Refining Prognostic Factors in Adult-Onset Multiple Sclerosis: A Narrative Review of Current Insights
Abstract
1. Introduction
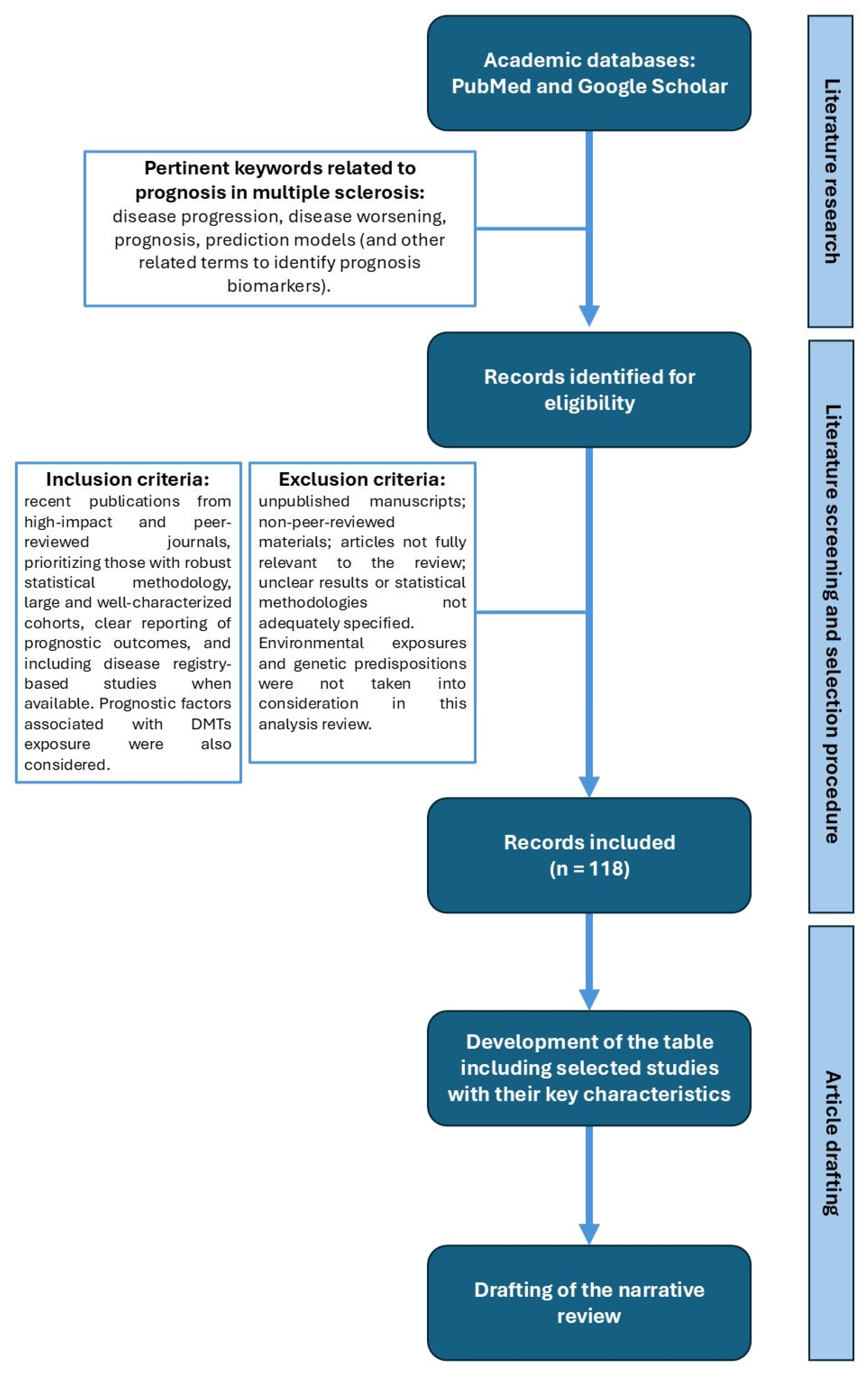
2. Methods
2.1. Aims and Research Planning
2.2. Study Selection
2.3. Role of the Funding Source
3. Demographic and Clinical Prognostic Factors
4. Radiological Predictors
5. Fluid Biomarkers
6. Therapies and Prognosis
7. Other Biomarkers with a Prognostic Value
8. The Strategic Role of Prognostic Algorithms in Clinical Decision-Making and Research
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aHR | adjusted Hazard Ratio |
| APRIL | A proliferation-inducing ligand |
| AUC | Area Under the Curve |
| BAFF | B-cell activating factor |
| CAL | chronic active lesions |
| CIS | clinical isolated syndrome |
| CSF | cerebrospinal fluid |
| CXCL | chemokine ligand |
| DMTs | disease-modifying therapies |
| EDSS | Expanded Disability Status Scale |
| EIT | early intensive therapy |
| ESC | escalation strategy |
| Gd | gadolinium |
| mGCIPL | macular ganglion cell and inner plexiform layers |
| GM | grey matter |
| HAMP | hepcidin antimicrobial peptide |
| HMOX1 | heme oxygenase 1 |
| HE DMTs | high-efficacy DMTs |
| IL | interleukin |
| KFLC | kappa free light chain |
| MAGNIMS | magnetic resonance imaging in MS |
| MMP-2 | matrix metalloproteinase-2 |
| ME DMTs | moderate-efficacy DMTs |
| MRI | magnetic resonance imaging |
| mRNA | messenger RNA |
| mtDNA | mitochondrial DNA |
| NEDA | no evidence of disease activity |
| NfL | neurofilament light chains |
| OCBs | oligoclonal bands |
| OND | optic nerve diameter |
| PRL | paramagnetic rim lesions |
| pRNFL | peripapillary retinal nerve fiber layer |
| PIRA | progression independent of relapse activity |
| PPMS | primary progressive multiple sclerosis |
| RAW | relapse-related worsening |
| RRMS | relapsing–remitting multiple sclerosis |
| SPMS | secondary progressive multiple sclerosis |
| SC | spinal cord |
| SELs | slowly expanding lesions |
| TNF | tumor necrosis factor |
| TWEAK | TNF-related weak inducer of apoptosis |
| TSPO | 18-kDa translocator protein |
| WM | white matter |
References
- Calabrese, M.; Preziosa, P.; Scalfari, A.; Colato, E.; Marastoni, D.; Absinta, M.; Battaglini, M.; De Stefano, N.; Di Filippo, M.; Hametner, S.; et al. Determinants and biomarkers of progression independent of relapses in multiple sclerosis. Ann. Neurol. 2024, 96, 1–20. [Google Scholar] [CrossRef]
- Jakimovski, D.; Bittner, S.; Zivadinov, R.; Morrow, S.A.; Benedict, R.H.; Zipp, F.; Weinstock-Guttman, B. Multiple sclerosis. Lancet 2024, 403, 183–202. [Google Scholar] [CrossRef]
- Guerra, T.; Iaffaldano, P.A. Window into New Insights on Progression Independent of Relapse Activity in Multiple Sclerosis: Role of Therapies and Current Perspective. Int. J. Mol. Sci. 2025, 26, 884. [Google Scholar] [CrossRef]
- Rocca, M.A.; Preziosa, P.; Barkhof, F.; Brownlee, W.; Calabrese, M.; De Stefano, N.; Granziera, C.; Ropele, S.; Toosy, A.T.; Vidal-Jordana, À.; et al. Current and future role of MRI in the diagnosis and prognosis of multiple sclerosis. Lancet Reg. Health Eur. 2024, 44, 100978. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Amato, M.P.; Avolio, C.; Gallo, P.; Gasperini, C.; Inglese, M.; Marfia, G.A.; Patti, F. Towards a biological view of multiple sclerosis from early subtle to clinical progression: An expert opinion. J. Neurol. 2025, 272, 179. [Google Scholar] [CrossRef] [PubMed]
- Dalla Costa, G.; Leocani, L.; Rodegher, M.; Chiveri, L.; Gradassi, A.; Comi, G. An overview on disease modifying and symptomatic drug treatments for multiple sclerosis. Expert Rev. Clin. Pharmacol. 2024, 17, 901–921. [Google Scholar] [CrossRef] [PubMed]
- Portaccio, E.; Magyari, M.; Havrdova, E.K.; Ruet, A.; Brochet, B.; Scalfari, A.; Di Filippo, M.; Tur, C.; Montalban, X.; Amato, M.P. Multiple sclerosis: Emerging epidemiological trends and redefining the clinical course. Lancet Reg. Health Eur. 2024, 44, 100977. [Google Scholar] [CrossRef] [PubMed]
- Scalfari, A.; Traboulsee, A.; Oh, J.; Airas, L.; Bittner, S.; Calabrese, M.; Garcia Dominguez, J.M.; Granziera, C.; Greenberg, B.; Hellwig, K.; et al. Smouldering-Associated Worsening in Multiple Sclerosis: An International Consensus Statement on Definition, Biology, Clinical Implications, and Future Directions. Ann. Neurol. 2024, 96, 826–845. [Google Scholar] [CrossRef]
- Lublin, F.D.; Häring, D.A.; Ganjgahi, H.; Ocampo, A.; Hatami, F.; Čuklina, J.; Aarden, P.; Dahlke, F.; Arnold, D.L.; Wiendl, H.; et al. How patients with multiple sclerosis acquire disability. Brain 2022, 145, 3147–3161. [Google Scholar] [CrossRef]
- Huang, Y. Predictive factors for the progression of multiple sclerosis: A meta-analysis. Neurol. Sci. 2025, 46, 3765–3773. [Google Scholar] [CrossRef]
- Comi, G.; Dalla Costa, G.; Stankoff, B.; Hartung, H.P.; Soelberg Sørensen, P.; Vermersch, P.; Leocani, L. Assessing disease progression and treatment response in progressive multiple sclerosis. Nat. Rev. Neurol. 2024, 20, 573–586. [Google Scholar] [CrossRef]
- Espiritu, A.; Oh, J. Prognostic Factors in Multiple Sclerosis. Prediction of Highly Active Disease and Rapid Disability Accumulation Is Crucial to Optimizing Clinical Outcomes over Time. Available online: https://practicalneurology.com/diseases-diagnoses/ms-immune-disorders/prognostic-factors-in-multiple-sclerosis/31874/ (accessed on 9 June 2025).
- Zeng, X.; Zhang, Y.; Kwong, J.S.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid. Based Med. 2015, 8, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Kalincik, T.; Vivek, V.; Jokubaitis, V.; Lechner-Scott, J.; Trojano, M.; Izquierdo, G.; Lugaresi, A.; Grand’maison, F.; Hupperts, R.; Oreja-Guevara, C.; et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain 2013, 136, 3609–3617. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, L.; Ruggieri, S.; Haggiag, S.; Tortorella, C.; Pozzilli, C.; Gasperini, C. Prognostic Accuracy of NEDA-3 in Long-term Outcomes of Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1059. [Google Scholar] [CrossRef]
- Signori, A.; Schiavetti, I.; Gallo, F.; Sormani, M.P. Subgroups of multiple sclerosis patients with larger treatment benefits: A meta-analysis of randomized trials. Eur. J. Neurol. 2015, 22, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Thränhardt, P.; Veselaj, A.; Friedli, C.; Wagner, F.; Marti, S.; Diem, L.; Hammer, H.; Radojewski, P.; Wiest, R.; Chan, A.; et al. Sex differences in multiple sclerosis relapse presentation and outcome: A retrospective, monocentric study of 134 relapse events. Ther. Adv. Neurol. Disord. 2024, 17, 17562864241237853. [Google Scholar] [CrossRef]
- Briggs, F.B.S.; Thompson, N.R.; Conway, D.S. Prognostic factors of disability in relapsing remitting multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 30, 9–16. [Google Scholar] [CrossRef]
- Kister, I.; Spelman, T.; Patti, F.; Duquette, P.; Trojano, M.; Izquierdo, G.; Lugaresi, A.; Grammond, P.; Sola, P.; Ferraro, D.; et al. Predictors of relapse and disability progression in MS patients who discontinue disease-modifying therapy. J. Neurol. Sci. 2018, 391, 72–76. [Google Scholar] [CrossRef]
- Tedeholm, H.; Skoog, B.; Lisovskaja, V.; Runmarker, B.; Nerman, O.; Andersen, O. The outcome spectrum of multiple sclerosis: Disability, mortality, and a cluster of predictors from onset. J. Neurol. 2015, 262, 1148–1163. [Google Scholar] [CrossRef]
- Bose, G.; Healy, B.C.; Barro, C.; Glanz, B.I.; Lokhande, H.A.; Polgar-Turcsanyi, M.; Guttmann, C.R.; Bakshi, R.; Weiner, H.L.; Chitnis, T. Younger age at multiple sclerosis onset is associated with worse outcomes at age 50. J. Neurol. Neurosurg. Psychiatry, 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Iaffaldano, P.; Lucisano, G.; Patti, F.; Brescia Morra, V.; De Luca, G.; Lugaresi, A.; Zaffaroni, M.; Inglese, M.; Salemi, G.; Cocco, E.; et al. Transition to secondary progression in relapsing-onset multiple sclerosis: Definitions and risk factors. Mult. Scler. 2021, 27, 430–438. [Google Scholar] [CrossRef]
- Tur, C.; Carbonell-Mirabent, P.; Cobo-Calvo, Á.; Otero-Romero, S.; Arrambide, G.; Midaglia, L.; Castilló, J.; Vidal-Jordana, Á.; Rodríguez-Acevedo, B.; Zabalza, A.; et al. Association of Early Progression Independent of Relapse Activity with Long-term Disability After a First Demyelinating Event in Multiple Sclerosis. JAMA Neurol. 2023, 80, 151–160. [Google Scholar] [CrossRef]
- Guillemin, F.; Baumann, C.; Epstein, J.; Kerschen, P.; Garot, T.; Mathey, G.; Debouverie, M.; LORSEP Group. Older Age at Multiple Sclerosis Onset Is an Independent Factor of Poor Prognosis: A Population-Based Cohort Study. Neuroepidemiology 2017, 48, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Vural, A.; Derle, E.; Sayat-Gürel, G.; Karabudak, R.; Tuncer, A. Predictors of progression in primary progressive multiple sclerosis in a large Turkish cohort. Mult. Scler. Relat. Disord. 2020, 38, 101520. [Google Scholar] [CrossRef]
- Iaffaldano, P.; Portaccio, E.; Lucisano, G.; Simone, M.; Manni, A.; Guerra, T.; Paolicelli, D.; Betti, M.; De Meo, E.; Pastò, L.; et al. Multiple Sclerosis Progression and Relapse Activity in Children. JAMA Neurol. 2024, 81, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.; Xu, T.; Yin, H.; Zhu, Y.; Peng, B.; Cui, L. Prediction of long-term disability in Chinese patients with multiple sclerosis: A prospective cohort study. Mult. Scler. Relat. Disord. 2020, 46, 102461. [Google Scholar] [CrossRef]
- Brown, F.S.; Glasmacher, S.A.; Kearns, P.K.A.; MacDougall, N.; Hunt, D.; Connick, P.; Chandran, S. Systematic review of prediction models in relapsing remitting multiple sclerosis. PLoS ONE 2020, 15, e0233575. [Google Scholar] [CrossRef]
- Preziosa, P.; Pagani, E.; Meani, A.; Moiola, L.; Rodegher, M.; Filippi, M.; Rocca, M.A. Slowly Expanding Lesions Predict 9-Year Multiple Sclerosis Disease Progression. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1139. [Google Scholar] [CrossRef] [PubMed]
- Traboulsee, A.L.; Cornelisse, P.; Sandberg-Wollheim, M.; Uitdehaag, B.M.; Kappos, L.; Jongen, P.J.; Constantinescu, C.S.; di Cantogno, E.V.; Li, D.K. Prognostic factors for long-term outcomes in relapsing-remitting multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2016, 2, 2055217316666406. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, C.; Prosperini, L.; Rovira, À.; Tintoré, M.; Sastre-Garriga, J.; Tortorella, C.; Haggiag, S.; Galgani, S.; Capra, R.; Pozzilli, C.; et al. Scoring the 10-year risk of ambulatory disability in multiple sclerosis: The RoAD score. Eur. J. Neurol. 2021, 28, 2533–2542. [Google Scholar] [CrossRef]
- Brownlee, W.J.; Altmann, D.R.; Prados, F.; Miszkiel, K.A.; Eshaghi, A.; Gandini Wheeler-Kingshott, C.A.M.; Barkhof, F.; Ciccarelli, O. Early imaging predictors of long-term outcomes in relapse-onset multiple sclerosis. Brain 2019, 142, 2276–2287. [Google Scholar] [CrossRef]
- Tintore, M.; Rovira, À.; Río, J.; Otero-Romero, S.; Arrambide, G.; Tur, C.; Comabella, M.; Nos, C.; Arévalo, M.J.; Negrotto, L.; et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain 2015, 138, 1863–1874. [Google Scholar] [CrossRef]
- Cavaco, S.; Ferreira, I.; Moreira, I.; Santos, E.; Samões, R.; Sousa, A.P.; Pinheiro, J.; Teixeira-Pinto, A.; Martins da Silva, A. Cognitive dysfunction and mortality in multiple sclerosis: Long-term retrospective review. Mult. Scler. 2022, 28, 1382–1391. [Google Scholar] [CrossRef]
- Bsteh, G.; Ehling, R.; Lutterotti, A.; Hegen, H.; Di Pauli, F.; Auer, M.; Deisenhammer, F.; Reindl, M.; Berger, T. Long Term Clinical Prognostic Factors in Relapsing-Remitting Multiple Sclerosis: Insights from a 10-Year Observational Study. PLoS ONE 2016, 11, e0158978. [Google Scholar] [CrossRef] [PubMed]
- Haider, L.; Prados, F.; Chung, K.; Goodkin, O.; Kanber, B.; Sudre, C.; Yiannakas, M.; Samson, R.S.; Mangesius, S.; Thompson, A.J.; et al. Cortical involvement determines impairment 30 years after a clinically isolated syndrome. Brain 2021, 144, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Treaba, C.A.; Granberg, T.E.; Sormani, M.P.; Herranz, E.; Ouellette, R.A.; Louapre, C.; Sloane, J.A.; Kinkel, R.P.; Mainero, C. Longitudinal Characterization of Cortical Lesion Development and Evolution in Multiple Sclerosis with 7.0-T MRI. Radiology 2019, 291, 740–749. [Google Scholar] [CrossRef]
- Farina, G.; Magliozzi, R.; Pitteri, M.; Reynolds, R.; Rossi, S.; Gajofatto, A.; Benedetti, M.D.; Facchiano, F.; Monaco, S.; Calabrese, M. Increased cortical lesion load and intrathecal inflammation is associated with oligoclonal bands in multiple sclerosis patients: A combined CSF and MRI study. J. Neuroinflammation 2017, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Magliozzi, R.; Scalfari, A.; Pisani, A.I.; Ziccardi, S.; Marastoni, D.; Pizzini, F.B.; Bajrami, A.; Tamanti, A.; Guandalini, M.; Bonomi, S.; et al. The CSF Profile Linked to Cortical Damage Predicts Multiple Sclerosis Activity. Ann. Neurol. 2020, 88, 562–573. [Google Scholar] [CrossRef]
- Gębka-Kępińska, B.; Adamczyk, B.; Gębka, D.; Czuba, Z.; Szczygieł, J.; Adamczyk-Sowa, M. Cytokine Profiling in Cerebrospinal Fluid of Patients with Newly Diagnosed Relapsing-Remitting Multiple Sclerosis (RRMS): Associations between Inflammatory Biomarkers and Disease Activity. Int. J. Mol. Sci. 2024, 25, 7399. [Google Scholar] [CrossRef]
- Fonderico, M.; Portaccio, E.; Razzolini, L.; Pastò, L.; Bellinvia, A.; Addazio, I.; Betti, M.; Aprea, M.G.; Ballerini, C.; Biagioli, T.; et al. Cerebrospinal Fluid IgM and Oligoclonal IgG Bands in Multiple Sclerosis: A Meta-Analysis of Prevalence and Prognosis. Brain Sci. 2021, 11, 1444. [Google Scholar] [CrossRef]
- Scalfari, A.; Neuhaus, A.; Daumer, M.; Deluca, G.C.; Muraro, P.A.; Ebers, G.C. Early relapses, onset of progression, and late outcome in multiple sclerosis. JAMA Neurol. 2013, 70, 214–222. [Google Scholar] [CrossRef]
- Amato, M.P.; Fonderico, M.; Portaccio, E.; Pastò, L.; Razzolini, L.; Prestipino, E.; Bellinvia, A.; Tudisco, L.; Fratangelo, R.; Comi, G.; et al. Disease-modifying drugs can reduce disability progression in relapsing multiple sclerosis. Brain 2020, 143, 3013–3024. [Google Scholar] [CrossRef]
- Jokubaitis, V.G.; Spelman, T.; Kalincik, T.; Lorscheider, J.; Havrdova, E.; Horakova, D.; Duquette, P.; Girard, M.; Prat, A.; Izquierdo, G.; et al. Predictors of long-term disability accrual in relapse-onset multiple sclerosis. Ann. Neurol. 2016, 80, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Kalincik, T.; Diouf, I.; Sharmin, S.; Malpas, C.; Spelman, T.; Horakova, D.; Havrdova, E.K.; Trojano, M.; Izquierdo, G.; Lugaresi, A.; et al. Effect of Disease-Modifying Therapy on Disability in Relapsing-Remitting Multiple Sclerosis Over 15 Years. Neurology 2021, 96, e783–e797. [Google Scholar] [CrossRef]
- Invernizzi, P.; Bertolasi, L.; Bianchi, M.R.; Turatti, M.; Gajofatto, A.; Benedetti, M.D. Prognostic value of multimodal evoked potentials in multiple sclerosis: The EP score. J. Neurol. 2011, 258, 1933–1939. [Google Scholar] [CrossRef]
- Vecchio, D.; Barbero, P.; Galli, G.; Virgilio, E.; Naldi, P.; Comi, C.; Cantello, R. Prognostic Role of Visual Evoked Potentials in Non-Neuritic Eyes at Multiple Sclerosis Diagnosis. J. Clin. Med. 2023, 12, 2382. [Google Scholar] [CrossRef]
- Giffroy, X.; Maes, N.; Albert, A.; Maquet, P.; Crielaard, J.-M.; Dive, D. Multimodal evoked potentials for functional quantification and prognosis in multiple sclerosis. BMC Neurol. 2016, 16, 83. [Google Scholar] [CrossRef]
- Barro, C.; Healy, B.C.; Liu, Y.; Saxena, S.; Paul, A.; Polgar-Turcsanyi, M.; Guttmann, C.R.G.; Bakshi, R.; Kropshofer, H.; Weiner, H.L.; et al. Serum GFAP and NfL Levels Differentiate Subsequent Progression and Disease Activity in Patients With Progressive Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2022, 10, e200052. [Google Scholar] [CrossRef] [PubMed]
- Kassubek, R.; Gorges, M.; Schocke, M.; Hagenston, V.A.M.; Huss, A.; Ludolph, A.C.; Kassubek, J.; Tumani, H. GFAP in early multiple sclerosis: A biomarker for inflammation. Neurosci. Lett. 2017, 657, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Ayrignac, X.; Aouinti, S.; Vincent, T.; Carra-Dallière, C.; Charif, M.; Duflos, C.; Hirtz, C.; Santos, A.D.; de Champfleur, N.M.; Labauge, P.; et al. Serum NfL and GFAP are weak predictors of long-term multiple sclerosis prognosis: A 6-year follow-up. Mult. Scler. Relat. Disord. 2024, 89, 105747. [Google Scholar] [CrossRef]
- Filippi, M.; Preziosa, P.; Copetti, M.; Riccitelli, G.; Horsfield, M.A.; Martinelli, V.; Comi, G.; Rocca, M.A. Gray matter damage predicts the accumulation of disability 13 years later in MS. Neurology 2013, 81, 1759–1767. [Google Scholar] [CrossRef]
- Eijlers, A.J.C.; van Geest, Q.; Dekker, I.; Steenwijk, M.D.; Meijer, K.A.; Hulst, H.E.; Barkhof, F.; Uitdehaag, B.M.J.; Schoonheim, M.M.; Geurts, J.J.G. Predicting cognitive decline in multiple sclerosis: A 5-year follow-up study. Brain 2018, 141, 2605–2618. [Google Scholar] [CrossRef]
- Storelli, L.; Azzimonti, M.; Gueye, M.; Vizzino, C.; Preziosa, P.; Tedeschi, G.; De Stefano, N.; Pantano, P.; Filippi, M.; Rocca, M.A. A Deep Learning Approach to Predicting Disease Progression in Multiple Sclerosis Using Magnetic Resonance Imaging. Investig. Radiol. 2022, 57, 423–432. [Google Scholar] [CrossRef]
- Jacobsen, C.; Hagemeier, J.; Myhr, K.-M.; Nyland, H.; Lode, K.; Bergsland, N.; Ramasamy, D.P.; Dalaker, T.O.; Larsen, J.P.; Farbu, E.; et al. Brain atrophy and disability progression in multiple sclerosis patients: A 10-year follow-up study. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1109–1115. [Google Scholar] [CrossRef]
- Yavas, I.; Kaya, E.; Ozdogar, A.T. Determining the Prognostic Characteristics of People with Multiple Sclerosis with Bowel and Bladder Dysfunction as the Initial Presentation: A Cohort Study. J. Mult. Scler. Res. 2022, 2, 62–67. [Google Scholar] [CrossRef]
- Damasceno, A.; Von Glehn, F.; Brandão, C.O.; Damasceno, B.P.; Cendes, F. Prognostic indicators for long-term disability in multiple sclerosis patients. J. Neurol. Sci. 2013, 324, 29–33. [Google Scholar] [CrossRef]
- Novotna, M.; Paz Soldán, M.M.; Abou Zeid, N.; Kale, N.; Tutuncu, M.; Crusan, D.J.; Atkinson, E.J.; Siva, A.; Keegan, B.M.; Pirko, I.; et al. Poor early relapse recovery affects onset of progressive disease course in multiple sclerosis. Neurology 2015, 85, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Pérez Sánchez, S.; Eichau Madueño, S.; Rus Hidalgo, M.; Domínguez Mayoral, A.M.; Vilches-Arenas, A.; Navarro Mascarell, G.; Izquierdo, G. Usefulness of optic nerve ultrasound to predict clinical progression in multiple sclerosis. Neurologia 2021, 36, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Koraysha, N.A.; Kishk, N.; Hassan, A.; Samy El Gendy, N.M.; Shehata, H.S.; Al-Azayem, S.A.; Kamal, Y.S. Evaluating optic nerve diameter as a possible biomarker for disability in patients with multiple sclerosis. Neuropsychiatr. Dis. Treat. 2019, 15, 2571–2578. [Google Scholar] [CrossRef]
- Lorefice, L.; Piras, C.; Sechi, V.; Barracciu, M.A.; Cocco, E.; Fenu, G. Spinal cord MRI activity in multiple sclerosis: Predictive value for relapses and impact on treatment decisions. J. Neurol. Sci. 2024, 462, 123057. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Ramagopalan, S.; Davis, A.; Giovannoni, G. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: A meta-analysis of prevalence, prognosis and effect of latitude. J. Neurol. Neurosurg. Psychiatry 2013, 84, 909–914. [Google Scholar] [CrossRef]
- Karrenbauer, V.D.; Bedri, S.K.; Hillert, J.; Manouchehrinia, A. Cerebrospinal fluid oligoclonal immunoglobulin gamma bands and long-term disability progression in multiple sclerosis: A retrospective cohort study. Sci. Rep. 2021, 11, 14987. [Google Scholar] [CrossRef]
- Ben Noon, G.; Vigiser, I.; Shiner, T.; Kolb, H.; Karni, A.; Regev, K. Reinforcing the evidence of oligoclonal bands as a prognostic factor in patients with Multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 56, 103220. [Google Scholar] [CrossRef]
- Scalfari, A.; Neuhaus, A.; Degenhardt, A.; Rice, G.P.; Muraro, P.A.; Daumer, M.; Ebers, G.C. The natural history of multiple sclerosis: A geographically based study 10: Relapses and long-term disability. Brain 2010, 133 Pt 7, 1914–1929. [Google Scholar] [CrossRef]
- Thebault, S.; Abdoli, M.; Fereshtehnejad, S.M.; Tessier, D.; Tabard-Cossa, V.; Freedman, M.S. Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis. Sci. Rep. 2020, 10, 10381. [Google Scholar] [CrossRef]
- Benkert, P.; Meier, S.; Schaedelin, S.; Manouchehrinia, A.; Yaldizli, Ö.; Maceski, A.; Oechtering, J.; Achtnichts, L.; Conen, D.; Derfuss, T.; et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: A retrospective modelling and validation study. Lancet Neurol. 2022, 21, 246–257. [Google Scholar] [CrossRef]
- Kuhle, J.; Plavina, T.; Barro, C.; Disanto, G.; Sangurdekar, D.; Singh, C.M.; de Moor, C.; Engle, B.; Kieseier, B.C.; Fisher, E.; et al. Neurofilament light levels are associated with long-term outcomes in multiple sclerosis. Mult. Scler. J. 2020, 26, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Uher, T.; Havrdova, E.K.; Benkert, P.; Bergsland, N.; Krasensky, J.; Srpova, B.; Dwyer, M.; Tyblova, M.; Meier, S.; Vaneckova, M.; et al. Measurement of neurofilaments improves stratification of future disease activity in early multiple sclerosis. Mult. Scler. J. 2021, 27, 2001–2013. [Google Scholar] [CrossRef]
- Gottwald, N.S.; Asseyer, S.; Chien, C.; Brasanac, J.; Nauman, A.T.; Rust, R.; Schmitz-Hübsch, T.; Strobl, J.B.; Ruprecht, K.; Paul, F.; et al. Impact of sex on clinical outcome in early Multiple Sclerosis. Mult. Scler. Relat. Disord. 2024, 88, 105749. [Google Scholar] [CrossRef] [PubMed]
- Kearney, H.; Rocca, M.A.; Valsasina, P.; Balk, L.; Sastre-Garriga, J.; Reinhardt, J.; Ruggieri, S.; Rovira, A.; Stippich, C.; Kappos, L.; et al. Magnetic resonance imaging correlates of physical disability in relapse onset multiple sclerosis of long disease duration. Mult. Scler. J. 2014, 20, 72–80. [Google Scholar] [CrossRef]
- Lauerer, M.; McGinnis, J.; Bussas, M.; El Husseini, M.; Pongratz, V.; Engl, C.; Wuschek, A.; Berthele, A.; Riederer, I.; Kirschke, J.S.; et al. Prognostic value of spinal cord lesion measures in early relapsing-remitting multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2023, 95, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Coret, F.; Bosca, I.; Landete, L.; Magraner, M.J.; Navarré, A.; León, J.L.; Casanova, B. Early diffuse demyelinating lesion in the cervical spinal cord predicts a worse prognosis in relapsing-remitting multiple sclerosis. Mult. Scler. J. 2010, 16, 935–941. [Google Scholar] [CrossRef]
- Iaffaldano, P.; Lucisano, G.; Butzkueven, H.; Hillert, J.; Hyde, R.; Koch-Henriksen, N.; Magyari, M.; Pellegrini, F.; Spelman, T.; Sørensen, P.S.; et al. Early treatment delays long-term disability accrual in RRMS: Results from the BMSD network. Mult. Scler. 2021, 27, 1543–1555. [Google Scholar] [CrossRef]
- He, A.; Merkel, B.; Brown, J.W.L.; Zhovits Ryerson, L.; Kister, I.; Malpas, C.B.; Sharmin, S.; Horakova, D.; Kubala Havrdova, E.; Spelman, T.; et al. Timing of high-efficacy therapy for multiple sclerosis: A retrospective observational cohort study. Lancet Neurol. 2020, 19, 307–316. [Google Scholar] [CrossRef]
- Chalmer, T.A.; Baggesen, L.M.; Nørgaard, M.; Koch-Henriksen, N.; Magyari, M.; Sorensen, P.S.; Danish Multiple Sclerosis Group. Early versus later treatment start in multiple sclerosis: A register-based cohort study. Eur. J. Neurol. 2018, 25, 1262-e110. [Google Scholar] [CrossRef]
- He, A.; Spelman, T.; Manouchehrinia, A.; Ciccarelli, O.; Hillert, J.; McKay, K. Association between early treatment of multiple sclerosis and patient-reported outcomes: A nationwide observational cohort study. J. Neurol. Neurosurg. Psychiatry 2023, 94, 284–289. [Google Scholar] [CrossRef]
- Iaffaldano, P.; Lucisano, G.; Caputo, F.; Paolicelli, D.; Patti, F.; Zaffaroni, M.; Brescia Morra, V.; Pozzilli, C.; De Luca, G.; Inglese, M.; et al. Long-term disability trajectories in relapsing multiple sclerosis patients treated with early intensive or escalation treatment strategies. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211019574. [Google Scholar] [CrossRef]
- Hrnciarova, T.; Drahota, J.; Spelman, T.; Hillert, J.; Lycke, J.; Kubala Havrdova, E.; Recmanova, E.; Adamkova, J.; Mares, J.; Libertinova, J.; et al. Does initial high efficacy therapy in multiple sclerosis surpass escalation treatment strategy? A comparison of patients with relapsing-remitting multiple sclerosis in the Czech and Swedish national multiple sclerosis registries. Mult. Scler. Relat. Disord. 2023, 76, 104803. [Google Scholar] [CrossRef] [PubMed]
- Harding, K.; Williams, O.; Willis, M.; Hrastelj, J.; Rimmer, A.; Joseph, F.; Tomassini, V.; Wardle, M.; Pickersgill, T.; Robertson, N.; et al. Clinical Outcomes of Escalation vs. Early Intensive Disease-Modifying Therapy in Patients with Multiple Sclerosis. JAMA Neurol. 2019, 76, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Buron, M.D.; Chalmer, T.A.; Sellebjerg, F.; Barzinji, I.; Danny, B.; Christensen, J.R.; Christensen, M.K.; Hansen, V.; Illes, Z.; Jensen, H.B.; et al. Initial high-efficacy disease-modifying therapy in multiple sclerosis: A nationwide cohort study. Neurology 2020, 95, e1041–e1051. [Google Scholar] [CrossRef]
- Degenhardt, A.; Ramagopalan, S.V.; Scalfari, A.; Ebers, G.C. Clinical prognostic factors in multiple sclerosis: A natural history review. Nat. Rev. Neurol. 2009, 5, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Simone, M.; Lucisano, G.; Guerra, T.; Paolicelli, D.; Rocca, M.A.; Brescia Morra, V.; Patti, F.; Annovazzi, P.; Gasperini, C.; De Luca, G.; et al. Disability trajectories by progression independent of relapse activity status differ in pediatric, adult and late-onset multiple sclerosis. J. Neurol. 2024, 271, 6782–6790. [Google Scholar] [CrossRef]
- Graves, J.S.; Krysko, K.M.; Hua, L.H.; Absinta, M.; Franklin, R.J.M.; Segal, B.M. Ageing and multiple sclerosis. Lancet Neurol. 2023, 22, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Leray, E.; Yaouanq, J.; Le Page, E.; Coustans, M.; Laplaud, D.; Oger, J.; Edan, G. Evidence for a two-stage disability progression in multiple sclerosis. Brain 2010, 133 Pt 7, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; Vukusic, S.; Adeleine, P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: An amnesic process. Brain 2003, 126 Pt 4, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Lebrun-Frenay, C.; Kantarci, O.; Siva, A.; Sormani, M.P.; Pelletier, D.; Okuda, D.T.; 10-year RISC study group on behalf of SFSEP, OFSEP. Radiologically isolated syndrome: 10-year risk estimate of a clinical event. Ann. Neurol. 2020, 88, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Kuhle, J.; Multanen, J.; Kremenchutzky, M.; Verdun di Cantogno, E.; Cornelisse, P.; Lehr, L.; Casset-Semanaz, F.; Issard, D.; Uitdehaag, B.M. Factors influencing long-term outcomes in relapsing-remitting multiple sclerosis: PRISMS-15. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1202–1207. [Google Scholar] [CrossRef]
- Dzau, W.; Sharmin, S.; Patti, F.; Izquierdo, G.; Eichau, S.; Prat, A.; Girard, M.; Duquette, P.; Onofrj, M.; Lugaresi, A.; et al. Risk of secondary progressive multiple sclerosis after early worsening of disability. J. Neurol. Neurosurg. Psychiatry 2023, 94, 984–991. [Google Scholar] [CrossRef]
- Moccia, M.; Lanzillo, R.; Palladino, R.; Chang, K.C.; Costabile, T.; Russo, C.; De Rosa, A.; Carotenuto, A.; Saccà, F.; Maniscalco, G.T.; et al. Cognitive impairment at diagnosis predicts 10-year multiple sclerosis progression. Mult. Scler. 2016, 22, 659–667. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef]
- Li, J.; Vas, N.; Amezcua, L.; Rotstein, D.L. Multiple Sclerosis in People of Diverse Racial and Ethnic Backgrounds: Presentation, Disease Course, and Interactions with Disease-Modifying Therapy. CNS drugs, 2025; Advance online publication. [Google Scholar] [CrossRef]
- Amezcua, L.; McCauley, J.L. Race and ethnicity on MS presentation and disease course. Mult. Scler. 2020, 26, 561–567. [Google Scholar] [CrossRef]
- Fisniku, L.K.; Brex, P.A.; Altmann, D.R.; Miszkiel, K.A.; Benton, C.E.; Lanyon, R.; Thompson, A.J.; Miller, D. Disability and T2 MRI lesions: A 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain 2008, 131 Pt 3, 808–817. [Google Scholar] [CrossRef]
- Bagnato, F.; Jeffries, N.; Richert, N.D.; Stone, R.D.; Ohayon, J.M.; McFarland, H.F.; Frank, J.A. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain 2003, 126(Pt8), 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Sahraian, M.A.; Radue, E.W.; Haller, S.; Kappos, L. Black holes in multiple sclerosis: Definition, evolution, and clinical correlations. Acta Neurol. Scand. 2010, 122, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, A.; Moassefi, M.; Barati, E.; Ali Sahraian, M.; Aghajani, F.; Fattahi, M.R. Correlation between the clinical disability and T1 hypointense lesions’ volume in cerebral magnetic resonance imaging of multiple sclerosis patients: A systematic review and meta-analysis. CNS Neurosci. Ther. 2021, 27, 1268–1280. [Google Scholar] [CrossRef]
- Denis, M.; Woillez, J.P.; Smirnov, V.M.; Drumez, E.; Lannoy, J.; Boucher, J.; Zedet, M.; Pruvo, J.P.; Labreuche, J.; Zephir, H.; et al. Optic Nerve Lesion Length at the Acute Phase of Optic Neuritis Is Predictive of Retinal Neuronal Loss. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1135. [Google Scholar] [CrossRef]
- Bagnato, F.; Sati, P.; Hemond, C.C.; Elliott, C.; Gauthier, S.A.; Harrison, D.M.; Mainero, C.; Oh, J.; Pitt, D.; Shinohara, R.T.; et al. Imaging chronic active lesions in multiple sclerosis: A consensus statement. Brain 2024, 147, 2913–2933. [Google Scholar] [CrossRef]
- Absinta, M.; Sati, P.; Masuzzo, F.; Nair, G.; Sethi, V.; Kolb, H.; Ohayon, J.; Wu, T.; Cortese, I.C.M.; Reich, D.S. Association of Chronic Active Multiple Sclerosis Lesions with Disability In Vivo. JAMA Neurol. 2019, 76, 1474–1483. [Google Scholar] [CrossRef]
- Okar, S.V.; Dieckhaus, H.; Beck, E.S.; Gaitán, M.I.; Norato, G.; Pham, D.L.; Absinta, M.; Cortese, I.C.; Fletcher, A.; Jacobson, S.; et al. Highly Sensitive 3-Tesla Real Inversion Recovery MRI Detects Leptomeningeal Contrast Enhancement in Chronic Active Multiple Sclerosis. Investig. Radiol. 2024, 59, 243–251. [Google Scholar] [CrossRef]
- Wenzel, N.; Wittayer, M.; Weber, C.E.; Platten, M.; Gass, A.; Eisele, P. Multiple sclerosis iron rim lesions are linked to impaired cervical spinal cord integrity using the T1/T2-weighted ratio. J. Neuroimaging 2023, 33, 240–248. [Google Scholar] [CrossRef]
- Calvi, A.; Carrasco, F.P.; Tur, C.; Chard, D.T.; Stutters, J.; De Angelis, F.; John, N.; Williams, T.; Doshi, A.; Samson, R.S.; et al. Association of Slowly Expanding Lesions on MRI with Disability in People with Secondary Progressive Multiple Sclerosis. Neurology 2022, 98, e1783–e1793. [Google Scholar] [CrossRef] [PubMed]
- Calvi, A.; Tur, C.; Chard, D.; Stutters, J.; Ciccarelli, O.; Cortese, R.; Battaglini, M.; Pietroboni, A.; De Riz, M.; Galimberti, D.; et al. Slowly expanding lesions relate to persisting black-holes and clinical outcomes in relapse-onset multiple sclerosis. Neuroimage Clin. 2022, 35, 103048. [Google Scholar] [CrossRef]
- Hoffmann, O.; Gold, R.; Meuth, S.G.; Linker, R.A.; Skripuletz, T.; Wiendl, H.; Wattjes, M.P. Prognostic relevance of MRI in early relapsing multiple sclerosis: Ready to guide treatment decision making? Ther. Adv. Neurol. Disord. 2024, 17, 17562864241229325. [Google Scholar] [CrossRef]
- Calvi, A.; Mendelsohn, Z.; Hamed, W.; Chard, D.; Tur, C.; Stutters, J.; MacManus, D.; Kanber, B.; Wheeler-Kingshott, C.A.M.G.; Barkhof, F.; et al. Treatment reduces the incidence of newly appearing multiple sclerosis lesions evolving into chronic active, slowly expanding lesions: A retrospective analysis. Eur. J. Neurol. 2024, 31, e16092. [Google Scholar] [CrossRef] [PubMed]
- Traboulsee, A.; Li, D.K.B.; Cascione, M.; Fang, J.; Dangond, F.; Miller, A. Predictive value of early magnetic resonance imaging measures is differentially affected by the dose of interferon beta-1a given subcutaneously three times a week: An exploratory analysis of the PRISMS study. BMC Neurol. 2018, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, L.; Gallo, V.; Petsas, N.; Borriello, G.; Pozzilli, C. One-year MRI scan predicts clinical response to interferon beta in multiple sclerosis. Eur. J. Neurol. 2009, 16, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Min, M.; Spelman, T.; Lugaresi, A.; Boz, C.; Spitaleri, D.; Pucci, E.; Grand’Maison, F.; Granella, F.; Izquierdo, G.; Butzkueven, H.; et al. Silent lesions on MRI imaging—Shifting goal posts for treatment decisions in multiple sclerosis. Mult. Scler. 2018, 24, 1569–1577. [Google Scholar] [CrossRef]
- Gajofatto, A.; Calabrese, M.; Benedetti, M.D.; Monaco, S. Clinical, MRI, and CSF markers of disability progression in multiple sclerosis. Dis. Markers 2013, 35, 687–699. [Google Scholar] [CrossRef]
- Ludwig, N.; Cucinelli, S.; Hametner, S.; Muckenthaler, M.U.; Schirmer, L. Iron scavenging and myeloid cell polarization. Trends Immunol. 2024, 45, 625–638. [Google Scholar] [CrossRef]
- Hofmann, A.; Krajnc, N.; Dal-Bianco, A.; Riedl, C.J.; Zrzavy, T.; Lerma-Martin, C.; Kasprian, G.; Weber, C.E.; Pezzini, F.; Leutmezer, F.; et al. Myeloid cell iron uptake pathways and paramagnetic rim formation in multiple sclerosis. Acta Neuropathol. 2023, 146, 707–724. [Google Scholar] [CrossRef]
- Zhan, J.; Kipp, M.; Han, W.; Kaddatz, H. Ectopic lymphoid follicles in progressive multiple sclerosis: From patients to animal models. Immunology 2021, 164, 450–466. [Google Scholar] [CrossRef]
- Magliozzi, R.; Howell, O.W.; Reeves, C.; Roncaroli, F.; Nicholas, R.; Serafini, B.; Aloisi, F.; Reynolds, R. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann. Neurol. 2010, 68, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Reali, C.; Magliozzi, R.; Roncaroli, F.; Nicholas, R.; Howell, O.W.; Reynolds, R. B cell rich meningeal inflammation associates with increased spinal cord pathology in multiple sclerosis. Brain Pathol. 2020, 30, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Lenhart, A.; Rosenwald, A.; Monoranu, C.M.; Berberich-Siebelt, F. Lymphoid aggregates in the CNS of progressive multiple sclerosis patients lack regulatory T cells. Front. Immunol. 2019, 10, 3090. [Google Scholar] [CrossRef]
- Kosa, P.; Barbour, C.; Varosanec, M.; Wichman, A.; Sandford, M.; Greenwood, M.; Bielekova, B. Molecular models of multiple sclerosis severity identify heterogeneity of pathogenic mechanisms. Nat. Commun. 2022, 13, 7670. [Google Scholar] [CrossRef]
- Di Filippo, M.; Gaetani, L.; Centonze, D.; Hegen, H.; Kuhle, J.; Teunissen, C.E.; Tintoré, M.; Villar, L.M.; Willemse, E.A.J.; Zetterberg, H.; et al. Fluid biomarkers in multiple sclerosis: From current to future applications. Lancet Reg. Health Eur. 2024, 44, 101009. [Google Scholar] [CrossRef]
- Marastoni, D.; Sicchieri, M.; Pizzini, F.B.; Scartezzini, A.; Virla, F.; Turano, E.; Anni, D.; Bertolazzo, M.; Ziccardi, S.; Camera, V.; et al. Multiple sclerosis diagnosis and its differential diagnosis in patients presenting with type four ‘mirror pattern’ CSF oligoclonal bands. J. Neurol. 2025, 272, 207. [Google Scholar] [CrossRef]
- Abdelhak, A.; Benkert, P.; Schaedelin, S.; Boscardin, W.J.; Cordano, C.; Oechtering, J.; Ananth, K.; Granziera, C.; Melie-Garcia, L.; Montes, S.C.; et al. Neurofilament light chain elevation and disability progression in multiple sclerosis. JAMA Neurol. 2023, 80, 1317. [Google Scholar] [CrossRef] [PubMed]
- Toscano, S.; Oteri, V.; Chisari, C.G.; Finocchiaro, C.; Lo Fermo, S.; Valentino, P.; Bertolotto, A.; Zappia, M.; Patti, F. Cerebrospinal fluid neurofilament light chains predict early disease activity in Multiple Sclerosis. Mult. Scler. Relat. Disord. 2023, 80, 105131. [Google Scholar] [CrossRef]
- Meier, S.; Willemse, E.A.J.; Schaedelin, S.; Oechtering, J.; Lorscheider, J.; Melie-Garcia, L.; Cagol, A.; Barakovic, M.; Galbusera, R.; Subramaniam, S.; et al. Serum glial fibrillary acidic protein compared with neurofilament light chain as a biomarker for disease progression in multiple sclerosis. JAMA Neurol. 2023, 80, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.H.; Gelfand, J.M.; Thebault, S.; Bennett, J.L.; von Büdingen, H.C.; Cameron, B.; Carruthers, R.; Edwards, K.; Fallis, R.; Gerstein, R.; et al. Emerging Cerebrospinal Fluid Biomarkers of Disease Activity and Progression in Multiple Sclerosis. JAMA Neurol. 2024, 81, 373–383. [Google Scholar] [CrossRef]
- Ziccardi, S.; Tamanti, A.; Ruggieri, C.; Guandalini, M.; Marastoni, D.; Camera, V.; Montibeller, L.; Mazziotti, V.; Rossi, S.; Calderone, M.; et al. CSF Parvalbumin Levels at Multiple Sclerosis Diagnosis Predict Future Worse Cognition, Physical Disability, Fatigue, and Gray Matter Damage. Neurol. Neuroimmunol. Neuroinflamm. 2024, 11, e200301. [Google Scholar] [CrossRef] [PubMed]
- Hegen, H.; Walde, J.; Berek, K.; Arrambide, G.; Gnanapavan, S.; Kaplan, B.; Khalil, M.; Saadeh, R.; Teunissen, C.; Tumani, H.; et al. Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. 2023, 29, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Rosenstein, I.; Axelsson, M.; Novakova, L.; Malmeström, C.; Blennow, K.; Zetterberg, H.; Lycke, J. Intrathecal kappa free light chain synthesis is associated with worse prognosis in relapsing-remitting multiple sclerosis. J. Neurol. 2023, 270, 4800–4811. [Google Scholar] [CrossRef]
- Hegen, H.; Berek, K.; Bsteh, G.; Auer, M.; Altmann, P.; Di Pauli, F.; Grams, A.; Milosavljevic, D.; Ponleitner, M.; Poskaite, P.; et al. Kappa free light chain and neurofilament light independently predict early multiple sclerosis disease activity-a cohort study. eBioMedicine 2023, 91, 104573. [Google Scholar] [CrossRef]
- Berek, K.; Bsteh, G.; Auer, M.; Di Pauli, F.; Grams, A.; Milosavljevic, D.; Poskaite, P.; Schnabl, C.; Wurth, S.; Zinganell, A.; et al. Kappa-Free Light Chains in CSF Predict Early Multiple Sclerosis Disease Activity. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1005. [Google Scholar] [CrossRef]
- Huss, A.; Otto, M.; Senel, M.; Ludolph, A.C.; Abdelhak, A.; Tumani, H. A Score Based on NfL and Glial Markers May Differentiate Between Relapsing-Remitting and Progressive MS Course. Front. Neurol. 2020, 11, 608. [Google Scholar] [CrossRef]
- Magliozzi, R.; Howell, O.W.; Nicholas, R.; Cruciani, C.; Castellaro, M.; Romualdi, C.; Rossi, S.; Pitteri, M.; Benedetti, M.D.; Gajofatto, A.; et al. Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann. Neurol. 2018, 83, 739–755. [Google Scholar] [CrossRef]
- Leurs, C.E.; Podlesniy, P.; Trullas, R.; Balk, L.; Steenwijk, M.D.; Malekzadeh, A.; Piehl, F.; Uitdehaag, B.M.; Killestein, J.; van Horssen, J.; et al. Cerebrospinal fluid mtDNA concentration is elevated in multiple sclerosis disease and responds to treatment. Mult. Scler. 2018, 24, 472–480. [Google Scholar] [CrossRef]
- Albanese, M.; Zagaglia, S.; Landi, D.; Boffa, L.; Nicoletti, C.G.; Marciani, M.G.; Mandolesi, G.; Marfia, G.A.; Buttari, F.; Mori, F.; et al. Cerebrospinal fluid lactate is associated with multiple sclerosis disease progression. J. Neuroinflammation 2016, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Amato, M.P.; Centonze, D.; Gallo, P.; Gasperini, C.; Inglese, M.; Patti, F.; Pozzilli, C.; Preziosa, P.; Trojano, M. Early use of high-efficacy disease modifying therapies makes the difference in people with multiple sclerosis: An expert opinion. J. Neurol. 2022, 269, 5382–5394. [Google Scholar] [CrossRef]
- Puthenparampil, M.; Gaggiola, M.; Ponzano, M.; Zanotelli, G.; Miscioscia, A.; Nosadini, M.; Di Paola, A.; Sartori, S.; Perini, P.; Rinaldi, F.; et al. High NEDA and No PIRA in Natalizumab-Treated Patients with Pediatric-Onset Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2024, 11, e200303. [Google Scholar] [CrossRef]
- Chisari, C.G.; Aguglia, U.; Amato, M.P.; Bergamaschi, R.; Bertolotto, A.; Bonavita, S.; Morra, V.B.; Cavalla, P.; Cocco, E.; Conte, A.; et al. Long-term effectiveness of natalizumab in secondary progressive multiple sclerosis: A propensity-matched study. Neurotherapeutics 2024, 21, e00363. [Google Scholar] [CrossRef]
- Montobbio, N.; Cordioli, C.; Signori, A.; Bovis, F.; Capra, R.; Sormani, M.P. Relapse-Associated and Relapse-Independent Contribution to Overall Expanded Disability Status Scale Progression in Multiple Sclerosis Patients Diagnosed in Different Eras. Ann. Neurol. 2024, 91, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Paul, F.; Calabresi, P.A.; Barkhof, F.; Green, A.J.; Kardon, R.; Sastre-Garriga, J.; Schippling, S.; Vermersch, P.; Saidha, S.; Gerendas, B.S.; et al. Optical coherence tomography in multiple sclerosis: A 3-year prospective multicenter study. Ann. Clin. Transl. Neurol. 2021, 8, 2235–2251. [Google Scholar] [CrossRef] [PubMed]
- Albano, V.; Dammacco, R.; Manni, A.; Sisto, D.; Iaffaldano, A.; Mavilio, A.; Alessio, G.; Trojano, M.; Paolicelli, D. Macular ganglion cell-inner plexiform layer defect patterns in multiple sclerosis patients without optic neuritis: A Spectral-Domain-Optical Coherence Tomography Cross-Sectional, Case-Control, Pilot Study. Eur. J. Ophthalmol. 2023, 33, 546–555. [Google Scholar] [CrossRef]
- Swinnen, S.; De Wit, D.; Van Cleemput, L.; Cassiman, C.; Dubois, B. Optical coherence tomography as a prognostic tool for disability progression in MS: A systematic review. J. Neurol. 2023, 270, 1178–1186. [Google Scholar] [CrossRef]
- Pistor, M.; Hammer, H.; Salmen, A.; Hoepner, R.; Friedli, C. Application of the “risk of ambulatory disability” (RoAD) score in a “real-world” single-center multiple sclerosis cohort. CNS Neurosci. Ther. 2022, 28, 792–795. [Google Scholar] [CrossRef]
- Freedman, M.S.; Devonshire, V.; Duquette, P.; Giacomini, P.S.; Giuliani, F.; Levin, M.C.; Montalban, X.; Morrow, S.A.; Oh, J.; Rotstein, D.; et al. Treatment Optimization in Multiple Sclerosis: Canadian MS Working Group Recommendations. Can. J. Neurol. Sci. 2020, 47, 437–455. [Google Scholar] [CrossRef] [PubMed]
- Sormani, M.P.; Rio, J.; Tintorè, M.; Signori, A.; Li, D.; Cornelisse, P.; Stubinski, B.; Stromillo, M.l.; Montalban, X.; De Stefano, N. Scoring treatment response in patients with relapsing multiple sclerosis. Mult. Scler. 2013, 19, 605–612. [Google Scholar] [CrossRef]
- Jamroz-Wiśniewska, A.; Zajdel, R.; Słowik, A.; Marona, M.; Wnuk, M.; Adamczyk-Sowa, M.; Adamczyk, B.; Lasek-Bal, A.; Puz, P.; Stęposz, A.; et al. Modified Rio Score with Platform Therapy Predicts Treatment Success with Fingolimod and Natalizumab in Relapsing-Remitting Multiple Sclerosis Patients. J. Clin. Med. 2021, 10, 1830. [Google Scholar] [CrossRef]
- Sormani, M.P.; Truffinet, P.; Thangavelu, K.; Rufi, P.; Simonson, C.; De Stefano, N. Predicting long-term disability outcomes in patients with MS treated with teriflunomide in TEMSO. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e379. [Google Scholar] [CrossRef] [PubMed]
- Sormani, M.P.; Freedman, M.S.; Aldridge, J.; Marhardt, K.; Kappos, L.; De Stefano, N. MAGNIMS score predicts long-term clinical disease activity-free status and confirmed disability progression in patients treated with subcutaneous interferon beta-1a. Mult. Scler. Relat. Disord. 2021, 49, 102790. [Google Scholar] [CrossRef]
- Hapfelmeier, A.; On, B.I.; Mühlau, M.; Kirschke, J.S.; Berthele, A.; Gasperi, C.; Mansmann, U.; Wuschek, A.; Bussas, M.; Boeker, M.; et al. Retrospective cohort study to devise a treatment decision score predicting adverse 24-month radiological activity in early multiple sclerosis. Ther. Adv. Neurol. Disord. 2023, 16, 17562864231161892. [Google Scholar] [CrossRef]
- Giovannoni, G.; Turner, B.; Gnanapavan, S.; Offiah, C.; Schmierer, K.; Marta, M. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult. Scler. Relat. Disord. 2015, 4, 329–333. [Google Scholar] [CrossRef]
- Kappos, L.; De Stefano, N.; Freedman, M.S.; Cree, B.A.; Radue, E.W.; Sprenger, T.; Sormani, M.P.; Smith, T.; Häring, D.A.; Piani Meier, D.; et al. Inclusion of brain volume loss in a revised measure of ‘no evidence of disease activity’ (NEDA-4) in relapsing-remitting multiple sclerosis. Mult. Scler. 2016, 22, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, D.; Solomon, J.M.; Sormani, M.P.; Montalban, X.; Ye, X.Y.; Dababneh, D.; Muccilli, A.; Saab, G.; Shah, P. Association of NEDA-4 with No Long-term Disability Progression in Multiple Sclerosis and Comparison with NEDA-3: A Systematic Review and Meta-analysis. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e200032. [Google Scholar] [CrossRef]
- Wattjes, M.P.; Rovira, À.; Miller, D.; Yousry, T.A.; Sormani, M.P.; de Stefano, M.P.; Tintoré, M.; Auger, C.; Tur, C.; Filippi, M.; et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—Establishing disease prognosis and monitoring patients. Nat. Rev. Neurol. 2015, 11, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Andorra, M.; Freire, A.; Zubizarreta, I.; de Rosbo, N.K.; Bos, S.D.; Rinas, M.; Høgestøl, E.A.; de Rodez Benavent, S.A.; Berge, T.; Brune-Ingebretse, S.; et al. Predicting disease severity in multiple sclerosis using multimodal data and machine learning. J. Neurol. 2024, 271, 1133–1149. [Google Scholar] [CrossRef]
- Pilehvari, S.; Morgan, Y.; Peng, W. An analytical review on the use of artificial intelligence and machine learning in diagnosis, prediction, and risk factor analysis of multiple sclerosis. Mult. Scler. Relat. Disord. 2024, 89, 105761. [Google Scholar] [CrossRef]
- Seccia, R.; Romano, S.; Salvetti, M.; Crisanti, A.; Palagi, L.; Grassi, F. Machine Learning Use for Prognostic Purposes in Multiple Sclerosis. Life 2021, 11, 122. [Google Scholar] [CrossRef]
- Tur, C.; Carbonell-Mirabent, P.; Otero-Romero, S.; Cobo-Calvo, Á.; Arévalo, M.J.; Ariñoa, H.; Arrambide, G.; Auger, C.; Carvajal, R.; Castilló, J. The Barcelona baseline risk score to predict long-term prognosis after a first demyelinating event: A prospective observational study. Lancet Reg. Health Eur. 2025, 53, 101302. [Google Scholar] [CrossRef] [PubMed]
- Iaffaldano, P.; Lucisano, G.; Guerra, T.; Caputo, F.; Simone, M.; Copetti, M.; Paolicelli, D.; Portaccio, E.; Patti, F.; Perini, P.; et al. Early Intensive Versus Escalation Approach: Ten-Year Impact on Disability in Relapsing Multiple Sclerosis. Ann. Clin. Transl. Neurol. 2025; Advance online publication. [Google Scholar] [CrossRef]
- Trojano, M.; Kalincik, T.; Iaffaldano, P.; Amato, M.P. Interrogating large multiple sclerosis registries and databases: What information can be gained? Curr. Opin. Neurol. 2022, 35, 271–277. [Google Scholar] [CrossRef] [PubMed]

| Prognostic Factor | Year of Publication | Study Design | Population Size | Outcome(s) | Effect Measure | Evidence of Relevance to MS Progression/Worsening | References | |
|---|---|---|---|---|---|---|---|---|
| Demographic and clinical prognostic factors | Age | 2013 | Retrospective multi-center cohort study | 12,570 relapse-onset patients and 881 patients with PPMS | Relapse incidence | HR = 0.95, 95% CI = 0.949–0.953, p < 10−12. Tweedie model: rate ratio = 0.98, 95% CI = 0.982–0.985, p < 10−12 | Patient age is the most important determinant of decline in relapse incidence. | [14] |
| Demographic and clinical prognostic factors | Age | 2021 | Retrospective 2-center cohort study | 687 RRMS patients | PIRA | Sub distribution HR = 1.05 (1.01–1.10) for each year increase, p = 0.036 | The risk of PIRA was associated with increasing age. | [15] |
| Demographic and clinical prognostic factors | Age | 2015 | Metanalysis: 6 trials | 6693 RRMS | Treatment effectiveness: ARR; disability progression (EDSS worsening sustained for 12 or 24 weeks). Relative effect (RE) | Treatment effects on ARR (RE = 0.83 vs. RE = 1.30, p < 0.001) and on disability progression (RE = 0.82 vs. RE = 1.28, p = 0.017) were significantly higher in younger subjects. | In RRMS, lower age is associated with higher treatment effects. | [16] |
| Demographic and clinical prognostic factors | Age | 2024 | Retrospective cohort | 114 RMS | Change in EDSS from first assessment at relapse to EDSS after the last relapse treatment | Regression coefficient (95% CI): 0.04 (0.02, 0.06) p < 0.001 | Female sex, younger age, and a higher EDSS during relapse as factors associated with a higher chance of EDSS improvement after relapse treatment. | [17] |
| Demographic and clinical prognostic factors | Age | 2019 | Retrospective cohort | 2083 RRMS | Global disability (eight Performance Scales (PSS-8) and the PHQ-9) | PSS-8: 0.65 (0.49, 0.82) < 0.001; PHQ-9: 0.39 (−0.58, −0.19) < 0.001 | Older age is associated with higher global disability. | [18] |
| Demographic and clinical prognostic factors | Age | 2018 | Retrospective multi-center cohort study | 4842 MS patients | Risk of relapses after DMT discontinuation | HR (95% CI) p-value: 0.97 (0.97, 0.98) < 0.001 | In younger patients the risk of relapses after DMT suspension is higher. | [19] |
| Demographic and clinical prognostic factors | Age at onset | 2015 | Prospective study | 305 MS patients | SPMS conversion | HR: 1.049; p = 0.00426 | The factor “age at onset” was significant for risk of SP in men. | [20] |
| Demographic and clinical prognostic factors | Age at onset | 2015 | Prospective study | 305 MS patients | Death (EDSS 10) | HR: 1.061; p = 0.0135 | In men, age at onset remained a significant predictor of EDSS10. | [20] |
| Demographic and clinical prognostic factors | Age at onset | 2022 | Retrospective cohort study | 661 MS patients | EDSS worsening | 95% CI: 0.04 to 0.40; p = 0.015 | For every 5 years earlier, the EDSS was 0.22 points worse. | [21] |
| Demographic and clinical prognostic factors | Age at onset | 2022 | Retrospective cohort study | 661 MS patients | SPMS conversion | 95% CI: 1.08 to 1.64; p = 0.008 | For every 5 years earlier, odds of SPMS 1.33 times higher. | [21] |
| Demographic and clinical prognostic factors | Age at onset | 2022 | Retrospective cohort study | 661 MS patients | Brain T2-lesion volume (T2LV) | 95% CI: 1.02 to 2.70; p < 0.001 | For every 5 years earlier, odds of T2LV 1.86 mL higher. | [21] |
| Demographic and clinical prognostic factors | Age at onset | 2020 | Retrospective multicenter cohort study | 19,318 RRMS; 2343 SPMS identified with the DDA definition and 3868 identified with the neurologist definition (ND) | SPMS conversion (SPMS definition according to ND and DDA) | DDA group: HR (95% CI): 2.26 (1.92–2.67), p < 0.0001; ND group: 1.85 (1.63–2.09), p < 0.0001 | Age at onset > 40 years is associated with higher risk of SPMS | [22] |
| Demographic and clinical prognostic factors | Age at onset | 2023 | Retrospective analysis of data from patients prospectively included (patients with a first demyelinating attack) | 1128 patients | PIRA | HR, 1.43; 95% CI, 1.23–1.65; p < 0.001 for each older decade | Older age at the first attack is a predictor of PIRA. | [23] |
| Demographic and clinical prognostic factors | Age at onset | 2017 | Monocentric retrospective study | 3597 pMS | Time to EDSS 4.0 and 6.0 | HR 2.0 [95% CI 1.7–2.4] and 2.3 [1.9–2.9] | Worst outcomes with LOMS (≥50 years) (independent of PP course or male gender). | [24] |
| Demographic and clinical prognostic factors | Age at onset | 2020 | Retrospective monocentric study | 157 PPMS patients | Time to EDSS 6.0 | HR (95% CI): 1.03 (1.006–1.053); p = 0.012 | Older age of onset was associated with a shorter time to EDSS6 | [25] |
| Demographic and clinical prognostic factors | Age at onset | 2023 | Retrospective analysis of data from patients prospectively included (patients with a first demyelinating attack) | 1128 patients | Adjusted yearly EDSS increase rates | HR 0.18; 95% CI, 0.16–0.20 vs. 0.04; 95% CI, 0.02–0.05; p < 0.001 | Older age at the first attack is a predictor of PIRA. | [23] |
| Demographic and clinical prognostic factors | Age at onset | 2023 | Retrospective multicenter cohort study | 16,130 MS patients | PIRA | AOMS vs. POMS HR, 1.42; 95% CI, 1.30–1.55; LOMS vs. POMS HR, 2.98; 95% CI, 2.60–3.41; p < 0.001. | Older age at onset was associated with a higher risk of PIRA events. | [26] |
| Demographic and clinical prognostic factors | Age at onset | 2020 | Prospective study | 415 MS patients | Risk of EDSS 6.0 | HR 3.846, 95% CI 1.240–11.932, p = 0.020 | Age at disease onset greater than 50 years was significantly associated with a higher HR to reach an EDSS of 6.0. | [27] |
| Demographic and clinical prognostic factors | Baseline EDSS score | 2020 | Systematic review (30 studies). Data collection was guided by the checklist CHARMS and PROBAST. | N/A | N/A | N/A | The single most common clinical predictor was baseline EDSS (n = 11). | [28] |
| Demographic and clinical prognostic factors | Baseline EDSS score | 2020 | Retrospective multicenter cohort study (RISM) | 19,318 RRMS; 2343 SPMS identified with the DDA definition and 3868 identified with the neurologist definition (ND) | SPMS conversion (SPMS definition according to ND and DDA) | DDA group: HR (95% CI) 1.41 (1.38–1.44), p < 0.0001; ND group: 1.50 (1.48–1.53), p < 0.0001 | A higher baseline EDSS score is associated with higher risk of SPMS | [22] |
| Demographic and clinical prognostic factors | Baseline EDSS score | 2022 | Prospective, longitudinal cohort study | 53 RRMS | EDSS score increase of ≥1.5, 1.0, or 0.5, confirmed after a 3-month relapse-free period, when the baseline EDSS score was 0, ≤5.5, or ≥6.0, respectively | A higher baseline EDSS score (OR = 3.15 [95% CI = 1.61; 8.38], p = 0.003) is a significant independent predictor of EDSS score worsening at follow-up (C-index = 0.892) | A higher baseline EDSS score is a predictor of EDSS worsening. | [29] |
| Demographic and clinical prognostic factors | Baseline EDSS score | 2022 | Prospective, longitudinal cohort study | 53 RRMS | SPMS conversion | A higher baseline EDSS score (for each point higher: OR = 6.37 [1.98; 20.53], p = 0.002) independently predicted SPMS conversion (C-index = 0.947). | A higher baseline EDSS score is predictor of SPMS conversion | [29] |
| Demographic and clinical prognostic factors | Baseline EDSS score | 2016 | Post hoc analysis PRISMS long-term follow-up | 382 patients | Risk of EDSS 6.0 and time to EDSS 6.0 | R2 1.4125, 1.0862 | There is an association between EDSS at baseline and EDSS 6.0 and time to EDSS 6.0. | [30] |
| Demographic and clinical prognostic factors | Baseline EDSS score | 2016 | Post hoc analysis PRISMS long-term follow-up | 382 patients | SPMS conversion and time to SPMS | R2 0.8634; 0.6477 | There is an association between EDSS at baseline and SPMS conversion and time to SPMS conversion | [30] |
| Demographic and clinical prognostic factors | Baseline EDSS score | 2018 | Retrospective multi-center cohort study | 4842 MS patients | CDP | HR (95% CI) p-value: EDSS 2–3.5 1.79 (1.47, 2.17) < 0.001; EDSS 4.0–5.5 2.20 (1.77, 2.75) < 0.001; EDSS 6 + 2.62 (2.09, 3.28) < 0.001 | Hazard of CDP increased with increasing disability at baseline. | [19] |
| Demographic and clinical prognostic factors | Baseline EDSS score | 2021 | Observational cohort study | 2649 MS patients | MSIS physical score and psychological score worsening | Each year of treatment delay was associated with a worse MSIS physical score by 2.75 points (95% CI 1.29 to 4.20), and worse MSIS psychological score by 2.02 points (95% CI 0.03 to 3.78) | Earlier commencement of DMT was associated with better patient-reported physical symptoms. | [31] |
| Radiological predictors | Baseline gadolinium-enhancing lesions | 2019 | Prospective study | 180 MS patients | EDSS correlation | (≥1) β = 1.32, p < 0.01; (≥2) OR: 3.16, 1.08, 9.23; p = 0.035 | Baseline gadolinium-enhancing showed a consistent association with Expanded Disability Status Scale at 15 years. | [32] |
| Radiological predictors | Baseline gadolinium-enhancing lesions | 2021 | Retrospective 2-center cohort study | 687 RRMS patients | RAW | Sub distribution HR = 2.38 (1.01–5.63), p = 0.047 | RAW was predicted by the presence of contrast-enhancing lesions on baseline MRI | [15] |
| Radiological predictors | Brain T2 lesions at baseline MRI | 2015 | Observational study based on a prospective, open cohort | 1018 CIS | Risk of reaching EDSS score of at least 3.0 in 2 evaluations (defined “disability accumulation”) | Adjusted HR scores of 2.9 (95% CI 1.4–6.0) | The presence ≥10 brain T2 lesions on the baseline MRI was associated with a higher risk of the accumulation of disability | [33] |
| Radiological predictors | Brain T2 lesions at baseline MRI | 2021 | Retrospective 2-center cohort study | 687 RRMS patients | RAW | sHR = 3.92 (1.36–11.29), p = 0.012 | RAW was predicted by the presence of >9 T2 lesions on baseline MRI | [15] |
| Radiological predictors | CALs | 2022 | Prospective, longitudinal cohort study | 54 RRMS | EDSS score increase of ≥1.5, 1.0, or 0.5, confirmed after a 3-month relapse-free period, when the baseline EDSS score was 0, ≤5.5, or ≥6.0, respectively | A lower baseline MTR values of SELs (for each % higher: OR = 0.66 [0.41; 0.92], p = 0.033) is a significant independent predictor of EDSS score worsening at follow-up (C-index = 0.892) | Lower baseline MTR values of SELs are predictor of EDSS worsening. | [29] |
| Radiological predictors | CALs | 2022 | Prospective, longitudinal cohort study | 56 RRMS | SPMS conversion | A lower baseline MTR values of SELs (for each % higher: OR = 0.48 [0.25; 0.89], p = 0.02) independently predicted SPMS conversion (C-index = 0.947). | A lower baseline MTR values of SELs are predictor of SPMS conversion | [29] |
| Radiological predictors | CALs | 2022 | Prospective, longitudinal cohort study | 52 RRMS | EDSS score increase of ≥1.5, 1.0, or 0.5, confirmed after a 3-month relapse-free period, when the baseline EDSS score was 0, ≤5.5, or ≥6.0, respectively | A higher proportion of SELs among baseline lesions (OR = 1.22 [95% CI = 1.04; 1.58], p = 0.04) is a significant independent predictor of EDSS score worsening at follow-up (C-index = 0.892) | A higher proportion of SELs among baseline lesions is a predictor of EDSS worsening. | [29] |
| Demographic and clinical prognostic factors | Cognitive disfunction | 2022 | Retrospective study | 408 MS patients | SPMS conversion | OR = 2.29, p = 0.043 | Cognitive dysfunction was associated with higher odds of transitioning from relapsing–remitting course to a progressive disease course | [34] |
| Demographic and clinical prognostic factors | Cognitive disfunction | 2022 | Retrospective study | 409 MS patients | Mortality | aHR = 3.07, p = 0.006 | Cognitive dysfunction was associated with higher hazard of death in the total sample | [34] |
| Demographic and clinical prognostic factors | Cognitive disfunction | 2016 | Prospective observational study | 793 MS patients | Reaching severe disability: EDSS 6.0 and higher | HR 4.64; CI 1.11–19.50; p = 0.036 | Cognitive dysfunction 10 years after disease onset was associated with severe disability. | [35] |
| Radiological predictors | Cortical lesions | 2021 | Prospective study | 63 MS patients | EDSS | 0.37 (0.23 to 0.508); cortical lesions were higher in SPMS (100% sensitivity and 88% specificity) | Cortical lesions, grey matter volume and cervical cord volume explained 60% of the variance of EDSS; cortical lesions alone explained 43%. | [36] |
| Radiological predictors | Cortical lesions | 2022 | Prospective study | 20 RRMS patients, 13 SPMS patients, along with 10 age-matched healthy controls | MS progression | 3.6 lesions/year ± 4.2 vs. 1.1 lesions/year ± 0.9, respectively; p = 0.03 | Cortical lesion accrual was greater in participants with SPMS than with RRMS. | [37] |
| Radiological predictors | Cortical lesions | 2022 | Prospective study | 20 RRMS patients, 13 SPMS patients, along with 10 age-matched healthy controls | EDSS changes | β = 0.5, p = 0.003 | Total cortical lesion volume independently predicted baseline EDSS and EDSS changes at follow-up | [37] |
| Radiological predictors | Cortical lesions | 2017 | 10-year observational, cross-sectional study | 40 OCB-negative and 50 OCB-positive MS | Presence of cytokines | CXCL13 (r = 0.922; p < 0.001), CXCL12 (r = 0.678; p = 0.022), OPN (r = 0.692; p = 0.018), IL6 (r = 0.628; p = 0.039), TWEAK (r = 0.629; p = 0.038) | CL load significantly correlated with levels of several molecules linked to the B cell immune response. | [38] |
| Fluid biomarkers | CSF biomarkers | 2020 | Longitudinal 4-yearprospective study | 99 RRMS patients (treatment-naive) | Risk of EDA (measures of disease activity: (1) evidence of relapses; (2) confirmed disability progression as assessed by an increase of the EDSS score by at least 1 point sustained over 6 months; and (3) evidence of new or newly enlarging WMT2 lesions). | HR = 1.78; p = 0.0001 | CXCL13, LIGHT and APRIL were the CSF molecules more strongly associated with the risk of EDA. | [39] |
| Fluid biomarkers | CSF biomarkers | 2020 | Longitudinal 4-year prospective study | 99 RRMS patients (treatment-naive) | Risk of cortical thinning. | β = 4.7 × 10−4; p < 0.001 | Higher CSF levels of CXCL13 were associated with more severe cortical thinning. | [39] |
| Fluid biomarkers | CSF biomarkers | 2024 | Prospective study | 118 de novo diagnosed RRMS patients and 112 controls | Correlation with number of T2 and Gd(+) lesions on head MRI in patients with newly diagnosed RRMS. | R Spearman: 0.2434; t (N-2) 2.2165; p = 0.0296 | TNF-α levels positively correlated with post-contrast-enhancing brain lesions. | [40] |
| Fluid biomarkers | CSF biomarkers | 2024 | Prospective study | 118 de novo diagnosed RRMS patients and 112 controls | Correlation with number of T2 and Gd (+) lesions on C-spine MRI in patients with newly diagnosed RRMS. | R Spearman: −0.2730; t (N-2) −2.5058; p = 0.0143 | IL-15 levels in CSF correlated negatively with both the number of T2 lesions in C spine MRI and the number of Gd(+) lesions in C spine MRI | [40] |
| Fluid biomarkers | CSF biomarkers | 2021 | Meta-analysis | Six longitudinal studies, 1221 CIS/early RRMS patients | Risk of a second clinical relapse. | HR = 3.62, 95% CI 1.75–7.48, I2 = 88%, p = 0.0005 | The pooled analysis confirmed that the presence of intrathecal IgM synthesis is a risk factor for a second clinical relapse. | [41] |
| Demographic and clinical prognostic factors | Disease duration | 2023 | Retrospective multicenter cohort study (RISM) | 16,130 MS patients | PIRA | HR, 1.04; 95% CI, 1.04–1.05; p < 0.001 | A longer disease duration was associated with a higher risk of PIRA events. | [26] |
| Demographic and clinical prognostic factors | Disease duration | 2019 | Retrospective cohort | 2083 RRMS | Walking speed (T25FW speed) | HR −0.05 95% CI (−0.08, −0.02) < 0.001 | Walking speed is slower in patients with a longer disease duration (per 5 years). | [18] |
| Demographic and clinical prognostic factors | Disease duration | 2013 | Retrospective study (London Multiple Sclerosis Clinic database) | 730 MS patients | DSS 6 | OR, 0.76 [95% CI, 0.69–0.84] and 0.44 [95% CI, 0.37–0.52] for 5- and 15-year latency, respectively) | Longer latency to progression was associated with lower probability of attaining DSS 6. | [42] |
| Treatment | DMT exposure | 2023 | Retrospective multicenter cohort study (RISM) | 16,130 MS patients | PIRA | HR, 0.69; 95% CI, 0.64–0.74; p < 0.001 | A shorter DMT exposure as associated with a higher risk of PIRA events. | [26] |
| Treatment | DMT exposure | 2021 | Retrospective 2-center cohort study | 687 RRMS patients | RAW | sHR = 1.11 (1.02–1.21), p = 0.015 | RAW was predicted by the temporary or permanent discontinuation of the initial DMT | [15] |
| Treatment | DMT exposure | 2020 | Retrospective multicenter cohort study (RISM) | 646 POMS, 8473 AOMS and 382 LOMS patients at the first demyelinating event | Risk of 12-month confirmed disability worsening | aHR in non-exposed versus exposed: 6.3 (4.9–8.0) for adult-onset, p < 0.0001; LOMS 1.9 (0.9–4.1), p = 0.07. | DMT exposure reduced the risk of 12-month CDW, with a progressive risk reduction in different quartiles of exposure in paediatric-onset and adult-onset patients. | [43] |
| Treatment | DMT exposure | 2020 | Retrospective multicenter cohort study (RISM) | 646 POMS, 8473 AOMS and 382 LOMS patients at the first demyelinating event | Risk of sustained EDSS 4.0 | aHR in non-exposed versus exposed: 6.3 (4.9–8.0) for adult-onset, p < 0.0001; LOMS 1.9 (0.9–4.1), p = 0.07. | DMT exposure reduced the risk of sustained EDSS score of 4.0 | [43] |
| Treatment | DMT exposure | 2016 | Retrospective multi-center cohort study | 2466 MS patients | EDSS at 10 years | Coeff = −0.86, p = 1.3 × 10−9. | Cumulative treatment exposure was independently associated with lower EDSS at 10 years. | [44] |
| Treatment | DMT exposure | 2020 | Retrospective multi-center cohort study (MSBase registry) | 1085 patients with ≥15-year follow-up | Risk of relapses | HR 0.59, 95% CI 0.50–0.70, p = 10−9 | Treated patients were less likely to experience relapses (0.59, 0.50–0.70, p = 10−9) and worsening of disability | [45] |
| Treatment | DMT exposure | 2020 | Retrospective multi-center cohort study (MSBase registry) | 1085 patients with ≥15-year follow-up | Risk of EDSS worsening | HR 0.81, 95% CI 0.67–0.99, p = 0.043 | Treated patients were less likely to experience relapses (0.59, 0.50–0.70, p = 10−9) and worsening of disability | [45] |
| Treatment | DMT exposure | 2020 | Retrospective multicenter cohort study (RISM) | 19,318 RRMS; 2343 SPMS identified with the DDA definition and 3868 identified with the neurologist | SPMS conversion (SPMS definition according to ND and DDA) | DDA group: HR (95% CI) 0.43 (0.36–0.50) p < 0.0001 | A longer exposure to DMT is associated with lower risk of SPMS | [22] |
| Other biomarkers | Evoked potentials | 2011 | Retrospective monocentric study | 80 MS patients | Risk of EDSS 4.0 and 6.0 | log-rank test: p < 0.001 | Increased risk of disability in patients with EP score higher than the median value. EP score of 8 or 9 showed the highest sensitivity and specificity in predicting EDSS 4.0 and 6.0 | [46] |
| Other biomarkers | Evoked potentials | 2023 | Prospective monocentric study | 181 MS patients | Risk of MSSS worsening | OR 0.04; IC 95% 0.01–0.06; p-Value 0.002 | P100 latency resulted in a predictor for disability over time (MSSS). | [47] |
| Other biomarkers | Evoked potentials | 2016 | Retrospective monocentric study | 100 MS patients | EDSS worsening from baseline data | OR = 1.2; 95 % CI 1.1–1.3; p = 0.0012 | Baseline global EP score was a highly significant predictor of EDSS progression 6 years later. | [48] |
| Fluid biomarkers | GFAP | 2022 | Prospective cohort study (Comprehensive Longitudinal Investigation of MS at the Brigham and Women’s Hospital -climbstudy.org) | 257 MS patients | 6-months confirmed disability progression (6mCDP). EDSS progression was defined as an increase in the EDSS score since the previous visit of ≥1.0 point from an EDSS score of 1.0–5.0 or ≥0.5 point from an EDSS score of ≥5.5.6mCDP was defined as EDSS progression that was sustained for at least 180 days. | HR = 1.71; 95% CI = 1.19–2.45; p = 0.004 | Higher sGFAP levels were associated with higher risk of 6mCDP. The association was stronger in patients with low sNfL (aHR = 2.44; 95% CI 1.32–4.52; p = 0.005) and patients who were nonactive in the 2 years prior or after the sample. | [49] |
| Fluid biomarkers | GFAP | 2017 | Retrospective monocentric study | GFAP levels in the CSF from 18 patients with RRMS, 8 patients with CIS and 35 healthy controls | Infratentorial chronic inflammatory lesion load | r = 0.55, p = 0.004 | GFAP concentrations significantly correlated with infratentorial chronic, post-inflammatory lesion load | [50] |
| Fluid biomarkers | GFAP | 2017 | Retrospective monocentric study | GFAP levels in the CSF from 18 patients with RRMS, 8 patients with CIS and 35 healthy controls | Infratentorial chronic inflammatory lesion load | r = 0.71, p = 0.0002 | GFAP concentrations significantly correlated with the intensity of gadolinium-enhancement as a parameter for the acute activity of inflammatory processes. | [50] |
| Fluid biomarkers | GFAP | 2024 | Retrospective study (but with prospective data collection) | 133 RRMS patients | SPMS conversion | c β: 0.34 [−0.78;1.46]; p = 0.555 | GFAP was not associated with conversion to SPMS. | [51] |
| Fluid biomarkers | GFAP | 2024 | Retrospective study (but with prospective data collection) | 133 RRMS patients | EDSS score worsening | c β: 0.34 [−0.78;1.46]; p = 0.556 | GFAP was not associated with disability progression. | [51] |
| Radiological predictors | Gray matter pathology | 2014 | Prospective cohort study | 73 MS patients | EDSS worsening | OR = 0.79, p = 0.01; C-index = 0.69 | Baseline GMF is predictor of worsening of disability in the long term. | [52] |
| Radiological predictors | Gray matter pathology | 2021 | Prospective study | 332 MS patients, 96 healthy controls | Cognitive decline (test-defined assessment) | Nagelkerke R2 = 0.22, p < 0.001 | A prediction model that included only whole-brain MRI measures showed cortical grey matter volume as the only significant MRI predictor of cognitive decline. | [53] |
| Radiological predictors | Gray matter pathology | 2021 | Prospective study | 63 MS patients | EDSS | −0.26 (−0.444 to −0.074) | Across all subjects, cortical lesions, grey matter volume and cervical cord volume explained 60% of the variance of the Expanded Disability Status Scale. | [36] |
| Radiological predictors | Gray matter pathology | 2022 | Retrospective multi-center cohort study | 373 MS patients | Difference in mean EDSS score over the years of follow-up | A deep learning architecture based on convolutional neural networks was implemented to predict: (1) clinical worsening (EDSS-based model), (2) cognitive deterioration (SDMT-based model), or (3) both (EDSS + SDMT-based model). | The convolutional neural network model showed high predictive accuracy for clinical (83.3%) and cognitive (67.7%) worsening, although the highest accuracy was reached when training the algorithm using both EDSS and SDMT information (85.7%). | [54] |
| Radiological predictors | Gray matter pathology | 2014 | Prospective study | 81 MS patients | Disease progression | Patients with disability Progression showed significantly increased loss of whole brain (−3.8% vs. −2.0%, p < 0.001), cortical (−3.4% vs. −1.8%, p = 0.009) compared to patients with no progression. | GM atrophy showed association with disease progression | [55] |
| Demographic and clinical prognostic factors | Onset type | 2022 | Retrospective study | 21 RRMS patients, 13 SPMS patients, along with 10 age-matched healthy controls | SPMS conversion | p > 0.05 | Affected bowel and bladder functions during the first relapse were ineffective in predicting the transition to the SPMS course. | [56] |
| Demographic and clinical prognostic factors | Onset type | 2015 | Observational study based on a prospective, open cohort | 1016 CIS | Risk of reaching EDSS score of at least 3.0 in 2 evaluations (defined “disability accumulation”) | HR 0.5; 95% CI 0.3–0.8 | Patients presenting CIS with optic neuritis appeared to display a lower risk of reaching an EDSS score of 3.0. | [33] |
| Demographic and clinical prognostic factors | Onset type | 2016 | Prospective observational study | 793 MS patients | Reaching moderate disability: EDSS 3.0–5.5 | HR 0.42; CI 0.23–0.77; p = 0.005 | Complete remission of neurological symptoms at onset reduced the risk of moderate disability. | [35] |
| Demographic and clinical prognostic factors | Onset type | 2020 | Retrospective multicenter cohort study (RISM) | 19,318 RRMS; 2343 SPMS identified with dhe DDA definition and 3868 identified with the neurologist definition (ND) | SPMS conversion (SPMS definition according to ND and DDA) | DDA group: HR (95% CI) 1.26 (1.12–1.40), p < 0.0001; ND group: 1.13 (1.03–1.23), p = 0.011 | Multifocal onset is associeted with higher risk of SPMS | [22] |
| Demographic and clinical prognostic factors | Onset type | 2020 | Retrospective monocentric study | 157 PPMS patients | Time to EDSS 6.0 | HR (95% CI): 2.13 (1.24–3.63); p= 0.006 | The presence of spinal motor symptoms at onset were associated with a shorter time to EDSS6 | [25] |
| Demographic and clinical prognostic factors | Onset type | 2013 | Retrospective monocentric study | 197 MS patients | Risk of EDSS 6.0 | 8.1 and 13.1 fold increased risk to EDSS 6, respectively (p = 0.04 and p = 0.01). | Motor and brainstem symptoms at onset were also associated with higher risk of EDSS 6.0 | [57] |
| Demographic and clinical prognostic factors | Onset type | 2020 | Prospective study | 415 MS patients | Risk of EDSS 6.0 | HR 2.107, 95% CI 1.168–3.800, p = 0.013 | An incomplete recovery from first attack was significantly associated with a higher HR to reach an EDSS of 6.0. | [27] |
| Demographic and clinical prognostic factors | Onset type | 2015 | Population-based cohort (retrospective - prospective) | Population-based cohort (105 patients with relapsing-remitting MS, 86 with bout-onset progressive MS) and a clinic-based cohort (415 patients with bout-onset progressive MS) | Recovery from first relapse | p = 0.001 | A brainstem, cerebellar, or spinal cord syndrome was associated with a poor recovery from the initial relapse. | [58] |
| Other biomarkers | Optic nerve diameter | 2021 | Prospective study | 63 MS patients | Disease progression | p = 0.041 for the right eye and p = 0.037 for the left eye | Smaller diameters of optic nerve are associated with poor clinical progression and greater disability (measured by EDSS). | [59] |
| Other biomarkers | Optic nerve diameter | 2021 | Prospective study | 63 MS patients | Sustained increase (>3 months) of over 0.5 points on the EDSS. | p = 0.07 for the right eye and p = 0.043 for the left eye | Smaller diameters of optic nerve are associated with poor clinical progression and greater disability (measured by EDSS). | [59] |
| Other biomarkers | Optic nerve diameter | 2019 | Prospective study | 49 RRMS patients, 50 matched healthy controls | Sustained EDSS > 2 | p = 0.044, OR = 0.000, 95% CI = 0.000–0.589 | Optic nerve diameter was an independent predictor of EDSS > 2 | [60] |
| Demographic and clinical prognostic factors | PIRA | 2023 | Retrospective analysis of data from patients prospectively included (patients with a first demyelinating attack) | 1128 patients | Adjusted yearly EDSS increase rates | 0.31; 95% CI, 0.26–0.35 vs. 0.13; 95% CI, 0.10–0.16; p < 0.001 | Early PIRA had steeper EDSS yearly increase rates than late PIRA. | [23] |
| Demographic and clinical prognostic factors | PIRA | 2023 | Retrospective analysis of data from patients prospectively included (patients with a first demyelinating attack) | 1128 patients | Risk of reaching EDSS 6.0 | HR, 26.21; 95% CI, 2.26–303.95; p = 0.009 | Early PIRA had a 26-fold greater risk of reaching EDSS 6.0 from the first attack (HR, 26.21; 95% CI, 2.26–303.95; p = 0.009). | [23] |
| Demographic and clinical prognostic factors | PIRA | 2023 | Retrospective analysis of data from patients prospectively included (patients with a first demyelinating attack) | 1128 patients | Risk of reaching EDSS 6.0 | HR, 7.93; 95% CI, 2.25–27.96; p = 0.001 | Patients with PIRA had an 8-fold greater risk of reaching EDSS 6.0. | [23] |
| Radiological predictors | Presence of new Gd + SC lesions | 2018 | Single-centre retrospective study | 201 RRMS patients | Relapse occurrence (clinical relapses within 3 months) | B 1.113, Exp (B), 95% CI for EXP(B) 3.042, 1.158–7.995; p = 0.024 | A significant association between new Gd + SC lesions and clinical relapses within 3 months was found. | [61] |
| Radiological predictors | Presence of new Gd + SC lesions | 2018 | Single-centre retrospective study | 201 RRMS patients | DMT changes within 3 months | B 1.482, Exp (B), 95% CI for EXP(B) 4.402, 1.642–11.799; p = 0.003 | Even without clinical symptoms, worsening SC findings significantly predicted treatment changes. | [61] |
| Fluid biomarkers | Presence of OCBs | 2013 | Meta analysis: 71 studies | 12,253 MS patients, | EDSS worsening, EDSS disability milestones | 1.96 (95% CI 1.31 to 2.94; p = 0.001) with no between-study heterogeneity (I2 = 0%; X2 = 2.95, df = 3, p = 0.40) | OCB-positive MS patients had an OR of 1.96 of reaching disability outcomes. | [62] |
| Fluid biomarkers | Presence of OCBs | 2021 | Retrospective registry-based study | 7322 patients, 6494 OCB+ | Risk of reaching sustained EDSS score milestones 3.0, 4.0 and 6.0 | EDSS 3.0 (HR = 1.29, 95% CI 1.12 to 1.48, p < 0.001) and 4.0 (HR = 1.38, 95% CI 1.17 to 1.63, p < 0.001). | CSF-OCB presence is associated with higher risk of reaching EDSS milestones 3.0 anf 4.0. | [63] |
| Fluid biomarkers | Presence of OCBs | 2021 | Retrospective registry-based study | 7322 patients, 6494 OCB+ | SPMS conversion. | HR: 1.20, 95% CI 1.02 to 1.41, p = 0.03, n = 5721 | OCB positivity IS associated with increased risk of conversion to SPMS. | [63] |
| Fluid biomarkers | Presence of OCBs | 2015 | Observational study based on a prospective, open cohort | 1017 CIS | Risk of reaching EDSS score of at least 3.0 in 2 evaluations (defined “disability accumulation”) | adjusted HR scores of 2.0 (95% CI 1.2–3.6) | The presence of OCBs was associated with a higher risk of the accumulation of disability | [33] |
| Fluid biomarkers | Presence of OCBs | 2021 | Meta-analysis | Six longitudinal studies, 1221 CIS/early RRMS patients | Risk of a second clinical relapse. | HR = 2.18, 95% CI 1.24–3.82, I2 = 73%, p = 0.007 | The pooled analysis confirmed that the presence of OCBs (IgG) is a risk factor for a second clinical relapse. | [41] |
| Fluid biomarkers | Presence of OCBs | 2021 | Retrospective monocentric study | 358 patients, 287 OCB positive | MSSS | OCB + vs. OCB - (2.10 vs. 0.94, p value = 0.023) | Median MSSS was significantly higher in the OCB positive group (2.10 vs. 0.94, p value = 0.023) and remained significant when controlling for age at EDSS. | [64] |
| Fluid biomarkers | Presence of OCBs | 2017 | 10-year observational, cross-sectional study | 40 OCB-negative and 50 OCB-positive MS | Presence of cortical lesions | mean ± standard deviation: OCB + 6.1 ± 6.1 (0–24), ocb − 2.2 ± 2.8 (0–11), p < 0.0001 | Increased number of CLs was found in OCB+ compared to OCB− patients. | [38] |
| Demographic and clinical prognostic factors | Relapses | 2015 | Population-based cohort (retrospective-prospective) | Population-based cohort (105 patients with relapsing-remitting MS, 86 with bout-onset progressive MS) and a clinic-based cohort (415 patients with bout-onset progressive MS) | SPMS conversion | Half of the good recoverers developed progressive MS by 30.2 years after MS onset, whereas half of the poor recoverers developed progressive MS by 8.3 years after MS onset (p = 0.001). | Patients with MS with poor recovery from early relapses will develop progressive disease course earlier than those with good recovery. | [58] |
| Demographic and clinical prognostic factors | Relapses | 2020 | Prospective study | 415 MS patients | Risk of EDSS 6.0 | HR 2.217, 95% CI 1.148–4.281, p = 0.018 | ≥2 relapses during the first 2 years after onset were significantly associated with a higher HR to reach an EDSS of 6.0. | [27] |
| Demographic and clinical prognostic factors | Relapses | 2015 | Population-based cohort (retrospective-prospective) | Population-based cohort (105 patients with relapsing-remitting MS, 86 with bout-onset progressive MS) and a clinic-based cohort (415 patients with bout-onset progressive MS) | Recovery from first relapse | p = 0.001 | A fulminant relapse was associated with a poor recovery from the initial relapse. | [58] |
| Demographic and clinical prognostic factors | Relapses | 2010 | Retrospective study (London Multiple Sclerosis Clinic database) | 806 RTMS patients | DSS 6, 8, 10 | various OR | Frequent relapses in the first 2 years and shorter first inter-attack intervals predicted shorter times to reach hard disability endpoints. | [65] |
| Demographic and clinical prognostic factors | Relapses | 2013 | Retrospective monocentric study | 197 MS patients | Risk of EDSS 8.0 | 1.28 (5 years) and 1.19 (10 years), respectively p = 0.032 and p = 0.015 | The number of relapses in five and ten years of disease onset was associated with a slightly increased risk to EDSS 8 | [57] |
| Demographic and clinical prognostic factors | Relapses | 2024 | Retrospective cohort | 115 RMS | Change in EDSS from first assessment at relapse to EDSS after the last relapse treatment for all relapse events: EDSS improvement (after relapse treatment with steroid or PLEX) | Regression coefficient (95% CI): −0.32 (−0.44, −0.19) p < 0.001 | Female sex, younger age, and a higher EDSS during relapse as factors associated with a higher chance of EDSS improvement after relapse treatment. | [17] |
| Demographic and clinical prognostic factors | Relapses | 2020 | Retrospective multicenter cohort study (RISM) | 19,318 RRMS; 2343 SPMS identified with the DDA definition and 3868 identified with the neurologist definition (ND) | SPMS conversion (SPMS definition according to ND and DDA) | DDA group: HR (95% CI) 2.90 (2.54–3.30), p < 0.0001; ND group: 1.78 (1.64–1.94), p < 0.0001 | A higher number of relapses during RRMS phase is associated with higher risk of SPMS | [22] |
| Demographic and clinical prognostic factors | Relapses | 2020 | Retrospective multicenter cohort study (RISM) | 646 POMS, 8473 AOMS and 382 LOMS patients at the first demyelinating event | Risk of 12-month confirmed disability worsening | aHR: AOMS 1.37 (1.36–1.39); LOMS 1.40 (1.31–1.49) | Relapses were a risk factor for 12-month confirmed disability worsening in all three cohorts | [43] |
| Fluid biomarkers | Serum NfL level | 2020 | Prospective cohort study | 258 MS patients | Conversion to clinically diagnosed progressive MS | AUC of 0.744 (95% CI 0.61–0.88, p = 0.054). | MS patients with low serum NfL values (<7.62 pg/mL) at the baseline were 7.1 times less likely to develop progressive MS. | [66] |
| Fluid biomarkers | Serum NfL level | 2020 | Prospective cohort study | 258 MS patients | Annual rate of EDSS progression | 0.17 units/year, Kruskal–Wallis p = 0.020, df 2 | Patients with the highest NfL levels (>13.2 pg/mL) progressed most rapidly with an EDSS annual rate of 0.16 (p = 0.004), remaining significant after adjustment for sex, age, and disease-modifying treatment (p = 0.022) | [66] |
| Fluid biomarkers | Serum NfL level | 2022 | Case-control | 5390 control, 1313 MS patients | Risk of relapse | OR 1.41, 95% CI 1.30–1.54; p < 0.0001 | Patients with higher sNfL Z scores showed a greater probability of relapses in the following year, based on a model with Z score as a continuous predictor. | [67] |
| Fluid biomarkers | Serum NfL level | 2022 | Case-control | 5390 control, 1313 MS patients | EDSS worsening | OR 1.11, 1.03–1.21; p = 0.0093 | People with higher sNfL Z scores showed a greater probability of EDSS worsening in the following year, based on a model with Z score as a continuous predictor. | [67] |
| Fluid biomarkers | Serum NfL level | 2022 | Case-control | 5390 control, 1313 MS patients | EDA 3 | OR 1.43, 1.31–1.57; p < 0.0001 | People with higher sNfL Z scores showed a greater probability of EDA-3 in the following year, based on a model with Z score as a continuous predictor | [67] |
| Fluid biomarkers | Serum NfL level | 2022 | Case-control | 5390 control, 1313 MS patients | Clinical or MRI disease activity | All people with multiple sclerosis: OR 3.15, 95% CI 2.35–4.23; p < 0.0001); people considered stable with no evidence of disease activity (2.66, 1.08–6.55; p = 0.034) | A sNfL Z score above 1.5 was associated with an increased risk of future clinical or MRI disease activity in all people with multiple sclerosis and in people considered stable with no evidence of disease activity. | [67] |
| Fluid biomarkers | Serum NfL level | 2019 | Prospective study | 235 MS patients in a 2-year RCT of intramuscular interferon β-1a, and in serum (n = 164) from the extension study. | Risk of EDSS ≥ 6.0 at Year 8 | OR = 3.4; 95% CI = 1.2–9.9, p < 0.05 | Year 2 CSF levels were predictive of reaching EDSS ≥ 6.0 at Year 8 | [68] |
| Fluid biomarkers | Serum NfL level | 2019 | Prospective study | 235 MS patients in a 2-year RCT of intramuscular interferon β-1a, and in serum (n = 164) from the extension study. | Risk of EDSS ≥ 6.0 at Year 8 | OR = 11.0, 95% CI = 2.0–114.6; p < 0.01 | Year 3 serum levels were predictive of reaching EDSS ≥ 6.0 at Year 9 | [68] |
| Fluid biomarkers | Serum NfL level | 2019 | Prospective study | 235 MS patients in a 2-year RCT of intramuscular interferon β-1a, and in serum (n = 164) from the extension study. | Risk of EDSS ≥ 6.0 at Year 15 | OR (upper vs. lower tertile) = 4.9; 95% CI = 1.4–20.4; p < 0.05 | Year 4 serum levels were predictive of reaching EDSS ≥ 6.0 at Year 15 | [68] |
| Fluid biomarkers | Serum NfL level | 2021 | Prospective study | 369 blood samples from 155 early relapsing-remitting MS patients on interferon beta-1a. | Odds of EDA-3 | upper vs. lower 86.5% vs. 57.9%; OR = 4.25, 95% CI: [2.02, 8.95]; p = 0.0001 | In patients with disease activity (EDA-3), those with higher sNFL had higher odds of EDA-3 in the following year than those with low sNFL. | [69] |
| Fluid biomarkers | Serum NfL level | 2021 | Prospective study | 369 blood samples from 155 early relapsing-remitting MS patients on interferon beta. | BVL (brain volume loss) | β = −0.36%; 95% CI = [−0.60, −0.13]; p = 0.002 | In patients with disease activity (EDA-3), those with higher sNFL had greater whole brain volume loss during the following year. | [69] |
| Fluid biomarkers | Serum NfL level | 2020 | Prospective cohort study | 258 MS patients | EDSS score of ≥ 4 | 2nd tertile (>7.62 pg/mL): HR = 5.5 (95% CI 1.4–21.0), p = 0.012; 3rd tertile (>13.2 pg/mL): HR = 5.2 (95% CI 1.5–18.6), p = 0.010. AUC of 0.734 (95% CI 0.63–0.84, p = 0.001). | MS patients with higher serum NfL values (>7.62 pg/mL) at the baseline had a significantly higher risk of developing an EDSS ≥ 4, showing that they were on average > 5-times at higher risk of developing EDSS ≥ 4 over the follow-up. | [66] |
| Fluid biomarkers | Serum NfL level | 2024 | Retrospective study (but with prospective data collection) | 133 RRMS patients | SPMS conversion | c β [95% CI]: 9.92 [0.62;19.21]; p = 0.037 | sNfL was associated with conversion to SPMS, | [51] |
| Fluid biomarkers | Serum NfL level | 2024 | Retrospective study (but with prospective data collection) | 133 RRMS patients | EDSS score worsening | c β: 0.34 [−0.78;1.46]; p = 0.554 | sNfL was not associated with disability progression. | [51] |
| Demographic and clinical prognostic factors | Sex | 2013 | Retrospective multi-center cohort study (MSBase registry) | 11,570 relapse-onset patients and 881 patients with PPMS | Relapse incidence | Relapse frequency was 17.7% higher in females compared with males. | Within the initial 5 years, the female-to-male ratio increased from 2.3:1 to 3.3:1 in patients with 0 versus ≥4 relapses per year, respectively. | [14] |
| Demographic and clinical prognostic factors | Sex | 2015 | Observational study based on a prospective, open cohort | 1015 CIS | Risk of reaching EDSS score of at least 3.0 in 2 evaluations (defined “disability accumulation”) | HR 0.7; 95% CI 0.5–1.1 | Female sex appeared to display a lower risk of reaching an EDSS score of 3.0. | [33] |
| Demographic and clinical prognostic factors | Sex | 2024 | Retrospective cohort | 113 RMS | Change in EDSS from first assessment at relapse to EDSS after the last relapse treatment for all relapse events: EDSS improvement (after relapse treatment with steroid or PLEX) | Regression coefficient (95% CI): −0.60 (−0.98, −0.21) p < 0.01 | Female sex, younger age, and a higher EDSS during relapse as factors associated with a higher chance of EDSS improvement after relapse treatment. | [17] |
| Demographic and clinical prognostic factors | Sex | 2019 | Retrospective cohort | 2083 RRMS | Walking speed (T25FW speed) | HR 0.28, 95% CI 0.20–0.36, <0.001 | Walking speed is slower in females. | [18] |
| Demographic and clinical prognostic factors | Sex | 2013 | Retrospective monocentric study | 197 MS patients | Risk of EDSS 6.0, 7.0 | 4.63-fold increased risk to EDSS 6 (p < 0.001); 4.69-fold increased risk to EDSS 7 (p = 0.006). | Male sex was associated with a higher risk to EDSS 6 and 7. | [57] |
| Demographic and clinical prognostic factors | Sex | 2024 | Prospective study | 149 MS patients | time-to-relapse | HR = 0.91; 95 %CI = 0.53–1.58 | No sex differences in time-to-relapse emerged. | [70] |
| Demographic and clinical prognostic factors | Sex | 2024 | Prospective study | 149 MS patients | EDSS worsening | OR = 0.75; 95% CI = 0.21–2.35 | Males had no increased risk of EDSS worsening compared to females. | [70] |
| Demographic and clinical prognostic factors | Sex | 2020 | Retrospective multicenter cohort study (RISM) | 646 POMS, 8473 AOMS and 382 LOMS patients at the first demyelinating event | Risk of 12-month confirmed disability worsening | aHR: LOMS, female sex 0.74 (0.53–1.04) | Female sex exerted a protective role in the late-onset cohort for the risk of confirmed 12-months CDW. | [43] |
| Radiological predictors | Spinal cord atrophy | 2014 | Prospective study | 159 MS patients | Risk of EDSS score of at least 6.0 (requirement of a walking aid) | OR = 0.57 per 1 SD higher cord area; 95% CI 0.37, 0.86; p = 0.01 | Long-term physical disability was independently linked with atrophy of the spinal cord | [71] |
| Radiological predictors | Spinal cord atrophy | 2021 | Prospective study | 63 MS patients | EDSS | −0.27 (−0.421 to −0.109) | Across all subjects, cortical lesions, grey matter volume and cervical cord volume explained 60% of the variance of the Expanded Disability Status Scale. | [36] |
| Radiological predictors | Spinal cord lesions | 2024 | Monocentric retrospective study | 205 RRMS | CDA occurrence. CDA was defined as an EDSS increase of 1.5 for baseline EDSS scores of 0, an increase of 1 for baseline EDSS scores between 1.0 and 5.0, and an increase of 0.5 for baseline EDSS scores of 5.5 or higher; the increase had to be confirmed on clinical follow-up over at least 6 months; PIRA and RAW | Spearman’s rank correlation coefficient (rs): SCLN and SCLV were closely correlated (rs = 0.91, p < 0.001) and were both significantly associated with CDA on follow-up (p < 0.001). Subgroup analyses confirmed this association for patients with PIRA on CDA (34 events, p < 0.001) | The number of SC lesions on MRI is associated with future accumulation of disability largely independent of relapses. | [72] |
| Radiological predictors | Spinal cord lesions | 2019 | Prospective study | 178 MS patients | EDSS correlation | β = 1.53, p < 0.01 | Spinal cord lesions showed a consistent association with Expanded Disability Status Scale at 15 years. | [32] |
| Radiological predictors | Spinal cord lesions | 2019 | Prospective study | 178 MS patients | SPMS conversion at 15 years follow-up | OR 4.71, 1.72, 12.92; p = 0.003 | Spinal cord lesions were independently associated with secondary progressive multiple sclerosis at 15 years. | [32] |
| Radiological predictors | Spinal cord lesions | 2021 | Retrospective 2-center cohort study | 687 RRMS patients | CDA occurrence | Sub distribution HR = 4.08 (1.29–12.87), p = 0.016 | The risk of PIRA was associated with the presence of spinal cord lesions at baseline MRI scan. | [15] |
| Radiological predictors | Spinal cord lesions | 2024 | Monocentric retrospective study | 204 RRMS | CDA occurrence | OR 5.8, 95% CI 2.1 to 19.10 | The volume of SC lesions on MRI is associated with future accumulation of disability largely independent of relapses. | [72] |
| Radiological predictors | Spinal cord lesions | 2010 | Retrospective cohort study | 25 RRMS patients | EDSS 4.0 | HR 7.2, 95% confidence interval 1.4–36.4 | The diffuse abnormality in cervical spinal cord at the beginning of the disease is persistent and predicts a worse prognosis in RRMS patients. | [73] |
| Radiological predictors | Spinal cord lesions | 2024 | Monocentric retrospective study | 204 RRMS | CDA occurrence | OR 5.8, 95% CI 2.1 to 19.8 | Patients without any SC lesions experienced significantly less CDA | [72] |
| Treatment | Time to DMT initiation | 2023 | Retrospective multicenter cohort study (RISM) | 16,130 MS patients | Risk of PIRA | HR, 1.16; 95% CI, 1.00–1.34; p = 0.04 | Delayed DMT initiation was associated with higher risk of PIRA events. | [26] |
| Treatment | Time to DMT initiation | 2023 | Retrospective multicenter cohort study (RISM) | 16,130 MS patients | Risk of RAW | HR, 1.75; 95% CI, 1.28–2.39; p = 0.001 | Delayed DMT initiation was associated with higher risk of RAW events. | [26] |
| Treatment | Time to DMT initiation | 2021 | Retrospective multi-center cohort study (BMSD) | 11,871 RRMS patients | 3-month CDW | Retrospective 2-center cohort study+G91 | The time interval between disease onset and the first DMT start is a strong predictor of disability accumulation, independent of relapse activity, over the long-term. | [74] |
| Treatment | Time to DMT initiation | 2021 | Retrospective multi-center cohort study (BMSD) | 11,871 RRMS patients | 12-month CDW | HR 95% CI 1.21 (1.09–1.35) p = 0.0004 | The time interval between disease onset and the first DMT start is a strong predictor of disability accumulation, independent of relapse activity, over the long-term. | [74] |
| Treatment | Time to DMT initiation | 2020 | Retrospective multi-center cohort study (MSBase registry; Swedish MS registry) | 308 in the MSBase registry and 236 in the Swedish MS registry | Difference in mean EDSS score over the years of follow-up | Mean EDSS 2.2 (SD 1.6) in the early group compared with 2.9 (SD 1.8) in the late group (p < 0.0001). All follow-up years (mean EDSS score 2.3 [SD 1.8] vs. 3.5 [SD 2.1]; p < 0.0001), with a difference between groups of −0.98 (95% CI −1.51 to −0.45; p < 0.0001) | High-efficacy therapy commenced within 2 years of disease onset is associated with less disability after 6–10 years than when commenced later in the disease course. | [75] |
| Treatment | Time to DMT initiation | 2018 | Retrospective multi-center cohort study | 3795 MS patients (Danish MS Register) | Risk of reaching EDSS 6.0 | HR, 1.42; 95% confidence interval (CI), 1.18–1.70; p < 0.001 | Patients who started treatment with DMT later reached an EDSS score of 6 more quickly compared with patients who started early | [76] |
| Treatment | Time to DMT initiation | 2018 | Retrospective multi-center cohort study | 3795 MS patients (Danish MS Register) | Mortality | HR, 1.38; 95% CI, 0.96–1.99; p = 0.08 | Mortality increased by 38% in later DMT starters. | [76] |
| Treatment | Time to DMT initiation | 2023 | Observational cohort study | 2648 MS patients | MSIS physical score and psychological score worsening | Worsening of MSIS physical score worsening by 2.75 points (95% CI 1.29 to 4.20), and MSIS psychological score by 2.02 points (95% CI 0.03 to 3.78) | Earlier commencement of disease-modifying treatment was associated with better patient-reported physical symptoms | [77] |
| Treatment | Treatment strategy | 2021 | Retrospective multicenter cohort study (RISM) | 2702 RRMS patients (PS matching: 363 pairs) | Mean annual delta-EDSS | Mean annual delta-EDSS values were all significantly (p < 0.02) higher in the ESC group compared with the EIT group. In particular, the mean delta-EDSS differences between the two groups tended to increase from 0.1 (0.01–0.19, p = 0.03) at 1 year to 0.30 (0.07–0.53, p = 0.009) at 5 years and to 0.67 (0.31–1.03, p = 0.0003) at 10 years. | EIT strategy is more effective than ESC strategy in controlling disability progression over time. | [78] |
| Treatment | Treatment strategy | 2023 | Retrospective multi-center cohort study (Swedish MS registry, Czech national MS registry) | 6410 MS patients | Risk of reaching EDSS 4 | HR 0.74, 95% CI 0.6–0.91, p-value 0.0327) | The risk of reaching EDSS 4.0 was reduced by 26% in patients starting with HE DMTs. | [79] |
| Treatment | Treatment strategy | 2023 | Retrospective multi-center cohort study (Swedish MS registry, Czech national MS registry) | 6410 MS patients | Risk of relapses | HR 0.34, 95% CI 0.3–0.39, p-value < 0.001) | The risk of relapses was reduced by 66% (HR 0.34, 95% CI 0.3–0.39, p-value < 0.001) | [79] |
| Treatment | Treatment strategy | 2023 | Retrospective multi-center cohort study (Swedish MS registry, Czech national MS registry) | 6410 MS patients | Probability of confirmed disability improvement (CDI) | HR 3.04, 95% CI 2.37–3.9, p-value < 0.001 | The probability of CDI was three times higher. | [79] |
| Treatment | Treatment strategy | 2019 | Retrospective cohort study | 592 MS patients | Mean (SD) 5-year change in EDSS score | EIT group vs. the ESC group (0.3 [1.5] vs. 1.2 [1.5]). β = −0.85; 95% CI, −1.38 to −0.32; p = 0.002) | Mean (SD) 5-year change in EDSS score was lower in the EIT group than the ESC group | [80] |
| Treatment | Treatment strategy | 2019 | Retrospective cohort study | 593 MS patients | Median time to sustained accumulation of disability (SAD) | 6.0 (3.17–9.16) years for EIT and 3.14 (2.77–4.00) years for ESC (p = 0.05). | Median time to SAD was longer for the EIT group | [80] |
| Treatment | Treatment strategy | 2020 | Retrospective cohort study (Danish MS Register) | 388 patients in the study: 194 starting initial therapy with heDMT matched to 194 patients starting meDMT. | 6-month confirmed EDSS score worsening | 16.7% (95% CI 10.4–23.0%) and 30.1% (95% CI 23.1–37.1%) for heDMT and meDMT initiators, respectively (HR 0.53, 95% CI 0.33–0.83, p = 0.006). | A lower probability of 6-month confirmed EDSS score worsening was found in patients starting a heDMT as first therapy, compared to a matched sample starting meDMT. | [81] |
| Treatment | Treatment strategy | 2020 | Retrospective cohort study (Danish MS Register) | 388 patients in the study: 194 starting initial therapy with heDMT matched to 194 patients starting meDMT. | Risk of first relapse after treatment start | HR 0.50, 95% CI 0.37–0.67 | A lower probability of a first relapse was found in patients starting a heDMT as first therapy, compared to a matched sample starting meDMT. | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra, T.; Copetti, M.; Achille, M.; Ferri, C.; Simone, M.; D’Alfonso, S.; Pugliatti, M.; Iaffaldano, P. Refining Prognostic Factors in Adult-Onset Multiple Sclerosis: A Narrative Review of Current Insights. Int. J. Mol. Sci. 2025, 26, 7756. https://doi.org/10.3390/ijms26167756
Guerra T, Copetti M, Achille M, Ferri C, Simone M, D’Alfonso S, Pugliatti M, Iaffaldano P. Refining Prognostic Factors in Adult-Onset Multiple Sclerosis: A Narrative Review of Current Insights. International Journal of Molecular Sciences. 2025; 26(16):7756. https://doi.org/10.3390/ijms26167756
Chicago/Turabian StyleGuerra, Tommaso, Massimiliano Copetti, Mariaclara Achille, Caterina Ferri, Marta Simone, Sandra D’Alfonso, Maura Pugliatti, and Pietro Iaffaldano. 2025. "Refining Prognostic Factors in Adult-Onset Multiple Sclerosis: A Narrative Review of Current Insights" International Journal of Molecular Sciences 26, no. 16: 7756. https://doi.org/10.3390/ijms26167756
APA StyleGuerra, T., Copetti, M., Achille, M., Ferri, C., Simone, M., D’Alfonso, S., Pugliatti, M., & Iaffaldano, P. (2025). Refining Prognostic Factors in Adult-Onset Multiple Sclerosis: A Narrative Review of Current Insights. International Journal of Molecular Sciences, 26(16), 7756. https://doi.org/10.3390/ijms26167756







