The Immune/Inflammatory Underpinnings of Neurodevelopmental Disorders and Pediatric Acute-Onset Neuropsychiatric Syndrome: A Scoping Review
Abstract
1. Introduction
Aim of Review
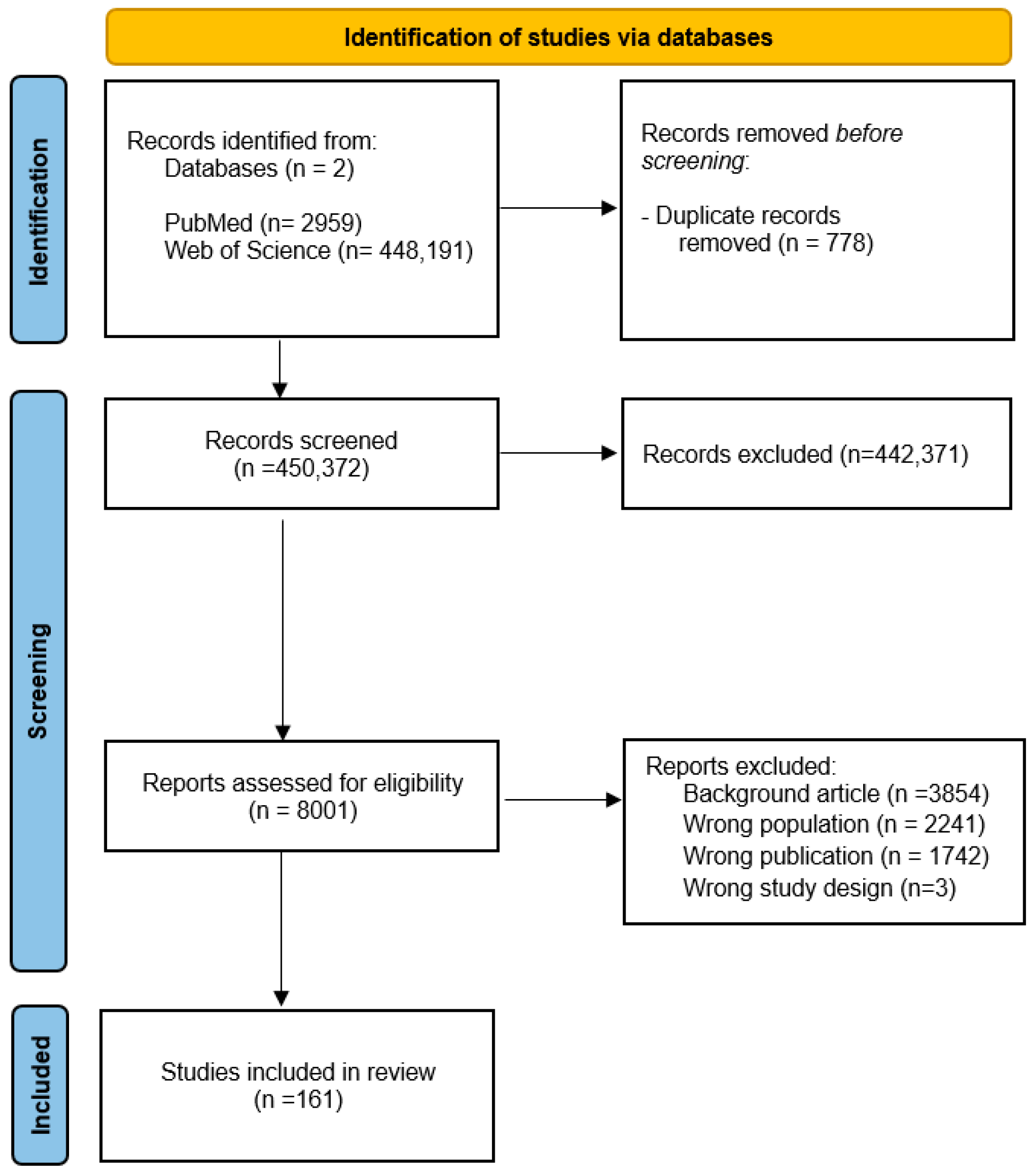
2. Materials and Methods
2.1. Data Source and Search Strategy
2.2. Selection Procedures
2.3. Data Extraction and Evaluation
3. Results
3.1. Evidence of Immune Dysregulation and Neuroinflammation in Neurodevelopmental Disorders
3.2. Attention Deficit Hyperactivity Disorder
3.3. Autism Spectrum Disorder
3.4. Tics and Tourette’s Disorder
3.5. Intellectual Disability
3.6. Schizophrenia Spectrum (SS) and Other Psychotic Disorders (PDs)
3.7. Obsessive–Compulsive Disorder
3.8. Pediatric Acute-Onset Neuropsychiatric Syndrome
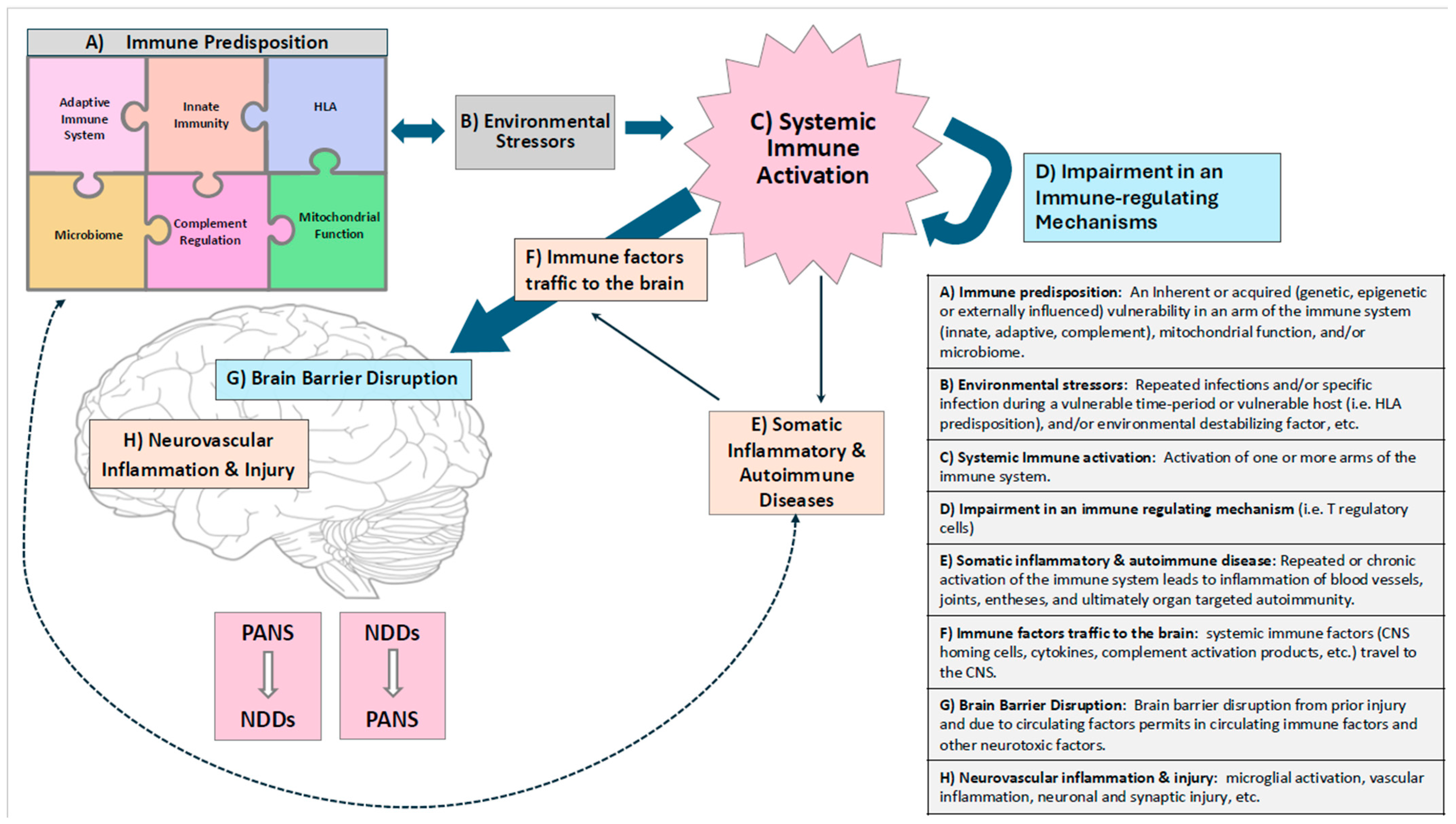
4. Discussion
Forthcoming Issues
5. Limitations
6. Future Directions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABGA | Anti-Basal Ganglia Antibody |
| ADHD | Attention Deficit Hyperactivity Disorder |
| AE | Autoimmune Encephalitis |
| ASD | Autism Spectrum Disorder |
| BBB | Blood–Brain Barrier |
| CD | Communication Disorder |
| CINs | Cholinergic Interneurons |
| CNS | Central Nervous System |
| CSF | Cerebrospinal Fluid |
| D2R | Anti-D2 Dopamine Receptor |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorder-5 |
| EEG | Electroencephalogram |
| GABHS | Group A-beta-hemolytic Streptococcus |
| GAS | Group A Streptococcus |
| GWAS | Genome-Wide Association Study |
| HLA | Human Leukocyte Antigen |
| HPA | Activation of the Hypothalamus–Pituitary–Adrenal |
| ID | Intellectual Disability |
| IFN-γ | Interferon-gamma |
| Ig | Immunoglobulin |
| M1 | Microglia Phenotype Proinflammatory |
| M2 | Microglia Phenotype Anti-Inflammatory |
| MIA | Maternal Immune Activation |
| MRI | Magnetic Resonance Imaging |
| NDDs | Neurodevelopmental Disorders |
| NMDAR | Anti-N-Methyl-D-Aspartate Receptor |
| NSE | Neuron-Specific Enolase |
| OCD | Obsessive–Compulsive Disorder |
| PANDAS | Pediatric Autoimmune Neuropsychiatric Disorders Associated With Group A β-hemolytic Streptococcus |
| PANS | Pediatric Acute-Onset Neuropsychiatric Syndrome |
| PD | Psychotic Disorder |
| PIDs | Primary Immunodeficiency Disorders |
| SAA | Serum Amyloid A |
| SCZ | Schizophrenia |
| SHH | Sonic Hedgehog Protein |
| SLD | Specific Learning Disorder |
| SNP | Single-Nucleotide Polymorphism |
| SS | Schizophrenia Spectrum |
| TD | Tic Disorder |
| TGF-β | Transforming Growth Factor-beta |
| TNF α | Tumor Necrosis Factor-alpha |
| TS | Tourette Syndrome |
| WES | Whole-Exome Study |
| WGS | Whole-Genome Sequencing Study |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Mullin, A.P.; Gokhale, A.; Moreno-De-Luca, A.; Sanyal, S.; Waddington, J.L.; Faundez, V. Neurodevelopmental Disorders: Mechanisms and Boundary Definitions from Genomes, Interactomes and Proteomes. Transl. Psychiatry 2013, 3, e329. [Google Scholar] [CrossRef]
- Parenti, I.; Rabaneda, L.G.; Schoen, H.; Novarino, G. Neurodevelopmental Disorders: From Genetics to Functional Pathways. Trends Neurosci. 2020, 43, 608–621. [Google Scholar] [CrossRef]
- Mottahedin, A.; Ardalan, M.; Chumak, T.; Riebe, I.; Ek, J.; Mallard, C. Effect of Neuroinflammation on Synaptic Organization and Function in the Developing Brain: Implications for Neurodevelopmental and Neurodegenerative Disorders. Front. Cell. Neurosci. 2017, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Martino, D.; Johnson, I.; Leckman, J.F. What Does Immunology Have to Do with Normal Brain Development and the Pathophysiology Underlying Tourette Syndrome and Related Neuropsychiatric Disorders? Front. Neurol. 2020, 11, 567407. [Google Scholar] [CrossRef] [PubMed]
- Shohat, S.; Amelan, A.; Shifman, S. Convergence and Divergence in the Genetics of Psychiatric Disorders from Pathways to Developmental Stages. Biol. Psychiatry 2021, 89, 32–40. [Google Scholar] [CrossRef]
- Smoller, J.W.; Andreassen, O.A.; Edenberg, H.J.; Faraone, S.V.; Glatt, S.J.; Kendler, K.S. Psychiatric Genetics and the Structure of Psychopathology. Mol. Psychiatry 2019, 24, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, F.A.C.; Carvalho, L.R.B.; Grinberg, L.T.; Farfel, J.M.; Ferretti, R.E.L.; Leite, R.E.P.; Filho, W.J.; Lent, R.; Herculano-Houzel, S. Equal Numbers of Neuronal and Nonneuronal Cells Make the Human Brain an Isometrically Scaled-Up Primate Brain. J. Comp. Neurol. 2009, 513, 532–541. [Google Scholar] [CrossRef]
- Mondelli, V.; Dazzan, P.; Pariante, C.M. Immune Abnormalities Across Psychiatric Disorders: Clinical Relevance; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Pape, K.; Tamouza, R.; Leboyer, M.; Zipp, F. Immunoneuropsychiatry—Novel Perspectives on Brain Disorders. Nat. Rev. Neurol. 2019, 15, 317–328. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Solmi, M.; Sanches, M.; Machado, M.O.; Stubbs, B.; Ajnakina, O.; Sherman, C.; Sun, Y.R.; Liu, C.S.; Brunoni, A.R.; et al. Evidence-Based Umbrella Review of 162 Peripheral Biomarkers for Major Mental Disorders. Transl. Psychiatry 2020, 10, 152. [Google Scholar] [CrossRef]
- Goldsmith, D.R.; Bekhbat, M.; Mehta, N.D.; Felger, J.C. Inflammation-Related Functional and Structural Dysconnectivity as a Pathway to Psychopathology. Biol. Psychiatry 2023, 93, 405–418. [Google Scholar] [CrossRef]
- Merzon, E.; Israel, A.; Ashkenazi, S.; Rotem, A.; Schneider, T.; Faraone, S.V.; Biederman, J.; Green, I.; Golan-Cohen, A.; Vinker, S.; et al. Attention-Deficit/Hyperactivity Disorder Is Associated with Increased Rates of Childhood Infectious Diseases: A Population-Based Case-Control Study. J. Am. Acad. Child Adolesc. Psychiatry 2023, 62, 253–260.e1. [Google Scholar] [CrossRef]
- Lydholm, C.N.; Köhler-Forsberg, O.; Nordentoft, M.; Yolken, R.H.; Mortensen, P.B.; Petersen, L.; Benros, M.E. Parental Infections before, during, and after Pregnancy as Risk Factors for Mental Disorders in Childhood and Adolescence: A Nationwide Danish Study. Biol. Psychiatry 2019, 85, 317–325. [Google Scholar] [CrossRef]
- Black, M.M.; Walker, S.P.; Fernald, L.C.H.; Andersen, C.T.; DiGirolamo, A.M.; Lu, C.; McCoy, D.C.; Fink, G.; Shawar, Y.R.; Shiffman, J.; et al. Early Childhood Development Coming of Age: Science through the Life Course. Lancet 2017, 389, 77–90. [Google Scholar] [CrossRef]
- Tioleco, N.; Silberman, A.E.; Stratigos, K.; Banerjee-Basu, S.; Spann, M.N.; Whitaker, A.H.; Turner, J.B. Prenatal Maternal Infection and Risk for Autism in Offspring: A Meta-Analysis. Autism Res. 2021, 14, 1296–1316. [Google Scholar] [CrossRef] [PubMed]
- Walle, K.M.; Askeland, R.B.; Gustavson, K.; Mjaaland, S.; Ystrom, E.; Lipkin, W.I.; Magnus, P.; Stoltenberg, C.; Susser, E.; Bresnahan, M.; et al. Risk of Attention-Deficit Hyperactivity Disorder in Offspring of Mothers with Infections during Pregnancy. JCPP Adv. 2022, 2, e12070. [Google Scholar] [CrossRef]
- Brown, A.S.; Derkits, E.J. Prenatal Infection and Schizophrenia: A Review of Epidemiologic and Translational Studies. Am. J. Psychiatry 2010, 167, 261–280. [Google Scholar] [CrossRef]
- Jones, H.F.; Han, V.X.; Patel, S.; Gloss, B.S.; Soler, N.; Ho, A.; Sharma, S.; Kothur, K.; Nosadini, M.; Wienholt, L.; et al. Maternal autoimmunity and inflammation are associated with childhood tics and obsessive-compulsive disorder: Transcriptomic data show common enriched innate immune pathways. Brain Behav. Immun. 2021, 94, 308–317. [Google Scholar] [CrossRef]
- Sotgiu, S.; Manca, S.; Gagliano, A.; Minutolo, A.; Melis, M.C.; Pisuttu, G.; Scoppola, C.; Bolognesi, E.; Clerici, M.; Guerini, F.R.; et al. Immune Regulation of Neurodevelopment at the Mother–Foetus Interface: The Case of Autism. Clin. Transl. Immunol. 2020, 9, e1211. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, Z.; Lennox, B. Maternal Immune Activation and Schizophrenia—Evidence for an Immune Priming Disorder. Front. Psychiatry 2021, 12, 585742. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, J.H.; Jarskog, L.F. Exposure to infection and brain development: Cytokines in the pathogenesis of schizophrenia. Schizophr. Res. 1997, 24, 365–367. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Maternal Immune Activation: Implications for Neuropsychiatric Disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef]
- Weber-Stadlbauer, U. Epigenetic and Transgenerational Mechanisms in Infection-Mediated Neurodevelopmental Disorders. Transl. Psychiatry 2017, 7, e1113. [Google Scholar] [CrossRef]
- Graus, F.; Titulaer, M.J.; Balu, R.; Benseler, S.; Bien, C.G.; Cellucci, T.; Cortese, I.; Dale, R.C.; Gelfand, J.M.; Geschwind, M.; et al. A Clinical Approach to Diagnosis of Autoimmune Encephalitis. Lancet Neurol. 2016, 15, 391–404. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dalmau, J.; Graus, F. Antibody-Mediated Encephalitis. N. Engl. J. Med. 2018, 378, 840–851. [Google Scholar] [CrossRef]
- Cellucci, T.; Van Mater, H.; Graus, F.; Muscal, E.; Gallentine, W.; Klein-Gitelman, M.S.; Benseler, S.M.; Frankovich, J.; Gorman, M.P.; Van Haren, K.; et al. Clinical approach to the diagnosis of autoimmune encephalitis in the pediatric patient. Neurol. Neuroimmunol. Neuroinflammation 2020, 7, e663, Erratum in Neurol Neuroimmunol. Neuroinflamm. 2020, 7, e730. https://doi.org/10.1212/NXI.0000000000000730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Armangue, T.; Spatola, M.; Vlagea, A.; Mattozzi, S.; Cárceles-Cordon, M.; Martinez-Heras, E.; Llufriu, S.; Muchart, J.; Erro, M.E.; Abraira, L.; et al. Frequency, Symptoms, Risk Factors, and Outcomes of Autoimmune Encephalitis after Herpes Simplex Encephalitis: A Prospective Observational Study and Retrospective Analysis. Lancet Neurol. 2018, 17, 760–772. [Google Scholar] [CrossRef]
- Uy, C.E.; Binks, S.; Irani, S.R. Autoimmune Encephalitis: Clinical Spectrum and Management. Pract. Neurol. 2021, 21, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Bien, C.G.; Vincent, A.; Barnett, M.H.; Becker, A.J.; Blümcke, I.; Graus, F.; Jellinger, K.A.; Reuss, D.E.; Ribalta, T.; Schlegel, J.; et al. Immunopathology of Autoantibody-Associated Encephalitides: Clues for Pathogenesis. Brain 2012, 135, 1622–1638. [Google Scholar] [CrossRef]
- Ehrenreich, H. Autoantibodies against the N-Methyl-d-Aspartate Receptor Subunit NR1: Untangling Apparent Inconsistencies for Clinical Practice. Front. Immunol. 2017, 8, 181. [Google Scholar] [CrossRef]
- Makrides, V.; Dolgodilina, E.; Virgintino, D. Blood–Brain Barrier Transporters and Neuroinflammation: Partners in Neuroprotection and in Pathology. In The Blood Brain Barrier and Inflammation; Springer: Cham, Switzerland, 2017; pp. 103–151. [Google Scholar] [CrossRef]
- Dileepan, T.; Smith, E.D.; Knowland, D.; Hsu, M.; Platt, M.; Bittner-Eddy, P.; Cohen, B.; Southern, P.; Latimer, E.; Harley, E.; et al. Group A Streptococcus Intranasal Infection Promotes CNS Infiltration by Streptococcal-Specific Th17 Cells. J. Clin. Investig. 2016, 126, 303–317. [Google Scholar] [CrossRef]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional Morphology of the Blood–Brain Barrier in Health and Disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef]
- Platt, M.P.; Agalliu, D.; Cutforth, T. Hello from the Other Side: How Autoantibodies Circumvent the Blood–Brain Barrier in Autoimmune Encephalitis. Front. Immunol. 2017, 8, 442. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Ferretti, M.T. Function and Dysfunction of Microglia during Brain Development: Consequences for Synapses and Neural Circuits. Front. Synaptic Neurosci. 2017, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Mehl, L.C.; Manjally, A.V.; Bouadi, O.; Gibson, E.M.; Tay, T.L. Microglia in Brain Development and Regeneration. Development 2022, 149, dev200425. [Google Scholar] [CrossRef] [PubMed]
- Komada, M.; Nishimura, Y. Epigenetics and Neuroinflammation Associated with Neurodevelopmental Disorders: A Microglial Perspective. Front. Cell Dev. Biol. 2022, 10, 852752. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, A.; Carta, A.; Tanca, M.G.; Sotgiu, S. Pediatric Acute-Onset Neuropsychiatric Syndrome: Current Perspectives. Neuropsychiatr. Dis. Treat. 2023, 19, 1221–1250. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A Sensor for Pathological Events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kaushik, D.K.; Gupta, M.; Basu, A. Inflammasome Signaling at the Heart of Central Nervous System Pathology. J. Neurosci. Res. 2010, 88, 1615–1631. [Google Scholar] [CrossRef]
- Singhal, G.; Jaehne, E.J.; Corrigan, F.; Toben, C.; Baune, B.T. Inflammasomes in Neuroinflammation and Changes in Brain Function: A Focused Review. Front. Neurosci. 2014, 8, 315. [Google Scholar] [CrossRef]
- Heneka, M.T.; McManus, R.M.; Latz, E. Inflammasome Signalling in Brain Function and Neurodegenerative Disease. Nat. Rev. Neurosci. 2018, 19, 610–621. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 Polarization and Metabolic States. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Stratoulias, V.; Venero, J.L.; Tremblay, M.È.; Joseph, B. Microglial Subtypes: Diversity within the Microglial Community. EMBO J. 2019, 38, e101997. [Google Scholar] [CrossRef]
- Thion, M.S.; Garel, S. Microglial Ontogeny, Diversity and Neurodevelopmental Functions. Curr. Opin. Genet. Dev. 2020, 65, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Glatt, S.J.; Faraone, S.V.; Tsuang, M.T. Is Schizophrenia a Neurodevelopmental Disorder? In Schizophrenia; Oxford University Press: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Burchi, E.; Pallanti, S. Diagnostic Issues in Early-Onset Obsessive-Compulsive Disorder and Their Treatment Implications. Curr. Neuropharmacol. 2019, 17, 672–680. [Google Scholar] [CrossRef]
- Gagliano, A.; Galati, C.; Ingrassia, M.; Ciuffo, M.; Alquino, M.A.; Tanca, M.G.; Carucci, S.; Zuddas, A.; Grossi, E. Pediatric Acute-Onset Neuropsychiatric Syndrome: A Data Mining Approach to a Very Specific Constellation of Clinical Variables. J. Child Adolesc. Psychopharmacol. 2020, 30, 495–511. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Buske-Kirschbaum, A.; Schmitt, J.; Plessow, F.; Romanos, M.; Weidinger, S.; Roessner, V. Psychoendocrine and Psychoneuroimmunological Mechanisms in the Comorbidity of Atopic Eczema and ADHD. Psychoneuroendocrinology 2013, 38, 12–23. [Google Scholar] [CrossRef]
- Hegvik, T.A.; Chen, Q.; Kuja-Halkola, R.; Klungsøyr, K.; Butwicka, A.; Lichtenstein, P.; Almqvist, C.; Faraone, S.V.; Haavik, J.; Larsson, H. Familial co-aggregation of attention-deficit/hyperactivity disorder and autoimmune diseases: A cohort study based on Swedish population-wide registers. Int. J. Epidemiol. 2022, 51, 898–909. [Google Scholar] [CrossRef]
- Liao, T.C.; Lien, Y.T.; Wang, S.; Huang, S.L.; Chen, C.Y. Comorbidity of atopic disorders with autism spectrum disorder and attention deficit/hyperactivity disorder. J. Pediatr. 2016, 171, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Chen, Y.C.; Gau, S.S.; Yeh, T.H.; Fan, H.Y.; Hwang, Y.Y.; Lee, Y.L. Associations between allergic diseases and attention deficit hyperactivity/oppositional defiant disorders in children. Pediatr. Res. 2016, 80, 480–485. [Google Scholar] [CrossRef]
- Fasmer, O.B.; Halmøy, A.; Eagan, T.M.; Oedegaard, K.J.; Haavik, J. Adult attention deficit hyperactivity disorder is associated with asthma. BMC Psychiatry 2011, 11, 128. [Google Scholar] [CrossRef]
- Hegvik, T.A.; Instanes, J.T.; Haavik, J.; Klungsøyr, K.; Engeland, A. Associations between attention-deficit/hyperactivity disorder and autoimmune diseases are modified by sex: A population-based cross-sectional study. Eur. Child Adolesc. Psychiatry 2018, 27, 663–675. [Google Scholar] [CrossRef]
- Segman, R.H.; Meltzer, A.; Gross-Tsur, V.; Kosov, A.; Frisch, A.; Inbar, E.; Darvasi, A.; Levy, S.; Goltser, T.; Weizman, A.; et al. Preferential transmission of interleukin-1 receptor antagonist alleles in attention deficit hyperactivity disorder. Mol. Psychiatry 2002, 7, 72–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giana, G.; Romano, E.; Porfirio, M.C.; D’Ambrosio, R.; Giovinazzo, S.; Troianiello, M.; Barlocci, E.; Travaglini, D.; Granstrem, O.; Pascale, E.; et al. Detection of auto-antibodies to DAT in the serum: Interactions with DAT genotype and psycho-stimulant therapy for ADHD. J. Neuroimmunol. 2015, 278, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Toto, M.; Margari, F.; Simone, M.; Craig, F.; Petruzzelli, M.G.; Tafuri, S.; Margari, L. Antibasal Ganglia Antibodies and Antistreptolysin O in Noncomorbid ADHD. J. Atten. Disord. 2015, 19, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Instanes, J.T.; Halmøy, A.; Engeland, A.; Haavik, J.; Furu, K.; Klungsøyr, K. Attention-Deficit/Hyperactivity Disorder in Offspring of Mothers With Inflammatory and Immune System Diseases. Biol. Psychiatry 2017, 81, 452–459. [Google Scholar] [CrossRef]
- Leffa, D.T.; Torres, I.L.S.; Rohde, L.A. A review on the role of inflammation in attention-deficit/hyperactivity disorder. Neuroimmunomodulation 2019, 25, 328–333. [Google Scholar] [CrossRef]
- Zayats, T.; Athanasiu, L.; Sonderby, I.; Djurovic, S.; Westlye, L.T.; Tamnes, C.K.; Fladby, T.; Aase, H.; Zeiner, P.; Reichborn-Kjennerud, T.; et al. Genome-Wide Analysis of Attention Deficit Hyperactivity Disorder in Norway. PLoS ONE 2015, 10, e0122501. [Google Scholar] [CrossRef]
- Faraone, S.V.; Asherson, P.; Banaschewski, T.; Biederman, J.; Buitelaar, J.K.; Ramos-Quiroga, J.A.; Rohde, L.A.; Sonuga-Barke, E.J.; Tannock, R.; Franke, B. Attention-Deficit/Hyperactivity Disorder. Nat. Rev. Dis. Primers 2015, 1, 15020. [Google Scholar] [CrossRef]
- Li, D.J.; Tsai, C.S.; Hsiao, R.C.; Chen, Y.L.; Yen, C.F. Associations between allergic and autoimmune diseases with autism spectrum disorder and attention-deficit/hyperactivity disorder within families: A population-based cohort study. Int. J. Environ. Res. Public Health 2022, 19, 4503. [Google Scholar] [CrossRef]
- Dunn, G.; Nigg, J.; Sontag-Padilla, L.; Seligman, M. Neuroinflammation as a Risk Factor for Attention Deficit Hyperactivity Disorder. Physiol. Behav. 2019, 198, 112–120. [Google Scholar] [CrossRef]
- Aguilar-Valles, A.; Rodrigue, B.; Matta-Camacho, E. Maternal Immune Activation and the Development of Dopaminergic Neurotransmission of the Offspring: Relevance for Schizophrenia and Other Psychoses. Front. Psychiatry 2020, 11, 852. [Google Scholar] [CrossRef]
- Rosenberg, J.B.; Jepsen, J.R.M.; Mohammadzadeh, P.; Sevelsted, A.; Vinding, R.; Sørensen, M.E.; Horner, D.; Aagaard, K.; Fagerlund, B.; Brix, S.; et al. Maternal Inflammation during Pregnancy Is Associated with Risk of ADHD in Children at Age 10. Brain Behav. Immun. 2024, 115, 450–457. [Google Scholar] [CrossRef]
- Anand, D.; Colpo, G.D.; Zeni, G.; Zeni, C.P.; Teixeira, A.L. Attention-Deficit/Hyperactivity Disorder and Inflammation: What Does Current Knowledge Tell Us? A Systematic Review. Front. Psychiatry 2017, 8, 228. [Google Scholar] [CrossRef]
- Donfrancesco, R.; Nativio, P.; Di Benedetto, A.; Villa, M.P.; Andriola, E.; Melegari, M.G.; Cipriano, E.; Di Trani, M. Anti-Yo Antibodies in Children with ADHD: First Results about Serum Cytokines. J. Atten. Disord. 2020, 24, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Oades, R.D.; Dauvermann, M.R.; Schimmelmann, B.G.; Schwarz, M.J.; Myint, A.M. Attention-Deficit Hyperactivity Disorder (ADHD) and Glial Integrity: S100B, Cytokines and Kynurenine Metabolism—Effects of Medication. Behav. Brain Funct. 2010, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Corona, J.C. Role of Oxidative Stress and Neuroinflammation in Attention-Deficit/Hyperactivity Disorder. Antioxidants 2020, 9, 1039. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-González, D.; Carreón-Trujillo, S.; Alvarez-Arellano, L.; Abarca-Merlin, D.M.; Domínguez-López, P.; Salazar-García, M.; Corona, J.C. A Potential Role for Neuroinflammation in ADHD. In Neuroinflammation, Gut–Brain Axis and Immunity in Neuropsychiatric Disorders; Springer: Singapore, 2023; pp. 327–356. [Google Scholar] [CrossRef]
- Lai, M.-C.; Kassee, C.; Besney, R.; Bonato, S.; Hull, L.; Mandy, W.; Szatmari, P.; Ameis, S.H. Prevalence of Co-Occurring Mental Health Diagnoses in the Autism Population: A Systematic Review and Meta-Analysis. Lancet Psychiatry 2019, 6, 819–829. [Google Scholar] [CrossRef]
- Genovese, A.; Butler, M.G. The Autism Spectrum: Behavioral, Psychiatric and Genetic Associations. Genes 2023, 14, 677. [Google Scholar] [CrossRef]
- Wiznitzer, M. Autism and Tuberous Sclerosis. J. Child Neurol. 2004, 19, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Bergbaum, A.; Ogilvie, C.M. Autism and Chromosome Abnormalities—A Review. Clin. Anat. 2016, 29, 620–627. [Google Scholar] [CrossRef]
- Keski-Rahkonen, A.; Ruusunen, A. Avoidant-Restrictive Food Intake Disorder and Autism: Epidemiology, Etiology, Complications, Treatment, and Outcome. Curr. Opin. Psychiatry 2023, 36, 438–442. [Google Scholar] [CrossRef]
- Madra, M.; Ringel, R.; Margolis, K.G. Gastrointestinal Issues and Autism Spectrum Disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2020, 29, 501–513. [Google Scholar] [CrossRef]
- Capal, J.K.; Jeste, S.S. Autism and Epilepsy. Pediatr. Clin. N. Am. 2024, 71, 241–252. [Google Scholar] [CrossRef]
- Johnson, K.P.; Zarrinnegar, P. Autism Spectrum Disorder and Sleep. Child Adolesc. Psychiatr. Clin. N. Am. 2021, 30, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Voineagu, I.; Wang, X.; Johnston, P.; Lowe, J.K.; Tian, Y.; Horvath, S.; Mill, J.; Cantor, R.M.; Blencowe, B.J.; Geschwind, D.H. Transcriptomic Analysis of Autistic Brain Reveals Convergent Molecular Pathology. Nature 2011, 474, 380–384. [Google Scholar] [CrossRef]
- Gładysz, D.; Krzywdzińska, A.; Hozyasz, K.K. Immune Abnormalities in Autism Spectrum Disorder—Could They Hold Promise for Causative Treatment? Mol. Neurobiol. 2018, 55, 6387–6435. [Google Scholar] [CrossRef]
- Hughes, H.K.; Moreno, R.J.; Ashwood, P. Innate Immune Dysfunction and Neuroinflammation in Autism Spectrum Disorder (ASD). Brain Behav. Immun. 2023, 108, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Usui, N.; Kobayashi, H.; Shimada, S. Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder. Int. J. Mol. Sci. 2023, 24, 5487. [Google Scholar] [CrossRef]
- Villarreal, V.R.; Katusic, M.Z.; Myers, S.M.; Weaver, A.L.; Nocton, J.J.; Voigt, R.G. Risk of Autoimmune Disease in Research-Identified Cases of Autism Spectrum Disorder: A Longitudinal, Population-Based Birth Cohort Study. J. Dev. Behav. Pediatr. 2024, 45, E46–E53. [Google Scholar] [CrossRef]
- Zerbo, O.; Leong, A.; Barcellos, L.; Bernal, P.; Fireman, B.; Croen, L.A. Immune Mediated Conditions in Autism Spectrum Disorders. Brain Behav. Immun. 2015, 46, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, B.J.S.; Jacobsson, B.; Chabra, S.; Modzelewska, D.; Olson, E.M.; Bernier, R.; Enquobahrie, D.A.; Hagberg, H.; Östling, S.; Rajagopal, L.; et al. Long-term risk of neuropsychiatric disease after exposure to infection in utero. JAMA Psychiatry 2019, 76, 594–602. [Google Scholar] [CrossRef]
- Croen, L.A.; Ames, J.L.; Qian, Y.; Alexeeff, S.; Ashwood, P.; Gunderson, E.P.; Wu, Y.W.; Boghossian, A.S.; Yolken, R.; Van de Water, J.; et al. Inflammatory conditions during pregnancy and risk of autism and other neurodevelopmental disorders. Biol. Psychiatry Glob. Open Sci. 2024, 4, 39–50. [Google Scholar] [CrossRef]
- Brown, A.S.; Surcel, H.M.; Hinkka-Yli-Salomäki, S.; Cheslack-Postava, K.; Bao, Y.; Sourander, A. Maternal Thyroid Autoantibody and Elevated Risk of Autism in a National Birth Cohort. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 57, 86–92. [Google Scholar] [CrossRef]
- Nielsen, T.C.; Nassar, N.; Shand, A.W.; Jones, H.F.; Han, V.X.; Patel, S.; Guastella, A.J.; Dale, R.C.; Lain, S.J. Association of Maternal Autoimmune Disease and Early Childhood Infections with Offspring Autism Spectrum Disorder: A Population-Based Cohort Study. Autism Res. 2022, 15, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.; Croen, L.A.; Yoshida, C.K.; Heuer, L.S.; Hansen, R.L.; Zerbo, O.; DeLorenze, G.N.; Kharrazi, M.; Yolken, R.; Ashwood, P.; et al. Autism with Intellectual Disability Is Associated with Increased Levels of Maternal Cytokines and Chemokines during Gestation. Mol. Psychiatry 2017, 22, 273–279. [Google Scholar] [CrossRef]
- Abdallah, M.W.; Larsen, N.; Grove, J.; Nørgaard-Pedersen, B.; Thorsen, P.; Mortensen, E.L.; Hougaard, D.M. Amniotic fluid chemokines and autism spectrum disorders: An exploratory study utilizing a Danish Historic Birth Cohort. Brain Behav. Immun. 2012, 26, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, M.D.; Graham, A.M.; Feczko, E.; Miranda-Dominguez, O.; Rasmussen, J.M.; Nardos, R.; Entringer, S.; Wadhwa, P.D.; Buss, C.; Fair, D.A. Maternal IL-6 during Pregnancy Can Be Estimated from Newborn Brain Connectivity and Predicts Future Working Memory in Offspring. Nat. Neurosci. 2018, 21, 765–772. [Google Scholar] [CrossRef]
- Graham, A.M.; Rasmussen, J.M.; Rudolph, M.D.; Heim, C.M.; Gilmore, J.H.; Styner, M.; Potkin, S.G.; Entringer, S.; Wadhwa, P.D.; Fair, D.A.; et al. Maternal Systemic Interleukin-6 During Pregnancy Is Associated with Newborn Amygdala Phenotypes and Subsequent Behavior at 2-Years-of-Age. Biol. Psychiatry 2018, 83, 109–119. [Google Scholar] [CrossRef]
- Abdallah, M.W.; Larsen, N.; Grove, J.; Bonefeld-Jørgensen, E.C.; Nørgaard-Pedersen, B.; Hougaard, D.M.; Mortensen, E.L. Neonatal Chemokine Levels and Risk of Autism Spectrum Disorders: Findings from a Danish Historic Birth Cohort Follow-up Study. Cytokine 2013, 61, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Krakowiak, P.; Goines, P.E.; Tancredi, D.J.; Ashwood, P.; Hansen, R.L.; Hertz-Picciotto, I.; Van de Water, J. Neonatal Cytokine Profiles Associated with Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Heuer, L.S.; Croen, L.A.; Jones, K.L.; Yoshida, C.K.; Hansen, R.L.; Yolken, R.; Zerbo, O.; DeLorenze, G.; Kharrazi, M.; Ashwood, P.; et al. An Exploratory Examination of Neonatal Cytokines and Chemokines as Predictors of Autism Risk: The Early Markers for Autism Study. Biol. Psychiatry 2019, 86, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Oskvig, D.B.; Elkahloun, A.G.; Johnson, K.R.; Phillips, T.M.; Herkenham, M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav. Immun. 2012, 26, 623–634. [Google Scholar] [CrossRef]
- O’Loughlin, E.; Pakan, J.M.P.; Yilmazer-Hanke, D.; McDermott, K.W. Acute in Utero Exposure to Lipopolysaccharide Induces Inflammation in the Pre- and Postnatal Brain and Alters the Glial Cytoarchitecture in the Developing Amygdala. J. Neuroinflammation 2017, 14, 212. [Google Scholar] [CrossRef]
- Cieślik, M.; Gąssowska-Dobrowolska, M.; Jęśko, H.; Czapski, G.A.; Wilkaniec, A.; Zawadzka, A.; Dominiak, A.; Polowy, R.; Filipkowski, R.K.; Boguszewski, P.M.; et al. Maternal Immune Activation Induces Neuroinflammation and Cortical Synaptic Deficits in the Adolescent Rat Offspring. Int. J. Mol. Sci. 2020, 21, 4097. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal Immune Activation and Neuroinflammation in Human Neurodevelopmental Disorders. Nat. Rev. Neurol. 2021, 17, 564–579. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, J.; Li, Y. Microglia and Astrocytes Underlie Neuroinflammation and Synaptic Susceptibility in Autism Spectrum Disorder. Front. Neurosci. 2023, 17, 1125428. [Google Scholar] [CrossRef]
- Lampiasi, N.; Bonaventura, R.; Deidda, I.; Zito, F.; Russo, R. Inflammation and the Potential Implication of Macrophage-Microglia Polarization in Human ASD: An Overview. Int. J. Mol. Sci. 2023, 24, 2703. [Google Scholar] [CrossRef]
- Rodriguez, J.I.; Kern, J.K. Evidence of Microglial Activation in Autism and Its Possible Role in Brain Underconnectivity. Neuron Glia Biol. 2011, 7, 205–213. [Google Scholar] [CrossRef]
- Hu, C.; Li, H.; Li, J.; Luo, X.; Hao, Y. Microglia: Synaptic Modulator in Autism Spectrum Disorder. Front. Psychiatry 2022, 13, 958661. [Google Scholar] [CrossRef]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial Activation and Neuroinflammation in the Brain of Patients with Autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Than, U.T.T.; Nguyen, L.T.; Nguyen, P.H.; Nguyen, X.H.; Trinh, D.P.; Hoang, D.H.; Nguyen, P.A.T.; Dang, V.D. Inflammatory Mediators Drive Neuroinflammation in Autism Spectrum Disorder and Cerebral Palsy. Sci. Rep. 2023, 13, 22587. [Google Scholar] [CrossRef]
- De Giacomo, A.; Gargano, C.D.; Simone, M.; Petruzzelli, M.G.; Pedaci, C.; Giambersio, D.; Margari, L.; Ruggieri, M. B and T Immunoregulation: A New Insight of B Regulatory Lymphocytes in Autism Spectrum Disorder. Front. Neurosci. 2021, 15, 732611. [Google Scholar] [CrossRef]
- Geller, H.; Williams, K.A. 6.34 Comparison of Primary Humoral Immunodeficiencies in Autism Spectrum Disorder (ASD) and Other Pediatric-Onset Psychiatric Disorders. J. Am. Acad. Child Adolesc. Psychiatry 2021, 60, S169. [Google Scholar] [CrossRef]
- Bjørklund, G.; Saad, K.; Chirumbolo, S.; Kern, J.K.; Geier, D.A.; Geier, M.R.; Urbina, M.A. Immune Dysfunction and Neuroinflammation in Autism Spectrum Disorder. Acta Neurobiol. Exp. 2016, 76, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.M.; Eapen, V.; Singer, H.S.; Martino, D.; Scharf, J.M.; Paschou, P.; Roessner, V.; Woods, D.W.; Hariz, M.; Mathews, C.A.; et al. Gilles de la Tourette Syndrome. Nat. Rev. Dis. Primers 2017, 3, 16097. [Google Scholar] [CrossRef] [PubMed]
- Knight, T.; Steeves, T.; Day, L.; Lowerison, M.; Jette, N.; Pringsheim, T. Prevalence of Tic Disorders: A Systematic Review and Meta-Analysis. Pediatr. Neurol. 2012, 47, 77–90. [Google Scholar] [CrossRef]
- Jellinger, K.A. Neuropathology and Pathogenesis of Extrapyramidal Movement Disorders: A Critical Update. II. Hyperkinetic Disorders. J. Neural Transm. 2019, 126, 997–1027. [Google Scholar] [CrossRef]
- Swedo, S.E.; Leonard, H.L.; Garvey, M.; Mittleman, B.; Allen, A.J.; Perlmutter, S.; Lougee, L.; Dow, S.; Zamkoff, J.; Dubbert, B.K. Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections: Clinical Description of the First 50 Cases. In Obsessive-Compulsive Disorder and Tourette’s Syndrome; Routledge: Oxfordshire, UK, 1998; Volume 155, pp. 264–271. [Google Scholar] [CrossRef]
- Mataix-Cols, D.; Frans, E.; Pérez-Vigil, A.; Kuja-Halkola, R.; Gromark, C.; Isomura, K.; Fernández de la Cruz, L.; Serlachius, E.; Leckman, J.F.; Crowley, J.J.; et al. A Total-Population Multigenerational Family Clustering Study of Autoimmune Diseases in Obsessive–Compulsive Disorder and Tourette’s/Chronic Tic Disorders. Mol. Psychiatry 2018, 23, 1652–1658. [Google Scholar] [CrossRef]
- Keszler, G.; Kruk, E.; Kenezloi, E.; Tarnok, Z.; Sasvari-Szekely, M.; Nemoda, Z. Association of the Tumor Necrosis Factor -308 A/G Promoter Polymorphism with Tourette Syndrome. Int. J. Immunogenet. 2014, 41, 493–498. [Google Scholar] [CrossRef]
- Tylee, D.S.; Sun, J.; Hess, J.L.; Tahir, M.A.; Sharma, E.; Malik, R.; Worrall, B.B.; Levine, A.J.; Martinson, J.J.; Nejentsev, S.; et al. Genetic Correlations among Psychiatric and Immune-related Phenotypes Based on Genome-wide Association Data. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2018, 177, 641–657. [Google Scholar] [CrossRef]
- Tsetsos, F.; Yu, D.; Sul, J.H.; Huang, A.Y.; Illmann, C.; Osiecki, L.; Darrow, S.M.; Hirschtritt, M.E.; Greenberg, E.; Muller-Vahl, K.R.; et al. Synaptic Processes and Immune-Related Pathways Implicated in Tourette Syndrome. Transl. Psychiatry 2021, 11, 56. [Google Scholar] [CrossRef]
- Elamin, I.; Edwards, M.J.; Martino, D. Immune Dysfunction in Tourette Syndrome. Behav. Neurol. 2013, 27, 23–32. [Google Scholar] [CrossRef]
- Martino, D.; Zis, P.; Buttiglione, M. The Role of Immune Mechanisms in Tourette Syndrome. Brain Res. 2015, 1617, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Leckman, J.F.; Katsovich, L.; Kawikova, I.; Lin, H.; Zhang, H.; Krönig, H.; Morshed, S.; Parveen, S.; Grantz, H.; Lombroso, P.J.; et al. Increased Serum Levels of Interleukin-12 and Tumor Necrosis Factor-Alpha in Tourette’s Syndrome. Biol. Psychiatry 2005, 57, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.; Mok, Y.E.; Kang, J.; Gim, J.A.; Han, C.; Lee, M.S. Cytokine Levels Reflect Tic Symptoms More Prominently during Mild Phases. BMC Neurosci. 2023, 24, 14. [Google Scholar] [CrossRef]
- Tao, Y.; Xu, P.; Zhu, W.; Chen, Z.; Tao, X.; Liu, J.; Xue, Z.; Zhu, T.; Jiang, P. Changes of Cytokines in Children With Tic Disorder. Front. Neurol. 2022, 12, 800189. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Yang, H.; Li, Y.; Gui, J.; Cui, Y. Profiles of Proinflammatory Cytokines and T Cells in Patients With Tourette Syndrome: A Meta-Analysis. Front. Immunol. 2022, 13, 843247. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, E.; Krause, D.; Wildenauer, A.; Meyer, S.; Gruber, R.; Schwarz, M.J.; Müller, N. Impaired Activation of the Innate Immune Response to Bacterial Challenge in Tourette Syndrome. World J. Biol. Psychiatry 2014, 15, 453–458. [Google Scholar] [CrossRef]
- Kawikova, I.; Leckman, J.F.; Kronig, H.; Katsovich, L.; Bessen, D.E.; Ghebremichael, M.; Bothwell, A.L. Decreased Numbers of Regulatory T Cells Suggest Impaired Immune Tolerance in Children with Tourette Syndrome: A Preliminary Study. Biol. Psychiatry 2007, 61, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Dale, R.C.; Merheb, V.; Pillai, S.; Wang, D.; Cantrill, L.; Murphy, T.K.; Ben-Pazi, H.; Varadkar, S.; Aumann, T.D.; Horne, M.K.; et al. Antibodies to Surface Dopamine-2 Receptor in Autoimmune Movement and Psychiatric Disorders. Brain 2012, 135, 3453–3468. [Google Scholar] [CrossRef]
- Addabbo, F.; Baglioni, V.; Schrag, A.; Schwarz, M.J.; Dietrich, A.; Hoekstra, P.J.; Martino, D.; Buttiglione, M.; Emtics Collaborative Group. Anti-dopamine D2 Receptor Antibodies in Chronic Tic Disorders. Dev. Med. Child Neurol. 2020, 62, 1205–1212. [Google Scholar] [CrossRef]
- Bos-Veneman, N.G.; Olieman, R.; Tobiasova, Z.; Hoekstra, P.J.; Katsovich, L.; Bothwell, A.L.; Leckman, J.F.; Kawikova, I. Altered Immunoglobulin Profiles in Children with Tourette Syndrome. Brain Behav. Immun. 2011, 25, 532–538. [Google Scholar] [CrossRef]
- Harris, J. Intellectual Disability: Understanding Its Development, Causes, Classification, Evaluation, and Treatment; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Brynge, M.; Sjöqvist, H.; Gardner, R.M.; Lee, B.K.; Dalman, C.; Karlsson, H. Maternal Infection during Pregnancy and Likelihood of Autism and Intellectual Disability in Children in Sweden: A Negative Control and Sibling Comparison Cohort Study. Lancet Psychiatry 2022, 9, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Rezaeinejad, M.; Riahi, S.M.; Moghadam, K.B.; Tadi, M.J.; Geraili, Z.; Parsa, H.; Marhoommirzabak, E.; Nourollahpour Shiadeh, M.; Khatir, A.A. The Association between Maternal Infection and Intellectual Disability in Children: A Systematic Review and Meta-Analysis. PLoS ONE 2023, 18, e0292226. [Google Scholar] [CrossRef]
- Benmakhlouf, Y.; Zian, Z.; Nourouti, N.G.; Barakat, A.; Mechita, M.B. Potential Cytokine Biomarkers in Intellectual Disability. Endocr. Metab. Immune Disord.—Drug Targets 2020, 21, 569–576. [Google Scholar] [CrossRef]
- Mekori-Domachevsky, E.; Taler, M.; Shoenfeld, Y.; Gurevich, M.; Sonis, P.; Weisman, O.; Weizman, A.; Gothelf, D. Elevated Proinflammatory Markers in 22q11.2 Deletion Syndrome Are Associated with Psychosis and Cognitive Deficits. J. Clin. Psychiatry 2017, 78, e1219–e1225. [Google Scholar] [CrossRef]
- Fan, X.Y.; Shi, G.; Zhao, P. Neonatal Sevoflurane Exposure Impairs Learning and Memory by the Hypermethylation of Hippocampal Synaptic Genes. Mol. Neurobiol. 2021, 58, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hussain, B.; Chang, J. Peripheral Inflammation and Blood–Brain Barrier Disruption: Effects and Mechanisms. CNS Neurosci. Ther. 2021, 27, 36–47. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, J.; Wang, X.; Han, M.; Fei, Y.; Wang, J. Blood–Brain Barrier Disruption in Schizophrenia: Insights, Mechanisms, and Future Directions. Int. J. Mol. Sci. 2025, 26, 873. [Google Scholar] [CrossRef]
- Al-Diwani, A.; Handel, A.; Townsend, L.; Pollak, T.; Leite, M.I.; Harrison, P.J.; Lennox, B.R.; Okai, D.; Manohar, S.G.; Irani, S.R. The psychopathology of NMDAR-antibody encephalitis in adults: A systematic review and phenotypic analysis of individual patient data. Lancet Psychiatry 2019, 6, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.; Steiner, J.; Najjar, A.; Bechter, K. A clinical approach to new-onset psychosis associated with immune dysregulation: The concept of autoimmune psychosis. J. Neuroinflammation 2018, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- McKeon, A.; Dubey, D.; Flanagan, E.; Pittock, S.; Zekeridou, A. Autoimmune psychosis. Lancet Psychiatry 2020, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar] [CrossRef]
- Bechter, K.; Reiber, H.; Herzog, S.; Fuchs, D.; Tumani, H.; Maxeiner, H.G. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: Identification of subgroups with immune responses and blood-CSF barrier dysfunction. J. Psychiatr. Res. 2010, 44, 321–330. [Google Scholar] [CrossRef]
- Endres, D.; Perlov, E.; Baumgartner, A.; Hottenrott, T.; Dersch, R.; Stich, O.; Tebartz van Elst, L. Immunological findings in psychotic syndromes: A tertiary care hospital’s CSF sample of 180 patients. Front. Hum. Neurosci. 2015, 9, 476. [Google Scholar] [CrossRef]
- Upthegrove, R.; Manzanares-Teson, N.; Barnes, N.M. Cytokine function in medication-naive first-episode psychosis: A systematic review and meta-analysis. Schizophr Res. 2014, 155, 101–108. [Google Scholar] [CrossRef]
- Nayak, U.; Manikkath, J.; Arora, D.; Mudgal, J. Impact of neuroinflammation on brain glutamate and dopamine signalling in schizophrenia: An update. Metab. Brain Dis. 2025, 40, 119. [Google Scholar] [CrossRef]
- Miller, B.J.; Buckley, P.; Seabolt, W.; Mellor, A.; Kirkpatrick, B. Meta-Analysis of Cytokine Alterations in Schizophrenia: Clinical Status and Antipsychotic Effects. Biol. Psychiatry 2011, 70, 663–671. [Google Scholar] [CrossRef]
- Gallego, J.A.; Blanco, E.A.; Husain-Krautter, S.; Fagen, E.M.; Moreno-Merino, P.; del Ojo-Jiménez, J.A.; Ahmed, A.; Rothstein, T.L.; Lencz, T.; Malhotra, A.K. Cytokines in cerebrospinal fluid of patients with schizophrenia spectrum disorders: New data and an updated meta-analysis. Schizophr Res. 2018, 202, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.M.; Pearson, R.M.; Zammit, S.; Lewis, G.; Jones, P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: A population-based longitudinal study. JAMA Psychiatry 2014, 71, 1121–1128. [Google Scholar] [CrossRef]
- Metcalf, S.A.; Jones, P.B.; Nordstrom, T.; Timonen, M.; Mäki, P.; Miettunen, J.; Jääskeläinen, E.; Järvelin, M.R.; Stochl, J.; Murray, G.K.; et al. Serum C-reactive protein in adolescence and risk of schizophrenia in adulthood: A prospective birth cohort study. Brain Behav. Immun. 2017, 59, 253–259. [Google Scholar] [CrossRef]
- Dunleavy, C.; Elsworthy, R.J.; Upthegrove, R.; Wood, S.J.; Aldred, S. Inflammation in first-episode psychosis: The contribution of inflammatory biomarkers to the emergence of negative symptoms, a systematic review and meta-analysis. Acta Psychiatr. Scand. 2022, 146, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Pollak, T.A.; Lennox, B.R.; Müller, S.; Benros, M.E.; Prüss, H.; Tebartz van Elst, L.; Klein, H.; Steiner, J.; Frodl, T.; Bogerts, B.; et al. Autoimmune psychosis: An international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry 2020, 7, 93–108. [Google Scholar] [CrossRef]
- Pollak, T.; Drndarski, S.; Stone, J.M.; David, A.S.; McGuire, P.K.; Abbott, N.J. The blood–brain barrier in psychosis. Lancet Psychiatry 2018, 5, 79–92. [Google Scholar] [CrossRef]
- Trépanier, M.O.; Hopperton, K.E.; Mizrahi, R.; Mechawar, N.; Bazinet, R.P. Postmortem evidence of cerebral inflammation in schizophrenia: A systematic review. Mol. Psychiatry 2016, 21, 1009–1026. [Google Scholar] [CrossRef]
- van Kesteren, C.F.M.G.; Gremmels, H.; de Witte, L.D.; Hol, E.M.; Van Gool, A.R.; Falkai, P.G.; Kahn, R.S.; Sommer, I.E.C. Immune involvement in the pathogenesis of schizophrenia: A meta-analysis on postmortem brain studies. Transl. Psychiatry 2017, 7, e1075. [Google Scholar] [CrossRef]
- Kayser, M.S.; Dalmau, J. Anti-NMDA receptor encephalitis, autoimmunity, and psychosis. Schizophr. Res. 2016, 176, 36–40. [Google Scholar] [CrossRef]
- Subeh, G.K.; Lajber, M.; Patel, T.; Mostafa, J.A. Anti-N-methyl-D-aspartate receptor encephalitis: A detailed review of the different psychiatric presentations and red flags to look for in suspected cases. Cureus 2021, 13, e15188. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D. Autoimmune encephalitis in children. Pediatr. Neurol. 2022, 132, 56–66. [Google Scholar] [CrossRef]
- Giri, Y.R.; Parrill, A.; Damodar, S.; Fogel, J.; Ayed, N.; Syed, M.; Korie, I.; Ayyanar, S.; Typhair, C.; Hashmi, S. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents: A systematic review and quantitative analysis of reported cases. J. Can. Acad. Child Adolesc. Psychiatry 2021, 30, 236–248. [Google Scholar] [PubMed]
- Ahmad, S.A.; Archer, H.A.; Rice, C.M.; Gerhand, S.; Bradley, M.; Wilkins, A. Seronegative limbic encephalitis: Case report, literature review and proposed treatment algorithm. Pract. Neurol. 2011, 11, 355–361. [Google Scholar] [CrossRef]
- Shah, K.; Iloh, N.; Tabares, P.; Nnadi, C.; Sharif, Z.; Macaluso, C. Limbic encephalitis and psychosis. Gen. Hosp. Psychiatry 2013, 35, 682.e1–682.e2. [Google Scholar] [CrossRef]
- Eaton, W.W.; Byrne, M.; Ewald, H.; Mors, O.; Chen, C.Y.; Agerbo, E.; Mortensen, P.B. Association of schizophrenia and autoimmune diseases: Linkage of Danish national registers. Am. J. Psychiatry 2006, 163, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Benros, M.E.; Pedersen, M.G.; Rasmussen, H.; Eaton, W.W.; Nordentoft, M.; Mortensen, P.B. A nationwide study on the risk of autoimmune diseases in individuals with a personal or a family history of schizophrenia and related psychosis. Am. J. Psychiatry 2014, 171, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Benros, M.E.; Nielsen, P.R.; Nordentoft, M.; Eaton, W.W.; Dalton, S.O.; Mortensen, P.B. Autoimmune diseases and severe infections as risk factors for schizophrenia: A 30-year population-based register study. Am. J. Psychiatry 2011, 168, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Yolken, R.H.; Torrey, E.F. Are some cases of psychosis caused by microbial agents? A review of the evidence. Mol. Psychiatry 2008, 13, 470–479. [Google Scholar] [CrossRef]
- Benros, M.E.; Waltoft, B.L.; Nordentoft, M.; Ostergaard, S.D.; Eaton, W.W.; Krogh, J.; Mortensen, P.B. Autoimmune diseases and severe infections as risk factors for mood disorders: A nationwide study. JAMA Psychiatry 2013, 70, 812–820. [Google Scholar] [CrossRef]
- Girgis, R.R.; Ciarleglio, A.; Choo, T.; Haynes, G.; Bathon, J.M.; Cremers, S.; Kantrowitz, J.T.; Lieberman, J.A.; Brown, A.S. A randomized, double-blind, placebo-controlled clinical trial of tocilizumab, an interleukin-6 receptor antibody, for residual symptoms in schizophrenia. Neuropsychopharmacology 2018, 43, 1317–1323. [Google Scholar] [CrossRef]
- Ruscio, A.M.; Stein, D.J.; Chiu, W.T.; Kessler, R.C. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol. Psychiatry 2010, 15, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Robbins, T.W.; Vaghi, M.M.; Banca, P. Obsessive-compulsive disorder: Puzzles and prospects. Neuron 2019, 102, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Frick, L.; Pittenger, C. Microglial dysregulation in OCD, Tourette syndrome, and PANDAS. J. Immunol. Res. 2016, 2016, 8606057. [Google Scholar] [CrossRef]
- Gerentes, M.; Pelissolo, A.; Rajagopal, K.; Tamouza, R.; Hamdani, N. Obsessive-compulsive disorder: Autoimmunity and neuroinflammation. Curr. Psychiatry Rep. 2019, 21, 78. [Google Scholar] [CrossRef]
- Snider, L.A.; Swedo, S.E. PANDAS: Current status and directions for research. Mol. Psychiatry 2004, 9, 900–907. [Google Scholar] [CrossRef]
- Orlovska, S.; Vestergaard, C.H.; Bech, B.H.; Nordentoft, M.; Vestergaard, M.; Benros, M.E. Association of Streptococcal Throat Infection With Mental Disorders: Testing Key Aspects of the PANDAS Hypothesis in a Nationwide Study. JAMA Psychiatry 2017, 74, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, R.J.; Fahey, S.; Frick, L.; Leckman, J.; Vaccarino, F.; Duman, R.S.; Williams, K.; Swedo, S.; Pittenger, C. Antibodies from children with PANDAS bind specifically to striatal cholinergic interneurons and alter their activity. Am. J. Psychiatry 2021, 178, 48–64. [Google Scholar] [CrossRef]
- Xu, M.; Kobets, A.; Du, J.C.; Lennington, J.; Li, L.; Banasr, M.; Duman, R.S.; Vaccarino, F.M.; DiLeone, R.J.; Pittenger, C. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 893–898. [Google Scholar] [CrossRef]
- Zhang, T.; Brander, G.; Isung, J.; Isomura, K.; Sidorchuk, A.; Larsson, H.; Chang, Z.; Mataix-Cols, D.; Fernández de la Cruz, L. Prenatal and early childhood infections and subsequent risk of obsessive-compulsive disorder and tic disorders: A nationwide, sibling-controlled study. Biol. Psychiatry 2023, 93, 1023–1030. [Google Scholar] [CrossRef]
- Cainelli, E.; Nosadini, M.; Sartori, S.; Suppiej, A. Neuropsychological and psychopathological profile of anti-NMDAR encephalitis: A possible pathophysiological model for pediatric neuropsychiatric disorders. Arch. Clin. Neuropsychol. 2019, 34, 1309–1319. [Google Scholar] [CrossRef]
- Foroughipour, M.; Behdani, F.; Hebrani, P.; Marvast, M.N.; Esmatinia, F.; Akhavanrezayat, A. Frequency of obsessive-compulsive disorder in patients with multiple sclerosis: A cross-sectional study. J. Res. Med. Sci. 2012, 17, 248–253. [Google Scholar]
- Wang, L.Y.; Chen, S.F.; Chiang, J.H.; Hsu, C.Y.; Shen, Y.C. Systemic autoimmune diseases are associated with an increased risk of obsessive-compulsive disorder: A nationwide population-based cohort study. Soc. Psychiatry Psychiatr. Epidemiol. 2019, 54, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Lüngen, E.M.; Maier, V.; Venhoff, N.; Salzer, U.; Dersch, R.; Berger, B.; Riering, A.N.; Nickel, K.; Fiebich, B.L.; Süß, P.; et al. Systemic lupus erythematosus with isolated psychiatric symptoms and antinuclear antibody detection in the cerebrospinal fluid. Front. Psychiatry 2019, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, J.F.; Ribeiro, F.M. Sjögren syndrome associated with obsessive-compulsive disorder. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11801–11803. [Google Scholar]
- Endres, D.; Pollak, T.A.; Bechter, K.; Denzel, D.; Pitsch, K.; Nickel, K.; Runge, K.; Pankratz, B.; Klatzmann, D.; Tamouza, R.; et al. Immunological causes of obsessive-compulsive disorder: Is it time for the concept of an “autoimmune OCD” subtype? Transl Psychiatry 2022, 12, 5. [Google Scholar] [CrossRef]
- Pearlman, D.M.; Vora, H.S.; Marquis, B.G.; Najjar, S.; Dudley, L.A. Anti-basal ganglia antibodies in primary obsessive-compulsive disorder: Systematic review and meta-analysis. Br. J. Psychiatry 2014, 205, 8–16. [Google Scholar] [CrossRef]
- Gray, S.M.; Bloch, M.H. Systematic review of proinflammatory cytokines in obsessive-compulsive disorder. Curr. Psychiatry Rep. 2012, 14, 220–228. [Google Scholar] [CrossRef]
- Tamouza, R.; Krishnamoorthy, R.; Leboyer, M. Understanding the genetic contribution of the human leukocyte antigen system to common major psychiatric disorders in a world pandemic context. Brain Behav. Immun. 2021, 91, 731–739. [Google Scholar] [CrossRef]
- Costas, J.; Carrera, N.; Alonso, P.; Gurriarán, X.; Segalàs, C.; Real, E.; López-Solà, C.; Mas, S.; Gassó, P.; Domènech, L.; et al. Exon-focused genome-wide association study of obsessive-compulsive disorder and shared polygenic risk with schizophrenia. Transl. Psychiatry 2016, 6, e768. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.; Morer, A.; González-Navarro, E.A.; Gassó, P.; Boloc, D.; Serra-Pagès, C.; Lafuente, A.; Lazaro, L.; Mas, S. Human-leukocyte antigen class II genes in early-onset obsessive-compulsive disorder. World J. Biol. Psychiatry 2019, 20, 352–358. [Google Scholar] [CrossRef]
- Noble, J.A. Immunogenetics of type 1 diabetes: A comprehensive review. J. Autoimmun. 2015, 64, 101–112. [Google Scholar] [CrossRef]
- Jiang, C.; Ma, X.; Qi, S.; Han, G.; Li, Y.; Liu, Y.; Liu, L. Association between TNF-α-238G/A gene polymorphism and OCD susceptibility: A meta-analysis. Medicine 2018, 97, e9769. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Q.; Xu, T.; Gu, Q.; Liu, Q.; Wang, Y.; Lin, G.N.; Wang, Z. Interaction between PGRN gene and the early trauma on clinical characteristics in patients with obsessive-compulsive disorder. J. Affect. Disord. 2020, 263, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Swedo, S.E.; Leckman, J.F.; Rose, N.R. From research subgroup to clinical syndrome: Modifying the PANDAS criteria to describe PANS (Pediatric Acute-Onset Neuropsychiatric Syndrome). Pediatr. Ther. 2012, 2, 1000113. [Google Scholar] [CrossRef]
- Chang, K.; Frankovich, J.; Cooperstock, M.; Cunningham, M.W.; Latimer, M.E.; Murphy, T.K.; Pasternack, M.; Thienemann, M.; Williams, K.; Walter, J.; et al. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): Recommendations from the 2013 PANS Consensus Conference. J. Child Adolesc. Psychopharmacol. 2015, 25, 3–13. [Google Scholar] [CrossRef]
- Frankovich, J.; Thienemann, M.; Pearlstein, J.; Crable, A.; Brown, K.; Chang, K. Multidisciplinary clinic dedicated to treating youth with pediatric acute-onset neuropsychiatric syndrome: Presenting characteristics of the first 47 consecutive patients. J. Child Adolesc. Psychopharmacol. 2015, 25, 38–47. [Google Scholar] [CrossRef]
- Gagliano, A.; Puligheddu, M.; Ronzano, N.; Congiu, P.; Tanca, M.G.; Cursio, I.; Carucci, S.; Sotgiu, S.; Grossi, E.; Zuddas, A. Artificial neural networks analysis of polysomnographic and clinical features in pediatric acute-onset neuropsychiatric syndrome (PANS): From sleep alteration to “Brain Fog”. Nat. Sci. Sleep. 2021, 13, 1209–1224. [Google Scholar] [CrossRef]
- Chan, A.; Gao, J.; Houston, M.; Willett, T.; Farhadian, B.; Silverman, M.; Tran, P.; Jaradeh, S.; Thienemann, M.; Frankovich, J. Children With PANS May Manifest POTS. Front. Neurol. 2022, 13, 819636. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santoro, J.D.; Frankovich, J.; Bhargava, S. Continued Presence of Period Limb Movements During REM Sleep in Patients With Chronic Static Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS). J. Clin. Sleep Med. 2018, 14, 1187–1192. [Google Scholar] [CrossRef]
- Gaughan, T.; Buckley, A.; Hommer, R.; Grant, P.; Williams, K.; Leckman, J.F.; Swedo, S.E. Rapid Eye Movement Sleep Abnormalities in Children with Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS). J. Clin. Sleep Med. 2016, 12, 1027–1032. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calaprice, D.; Tona, J.; Parker-Athill, E.C.; Murphy, T.K. A survey of pediatric acute-onset neuropsychiatric syndrome characteristics and course. J. Child Adolesc. Psychopharmacol. 2017, 27, 607–618. [Google Scholar] [CrossRef]
- Pavone, P.; Parano, E.; Battaglia, C.; Marino, S.; Trifiletti, R.R.; Marino, S.D.; Falsaperla, R. Severe psychotic symptoms in youth with PANS/PANDAS: Case-series. J. Child Adolesc. Psychopharmacol. 2020, 30, 567–571. [Google Scholar] [CrossRef]
- Silverman, M.; Frankovich, J.; Nguyen, E.; Leibold, C.; Yoon, J.; Mark Freeman, G., Jr.; Karpel, H.; Thienemann, M. Psychotic symptoms in youth with Pediatric Acute-onset Neuropsychiatric Syndrome (PANS) may reflect syndrome severity and heterogeneity. J. Psychiatr. Res. 2019, 110, 93–102. [Google Scholar] [CrossRef]
- Kalinowski, A.; Tian, L.; Pattni, R.; Ollila, H.; Khan, M.; Manko, C.; Silverman, M.; Ma, M.; Columbo, L.; Farhadian, B.; et al. Evaluation of C4 gene copy number in Pediatric Acute Neuropsychiatric Syndrome. Dev. Neurosci. 2023, 45, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Frankovich, J.; Liu, R.J.; Thienemann, M.; Silverman, M.; Farhadian, B.; Willett, T.; Manko, C.; Columbo, L.; Leibold, C.; et al. Elevated antibody binding to striatal cholinergic interneurons in patients with pediatric acute-onset neuropsychiatric syndrome. Brain Behav. Immun. 2024, 122, 241–255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, M.; Masterson, E.E.; Gao, J.; Karpel, H.; Chan, A.; Pooni, R.; Sandberg, J.; Rubesova, E.; Farhadian, B.; Willet, T.; et al. Development of Autoimmune Diseases Among Children With Pediatric Acute-Onset Neuropsychiatric Syndrome. JAMA Netw. Open. 2024, 7, e2421688. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melamed, I.; Rahman, S.; Pein, H.; Heffron, M.; Frankovich, J.; Kreuwel, H.; Mellins, E.D. IVIG response in pediatric acute-onset neuropsychiatric syndrome correlates with reduction in pro-inflammatory monocytes and neuropsychiatric measures. Front. Immunol. 2024, 15, 1383973. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.K.; Storch, E.A.; Lewin, A.B.; Edge, P.J.; Goodman, W.K. Clinical factors associated with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J. Pediatr. 2012, 160, 314–319. [Google Scholar] [CrossRef]
- Pavone, P.; Ceccarelli, M.; Marino, S.; Caruso, D.; Falsaperla, R.; Berretta, M.; Rullo, E.V.; Nunnari, G. SARS-CoV-2 related paediatric acute-onset neuropsychiatric syndrome. Lancet Child Adolesc. Health 2021, 5, e19–e21. [Google Scholar] [CrossRef]
- Berloffa, S.; Salvati, A.; Pantalone, G.; Falcioni, L.; Rizzi, M.M.; Naldini, F.; Masi, G.; Gagliano, A. Steroid treatment response to post SARS-CoV-2 PANS symptoms: Case series. Front. Neurol. 2023, 14, 1085948. [Google Scholar] [CrossRef]
- Saini, T.; Ma, M.; Sandberg, J.; Farhadian, B.; Manko, C.; Xie, Y.; Madan, J.; Bauer, K.; Tran, P.; Frankovich, J. New-onset OCD and juvenile enthesitis-related arthritis after COVID-19 (Three Cases). Dev. Neurosci. 2025, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Pallanti, S.; Di Ponzio, M. PANDAS/PANS in the COVID-19 age: Autoimmunity and Epstein–Barr virus reactivation as trigger agents? Children 2023, 10, 648. [Google Scholar] [CrossRef]
- Zheng, J.; Frankovich, J.; McKenna, E.; Rowe, N.; MacEachern, S.; Ng, N.; Tam, L.; Moon, P.; Gao, J.; Thienemann, M.; et al. Association of Pediatric Acute-Onset Neuropsychiatric Syndrome With Microstructural Differences in Brain Regions Detected via Diffusion-Weighted Magnetic Resonance Imaging. J. Am. Med. Assoc. (JAMA) Netw. Open 2020, 3, e204063. [Google Scholar] [CrossRef]
- Cabrera, B.; Romero-Rebollar, C.; Jiménez-Ángeles, L.; Genis-Mendoza, A.D.; Flores, J.; Lanzagorta, N.; Arroyo, M.; de la Fuente-Sandoval, C.; Santana, D.; Medina-Bañuelos, V.; et al. Neuroanatomical features and its usefulness in classification of patients with PANDAS. CNS Spectr. 2019, 24, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Williams, M.T.; Chugani, H.T. Evaluation of Basal Ganglia and Thalamic Inflammation in Children With Pediatric Autoimmune Neuropsychiatric Disorders Associated With Streptococcal Infection and Tourette Syndrome: A Positron Emission Tomographic (PET) Study Using 11C-[R]-PK11195. J. Child Neurol. 2015, 30, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Giedd, J.N.; Rapoport, J.L.; Garvey, M.A.; Perlmutter, S.; Swedo, S.E. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. Am. J. Psychiatry 2000, 157, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Congiu, P.; Gagliano, A.; Carucci, S.; Lanza, G.; Ferri, R.; Puligheddu, M. REM sleep atonia in patients with pediatric acute-onset neuropsychiatric syndrome: Implications for pathophysiology. J. Clin. Sleep Med. 2025, 21, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Zebrack, J.E.; Gao, J.; Verhey, B.; Tian, L.; Stave, C.; Farhadian, B.; Ma, M.; Silverman, M.; Xie, Y.; Tran, P.; et al. Neurological Soft Signs at Presentation in Patients With Pediatric Acute-Onset Neuropsychiatric Syndrome. JAMA Netw. Open. 2025, 8, e250314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frick, L.R.; Rapanelli, M.; Jindachomthong, K.; Grant, P.; Leckman, J.F.; Swedo, S.; Williams, K.; Pittenger, C. Differential binding of antibodies in PANDAS patients to cholinergic interneurons in the striatum. Brain Behav. Immun. 2018, 69, 304–311. [Google Scholar] [CrossRef]
- Chain, J.L.; Alvarez, K.; Mascaro-Blanco, A.; Reim, S.; Bentley, R.; Hommer, R.; Grant, P.; Leckman, J.F.; Kawikova, I.; Williams, K.; et al. Autoantibody Biomarkers for Basal Ganglia Encephalitis in Sydenham Chorea and Pediatric Autoimmune Neuropsychiatric Disorder Associated With Streptococcal Infections. Front. Psychiatry 2020, 11, 564. [Google Scholar] [CrossRef]
- Foiadelli, T.; Loddo, N.; Sacchi, L.; Santi, V.; D’Imporzano, G.; Spreafico, E.; Orsini, A.; Ferretti, A.; De Amici, M.; Testa, G.; et al. IL-17 in serum and cerebrospinal fluid of pediatric patients with acute neuropsychiatric disorders: Implications for PANDAS and PANS. Eur. J. Paediatr. Neurol. 2025, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, L.; Perna, C.; Bernabei, I.; Fiore, M.; Ma, M.; Frankovich, J.; Tarani, L.; Spalice, A. Pediatric acute-onset neuropsychiatric syndrome (PANS) and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS): Immunological Features Underpinning Controversial Entities. Children 2024, 11, 1043. [Google Scholar] [CrossRef]
- Gromark, C.; Hesselmark, E.; Djupedal, I.G.; Silverberg, M.; Horne, A.; Harris, R.A.; Serlachius, E.; Mataix-Cols, D. A two-to-five year follow-up of a pediatric acute-onset neuropsychiatric syndrome cohort. Child Psychiatry Hum. Dev. 2022, 53, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.K.; Lewin, A.B.; Parker-Athill, E.C.; Storch, E.A.; Mutch, P.J. Tonsillectomies and adenoidectomies do not prevent the onset of pediatric autoimmune neuropsychiatric disorder associated with group A streptococcus. Pediatr. Infect. Dis. J. 2013, 32, 834–838. [Google Scholar] [CrossRef]
- Gromark, C.; Harris, R.A.; Wickström, R.; Horne, A.; Silverberg-Mörse, M.; Serlachius, E.; Mataix-Cols, D. Establishing a pediatric acute-onset neuropsychiatric syndrome clinic: Baseline clinical features of the pediatric acute-onset neuropsychiatric syndrome cohort at Karolinska Institutet. J. Child Adolesc. Psychopharmacol. 2019, 29, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Stagi, S.; Rigante, D.; Lepri, G.; Bertini, F.; Matucci-Cerinic, M.; Falcini, F. Evaluation of autoimmune phenomena in patients with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). Autoimmun. Rev. 2014, 13, 1236–1240. [Google Scholar] [CrossRef]
- Williams, K.A.; Swedo, S.E.; Farmer, C.A.; Grantz, H.; Grant, P.J.; D’Souza, P.; Hommer, R.; Katsovich, L.; King, R.A.; Leckman, J.F. Randomized, controlled trial of intravenous immunoglobulin for pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 860–867.e2. [Google Scholar] [CrossRef]
- Johnson, M.; Fernell, E.; Preda, I.; Wallin, L.; Fasth, A.; Gillberg, C.; Gillberg, C. Paediatric acute-onset neuropsychiatric syndrome in children and adolescents: An observational cohort study. Lancet Child Adolesc. Health 2019, 3, 175–180. [Google Scholar] [CrossRef]
- Pohlman, D. PANDAS Network: PN 2018 State of Our Children SURVEY 2018 [Internet]. PANDAS Network; Downloaded by East Carolina University. 2018. Available online: https://pandasnetwork.org/wp-content/uploads/2018/10/PN-SOOC-SURVEY_2018.pdf (accessed on 5 August 2025).
- Chan, A.; Phu, T.; Farhadian, B.; Willett, T.; Thienemann, M.; Frankovich, J. Familial Clustering of Immune-Mediated Diseases in Children with Abrupt-Onset Obsessive Compulsive Disorder. J. Child Adolesc. Psychopharmacol. 2020, 30, 345–346. [Google Scholar] [CrossRef]
- Trifiletti, R.; Lachman, H.M.; Manusama, O.; Zheng, D.; Spalice, A.; Chiurazzi, P.; Schornagel, A.; Serban, A.M.; Van Wijck, R.; Cunningham, J.L.; et al. Identification of Ultra-Rare Genetic Variants in Pediatric Acute Onset Neuropsychiatric Syndrome (PANS) by Exome and Whole Genome Sequencing. Sci. Rep. 2022, 12, 11106. [Google Scholar] [CrossRef]
- Wang, Y.; Du, W.; Hu, X.; Yu, X.; Guo, C.; Jin, X.; Wang, W. Targeting the blood-brain barrier to delay aging-accompanied neurological diseases by modulating gut microbiota, circadian rhythms, and their interplays. Acta Pharm. Sin. B 2023, 13, 4667–4687. [Google Scholar] [CrossRef] [PubMed]
- Tărlungeanu, D.C.; Deliu, E.; Dotter, C.P.; Kara, M.; Janiesch, P.C.; Scalise, M.; Galluccio, M.; Tesulov, M.; Morelli, E.; Sonmez, F.M.; et al. Impaired amino acid transport at the blood brain barrier is a cause of autism spectrum disorder. Cell 2016, 167, 1481–1494.e18. [Google Scholar] [CrossRef]
- Xiu, Z.; Sun, L.; Liu, K.; Cao, H.; Qu, H.Q.; Glessner, J.T.; Ding, Z.; Zheng, G.; Wang, N.; Xia, Q.; et al. Shared Molecular Mechanisms and Transdiagnostic Potential of Neurodevelopmental Disorders and Immune Disorders. Brain Behav. Immun. 2024, 119, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-J.; Sun, C.-K.; Tsai, S.-J.; Bai, Y.-M.; Hung, K.-C.; Hsu, J.-W.; Huang, K.-L.; Su, T.-P.; Chen, T.-J.; Sun, A.; et al. A Nationwide Study of the Risks of Major Mental Disorders among the Offspring of Parents with Rheumatoid Arthritis. Sci. Rep. 2022, 12, 4962. [Google Scholar] [CrossRef]
- Arrondo, G.; Solmi, M.; Dragioti, E.; Eudave, L.; Ruiz-Goikoetxea, M.; Ciaurriz-Larraz, A.M.; Magallon, S.; Carvalho, A.F.; Cipriani, A.; Fusar-Poli, P.; et al. Associations between Mental and Physical Conditions in Children and Adolescents: An Umbrella Review. Neurosci. Biobehav. Rev. 2022, 137, 104662. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and Macrophages in Brain Homeostasis and Disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Lenz, K.M.; Nelson, L.H. Microglia and Beyond: Innate Immune Cells as Regulators of Brain Development and Behavioral Function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef]
- Lussier, A.A.; Bodnar, T.S.; Weinberg, J. Intersection of Epigenetic and Immune Alterations: Implications for Fetal Alcohol Spectrum Disorder and Mental Health. Front. Neurosci. 2021, 15, 788630. [Google Scholar] [CrossRef]
- Asslih, S.; Damri, O.; Agam, G. Neuroinflammation as a Common Denominator of Complex Diseases (Cancer, Diabetes Type 2, and Neuropsychiatric Disorders). Int. J. Mol. Sci. 2021, 22, 6138. [Google Scholar] [CrossRef]
- Masterson, E.E.; Miles, K.; Schlenk, N.; Manko, C.; Ma, M.; Farhadian, B.; Chang, K.; Silverman, M.; Thienemann, M.; Frankovich, J. Defining Clinical Course of Patients Evaluated for Pediatric Acute-Onset Neuropsychiatric Syndrome: Phenotypic Classification Based on 10 Years of Clinical Data. Dev. Neurosci. 2025, 270–286. [Google Scholar] [CrossRef] [PubMed]
- Maser, J.D.; Akiskal, H.S. Spectrum concepts in major mental disorders. Psychiatr. Clin. N. Am. 2002, 25, xi–xiii. [Google Scholar] [CrossRef]
- Hopwood, C.J.; Morey, L.C.; Markon, K.E. What is a psychopathology dimension? Clin. Psychol. Rev. 2023, 106, 102356. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.; Garvey, M.A.; Sarampote, C.S.; Cohen, E.D.; Murphy, E.R.; Friedman-Hill, S.R. Annual Research Review: The contributions of the RDoC research framework on understanding the neurodevelopmental origins, progression and treatment of mental illnesses. J. Child Psychol. Psychiatry 2022, 63, 360–376. [Google Scholar] [CrossRef]
- Arnsten, A.F.; Rubia, K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: Disruptions in neurodevelopmental psychiatric disorders. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Vreeland, A.; Calaprice, D.; Or-Geva, N.; Frye, R.E.; Agalliu, D.; Lachman, H.M.; Pittenger, C.; Pallanti, S.; Williams, K.; Ma, M.; et al. Postinfectious Inflammation, Autoimmunity, and Obsessive-Compulsive Disorder: Sydenham Chorea, Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcal Infection, and Pediatric Acute-Onset Neuropsychiatric Disorder. Dev. Neurosci. 2023, 45, 361–374. [Google Scholar] [CrossRef]
- Karakochuk, C.; Whitfield, K.; Green, T.; Kraemer, K. The Biology of the First 1000 Days; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Wang, Q.; Yang, Q.; Liu, X. The Microbiota–Gut–Brain Axis and Neurodevelopmental Disorders. Protein Cell 2023, 14, 762–775. [Google Scholar] [CrossRef]
- Murgia, F.; Gagliano, A.; Tanca, M.G.; Or-Geva, N.; Hendren, A.; Carucci, S.; Pintor, M.; Cera, F.; Cossu, F.; Sotgiu, S.; et al. Metabolomic Characterization of Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS). Front. Neurosci. 2021, 15, 645267. [Google Scholar] [CrossRef]
- Likhitweerawong, N.; Thonusin, C.; Boonchooduang, N.; Louthrenoo, O.; Nookaew, I.; Chattipakorn, N.; Chattipakorn, S.C. Profiles of Urine and Blood Metabolomics in Autism Spectrum Disorders. Metab. Brain Dis. 2021, 36, 1641–1671. [Google Scholar] [CrossRef]
- Gagliano, A.; Murgia, F.; Capodiferro, A.M.; Tanca, M.G.; Hendren, A.; Falqui, S.G.; Aresti, M.; Comini, M.; Carucci, S.; Cocco, E.; et al. 1H-NMR-Based Metabolomics in Autism Spectrum Disorder and Pediatric Acute-Onset Neuropsychiatric Syndrome. J. Clin. Med. 2022, 11, 6493. [Google Scholar] [CrossRef]

| NDDs | Blood–Brain Barrier Dysfunction | Microglial Dysregulation | Inflammatory Biomarkers | Maternal Immune Activation (MIA) | Family History of Autoimmune or Allergic Disorders | Genetic Immune-Related Alterations |
|---|---|---|---|---|---|---|
| ADHD | Yes: In SHR models, chronic inflammation and altered autophagy impair BBB integrity, with reduced expression of tight junction proteins such as occludin and ZO-1 [229]. | Limited evidence: Prenatal immune activation and antibody response against dopaminergic structures suggest early microglial dysfunction, affecting cortical development and synaptic dopamine regulation [66,69]. | Yes: ↑ IL-6, TNF-α, and CRP, and the presence of autoantibodies against basal ganglia and dopamine transporters. The imbalance between pro- and anti-inflammatory cytokines correlates with clinical severity [69,70,71]. | Yes: In mouse models, MIA reduces prefrontal cortex volume and alters the development of dopaminergic neurons via dysregulation of the Sonic Hedgehog (SHH) pathway [66]. In a Danish mother–child cohort, ↑ maternal hsCRP levels during pregnancy were associated with an increased risk of ADHD at age 10 [68]. | Yes: The prevalence of inflammatory and autoimmune diseases such as asthma, eczema, rheumatoid arthritis, psoriasis, type 1 diabetes, and IBD among relatives—especially mothers—of individuals with ADHD [57,61,125]. | Yes: Polymorphisms in genes involved in the inflammatory response, such as TNF-α, IL-1β, and IL-6 [62]. GWASs confirmed the association with genes regulating the inflammatory cascade [63,64]. |
| ASD | Yes: Defects in amino acid transport across the blood–brain barrier have been linked to impaired neurological development in ASD [230]. | Yes: Postmortem studies show microglial and astrocyte activation in cortex, white matter, and cerebellum, linked to neuroinflammation and synaptic dysregulation. MIA triggers brain inflammation, oxidative stress, and synaptic ultrastructural changes [101,103,104,107]. | Yes: ↑ maternal and neonatal levels of IL-6, IL-4, IL-21, TNF-α, BAFF, CCL2, and CXCL8, associated with altered amygdala connectivity and later cognitive impairments [93,96,97,108]. | Yes: Infections, asthma, maternal obesity, thyroid autoimmunity (e.g., TPO-Ab), and elevated CRP during pregnancy increase the risk of ASD in offspring [68,88,89,90,92,95]. | Yes: Familial co-occurrence of allergies, autoimmune diseases, and psoriasis in parents of individuals with ASD, especially mothers. One study reported a significantly increased risk with combined maternal asthma and obesity [89,90]. | Yes: ↑ autoantibodies, immunoglobulins, and peripheral cytokines; ↓ regulatory T and B cells. Polymorphisms and abnormalities in genes related to immunity and inflammation, including TPO-Ab and immune signaling proteins [90,109,111]. |
| TIC/TD | Evidence not available: Preliminary evidence suggests that systemic inflammation may impair the BBB, but further studies are needed [5]. | Indirect evidence: Studies showing reduced regulatory T cells (Tregs) and altered innate immune responses suggest microglial activation as an inflammatory mediator [5,126,127]. | Yes: ↑ levels of TNF-α, IL-6, IL-12p40, IL-4, and IL-8 correlate with tic severity. ↑ anti-D2R autoantibodies during clinical exacerbations [122,123,129]. | Limited evidence: Prenatal immune activation is hypothesized as a trigger of alterations in the neural circuits underlying tics [19,176]. | Yes: ↑ prevalence of rheumatoid arthritis and lupus in first-degree relatives of individuals with TD [116]. | Yes: SNP in the TNF-α gene (-308 A/G) associated with TD. GWASs show correlations with immune-related variants (FLT3), HLA system, and IL-1α [116,117,119]. |
| ID | No studies identified: No direct data are available on BBB alterations in individuals with ID. | Evidence not available: No direct evidence of microglial involvement in individuals with ID. | Yes: Individuals with ID (including syndromic forms) show altered cytokine profiles, with ↑ levels of IL-6, TNF-α, IL-10, and CRP. Systemic inflammation has been correlated with cognitive severity [134,135]. | Yes: Maternal infections during pregnancy increase the risk of ID in the child. Beyond direct damage from infectious agents, maternal immune activation plays a key role through the production of proinflammatory cytokines [131,132,133]. | Indirect evidence: No consistent data available on familial autoimmunity in non-syndromic forms. However, in certain syndromic forms (e.g., Down syndrome, 22q11.2DS), a shared immunogenetic component has been hypothesized [135]. | Indirect evidence: Evidence from individuals with 22q11.2 deletion syndrome shows that a higher IL-6/IL-10 ratio correlates with cognitive and psychotic symptom severity. While not directly studied in idiopathic schizophrenia, this supports the hypothesis of immune involvement [135]. |
| SS/PD | Yes: Neurovascular inflammation and increased BBB permeability facilitate the entry of immune mediators into the brain, contributing to chronic neuroinflammation [152,153]. | Yes: Postmortem evidence of activated microglia and upregulation of proinflammatory genes in individuals with schizophrenia [154,155]. | Yes: Elevated serum and CSF levels of IL-6, IL-1β, IL-12, TNF-α, TGF-β, and IFN-γ. Some cytokines (e.g., IL-6, IL-1β) are increased only during acute episodes, while TNF-α, IFN-γ, and sIL-2R remain persistently elevated (state vs. trait markers) [142,147,148]. | Yes: Maternal infections and severe early-life infections increase the risk of schizophrenia. The risk is further amplified when autoimmune diseases are also present [164,165,166]. | Yes: The risk of schizophrenia is significantly increased in individuals with a personal or family history of autoimmune diseases [162,163]. | Yes: HLA polymorphisms (e.g., HLA-DQB1) are associated with schizophrenia risk and immune dysregulation. Additional associations have been described between schizophrenia and alleles linked to autoimmune encephalitis (e.g., NMDAR) [156,186]. |
| OCD | Indirect evidence: Not directly reported. However, in individuals with neuronal antibodies (e.g., PANDAS), altered BBB permeability has been hypothesized to allow for IgG entry into the brain [174]. | No studies identified: No direct evidence specific to OCD, but microglial involvement is hypothesized in autoimmune-related subtypes such as PANDAS, which share overlapping features. | Yes: ABGAs (anti-basal ganglia antibodies) are up to 5 times higher in OCD patients compared to controls. Some studies report ↑ TNF-α and ↓ IL-1β and IL-6, especially in children [183,184]. | Yes: Prenatal or early childhood infections increase the risk of OCD, but not paternal or sibling infections, suggesting vulnerability linked to early immune exposure and genetic predisposition [176]. | Yes: Frequently co-occurs in individuals with lupus, multiple sclerosis, and dermatomyositis, suggesting a possible shared autoimmune background in predisposed families [179,180,181]. | Yes: GWASs identified two HLA regions associated with OCD risk, shared with schizophrenia. The HLA-DRB1*04 allele has been linked to early-onset OCD. Alterations in the PGRN gene (progranulin), have also been reported in patients with OCD and a history of childhood trauma [186,187,190]. |
| PANS | Yes: Genetic variants in vascular genes and increased cytokines suggest heightened BBB permeability. The presence of IL-17 in CSF supports the hypothesis of central immune infiltration [35,228]. | Yes: Th17/IL-17 response suggests active neuroglial inflammation. Direct microglial involvement has been observed in neuroinflammation and synaptic dysregulation [33,35]. | Yes: Elevated serum and CSF levels of IL-1β, IL-6, TNF-α, and NSE have been reported in PANS patients, along with increased CRP, SAA, and complement activation [220,221]. | Indirect evidence: No direct studies exist, but candidate genes for PANS are expressed in the choroid plexus and cerebral vessels, supporting a possible early role of MIA in vulnerability [19,228]. | Yes: ↑ About 70% of individuals with PANS have a family history of autoimmune disorders. Mothers show a 20% prevalence of severe autoimmune diseases, suggesting genetic predisposition to autoimmunity [50,198,225]. | Yes: WES/WGS studies identified ultra-rare variants in 11 genes (e.g., PPM1D, SYNGAP1, NLRC4, SHANK3, RAG1) involved in immune response, synaptogenesis, blood–brain barrier integrity, and the enteric nervous system [228]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagliano, A.; Cucinotta, F.; Giunta, I.; Di Modica, I.; De Domenico, C.; Costanza, C.; Germanò, E.; Frankovich, J. The Immune/Inflammatory Underpinnings of Neurodevelopmental Disorders and Pediatric Acute-Onset Neuropsychiatric Syndrome: A Scoping Review. Int. J. Mol. Sci. 2025, 26, 7767. https://doi.org/10.3390/ijms26167767
Gagliano A, Cucinotta F, Giunta I, Di Modica I, De Domenico C, Costanza C, Germanò E, Frankovich J. The Immune/Inflammatory Underpinnings of Neurodevelopmental Disorders and Pediatric Acute-Onset Neuropsychiatric Syndrome: A Scoping Review. International Journal of Molecular Sciences. 2025; 26(16):7767. https://doi.org/10.3390/ijms26167767
Chicago/Turabian StyleGagliano, Antonella, Francesca Cucinotta, Ivana Giunta, Irene Di Modica, Carmela De Domenico, Carola Costanza, Eva Germanò, and Jennifer Frankovich. 2025. "The Immune/Inflammatory Underpinnings of Neurodevelopmental Disorders and Pediatric Acute-Onset Neuropsychiatric Syndrome: A Scoping Review" International Journal of Molecular Sciences 26, no. 16: 7767. https://doi.org/10.3390/ijms26167767
APA StyleGagliano, A., Cucinotta, F., Giunta, I., Di Modica, I., De Domenico, C., Costanza, C., Germanò, E., & Frankovich, J. (2025). The Immune/Inflammatory Underpinnings of Neurodevelopmental Disorders and Pediatric Acute-Onset Neuropsychiatric Syndrome: A Scoping Review. International Journal of Molecular Sciences, 26(16), 7767. https://doi.org/10.3390/ijms26167767







