanti, S.; Stephenson, E.; Rocha-Vieira, E.; Protonotarios, A.; Kanoni, S.; Shahaj, E.; Longhi, M.P.; Vyas, V.S.; Dyer, C.; Pontarini, E.; et al. Circulating c-Met-Expressing Memory T Cells Define Cardiac Autoimmunity. Circulation 2022, 146, 1930–1945. [Google Scholar] [CrossRef]
- Hayashi, T.; Tiwary, S.K.; Lavine, K.J.; Acharya, S.; Brent, M.; Adamo, L.; Kovacs, A.; Mann, D.L. The Programmed Death-1 Signaling Axis Modulates Inflammation and LV Structure/Function in a Stress-Induced Cardiomyopathy Model. JACC Basic Transl. Sci. 2022, 7, 1120–1139. [Google Scholar] [CrossRef]
- Cozma, A.; Sporis, N.D.; Lazar, A.L.; Buruiana, A.; Ganea, A.M.; Malinescu, T.V.; Berechet, B.M.; Fodor, A.; Sitar-Taut, A.V.; Vlad, V.C.; et al. Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 10948. [Google Scholar] [CrossRef]
- Rao, M.; Wang, X.; Guo, G.; Wang, L.; Chen, S.; Yin, P.; Chen, K.; Chen, L.; Zhang, Z.; Chen, X.; et al. Resolving the intertwining of inflammation and fibrosis in human heart failure at single-cell level. Basic Res. Cardiol. 2021, 116, 55. [Google Scholar] [CrossRef]
- Liu, M.; Xia, N.; Zha, L.; Yang, H.; Gu, M.; Hao, Z.; Zhu, X.; Li, N.; He, J.; Tang, T.; et al. Increased expression of protein tyrosine phosphatase nonreceptor type 22 alters early T-cell receptor signaling and differentiation of CD4+ T cells in chronic heart failure. FASEB J. 2024, 38, e23386. [Google Scholar] [CrossRef] [PubMed]
- Martín, P.; Sánchez-Madrid, F. T cells in cardiac health and disease. J. Clin. Investig. 2025, 135, e185218. [Google Scholar] [CrossRef]
- Nowakowska, D.J.; Kissler, S. Ptpn22 Modifies Regulatory T Cell Homeostasis via GITR Upregulation. J. Immunol. 2016, 196, 2145–2152. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, R.J.; Salmond, R.J. Regulation of T Cell Signaling and Immune Responses by PTPN22. Mol. Cell. Biol. 2024, 44, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, F.; Hu, G.; Li, X.; Wang, L.; Li, C.; Huo, C.; Xu, R.; Hou, L.; Wang, N.; et al. LRRC8A critically regulates myofibroblast phenotypes and fibrotic remodeling following myocardial infarction. Theranostics 2022, 12, 5824–5835. [Google Scholar] [CrossRef]
- McCarthy-Leo, C.; Darwiche, F.; Tainsky, M.A. DNA Repair Mechanisms, Protein Interactions, and Therapeutic Targeting of the MRN Complex. Cancers 2022, 14, 5278. [Google Scholar] [CrossRef]
- Ong, S.; Rose, N.R.; Čiháková, D. Natural Killer Cells in Inflammatory Heart Disease. Clin. Immunol. 2017, 175, 26–33. [Google Scholar] [CrossRef]
- Shamlou, S.; Kiani, J.; Hasanpoor, Z.; Tollabi, M.; Norooznezhad, F.; Torabi Goudarzi, S.; Verdi, J.; Vousooghi, N. Natural Killer Cells as Critical Modulators of Heart Disease: Exploring Pathophysiological Mechanisms and Therapeutic Perspectives. Iran. J. Allergy Asthma Immunol. 2025, 24, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, Y.; Li, H.; Zhang, Z.; Guo, S.; Zhu, S.; Guo, Q.; Li, P.; Min, L.; Zhang, S. FAM175B Promotes Apoptosis by Inhibiting ATF4 Ubiquitination in Esophageal Squamous Cell Carcinoma. Mol. Oncol. 2019, 13, 1150–1165. [Google Scholar] [CrossRef] [PubMed]
- Kachanova, O.; Lobov, A.; Malashicheva, A. The Role of the Notch Signaling Pathway in Recovery of Cardiac Function after Myocardial Infarction. Int. J. Mol. Sci. 2022, 23, 12509. [Google Scholar] [CrossRef]
- Maggiorani, D.; Santin, Y.; Formoso, K.; Drapé, E.; Martini, H.; Brun, S.; Cousin, G.; Lairez, O.; Lezoualc’h, F.; Parini, A.; et al. Identification of Prominin-2 as a New Player of Cardiomyocyte Senescence in the Aging Heart. Aging Cell 2024, 23, e14204. [Google Scholar] [CrossRef]
- Hanieh, H.; Alfwuaires, M.A.; Abduh, M.S.; Abdrabu, A.; Qinna, N.A.; Alzahrani, A.M. Protective Effects of a Dihydrodiazepine Against Endotoxin Shock Through Suppression of TLR4/NF-κB/IRF3 Signaling Pathways. Inflammation, 2024; epub ahead of printing. [Google Scholar] [CrossRef]
- Gong, C.; Chang, L.; Huang, R.; Sun, X.; Liu, Y.; Wu, S.; Wang, L.; Xu, B.; Wang, L. LIM Kinase 2 Activates Cardiac Fibroblasts and Exacerbates Postinfarction Left Ventricular Remodeling via Crosstalk Between the Canonical and Non-Canonical Wnt Pathways. Pharmacol. Res. 2024, 208, 107347. [Google Scholar] [CrossRef]
- Pandey, S.N.; Afzal, M.; Uikey, J.; Ganesan, S.; Mishra, S.; Bansal, P.; Kazmi, I.; Alzarea, S.I.; Almalk, W.H.; Goyal, K.; et al. ATM and p53 in Aging and Cancer: A Double-Edged Sword in Genomic Integrity. Biogerontology 2025, 26, 102. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, J.; Zhou, Z.; Guo, X.; Xu, Y.; Huang, T.; Meng, S.; Cao, Z.; Xu, D.; Zhao, Q.; et al. Persistent Hypertension Induces Atrial Remodeling and Atrial Fibrillation Through DNA Damage and ATM/CHK2/p53 Signaling Pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167534. [Google Scholar] [CrossRef]
- Miranda, E.C.; Castor, R.G.; Bruno, A.S.; Pereira, C.A.; Bello, F.L.M.; Rodrigues, Y.B.; Silva, M.G.; Bernardes, S.S.; Castor, M.G.E.; Ferreira, A.J.; et al. Glibenclamide Reverses Cardiac Damage and NLRP3 Inflammasome Activation Associated with a High Refined Sugar Diet. Eur. J. Pharmacol. 2024, 984, 177035. [Google Scholar] [CrossRef]
- Gandhi, T.; Patel, A.; Gupta, D.; Pandya, H.; Chandel, A. Repositioning Glibenclamide in Cardiac Fibrosis by Targeting the TGF-β1-pSmad2/3-NLRP3 Cascade. Mol. Cell. Biochem. 2023, 478, 2281–2295. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Morón, A.; Requena, S.; Roda-Navarro, P.; Martín-Cófreces, N.B. Activation Kinetics of Regulatory Molecules During Immunological Synapse in T Cells. Methods Cell Biol. 2023, 178, 149–171. [Google Scholar] [CrossRef]
- Wheatley, B.A.; Rey-Suarez, I.; Hourwitz, M.J.; Kerr, S.; Shroff, H.; Fourkas, J.T.; Upadhyaya, A. Nanotopography Modulates Cytoskeletal Organization and Dynamics During T Cell Activation. Mol. Biol. Cell 2022, 33, ar88. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Wang, Z.; An, T. T-Cell Metabolic Reprogramming in Atherosclerosis. Biomedicines 2024, 12, 1844. [Google Scholar] [CrossRef]
- Ahmed, A.; Syed, J.N.; Chi, L.; Wang, Y.; Perez-Romero, C.; Lee, D.; Kocaqi, E.; Caballero, A.; Yang, J.; Escalante-Covarrubias, Q.; et al. KDM8 Epigenetically Controls Cardiac Metabolism to Prevent Initiation of Dilated Cardiomyopathy. Nat. Cardiovasc. Res. 2023, 2, 174–191. [Google Scholar] [CrossRef]
- Shipra; Tembhre, M.K.; Hote, M.P.; Bhari, N.; Lakshmy, R.; Kumaran, S.S. PGC-1α Agonist Rescues Doxorubicin-Induced Cardiomyopathy by Mitigating Oxidative Stress and Necroptosis. Antioxidants 2023, 12, 1720. [Google Scholar] [CrossRef]
- Huang, G.; Liu, J.; Yang, C.; Xiang, Y.; Wang, Y.; Wang, J.; Cao, M.; Yang, W. RNA Sequencing Discloses the Genome-Wide Profile of Long Noncoding RNAs in Dilated Cardiomyopathy. Mol. Med. Rep. 2019, 19, 2569–2580. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for Functional Genomics Data Sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef]
- Zeng, D.; Ye, Z.; Shen, R.; Yu, G.; Wu, J.; Xiong, Y.; Zhou, R.; Qiu, W.; Huang, N.; Sun, L.; et al. IOBR: Multi-Omics Immuno-Oncology Biological Research to Decode Tumor Microenvironment and Signatures. Front. Immunol. 2021, 12, 687975. [Google Scholar] [CrossRef]
- Gribov, A.; Sill, M.; Lück, S.; Rücker, F.; Döhner, K.; Bullinger, L.; Benner, A.; Unwin, A. SEURAT: Visual Analytics for the Integrated Analysis of Microarray Data. BMC Med. Genom. 2010, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.X.; Zhang, Y.N.; Lai, Z.; Li, W.; Zhou, L.; Zhong, F. Principal Component Analysis Based on Nuclear Norm Minimization. Neural Netw. 2019, 118, 1–16. [Google Scholar] [CrossRef]
- Korsunsky, I.; Millard, N.; Fan, J.; Slowikowski, K.; Zhang, F.; Wei, K.; Baglaenko, Y.; Brenner, M.; Loh, P.R.; Raychaudhuri, S. Fast, Sensitive and Accurate Integration of Single-Cell Data with Harmony. Nat. Methods 2019, 16, 1289–1296. [Google Scholar] [CrossRef]
- Kobak, D.; Berens, P. The Art of Using t-SNE for Single-Cell Transcriptomics. Nat. Commun. 2019, 10, 5416. [Google Scholar] [CrossRef]
- Hu, C.; Li, T.; Xu, Y.; Zhang, X.; Li, F.; Bai, J.; Chen, J.; Jiang, W.; Yang, K.; Ou, Q.; et al. CellMarker 2.0: An Updated Database of Manually Curated Cell Markers in Human/Mouse and Web Tools Based on scRNA-seq Data. Nucleic Acids Res. 2023, 51, D870–D876. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Cacchiarelli, D.; Grimsby, J.; Pokharel, P.; Li, S.; Morse, M.; Lennon, N.J.; Livak, K.J.; Mikkelsen, T.S.; Rinn, J.L. The Dynamics and Regulators of Cell Fate Decisions Are Revealed by Pseudotemporal Ordering of Single Cells. Nat. Biotechnol. 2014, 32, 381–386. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Piñero, J.; Bravo, À.; Queralt-Rosinach, N.; Gutiérrez-Sacristán, A.; Deu-Pons, J.; Centeno, E.; García-García, J.; Sanz, F.; Furlong, L.I. DisGeNET: A Comprehensive Platform Integrating Information on Human Disease-Associated Genes and Variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R Package for Weighted Correlation Network Analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying Hub Objects and Sub-Networks from Complex Interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef] [PubMed]
- Meier, L.; Van De Geer, S.; Bühlmann, P. The Group Lasso for Logistic Regression. J. R. Stat. Soc. Ser. B Stat. Methodol. 2008, 70, 53–71. [Google Scholar] [CrossRef]
- Sanz, H.; Valim, C.; Vegas, E.; Oller, J.M.; Reverter, F. SVM-RFE: Selection and Visualization of the Most Relevant Features Through Non-Linear Kernels. BMC Bioinform. 2018, 19, 432. [Google Scholar] [CrossRef] [PubMed]
- Kursa, M.B.; Jankowski, A.; Rudnicki, W.R. Boruta: A System for Feature Selection. Fundam. Inform. 2010, 101, 271–285. [Google Scholar] [CrossRef]
- McEligot, A.J.; Poynor, V.; Sharma, R.; Panangadan, A. Logistic LASSO Regression for Dietary Intakes and Breast Cancer. Nutrients 2020, 12, 2652. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Singh, P.; Nath, M.; Bhalla, D.; Sengupta, S.; Kumar, A.; Pandit, A.K.; Aggarwal, P.; Srivastava, A.K.; Mohania, D.; et al. Blood-Based Protein Biomarkers for the Diagnosis of Acute Stroke: A Discovery-Based SWATH-MS Proteomic Approach. Front. Neurol. 2022, 13, 989856. [Google Scholar] [CrossRef]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Yoo, M.; Shin, J.; Kim, J.; Ryall, K.A.; Lee, K.; Lee, S.; Jeon, M.; Kang, J.; Tan, A.C. DSigDB: Drug Signatures Database for Gene Set Analysis. Bioinformatics 2015, 31, 3069–3071. [Google Scholar] [CrossRef]
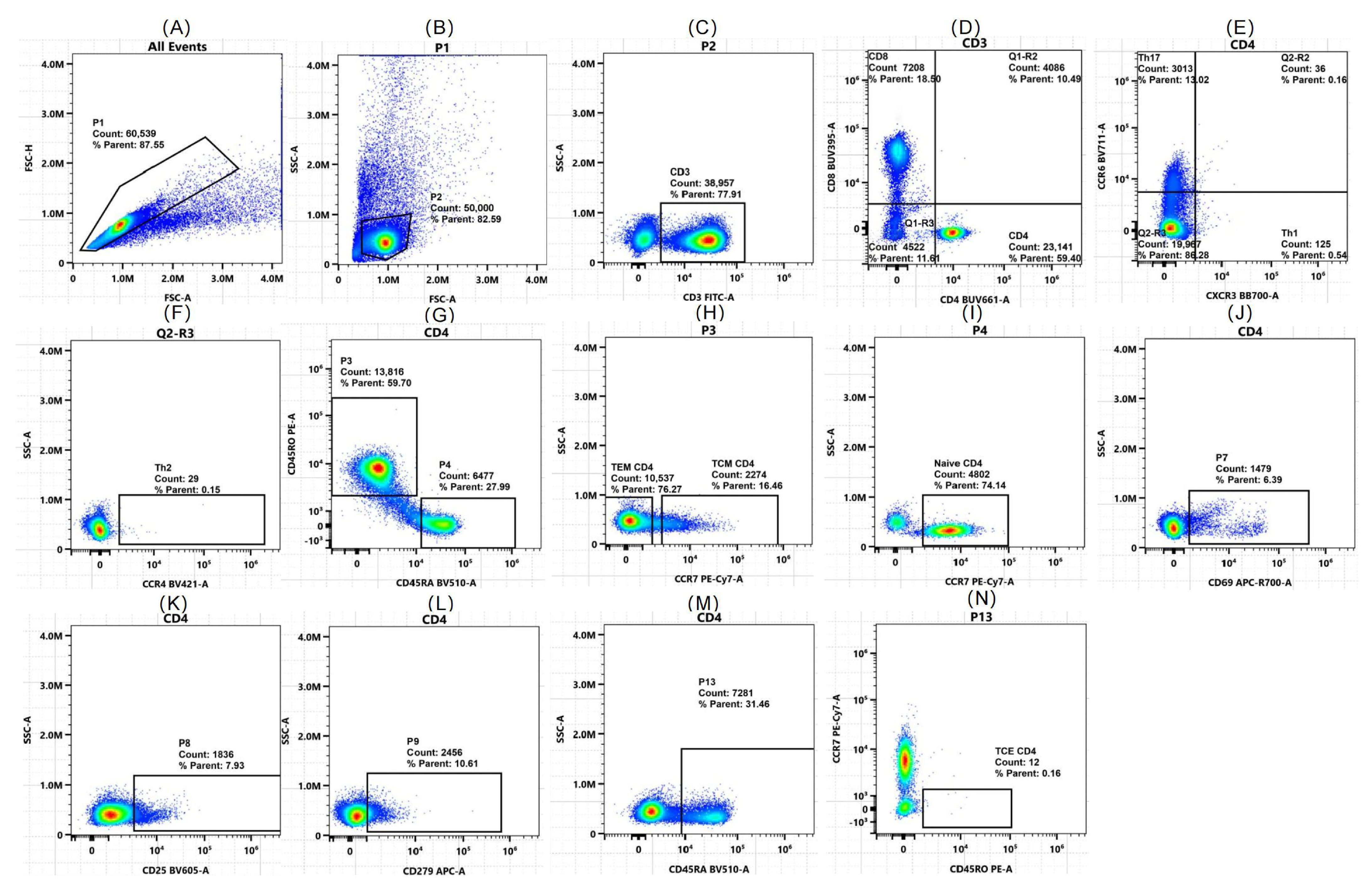
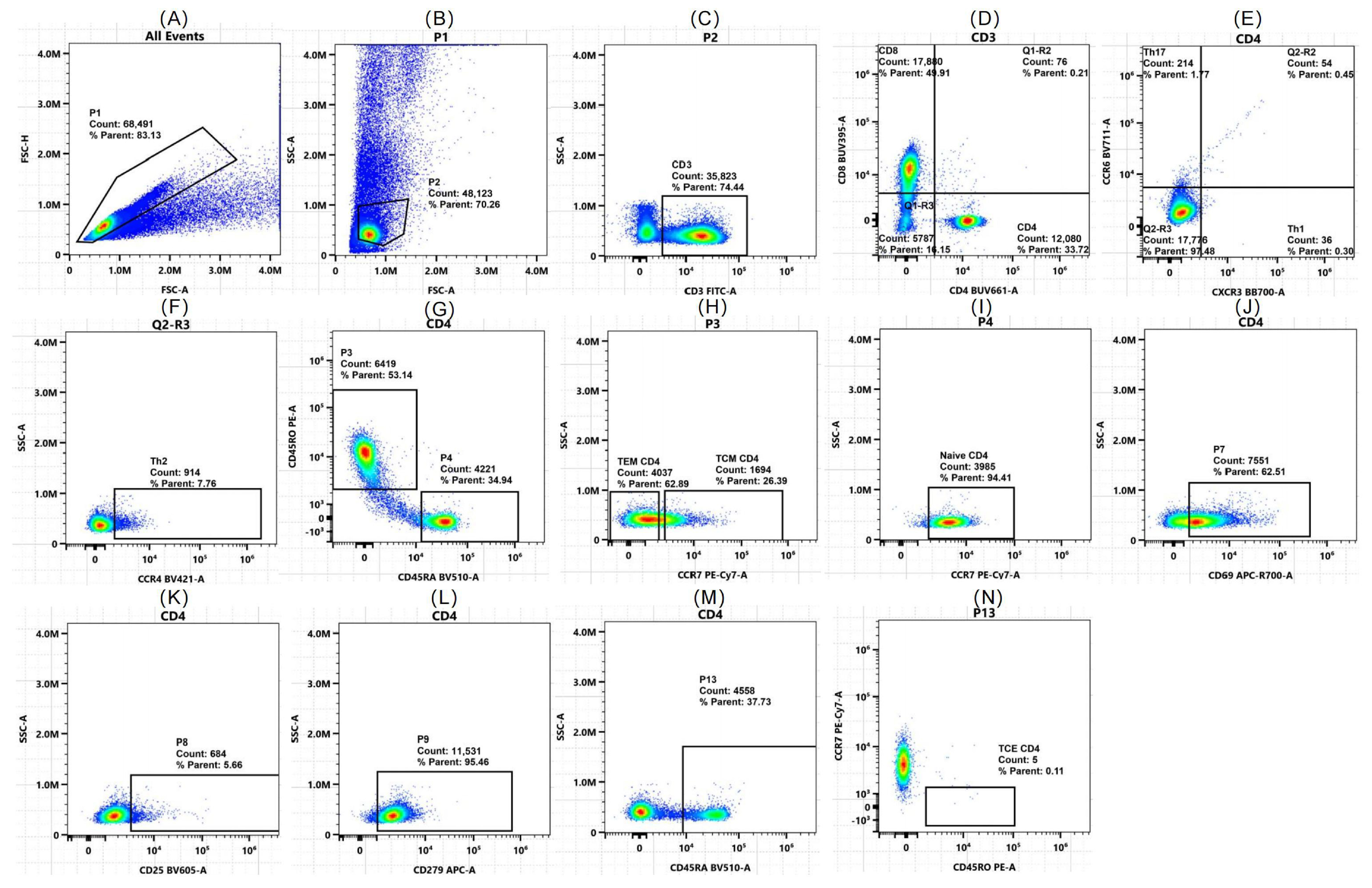




| Parameter | DCM (n = 40) | Healthy (n = 40) | p Value |
|---|---|---|---|
| CD3+ T cells (% of lymphocytes) | 37.10 ± 16.93 | 30.16 ± 19.5 | 0.203 |
| PD-1+ exhausted CD4+ T cells (% of CD4+) | 15.95 (8.13, 33.55) | 13.8 (8.31, 56.7) | 0.773 |
| Th2 cells (% of CD4+ T cells) | 11.95 (8.32, 18.42) | 12 (5.09, 14.77) | 0.282 |
| Th1 cells (% of CD4+ T cells) | 34.4 (13.8, 62.6) | 46.4 (40.05, 75.32) | 0.034 * |
| CD4+ T cells (% of CD3+) | 54.15 (41.87, 61.47) | 49.55 (4.66, 56.4) | 0.043 * |
| CD4+ TCM cells (% of CD4+) | 21.63 ± 10.93 | 14.19 ± 7.81 | 0.013 * |
| Late-activated CD4+ T cells (CD25+, % of CD4+) | 5.24 (0.89, 9.9) | 4.44 (2.67, 35.2) | 0.268 |
| Naive CD4+ T cells (% of CD4+) | 17.5 (7.28, 28.42) | 27.8 (19.97, 36.35) | 0.039 * |
| Early-activated CD4+ T cells (CD69+, % of CD4+) | 3.59 (1.39, 19.95) | 1.2 (0.62, 1.85) | 0.001 * |
| CD4+ TCE cells (% of CD4+) | 0.77 (0.31, 2.32) | 0.81 (0.2, 15.68) | 0.485 |
| CD4+ TEM cells (% of CD4+) | 7.54 (4.57, 18.85) | 3.96 (1.53, 5.30) | 0.001 * |
| CD8+ T cells (% of CD3+) | 38.74 ± 15.19 | 24.48 ± 8.34 | <0.001 * |
| Late-activated CD8+ T cells (CD25+, % of CD8+) | 5.02 (0.66, 17.98) | 2.18 (0.41, 38.93) | 0.851 |
| Early-activated CD8+ T cells (CD69+, % of CD8+) | 11.65 (5.50, 21.4) | 4.61 (3.45, 11.55) | 0.012 * |
| PD-1+ exhausted CD8+ T cells (% of CD8+) | 27.25 (5.9, 51.08) | 25.85 (16.73, 87.68) | 0.223 |
| CD8+ TCM cells (% of CD8+) | 1.07 (0.44, 2.37) | 0.95 (0.52, 1.19) | 0.912 |
| Naive CD8+ T cells (% of CD8+) | 19.27 ± 11.68 | 27.98 ± 17.54 | 0.049 * |
| CD8+ TCE cells (% of CD8+) | 3.01 (0.44, 42.42) | 1.35 (0.43, 3.06) | 0.147 |
| CD8+ TEM cells (% of CD8+) | 27.5 (1.11, 31.37) | 24.3 (15.92, 31.95) | 0.528 |
| Cell Surface Markers | Fluorescent Dyes | Clone Number | Species Source | Antibody Vendor |
|---|---|---|---|---|
| CCR4 | BV421 | 1G1 | Mouse | BD Pharmingen |
| CD45RA | BV510 | HI100 | Mouse | BD Pharmingen |
| CD279 | BV605 | MIH4 | Mouse | Biolegend |
| CD25 | BV711 | 2A3 | Mouse | BD Pharmingen |
| CD3 | FITC | 7F5 | Mouse | Absin |
| CD45RO | PerCP-Cy5.5 | UCHL1 | Mouse | BD Pharmingen |
| CCR7 | PE | 3D12 | Rat | BD Pharmingen |
| CXCR3 | PE-Cy7 | 1C6/CXCR3 | Mouse | BD Pharmingen |
| CCR6 | APC | G034E3 | Mouse | BD Pharmingen |
| CD69 | APC-R700 | FN50 | Mouse | BD Pharmingen |
| CD8 | BUV395 | RPA-T8 | Mouse | BD Pharmingen |
| CD4 | BUV661 | RPA-T4 | Mouse | Thermo |
| Cell Type/Functional State | Surface Markers |
|---|---|
| Total T cells | CD3+ |
| CD4+ T cells | CD4+ |
| Tc cells | CD8+ |
| Th1 cells | CD4+ CXCR3+ CCR6 |
| Th2 cells | CD4+ CXCR3− CCR6− CCR4+ |
| Naive T cells | CD45RA+ CD45RO− CCR7+ |
| TCM cells | CD45RA− CD45RO+ CCR7+ |
| TEM cells | CD45RA− CD45RO+ CCR7− |
| TCE cells | CD45RA+ CD45RO− CCR7− |
| Early Activation Marker | CD69+ |
| Late Activation Marker | CD25+ |
| Exhausted T cells | PD-1+ (CD279+) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhou, J.; Kang, Y.; Chen, X.; Yang, Z.; Xie, Y.; Liu, T.; Liu, X.; Zhang, Q. CD4+ T Cell Subsets and PTPN22 as Novel Biomarkers of Immune Dysregulation in Dilated Cardiomyopathy. Int. J. Mol. Sci. 2025, 26, 7806. https://doi.org/10.3390/ijms26167806
Zhang X, Zhou J, Kang Y, Chen X, Yang Z, Xie Y, Liu T, Liu X, Zhang Q. CD4+ T Cell Subsets and PTPN22 as Novel Biomarkers of Immune Dysregulation in Dilated Cardiomyopathy. International Journal of Molecular Sciences. 2025; 26(16):7806. https://doi.org/10.3390/ijms26167806
Chicago/Turabian StyleZhang, Xinyu, Junteng Zhou, Yu Kang, Xiaojing Chen, Zixuan Yang, Yingjing Xie, Ting Liu, Xiaojing Liu, and Qing Zhang. 2025. "CD4+ T Cell Subsets and PTPN22 as Novel Biomarkers of Immune Dysregulation in Dilated Cardiomyopathy" International Journal of Molecular Sciences 26, no. 16: 7806. https://doi.org/10.3390/ijms26167806
APA StyleZhang, X., Zhou, J., Kang, Y., Chen, X., Yang, Z., Xie, Y., Liu, T., Liu, X., & Zhang, Q. (2025). CD4+ T Cell Subsets and PTPN22 as Novel Biomarkers of Immune Dysregulation in Dilated Cardiomyopathy. International Journal of Molecular Sciences, 26(16), 7806. https://doi.org/10.3390/ijms26167806







