Role of Different Enzymes in H2O2 Neutralization and Cellular Radioresistance, Estimated by Mathematical Modeling
Abstract
1. Introduction
2. Results and Discussion
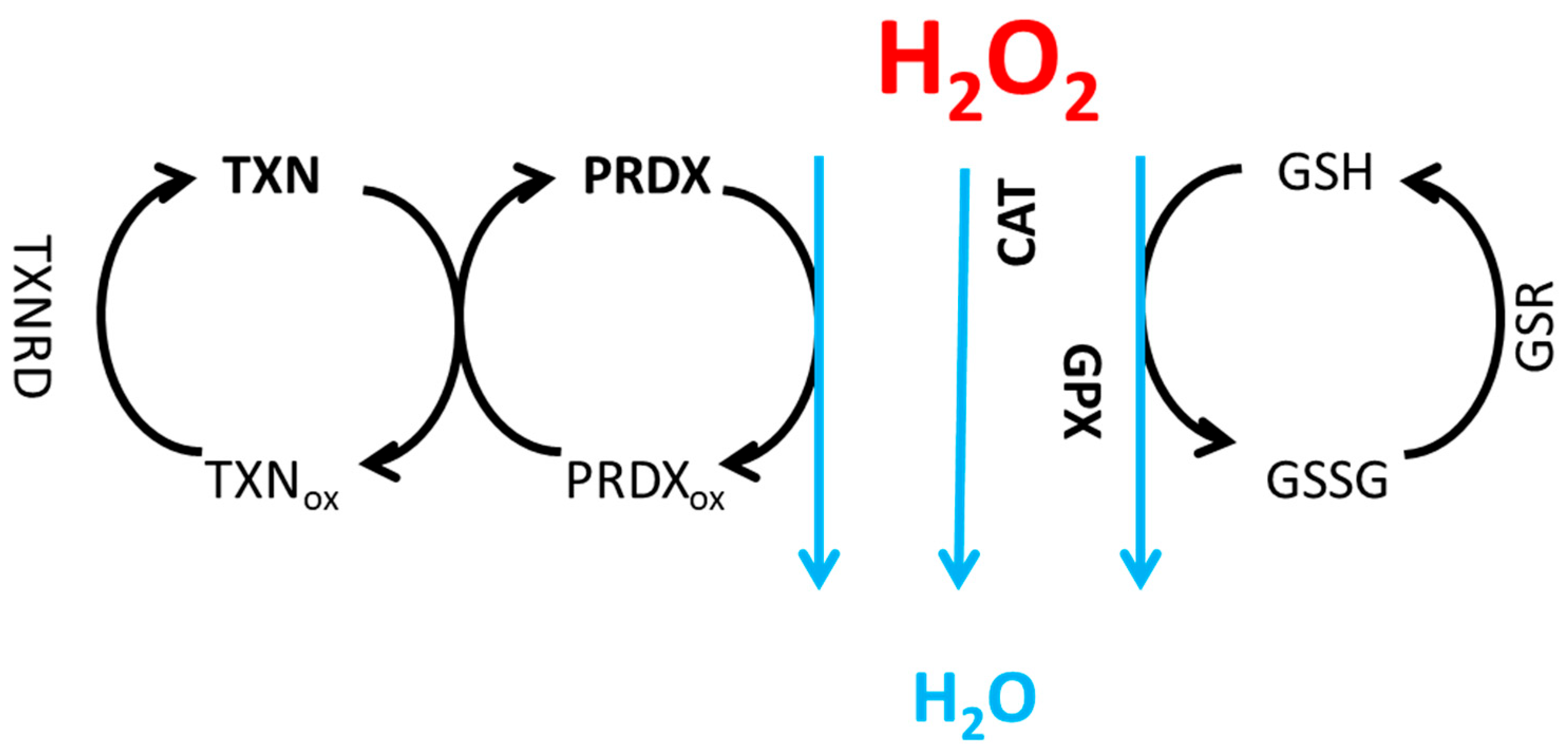
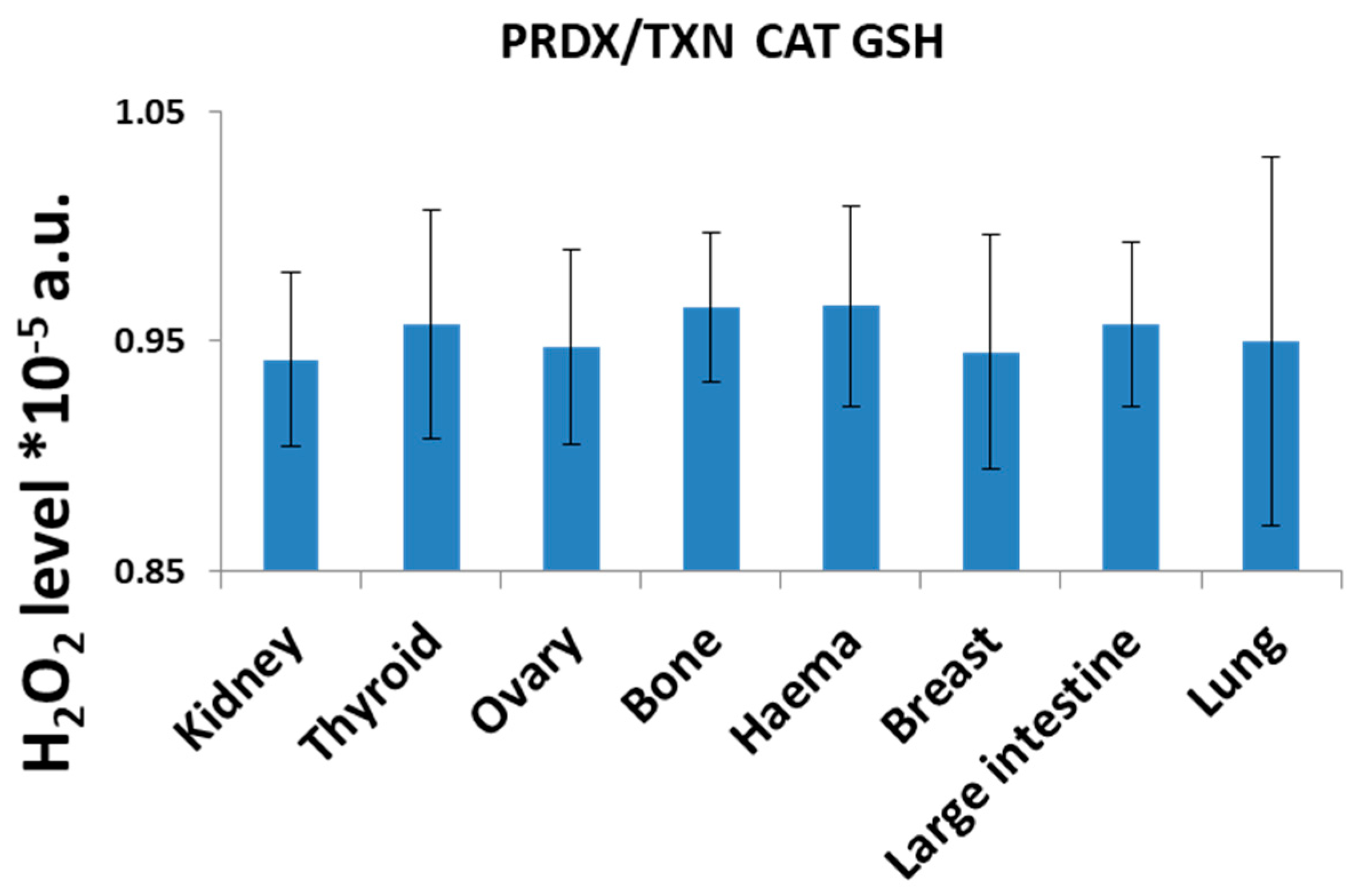
H2O2 Neutralization Pathways and Their Connection to Radioresistance
3. Materials and Methods
Model Equations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarsour, E.H.; Kumar, M.G.; Chaudhuri, L.; Kalen, A.L.; Goswami, P.C. Redox control of the cell cycle in health and disease. Antioxid. Redox Signal. 2009, 11, 2985–3011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xing, D.; Gao, X. Low-power laser irradiation activates Src tyrosine kinase through reactive oxygen species-mediated signaling pathway. J. Cell. Physiol. 2008, 217, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Feinendegen, L.; Pollycove, M.; Sondhaus, C.A. Responses to Low Doses of Ionizing Radiation in Biological Systems. Nonlinearity Biol. Toxicol. Med. 2004, 2, 154014204905074. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Valle, N.R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta (BBA)—Biomembr. 2006, 1758, 994–1003. [Google Scholar] [CrossRef]
- Stone, J.R.; Yang, S. Hydrogen Peroxide: A Signaling Messenger. Antioxid. Redox Signal. 2006, 8, 243–270. [Google Scholar] [CrossRef]
- Antunes, F.; Cadenas, E. Cellular titration of apoptosis with steady state concentrations of H2O2: Submicromolar levels of H2O2 induce apoptosis through fenton chemistry independent of the cellular thiol state. Free Radic. Biol. Med. 2001, 30, 1008–1018. [Google Scholar] [CrossRef]
- Gülden, M.; Jess, A.; Kammann, J.; Maser, E.; Seibert, H. Cytotoxic potency of H2O2 in cell cultures: Impact of cell concentration and exposure time. Free Radic. Biol. Med. 2010, 49, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Burdon, R. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic. Biol. Med. 1995, 18, 775–794. [Google Scholar] [CrossRef]
- Lee, J.; Koo, N.; Min, D.B. Reactive Oxygen Species, Aging, and Antioxidative Nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Matsunaga, S.; Kohda, A.; Kamakura, S.; Hayase, J.; Miyano, K.; Shiose, A.; Sumimoto, H. Hypoxia stabilizes the H2O2-producing oxidase Nox4 in cardiomyocytes via suppressing autophagy-related lysosomal degradation. Genes Cells 2024, 29, 63–72. [Google Scholar] [CrossRef]
- Radi, R.; Cassina, A.; Hodara, R.; Quijano, C.; Castro, L. Peroxynitrite reactions and formation in mitochondria. Free Radic. Biol. Med. 2002, 33, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Henle, E.; Linn, S. Formation, prevention, and repair of DNA damage by iron/hydrogen peroxide. J. Biol. Chem. 1997, 272, 19095–19098. [Google Scholar] [CrossRef] [PubMed]
- Florence, T.M. The production of hydroxyl radical from hydrogen peroxide. J. Inorg. Biochem. 1984, 22, 221–230. [Google Scholar] [CrossRef]
- Puppo, A.; Halliwellt, B. Formation of hydroxyl radicals from hydrogen peroxide in the presence of iron Is haemoglobin a biological Fenton reagent? Biochem. J. 1988, 249, 185–190. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Meister, A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988, 263, 17205–17208. [Google Scholar] [CrossRef]
- Shelly, C.L. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Adamiec, M.; Skonieczna, M. UV radiation in HCT 116 cells influences intracellular H2O2 and glutathione levels, antioxidant expression, and protein glutathionylation. Acta Biochim. Pol. 2019, 66, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Immenschuh, S.; Baumgart-Vogt, E. Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid. Redox Signal. 2005, 7, 768–777. [Google Scholar] [CrossRef]
- Poynton, R.A.; Hampton, M.B. Peroxiredoxins as biomarkers of oxidative stress. Biochim. Biophys. Acta—Gen. Subj. 2014, 1840, 906–912. [Google Scholar] [CrossRef]
- Sue, G.R.; Ho, Z.C.; Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005, 38, 1543–1552. [Google Scholar] [CrossRef]
- Hewitt, O.H.; Degnan, S.M. Antioxidant enzymes that target hydrogen peroxide are conserved across the animal kingdom, from sponges to mammals. Sci. Rep. 2023, 13, 2510. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Cederbaum, A.I. Mitochondrial catalase and oxidative injury. NeuroSignals 2001, 10, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione peroxidase family—An evolutionary overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef]
- Powis, G.; Montfort, W.R. Properties and biological activities of thioredoxins. Annu. Rev. Biophys. Biomol. Struct. 2001, 30, 421–455. [Google Scholar] [CrossRef]
- Arnér, E.S.J.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. [Google Scholar] [CrossRef]
- Melo, D.; Coimbra, S.; Rocha, S.; Santos-Silva, A. Inhibition of erythrocyte’s catalase, glutathione peroxidase or peroxiredoxin 2—Impact on cytosol and membrane. Arch. Biochem. Biophys. 2023, 739, 109569. [Google Scholar] [CrossRef]
- Mitozo, P.A.; de Souza, L.F.; Loch-Neckel, G.; Flesch, S.; Maris, A.F.; Figueiredo, C.P.; dos Santos, A.R.S.; Farina, M.; Dafre, A.L. A study of the relative importance of the peroxiredoxin-, catalase-, and glutathione-dependent systems in neural peroxide metabolism. Free Radic. Biol. Med. 2011, 51, 69–77. [Google Scholar] [CrossRef]
- Ciesielska, S.; Bil, P.; Gajda, K.; Poterala-Hejmo, A.; Hudy, D.; Rzeszowska-Wolny, J. Cell type-specific differences in redox regulation and proliferation after low UVA doses. PLoS ONE 2019, 14, e0205215. [Google Scholar] [CrossRef]
- Bil, P.; Ciesielska, S.; Jaksik, R.; Rzeszowska-Wolny, J. Circuits Regulating Superoxide and Nitric Oxide Production and Neutralization in Different Cell Types: Expression of Participating Genes and Changes Induced by Ionizing Radiation. Antioxidants 2020, 9, 701. [Google Scholar] [CrossRef]
- Aon, M.A.; Stanley, B.A.; Sivakumaran, V.; Kembro, J.M.; O’Rourke, B.; Paolocci, N.; Cortassa, S. Glutathione/thioredoxin systems modulate mitochondrial H2O2 emission: An experimental-computational study. J. Gen. Physiol. 2012, 139, 479. [Google Scholar] [CrossRef]
- Cortassa, S.; Aon, M.A.; Winslow, R.L.; O’Rourke, B. A Mitochondrial Oscillator Dependent on Reactive Oxygen Species. Biophys. J. 2004, 87, 2060–2073. [Google Scholar] [CrossRef]
- Kembro, J.M.; Aon, M.A.; Winslow, R.L.; O’Rourke, B.; Cortassa, S. Integrating Mitochondrial Energetics, Redox and ROS Metabolic Networks: A Two-Compartment Model. Biophys. J. 2013, 104, 332–343. [Google Scholar] [CrossRef]
- Ng, C.F.; Schafer, F.Q.; Buettner, G.R.; Rodgers, V.G.J. The rate of cellular hydrogen peroxide removal shows dependency on GSH: Mathematical insight into in vivo H2O2 and GPx concentrations. Free Radic. Res. 2007, 41, 1201. [Google Scholar] [CrossRef]
- Weaver, K.; Skouta, R. The Selenoprotein Glutathione Peroxidase 4: From Molecular Mechanisms to Novel Therapeutic Opportunities. Biomedicines 2022, 10, 891. [Google Scholar] [CrossRef]
- Yang, H.J.; Kim, N.; Seong, K.M.; Youn, H.; Youn, B. Investigation of Radiation-induced Transcriptome Profile of Radioresistant Non-small Cell Lung Cancer A549 Cells Using RNA-seq. PLoS ONE 2013, 8, e59319. [Google Scholar] [CrossRef]
- Amornwichet, N.; Oike, T.; Shibata, A.; Nirodi, C.S.; Ogiwara, H.; Makino, H.; Kimura, Y.; Hirota, Y.; Isono, M.; Yoshida, Y.; et al. The EGFR mutation status affects the relative biological effectiveness of carbon-ion beams in non-small cell lung carcinoma cells. Sci. Rep. 2015, 5, 11305. [Google Scholar] [CrossRef]
- Das, A.K.; Bell, M.H.; Nirodi, C.S.; Story, M.D.; Minna, J.D. Radiogenomics- predicting tumor responses to radiotherapy in lung cancer. Semin. Radiat. Oncol. 2010, 20, 149–155. [Google Scholar] [CrossRef]
- Karagounis, I.V.; Kalamida, D.; Mitrakas, A.; Pouliliou, S.; Liousia, M.V.; Giatromanolaki, A.; Koukourakis, M.I. Repression of the autophagic response sensitises lung cancer cells to radiation and chemotherapy. Br. J. Cancer 2016, 115, 312–321. [Google Scholar] [CrossRef]
- Schilling, D.; Bayer, C.; Li, W.; Molls, M.; Vaupel, P.; Multhoff, G. Radiosensitization of Normoxic and Hypoxic H1339 Lung Tumor Cells by Heat Shock Protein 90 Inhibition Is Independent of Hypoxia Inducible Factor-1α. PLoS ONE 2012, 7, e31110. [Google Scholar] [CrossRef]
- Kang, J.; Kim, W.; Kwon, T.; Youn, H.; Kim, J.S.; Youn, B. Plasminogen activator inhibitor-1 enhances radioresistance and aggressiveness of non-small cell lung cancer cells. Oncotarget 2016, 7, 23961–23974. [Google Scholar] [CrossRef]
- Liberal, F.D.C.G.; McMahon, S.J. Characterization of Intrinsic Radiation Sensitivity in a Diverse Panel of Normal, Cancerous and CRISPR-Modified Cell Lines. Int. J. Mol. Sci. 2023, 24, 7861. [Google Scholar] [CrossRef]
- Lüdeking, M.; Stemwedel, K.; Ramachandran, D.; Grosche, S.; Christiansen, H.; Merten, R.; Henkenberens, C.; Bogdanova, N.V. Efficiency of moderately hypofractionated radiotherapy in NSCLC cell model. Front. Oncol. 2024, 14, 1293745. [Google Scholar] [CrossRef]
- Carmichael, J.; Degraff, W.G.; Gamson, J.; Russo, D.; Gazdar, A.F.; Levitt, M.L.; Minna, J.D.; Mitchell, J.B. Radiation sensitivity of human lung cancer cell lines. Eur. J. Cancer Clin. Oncol. 1989, 25, 527–534. [Google Scholar] [CrossRef]
- Yun, H.S.; Baek, J.-H.; Yim, J.-H.; Um, H.-D.; Park, J.K.; Song, J.-Y.; Park, I.-C.; Kim, J.-S.; Lee, S.-J.; Lee, C.-W.; et al. Radiotherapy diagnostic biomarkers in radioresistant human H460 lung cancer stem-like cells. Cancer Biol. Ther. 2016, 17, 208–218. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Casciati, A.; Bakar, Z.A.; Hamzah, H.; Ahmad, T.A.T.; Noor, M.H.M. The Detection of DNA Damage Response in MCF7 and MDA-MB-231 Breast Cancer Cell Lines after X-ray Exposure. Genome Integr. 2023, 14, 20220001. [Google Scholar] [CrossRef]
- Bristol, M.L.; Di, X.; Beckman, M.J.; Wilson, E.N.; Henderson, S.C.; Maiti, A.; Fan, Z.; Gewirtz, D.A. Dual functions of autophagy in the response of breast tumor cells to radiation. Autophagy 2012, 8, 739–753. [Google Scholar] [CrossRef]
- Bruss, C.; Albert, V.; Seitz, S.; Blaimer, S.; Kellner, K.; Pohl, F.; Ortmann, O.; Brockhoff, G.; Wege, A.K. Neoadjuvant radiotherapy in ER+, HER2+, and triple-negative -specific breast cancer based humanized tumor mice enhances anti-PD-L1 treatment efficacy. Front. Immunol. 2024, 15, 1355130. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Lee, M.G.; Kim, N.Y.; Nam, G.S.; Nam, K.S.; Jang, H.; Kim, S. Overcoming radioresistance of breast cancer cells with MAP4K4 inhibitors. Sci. Rep. 2024, 14, 7410. [Google Scholar] [CrossRef]
- Gray, M.; Turnbull, A.K.; Ward, C.; Meehan, J.; Martínez-Pérez, C.; Bonello, M.; Pang, L.Y.; Langdon, S.P.; Kunkler, I.H.; Murray, A.; et al. Development and characterisation of acquired radioresistant breast cancer cell lines. Radiat. Oncol. 2019, 14, 64. [Google Scholar] [CrossRef]
- Lafontaine, J.; Boisvert, J.-S.; Glory, A.; Coulombe, S.; Wong, P. Synergy between Non-Thermal Plasma with Radiation Therapy and Olaparib in a Panel of Breast Cancer Cell Lines. Cancers 2020, 12, 348. [Google Scholar] [CrossRef]
- Anastasov, N.; Höfig, I.; Vasconcellos, I.G.; Rappl, K.; Braselmann, H.; Ludyga, N.; Auer, G.; Aubele, M.; Atkinson, M.J. Radiation resistance due to high expression of miR-21 and G2/M checkpoint arrest in breast cancer cells. Radiat. Oncol. 2012, 7, 206. [Google Scholar] [CrossRef]
- Aschenbrenner, B.; Negro, G.; Savic, D.; Sorokin, M.; Buzdin, A.; Ganswindt, U.; Cemazar, M.; Sersa, G.; Skvortsov, S.; Skvortsova, I. Simvastatin Is Effective in Killing the Radioresistant Breast Carcinoma Cells. Radiol. Oncol. 2021, 55, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Rajagopalan, D.; Hora, S.; Jadhav, S.P. Breast Cancer: From Transcriptional Control to Clinical Outcome. In Breast Cancer—From Biology to Medicine; Pham, P.V., Ed.; InTech: Nappanee, IN, USA, 2017; ISBN 978-953-51-2999-8. [Google Scholar]
- HCC70: A Model of Triple Negative Breast Cancer. Available online: https://oncology.labcorp.com/hcc70-model-triple-negative-breast-cancer (accessed on 22 March 2025).
- Steffen, A.-C.; Göstring, L.; Tolmachev, V.; Palm, S.; Stenerlöw, B.; Carlsson, J. Differences in radiosensitivity between three HER2 overexpressing cell lines. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, A.; Nasonova, E.; Kutsalo, P.; Czerski, K. Chromosomal radiosensitivity of human breast carcinoma cells and blood lymphocytes following photon and proton exposures. Radiat. Environ. Biophys. 2023, 62, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Schröder-Heurich, B.; Bogdanova, N.; Wieland, B.; Xie, X.; Noskowicz, M.; Park-Simon, T.W.; Dörk, T. Functional deficiency of NBN, the Nijmegen breakage syndrome protein, in a p.R215W mutant breast cancer cell line. BMC Cancer 2014, 14, 434. [Google Scholar] [CrossRef]
- Kobunai, T.; Watanabe, T.; Fukusato, T. REG4, NEIL2, and BIRC5 Gene Expression Correlates with Gamma-radiation Sensitivity in Patients with Rectal Cancer Receiving Radiotherapy. Anticancer Res. 2011, 31, 4147–4153. [Google Scholar]
- Guardamagna, I.; Lonati, L.; Savio, M.; Stivala, L.A.; Ottolenghi, A.; Baiocco, G. An Integrated Analysis of the Response of Colorectal Adenocarcinoma Caco-2 Cells to X-Ray Exposure. Front. Oncol. 2021, 11, 688919. [Google Scholar] [CrossRef]
- Morini, J.; Babini, G.; Barbieri, S.; Baiocco, G.; Ottolenghi, A. The Interplay between Radioresistant Caco-2 Cells and the Immune System Increases Epithelial Layer Permeability and Alters Signaling Protein Spectrum. Front. Immunol. 2017, 8, 223. [Google Scholar] [CrossRef]
- Dunne, A.L.; Price, M.E.; Mothersill, C.; McKeown, S.R.; Robson, T.; Hirst, D.G. Relationship between clonogenic radiosensitivity, radiation-induced apoptosis and DNA damage/repair in human colon cancer cells. Br. J. Cancer 2003, 89, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Rödel, C.; Haas, J.; Groth, A.; Grabenbauer, G.G.; Sauer, R.; Rödel, F. Spontaneous and radiation-induced apoptosis in colorectal carcinoma cells with different intrinsic radiosensitivities: Survivin as a radioresistance factor. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Clifford, R.E.; Govindarajah, N.; Bowden, D.; Sutton, P.; Glenn, M.; Darvish-Damavandi, M.; Buczacki, S.; McDermott, U.; Szulc, Z.; Ogretmen, B.; et al. Targeting Acid Ceramidase to Improve the Radiosensitivity of Rectal Cancer. Cells 2020, 9, 2693. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, C.; Yang, X.; Zhang, Q.; Chen, F. Prognostic roles of mRNA expression of peroxiredoxins in lung cancer. OncoTargets Ther. 2018, 11, 8381–8388. [Google Scholar] [CrossRef]
- Ciesielska, S.; Slezak-Prochazka, I.; Bil, P.; Rzeszowska-Wolny, J. Micro RNAs in Regulation of Cellular Redox Homeostasis. Int. J. Mol. Sci. 2021, 22, 6022. [Google Scholar] [CrossRef]
- Heo, S.; Kim, S.; Kang, D. The Role of Hydrogen Peroxide and Peroxiredoxins throughout the Cell Cycle. Antioxidants 2020, 9, 280. [Google Scholar] [CrossRef]
- Liu, C.; Nie, J.; Wang, R.; Mao, W. The Cell Cycle G2/M Block Is an Indicator of Cellular Radiosensitivity. Dose-Response 2019, 17, 1559325819891008. [Google Scholar] [CrossRef]
- Tamamoto, T.; Ohnishi, K.; Takahashi, A.; Wang, X.; Yosimura, H.; Ohishi, H.; Uchida, H.; Ohnishi, T. Correlation between γ-ray-induced G2 arrest and radioresistance in two human cancer cells. Int. J. Radiat. Oncol. *Biol. *Phys. 1999, 44, 905–909. [Google Scholar] [CrossRef]
- Smith-Pearson, P.S.; Kooshki, M.; Spitz, D.R.; Poole, L.B.; Zhao, W.; Robbins, M.E. Decreasing peroxiredoxin II expression decreases glutathione, alters cell cycle distribution, and sensitizes glioma cells to ionizing radiation and H2O2. Free Radic. Biol. Med. 2008, 45, 1178–1189. [Google Scholar] [CrossRef]
- Sciegienka, S.J.; Solst, S.R.; Falls, K.C.; Schoenfeld, J.D.; Klinger, A.R.; Ross, N.L.; Rodman, S.N.; Spitz, D.R.; Fath, M.A. D-penicillamine combined with inhibitors of hydroperoxide metabolism enhances lung and breast cancer cell responses to radiation and carboplatin via H2O2-mediated oxidative stress. Free Radic. Biol. Med. 2017, 108, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Oraki Kohshour, M.; Najafi, L.; Heidari, M.; Ghaffari Sharaf, M. Antiproliferative Effect of H2O2 against Human Acute Myelogenous Leukemia KG1 Cell Line. J. Acupunct. Meridian Stud. 2013, 6, 134–141. [Google Scholar] [CrossRef]
- Vilema-Enríquez, G.; Arroyo, A.; Grijalva, M.; Amador-Zafra, R.I.; Camacho, J. Molecular and Cellular Effects of Hydrogen Peroxide on Human Lung Cancer Cells: Potential Therapeutic Implications. Oxidative Med. Cell. Longev. 2016, 2016, 1908164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Coulter, J.A.; Yang, R. Octaarginine-modified gold nanoparticles enhance the radiosensitivity of human colorectal cancer cell line LS180 to megavoltage radiation. Int. J. Nanomed. 2018, 13, 3541–3552. [Google Scholar] [CrossRef] [PubMed]
- Ubezio, P.; Civoli, F. Flow cytometric detection of hydrogen peroxide production induced by doxorubicin in cancer cells. Free Radic. Biol. Med. 1994, 16, 509–516. [Google Scholar] [CrossRef]
- Arnold, R.; Shi, J.; Murad, E.; Whalen, A.; Sun, C.; Polavarapu, R.; Parthasarathy, S.; Petros, J.; Lambeth, J. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc. Natl. Acad. Sci. USA 2001, 98, 5550–5555. [Google Scholar] [CrossRef]
- Nguyen, L.; Dobiasch, S.; Schneider, G.; Schmid, R.M.; Azimzadeh, O.; Kanev, K.; Buschmann, D.; Pfaffl, M.W.; Bartzsch, S.; Schmid, T.E.; et al. Impact of DNA repair and reactive oxygen species levels on radioresistance in pancreatic cancer. Radiother. Oncol. 2021, 159, 265–276. [Google Scholar] [CrossRef]
- Conour, J.E.; Graham, W.V.; Gaskins, H.R. A combined in vitro/bioinformatic investigation of redox regulatory mechanisms governing cell cycle progression. Physiol. Genom. 2004, 18, 196–205. [Google Scholar] [CrossRef][Green Version]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of Reactive Oxygen Species Levels and Radioresistance in Cancer Stem Cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef]
- Emmink, B.L.; Laoukili, J.; Kipp, A.P.; Koster, J.; Govaert, K.M.; Fatrai, S.; Verheem, A.; Steller, E.J.A.; Brigelius-Flohé, R.; Jimenez, C.R.; et al. GPx2 Suppression of H2O2 Stress Links the Formation of Differentiated Tumor Mass to Metastatic Capacity in Colorectal Cancer. Cancer Res. 2014, 74, 6717–6730. [Google Scholar] [CrossRef]
- Reinema, F.V.; Hudson, N.; Adema, G.J.; Peeters, W.J.M.; Neuzil, J.; Stursa, J.; Werner, L.; Sweep, F.C.G.J.; Bussink, J.; Span, P.N. MitoTam induces ferroptosis and increases radiosensitivity in head and neck cancer cells. Radiother. Oncol. 2024, 200, 110503. [Google Scholar] [CrossRef]
- Park, W.H. Hydrogen peroxide inhibits the growth of lung cancer cells via the induction of cell death and G1-phase arrest. Oncol. Rep. 2018, 40, 1787–1794. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Fang, Y.; Moore, B.; Bai, Q.; Cook, K. Hydrogen Peroxide Enhances Radiation-induced Apoptosis and Inhibition of Melanoma Cell Proliferation | Request PDF. Anticancer Res. 2013, 33, 1799–1807. [Google Scholar] [PubMed]
- Hu, R.; Saito, A.I.; Mitsuhashi, T.; Inoue, T.; Ota, T.; Ujihira, T.; Yoshida, K.; Sasai, K. Radiosensitization using hydrogen peroxide in patients with cervical cancer. Mol. Clin. Oncol. 2021, 15, 1–7. [Google Scholar] [CrossRef]
- Shenton, D.; Smirnova, J.B.; Selley, J.N.; Carroll, K.; Hubbard, S.J.; Pavitt, G.D.; Ashe, M.P.; Grant, C.M. Global Translational Responses to Oxidative Stress Impact upon Multiple Levels of Protein Synthesis. J. Biol. Chem. 2006, 281, 29011–29021. [Google Scholar] [CrossRef] [PubMed]
- Busato, F.; Khouzai, B.E.; Mognato, M. Biological Mechanisms to Reduce Radioresistance and Increase the Efficacy of Radiotherapy: State of the Art. Int. J. Mol. Sci. 2022, 23, 10211. [Google Scholar] [CrossRef]
- Guichard, M.; Dertinger, H.; Malaise, E.P. Radiosensitivity of Four Human Tumor Xenografts. Influence of Hypoxia and Cell-Cell Contact. Radiat. Res. 1983, 95, 602. [Google Scholar] [CrossRef]
- Bolus, N.E. Basic Review of Radiation Biology and Terminology. J. Nucl. Med. Technol. 2017, 45, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Ghandi, M.; Huang, F.W.; Jané-Valbuena, J.; Kryukov, G.V.; Lo, C.C.; McDonald, E.R.; Barretina, J.; Gelfand, E.T.; Bielski, C.M.; Li, H.; et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 2019, 569, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Ambarsari, L.; Lindawati, E. Isolation, Fractionation and Characterization of Catalase from Neurospora crassa (InaCC F226). IOP Conf. Ser. Earth Environ. Sci. 2017, 58, 012068. [Google Scholar] [CrossRef]
- Makino, N.; Mochizuki, Y.; Bannai, S.; Sugita, Y. Kinetic Studies on the Removal of Extracellular Hydrogen Peroxide by Cultured Fibroblasts. J. Biol. Chem. 1994, 269, 1020–1025. [Google Scholar] [CrossRef]
- Karpenko, I.L.; Valuev-Elliston, V.T.; Ivanova, O.N.; Smirnova, O.A.; Ivanov, A.V. Peroxiredoxins-The Underrated Actors during Virus-Induced Oxidative Stress. Antioxidants 2021, 10, 977. [Google Scholar] [CrossRef]
- Manta, B.; Hugo, M.; Ortiz, C.; Ferrer-Sueta, G.; Trujillo, M.; Denicola, A. The peroxidase and peroxynitrite reductase activity of human erythrocyte peroxiredoxin 2’. Arch. Biochem. Biophys. 2009, 484, 146–154. [Google Scholar] [CrossRef]
- Lundberg, E.; Fagerberg, L.; Klevebring, D.; Matic, I.; Geiger, T.; Cox, J.; Uhlen, M. Defining the transcriptome and proteome in three functionally different human cell lines. Mol. Syst. Biol. 2010, 6, 450. [Google Scholar] [CrossRef]
- Edfors, F.; Danielsson, F.; Hallström, B.M.; Käll, L.; Lundberg, E.; Pontén, F.; Forsström, B.; Uhlén, M. Gene-specific correlation of RNA and protein levels in human cells and tissues. Mol. Syst. Biol. 2016, 12, 883. [Google Scholar] [CrossRef] [PubMed]



| Type of Cancer | Cell Line | Radiosensitive (RS)/ Radioresistant (RR) | Reference |
|---|---|---|---|
| Lung | A549 | RR | [39] |
| H1703 | RR | [40] | |
| H661 | RR | [41] | |
| H1299 | RR | [42] | |
| H1339 | RR | [43] | |
| H292 | RR | [44] | |
| H358 | RR | [44] | |
| H23 | RS | [44] | |
| H441 | RS | [45] | |
| H1650 | RS | [46] | |
| H522 | RS | [46] | |
| HCC827 | RS | [40] | |
| H69 | RS | [47] | |
| H460 | RS | [48] | |
| Breast | MCF-7 | RR | [49,50,51] |
| SK-BR-3 | RR | [52] | |
| ZR-751 | RR | [53] | |
| HCC1428 | RR | [54] | |
| T47D | RR | [55,56] | |
| HS578T | RR | [50] | |
| UACC-812 | RR | [57] | |
| MDA-MB-175VII | RR | [54] | |
| MDA-MB-361 | RS | [55] | |
| HCC70 | RS | [58] | |
| MDA-MB-231 | RS | [49,51,56] | |
| BT474 | RS | [50,59] | |
| JIMT-1 | RS | [51] | |
| CAL-51 | RS | [60] | |
| HCC1395 | RS | [61] | |
| Colorectal | HT115 | RR | [62] |
| DLD-1 | RR | [62] | |
| Lovo | RR | [62] | |
| HT29 | RR | [62] | |
| Caco-2 | RR | [63,64] | |
| SW480 | RR | [65,66] | |
| MDST8 | RR | [67] | |
| Colo-201 | RS | [62] | |
| Colo-205 | RS | [62] | |
| Colo-320 | RS | [62] | |
| HCT116 | RS | [62] | |
| SW48 | RS | [65,66] |
| Description | Symbol | Value 1 [mM] |
|---|---|---|
| CAT Concentration | CAT | 0.001 |
| PRDX Concentration | PRDX | 0.15 |
| TXN Concentration | TXN | 0.025 |
| TXNRD Concentration | TXNRD | 0.025 |
| GSH Concentration | GSH | 3.0 |
| GPX Concentration | GPX | 0.05 |
| GSR Concentration | GSR | 0.05 |
| Description | Symbol | Value [unit] |
|---|---|---|
| Rate Constant of CAT | kCAT | 0.034 [mM−1 ms−1] |
| Rate Constant of PRDX | kPRDX | 0.26 [mM−1 ms−1] |
| Rate Constant of TXN | kTXNox | 0.23 [mM−1 ms−1] |
| Rate Constant of TXNRD | kTXNRD | 0.31 [mM−1 ms−1] |
| Rate Constant of GSR | kGSR | 0.08 [mM−1 ms−1] |
| Rate Constant of GPX 1 | kGPX | 67 [mM−2 ms−1] |
| H2O2 Influx to the System 1 | H2O2IN | 10−5 [mM ms−1] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciesielska, S.; Mazur, K.; Fujarewicz, K.; Rzeszowska-Wolny, J. Role of Different Enzymes in H2O2 Neutralization and Cellular Radioresistance, Estimated by Mathematical Modeling. Int. J. Mol. Sci. 2025, 26, 7754. https://doi.org/10.3390/ijms26167754
Ciesielska S, Mazur K, Fujarewicz K, Rzeszowska-Wolny J. Role of Different Enzymes in H2O2 Neutralization and Cellular Radioresistance, Estimated by Mathematical Modeling. International Journal of Molecular Sciences. 2025; 26(16):7754. https://doi.org/10.3390/ijms26167754
Chicago/Turabian StyleCiesielska, Sylwia, Krzysztof Mazur, Krzysztof Fujarewicz, and Joanna Rzeszowska-Wolny. 2025. "Role of Different Enzymes in H2O2 Neutralization and Cellular Radioresistance, Estimated by Mathematical Modeling" International Journal of Molecular Sciences 26, no. 16: 7754. https://doi.org/10.3390/ijms26167754
APA StyleCiesielska, S., Mazur, K., Fujarewicz, K., & Rzeszowska-Wolny, J. (2025). Role of Different Enzymes in H2O2 Neutralization and Cellular Radioresistance, Estimated by Mathematical Modeling. International Journal of Molecular Sciences, 26(16), 7754. https://doi.org/10.3390/ijms26167754





