Silicon Carbide (SiC) and Silicon/Carbon (Si/C) Composites for High-Performance Rechargeable Metal-Ion Batteries
Abstract
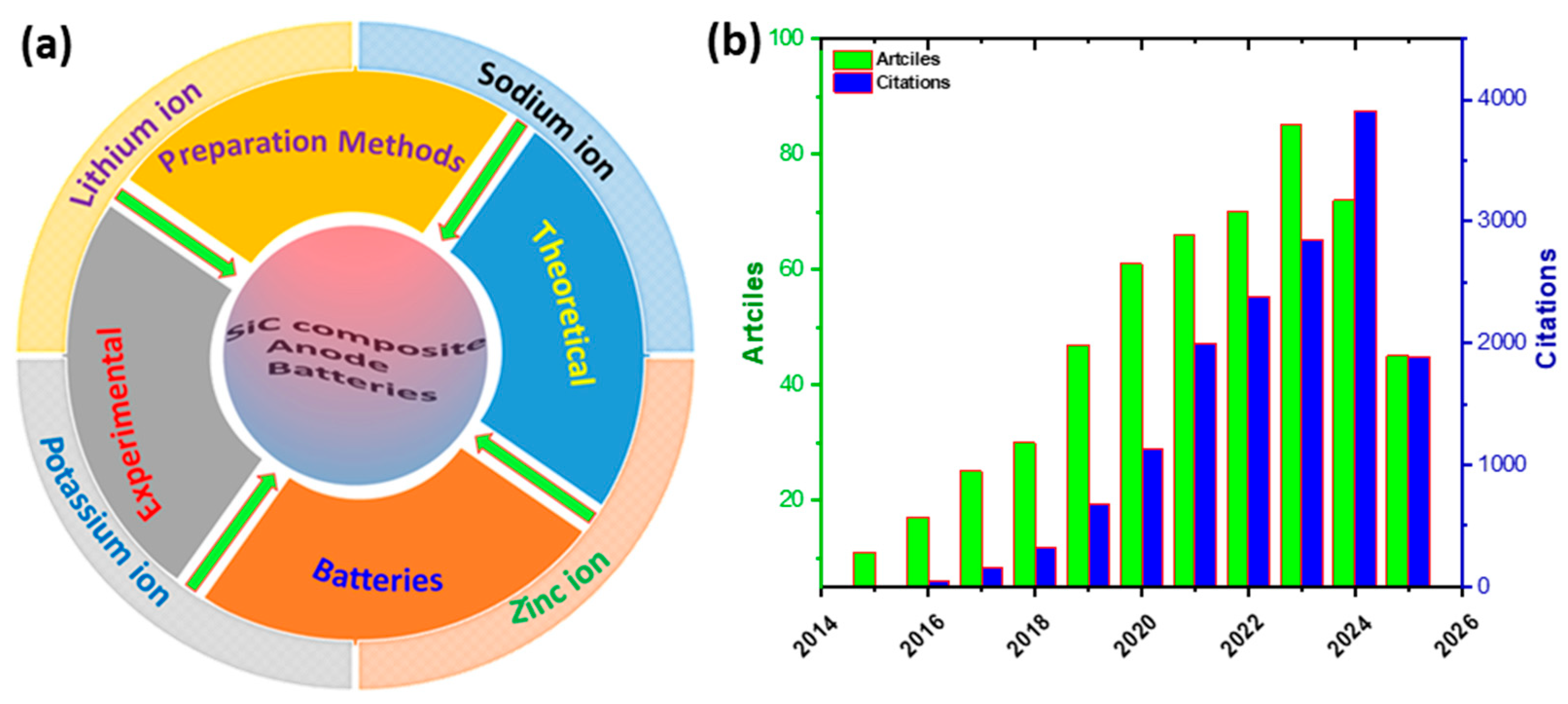
1. Introduction
2. Fabrication Methods of SiC and Si/C Composites for Metal-Ion Batteries
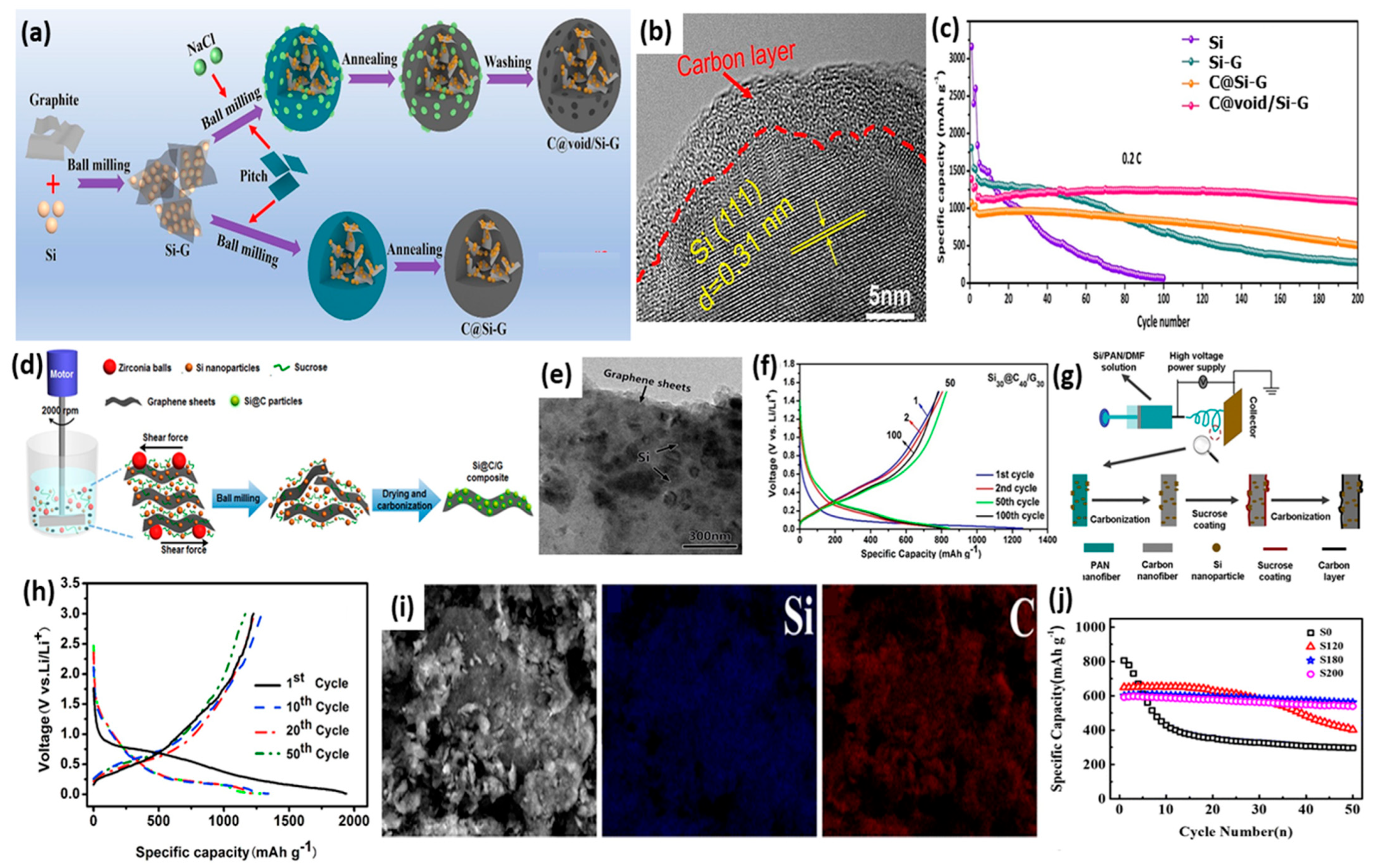
2.1. Ball Milling
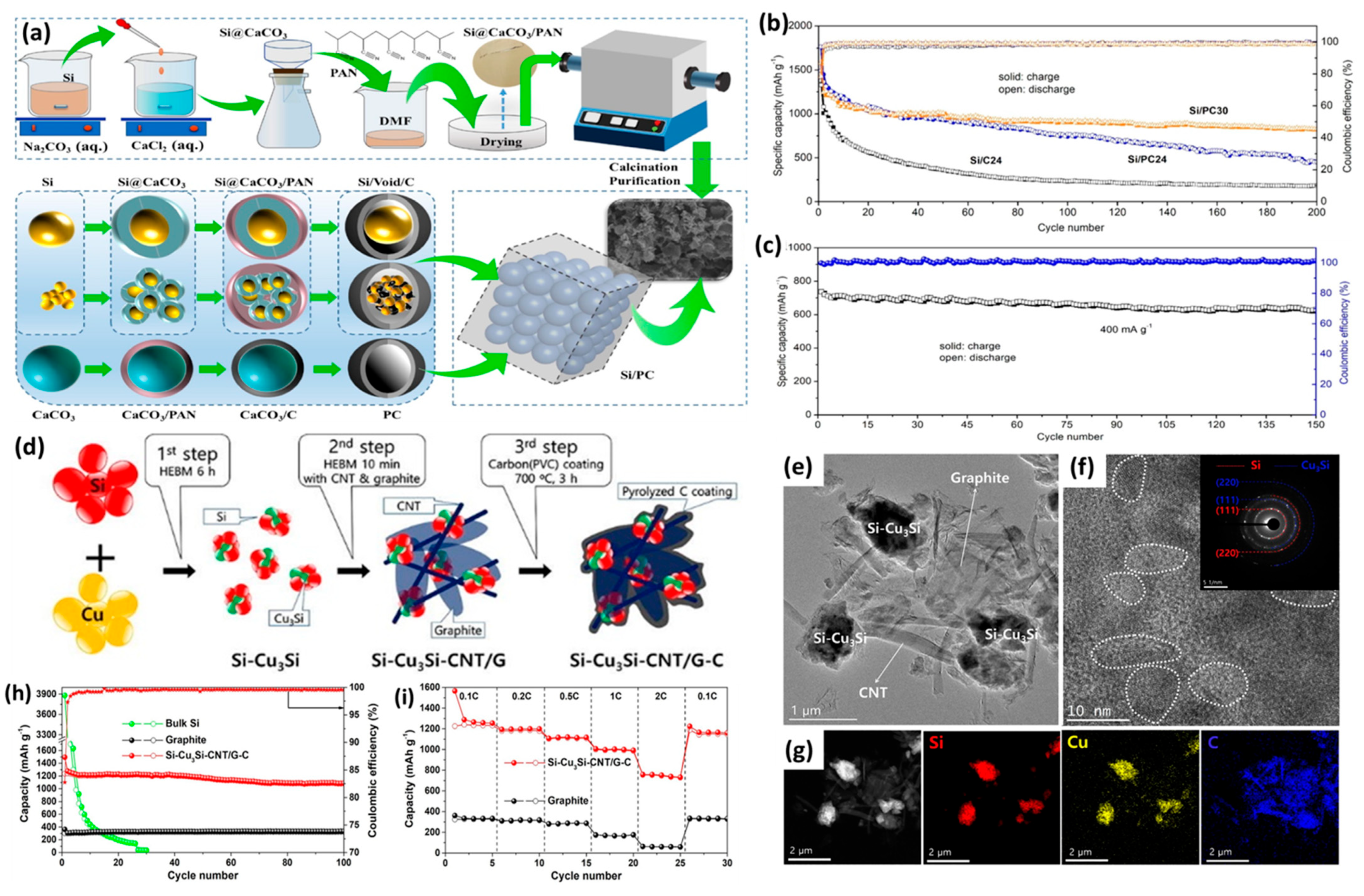
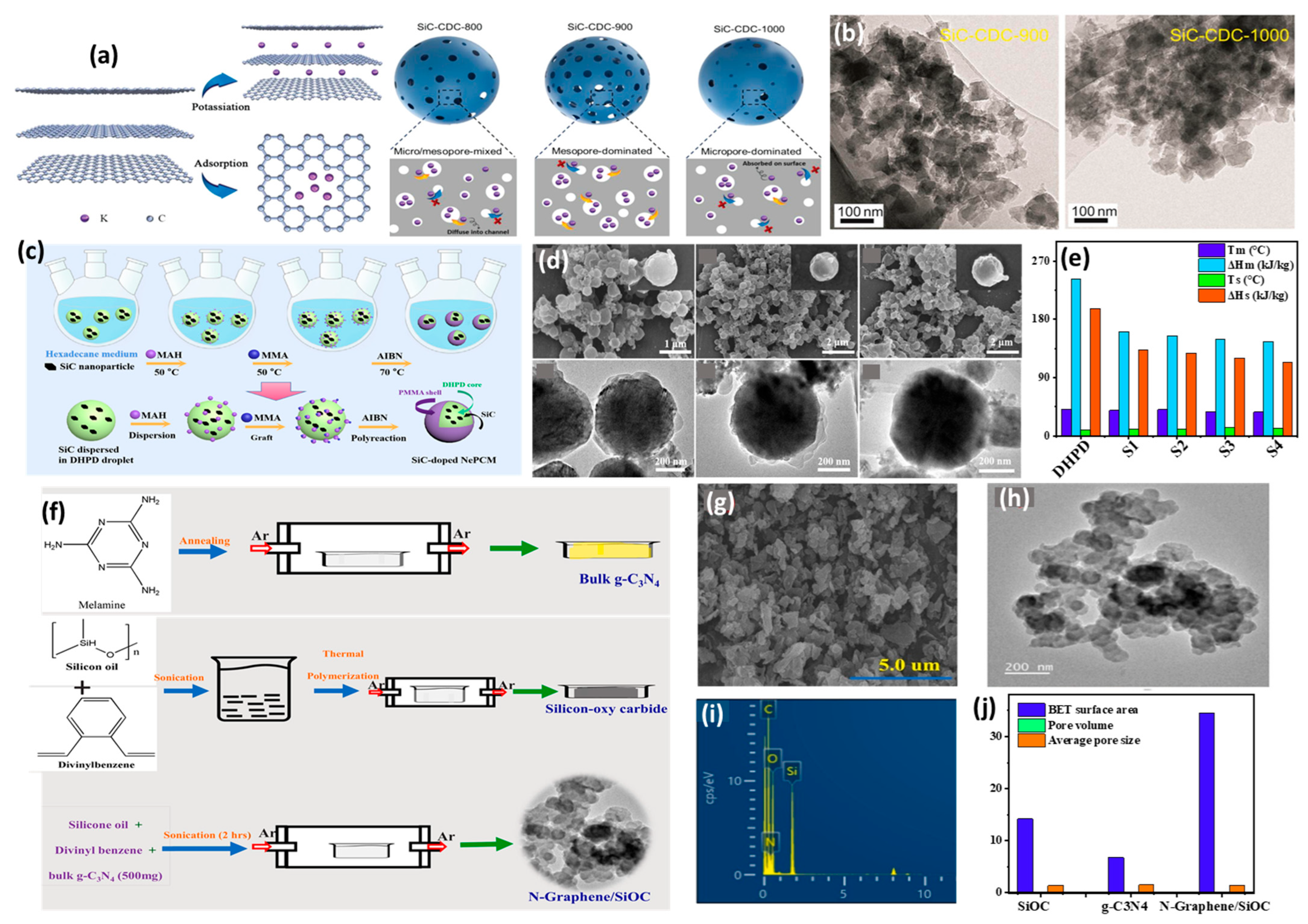
2.2. Pyrolysis
2.3. Spray Drying
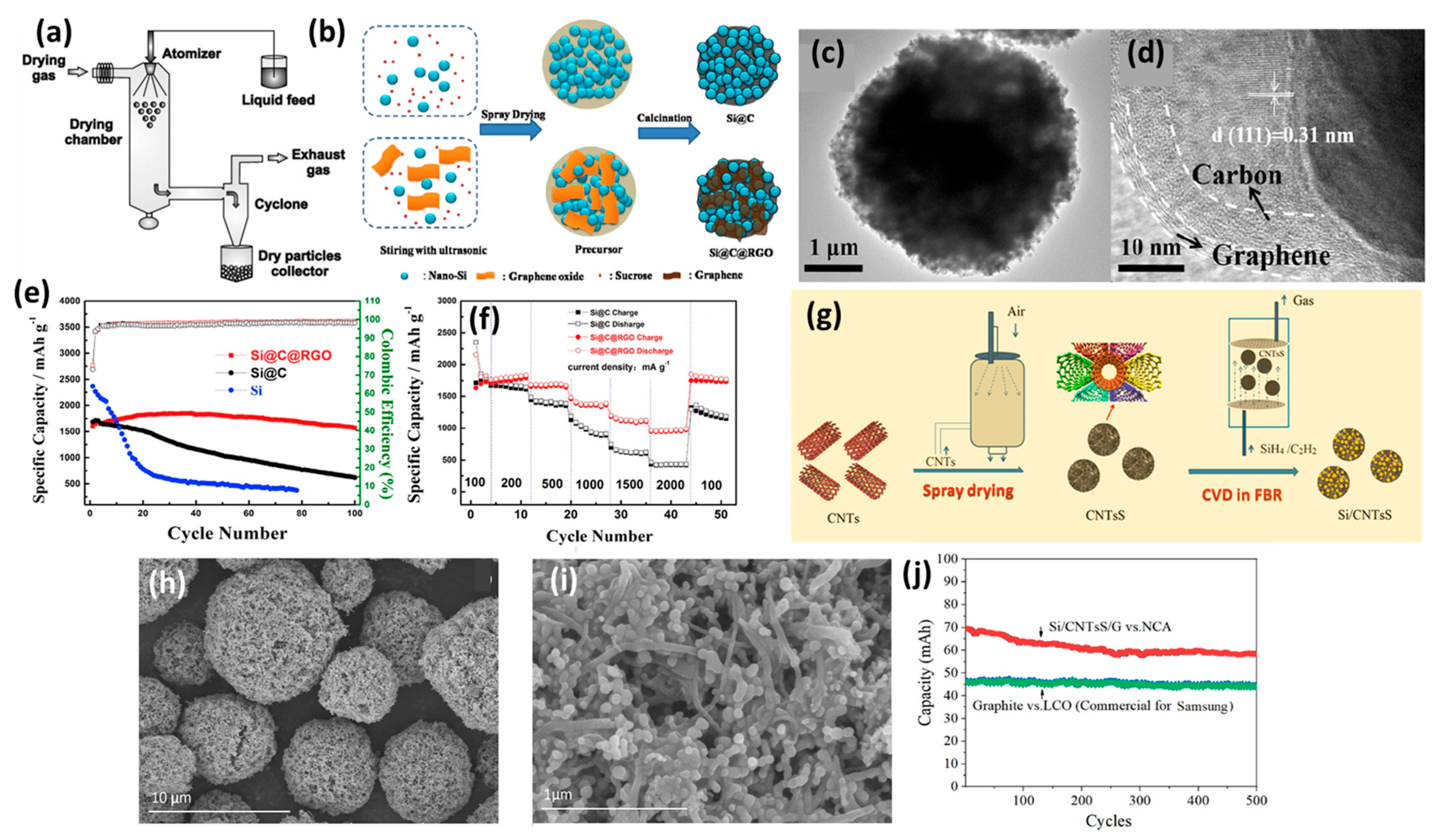
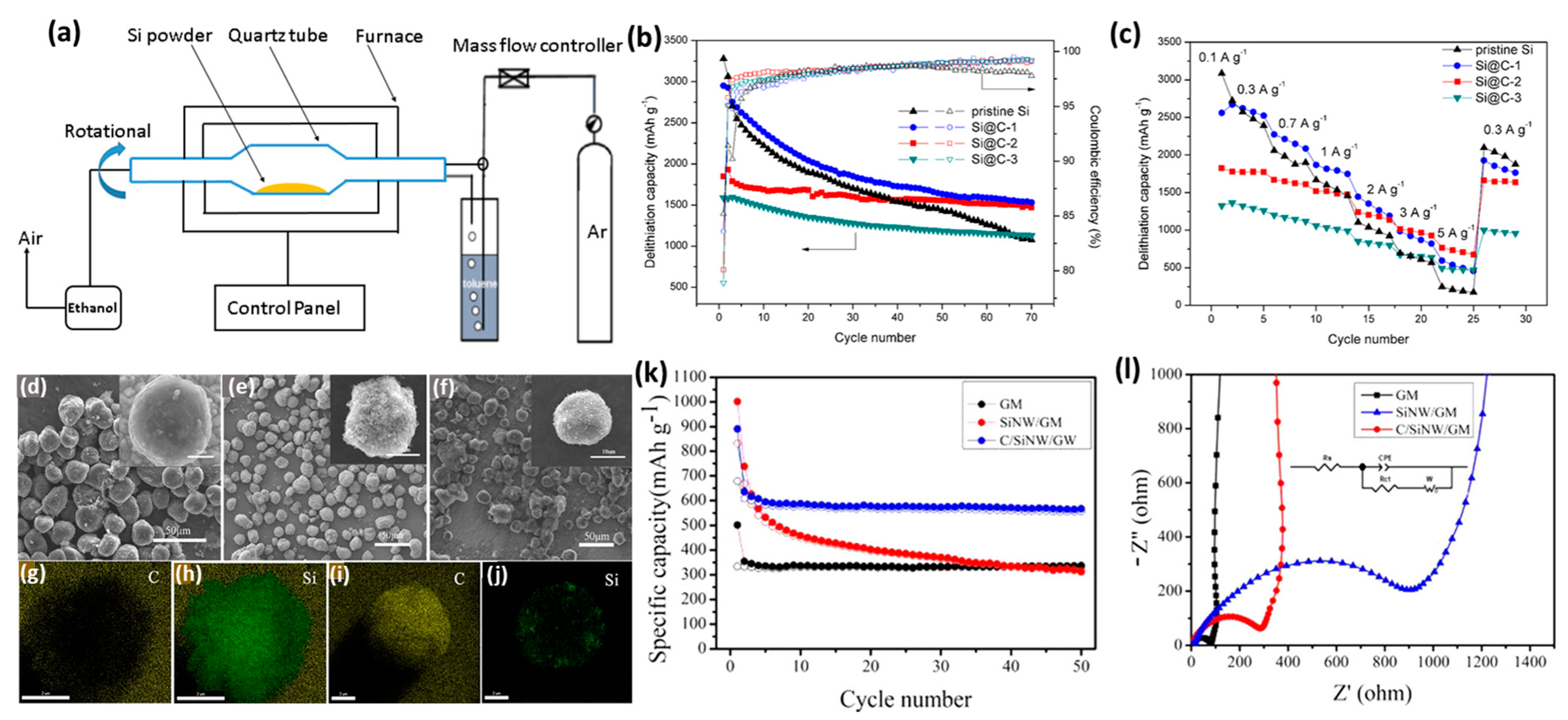
2.4. Chemical Vapor Deposition (CVD)
3. Doped SiC Anodes for Recharge Batteries
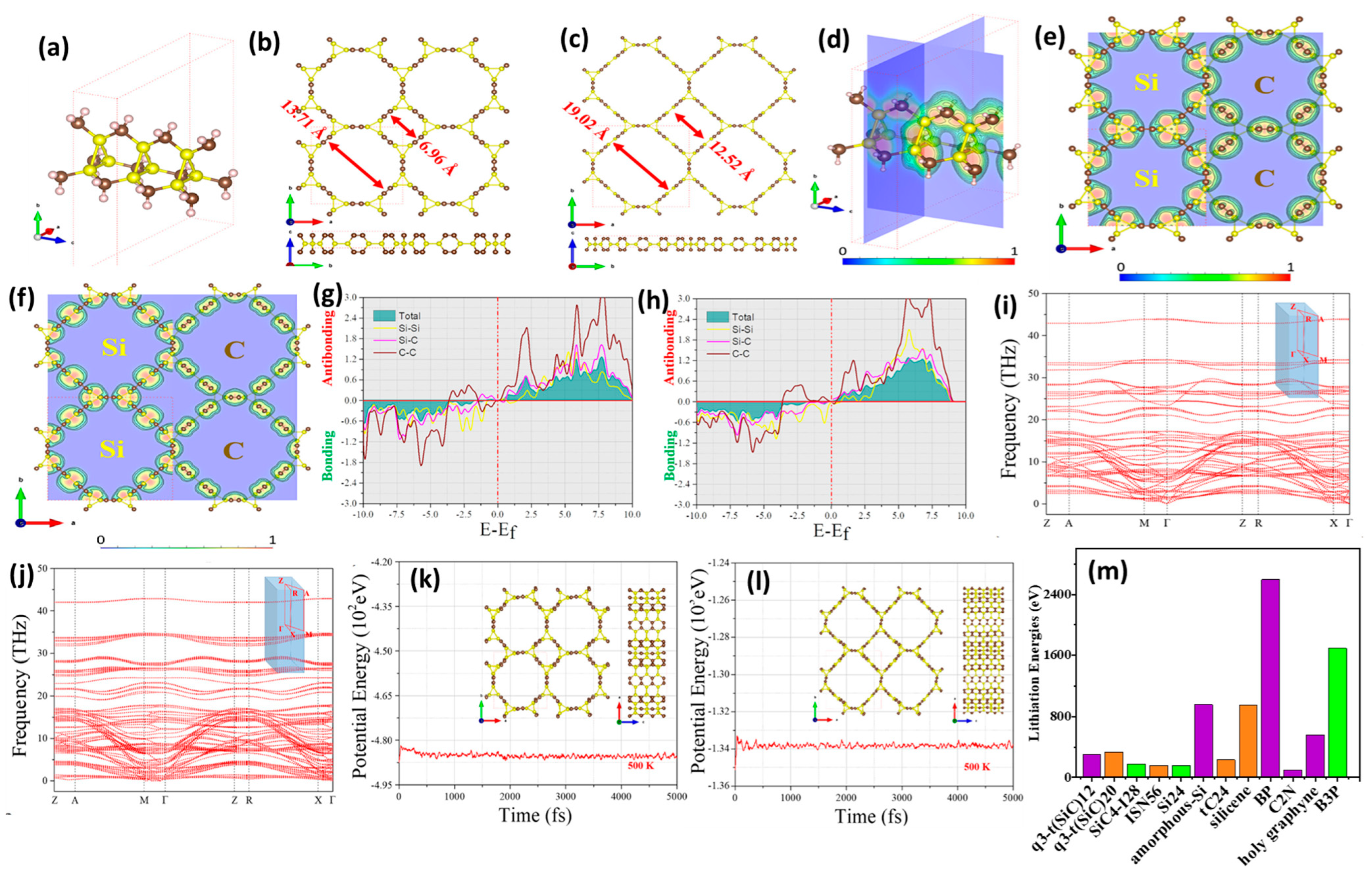
3.1. Theoretical Studies of Doped SiC Anodes
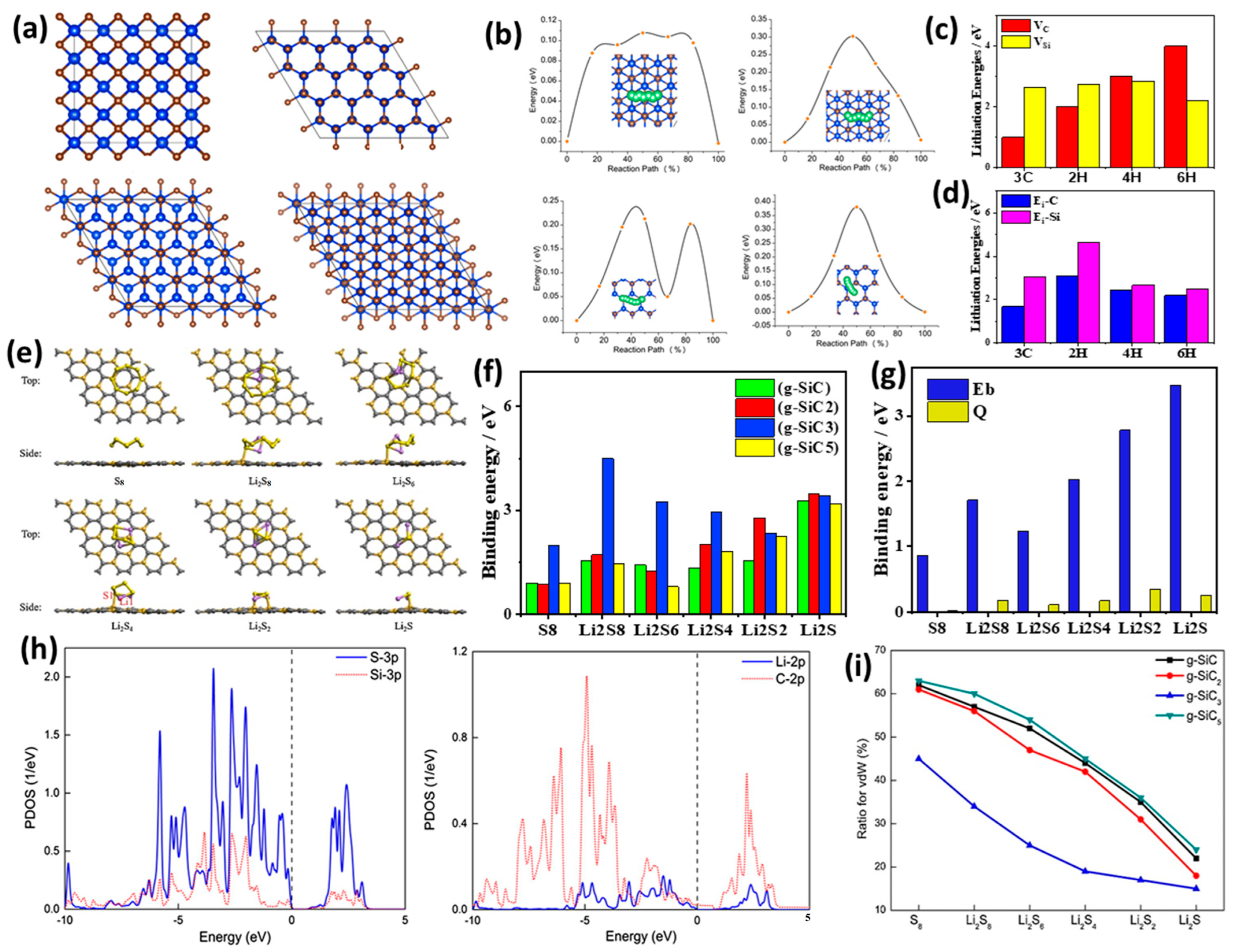
3.2. Experimental Studies of Doped SiC Anodes for LIBs
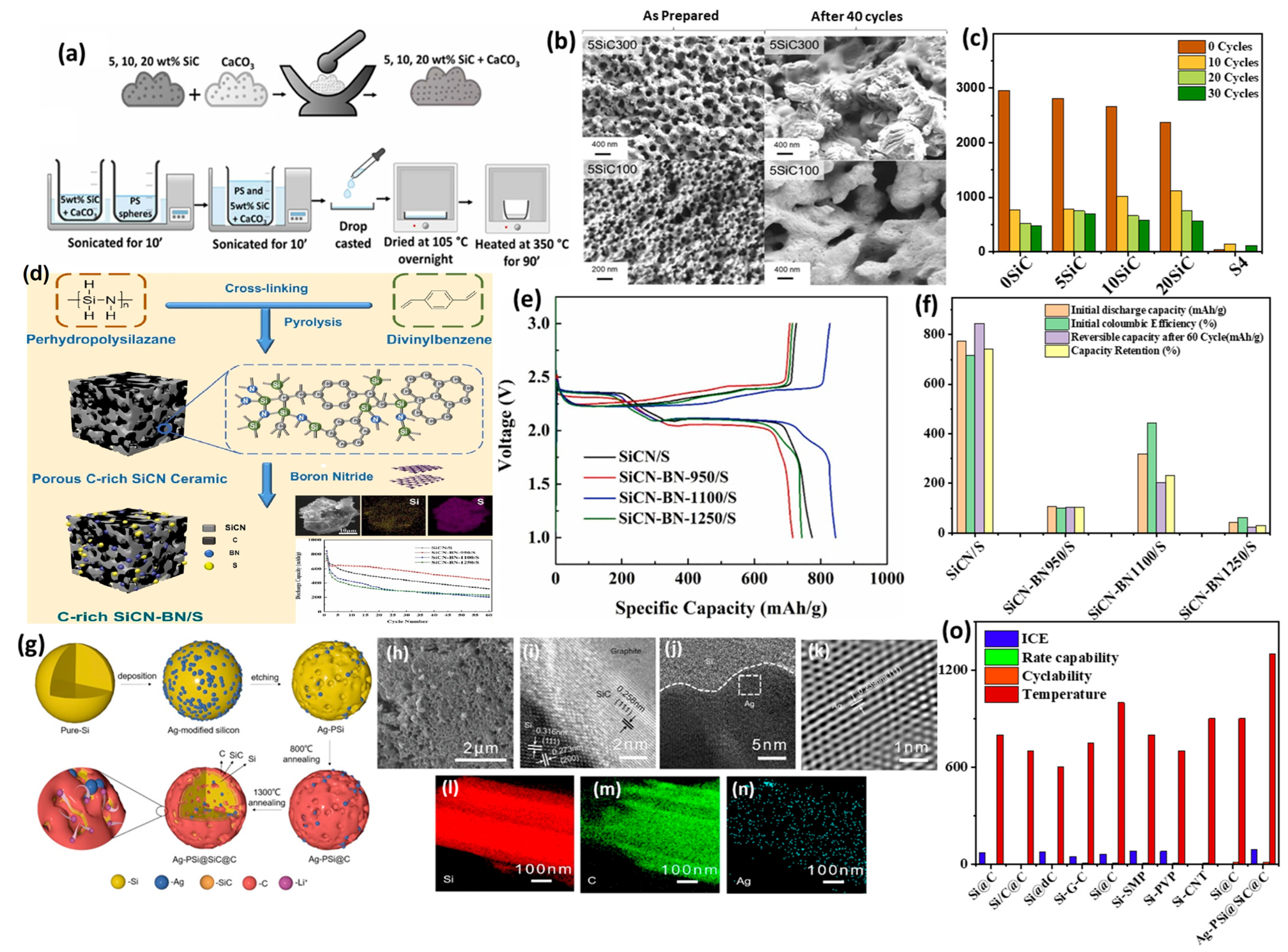
4. Conclusions and Future Perspectives
- Performance Limitations: The currently reported Li-storage capacities of SiC and Si/C materials (≤1500 mAh/g), as well as cycling stability (≤500 cycles), are still insufficient for commercial utilization. The practical industrial energy storage deployment of Si/C and SiC requires a high energy density, long cycle life, and high charging/discharging rates. Mainly, their capacity should reach tens or hundreds of kilowatt-hours per cycle life (≥3000 cycles). Overcoming these performance limitations may be solved via integrating high-capacity active materials such as porous-activated biochar [160], MXenes [161], and graphdiyne [162]. These materials offer outstanding surface area, electrical conductivity, specific capacity, huge Li-storage capacity, and physiochemical stability in addition to ease of preparation from earth-abundant materials, which are needed merits for the industrial battery devices.
- Synthesis Complexity: The methods of forming of SiC composites are not productive (up to the milligram range) and entail multiple reaction steps and heating to elevated temperatures, leading to improper and inhomogeneous distribution of Si within the skeleton structure of carbon [28,51,52,53]. Thus, these methods should be simplified and result in a higher yield (up to the kilogram range), besides providing a homogeneous coating of Si with carbon to fit scalable and practical usage. These issues could be resolved via the in situ growth of Si nanoparticles within carbon support by means of chemical reduction, seed-mediated, templates, and microwave methods, along with their coupling, to be feasible for commercial utilization.
- Unresolved Electrolyte Effects: The effect of electrolytes on the performance of SiC and Si/C anodes is still ambiguous and not yet resolved. Using organic electrolytes (i.e., ionic liquids and poly (ionic liquid) solid polymer electrolytes) [19,163] could probably widen the potential window and enhance the specific capacity and life cycle of SiC and Si/C anodes. Also, these electrolytes can improve safety and thermal stability, enhance energy density, and preclude Si-volume change or metal-ion dendrite growth, thus offering a pathway toward enhanced long-term cycling performance.
- Underexplored Doping Strategies: The effect of heteroatom doping (i.e., N, B, P, O, and halides) and metal atoms on the performance of SiC and Si/C anodes for metal-ion batteries is rarely reported; meanwhile, understanding distribution, interaction, and stability under cycling conditions remains a key challenge. The integration of these heteroatoms can pave the way for hot research directions and enhance the battery performance [164,165]. This is due to the significant effects of heteroatoms, resulting in improved electronic conductivity, enhanced metal-ion (Li, Na, and Zn) diffusion, structural stability, and the creation of active sites for metal-ion insertion/extraction kinetics [128,151]. In addition to increasing the wettability, providing pseudocapacitive behavior, and boosting capacity and rate performance, these heteroatoms improve SEI layer uniformity and stability.
- Scalability Challenges and Bottlenecks: From the industrial viewpoint, the key scalability hurdles include inconsistent material quality at the large scale, the high energy demand for synthesis, and integration challenges with current industrial fabrication processes for the electrodes of metal-ion batteries. The industrial case studies indicate that translating lab-scale pyrolysis or CVD methods into continuous, roll-to-roll production remains impractical, owing to reactor design limitations, gas management, and precursor control. Solving these barriers will require process optimization, modular reactor designs, and improved precursor delivery systems.
- Necessity of Using Machine Learning and Simulations: Machine learning (ML) offers transformative opportunities across multiple stages of SiC and Si/C anode development and performance predictions. Machine learning models can easily be used for the discovery of SiC and Si/C materials by predicting optimal dopant combinations or structural motifs with a high capacity and low volume expansion. In addition to performance prediction, they correlate synthesis parameters of Si/C and SiC with electrochemical performance. Meanwhile, ML can assist process optimization by fine-tuning preparation method conditions and factors to ensure consistent product quality. Also, ML-guided models can be used to predict optimal electrolyte conditions (i.e., type, composition, and pH) for metal-ion batteries. Coupling ML with density functional theory (DFT) calculations could accelerate the identification of high-performance compositions and interface chemistries, leading to more efficient SiC and Si/C material designs [166,167,168]. This endeavor works to optimize the performance of SiC and Si/C anodes (i.e., shape, composition, and strain/synergetic effect) for various metal rechargeable batteries, especially NIBs, ZnBs, and PIBs, to complement the extensive existing research on LIBs.
- Advanced Characterization Needs: In situ characterization techniques such as SEM, TEM, and XPS should be further integrated and synchronized during battery cycling to elucidate structural evolution, SEI layer formation, and degradation pathways [169]. Such insights will be crucial for rational SiC and Si/C anode design and lifespan extension.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, Z.; Yu, R.; Wang, Y.; Xu, K.; Eid, K.; Zhang, Y.; He, J.; Ning, F.; Liu, L.; Zhang, J.; et al. Cavities-Induced Compressive Strain in Unique Nanotubes Boosts the C1 Pathway of Ethanol Oxidation Electrocatalysis. ACS Nano 2025, 19, 7379–7390. [Google Scholar] [CrossRef]
- Gamal, A.; Eid, K.; Abdullah, A.M. Engineering of Pt-based nanostructures for efficient dry (CO2) reforming: Strategy and mechanism for rich-hydrogen production. Int. J. Hydrogen Energy 2022, 47, 5901–5928. [Google Scholar] [CrossRef]
- Varela, H.; Paredes-Salazar, E.A.; Lima, F.H.B.; Eid, K. Renewable methanol and the energy challenge: The role of electrocatalysis. Curr. Opin. Electrochem. 2024, 46, 101539. [Google Scholar] [CrossRef]
- Lu, Q.; Zhao, X.; Luque, R.; Eid, K. Structure-activity relationship of tri-metallic Pt-based nanocatalysts for methanol oxidation reaction. Coord. Chem. Rev. 2023, 493, 215280. [Google Scholar] [CrossRef]
- Ma, F.; Jin, R.; Zhou, K.; Zhu, Y.; Huang, T.; Lu, Q.; Gai, L.; Liu, L.; Varma, R.S.; Eid, K. Rational one-step synthesis of porous PtAg nanowires for methanol oxidation with a CO-poisoning tolerance: An experimental and theoretical study. Chem. Eng. J. 2024, 492, 151988. [Google Scholar] [CrossRef]
- Eid, K.; Ozoemena, K.I.; Varma, R.S. Unravelling the structure-activity relationship of porous binary metal-based electrocatalysts for green hydrogen evolution reaction. Coord. Chem. Rev. 2025, 523, 216238. [Google Scholar] [CrossRef]
- Salah, B.; Abdelgawad, A.; El-Demellawi, J.K.; Lu, Q.; Xia, Z.; Abdullah, A.M.; Eid, K. Scalable One-Pot Fabrication of Carbon-Nanofiber-Supported Noble-Metal-Free Nanocrystals for Synergetic-Dependent Green Hydrogen Production: Unraveling Electrolyte and Support Effects. ACS Appl. Mater. Interfaces 2024, 16, 18768–18781. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Mohamed, A.; Abdelgawad, A.M.; Eid, K.; Abdullah, A.M.; Elzatahry, A. The Recent Advances in the Mechanical Properties of Self-Standing Two-Dimensional MXene-Based Nanostructures: Deep Insights into the Supercapacitor. Nanomaterials 2020, 10, 1916. [Google Scholar] [CrossRef]
- Wu, J.; Chen, X.; Fan, W.; Li, X.; Mai, Y.W.; Chen, Y. Rationally designed alloy phases for highly reversible alkali metal batteries. Energy Storage Mater. 2022, 48, 223–243. [Google Scholar] [CrossRef]
- Wang, X.; Tang, S.; Guo, W.; Fu, Y.; Manthiram, A. Advances in multimetallic alloy-based anodes for alkali-ion and alkali-metal batteries. Mater. Today 2021, 50, 259–275. [Google Scholar] [CrossRef]
- Pirayesh, P.; Tantratian, K.; Amirmaleki, M.; Yang, F.; Jin, E.; Wang, Y.; Goncharova, L.V.; Guo, J.; Filleter, T.; Chen, L.; et al. From Nanoalloy to Nano-Laminated Interfaces for Highly Stable Alkali-Metal Anodes. Adv. Mater. 2023, 35, 2301414. [Google Scholar] [CrossRef]
- Ipadeola, A.K.; Haruna, A.B.; Gaolatlhe, L.; Lebechi, A.K.; Meng, J.; Pang, Q.; Eid, K.; Abdullah, A.M.; Ozoemena, K.I. Efforts at Enhancing Bifunctional Electrocatalysis and Related Events for Rechargeable Zinc-Air Batteries. ChemElectroChem 2021, 8, 3998–4018. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, Y.; Hou, Z.; Jiang, M.; He, W.; Su, D.; Wang, M.; Wang, J.G. Lignocellulosic oxidation bridging to modulate pseudographitic domain of hard carbon toward boosted sodium storage. J. Energy Storage 2025, 130, 117496. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, F.; Jiang, M.; Hou, Z.; He, W.; Su, D.; Xu, F.; Wang, J.G. Yolk-Shell Sodium Iron Sulfate@Carbon for Advanced Sodium Storage with Enhanced Capacity and Stability. Small 2025, 2506866. [Google Scholar] [CrossRef]
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building Safe Lithium-Ion Batteries for Electric Vehicles: A Review. Electrochem. Energy Rev. 2020, 3, 1–42. [Google Scholar] [CrossRef]
- Hu, C.; Ye, H.; Jain, G.; Schmidt, C. Remaining useful life assessment of lithium-ion batteries in implantable medical devices. J. Power Sources 2018, 375, 118–130. [Google Scholar] [CrossRef]
- Parveez, B.; Kittur, M.I.; Badruddin, I.A.; Kamangar, S.; Hussien, M.; Umarfarooq, M.A. Scientific Advancements in Composite Materials for Aircraft Applications: A Review. Polymers 2022, 14, 5007. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Rehman, J.; Eid, K.; Alofi, A.S.; Laref, A.; Albaqami, M.D.; Alotabi, R.G.; Shibl, M.F. Vanadium Carbide (V4C3) MXene as an Efficient Anode for Li-Ion and Na-Ion Batteries. Nanomaterials 2022, 12, 2825. [Google Scholar] [CrossRef]
- Ma, F.; Liu, Y.; Huang, T.; Du, X.; Lu, Q.; Kid, K. Facile in situ polymerization synthesis of poly(ionic liquid)-based polymer electrolyte for high-performance solid-state batteries. Energy Convers. Manag. X 2024, 22, 100570. [Google Scholar] [CrossRef]
- Adewale, K.I.; Aboubakr, M.A.; Kamel, E. Recent advances in porous multimetallic alloy-based anodes for rechargeable alkali metal-ion batteries. Energy Mater. 2024, 4, 400079. [Google Scholar]
- Ipadeola, A.K.; Eid, K.; Abdullah, A.M. Porous transition metal-based nanostructures as efficient cathodes for aluminium-air batteries. Curr. Opin. Electrochem. 2023, 37, 101198. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Shao, R.; Wu, J.; Jiang, R.; Jin, Z. Recent progress and future perspective on practical silicon anode-based lithium ion batteries. Energy Storage Mater. 2022, 46, 482–502. [Google Scholar] [CrossRef]
- Preman, A.N.; Vo, T.N.; Choi, S.; Lee, H.; Lim, Y.E.; Kim, I.T.; Ahn, S.K. Self-Healable Poly(Acrylic Acid) Binder toward Optimized Electrochemical Performance for Silicon Anodes: Importance of Balanced Properties. ACS Appl. Energy Mater. 2024, 7, 749–759. [Google Scholar] [CrossRef]
- Gu, M.; He, Y.; Zheng, J.; Wang, C. Nanoscale silicon as anode for Li-ion batteries: The fundamentals, promises, and challenges. Nano Energy 2015, 17, 366–383. [Google Scholar] [CrossRef]
- Li, P.; Kim, H.; Myung, S.T.; Sun, Y.K. Diverting Exploration of Silicon Anode into Practical Way: A Review Focused on Silicon-Graphite Composite for Lithium Ion Batteries. Energy Storage Mater. 2021, 35, 550–576. [Google Scholar] [CrossRef]
- Franco Gonzalez, A.; Yang, N.H.; Liu, R.S. Silicon Anode Design for Lithium-Ion Batteries: Progress and Perspectives. J. Phys. Chem. C 2017, 121, 27775–27787. [Google Scholar] [CrossRef]
- Lee, G.; Kim, I.T.; Hur, J. Highly conductive and robust telluride-carbon hybrid matrix for enhanced copper diphosphide anode in Li-ion batteries. J. Alloys Compd. 2023, 950, 169914. [Google Scholar] [CrossRef]
- Li, P.; Zhao, G.; Zheng, X.; Xu, X.; Yao, C.; Sun, W.; Dou, S.X. Recent progress on silicon-based anode materials for practical lithium-ion battery applications. Energy Storage Mater. 2018, 15, 422–446. [Google Scholar] [CrossRef]
- Ozanam, F.; Rosso, M. Silicon as anode material for Li-ion batteries. Mater. Sci. Eng. B 2016, 213, 2–11. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and Recent Progress in the Development of Si Anodes for Lithium-Ion Battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef]
- Shen, T.; Yao, Z.; Xia, X.; Wang, X.; Gu, C.; Tu, J. Rationally Designed Silicon Nanostructures as Anode Material for Lithium-Ion Batteries. Adv. Eng. Mater. 2018, 20, 1700591. [Google Scholar] [CrossRef]
- Rahman, M.A.; Song, G.; Bhatt, A.I.; Wong, Y.C.; Wen, C. Nanostructured Silicon Anodes for High-Performance Lithium-Ion Batteries. Adv. Funct. Mater. 2016, 26, 647–678. [Google Scholar] [CrossRef]
- Andersen, H.F.; Foss, C.E.L.; Voje, J.; Tronstad, R.; Mokkelbost, T.; Vullum, P.E.; Ulvestad, A.; Kirkengen, M.; Mæhlen, J.P. Silicon-Carbon composite anodes from industrial battery grade silicon. Sci. Rep. 2019, 9, 14814. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, J.; Li, H.; Nuli, Y.; Wang, J. Electrolytes for advanced lithium ion batteries using silicon-based anodes. J. Mater. Chem. A 2019, 7, 9432–9446. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Q.; Zhang, H.; Cheng, J.; Li, Y.; Zeng, Z.; Zhang, S.; Xu, X.; Ji, F.; Li, D.; et al. The application road of silicon-based anode in lithium-ion batteries: From liquid electrolyte to solid-state electrolyte. Energy Storage Mater. 2023, 55, 244–263. [Google Scholar] [CrossRef]
- Cao, Z.; Zheng, X.; Qu, Q.; Huang, Y.; Zheng, H. Electrolyte Design Enabling a High-Safety and High-Performance Si Anode with a Tailored Electrode–Electrolyte Interphase. Adv. Mater. 2021, 33, 2103178. [Google Scholar] [CrossRef]
- Preman, A.N.; Lee, H.; Yoo, J.; Kim, I.T.; Saito, T.; Ahn, S.K. Progress of 3D network binders in silicon anodes for lithium ion batteries. J. Mater. Chem. A 2020, 8, 25548–25570. [Google Scholar] [CrossRef]
- Wang, L.; Yu, J.; Li, S.; Xi, F.; Ma, W.; Wei, K.; Lu, J.; Tong, Z.; Liu, B.; Luo, B. Recent advances in interface engineering of silicon anodes for enhanced lithium-ion battery performance. Energy Storage Mater. 2024, 66, 103243. [Google Scholar] [CrossRef]
- Wang, L.; Lu, J.J.; Li, S.Y.; Xi, F.S.; Tong, Z.Q.; Chen, X.H.; Wei, K.X.; Ma, W.H. Controllable Interface Engineering for the Preparation of High Rate Silicon Anode. Adv. Funct. Mater. 2024, 34, 2403574. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Wang, J.Z.; Zhong, C.; Chou, S.L.; Liu, H.K. Flexible free-standing graphene-silicon composite film for lithium-ion batteries. Electrochem. Commun. 2010, 12, 1467–1470. [Google Scholar] [CrossRef]
- Ramar, A.; Sanjana, K.; Wang, F.M. Carbides and Nitrides: Adv. Mater. for engineering the electrochemistry of silicon anodes for high energy density Lithium-Ion battery. Chem. Eng. J. 2024, 491, 151921. [Google Scholar] [CrossRef]
- Shen, X.; Tian, Z.; Fan, R.; Shao, L.; Zhang, D.; Cao, G.; Kou, L.; Bai, Y. Research progress on silicon/carbon composite anode materials for lithium-ion battery. J. Energy Chem. 2018, 27, 1067–1090. [Google Scholar] [CrossRef]
- Cong, Q.; Zhu, X.; Ban, Z.; Li, J.; Cai, Z.; Pei, L. Silicon Carbide-based Materials from Rice Husk. Curr. Nanosci. 2025, 21, 585–595. [Google Scholar] [CrossRef]
- Shi, B.; Ramones, A.I.; Liu, Y.; Wang, H.; Li, Y.; Pischinger, S.; Andert, J. A review of silicon carbide MOSFETs in electrified vehicles: Application, challenges, and future development. IET Power Electron. 2023, 16, 2103–2120. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Aberoumand, S.; Dao, D.V. Advances in Si and SiC Materials for High-Performance Supercapacitors toward Integrated Energy Storage Systems. Small 2021, 17, 2101775. [Google Scholar] [CrossRef]
- Wu, C.Y.; Kuo, P.H.; Duh, J.G. Reviving of silicon waste with N-doped carbon core-shell structure prepared by vapor deposition polymerization of polypyrrole applied in lithium-ion battery. Surf. Coat. Technol. 2021, 421, 127418. [Google Scholar] [CrossRef]
- Hou, C.; Xie, H.; Qu, Y.; Tian, H.; Jiang, J.; Lu, H.; Yang, S.; Ma, Y. Rigid-flexible double coating silicon oxide composed of pitch pyrolytic carbon and polyvinyl alcohol/polyethyleneimine/carbon nanotubes as high-performance anode material for lithium-ion battery. Adv. Compos. Hybrid Mater. 2023, 6, 143. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material–fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energ. Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Shao, H.B.; Wang, J.M. A review of the carbon coating of the silicon anode in high-performance lithium-ion batteries. New Carbon Mater. 2024, 39, 896–917. [Google Scholar] [CrossRef]
- Zhao, X.; Lehto, V.P. Challenges and prospects of nanosized silicon anodes in lithium-ion batteries. Nanotechnology 2021, 32, 042002. [Google Scholar] [CrossRef]
- Feng, K.; Li, M.; Liu, W.; Kashkooli, A.G.; Xiao, X.; Cai, M.; Chen, Z. Silicon-Based Anodes for Lithium-Ion Batteries: From Fundamentals to Practical Applications. Small 2018, 14, 1702737. [Google Scholar] [CrossRef] [PubMed]
- Galashev, A.Y. Prospects for using silicene as an anode for lithium-ion batteries. A review. J. Energy Storage 2024, 93, 112281. [Google Scholar] [CrossRef]
- Fan, X.; Deng, D.; Li, Y.; Wu, Q.H. Recent Progress in SiC Nanostructures as Anode Materials for Lithium- Ion Batteries. Curr. Mater. Sci. 2023, 16, 18–29. [Google Scholar] [CrossRef]
- Park, I.; Lee, H.; Chae, O.B. Synthesis Methods of Si/C Composite Materials for Lithium-Ion Batteries. Batteries 2024, 10, 381. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Wu, T.; Huang, G. Roundly exploring the synthesis, structural design, performance modification, and practical applications of silicon-carbon composite anodes for lithium-ion batteries. J. Energy Storage 2024, 101, 113794. [Google Scholar] [CrossRef]
- Sadhu, K.K.; Prajapati, P.K.; Mandal, N.; Sahoo, R.R. Tribo-mechanical evaluation of SiC-reinforced wear-resistant aluminium composite fabricated through powder metallurgy. Mater. Today Commun. 2025, 42, 111184. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Song, J.; Zhang, Y.; Shi, Q.; Wang, J.; Tian, F.; Yuan, S.; Su, Z.; Zhou, C.; et al. Functionalization-assistant ball milling towards Si/graphene anodes in high performance Li-ion batteries. Carbon 2021, 181, 300–309. [Google Scholar] [CrossRef]
- Wang, D.; Gao, M.; Pan, H.; Wang, J.; Liu, Y. High performance amorphous-Si@SiOx/C composite anode materials for Li-ion batteries derived from ball-milling and in situ carbonization. J. Power Sources 2014, 256, 190–199. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, W.; Wang, D.; Wang, J.; Wang, C.; Xiong, Z.; Chen, F.R.; Dong, H.; Xu, B.; Yan, X. Facile preparation of silicon/carbon composite with porous architecture for advanced lithium-ion battery anode. J. Electroanal. Chem. 2023, 937, 117427. [Google Scholar] [CrossRef]
- Sun, W.; Hu, R.; Zhang, M.; Liu, J.; Zhu, M. Binding of carbon coated nano-silicon in graphene sheets by wet ball-milling and pyrolysis as high performance anodes for lithium-ion batteries. J. Power Sources 2016, 318, 113–120. [Google Scholar] [CrossRef]
- Overhoff, G.M.; Nölle, R.; Siozios, V.; Winter, M.; Placke, T. A Thorough Analysis of Two Different Pre-Lithiation Techniques for Silicon/Carbon Negative Electrodes in Lithium Ion Batteries. Batter. Supercaps 2021, 4, 1163–1174. [Google Scholar] [CrossRef]
- Monje, I.E.; Sanchez-Ramirez, N.; Santagneli, S.H.; Camargo, P.H.; Bélanger, D.; Schougaard, S.B.; Torresi, R.M. In situ-formed nitrogen-doped carbon/silicon-based materials as negative electrodes for lithium-ion batteries. J. Electroanal. Chem. 2021, 901, 115732. [Google Scholar] [CrossRef]
- Han, S.W.; Jung, D.W.; Jeong, J.H.; Oh, E.S. Effect of pyrolysis temperature on carbon obtained from green tea biomass for superior lithium ion battery anodes. Chem. Eng. J. 2014, 254, 597–604. [Google Scholar] [CrossRef]
- Chou, C.Y.; Kuo, J.R.; Yen, S.C. Silicon-Based Composite Negative Electrode Prepared from Recycled Silicon-Slicing Slurries and Lignin/Lignocellulose for Li-Ion Cells. ACS Sustain. Chem. Eng. 2018, 6, 4759–4766. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.G.; Zhang, H.L.; Zhao, Z.G.; Li, F.; Liu, C.; Cheng, H.M. Composite anode material of silicon/graphite/carbon nanotubes for Li-ion batteries. Electrochim. Acta 2006, 51, 4994–5000. [Google Scholar] [CrossRef]
- Han, J.; Zhao, C.; Wang, L.; Song, J.; Yang, D.; Tian, Q. Simple ball milling-assisted method enabling N-doped carbon embedded Si for high performance lithium-ion battery anode. J. Alloys Compd. 2023, 966, 171668. [Google Scholar] [CrossRef]
- Koraag, P.Y.E.; Firdaus, A.M.; Hawari, N.H.; Refino, A.D.; Dempwolf, W.; Iskandar, F.; Peiner, E.; Wasisto, H.S.; Sumboja, A. Covalently Bonded Ball-Milled Silicon/CNT Nanocomposite as Lithium-Ion Battery Anode Material. Batteries 2022, 8, 165. [Google Scholar] [CrossRef]
- Gu, P.; Cai, R.; Zhou, Y.; Shao, Z. Si/C composite lithium-ion battery anodes synthesized from coarse silicon and citric acid through combined ball milling and thermal pyrolysis. Electrochim. Acta 2010, 55, 3876–3883. [Google Scholar] [CrossRef]
- Cabello, M.; Gucciardi, E.; Herrán, A.; Carriazo, D.; Villaverde, A.; Rojo, T. Towards a High-Power Si@graphite Anode for Lithium Ion Batteries through a Wet Ball Milling Process. Molecules 2020, 25, 2494. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xiao, P.; Chu, F.; Chen, W.; Li, Y.; Wu, F. Constructing Si/6H-SiC Heterostructure As a High-Performance Anode for Boosting Lithium-Ion Storage. ACS Appl. Mater. Interfaces 2024, 16, 30088–30096. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Lan, J.L.; Xue, M.; Yu, Y.; Yang, X. Two-step ball-milling synthesis of a Si/SiOx/C composite electrode for lithium ion batteries with excellent long-term cycling stability. RSC Adv. 2017, 7, 36697–36704. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Shao, J.; Shen, Z.; Chen, R.; Zhang, X.; He, X.; Song, Y.; Xing, X. Pyrolytic carbon-coated silicon/carbon nanofiber composite anodes for high-performance lithium-ion batteries. J. Power Sources 2015, 298, 130–137. [Google Scholar] [CrossRef]
- Su, M.; Wang, Z.; Guo, H.; Li, X.; Huang, S.; Gan, L. Silicon, flake graphite and phenolic resin-pyrolyzed carbon based Si/C composites as anode material for lithium-ion batteries. Adv. Powder Technol. 2013, 24, 921–925. [Google Scholar] [CrossRef]
- Wang, M.S.; Fan, L.Z. Silicon/carbon nanocomposite pyrolyzed from phenolic resin as anode materials for lithium-ion batteries. J. Power Sources 2013, 244, 570–574. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, Y.; Guo, H.; Wang, Z.; Li, X.; Zhou, Y.; Wang, J. Compact structured silicon/carbon composites as high-performance anodes for lithium ion batteries. Ionics 2018, 24, 3405–3411. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Zhou, Y.; Guo, H.; Li, X. Synthesis of porous Si/graphite/carbon nanotubes@C composites as a practical high-capacity anode for lithium-ion batteries. Mater. Lett. 2017, 199, 84–87. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Yang, J.; Ma, X.; Lu, S. Scalable synthesis of a novel structured graphite/silicon/pyrolyzed-carbon composite as anode material for high-performance lithium-ion batteries. J. Alloys Compd. 2016, 688, 1072–1079. [Google Scholar] [CrossRef]
- Hu, J.; Wang, M.; Meyer, A.W.; Huang, X.; Cheng, Y.T. Oxidative Pyrolysis of Si/Polyacrylonitrile Composites as an Unconventional Approach to Fabricate High Performance Lithium Ion Battery Negative Electrodes. J. Electrochem. Soc. 2019, 166, A3716. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, Z.; Lai, L.; Okubo, M.; Song, Y.; Zhou, Y.; Liu, X.; Huang, W. Sub-micron silicon/pyrolyzed carbon@natural graphite self-assembly composite anode material for lithium-ion batteries. Chem. Eng. J. 2017, 313, 187–196. [Google Scholar] [CrossRef]
- Xie, Q.; Qu, S.; Zhao, P. A facile fabrication of micro/nano-sized silicon/carbon composite with a honeycomb structure as high-stability anodes for lithium-ion batteries. J. Electroanal. Chem. 2021, 884, 115074. [Google Scholar] [CrossRef]
- Lee, S.S.; Nam, K.H.; Jung, H.; Park, C.M. Si-based composite interconnected by multiple matrices for high-performance Li-ion battery anodes. Chem. Eng. J. 2020, 381, 122619. [Google Scholar] [CrossRef]
- Huang, X.; Guo, R.; Lin, Y.; Cao, Y.; Wu, J. Compact homogeneous Si/SiC/C in-situ nanocomposite microspheres as anode materials for lithium-ion batteries. J. Energy Storage 2024, 87, 111374. [Google Scholar] [CrossRef]
- Chen, W.T.; Muruganantham, R.; Liu, W.R. Construction of 3D porous graphene aerogel wrapped silicon composite as anode materials for high-efficient lithium-ion storage. Surf. Coat. Technol. 2022, 434, 128147. [Google Scholar] [CrossRef]
- Muruganantham, R.; Yang, C.W.; Wang, H.J.; Huang, C.H.; Liu, W.R. Industrial Silicon-Wafer-Wastage-Derived Carbon-Enfolded Si/Si-C/C Nanocomposite Anode Material through Plasma-Assisted Discharge Process for Rechargeable Li-Ion Storage. Nanomaterials 2022, 12, 659. [Google Scholar] [CrossRef]
- Campbell, H.R.; Alsharif, F.M.; Marsac, P.J.; Lodder, R.A. The Development of a Novel Pharmaceutical Formulation of D-Tagatose for Spray-Drying. J. Pharm. Innov. 2022, 17, 194–206. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Guo, H.; Wang, Z.; Li, X.; Zhang, X.; Wu, F.; Yue, P. Preparation and characterization of flake graphite/silicon/carbon spherical composite as anode materials for lithium-ion batteries. J. Alloys Compd. 2012, 530, 30–35. [Google Scholar] [CrossRef]
- Wang, D.; Gao, M.; Pan, H.; Liu, Y.; Wang, J.; Li, S.; Ge, H. Enhanced cycle stability of micro-sized Si/C anode material with low carbon content fabricated via spray drying and in situ carbonization. J. Alloys Compd. 2014, 604, 130–136. [Google Scholar] [CrossRef]
- Paireau, C.; Jouanneau, S.; Ammar, M.R.; Simon, P.; Béguin, F.; Raymundo-Piñero, E. Si/C composites prepared by spray drying from cross-linked polyvinyl alcohol as Li-ion batteries anodes. Electrochim. Acta 2015, 174, 361–368. [Google Scholar] [CrossRef]
- Pan, Q.; Zuo, P.; Lou, S.; Mu, T.; Du, C.; Cheng, X.; Ma, Y.; Gao, Y.; Yin, G. Micro-sized spherical silicon@carbon@graphene prepared by spray drying as anode material for lithium-ion batteries. J. Alloys Compd. 2017, 723, 434–440. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Hou, X.; Fu, L.; Wang, S.; Hu, X.; Qin, H.; Wu, Y.; Ru, Q.; Liu, X.; et al. Mass-producible method for preparation of a carbon-coated graphite@plasma nano-silicon@carbon composite with enhanced performance as lithium ion battery anode. Electrochim. Acta 2017, 249, 113–121. [Google Scholar] [CrossRef]
- Su, M.; Liu, S.; Tao, L.; Tang, Y.; Dou, A.; Lv, J.; Liu, Y. Silicon@graphene composite prepared by spray–drying method as anode for lithium ion batteries. J. Electroanal. Chem. 2019, 844, 86–90. [Google Scholar] [CrossRef]
- Shih, J.Y.; Chen, Y.R.; James Li, Y.J.; Hung, T.F.; Hsu, L.F.; Tsai, Y.D.; Ramaraj, S.K.; Jose, R.; Karuppiah, C.; Yang, C.C. Suppressed Volume Change of a Spray-Dried 3D Spherical-like Si/Graphite Composite Anode for High-Rate and Long-Term Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2022, 10, 12706–12720. [Google Scholar] [CrossRef]
- Bai, Y.; Cao, X.; Tian, Z.; Yang, S.; Cao, G. A high-performance silicon/carbon composite as anode material for lithium ion batteries. Nano Express 2021, 2, 010021. [Google Scholar] [CrossRef]
- Zhao, H.; Tu, N.; Zhang, W.; Zhang, M.; Wang, J. Novel synthesis of Silicon/Carbon nanotubes microspheres as anode additives through chemical vapor deposition in fluidized bed reactors. Scr. Mater. 2021, 192, 49–54. [Google Scholar] [CrossRef]
- Wang, X.; Yushin, G. Chemical vapor deposition and atomic layer deposition for advanced lithium ion batteries and supercapacitors. Energy Environ. Sci. 2015, 8, 1889–1904. [Google Scholar] [CrossRef]
- Shi, Q.; Zhou, J.; Ullah, S.; Yang, X.; Tokarska, K.; Trzebicka, B.; Ta, H.Q.; Rümmeli, M.H. A review of recent developments in Si/C composite materials for Li-ion batteries. Energy Storage Mater. 2021, 34, 735–754. [Google Scholar] [CrossRef]
- Wang, W.; Kumta, P.N. Nanostructured Hybrid Silicon/Carbon Nanotube Heterostructures: Reversible High-Capacity Lithium-Ion Anodes. ACS Nano 2010, 4, 2233–2241. [Google Scholar] [CrossRef]
- Zhao, T.; She, S.; Ji, X.; Jin, W.; Dang, A.; Li, H.; Li, T.; Shang, S.; Zhou, Z. In-situ growth amorphous carbon nanotube on silicon particles as lithium-ion battery anode materials. J. Alloys Compd. 2017, 708, 500–507. [Google Scholar] [CrossRef]
- Fu, K.; Xue, L.; Yildiz, O.; Li, S.; Lee, H.; Li, Y.; Xu, G.; Zhou, L.; Bradford, P.D.; Zhang, X. Effect of CVD carbon coatings on Si@CNF composite as anode for lithium-ion batteries. Nano Energy 2013, 2, 976–986. [Google Scholar] [CrossRef]
- Yoshio, M.; Wang, H.; Fukuda, K.; Umeno, T.; Dimov, N.; Ogumi, Z. Carbon-Coated Si as a Lithium-Ion Battery Anode Material. J. Electrochem. Soc. 2002, 149, A1598. [Google Scholar] [CrossRef]
- Iwamura, S.; Nishihara, H.; Kyotani, T. Fast and reversible lithium storage in a wrinkled structure formed from Si nanoparticles during lithiation/delithiation cycling. J. Power Sources 2013, 222, 400–409. [Google Scholar] [CrossRef]
- Yu, J.; Yang, J.; Feng, X.; Jia, H.; Wang, J.; Lu, W. Uniform carbon coating on silicon nanoparticles by dynamic CVD process for electrochemical lithium storage. Ind. Eng. Chem. Res. 2014, 53, 12697–12704. [Google Scholar] [CrossRef]
- Liu, B.; Huang, P.; Xie, Z.; Huang, Q. Large-scale production of a silicon nanowire/graphite composites anode via the CVD method for high-performance lithium-ion batteries. Energy Fuels 2021, 35, 2758–2765. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Li, J.; Tian, S.; Fei, T.; Li, H. Effects of deposition temperature and time on HfC nanowires synthesized by CVD on SiC-coated C/C composites. Ceram. Int. 2016, 42, 5623–5628. [Google Scholar] [CrossRef]
- Shukrullah, S.; Mohamed, N.; Shaharun, M.; Saheed, M.; Irshad, M. Effect of CVD process temperature on activation energy and structural growth of MWCNTs. Metall. Mater. Trans. A 2016, 47, 1413–1424. [Google Scholar] [CrossRef]
- Jin, H.C.; Sun, Q.; Wang, J.T.; Ma, C.; Ling, L.C.; Qiao, W.M. Preparation and electrochemical properties of novel silicon-carbon composite anode materials with a core-shell structure. New Carbon Mater. 2021, 36, 390–400. [Google Scholar] [CrossRef]
- Lim, S.Y. Amorphous-silicon nanoshell on artificial graphite composite as the anode for lithium-ion battery. Solid State Sci. 2019, 93, 24–30. [Google Scholar] [CrossRef]
- Kim, N.; Chae, S.; Ma, J.; Ko, M.; Cho, J. Fast-charging high-energy lithium-ion batteries via implantation of amorphous silicon nanolayer in edge-plane activated graphite anodes. Nat. Commun. 2017, 8, 812. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Chae, S.; Ma, J.; Kim, N.; Lee, H.W.; Cui, Y.; Cho, J. Scalable synthesis of silicon-nanolayer-embedded graphite for high-energy lithium-ion batteries. Nat. Energy 2016, 1, 16113. [Google Scholar] [CrossRef]
- Chen, H.; Hua, Y.; Luo, N.; He, X.; Li, Y.; Zhang, Y.; Chen, W.; Huang, S. Lithiation Abilities of SiC bulks and surfaces: A first-principles study. J. Phys. Chem. C 2020, 124, 7031–7038. [Google Scholar] [CrossRef]
- Capitani, G.C.; Di Pierro, S.; Tempesta, G. The 6H-SiC structure model: Further refinement from SCXRD data from a terrestrial moissanite. Am. Mineral. 2007, 92, 403–407. [Google Scholar] [CrossRef]
- Stein, A.; Wang, Z.; Fierke, M.A. Functionalization of porous carbon materials with designed pore architecture. Adv. Mater. 2009, 21, 265–293. [Google Scholar] [CrossRef]
- Deshmukh, S.; Pawar, K.; Koli, V.; Pachfule, P. Emerging graphitic carbon nitride-based nanobiomaterials for biological applications. ACS Appl. Bio Mater. 2023, 6, 1339–1367. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Oshiyama, A. Electron doping through lithium intercalation to interstitial channels in tetrahedrally bonded SiC. J. Appl. Phys. 2015, 118, 175704. [Google Scholar] [CrossRef]
- Shi, W.; Lu, C.; Yang, S.; Deng, J. Study on adsorption and diffusion of lithium on nitrogen doped silicon carbide nanotubes by density functional theory. Comput. Theor. Chem. 2017, 1115, 169–174. [Google Scholar] [CrossRef]
- Zyubin, A.S.; Zyubina, T.S.; Dobrovol’skii, Y.A.; Volokhov, V.M. Quantum-chemical modeling of lithiation of a silicon–silicon carbide composite. Russ. J. Inorg. Chem. 2016, 61, 1423–1429. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Qie, Y.; Yu, J.; Sun, Q. A new porous metallic silicon dicarbide for highly efficient Li-ion battery anode identified by targeted structure search. Carbon 2018, 140, 680–687. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Li, H.; Tan, T.; Wang, X.; Zhao, Y. Carbon nanotubes/SiC prepared by catalytic chemical vapor deposition as scaffold for improved lithium-sulfur batteries. J. Nanopart. Res. 2019, 21, 113. [Google Scholar] [CrossRef]
- Majid, A.; Fatima, A.; Khan, S.U.D.; Khan, S. Layered silicon carbide: A novel anode material for lithium ion batteries. New J. Chem. 2021, 45, 19105–19117. [Google Scholar] [CrossRef]
- Fatima, A.; Majid, A.; Haider, S.; Akhtar, M.S.; Alkhedher, M. First principles study of layered silicon carbide as anode in lithium ion battery. Int. J. Quantum Chem. 2022, 122, e26895. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, J.; Cai, Q. SiC2 siligraphene as a promising anchoring material for lithium-sulfur batteries: A computational study. Appl. Surf. Sci. 2018, 440, 889–896. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Seh, Z.W.; Fu, Z.; Zhang, R.; Cui, Y. Understanding the anchoring effect of two-dimensional layered materials for lithium–sulfur batteries. Nano Lett. 2015, 15, 3780–3786. [Google Scholar] [CrossRef] [PubMed]
- Qie, Y.; Wang, S.; Sun, Q. Three dimensional metallic porous SiC4 allotropes: Stability and battery applications. Nano Energy 2019, 63, 103862. [Google Scholar] [CrossRef]
- Liu, Q.; Fu, C.; Xiao, B.; Li, Y.; Cheng, J.; Liu, Z.; Yang, X.; Xu, X.; Cui, H.; Li, Q. Graphitic SiC: A potential anode material for Na-ion battery with extremely high storage capacity. Int. J. Quantum Chem. 2021, 121, e26608. [Google Scholar] [CrossRef]
- Abbas, G.; Johansson, G.; Alay-e-Abbas, S.M.; Shi, Y.; Larsson, J.A. Quasi three-dimensional tetragonal SiC polymorphs as efficient anodes for sodium-ion batteries. ACS Appl. Energy Mater. 2023, 6, 8976–8988. [Google Scholar] [CrossRef]
- Jin, Y.; Yuan, A.; Jin, X.; Tan, L.; Tang, H.; Sun, R. High Performance Nitrogen-Doped Si/C as the Anode Material of Lithium-Ion Batteries. Russ. J. Electrochem. 2022, 58, 136–142. [Google Scholar]
- Chen, B.H.; Chuang, S.I.; Liu, W.R.; Duh, J.G. A revival of waste: Atmospheric pressure nitrogen plasma jet enhanced jumbo silicon/silicon carbide composite in lithium ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 28166–28176. [Google Scholar] [CrossRef]
- Tang, X.; Sun, Z.; Liang, J.; Zhao, J.; Cheng, H.M.; Zhuo, S.; Li, F. A high tenacity electrode by assembly of a soft sorbent and a hard skeleton for lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 22459–22464. [Google Scholar] [CrossRef]
- Huang, X.D.; Zhang, F.; Gan, X.F.; Huang, Q.A.; Yang, J.Z.; Lai, P.T.; Tang, W.M. Electrochemical characteristics of amorphous silicon carbide film as a lithium-ion battery anode. RSC Adv. 2018, 8, 5189–5196. [Google Scholar] [CrossRef]
- Lipson, A.L.; Chattopadhyay, S.; Karmel, H.J.; Fister, T.T.; Emery, J.D.; Dravid, V.P.; Thackeray, M.M.; Fenter, P.A.; Bedzyk, M.J.; Hersam, M.C. Enhanced lithiation of doped 6H silicon carbide (0001) via high temperature vacuum growth of epitaxial graphene. J. Phys. Chem. C 2012, 116, 20949–20957. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H. Nanocrystalline silicon carbide thin film electrodes for lithium-ion batteries. Solid State Ion. 2014, 263, 23–26. [Google Scholar] [CrossRef]
- Tesfaye, A.T.; Mashtalir, O.; Naguib, M.; Barsoum, M.W.; Gogotsi, Y.; Djenizian, T. Anodized Ti3SiC2 as an anode material for Li-ion microbatteries. ACS Appl. Mater. Interfaces 2016, 8, 16670–16676. [Google Scholar] [CrossRef]
- Tesfaye, A.T.; Gogotsi, Y.; Djenizian, T. Tailoring the morphological properties of anodized Ti3SiC2 for better power density of Li-ion microbatteries. Electrochem. Commun. 2017, 81, 29–33. [Google Scholar] [CrossRef]
- Yi, X.; He, W.; Zhang, X.; Yang, G.; Wang, Y. Hollow mesoporous MnO/MnS/SiC/S-CN composites prepared from soda pulping black liquor for lithium-ion batteries. J. Alloys Compd. 2018, 735, 1306–1313. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, J.; Zhou, X.; Jia, M.; Ren, Y.; Jiang, M.; Hu, T.; Yang, J. Optimized Porous Si/SiC Composite Spheres as High-Performance Anode Material for Lithium-Ion Batteries. ChemElectroChem 2019, 6, 450–455. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, W.; Kang, W.; Ye, Y.; Yuan, Y.; Qiu, Z.; Wang, C.; Zhang, X.; Ke, Y.; Tang, Y. Silicon-nanoparticle-based composites for advanced lithium-ion battery anodes. Nanoscale 2020, 12, 7461–7484. [Google Scholar] [CrossRef]
- Nangir, M.; Massoudi, A.; Tayebifard, S.A. Investigation of the lithium-ion depletion in the silicon-silicon carbide anode/electrolyte interface in lithium-ion battery via electrochemical impedance spectroscopy. J. Electroanal. Chem. 2020, 873, 114385. [Google Scholar] [CrossRef]
- Zhou, W.; Lian, Q.; Huang, X.; Ding, W.; Jiang, C.; Zou, Z.; Su, X. Introducing SiC/C dual-interface on porous silicon anode by a conventional exothermic displacement reaction for improved cycle performance. J. Power Sources 2021, 508, 230326. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Zhang, R.; Huang, P.; Liu, M.; Xie, Z. The preparation of carbon-coated Si nanofiber deposited on carbon nanofiber/natural microcrystalline graphite as the anode for lithium-ion batteries. Ionics 2022, 28, 657–668. [Google Scholar] [CrossRef]
- Tian, F.; Pang, Z.; Wang, F.; Nie, W.; Zhang, X.; Chen, S.; Xu, J.; Li, G.; Chen, C.; Xu, Q.; et al. Tailored Electrosynthesis of Highly Porous Ti3SiC2-Derived Carbon Anodes with Excellent Lithium Storage Properties. ACS Sustain. Chem. Eng. 2023, 11, 17320–17330. [Google Scholar] [CrossRef]
- Chen, Z.; Jia, H.; Hoeppener, S.; Friebe, C.; Wang, J.; Chanteux, G.; Xie, D.; Lu, Y.; Vlad, A.; Schubert, U.S.; et al. Hollow porous silicon nanospheres with 3D SiC@ C coating as high-performance anodes. Mater. Des. 2023, 226, 111624. [Google Scholar] [CrossRef]
- Leonova, A.M.; Bashirov, O.A.; Leonova, N.M.; Lebedev, A.S.; Trofimov, A.A.; Suzdaltsev, A.V. Synthesis of C/SiC Mixtures for Composite Anodes of Lithium-Ion Power Sources. Appl. Sci. 2023, 13, 901. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Li, Z.; Yang, C.; Zhong, W.; Li, W.; Zhang, C.; Yang, N.; Zhang, Q.; Li, X. Toward high-performance capacitive potassium-ion storage: A superior anode material from silicon carbide-derived carbon with a well-developed pore structure. Adv. Funct. Mater. 2020, 30, 2004348. [Google Scholar] [CrossRef]
- Li, J.; Chen, H.; Jia, L.; Chen, Y. Nanoencapsulation of silicon carbide-doped hydrated salt for safe and high-efficient battery thermal management. ACS Appl. Energy Mater. 2022, 5, 11581–11590. [Google Scholar] [CrossRef]
- Naveenkumar, P.; Maniyazagan, M.; Yang, H.W.; Kang, W.S.; Kim, S.J. Nitrogen-doped graphene/silicon-oxycarbide nanosphere as composite anode for high-performance lithium-ion batteries. J. Energy Storage 2023, 59, 106572. [Google Scholar] [CrossRef]
- Garamoun, A.; Schubert, M.B.; Werner, J.H. Thin-Film Silicon for Flexible Metal–Air Batteries. ChemSusChem 2014, 7, 3272–3274. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Pollard, T.P.; Borodin, O.; Mars, J.E.; Tsao, Y.; Lukatskaya, M.R.; Kasse, R.M.; Schroeder, M.A.; Xu, K.; Toney, M.F.; et al. Toward unraveling the origin of lithium fluoride in the solid electrolyte interphase. Chem. Mater. 2021, 33, 7315–7336. [Google Scholar] [CrossRef]
- Boonprachai, R.; Autthawong, T.; Namsar, O.; Yu, A.; Sarakonsri, T.; Chimupala, Y. Natural porous si-c composited with nitrogen-doped graphene as anode materials in lithium-ion batteries. J. Sci. Technol. 2021, 28, 030074. [Google Scholar]
- Sun, C.; Xu, X.; Gui, C.; Chen, F.; Wang, Y.; Chen, S.; Shao, M.; Wang, J. High-Quality Epitaxial N Doped Graphene on SiC with Tunable Interfacial Interactions via Electron/Ion Bridges for Stable Lithium-Ion Storage. Nano-Micro Lett. 2023, 15, 202. [Google Scholar] [CrossRef]
- Nandan, R.; Takamori, N.; Higashimine, K.; Badam, R.; Matsumi, N. Confronting the Issues Associated with the Practical Implementation of Zinc Blende-type SiC Anodes for Efficient and Reversible Storage of Lithium Ions. ACS Appl. Energy Mater. 2024, 7, 2088–2100. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhang, D.T.; Chen, H.; Li, M.P.; Tian, C.Y.; Liu, M.C. Anticorrosion and Hydrophobic Surface Protection Inducing Dendrite-Free Zn Anode for Aqueous Zn-Ion Batteries. Energy Technol. 2024, 12, 2300885. [Google Scholar] [CrossRef]
- Nangir, M.; Massoudi, A. Power and energy performance of porous silicon carbide anode in lithium-metal battery. Mater. Today Proc. 2021, 42, 1534–1537. [Google Scholar] [CrossRef]
- Wang, X.; Dong, H.; Ma, Q.; Chen, Y.; Zhao, X.; Chen, L. Nickel nanoparticle decorated silicon carbide as a thermal filler in thermal conductive aramid nanofiber-based composite films for heat dissipation applications. RSC Adv. 2023, 13, 20984–20993. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.; Vijayaraghavan, R.K.; McNally, P.J.; Sofianos, M.V. The effect of SiC additives on the cycling performance of CaCO3 when used as a high-temperature thermal battery. J. Alloys Compd. 2023, 934, 167844. [Google Scholar] [CrossRef]
- Qu, F.; Graczyk-Zajac, M.; Vrankovic, D.; Chai, N.; Yu, Z.; Riedel, R. Effect of morphology of C-rich silicon carbonitride ceramic on electrochemical properties of sulfur cathode for Li-S battery. Electrochim. Acta 2021, 384, 138265. [Google Scholar] [CrossRef]
- Qu, F.; Yu, Z.; Widenmeyer, M.; Tian, C.; Yan, R.; Tian, H.; Kempf, A.; De Carolis, D.M.; Hofmann, J.P.; Weidenkaff, A.; et al. In-situ formed porous silicon carbonitride/boron nitride composites to boost cathode performance in lithium sulfur batteries. J. Alloys Compd. 2024, 984, 174021. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, L.; Hu, Y.; Yang, X.; Liu, Y.; Li, J.; Tang, M.; Chen, R.; Ma, F.; Wang, J.; et al. Modulating porous silicon-carbon anode stability: Carbon/silicon carbide semipermeable layer mitigates silicon-fluorine reaction and enhances lithium-ion transport. J. Colloid Interface Sci. 2024, 674, 643–652. [Google Scholar] [CrossRef]
- Aslam, J.; Waseem, M.A.; Lu, X.M.; Sun, W.; Wang, Y. From biochar to battery electrodes: A pathway to green lithium and sodium-ion battery systems. Chem. Eng. J. 2025, 505, 159556. [Google Scholar] [CrossRef]
- Shi, J.; Du, M.; Chen, Y.; Li, Q.; Xu, D.; Zhang, G.; Pang, H. MXene functionalized cathodes, anodes, and separators for batteries. Chem. Eng. J. 2025, 507, 160809. [Google Scholar] [CrossRef]
- Xiong, Z.; Sun, H.; Su, W.; Jin, W.; Liu, H.; Huang, Y.; Liu, H. The Full-Graphdiyne-Based Fast-Charging Aqueous Zinc Ion Battery Toward Synergistically Boosted Capacity and Long Lifespan. Small 2025, 21, 2502191. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, C.; Adenusi, H.; Wu, Y.; Passerini, S. Development of PFAS-Free Locally Concentrated Ionic Liquid Electrolytes for High-Energy Lithium and Aluminum Metal Batteries. Acc. Chem. Res. 2025, 58, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lu, X.; Biney, B.W.; Li, J.; Yan, Y.; Chen, K. In-situ synthesis of heteroatom-doped hard carbon for sodium-ion batteries: Dual benefits for green energy and environment. J. Colloid Interface Sci. 2025, 677, 312–322. [Google Scholar] [CrossRef]
- Jannath, K.A.; Saputra, H.A. A Review on Recent Developments in Transition Metal and Heteroatom-Doped Carbon Catalysts for Oxygen Reduction Reaction. Electrochem. Sci. Adv. 2025, 5, e202400033. [Google Scholar] [CrossRef]
- Geng, H.; Zou, X.; Min, Y.; Bu, Y.; Lu, Q. Advances and Challenges in Perovskite Oxide Design for High-Performance Zinc–Air Batteries: Integrating Experimental Strategies and Machine Learning. Adv. Funct. Mater. 2025, 2500657. [Google Scholar] [CrossRef]
- Hu, E.; Choo, H.H.; Zhang, W.; Sumboja, A.; Anggraningrum, I.T.; Syahrial, A.Z.; Zhu, Q.; Xu, J.; Loh, X.J.; Pan, H.; et al. Integrating Machine Learning and Characterization in Battery Research: Toward Cognitive Digital Twins with Physics and Knowledge. Adv. Funct. Mater. 2025, 35, 2422601. [Google Scholar] [CrossRef]
- Mahendiran, D.; Murugan, P.; Spencer, M.J. Defective SiC Nanotubes: Promising Anode Materials for Alkali Metal Ion Batteries. ACS Appl. Energy Mater. 2025, 8, 2003–2010. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, T.; Lu, Y.; Zhang, W.; Li, Z. In Situ Synthesis of MoO3 by Surface Oxidation of Mo2C (MXene) for Stable Near-Surface Reactions in Aqueous Aluminum-Ion Battery. Angew. Chem. Int. Ed. 2025, 64, e202416032. [Google Scholar] [CrossRef]

| Method | Yield/Purity | Cost | Scalability | Particle Size | Method Performance | Battery-Relevant Performance |
|---|---|---|---|---|---|---|
| Pyrolysis | High/Moderate | Low | High | Controlled (10–100 nm) | Moderate |
|
| Ball Milling | Moderate/Low | Moderate | High | Non-controlled (Broad agglomerated NPs) | Low to Moderate |
|
| Spray Drying | Moderate/Moderate | High | Low | Controlled (depend on nozzle and drying control) | Moderate |
|
| Chemical Vapor Deposition (CVD) | Very Low/High | Very High | Low | Excellent control (<50 nm films or shells) | High (crystalline, phase-pure) |
|
| Composite | Modification | Advantages | Disadvantages | Electrochemical Performance | Ref. |
|---|---|---|---|---|---|
| C@void/Si-g | Carbon coating via pitch + NaCl template | Improved durability; Si isolation from electrolyte | Template removal; multistep | LIB: 1082.7 mAh/g after 200 cycles at 0.2 C | [60] |
| Si30@C40/G30 | Graphene + sucrose carbon coating | High capacity and cycle stability | High irreversible capacity | LIB: 1259 mAh/g at 0.2 A/g | [61] |
| Si/C-CNFs-20 | Carbon-coated nanofibers via electrospinning + pyrolysis | Buffers Si volume; good rate capability | Moderate capacity vs. others | LIB: 1215.2 mAh/g after 50 cycles at 5 A/g | [73] |
| SiC-Graphite-180 | Graphite + pitch pyrolysis | Enhanced conductivity and structure | Irregular morphology | LIB: 602.4 mAh/g; 93.4% retention after 50 cycles | [76] |
| Si/PC-30 | N-doped porous C via PAN + CaCO3 template | Porous shell, high conductivity | Complex synthesis | LIB: 830 mAh/g after 200 cycles | [81] |
| Si-Cu3Si-CNT/G-C | Cu3Si + CNT + graphite + C-coating | Excellent rate and cycle performance | Complex architecture | LIB: 1237 mAh/g; ~1000 mAh/g at 1C | [82] |
| Si@C@RGO | Dual carbon coating (rGO + C layer) | High capacity; reduced Si expansion | Synthesis complexity | LIB: 2124 mAh/g; 94.9% retention after 100 cycles at 200 mA/g | [91] |
| Si/graphene (1:4) | Graphene coating via spray drying + annealing | Improved rate capability | Less effective at high graphene % | LIB: 1298.1 mAh/g; better rate-capability at 1000 mA/g | [93] |
| Si/MWNTs | CVD of Si on MWNTs | Enhanced contact; high conductivity | Requires metal catalyst | LIB: 2049 mAh/g with only 19.7% capacity loss | [99] |
| Si@C-2 (CVD) | Uniform C-layer via rotational CVD | Stable at high rates; uniform coating | Longer CVD time | LIB: 1600 mAh/g (70 cycles); 750 mAh/g at 5 A/g | [104] |
| NG@SiC | N-doped graphene–SiC heterostructure | High rate capability and durability | Graphene prep needed | LIB: 1197.5 mAh/g (200 cycles); 447.8 mAh/g after 1000 cycles at 10 A/g | [151] |
| B-ASiCNR (DFT) | B-doping of armchair SiC ribbon | High Li affinity; strong bonding | Theoretical model only | LIB: 836 mAh/g (theoretical) | [115] |
| pSi/SiC spheres | Porous Si + SiC via magnesiothermic reduction | Excellent long-term durability; low resistance | Multistep synthesis | LIB: 1022 mAh/g (400 cycles at 2 A/g); 420 mAh/g after 2000 cycles at 5 A/g | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, S.A.; Mobarak, N.N.; Khudayberdieva, A.; Doghmane, M.; Chettibi, S.; Eid, K. Silicon Carbide (SiC) and Silicon/Carbon (Si/C) Composites for High-Performance Rechargeable Metal-Ion Batteries. Int. J. Mol. Sci. 2025, 26, 7757. https://doi.org/10.3390/ijms26167757
Mahmood SA, Mobarak NN, Khudayberdieva A, Doghmane M, Chettibi S, Eid K. Silicon Carbide (SiC) and Silicon/Carbon (Si/C) Composites for High-Performance Rechargeable Metal-Ion Batteries. International Journal of Molecular Sciences. 2025; 26(16):7757. https://doi.org/10.3390/ijms26167757
Chicago/Turabian StyleMahmood, Sara Adnan, Nadhratun Naiim Mobarak, Arofat Khudayberdieva, Malika Doghmane, Sabah Chettibi, and Kamel Eid. 2025. "Silicon Carbide (SiC) and Silicon/Carbon (Si/C) Composites for High-Performance Rechargeable Metal-Ion Batteries" International Journal of Molecular Sciences 26, no. 16: 7757. https://doi.org/10.3390/ijms26167757
APA StyleMahmood, S. A., Mobarak, N. N., Khudayberdieva, A., Doghmane, M., Chettibi, S., & Eid, K. (2025). Silicon Carbide (SiC) and Silicon/Carbon (Si/C) Composites for High-Performance Rechargeable Metal-Ion Batteries. International Journal of Molecular Sciences, 26(16), 7757. https://doi.org/10.3390/ijms26167757







