Reliable New Biomarkers of Mitochondrial Oxidative Stress and Neuroinflammation in Cerebrospinal Fluid and Plasma from Alzheimer’s Disease Patients: A Pilot Study
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Data
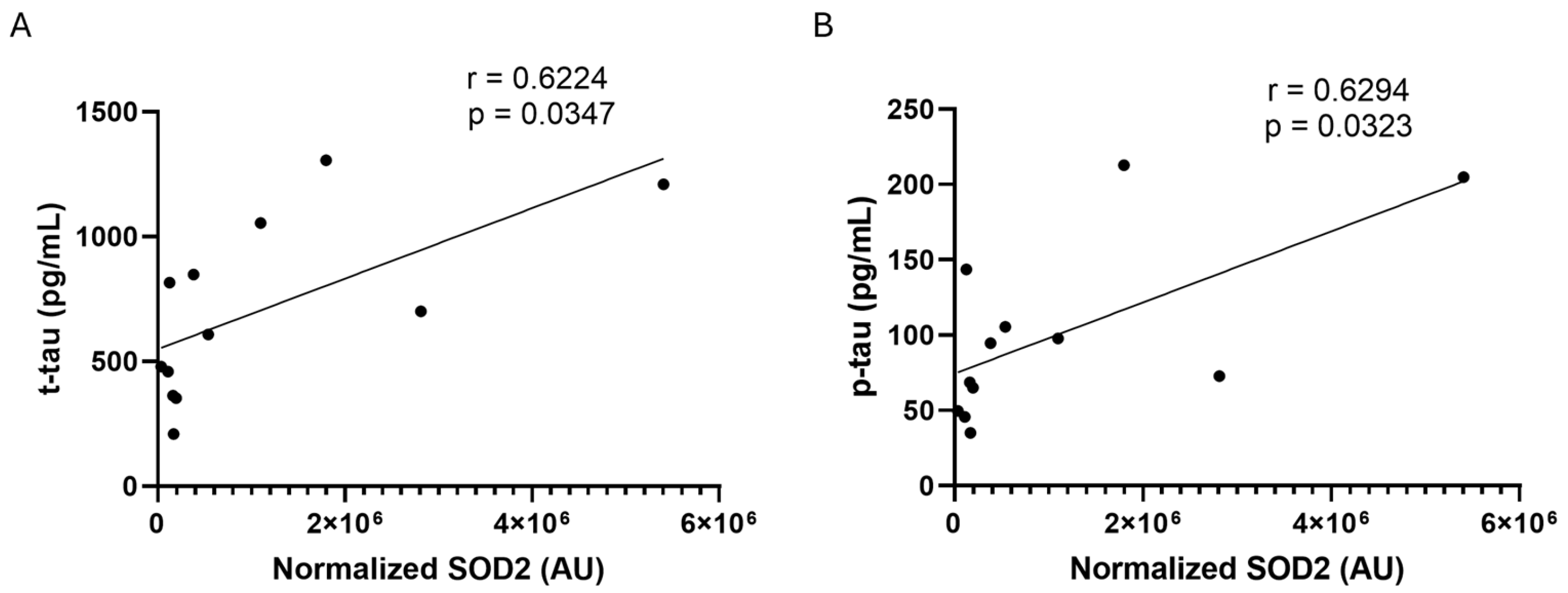
2.2. SOD2 Amount in CSF and Plasma Samples
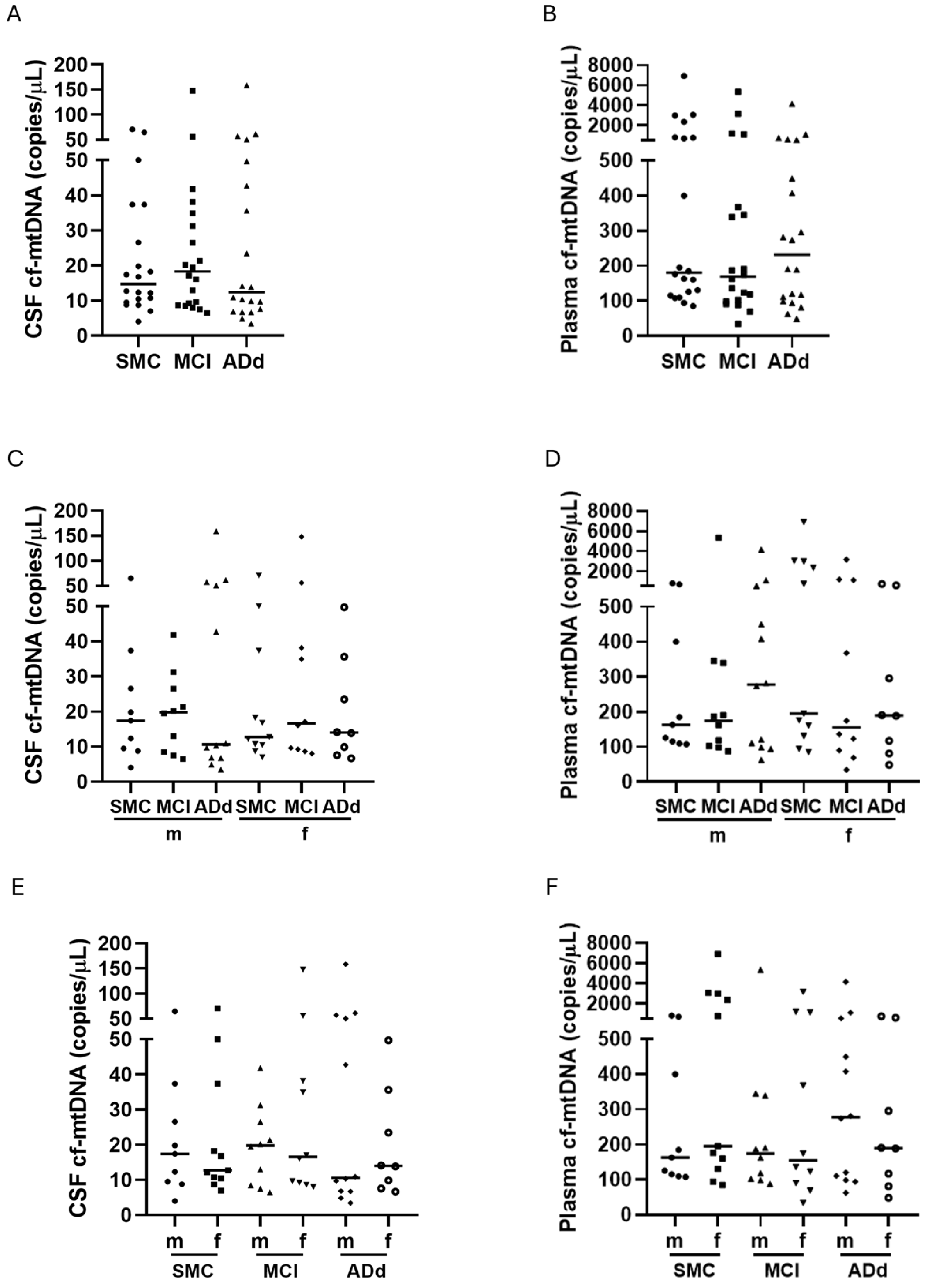
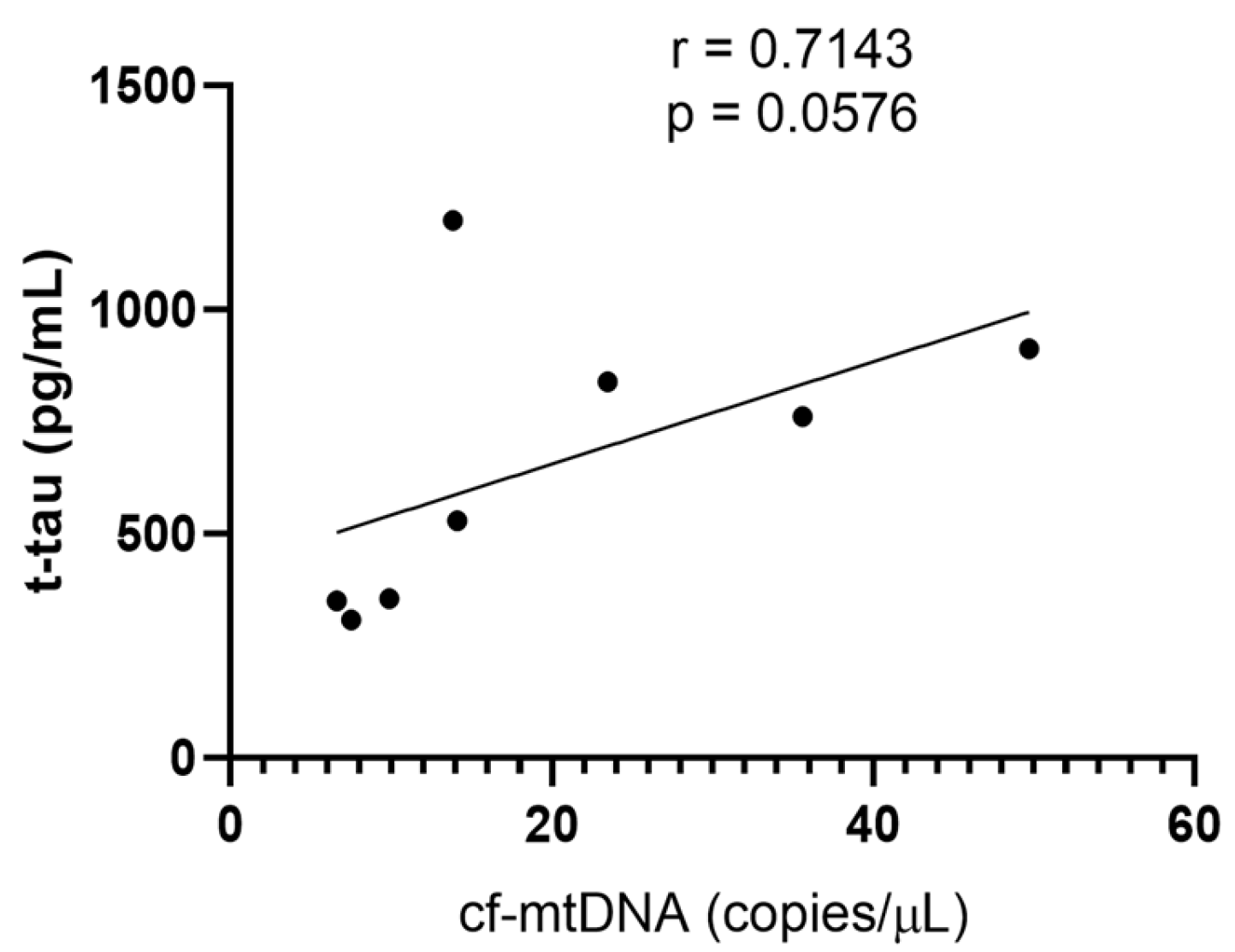
2.3. Cf-mtDNA in CSF and Plasma Samples
2.4. DNase Activity in CSF and Plasma Samples
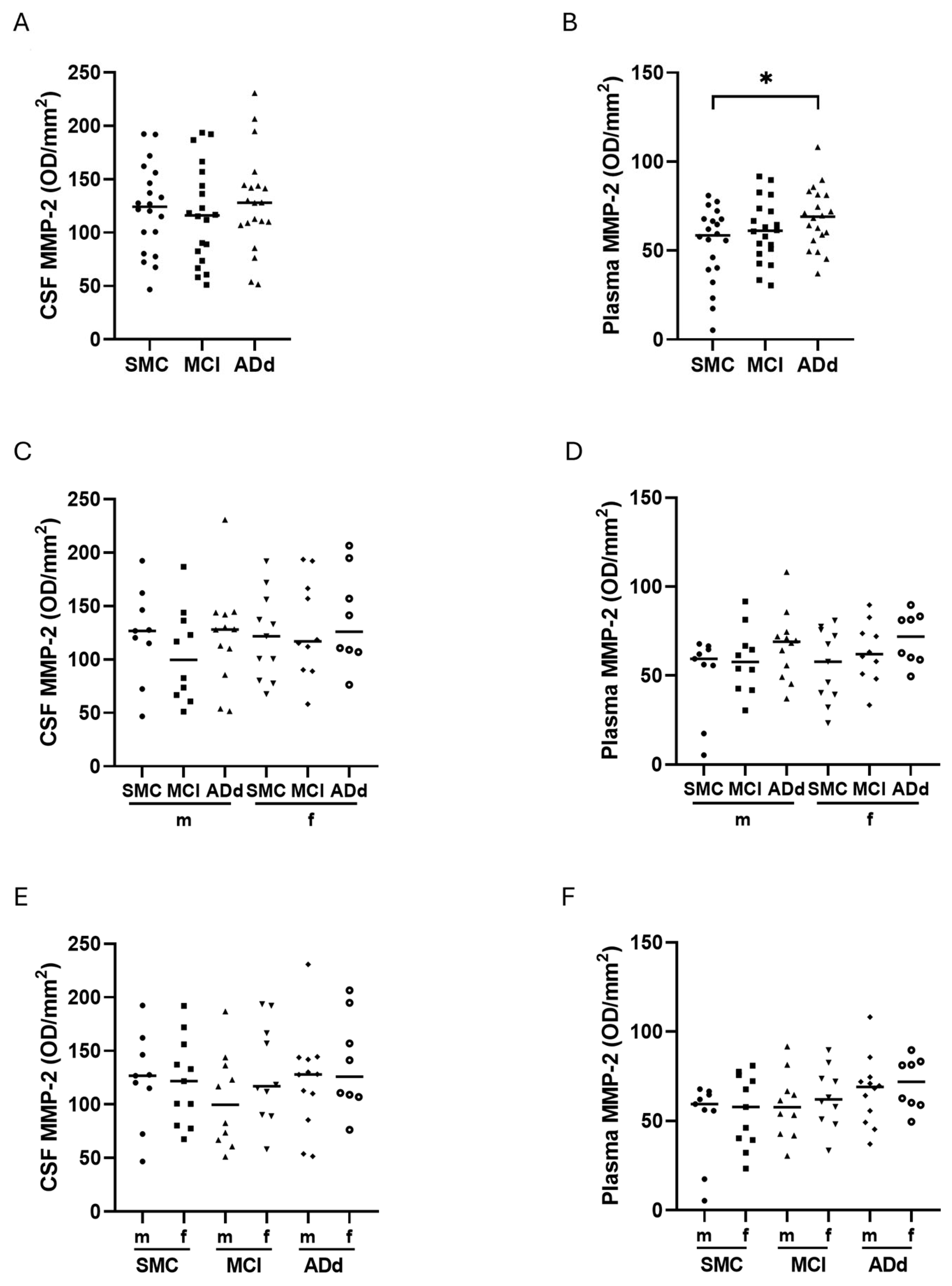
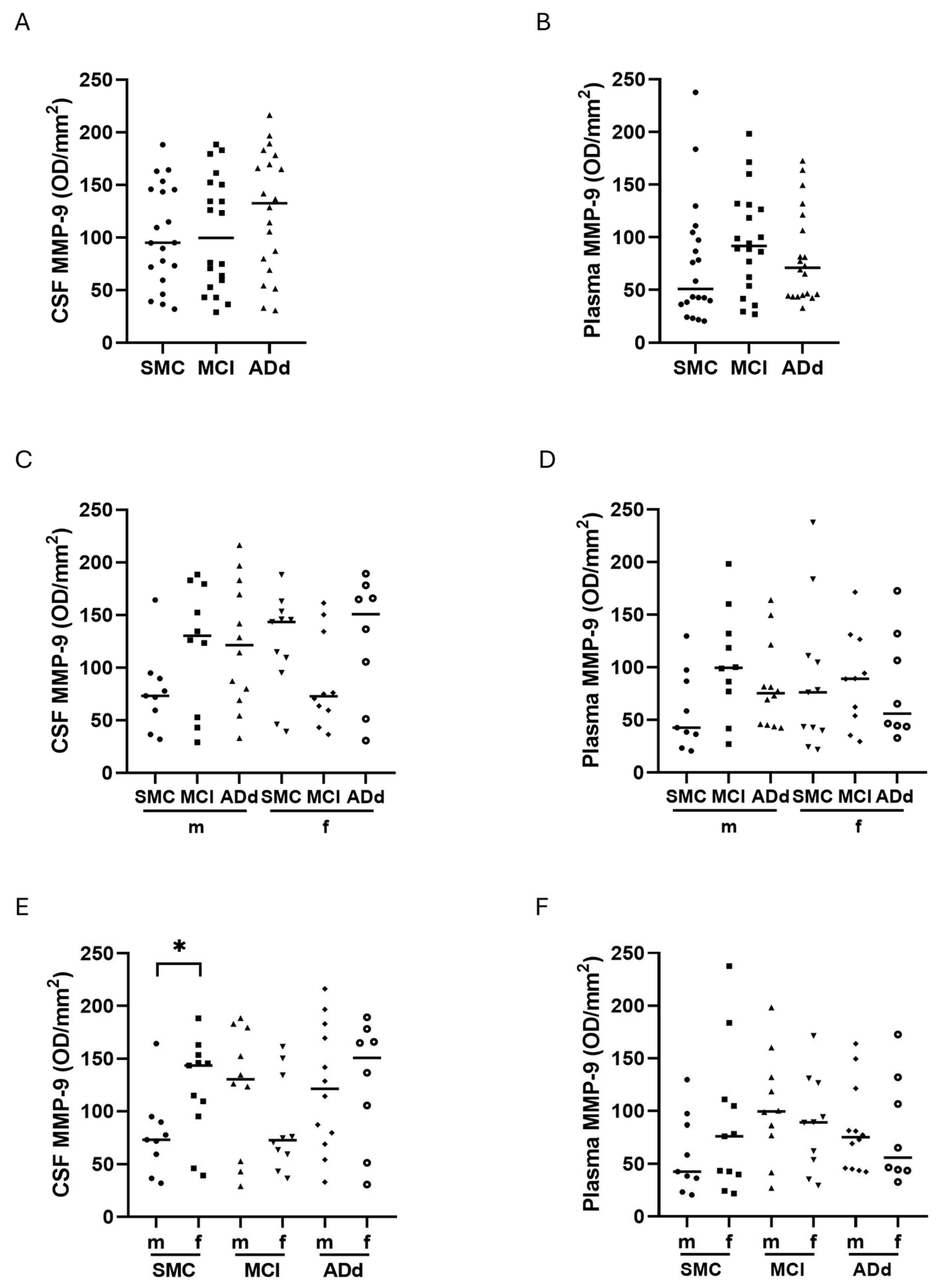
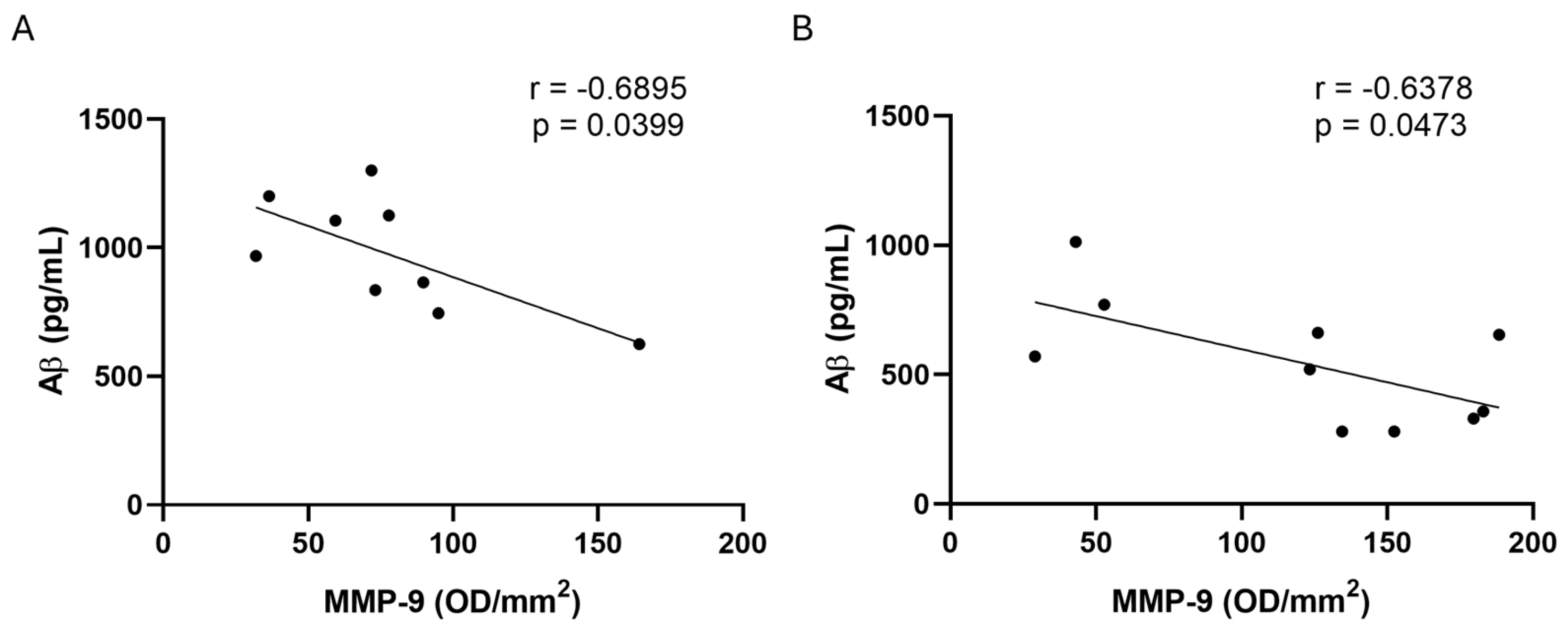
2.5. MMP-2 and MMP-9 Activities in CSF and Plasma Samples
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Sample Collection and CSF AD Biomarkers Analysis
4.3. Cf-mtDNA Quantification
4.4. Measurement of DNase Activity
4.5. Gel Electrophoresis and SOD2 Western Blotting
4.6. Detection of MMP Activity in Plasma and CSF Samples
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSF | cerebrospinal fluid |
| SMC | subjective memory complaints |
| MCI | mild cognitive impairment |
| AD | Alzheimer disease |
| ADd | Alzheimer disease dementia |
| Aβ | beta amyloid |
| t-tau | total tau |
| p-tau | hyperphosphorylated tau |
| CDR | Clinical Dementia Rating |
| MMSE | Mini Mental State Examination |
| NDAN | nondemented with Alzheimer’s neuropathology |
| SOD2 | manganese-containing superoxide dismutase 2 |
| cf-mtDNA | cell-free mitochondrial DNA |
| mtDNA | mitochondrial DNA |
| APP | amyloid precursor protein |
| DAMP | damage-associated molecular pattern |
| mtDAMP | mitochondrial damage-associated molecular pattern |
| BBB | blood brain barrier |
| TIMPs | tissue inhibitors of matrix metalloproteinases |
| MMP-2 | matrix metalloproteinases 2 |
| MMP-9 | matrix metalloproteinases 9 |
| EV | extracellular vesicles |
| MDVs | mitochondrial-derived vesicles |
| MRI | magnetic resonance imaging |
| FBB | 18F-florbetaben |
| PET | positron emission tomography |
| CLEIA | chemiluminescent immunoassay |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, 5th ed. |
| NIA-AA | National Institute on Aging-Alzheimer’s Association |
| ddPCR | digital droplet PCR |
| SDS-PAGE | sodium dodecyl sulphate–polyacrylamide gel electrophoresis |
References
- Crews, L.; Masliah, E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum. Mol. Genet. 2010, 19, R12–R20. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Grimm, A.; Friedland, K.; Eckert, A. Mitochondrial dysfunction: The missing link between aging and sporadic Alzheimer’s disease. Biogerontology 2016, 17, 281–296. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Singh, E. Antioxidants: An approach for restricting oxidative stress induced neurodegeneration in Alzheimer’s disease. Inflammopharmacology 2023, 31, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Abate, G.; Vezzoli, M.; Sandri, M.; Rungratanawanich, W.; Memo, M.; Uberti, D. Mitochondria and cellular redox state on the route from ageing to Alzheimer’s disease. Mech. Ageing Dev. 2020, 192, 111385. [Google Scholar] [CrossRef]
- Picca, A.; Fracasso, F.; Pesce, V.; Cantatore, P.; Joseph, A.M.; Leeuwenburgh, C.; Gadaleta, M.N.; Lezza, A.M.S. Age- and calorie restriction-related changes in rat brain mitochondrial DNA and TFAM binding. Age 2013, 35, 1607–1620. [Google Scholar] [CrossRef]
- Picca, A.; Pesce, V.; Fracasso, F.; Joseph, A.M.; Leeuwenburgh, C.; Lezza, A.M.S. Aging and calorie restriction oppositely affect mitochondrial biogenesis through TFAM binding at both origins of mitochondrial DNA replication in rat liver. PLoS ONE 2013, 8, e74644. [Google Scholar] [CrossRef]
- Ashleigh, T.; Swerdlow, R.H.; Beal, M.F. The role of mitochondrial dysfunction in Alzheimer’s disease pathogenesis. Alzheimers Dement. 2023, 19, 333–342. [Google Scholar] [CrossRef]
- Swerdlow, R.H.; Khan, S.M. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med. Hypotheses 2004, 63, 8–20. [Google Scholar] [CrossRef]
- Schmitt, K.; Grimm, A.; Kazmierczak, A.; Strosznajder, J.B.; Goetz, J.; Eckert, A. Insights into mitochondrial dysfunction: Aging, amyloid-beta, and tau-A deleterious trio. Antioxid. Redox Signal. 2012, 16, 1456–1466. [Google Scholar] [CrossRef]
- Lewczuk, P.; Riederer, P.; O’Bryant, S.E.; Verbeek, M.M.; Dubois, B.; Visser, P.J.; Jellinger, K.A.; Engelborghs, S.; Ramirez, A.; Parnetti, L.; et al. Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: An update of the Consensus of the Task Force on Biological Markers in Psychiatry of the World Federation of Societies of Biological Psychiatry. World. J. Biol. Psychiatry. 2018, 19, 244–328. [Google Scholar] [CrossRef]
- Dubois, B.; Villain, N.; Frisoni, G.B.; Rabinovici, G.D.; Sabbagh, M.; Cappa, S.; Bejanin, A.; Bombois, S.; Epelbaum, S.; Teichmann, M.; et al. Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurol. 2021, 20, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association Report. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Cummings, J.; Jack, C.R., Jr.; Morris, J.C.; Sperling, R.; Frölich, L.; Jones, R.W.; Dowsett, S.A.; Matthews, B.R.; Raskin, J.; et al. On the path to 2025: Understanding the Alzheimer’s disease continuum. Alzheimers Res. Ther. 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Wang, J.; Gu, B.J.; Masters, C.L.; Wang, Y.J. A systemic view of Alzheimer disease-insights from amyloid-β metabolism beyond the brain. Nat. Rev. Neurol. 2017, 13, 703. [Google Scholar] [CrossRef]
- Swerdlow, R.H. The mitochondrial hypothesis: Dysfunction, bioenergetic defects, and the metabolic link to Alzheimer’s disease. Int. Rev. Neurobiol. 2020, 154, 207–233. [Google Scholar] [CrossRef]
- Picca, A.; Lezza, A.M.S.; Leeuwenburgh, C.; Pesce, V.; Calvani, R.; Landi, F.; Bernabei, R.; Marzetti, E. Fueling Inflamm-Aging through Mitochondrial Dysfunction: Mechanisms and Molecular Targets. Int. J. Mol. Sci. 2017, 18, 933. [Google Scholar] [CrossRef] [PubMed]
- Chimienti, G.; Picca, A.; Fracasso, F.; Russo, F.; Orlando, A.; Riezzo, G.; Leeuwenburgh, C.; Pesce, V.; Lezza, A.M.S. The Age-Sensitive Efficacy of Calorie Restriction on Mitochondrial Biogenesis and mtDNA Damage in Rat Liver. Int. J. Mol. Sci. 2021, 22, 1665. [Google Scholar] [CrossRef] [PubMed]
- Valle, I.; Alvarez-Barrientos, A.; Arza, E.; Lamas, S.; Monsalve, M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 2005, 66, 562–573. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Suliman, H.B. Redox regulation of mitochondrial biogenesis. Free Radic. Biol. Med. 2012, 53, 2043–2053. [Google Scholar] [CrossRef]
- De Leo, M.E.; Borrello, S.; Passantino, M.; Palazzotti, B.; Mordente, A.; Daniele, A.; Filippini, V.; Galeotti, T.; Masullo, C. Oxidative stress and overexpression of manganese superoxide dismutase in patients with Alzheimer’s disease. Neurosci. Lett. 1998, 250, 173–176. [Google Scholar] [CrossRef]
- Fracassi, A.; Marcatti, M.; Zolochevska, O.; Tabor, N.; Woltjer, R.; Moreno, S.; Taglialatela, G. Oxidative Damage and Antioxidant Response in Frontal Cortex of Demented and Nondemented Individuals with Alzheimer’s Neuropathology. J. Neurosci. 2021, 41, 538–554. [Google Scholar] [CrossRef]
- Wang, H.; Dey, K.K.; Chen, P.C.; Li, Y.; Niu, M.; Cho, J.H.; Wang, X.; Bai, B.; Jiao, Y.; Chepyala, S.R.; et al. Integrated analysis of ultra-deep proteomes in cortex, cerebrospinal fluid and serum reveals a mitochondrial signature in Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 43. [Google Scholar] [CrossRef]
- Tijms, B.M.; Vromen, E.M.; Mjaavatten, O.; Holstege, H.; Reus, L.M.; van der Lee, S.; Wesenhagen, K.E.J.; Lorenzini, L.; Vermunt, L.; Venkatraghavan, V.; et al. Cerebrospinal fluid proteomics in patients with Alzheimer’s disease reveals five molecular subtypes with distinct genetic risk profiles. Nat. Aging 2024, 4, 33–47. [Google Scholar] [CrossRef]
- Picard, M.; Shirihai, O.S. Mitochondrial signal transduction. Cell Metab. 2022, 34, 1620–1653. [Google Scholar] [CrossRef]
- Giordano, L.; Gregory, A.D.; Pérez Verdaguer, M.; Ware, S.A.; Harvey, H.; DeVallance, E.; Brzoska, T.; Sundd, P.; Zhang, Y.; Sciurba, F.C.; et al. Extracellular Release of Mitochondrial DNA: Triggered by Cigarette Smoke and Detected in COPD. Cells 2022, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Tsilioni, I.; Natelson, B.; Theoharides, T.C. Exosome-associated mitochondrial DNA from patients with myalgic encephalomyelitis/chronic fatigue syndrome stimulates human microglia to release IL-1β. Eur. J. Neurosci. 2022, 56, 5784–5794. [Google Scholar] [CrossRef] [PubMed]
- Borah, S.; Mishra, R.; Dey, S.; Suchanti, S.; Bhowmick, N.A.; Giri, B.; Haldar, S. Corrigendum to “Prognostic value of circulating mitochondrial DNA in prostate cancer and underlying mechanism” [Mitochondrion 71 (2023) 40–49]. Mitochondrion 2023, 71, 63. [Google Scholar] [CrossRef]
- Gorham, I.K.; Barber, R.C.; Jones, H.P.; Phillips, N.R. Mitochondrial SOS: How mtDNA may act as a stress signal in Alzheimer’s disease. Alzheimers Res. Ther. 2023, 15, 171. [Google Scholar] [CrossRef]
- Grazioli, S.; Pugin, J. Mitochondrial Damage-Associated Molecular Patterns: From Inflammatory Signaling to Human Diseases. Front. Immunol. 2018, 9, 832. [Google Scholar] [CrossRef]
- Podlesniy, P.; Llorens, F.; Puigròs, M.; Serra, N.; Sepúlveda-Falla, D.; Schmidt, C.; Hermann, P.; Zerr, I.; Trullas, R. Cerebrospinal Fluid Mitochondrial DNA in Rapid and Slow Progressive Forms of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6298. [Google Scholar] [CrossRef]
- Klein, H.U.; Trumpff, C.; Yang, H.S.; Lee, A.J.; Picard, M.; Bennett, D.A.; De Jager, P.L. Characterization of mitochondrial DNA quantity and quality in the human aged and Alzheimer’s disease brain. Mol. Neurodegener. 2021, 16, 75. [Google Scholar] [CrossRef]
- Han, D.S.C.; Ni, M.; Chan, R.W.Y.; Chan, V.W.H.; Lui, K.O.; Chiu, R.W.K.; Lo, Y.M.D. The Biology of Cell-free DNA Fragmentation and the Roles of DNASE1, DNASE1L3, and DFFB. Am. J. Hum. Genet. 2020, 106, 202–214. [Google Scholar] [CrossRef]
- Sanders, O.D.; Rajagopal, L.; Rajagopal, J.A. The oxidatively damaged DNA and amyloid-β oligomer hypothesis of Alzheimer’s disease. Free Radic. Biol. Med. 2022, 179, 403–412. [Google Scholar] [CrossRef]
- Arvanitaki, E.S.; Goulielmaki, E.; Gkirtzimanaki, K.; Niotis, G.; Tsakani, E.; Nenedaki, E.; Rouska, I.; Kefalogianni, M.; Xydias, D.; Kalafatakis, I.; et al. Microglia-derived extracellular vesicles trigger age-related neurodegeneration upon DNA damage. Proc. Natl. Acad. Sci. USA 2024, 121, e2317402121. [Google Scholar] [CrossRef]
- Sanders, O.D. Virus-Like Cytosolic and Cell-Free Oxidatively Damaged Nucleic Acids Likely Drive Inflammation, Synapse Degeneration, and Neuron Death in Alzheimer’s Disease. J. Alzheimers Dis. Rep. 2023, 7, 1–19. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Coelho-Junior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial Dysfunction, Oxidative Stress, and Neuroinflammation: Intertwined Roads to Neurodegeneration. Antioxidants 2020, 9, 647. [Google Scholar] [CrossRef]
- Deus, C.M.; Tavares, H.; Beatriz, M.; Mota, S.; Lopes, C. Mitochondrial Damage-Associated Molecular Patterns Content in Extracellular Vesicles Promotes Early Inflammation in Neurodegenerative Disorders. Cells 2022, 11, 2364. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, G.; Sehgal, A.; Bhardwaj, S.; Singh, S.; Buhas, C.; Judea-Pusta, C.; Uivarosan, D.; Munteanu, M.A.; Bungau, S. Multifaceted Role of Matrix Metalloproteinases in Neurodegenerative Diseases: Pathophysiological and Therapeutic Perspectives. Int. J. Mol. Sci. 2021, 22, 1413. [Google Scholar] [CrossRef]
- Aksnes, M.; Capogna, E.; Vidal-Piñeiro, D.; Chaudhry, F.A.; Myrstad, M.; Idland, A.V.; Halaas, N.B.; Dakhil, S.; Blennow, K.; Zetterberg, H.; et al. Matrix metalloproteinases are associated with brain atrophy in cognitively unimpaired individuals. Neurobiol. Aging 2023, 131, 11–23. [Google Scholar] [CrossRef]
- Radosinska, D.; Radosinska, J. The Link Between Matrix Metalloproteinases and Alzheimer’s Disease Pathophysiology. Mol. Neurobiol. 2025, 62, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, L.; Vandooren, J.; Nencetti, S.; Orlandini, E. Natural Marine and Terrestrial Compounds as Modulators of Matrix Metalloproteinases-2 (MMP-2) and MMP-9 in Alzheimer’s Disease. Pharmaceuticals 2021, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Udeh-Momoh, C.; Watermeyer, T. Female Brain Health and Endocrine Research (FEMBER) consortium. Female specific risk factors for the development of Alzheimer’s disease neuropathology and cognitive impairment: Call for a precision medicine approach. Ageing Res. Rev. 2021, 71, 101459. [Google Scholar] [CrossRef]
- Flynn, J.M.; Melov, S. SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic. Biol. Med. 2013, 62, 4–12. [Google Scholar] [CrossRef]
- Zou, X.; Ratti, B.A.; O’Brien, J.G.; Lautenschlager, S.O.; Gius, D.R.; Bonini, M.G.; Zhu, Y. Manganese superoxide dismutase (SOD2): Is there a center in the universe of mitochondrial redox signaling? J. Bioenerg. Biomembr. 2017, 49, 325–333. [Google Scholar] [CrossRef]
- Costa, G.; Caputi, F.F.; Serra, M.; Simola, N.; Rullo, L.; Stamatakos, S.; Sanna, F.; Germain, M.; Martinoli, M.G.; Candeletti, S.; et al. Activation of Antioxidant and Proteolytic Pathways in the Nigrostriatal Dopaminergic System After 3,4-Methylenedioxymethamphetamine Administration: Sex-Related Differences. Front. Pharmacol. 2021, 12, 713486. [Google Scholar] [CrossRef]
- Dicarlo, M.; Pignataro, P.; Zecca, C.; Dell’Abate, M.T.; Urso, D.; Gnoni, V.; Giugno, A.; Borlizzi, F.; Zerlotin, R.; Oranger, A.; et al. Irisin Levels in Cerebrospinal Fluid Correlate with Biomarkers and Clinical Dementia Scores in Alzheimer Disease. Ann. Neurol. 2024, 96, 61–73. [Google Scholar] [CrossRef]
- Picca, A.; Guerra, F.; Calvani, R.; Coelho-Júnior, H.J.; Landi, F.; Bucci, C.; Marzetti, E. Mitochondrial-Derived Vesicles: The Good, the Bad, and the Ugly. Int. J. Mol. Sci. 2023, 24, 13835. [Google Scholar] [CrossRef]
- Podlesniy, P.; Figueiro-Silva, J.; Llado, A.; Antonell, A.; Sanchez-Valle, R.; Alcolea, D.; Lleo, A.; Molinuevo, J.L.; Serra, N.; Trullas, R. Low cerebrospinal fluid concentration of mitochondrial DNA in preclinical Alzheimer disease. Ann. Neurol. 2013, 74, 655–668. [Google Scholar] [CrossRef]
- Cervera-Carles, L.; Alcolea, D.; Estanga, A.; Ecay-Torres, M.; Izagirre, A.; Clerigué, M.; García-Sebastián, M.; Villanúa, J.; Escalas, C.; Blesa, R.; et al. Cerebrospinal fluid mitochondrial DNA in the Alzheimer’s disease continuum. Neurobiol. Aging 2017, 53, 192.e1–192.e4. [Google Scholar] [CrossRef]
- Mohanty, T.; Fisher, J.; Bakochi, A.; Neumann, A.; Cardoso, J.F.P.; Karlsson, C.A.Q.; Pavan, C.; Lundgaard, I.; Nilson, B.; Reinstrup, P.; et al. Neutrophil extracellular traps in the central nervous system hinder bacterial clearance during pneumococcal meningitis. Nat. Commun. 2019, 10, 1667. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, D.J.; Minahan, K.; Keely, S.; Lott, N.; Hansbro, P.; Smith, D.W.; Balogh, Z.J. Reduced deoxyribonuclease enzyme activity in response to high postinjury mitochondrial DNA concentration provides a therapeutic target for Systemic Inflammatory Response Syndrome. J. Trauma Acute Care Surg. 2018, 85, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Tetz, V.; Tetz, G. Effect of deoxyribonuclease I treatment for dementia in end-stage Alzheimer’s disease: A case report. J. Med. Case Rep. 2016, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Nalivaeva, N.N.; Turner, A.J. Targeting amyloid clearance in Alzheimer’s disease as a therapeutic strategy. Br. J. Pharmacol. 2019, 176, 3447–3463. [Google Scholar] [CrossRef]
- Yin, K.J.; Cirrito, J.R.; Yan, P.; Hu, X.; Xiao, Q.; Pan, X.; Bateman, R.; Song, H.; Hsu, F.F.; Turk, J.; et al. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J. Neurosci. 2006, 26, 10939–10948. [Google Scholar] [CrossRef]
- Loeffler, D.A. Experimental approaches for altering the expression of Abeta-degrading enzymes. J. Neurochem. 2023, 164, 725–763. [Google Scholar] [CrossRef]
- Horstmann, S.; Budig, L.; Gardner, H.; Koziol, J.; Deuschle, M.; Schilling, C.; Wagner, S. Matrix metalloproteinases in peripheral blood and cerebrospinal fluid in patients with Alzheimer’s disease. Int. Psychogeriatr. 2010, 22, 966–972. [Google Scholar] [CrossRef]
- Whelan, C.D.; Mattsson, N.; Nagle, M.W.; Vijayaraghavan, S.; Hyde, C.; Janelidze, S.; Stomrud, E.; Lee, J.; Fitz, L.; Samad, T.A.; et al. Multiplex proteomics identifies novel CSF and plasma biomarkers of early Alzheimer’s disease. Acta Neuropathol. Commun. 2019, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Lorenzl, S.; Albers, D.S.; Relkin, N.; Ngyuen, T.; Hilgenberg, S.L.; Chirichigno, J.; Cudkowicz, M.E.; Beal, M.F. Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer’s disease. Neurochem. Int. 2003, 43, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Lorenzl, S.; Buerger, K.; Hampel, H.; Beal, M.F. Profiles of matrix metalloproteinases and their inhibitors in plasma of patients with dementia. Int. Psychogeriatr. 2008, 20, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Liu, F.; Meng, M.; Zhang, L.; Gordon, M.L.; Wang, Y.; Cai, L.; Zhang, N. Elevated matrix metalloproteinase-9 levels in neuronal extracellular vesicles in Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2020, 7, 1681–1691. [Google Scholar] [CrossRef]
- Tsiknia, A.A.; Sundermann, E.E.; Reas, E.T.; Edland, S.D.; Brewer, J.B.; Galasko, D.; Banks, S.J. Alzheimer’s Disease Neuroimaging Initiative. Sex differences in Alzheimer’s disease: Plasma MMP-9 and markers of disease severity. Alzheimers Res. Ther. 2022, 14, 160. [Google Scholar] [CrossRef]
- Merlo, S.; Sortino, M.A. Estrogen activates matrix metalloproteinases-2 and -9 to increase beta amyloid degradation. Mol. Cell Neurosci. 2012, 49, 423–429. [Google Scholar] [CrossRef]
- Deb, S.; Wenjun Zhang, J.; Gottschall, P.E. Beta-amyloid induces the production of active, matrix-degrading proteases in cultured rat astrocytes. Brain Res. 2003, 970, 205–213. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®), 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Amariglio, R.E.; van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; van der Flier, W.M.; et al. Subjective Cognitive Decline Initiative (SCD-I) Working Group. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association Declaration of Helsinki. Law Med. Health Care 1991, 19, 264–265. [CrossRef] [PubMed]
- Vanderstichele, H.; Bibl, M.; Engelborghs, S.; Le Bastard, N.; Lewczuk, P.; Molinuevo, J.L.; Parnetti, L.; Perret-Liaudet, A.; Shaw, L.M.; Teunissen, C.; et al. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: A consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement. 2012, 8, 65–73. [Google Scholar] [CrossRef]
- Podlesniy, P.; Trullas, R. Biomarkers in Cerebrospinal Fluid: Analysis of Cell-Free Circulating Mitochondrial DNA by Digital PCR. Methods Mol. Biol. 2018, 1768, 111–126. [Google Scholar] [CrossRef]
- Chimienti, G.; Russo, F.; Bianco, A.; Maqoud, F.; De Virgilio, C.; Galeano, G.; Orlando, A.; Riezzo, G.; D’Attoma, B.; Ignazzi, A.; et al. Effect of a 12-Week Walking Program Monitored by Global Physical Capacity Score (GPCS) on Circulating Cell-Free mtDNA and DNase Activity in Patients with Irritable Bowel Syndrome. Int. J. Mol. Sci. 2024, 25, 4293. [Google Scholar] [CrossRef]
- Ondracek, A.S.; Aszlan, A.; Schmid, M.; Lenz, M.; Mangold, A.; Artner, T.; Emich, M.; Fritzer-Szekeres, M.; Strametz-Juranek, J.; Lang, I.M.; et al. Physical Exercise Promotes DNase Activity Enhancing the Capacity to Degrade Neutrophil Extracellular Traps. Biomedicines 2022, 10, 2849. [Google Scholar] [CrossRef]
- Colella, A.D.; Chegenii, N.; Tea, M.N.; Gibbins, I.L.; Williams, K.A.; Chataway, T.K. Comparison of Stain-Free gels with traditional immunoblot loading control methodology. Anal. Biochem. 2012, 430, 108–110. [Google Scholar] [CrossRef]
- Zuccalà, P.; Latronico, T.; Marocco, R.; Savinelli, S.; Vita, S.; Mengoni, F.; Tieghi, T.; Borgo, C.; Kertusha, B.; Carraro, A.; et al. Longitudinal Assessment of Multiple Immunological and Inflammatory Parameters during Successful DAA Therapy in HCV Monoinfected and HIV/HCV Coinfected Subjects. Int. J. Mol. Sci. 2022, 23, 11936. [Google Scholar] [CrossRef]
- Zingaropoli, M.A.; Iannetta, M.; Piermatteo, L.; Pasculli, P.; Latronico, T.; Mazzuti, L.; Campogiani, L.; Duca, L.; Ferraguti, G.; De Michele, M.; et al. Neuro-Axonal Damage and Alteration of Blood-Brain Barrier Integrity in COVID-19 Patients. Cells 2022, 11, 2480. [Google Scholar] [CrossRef]



| SMC (n = 20) | MCI (n = 20) | ADd (n = 20) | p | |
|---|---|---|---|---|
| Sex (Male/Female) | 9/11 | 10/10 | 12/8 | 0.6268 |
| Age (years) | 60.3 ± 11.2 | 65.7 ± 9.8 | 66.7 ± 9.3 | 0.1130 ° |
| CSF Aβ (pg/mL) | 937.7 (829.6–1171.0) | 518.0 (375.0–640.7) # | 442.0 (364.2–568.7) # | <0.0001 § |
| Male (pg/mL) | 968.7 (790.5–1163.0) | 546.0 (317.8–689.3) # | 501.9 (346.2–578.0) # | 0.0004 § |
| Female (pg/mL) | 910.0 (827.4–1186.0) | 511.5 (433.8–560.9) # | 389.3 (374.5–520.8) # | <0.0001 § |
| CSF t-tau (pg/mL) | 170.8 (134.2–234.5) | 398.5 (184.0–548.5) # | 654.2 (357.5–896.7) #,@ | <0.0001 § |
| Male (pg(mL) | 175.0 (138.4–217.0) | 313.0 (172.3–602.6) | 654.2 (387.0–1003.0) # | 0.0006 § |
| Female (pg/mL) | 166.7 (116.0–310.0) | 404.5 (192.0–575.0) | 645.7 (351.4–894.3) # | 0.0022 § |
| CSF p-tau (pg/mL) | 28.86 (19.98–36.40) | 55.80 (33.03–71,71) # | 82.50 (49.25–103.6) # | <0.0001 § |
| Male (pg/mL) | 25.2 (18.8–31.7) | 49.3 (35.9–70.2) # | 83.8 (53.6–134.1) # | 0.0001 § |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Lorenzo, R.; Zecca, C.; Chimienti, G.; Latronico, T.; Liuzzi, G.M.; Pesce, V.; Dell’Abate, M.T.; Borlizzi, F.; Giugno, A.; Urso, D.; et al. Reliable New Biomarkers of Mitochondrial Oxidative Stress and Neuroinflammation in Cerebrospinal Fluid and Plasma from Alzheimer’s Disease Patients: A Pilot Study. Int. J. Mol. Sci. 2025, 26, 7792. https://doi.org/10.3390/ijms26167792
Di Lorenzo R, Zecca C, Chimienti G, Latronico T, Liuzzi GM, Pesce V, Dell’Abate MT, Borlizzi F, Giugno A, Urso D, et al. Reliable New Biomarkers of Mitochondrial Oxidative Stress and Neuroinflammation in Cerebrospinal Fluid and Plasma from Alzheimer’s Disease Patients: A Pilot Study. International Journal of Molecular Sciences. 2025; 26(16):7792. https://doi.org/10.3390/ijms26167792
Chicago/Turabian StyleDi Lorenzo, Rosa, Chiara Zecca, Guglielmina Chimienti, Tiziana Latronico, Grazia Maria Liuzzi, Vito Pesce, Maria Teresa Dell’Abate, Francesco Borlizzi, Alessia Giugno, Daniele Urso, and et al. 2025. "Reliable New Biomarkers of Mitochondrial Oxidative Stress and Neuroinflammation in Cerebrospinal Fluid and Plasma from Alzheimer’s Disease Patients: A Pilot Study" International Journal of Molecular Sciences 26, no. 16: 7792. https://doi.org/10.3390/ijms26167792
APA StyleDi Lorenzo, R., Zecca, C., Chimienti, G., Latronico, T., Liuzzi, G. M., Pesce, V., Dell’Abate, M. T., Borlizzi, F., Giugno, A., Urso, D., Logroscino, G., & Lezza, A. M. S. (2025). Reliable New Biomarkers of Mitochondrial Oxidative Stress and Neuroinflammation in Cerebrospinal Fluid and Plasma from Alzheimer’s Disease Patients: A Pilot Study. International Journal of Molecular Sciences, 26(16), 7792. https://doi.org/10.3390/ijms26167792







