Evaluation of a Classifier Based on Calprotectin Concentration and Advanced Glycation End-Product Receptor as a Potential Biomarker for Abdominal Aortic Aneurysm
Abstract
1. Introduction
1.1. Background and Aims
1.2. Objective
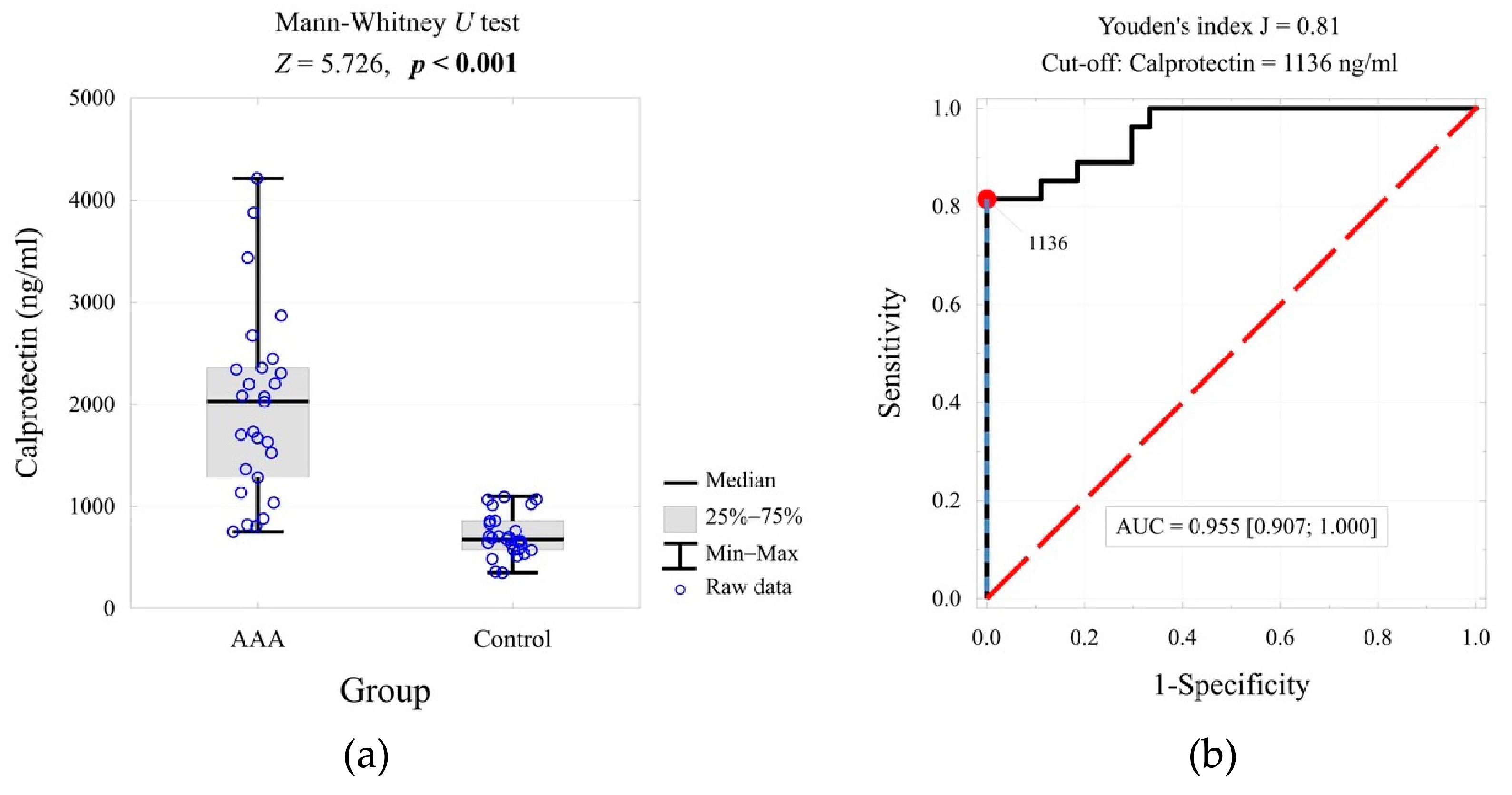
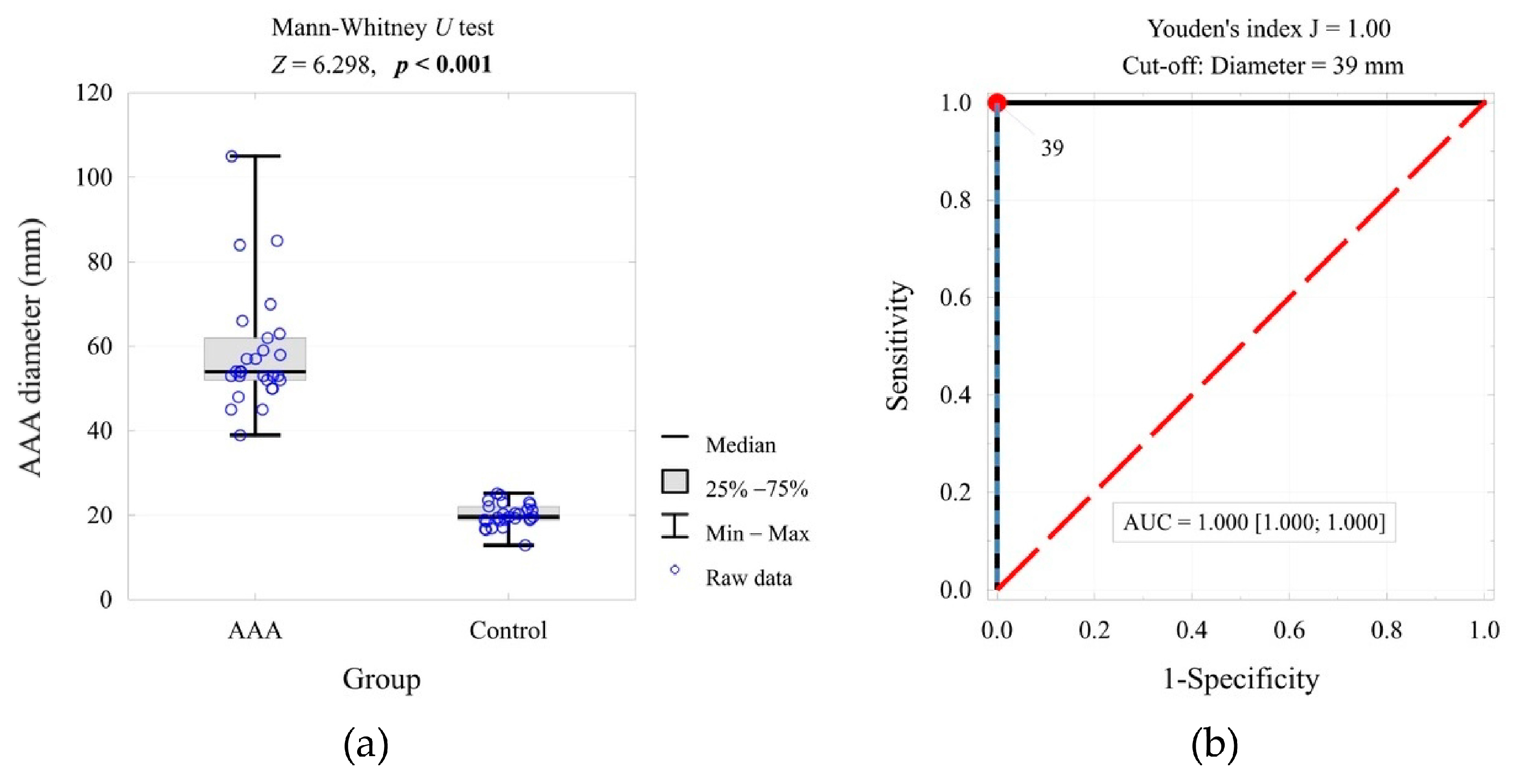
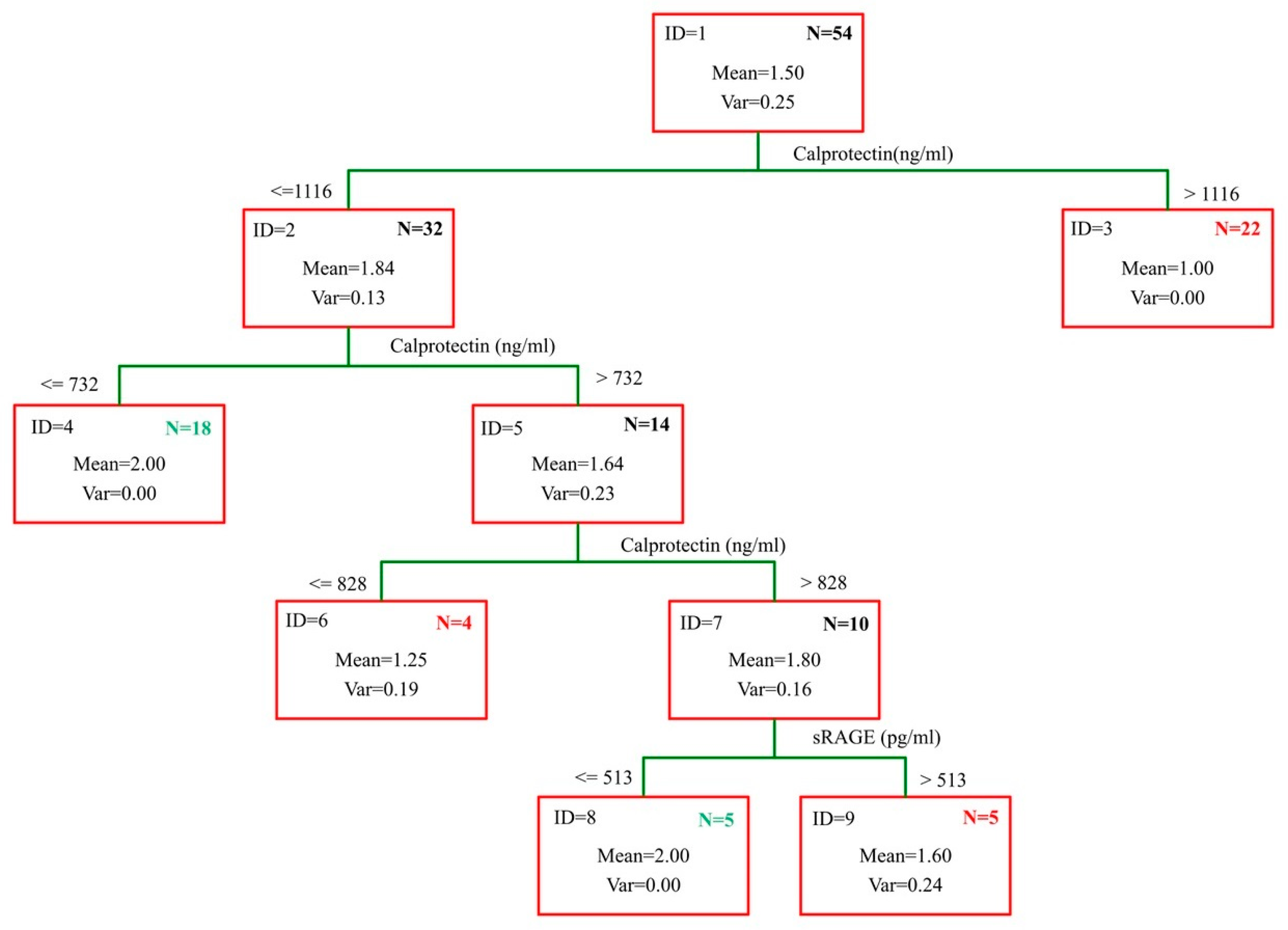
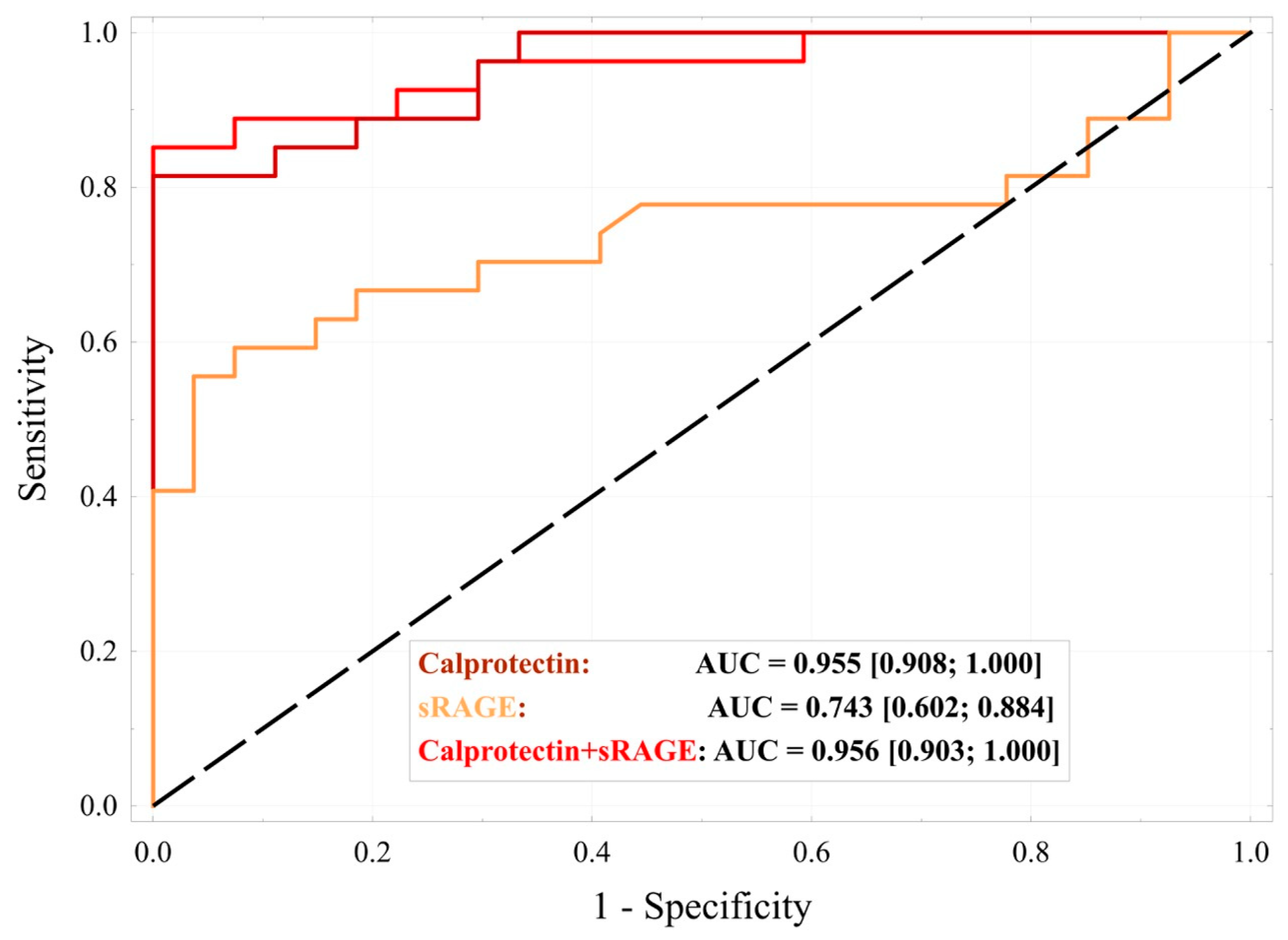
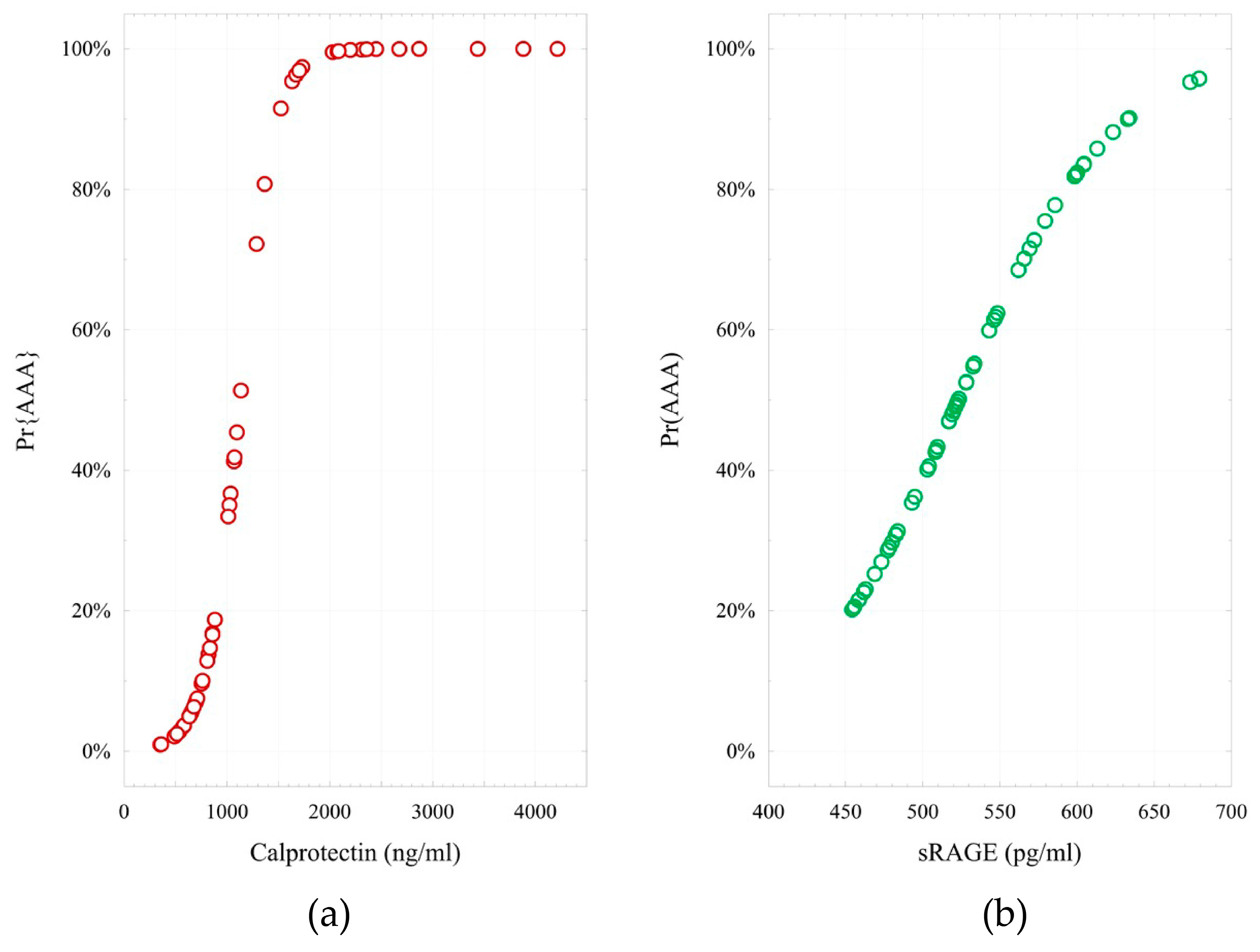
2. Results
- IF (Calprotectin > 1116; “AAA”; IF (Calprotectin > 732; “AAA”;
- IF (Calprotectin < 828; “no-AAA”; IF (sRAGE > 513; “AAA”; “no-AAA”))))
- logit (Pr{AAA} = 1|X) = −6.76 + 0.006 × (Calprotectin). H-L = 2.69; p = 0.952;
- logit (Pr{AAA} = 1|X) = −10.46 + 0.020 × (sRAGE). H-L = 12.8; p = 0.118;
- logit (Pr{AAA} = 1|X) = −19.32 + 0.007 × (Calprotectin) + 0.024 × (sRAGE). H-L = 4.81; p = 0.778.
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Study Group
4.2. Control Group
4.3. Sample Size
4.4. Study Material
4.5. Study Methods
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAA | Abdominal Aortic Aneurysm; |
| ACC | Accuracy; |
| AGE | Advanced Glycation End-Product; |
| AUC | Area Under the Curve; |
| ES | Effect Size; |
| H-L | Hosmer–Lemeshow; |
| HMGB1 | High Mobility Group Box 1; |
| LR | Likelihood Ratio; |
| mRAGE | Membrane-bound RAGE; |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| RAGE | Receptor for Advanced Glycation End-Products; |
| SENS | Sensitivity; |
| SPEC | Specificity; |
| sRAGE | Soluble RAGE. |
| TLR4 | Toll-like receptor 4 |
References
- Urbonavicius, S.; Urbonaviciene, G.; Honoré, B.; Henneberg, E.W.; Vorum, H.; Lindholt, J.S. Potential circulating biomarkers for abdominal aortic aneurysm expansion and rupture—A systematic review. Eur. J. Vasc. Endovasc. Surg. 2008, 36, 273–280, discussion 281–282. [Google Scholar] [CrossRef]
- Márquez-Sánchez, A.C.; Koltsova, E.K. Immune and inflammatory mechanisms of abdominal aortic aneurysm. Front. Immunol. 2022, 13, 989933. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Thanigaimani, S.; Powell, J.T.; Tsao, P.S. Pathogenesis and management of abdominal aortic aneurysm. Eur. Heart J. 2023, 44, 2682–2697. [Google Scholar] [CrossRef] [PubMed]
- Kruzliak, P.; Novák, J.; Novák, M.; Fodor, G.J. Role of calprotectin in cardiometabolic diseases. Cytokine Growth Factor Rev. 2014, 25, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Theocharis, S.; Davakis, S.; Patelis, N.; Agrogiannis, G.; Vlachos, I.S.; Spartalis, E.; Athanasiou, A.; Bakoyiannis, C.; Perrea, D.N.; et al. Serum Calprotectin as a Novel Biomarker in Abdominal Aortic Aneurysm Pathogenesis and Progression: Preliminary Data from Experimental Model in Rats. Curr. Vasc. Pharmacol. 2018, 16, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Plana, E.; Oto, J.; Herranz, R.; Medina, P.; Cana, F.; Miralles, M. Calprotectin as a new inflammatory marker of abdominal aortic aneurysm: A pilot study. Vasc. Med. 2024, 29, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Sarkar, A.; Zafar, M.A.; Shoker, A.; Moselhi, H.E.; Tranquilli, M.; Ziganshin, B.A.; Elefteriades, J.A. Advanced Glycation End Products and its Soluble Receptors in the Pathogenesis of Thoracic Aortic Aneurysm. Aorta 2016, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Park, I.H.; Yeon, S.I.; Youn, J.H.; Choi, J.E.; Sasaki, N.; Choi, I.H.; Shin, J.S. Expression of a novel secreted splice variant of the receptor for advanced glycation end products (RAGE) in human brain astrocytes and peripheral blood mononuclear cells. Mol. Immunol. 2004, 40, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Prasad, K.; Ziganshin, B.A.; Elefteriades, J.A. Reasons to Investigate the Soluble Receptor for Advanced Glycation End-Product (sRAGE) Pathway in Aortic Disease. Aorta 2013, 1, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Lindner, C.; Schneider, I.; Gonzalez, I.; Uribarri, J. The RAGE Axis: A Relevant Inflammatory Hub in Human Diseases. Biomolecules 2024, 14, 412. [Google Scholar] [CrossRef] [PubMed]
- Dobrucki, I.T.; Miskalis, A.; Nelappana, M.; Applegate, C.; Wozniak, M.; Czerwinski, A.; Kalinowski, L.; Dobrucki, L.W. Receptor for advanced glycation end-products: Biological significance and imaging applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1935. [Google Scholar] [CrossRef] [PubMed]
- Basta, G.; Lazzerini, G.; Massaro, M.; Simoncini, T.; Tanganelli, P.; Fu, C.; Kislinger, T.; Stern, D.M.; Schmidt, A.M.; De Caterina, R. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: A mechanism for amplification of inflammatory responses. Circulation 2002, 105, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Angel, K.; Provan, S.A.; Fagerhol, M.K.; Mowinckel, P.; Kvien, T.K.; Atar, D. Effect of 1-year anti-TNF-α therapy on aortic stiffness, carotid atherosclerosis, and calprotectin in inflammatory arthropathies: A controlled study. Am. J. Hypertens. 2012, 25, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tang, Y.; Jin, X.; Chen, C.; Lu, Y.; Liu, L.; Shen, C. Metformin Inhibits Advanced Glycation End Products-Induced Inflammatory Response in Murine Macrophages Partly through AMPK Activation and RAGE/NFκB Pathway Suppression. J. Diabetes Res. 2016, 2016, 4847812. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Yamagishi, S.-I.; Okuda, S. Association Between Metformin and Abdominal Aortic Aneurysm: A Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 908747. [Google Scholar] [CrossRef] [PubMed]
- Fukami, K.; Yamagishi, S.-I.; Okuda, S. Role of AGEs-RAGE system in cardiovascular disease. Curr. Pharm. Des. 2014, 20, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-H.; Wang, K.-C.; Kuo, C.-H.; Lee, F.-T.; Cheng, T.-L.; Chang, B.-I.; Yang, Y.-J.; Shi, G.-Y.; Wu, H.-L. Recombinant adeno-associated virus vector carrying the thrombomodulin lectin-like domain for the treatment of abdominal aortic aneurysm. Atherosclerosis 2017, 262, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhuang, J.; Li, Y.; Jing, B.; Li, H.; Li, J.; Shao, C.; Li, K.; Wang, H. Association of polymorphisms of the receptor for advanced glycation end products gene and susceptibility to sporadic abdominal aortic aneurysm. BioMed Res. Int. 2015, 2015, 394126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Kent, K.C.; Yamanouchi, D.; Zhang, Y.; Kato, K.; Tsai, S.; Nowygrod, R.; Schmidt, A.M.; Liu, B. Anti-receptor for advanced glycation end products therapies as novel treatment for abdominal aortic aneurysm. Ann. Surg. 2009, 250, 416–423. [Google Scholar] [CrossRef] [PubMed]


| Variable | Group | p-Value | ES | 1-β | |
|---|---|---|---|---|---|
| AAA | Control | ||||
| Calprotectin (ng/mL) | <0.001 | 2.53 | 1.000 | ||
| Mean ± SD | 1981 ± 902 | 715 ± 206 | |||
| Me [Q1; Q3] | 2028 [1286; 2358] | 678 [576; 858] | |||
| sRAGE (pg/mL) | 0.002 | 2.16 | 0.986 | ||
| Mean ± SD | 560 ± 66 | 508 ± 32 | |||
| Me [Q1; Q3] | 569 [516; 605] | 508 [483; 528] | |||
| AAA/aortic diameter (mm) | <0.001 | 4.81 | 1.000 | ||
| Mean ± SD | 58.5 ± 13.9 | 20.0 ± 2.7 | |||
| Me [Q1; Q3] | 54 [52; 62] | 20 [19; 22] | |||
| Calprotectin | Diameter (mm) | Test Result | OR [95% CI] | |
|---|---|---|---|---|
| ≥39 mm n = 27 | <39 mm n = 27 | |||
| ≥1136 ng/mL | 22 (81.5%) | 0 (0.0%) | p < 0.001 1-β = 1.00 | 225 [11.8; 4291] |
| <1136 ng/mL | 5 (18.5%) | 27 (100.0%) | 1.00 (ref.) | |
| sRAGE | Diameter (mm) | Test Result | OR [95% CI] | |
|---|---|---|---|---|
| ≥39 mm n = 27 | <39 mm n = 27 | |||
| ≥566 pg/mL | 14 (51.8%) | 1 (3.7%) | p < 0.001 1-β = 1.00 | 28 [3.31; 236.8] |
| <566 pg/mL | 13 (48.2%) | 26 (96.3%) | 1.00 (ref.) | |
| AAA Status | Diameter (mm) | Test Result | OR [95% CI] | |
|---|---|---|---|---|
| ≥39 mm n = 27 | <39 mm n = 27 | |||
| AAA | 22 (81.5%) | 5 (18.5%) | p < 0.001 1-β = 1.00 | 287 [14.7; 5617] |
| no-AAA | 0 (0.0%) | 27 (100.0%) | 1.00 (ref.) | |
| Decision Tree Classification | Diameter (mm) | Test Result | OR [95% CI] | |
|---|---|---|---|---|
| ≥39 mm n = 27 | <39 mm n = 27 | |||
| AAA | 27 (100.0%) | 9 (33.3%) | p < 0.001 1-β = 1.00 | 107 [5.86; 1955] |
| no-AAA | 0 (0.0%) | 18 (66.7%) | 1.00 (ref.) | |
| Decision Tree Rule Diagnosis | AAA n = 30 | no-AAA n = 40 | Test Result | OR [95% CI] |
|---|---|---|---|---|
| AAA | 30 (100.0%) | 20 (50.0%) | 0.001 | 61.0 [3.49; 1066] |
| no-AAA | 0 (0.0%) | 20 (50.0%) | 1.00 (ref.) |
| Variable | Group | p-Value | |
|---|---|---|---|
| AAA | Control | ||
| Age (years), mean ± SD | 69.0 ± 6.5 | 68.7 ± 6.3 | 0.866 |
| Type 2 diabetes (yes), n (%) | 6 (22.2) | 4 (14.8) | 0.728 |
| Arterial hypertension (yes), n (%) | 23 (85.2) | 22 (81.5) | 1.000 |
| Arterial disease *, n (%) | 6 (22.2) | 6 (22.2) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hauzer, W.; Hauzer, P.; Klimek, T.; Gnus, J.; Witkiewicz, W.; Jędruchniewicz, N. Evaluation of a Classifier Based on Calprotectin Concentration and Advanced Glycation End-Product Receptor as a Potential Biomarker for Abdominal Aortic Aneurysm. Int. J. Mol. Sci. 2025, 26, 7752. https://doi.org/10.3390/ijms26167752
Hauzer W, Hauzer P, Klimek T, Gnus J, Witkiewicz W, Jędruchniewicz N. Evaluation of a Classifier Based on Calprotectin Concentration and Advanced Glycation End-Product Receptor as a Potential Biomarker for Abdominal Aortic Aneurysm. International Journal of Molecular Sciences. 2025; 26(16):7752. https://doi.org/10.3390/ijms26167752
Chicago/Turabian StyleHauzer, Willy, Paula Hauzer, Tomasz Klimek, Jan Gnus, Wojciech Witkiewicz, and Natalia Jędruchniewicz. 2025. "Evaluation of a Classifier Based on Calprotectin Concentration and Advanced Glycation End-Product Receptor as a Potential Biomarker for Abdominal Aortic Aneurysm" International Journal of Molecular Sciences 26, no. 16: 7752. https://doi.org/10.3390/ijms26167752
APA StyleHauzer, W., Hauzer, P., Klimek, T., Gnus, J., Witkiewicz, W., & Jędruchniewicz, N. (2025). Evaluation of a Classifier Based on Calprotectin Concentration and Advanced Glycation End-Product Receptor as a Potential Biomarker for Abdominal Aortic Aneurysm. International Journal of Molecular Sciences, 26(16), 7752. https://doi.org/10.3390/ijms26167752







