Autoimmune Gastritis and Helicobacter pylori Infection: Molecular Mechanisms of Relationship
Abstract
1. Introduction
- Histone modifications may affect expression of genes regulating immune tolerance and inflammatory responses. Such changes may contribute to immune dysregulation and initiation of autoimmune reactions [26].
- H. pylori-negative AIG (naïve).
- Combination of AIG and H. pylori.
- AIG in the post-eradication period of H. pylori.
2. H. pylori-Negative AIG (Naïve)
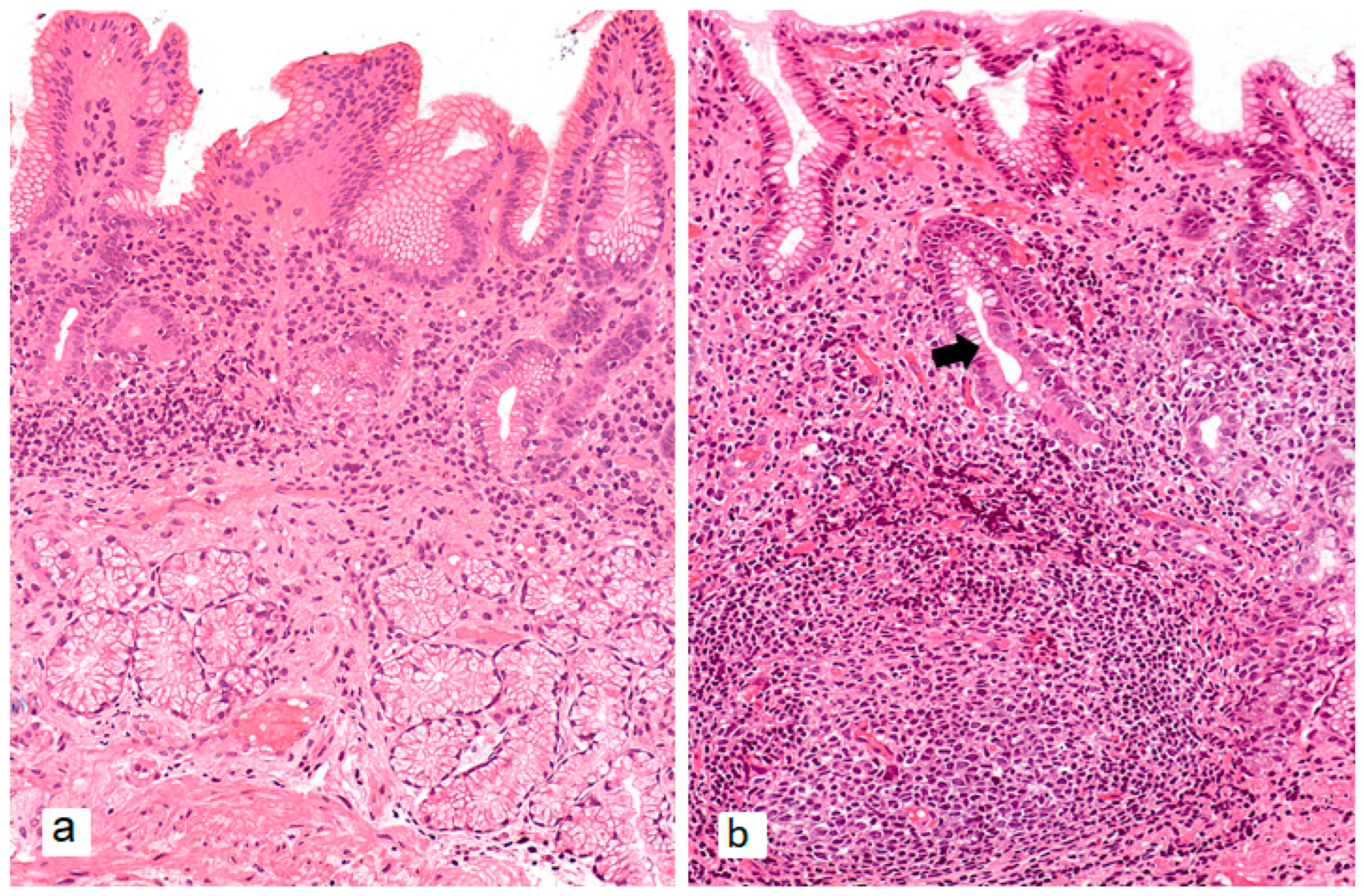
3. Combination of AIG and H. pylori Infection
4. AIG in the Post-Eradication Period of H. pylori
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rugge, M.; Genta, R.M.; Malfertheiner, P.; Dinis-Ribeiro, M.; El-Serag, H.; Graham, D.Y.; Kuipers, E.J.; Leung, W.K.; Park, J.Y.; Rokkas, T.; et al. RE.GA.IN.: The Real-World Gastritis Initiative-Updating the Updates. Gut 2024, 73, 407–441. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Arango, M.-T.; Perricone, C.; Kivity, S.; Cipriano, E.; Ceccarelli, F.; Valesini, G.; Shoenfeld, Y. HLA-DRB1 the Notorious Gene in the Mosaic of Autoimmunity. Immunol. Res. 2017, 65, 82–98. [Google Scholar] [CrossRef]
- Ungar, B.; Mathews, J.D.; Tait, B.D.; Cowling, D.C. HLA Patterns in Pernicious Anaemia. Br. Med. J. 1977, 1, 798–800. [Google Scholar] [CrossRef][Green Version]
- Ungar, B.; Mathews, J.D.; Tait, B.D.; Cowling, D.C. HLA-DR Patterns in Pernicious Anaemia. Br. Med. J. (Clin. Res. Ed.) 1981, 282, 768–770. [Google Scholar] [CrossRef]
- Lahner, E.; Spoletini, M.; Buzzetti, R.; Corleto, V.D.; Vannella, L.; Petrone, A.; Annibale, B. HLA-DRB1*03 and DRB1*04 Are Associated with Atrophic Gastritis in an Italian Population. Dig. Liver Dis. 2010, 42, 854–859. [Google Scholar] [CrossRef]
- Oksanen, A.M.; Haimila, K.E.; Rautelin, H.I.K.; Partanen, J.A. Immunogenetic Characteristics of Patients with Autoimmune Gastritis. World J. Gastroenterol. 2010, 16, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, X.; He, Y.; Lin, S.; Zhu, M.; Li, Y.; Wang, J.; Wang, J.; Ma, X.; Xu, J.; et al. Tumor Suppressor ATP4B Serve as a Promising Biomarker for Worsening of Gastric Atrophy and Poor Differentiation. Gastric Cancer 2021, 24, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Garelli, S.; Dalla Costa, M.; Sabbadin, C.; Barollo, S.; Rubin, B.; Scarpa, R.; Masiero, S.; Fierabracci, A.; Bizzarri, C.; Crinò, A.; et al. Autoimmune Polyendocrine Syndrome Type 1: An Italian Survey on 158 Patients. J. Endocrinol. Investig. 2021, 44, 2493–2510. [Google Scholar] [CrossRef] [PubMed]
- Fichna, M.; Żurawek, M.; Słomiński, B.; Sumińska, M.; Czarnywojtek, A.; Rozwadowska, N.; Fichna, P.; Myśliwiec, M.; Ruchała, M. Polymorphism in BACH2 Gene Is a Marker of Polyglandular Autoimmunity. Endocrine 2021, 74, 72–79. [Google Scholar] [CrossRef]
- Calvete, O.; Reyes, J.; Valdés-Socin, H.; Martin, P.; Marazuela, M.; Barroso, A.; Escalada, J.; Castells, A.; Torres-Ruiz, R.; Rodríguez-Perales, S.; et al. Alterations in SLC4A2, SLC26A7 and SLC26A9 Drive Acid-Base Imbalance in Gastric Neuroendocrine Tumors and Uncover a Novel Mechanism for a Co-Occurring Polyautoimmune Scenario. Cells 2021, 10, 3500. [Google Scholar] [CrossRef] [PubMed]
- Laisk, T.; Lepamets, M.; Koel, M.; Abner, E.; Estonian Biobank Research Team; Mägi, R. Genome-Wide Association Study Identifies Five Risk Loci for Pernicious Anemia. Nat. Commun. 2021, 12, 3761. [Google Scholar] [CrossRef]
- Săsăran, M.O.; Meliț, L.E.; Dobru, E.D. MicroRNA Modulation of Host Immune Response and Inflammation Triggered by Helicobacter Pylori. Int. J. Mol. Sci. 2021, 22, 1406. [Google Scholar] [CrossRef]
- Jiao, Y.; Yan, Z.; Yang, A. Mitochondria in Innate Immunity Signaling and Its Therapeutic Implications in Autoimmune Diseases. Front. Immunol. 2023, 14, 1160035. [Google Scholar] [CrossRef]
- Meliț, L.E.; Mărginean, C.O.; Mărginean, C.D.; Mărginean, M.O. The Relationship between Toll-like Receptors and Helicobacter Pylori-Related Gastropathies: Still a Controversial Topic. J. Immunol. Res. 2019, 2019, 8197048. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M. Role of Toll-like receptors in Helicobacter Pylori infection and immunity. World J. Gastrointest. Pathophysiol. 2014, 5, 133–146. [Google Scholar] [CrossRef]
- Rad, R.; Prinz, C.; Neu, B.; Neuhofer, M.; Zeitner, M.; Voland, P.; Becker, I.; Schepp, W.; Gerhard, M. Synergistic effect of Helicobacter pylori virulence factors and interleukin-1 polymorphisms for the development of severe histological changes in the gastric mucosa. J. Infect. Dis. 2003, 188, 272–281. [Google Scholar] [CrossRef]
- Backert, S.; Schmidt, T.P.; Harrer, A.; Wessler, S. Exploiting the gastric epithelial barrier: Helicobacter pylori’s attack on tight and adherens junctions. Curr. Top. Microbiol. Immunol. 2017, 400, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Solnick, J.V. Inflammation, Immunity, and Vaccine Development for Helicobacter Pylori. Helicobacter 2011, 16 (Suppl. 1), 26–32. [Google Scholar] [CrossRef]
- Moulton, V.R. Sex Hormones In Acquired Immunity And Autoimmune Disease. Front. Immunol. 2018, 9, 2279. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Eyerich, S.; Sarin, T.; Klatt, K.C. Nutrition And The Immune System: A Complicated Tango. Nutrients 2020, 12, 818. [Google Scholar] [CrossRef]
- Neumann, W.L.; Coss, E.; Rugge, M.; Genta, R.M. Autoimmune Atrophic Gastritis—Pathogenesis, Pathology And Management. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gorelik, G.; Strickland, F.M.; Richardson, B.C. Oxidative Stress, T Cell DNA methylation, and lupus. Arthritis Rheumatol. 2014, 66, 1574–1582. [Google Scholar] [CrossRef]
- Surace, A.E.A.; Hedrich, C.M. The Role Of Epigenetics In Autoimmune/Inflammatory Disease. Front. Immunol. 2019, 10, 1525. [Google Scholar] [CrossRef]
- Chen, Q.; Li, H.; Liu, Y.; Zhao, M. Epigenetic Regulation Of Immune And Inflammatory Responses In Rheumatoid Arthritis. Front. Immunol. 2022, 13, 881191. [Google Scholar] [CrossRef]
- Pauley, K.M.; Cha, S.; Chan, E.K.L. MicroRNA in Autoimmunity And Autoimmune Diseases. J. Autoimmun. 2009, 32, 189–194. [Google Scholar] [CrossRef]
- Salvi, V.; Gianello, V.; Tiberio, L.; Sozzani, S.; Bosisio, D. Cytokine targeting by miRNAs in autoimmune diseases. Front. Immunol. 2019, 10, 15. [Google Scholar] [CrossRef]
- Lenti, M.V.; Rugge, M.; Lahner, E.; Miceli, E.; Toh, B.-H.; Genta, R.M.; De Block, C.; Hershko, C.; Di Sabatino, A. Autoimmune gastritis. Nat. Rev. Dis. Primers 2020, 6, 56. [Google Scholar] [CrossRef]
- De Aizpurua, H.J.; Cosgrove, L.J.; Ungar, B.; Toh, B.H. Autoantibodies Cytotoxic To Gastric Parietal Cells In Serum Of Patients With Pernicious Anemia. N. Engl. J. Med. 1983, 309, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Glass, V.B. Effect of Prolonged Aministration of Parietal Cell Antibodies from Patients with Atrophic Gastritis and Pernicious Anemia on the Parietal Cell Mass and Hydrochloric Acid Output in Rats. Gastroenterology 1970, 58, 482–494. [Google Scholar] [CrossRef]
- Lenti, M.V.; Miceli, E.; Cococcia, S.; Klersy, C.; Staiani, M.; Guglielmi, F.; Giuffrida, P.; Vanoli, A.; Luinetti, O.; De Grazia, F.; et al. Determinants Of Diagnostic Delay In Autoimmune Atrophic Gastritis. Aliment. Pharmacol. Ther. 2019, 50, 167–175. [Google Scholar] [CrossRef] [PubMed]
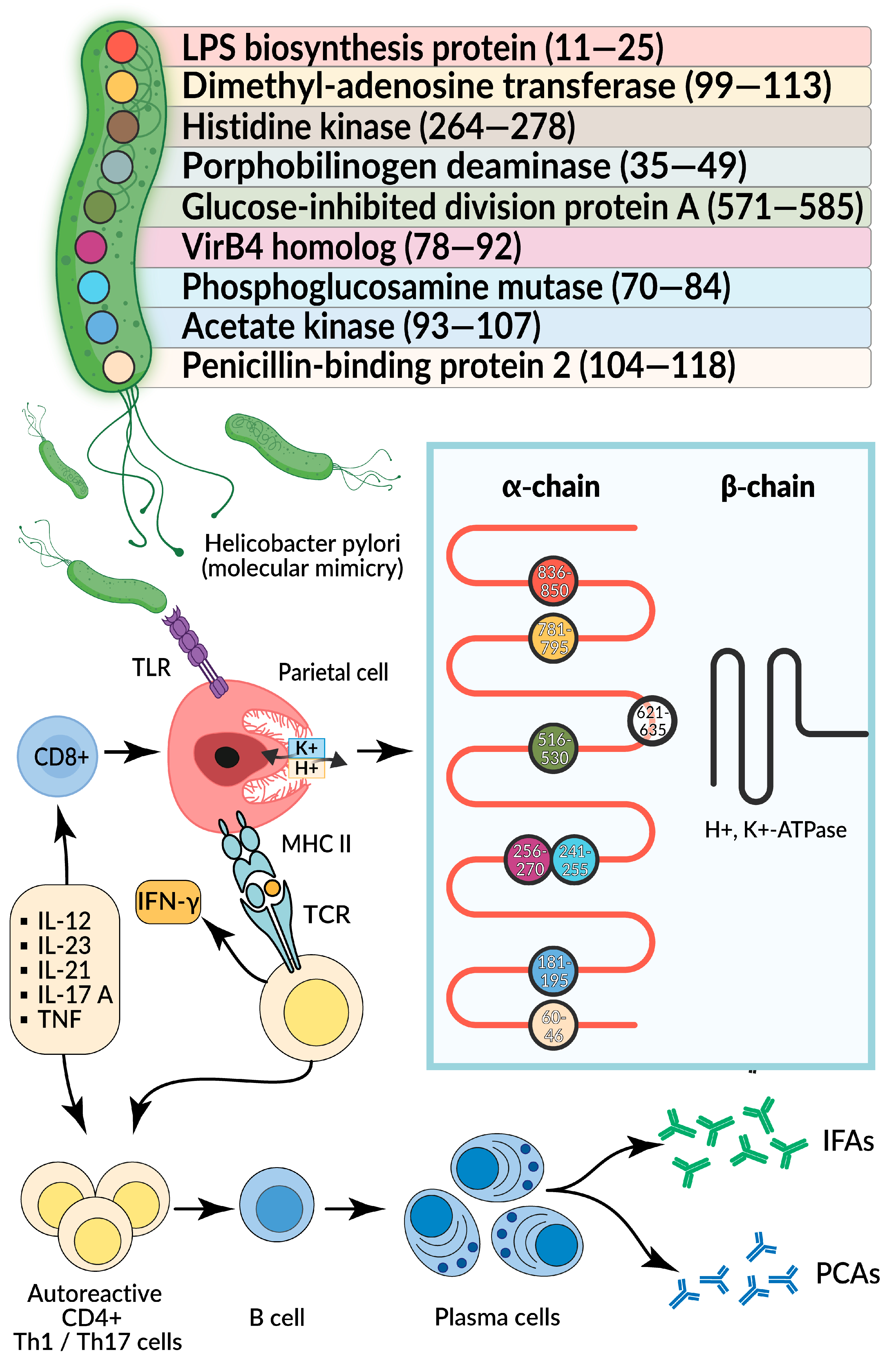
- D’Elios, M.M.; Bergman, M.P.; Azzurri, A.; Amedei, A.; Benagiano, M.; De Pont, J.J.; Cianchi, F.; Vandenbroucke-Grauls, C.M.; Romagnani, S.; Appelmelk, B.J.; et al. H(+),K(+)-Atpase (Proton Pump) Is the target autoantigen of Th1-Type Cytotoxic T cells in autoimmune gastritis. Gastroenterology 2001, 120, 377–386. [Google Scholar] [CrossRef]
- Iwamuro, M.; Tanaka, T.; Otsuka, M. Update in Molecular Aspects and Diagnosis of Autoimmune Gastritis. Curr. Issues Mol. Biol. 2023, 45, 5263–5275. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, X. Beyond Metaplasia: Unraveling the Complex Pathogenesis of Autoimmune Atrophic Gastritis and Its Implications for Gastric Cancer Risk. QJM 2025, 118, 203–247. [Google Scholar] [CrossRef]
- Goldenring, J.R. Pyloric Metaplasia, Pseudopyloric Metaplasia, Ulcer-Associated Cell Lineage and Spasmolytic Polypeptide-Expressing Metaplasia: Reparative Lineages in the Gastrointestinal Mucosa. J. Pathol. 2018, 245, 132–137. [Google Scholar] [CrossRef]
- Weis, V.G.; Sousa, J.F.; LaFleur, B.J.; Nam, K.T.; Weis, J.A.; Finke, P.E.; Ameen, N.A.; Fox, J.G.; Goldenring, J.R. Heterogeneity in Mouse Spasmolytic Polypeptide-Expressing Metaplasia Lineages Identifies Markers of Metaplastic Progression. Gut 2013, 62, 1270–1279. [Google Scholar] [CrossRef]
- Wada, Y.; Nakajima, S.; Kushima, R.; Takemura, S.; Mori, N.; Hasegawa, H.; Nakayama, T.; Mukaisho, K.-I.; Yoshida, A.; Umano, S.; et al. Pyloric, Pseudopyloric, and Spasmolytic Polypeptide-Expressing Metaplasias in Autoimmune Gastritis: A Case Series of 22 Japanese Patients. Virchows Arch. 2021, 479, 169–178. [Google Scholar] [CrossRef]
- Weis, V.G.; Goldenring, J.R. Current Understanding of SPEM and Its Standing in the Preneoplastic Process. Gastric Cancer 2009, 12, 189–197. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.A.C.; Greaves, L.C.; Gutierrez-Gonzalez, L.; Rodriguez-Justo, M.; Deheragoda, M.; Leedham, S.J.; Taylor, R.W.; Lee, C.Y.; Preston, S.L.; Lovell, M.; et al. Mechanisms of Field Cancerization in the Human Stomach: The Expansion and Spread of Mutated Gastric Stem Cells. Gastroenterology 2008, 134, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Burclaff, J.; Mills, J.C. Plasticity of Differentiated Cells in Wound Repair and Tumorigenesis, Part I: Stomach and Pancreas. Dis. Model. Mech. 2018, 11, dmm033373. [Google Scholar] [CrossRef] [PubMed]
- Willet, S.G.; Mills, J.C. Stomach Organ and Cell Lineage Differentiation: From Embryogenesis to Adult Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, J.B.; Mills, J.C. Acid and the Basis for Cellular Plasticity and Reprogramming in Gastric Repair and Cancer. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Okano, A.; Takakuwa, H.; Matsubayashi, Y. Parietal-Cell Hyperplasia Mimicking Sporadic Fundic Gland Polyps in the Atrophic Mucosa of Autoimmune Gastritis. Gastrointest. Endosc. 2007, 66, 394–395; discussion 395. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, G.; Dottori, L.; Fontana, F.; Cingolani, S.; Ligato, I.; Dilaghi, E.; Milani, C.; Ventura, M.; Borro, M.; Esposito, G.; et al. Gastric Microbiota Gender Differences in Subjects with Healthy Stomachs and Autoimmune Atrophic Gastritis. Microorganisms 2023, 11, 1938. [Google Scholar] [CrossRef]
- Marignani, M.; Delle Fave, G.; Mecarocci, S.; Bordi, C.; Angeletti, S.; D’Ambra, G.; Aprile, M.R.; Corleto, V.D.; Monarca, B.; Annibale, B. High Prevalence of Atrophic Body Gastritis in Patients with Unexplained Microcytic and Macrocytic Anemia: A Prospective Screening Study. Am. J. Gastroenterol. 1999, 94, 766–772. [Google Scholar] [CrossRef]
- De Block, C.E.; Van Campenhout, C.M.; De Leeuw, I.H.; Keenoy, B.M.; Martin, M.; Van Hoof, V.; Van Gaal, L.F. Soluble Transferrin Receptor Level: A New Marker of Iron Deficiency Anemia, a Common Manifestation of Gastric Autoimmunity in Type 1 Diabetes. Diabetes Care 2000, 23, 1384–1388. [Google Scholar] [CrossRef]
- Esposito, G.; Dottori, L.; Pivetta, G.; Ligato, I.; Dilaghi, E.; Lahner, E. Pernicious Anemia: The Hematological Presentation of a Multifaceted Disorder Caused by Cobalamin Deficiency. Nutrients 2022, 14, 1672. [Google Scholar] [CrossRef]
- Aditi, A.; Graham, D.Y. Vitamin C, Gastritis, and Gastric Disease: A Historical Review and Update. Dig. Dis. Sci. 2012, 57, 2504–2515. [Google Scholar] [CrossRef]
- Cavalcoli, F.; Zilli, A.; Conte, D.; Massironi, S. Micronutrient Deficiencies in Patients with Chronic Atrophic Autoimmune Gastritis: A Review. World J. Gastroenterol. 2017, 23, 563–572. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Konstantakis, C.; Triantos, C. Chronic Atrophic Autoimmune Gastritis: The Evolving Role of Vitamin D. Front. Biosci. (Landmark Ed.) 2024, 29, 252. [Google Scholar] [CrossRef]
- Johnson, C.R.; Thacher, T.D. Vitamin D: Immune Function, Inflammation, Infections and Auto-Immunity. Paediatr. Int. Child. Health 2023, 43, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Aranow, C. Vitamin D and the Immune System. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Scolletta, S.; Colletti, M.; Di Luigi, L.; Crescioli, C. Vitamin D Receptor Agonists Target CXCL10: New Therapeutic Tools for Resolution of Inflammation. Mediat. Inflamm. 2013, 2013, 876319. [Google Scholar] [CrossRef]
- Lemire, J.M.; Archer, D.C.; Beck, L.; Spiegelberg, H.L. Immunosuppressive Actions of 1,25-Dihydroxyvitamin D3: Preferential Inhibition of Th1 Functions. J. Nutr. 1995, 125, 1704S–1708S. [Google Scholar] [CrossRef]
- Cippitelli, M.; Santoni, A. Vitamin D3: A Transcriptional Modulator of the Interferon-Gamma Gene. Eur. J. Immunol. 1998, 28, 3017–3030. [Google Scholar] [CrossRef]
- Alroy, I.; Towers, T.L.; Freedman, L.P. Transcriptional Repression of the Interleukin-2 Gene by Vitamin D3: Direct Inhibition of NFATp/AP-1 Complex Formation by a Nuclear Hormone Receptor. Mol. Cell. Biol. 1995, 15, 5789–5799. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.K.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 Combine to Inhibit T Cell Production of Inflammatory Cytokines and Promote Development of Regulatory T Cells Expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef]
- Reichel, H.; Koeffler, H.P.; Tobler, A.; Norman, A.W. 1 Alpha,25-Dihydroxyvitamin D3 Inhibits Gamma-Interferon Synthesis by Normal Human Peripheral Blood Lymphocytes. Proc. Natl. Acad. Sci. USA 1987, 84, 3385–3389. [Google Scholar] [CrossRef]
- Waldum, H.; Mjønes, P. Towards Understanding of Gastric Cancer Based upon Physiological Role of Gastrin and ECL Cells. Cancers 2020, 12, 3477. [Google Scholar] [CrossRef] [PubMed]
- Isakov, V. Autoimmune Gastritis Studies and Gastric Cancer: True Renaissance or Bibliometric Illusion. World J. Gastroenterol. 2024, 30, 3783–3790. [Google Scholar] [CrossRef]
- Rugge, M.; Bricca, L.; Guzzinati, S.; Sacchi, D.; Pizzi, M.; Savarino, E.; Farinati, F.; Zorzi, M.; Fassan, M.; Dei Tos, A.P.; et al. Autoimmune Gastritis: Long-Term Natural History in Naïve Helicobacter Pylori-Negative Patients. Gut 2023, 72, 30–38. [Google Scholar] [CrossRef]
- Rugge, M.; Genta, R.M. Staging and Grading of Chronic Gastritis. Hum. Pathol. 2005, 36, 228–233. [Google Scholar] [CrossRef]
- Kishikawa, H.; Takarabe, S.; Ichikawa, M.; Sasaki, A.; Nishida, J. Gastric Adenocarcinoma in Helicobacter Pylori-Negative Autoimmune Gastritis: A Case Report and Literature Review. Cureus 2024, 16, e66910. [Google Scholar] [CrossRef]
- Lahner, E.; Esposito, G.; Pilozzi, E.; Purchiaroni, F.; Corleto, V.D.; Di Giulio, E.; Annibale, B. Occurrence of Gastric Cancer and Carcinoids in Atrophic Gastritis during Prospective Long-Term Follow Up. Scand. J. Gastroenterol. 2015, 50, 856–865. [Google Scholar] [CrossRef]
- Vannella, L.; Lahner, E.; Osborn, J.; Annibale, B. Systematic Review: Gastric Cancer Incidence in Pernicious Anaemia. Aliment. Pharmacol. Ther. 2013, 37, 375–382. [Google Scholar] [CrossRef]
- Song, M.; Latorre, G.; Ivanovic-Zuvic, D.; Camargo, M.C.; Rabkin, C.S. Autoimmune Diseases and Gastric Cancer Risk: A Systematic Review and Meta-Analysis. Cancer Res. Treat. 2019, 51, 841–850. [Google Scholar] [CrossRef]
- Faller, G.; Winter, M.; Steininger, H.; Lehn, N.; Meining, A.; Bayerdörffer, E.; Kirchner, T. Decrease of Antigastric Autoantibodies in Helicobacter Pylori Gastritis after Cure of Infection. Pathol. Res. Pract. 1999, 195, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Amedei, A.; Bergman, M.P.; Appelmelk, B.J.; Azzurri, A.; Benagiano, M.; Tamburini, C.; van der Zee, R.; Telford, J.L.; Vandenbroucke-Grauls, C.M.J.E.; D’Elios, M.M.; et al. Molecular Mimicry between Helicobacter Pylori Antigens and H+, K+-Adenosine Triphosphatase in Human Gastric Autoimmunity. J. Exp. Med. 2003, 198, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Benoist, C.; Mathis, D. Autoimmunity Provoked by Infection: How Good Is the Case for T Cell Epitope Mimicry? Nat. Immunol. 2001, 2, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Wucherpfennig, K.W. Mechanisms for the Induction of Autoimmunity by Infectious Agents. J. Clin. Investig. 2001, 108, 1097–1104. [Google Scholar] [CrossRef]
- D’Elios, M.M.; Appelmelk, B.J.; Amedei, A.; Bergman, M.P.; Del Prete, G. Gastric Autoimmunity: The Role of Helicobacter Pylori and Molecular Mimicry. Trends Mol. Med. 2004, 10, 316–323. [Google Scholar] [CrossRef]
- Valnes, K.; Huitfeldt, H.S.; Brandtzaeg, P. Relation between T Cell Number and Epithelial HLA Class II Expression Quantified by Image Analysis in Normal and Inflamed Human Gastric Mucosa. Gut 1990, 31, 647–652. [Google Scholar] [CrossRef]
- Ye, G.; Barrera, C.; Fan, X.; Gourley, W.K.; Crowe, S.E.; Ernst, P.B.; Reyes, V.E. Expression of B7-1 and B7-2 Costimulatory Molecules by Human Gastric Epithelial Cells: Potential Role in CD4+ T Cell Activation during Helicobacter Pylori Infection. J. Clin. Investig. 1997, 99, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Barrera, C.; Ye, G.; Espejo, R.; Gunasena, S.; Almanza, R.; Leary, J.; Crowe, S.; Ernst, P.; Reyes, V.E. Expression of Cathepsins B, L, S, and D by Gastric Epithelial Cells Implicates Them as Antigen Presenting Cells in Local Immune Responses. Hum. Immunol. 2001, 62, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Toh, B.H.; van Driel, I.R.; Gleeson, P.A. Pernicious Anemia. N. Engl. J. Med. 1997, 337, 1441–1448. [Google Scholar] [CrossRef]
- Mårdh, S.; Song, Y.H. Characterization of Antigenic Structures in Auto-Immune Atrophic Gastritis with Pernicious Anaemia. The Parietal Cell H,K-ATPase and the Chief Cell Pepsinogen Are the Two Major Antigens. Acta Physiol. Scand. 1989, 136, 581–587. [Google Scholar] [CrossRef]
- Furuta, T.; Baba, S.; Yamade, M.; Uotani, T.; Kagami, T.; Suzuki, T.; Tani, S.; Hamaya, Y.; Iwaizumi, M.; Osawa, S.; et al. High Incidence of Autoimmune Gastritis in Patients Misdiagnosed with Two or More Failures of H. Pylori Eradication. Aliment. Pharmacol. Ther. 2018, 48, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, J.; Hall, S.; Castrodad-Rodriguez, C.A.; Westerhoff, M.; El Jabbour, T.; Jain, S.; Panarelli, N.C. Features That Aid Identification of Autoimmune Gastritis in a Background of Active Helicobacter Pylori Infection. Arch. Pathol. Lab. Med. 2021, 145, 1536–1543. [Google Scholar] [CrossRef]
- Rugge, M.; Fassan, M.; Pizzi, M.; Zorzetto, V.; Maddalo, G.; Realdon, S.; De Bernard, M.; Betterle, C.; Cappellesso, R.; Pennelli, G.; et al. Autoimmune Gastritis: Histology Phenotype and OLGA Staging. Aliment. Pharmacol. Ther. 2012, 35, 1460–1466. [Google Scholar] [CrossRef]
- Dinis-Ribeiro, M.; Libânio, D.; Uchima, H.; Spaander, M.C.W.; Bornschein, J.; Matysiak-Budnik, T.; Tziatzios, G.; Santos-Antunes, J.; Areia, M.; Chapelle, N.; et al. Management of Epithelial Precancerous Conditions and Early Neoplasia of the Stomach (MAPS III): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG) and European Society of Pathology (ESP) Guideline Update 2025. Endoscopy 2025, 57, 504–554. [Google Scholar] [CrossRef]
- Stolte, M.; Meier, E.; Meining, A. Cure of Autoimmune Gastritis by Helicobacter Pylori Eradication in a 21-Year-Old Male. Z. Gastroenterol. 1998, 36, 641–643. [Google Scholar] [PubMed]
- Müller, H.; Rappel, S.; Wündisch, T.; Bayerdörffer, E.; Stolte, M. Healing of Active, Non-Atrophic Autoimmune Gastritis by H. Pylori Eradication. Digestion 2001, 64, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Annibale, B.; Di Giulio, E.; Caruana, P.; Lahner, E.; Capurso, G.; Bordi, C.; Delle Fave, G. The Long-Term Effects of Cure of Helicobacter Pylori Infection on Patients with Atrophic Body Gastritis. Aliment. Pharmacol. Ther. 2002, 16, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Vannella, L.; Lahner, E.; Bordi, C.; Pilozzi, E.; Di Giulio, E.; Corleto, V.D.; Osborn, J.; Delle Fave, G.; Annibale, B. Reversal of Atrophic Body Gastritis after H. Pylori Eradication at Long-Term Follow-Up. Dig. Liver Dis. 2011, 43, 295–299. [Google Scholar] [CrossRef]
- Kotera, T.; Nishimi, Y.; Kushima, R.; Haruma, K. Regression of Autoimmune Gastritis after Eradication of Helicobacter Pylori. Case Rep. Gastroenterol. 2023, 17, 34–40. [Google Scholar] [CrossRef]
- Sumi, N.; Haruma, K.; Urata, N.; Tanikawa, T.; Nakamura, J.; Suehiro, M. Autoimmune gastritis with rapid development of corporal atrophy found after H. pylori eradication therapy, report of a case. Stomach Intest. 2019, 7, 1053–1057, (In Japanese, Abstract in English). [Google Scholar]
- Ihara, T.; Ihara, N.; Kushima, R.; Haruma, K. Rapid Progression of Autoimmune Gastritis after Helicobacter Pylori Eradication Therapy. Intern. Med. 2023, 62, 1603–1609. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordin, D.S.; Livzan, M.A.; Mozgovoi, S.I.; Gaus, O.V. Autoimmune Gastritis and Helicobacter pylori Infection: Molecular Mechanisms of Relationship. Int. J. Mol. Sci. 2025, 26, 7737. https://doi.org/10.3390/ijms26167737
Bordin DS, Livzan MA, Mozgovoi SI, Gaus OV. Autoimmune Gastritis and Helicobacter pylori Infection: Molecular Mechanisms of Relationship. International Journal of Molecular Sciences. 2025; 26(16):7737. https://doi.org/10.3390/ijms26167737
Chicago/Turabian StyleBordin, Dmitry S., Maria A. Livzan, Sergei I. Mozgovoi, and Olga V. Gaus. 2025. "Autoimmune Gastritis and Helicobacter pylori Infection: Molecular Mechanisms of Relationship" International Journal of Molecular Sciences 26, no. 16: 7737. https://doi.org/10.3390/ijms26167737
APA StyleBordin, D. S., Livzan, M. A., Mozgovoi, S. I., & Gaus, O. V. (2025). Autoimmune Gastritis and Helicobacter pylori Infection: Molecular Mechanisms of Relationship. International Journal of Molecular Sciences, 26(16), 7737. https://doi.org/10.3390/ijms26167737







