Network Pharmacology-Guided Discovery of Traditional Chinese Medicine Extracts for Alzheimer’s Disease: Targeting Neuroinflammation and Gut–Brain Axis Dysfunction
Abstract
1. Introduction
2. Results
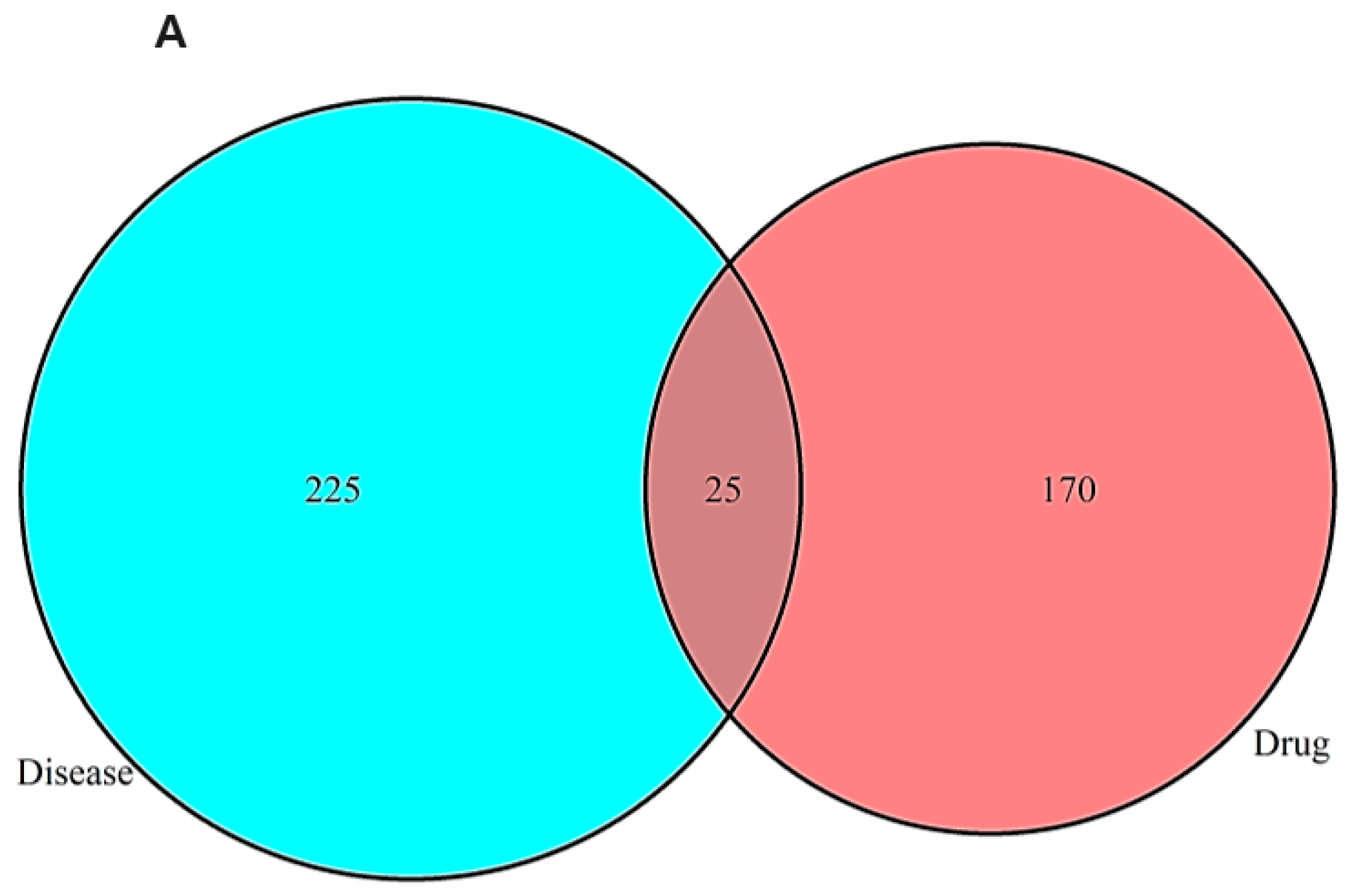
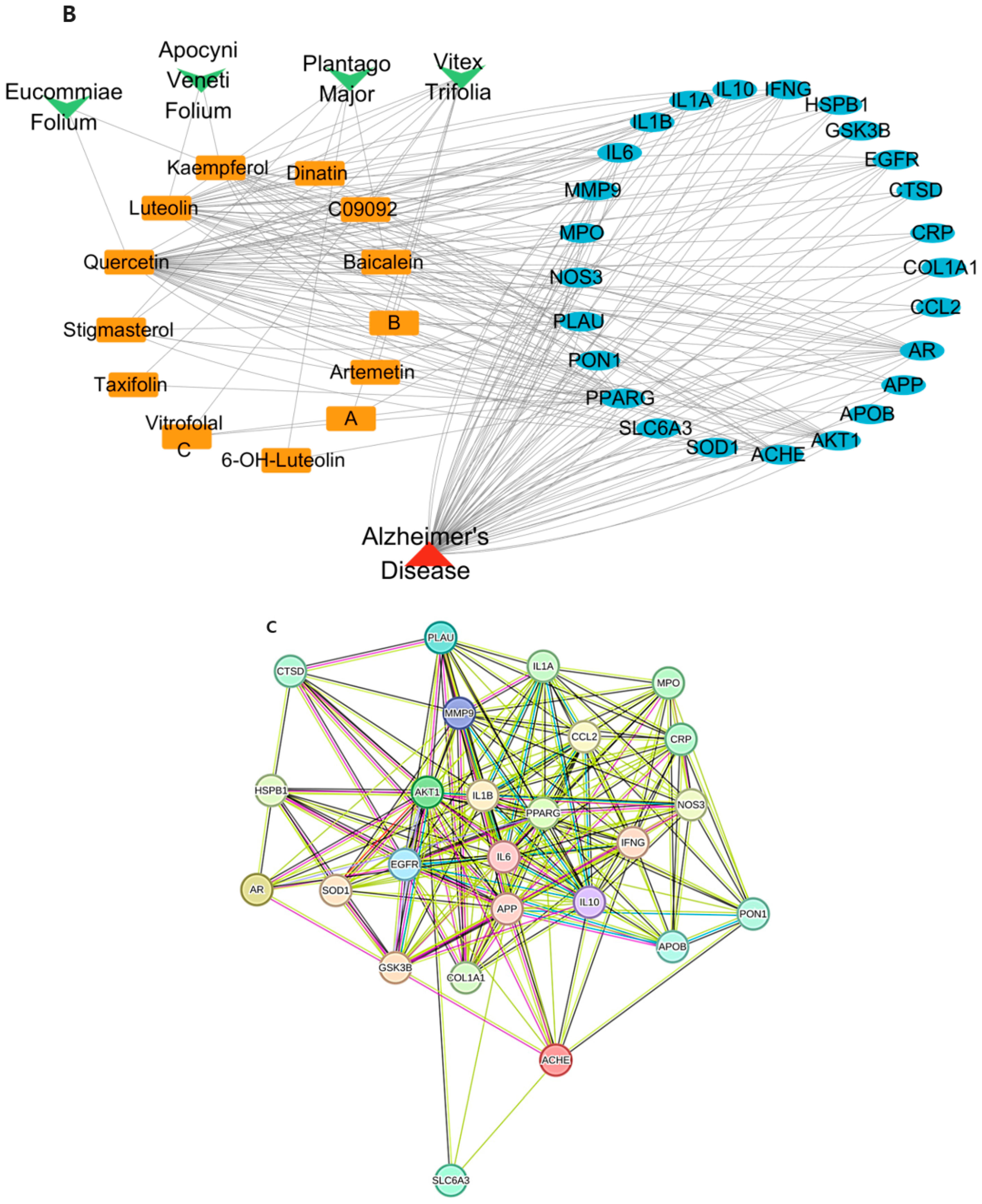
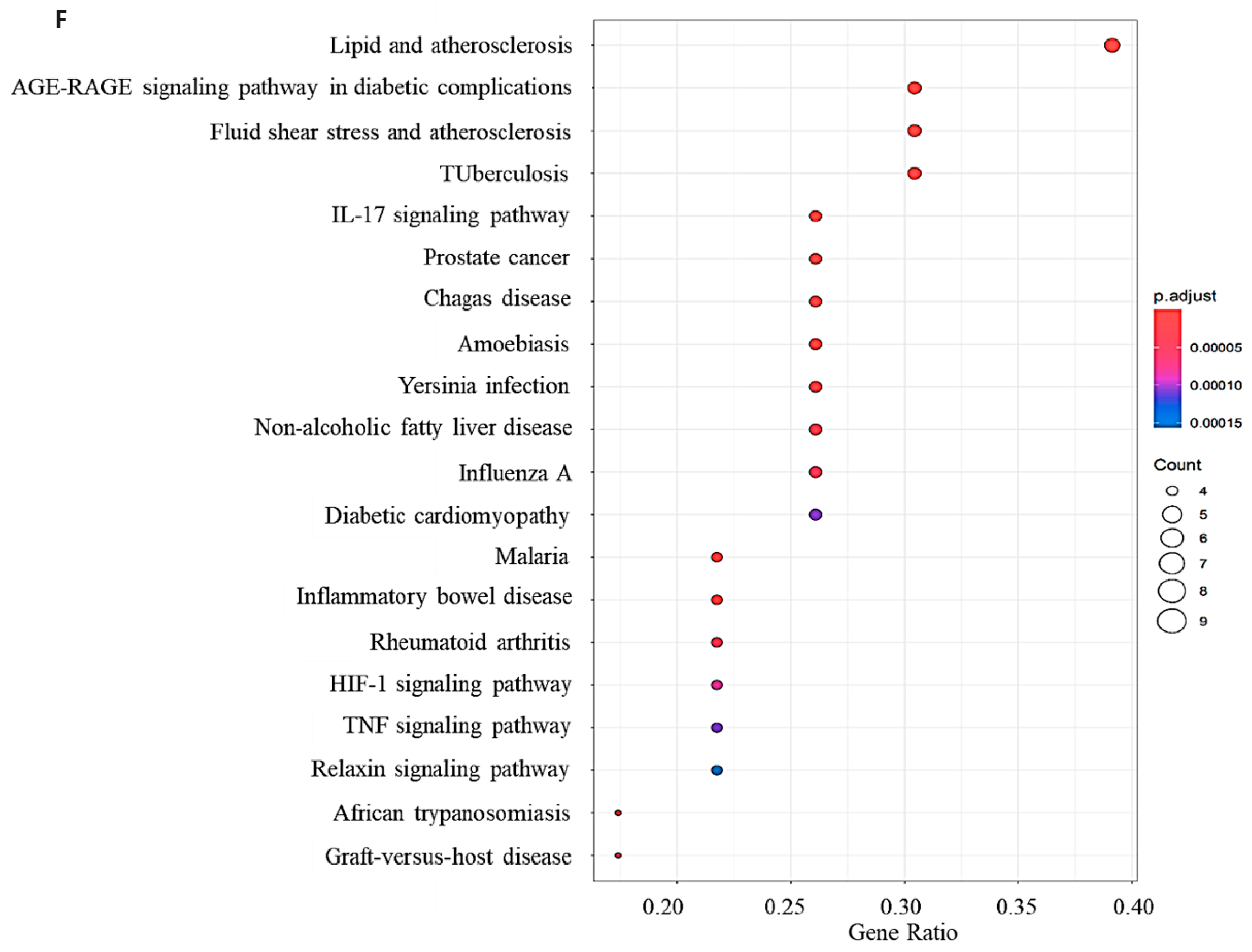
2.1. Network Pharmacology Results
2.2. Validation Through Molecular Docking
2.3. Phytochemical Composition of Herbal Extracts and Active Constituents
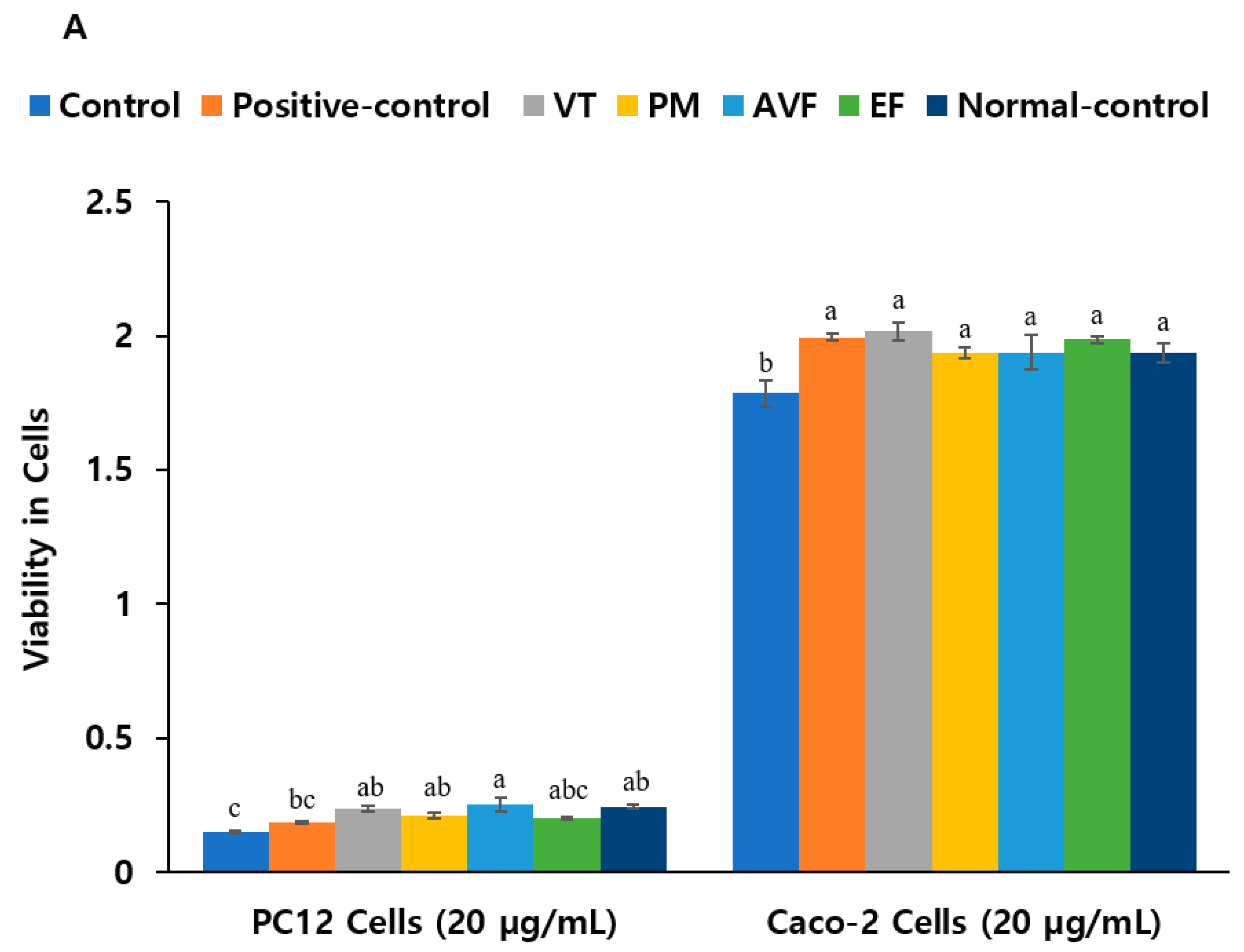
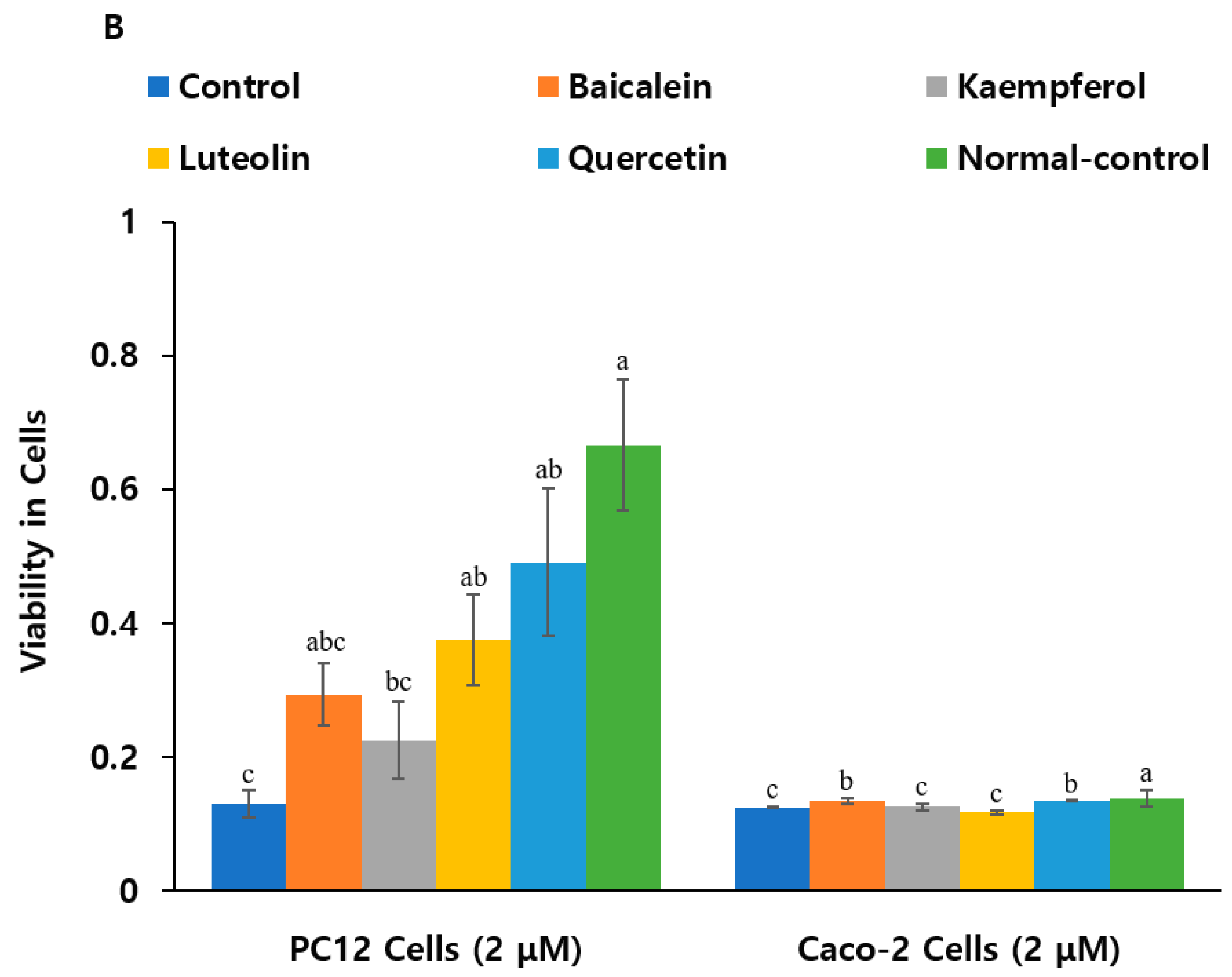
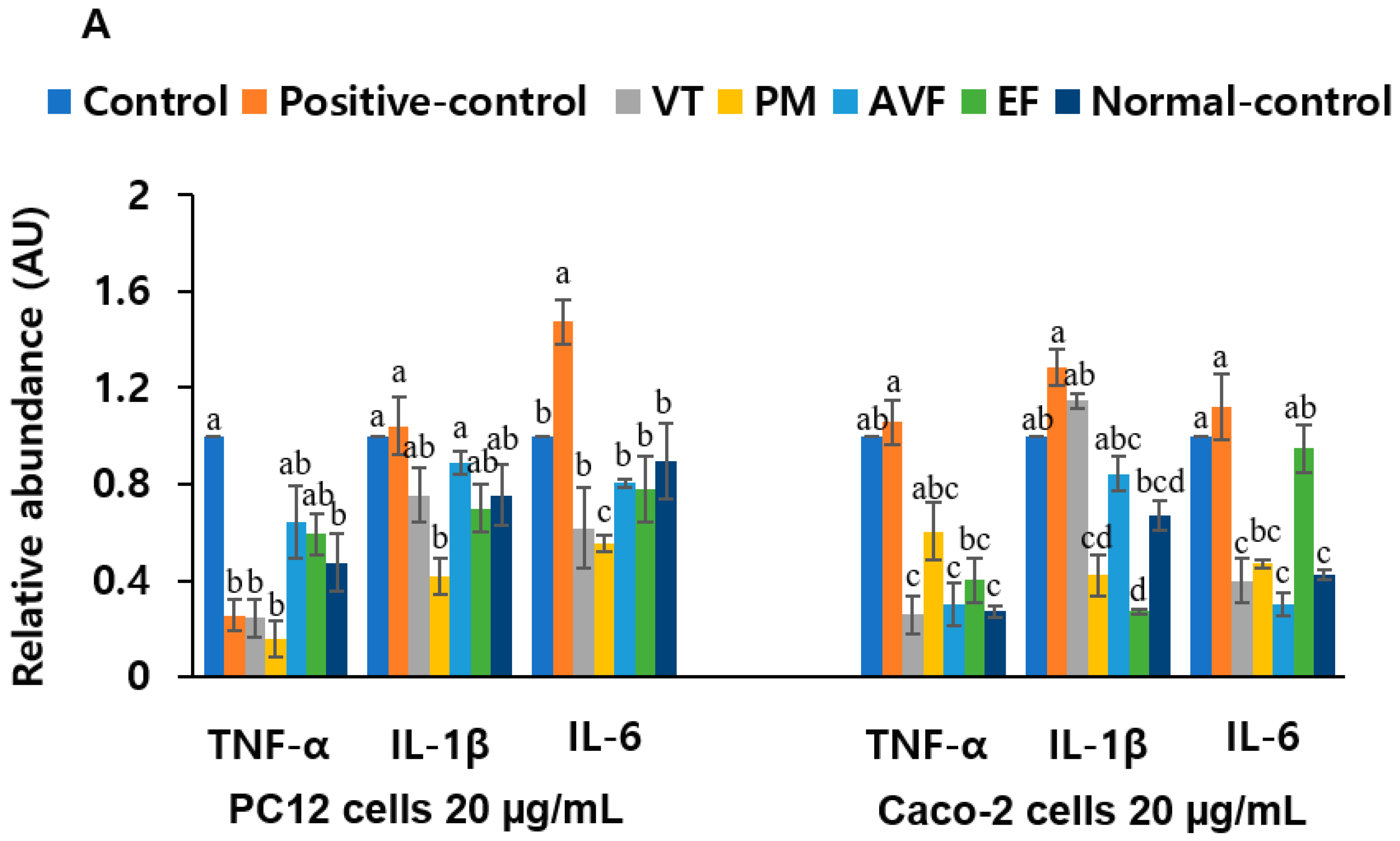
2.4. Neuroprotective Effects and Anti-Inflammatory Activity
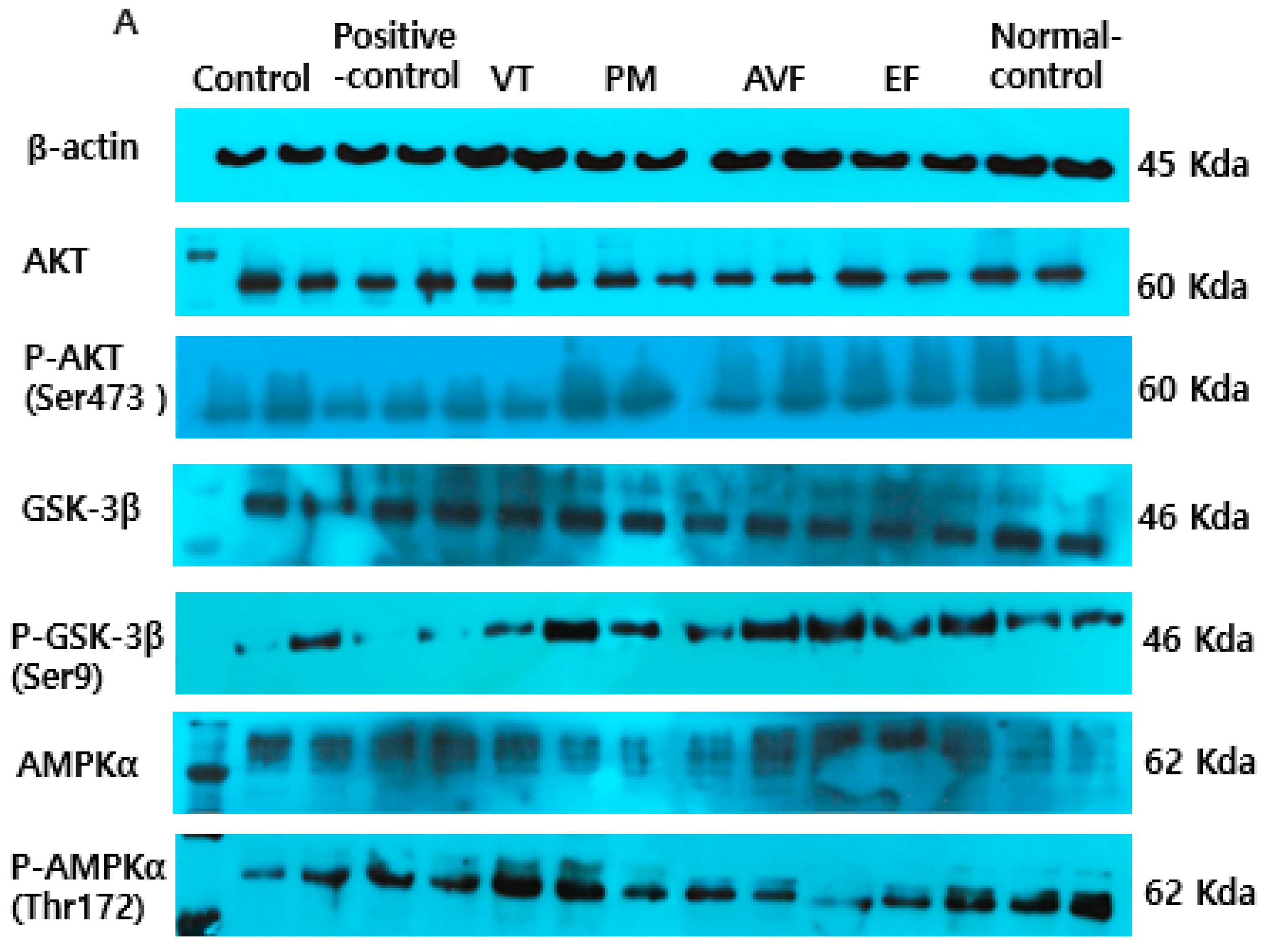
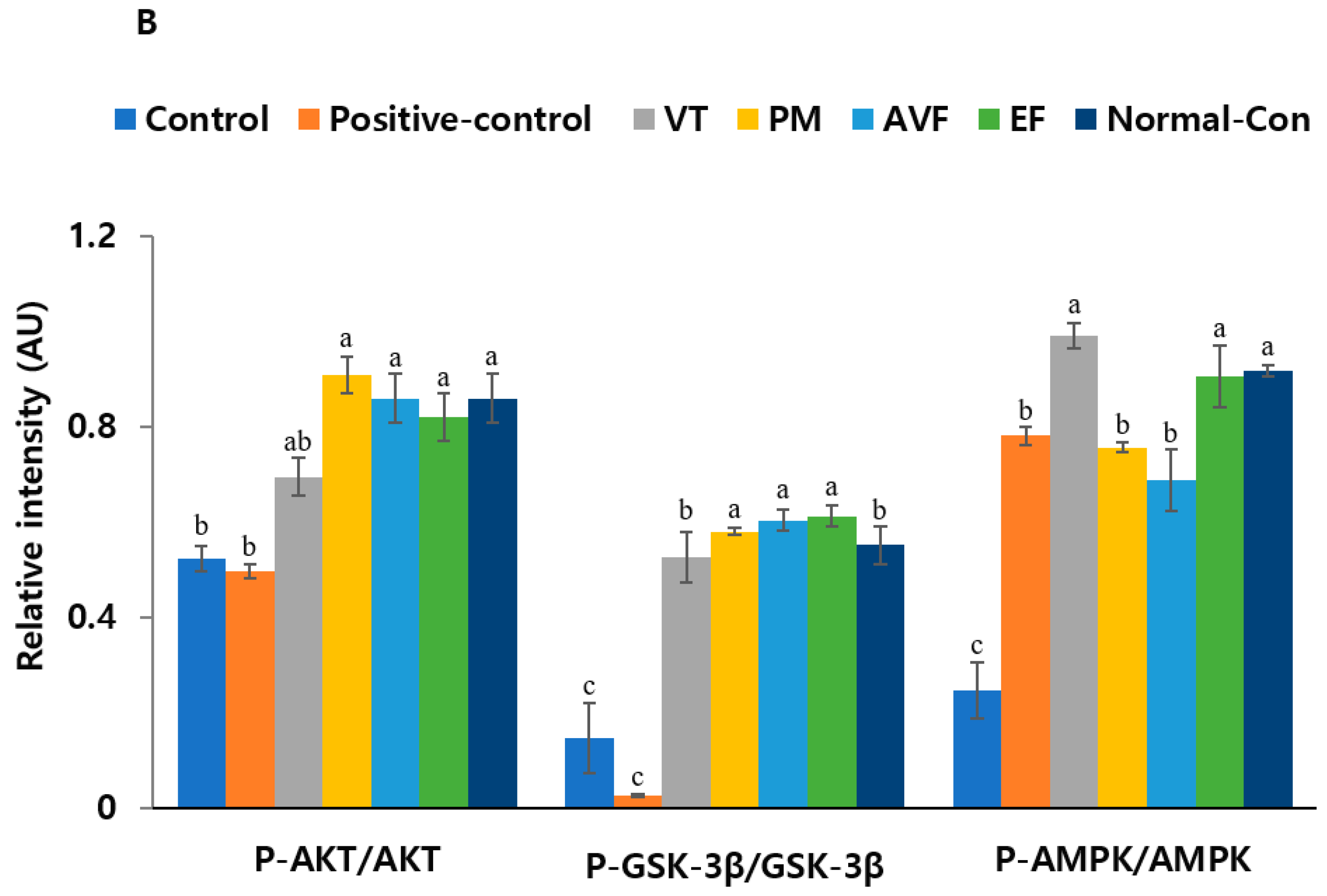
2.5. Molecular Mechanisms and Mitochondrial Function
3. Discussion
Limitations and Future Directions
4. Methods
4.1. Network Pharmacology Analysis
4.2. Molecular Docking
4.3. Preparation of Herbal Extract and Measurement of Index Bioactive Compounds
4.4. Co-Cell Culture and Cytotoxicity Assessment
4.5. TBARS and AChE Activity Assays
4.6. Molecular Analysis: Real-Time PCR Analysis and Western Blot Analysis
4.7. Assessment of Cellular Morphology and Mitochondrial Function
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Graff-Radford, J.; Yong, K.X.X.; Apostolova, L.G.; Bouwman, F.H.; Carrillo, M.; Dickerson, B.C.; Rabinovici, G.D.; Schott, J.M.; Jones, D.T.; Murray, M. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet. Neurol. 2021, 20, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Deng, T.; Zhai, Z.; Sun, T.; Xu, Y. The cellular model for Alzheimer’s disease research: PC12 cells. Front. Mol. Neurosci. 2022, 15, 1016559. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhao, Q.; Guan, Y.; Sun, Z.; Li, W.; Guo, S.; Zhang, A. The communication mechanism of the gut-brain axis and its effect on central nervous system diseases: A systematic review. Biomed. Pharmacother. 2024, 178, 117207. [Google Scholar] [CrossRef]
- Kearns, R. Gut–Brain Axis and Neuroinflammation: The Role of Gut Permeability and the Kynurenine Pathway in Neurological Disorders. Cell Mol. Neurobiol. 2024, 44, 64. [Google Scholar] [CrossRef]
- Kalyan, M.; Tousif, A.H.; Sonali, S.; Vichitra, C.; Sunanda, T.; Praveenraj, S.S.; Ray, B.; Gorantla, V.R.; Rungratanawanich, W.; Mahalakshmi, A.M.; et al. Role of Endogenous Lipopolysaccharides in Neurological Disorders. Cells 2022, 11, 4038. [Google Scholar] [CrossRef]
- Moysidou, C.M.; Owens, R.M. Advances in modelling the human microbiome-gut-brain axis in vitro. Biochem. Soc. Trans. 2021, 49, 187–201. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Drapaca, C.S.J.M.; Applications, C. A Mathematical Study of Effects of Alzheimer’s Drug Donepezil Hydrochloride on Neuronal Viscoelasticity and Action Potentials. Math. Comput. Appl. 2024, 29, 117. [Google Scholar] [CrossRef]
- Turgutalp, B.; Kizil, C. Multi-target drugs for Alzheimer’s disease. Trends Pharmacol. Sci. 2024, 45, 628–638. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, F.; Zhang, M. Therapeutic potential of plant-derived natural compounds in Alzheimer’s disease: Targeting microglia-mediated neuroinflammation. Biomed. Pharmacother. 2024, 178, 117235. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef]
- Wu, J.; Wu, J.; Li, Z.; Dong, X.; Yuan, S.; Liu, J.; Wang, L.; Modi, P. The Neuroprotective Effect of Shenmai Injection on Oxidative Stress Injury in PC12 Cells Based on Network Pharmacology. Evid. Based Complement. Altern. Med. 2022, 2022, 6969740. [Google Scholar] [CrossRef] [PubMed]
- Vitorakis, N.; Piperi, C. Pivotal role of AGE-RAGE axis in brain aging with current interventions. Ageing Res. Rev. 2024, 100, 102429. [Google Scholar] [CrossRef]
- Calderaro, A.; Patanè, G.T.; Tellone, E.; Barreca, D.; Ficarra, S.; Misiti, F.; Laganà, G. The Neuroprotective Potentiality of Flavonoids on Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 14835. [Google Scholar] [CrossRef]
- Daily, J.W.; Kang, S.; Park, S. Protection against Alzheimer’s disease by luteolin: Role of brain glucose regulation, anti-inflammatory activity, and the gut microbiota-liver-brain axis. BioFactors 2021, 47, 218–231. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Nejabati, H.R.; Roshangar, L. Kaempferol as a potential neuroprotector in Alzheimer’s disease. J. Food Biochem. 2022, 46, e14375. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, L.; Wu, T.; Yang, C.; Gao, L.; Cai, H.; Liu, J.; Fang, S.; Chen, Y.; Tan, W.; et al. Network pharmacology-based study on the mechanism of action for herbal medicines in Alzheimer treatment. J. Ethnopharmacol. 2017, 196, 281–292. [Google Scholar] [CrossRef]
- Kempuraj, D.; Ahmed, M.E.; Selvakumar, G.P.; Thangavel, R.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Burton, C.; James, D.; Zaheer, A. Mast Cell Activation, Neuroinflammation, and Tight Junction Protein Derangement in Acute Traumatic Brain Injury. Mediat. Inflamm. 2020, 2020, 4243953. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L. Mast cell-glia axis in neuroinflammation and therapeutic potential of the anandamide congener palmitoylethanolamide. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3312–3325. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S.; Kang, S.; Kim, H.J. The combination of luteolin and l-theanine improved Alzheimer disease-like symptoms by potentiating hippocampal insulin signaling and decreasing neuroinflammation and norepinephrine degradation in amyloid-β-infused rats. Nutr. Res. 2018, 60, 116–131. [Google Scholar] [CrossRef]
- Rarinca, V.; Nicoara, M.N.; Ureche, D.; Ciobica, A. Exploitation of Quercetin’s Antioxidative Properties in Potential Alternative Therapeutic Options for Neurodegenerative Diseases. Antioxidants 2023, 12, 1418. [Google Scholar] [CrossRef] [PubMed]
- Herrera, T.E.S.; Tello, I.P.S.; Mustafa, M.A.; Jamil, N.Y.; Alaraj, M.; Atiyah Altameem, K.K.; Alasheqi, M.Q.; Hamoody, A.-H.M.; Alkhafaji, A.T.; Shakir, M.N.; et al. Kaempferol: Unveiling its anti-inflammatory properties for therapeutic innovation. Cytokine 2025, 186, 156846. [Google Scholar] [CrossRef]
- Zaplatic, E.; Bule, M.; Shah, S.Z.A.; Uddin, M.S.; Niaz, K. Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci. 2019, 224, 109–119, Erratum in Life Sci. 2019, 231, 116616. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Valko, R.; Liska, J.; Nepovimova, E.; Kuca, K.; Valko, M. Flavonoids and their role in oxidative stress, inflammation, and human diseases. Chem. Biol. Interact. 2025, 413, 111489. [Google Scholar] [CrossRef]
- Park, S.; Wu, X. Modulation of the gut microbiota in memory impairment and Alzheimer’s disease via the inhibition of the parasympathetic nervous system. Int. J. Mol. Sci. 2022, 23, 13574. [Google Scholar] [CrossRef]
- Kim, C.K.; Lee, Y.R.; Ong, L.; Gold, M.; Kalali, A.; Sarkar, J. Alzheimer’s Disease: Key Insights from Two Decades of Clinical Trial Failures. J. Alzheimers Dis. 2022, 87, 83–100. [Google Scholar] [CrossRef]
- Brandscheid, C.; Schuck, F.; Reinhardt, S.; Schäfer, K.H.; Pietrzik, C.U.; Grimm, M.; Hartmann, T.; Schwiertz, A.; Endres, K. Altered Gut Microbiome Composition and Tryptic Activity of the 5xFAD Alzheimer’s Mouse Model. J. Alzheimers Dis. 2017, 56, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, D.; Xu, J.; Hu, J.; Wang, Y. Complex in vitro Model: A Transformative Model in Drug Development and Precision Medicine. Clin. Transl. Sci. 2023, 17, e13695. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, M.; Chen, S.; Li, M.; Zeng, J.; Wu, S.; Tong, X. Baicalin Mitigates the Neuroinflammation through the TLR4/MyD88/NF-κB and MAPK Pathways in LPS-Stimulated BV-2 Microglia. Biomed. Res. Int. 2022, 2022, 3263446. [Google Scholar] [CrossRef]
- Long, H.Z.; Cheng, Y.; Zhou, Z.W.; Luo, H.Y.; Wen, D.D.; Gao, L.C. PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer’s Disease and Parkinson’s Disease. Front. Pharmacol. 2021, 12, 648636. [Google Scholar] [CrossRef]
- Piletz, J.E.; Cooper, J.; Chidester, K.; Erson, K.; Melton, S.; Osemeka, A.; Patterson, M.; Strickland, K.; Wan, J.X.; Williams, K. Transepithelial Effect of Probiotics in a Novel Model of Gut Lumen to Nerve Signaling. Nutrients 2022, 14, 4856. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.R.; Barbosa, D.J.; Remião, F.; Silva, R. Co-Culture Models: Key Players in In Vitro Neurotoxicity, Neurodegeneration and BBB Modeling Studies. Biomedicines 2024, 12, 626. [Google Scholar] [CrossRef]
- Satsu, H.; Yokoyama, T.; Ogawa, N.; Fujiwara-Hatano, Y.; Shimizu, M. Effect of neuronal PC12 cells on the functional properties of intestinal epithelial Caco-2 cells. Biosci. Biotechnol. Biochem. 2003, 67, 1312–1318. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, L.; Siu, W.; Jin, Y.; Cao, M.; Li, W.; Chen, J.; Cong, W.; Ma, M.; Chen, K.; et al. A combined molecular biology and network pharmacology approach to investigate the multi-target mechanisms of Chaihu Shugan San on Alzheimer’s disease. Biomed. Pharmacother. 2019, 120, 109370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, C.; Yue, Y.; Yang, H.-J.; Ryu, M.S.; Wu, X.; Jeong, D.Y.; Park, S. Fermented red pepper paste (kochujang) modulates glucose metabolism and gut microbiota in parasympathetic suppression: Network pharmacology and In Vivo study. Food Biosci. 2024, 61, 104531. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Meng, M.; Zhang, L.; Ai, D.; Wu, H.; Peng, W. β-Asarone Ameliorates β-Amyloid-Induced Neurotoxicity in PC12 Cells by Activating P13K/Akt/Nrf2 Signaling Pathway. Front. Pharm. 2021, 12, 659955. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, X.; Yuan, H.; Huang, S.; Park, S. Mitigation of Memory Impairment with Fermented Fucoidan and λ-Carrageenan Supplementation through Modulating the Gut Microbiota and Their Metagenome Function in Hippocampal Amyloid-β Infused Rats. Cells 2022, 11, 2301. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Okada, K.; Hayashi, O. Immuno-regulatory and anti-inflammatory actions of phycocyanin on Caco-2/U937 cells co-culture as a model of the intestinal barrier. Funct. Food Health Dis. 2019, 9, 466–483. [Google Scholar] [CrossRef]





| A. Herbal extracts (20 μg/mL) | |||
| PC12 Cell TBARs (mg/dL) | Caco-2 Cell TBARs (mg/dL) | PC12 Cell AChE activity (U/mL) | |
| Control | 0.98 ± 0.008 a | 2.36 ± 0.001 a | 4.49 ± 0.022 a |
| Positive-control | 0.12 ± 0.002 b | 0.60 ± 0.002 b | 0.78 ± 0.019 c |
| VT | 0.24 ± 0.001 b | 0.76 ± 0.002 b | 2.97 ± 0.018 b |
| PM | 0.25 ± 0.004 b | 0.43 ± 0.002 b | 2.49 ± 0.005 b |
| AVF | 0.17 ± 0.002 b | 0.07 ± 0.000 c | 2.57 ± 0.024 b |
| EF | 0.26 ± 0.003 b | 0.56 ± 0.003 b | 2.15 ± 0.133 b |
| Normal-control | 0.13 ± 0.001 b | 0.43 ± 0.000 b | 2.46 ± 0.016 b |
| B. Index compounds (2 μM) | |||
| PC12 Cell TBARs (mg/dL) | Caco-2 Cell TBARs (mg/dL) | PC12 Cell AChE activity (U/mL) | |
| Control | 10.16 ± 0.021 a | 7.25 ± 0.028 a | 4.49 ± 0.023 a |
| Baicalein | 8.45 ± 0.011 b | 6.79 ± 0.011 b | 3.05 ± 0.033 b |
| Kaempferol | 6.12 ± 0.016 c | 6.93 ± 0.008 b | 2.46 ± 0.016 b |
| Luteolin | 8.73 ± 0.041 b | 6.51 ± 0.012 b | 2.87 ± 0.054 b |
| Quercetin | 9.60 ± 0.010 ab | 6.87 ± 0.023 b | 3.07 ± 0.024 b |
| Normal-control | 6.83 ± 0.000 c | 6.64 ± 0.017 b | 2.04 ± 0.018 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Park, S. Network Pharmacology-Guided Discovery of Traditional Chinese Medicine Extracts for Alzheimer’s Disease: Targeting Neuroinflammation and Gut–Brain Axis Dysfunction. Int. J. Mol. Sci. 2025, 26, 8545. https://doi.org/10.3390/ijms26178545
Zhang T, Park S. Network Pharmacology-Guided Discovery of Traditional Chinese Medicine Extracts for Alzheimer’s Disease: Targeting Neuroinflammation and Gut–Brain Axis Dysfunction. International Journal of Molecular Sciences. 2025; 26(17):8545. https://doi.org/10.3390/ijms26178545
Chicago/Turabian StyleZhang, Ting, and Sunmin Park. 2025. "Network Pharmacology-Guided Discovery of Traditional Chinese Medicine Extracts for Alzheimer’s Disease: Targeting Neuroinflammation and Gut–Brain Axis Dysfunction" International Journal of Molecular Sciences 26, no. 17: 8545. https://doi.org/10.3390/ijms26178545
APA StyleZhang, T., & Park, S. (2025). Network Pharmacology-Guided Discovery of Traditional Chinese Medicine Extracts for Alzheimer’s Disease: Targeting Neuroinflammation and Gut–Brain Axis Dysfunction. International Journal of Molecular Sciences, 26(17), 8545. https://doi.org/10.3390/ijms26178545







