Analysis of Beta-Dystroglycan in Different Cell Models of Senescence
Abstract
1. Introduction
2. Results
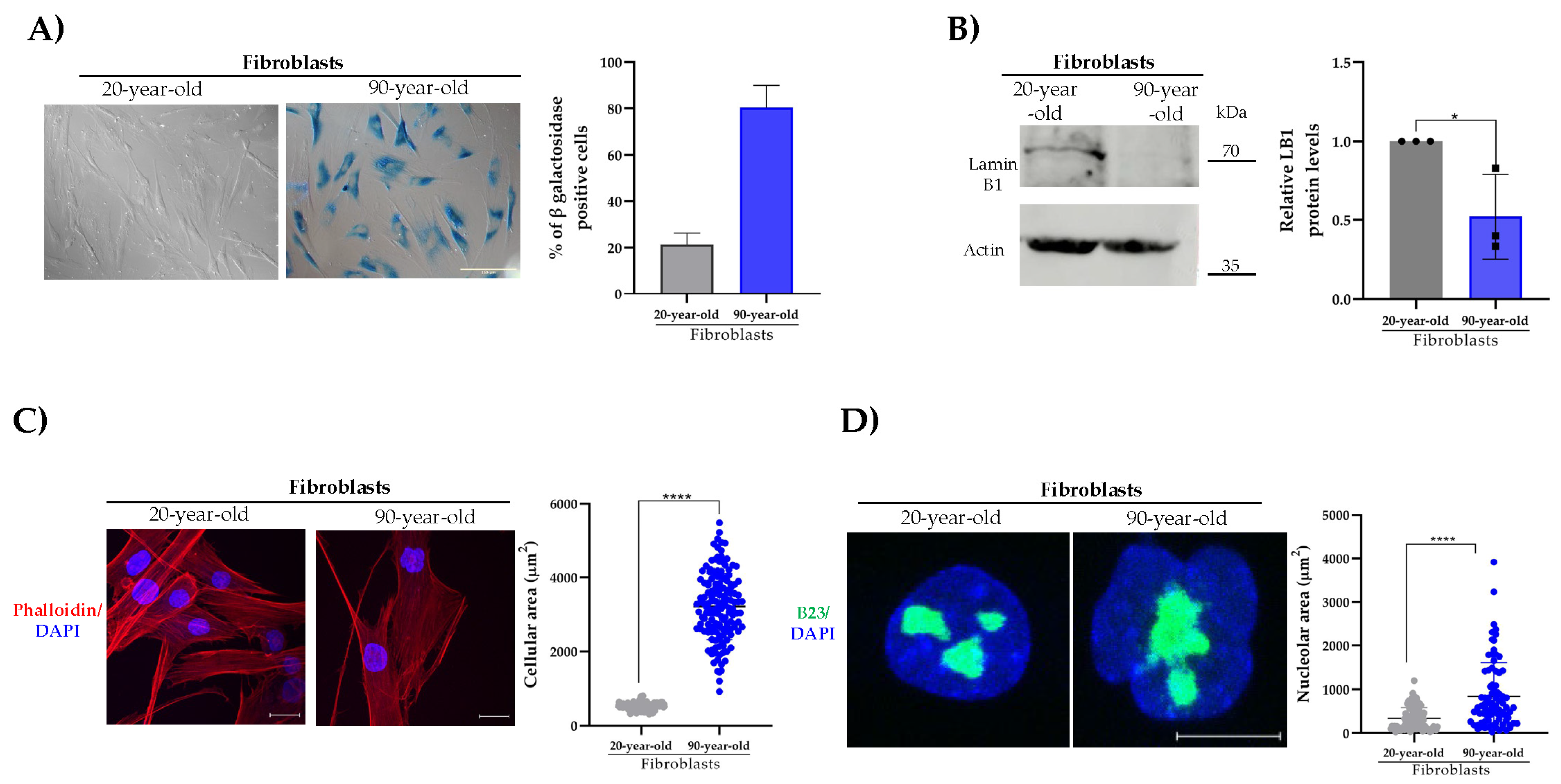
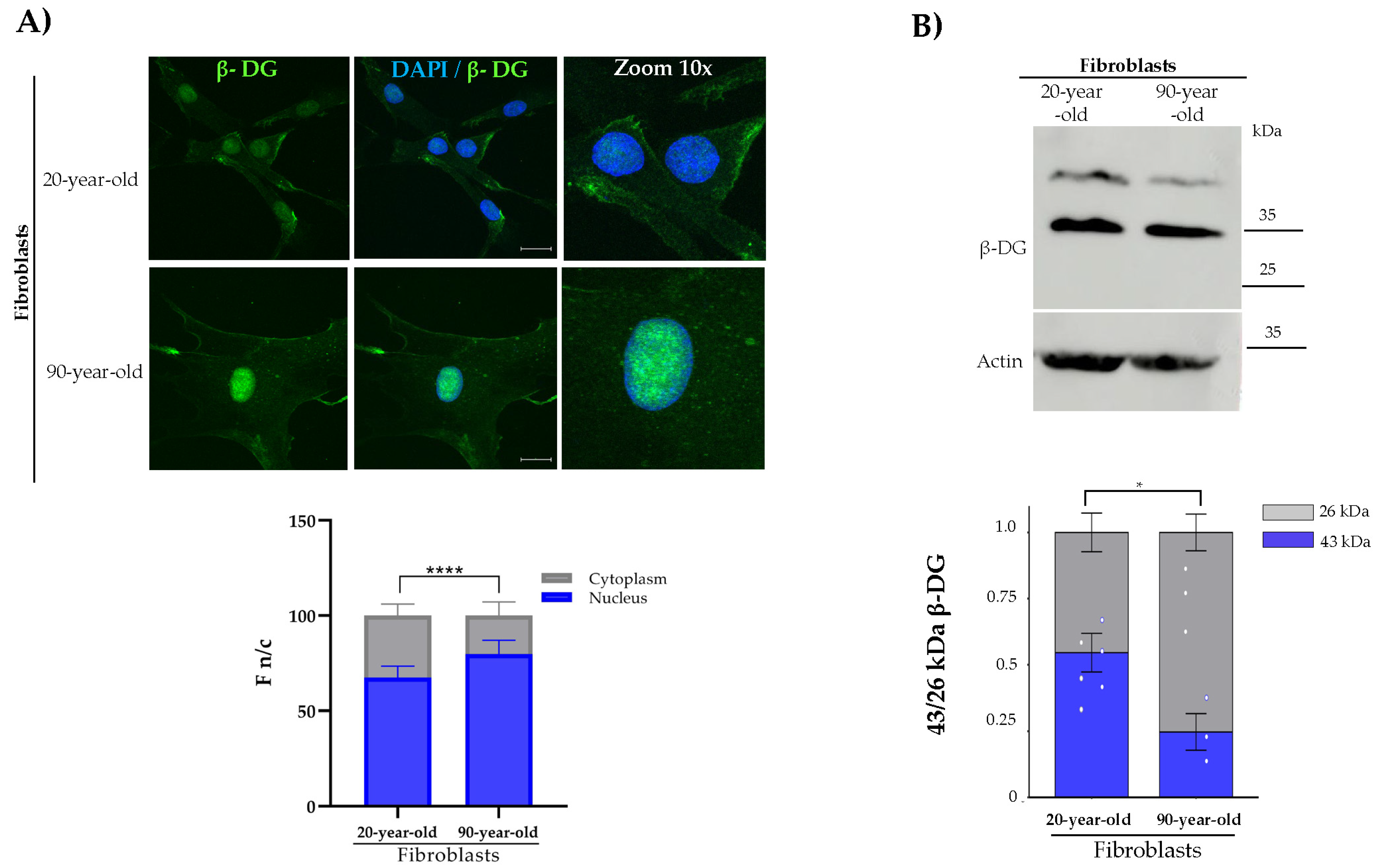
2.1. Analysis of β-DG in Physiological Aged Primary Fibroblasts
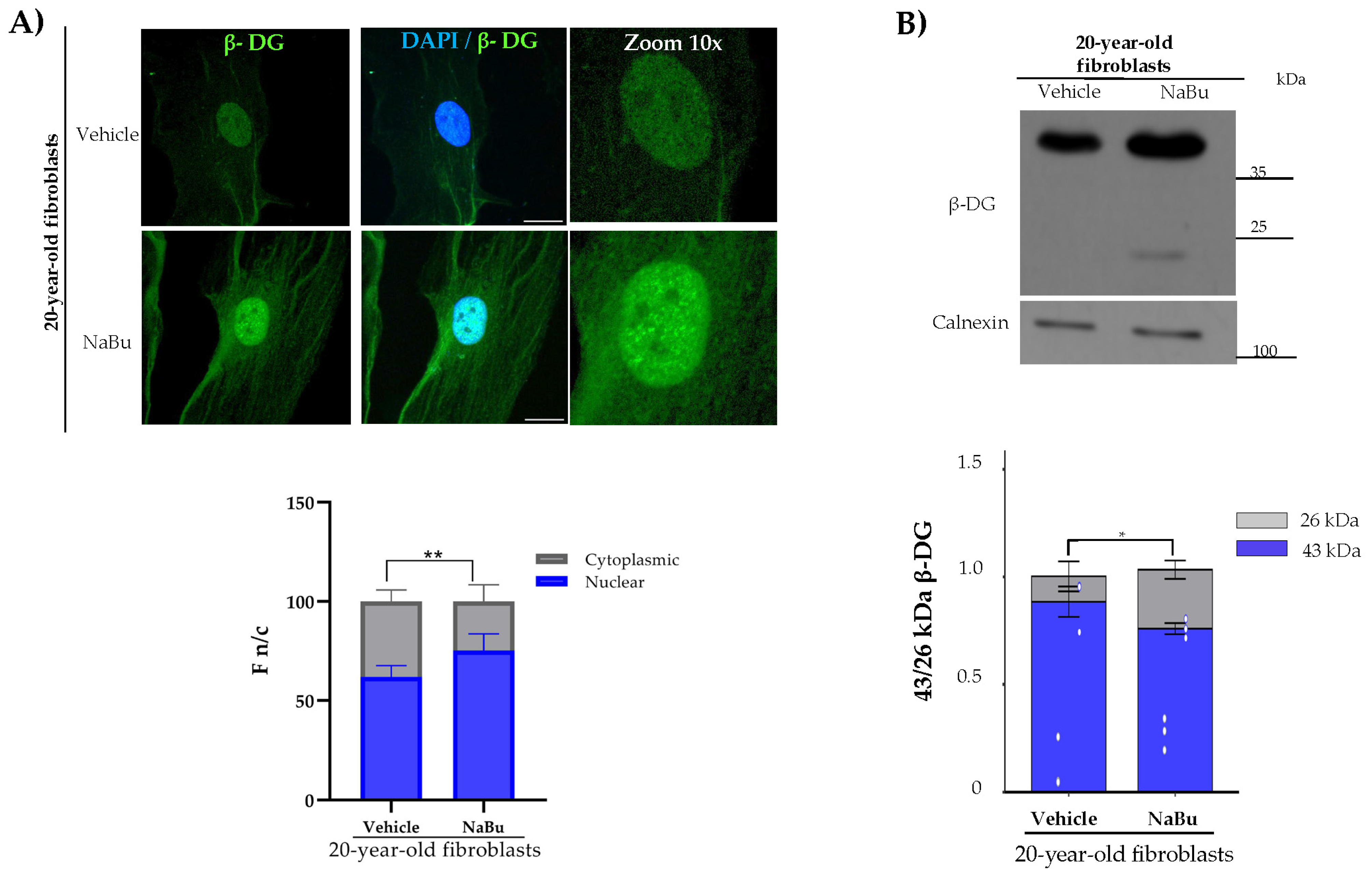
2.2. Evaluation of β-DG in Senescence-Induced Fibroblasts
2.3. Analysis of β-DG in HGPS Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Antibodies
4.3. Immunofluorescence and Confocal Microscopy Analysis
4.4. Senescence-Associated β-Galactosidase (SA-β-Gal) Assay
4.5. Western Blotting
4.6. Immunoprecipitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| β-DG | β-distroglycan |
| NaBu | Sodium butyrate |
| HGPS | Hutchinson–Gilford progeria syndrome |
| ICD | Intracellular domain |
| NE | Nuclear envelope |
| ETV1 | ETS (E twenty-six) variant 1 |
| MMP | Matrix metalloproteinase |
| UBF | Upstream binding factor |
| SA-(β-gal) | Senescence-associated β-galactosidase |
| LB1 | Lamin B1 |
| HDAC | Histone deacetylase |
| NLS | Nuclear import signal |
| NES | Nuclear export signal |
| F n/c | Nuclear–cytoplasmic fluorescence intensity ratio |
References
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Portincasa, P. The environment as a determinant of successful aging or frailty. Mech. Ageing Dev. 2020, 188, 111244. [Google Scholar] [CrossRef] [PubMed]
- Ogrodnik, M. Cellular aging beyond cellular senescence: Markers of senescence prior to cell cycle arrest in vitro and in vivo. Aging Cell 2021, 20, e13338. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Barresi, R.; Campbell, K.P. Dystroglycan: From biosynthesis to pathogenesis of human disease. J. Cell Sci. 2006, 119 Pt 2, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vieyra, I.A.; Vásquez-Limeta, A.; González-Ramírez, R.; Morales-Lázaro, S.L.; Mondragón, M.; Mondragón, R.; Ortega, A.; Winder, S.J.; Cisneros, B. A role for β-dystroglycan in the organization and structure of the nucleus in myoblasts. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Sbardella, D.; Inzitari, R.; Iavarone, F.; Gioia, M.; Marini, S.; Sciandra, F.; Castagnola, M.; Van den Steen, P.E.; Opdenakker, G.; Giardina, B.; et al. Enzymatic processing by MMP-2 and MMP-9 of wild-type and mutated mouse β-dystroglycan. IUBMB Life 2012, 64, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Azuara-Medina, P.M.; Sandoval-Duarte, A.M.; Morales-Lázaro, S.L.; Modragón-González, R.; Vélez-Aguilera, G.; Gómez-López, J.D.; Jiménez-Gutiérrez, G.E.; Tiburcio-Félix, R.; Martínez-Vieyra, I.; Suárez-Sánchez, R.; et al. The intracellular domain of β-dystroglycan mediates the nucleolar stress response by suppressing UBF transcriptional activity. Cell Death. Dis. 2019, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gutierrez, G.E.; Mondragon-Gonzalez, R.; Soto-Ponce, L.A.; Gómez-Monsiváis, W.L.; García-Aguirre, I.; Pacheco-Rivera, R.A.; Suárez-Sánchez, R.; Brancaccio, A.; Magaña, J.J.; CR Perlingeiro, R.; et al. Loss of Dystroglycan Drives Cellular Senescence via Defective Mitosis-Mediated Genomic Instability. Int. J. Mol. Sci. 2020, 21, 4961. [Google Scholar] [CrossRef] [PubMed]
- Munro, J.; Barr, N.I.; Ireland, H.; Morrison, V.; Parkinson, E.K. Histone deacetylase inhibitors induce a senescence-like state in human cells by a p16-dependent mechanism that is independent of a mitotic clock. Exp. Cell Res. 2004, 295, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Tigges, J.; Krutmann, J.; Fritsche, E.; Haendeler, J.; Schaal, H.; Fischer, J.W.; Kalfalah, F.; Reinke, H.; Reifenberger, G.; Stühler, K.; et al. The hallmarks of fibroblast ageing. Mech. Ageing Dev. 2014, 138, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Ashapkin, V.V.; Kutueva, L.I.; Kurchashova, S.Y.; Kireev, I.I. Are There Common Mechanisms Between the Hutchinson-Gilford Progeria Syndrome and Natural Aging? Front. Genet. 2019, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Sasagawa, S.; Itoh, K. Sodium butyrate induces senescence and inhibits the invasiveness of glioblastoma cells. Oncol. Lett. 2018, 15, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Jothi, D.; Kulka, L.A.M. Strategies for modeling aging and age-related diseases. Npj Aging 2024, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Lamis, A.; Siddiqui, S.J.W.; Ashok, T.; Patni, N.; Fatima, M.; Aneef, A.N. Hutchinson-Gilford Progeria Syndrome: A Literature Review. Cureus 2022, 14, e28629. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Rebehn, L.; Khalaji, S.; KleinJan, F.; Kleemann, A.; Port, F.; Paul, P.; Huster, C.; Nolte, U.; Singh, K.; Kwapich, L.; et al. The weakness of senescent dermal fibroblasts. Proc. Natl. Acad. Sci. USA 2023, 120, e2301880120. [Google Scholar] [CrossRef] [PubMed]
- Soto-Ponce, A.; De Ita, M.; Castro-Obregón, S.; Cortez, D.; Landesman, Y.; Magaña, J.J.; Gonzalo, S.; Zavaleta, T.; Soberano-Nieto, A.; Unzueta, J.; et al. Targeting CRM1 for Progeria Syndrome Therapy. Aging Cell 2025, 24, e14495. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jung, Y.S.; Yoon, M.H.; Kang, S.M.; Oh, A.Y.; Lee, J.H.; Jun, S.Y.; Woo, T.G.; Chun, H.Y.; Kim, S.K.; et al. Interruption of progerin-lamin A/C binding ameliorates Hutchinson-Gilford progeria syndrome phenotype. J. Clin. Investig. 2016, 126, 3879–3893. [Google Scholar] [CrossRef] [PubMed]
- Buchwalter, A.; Hetzer, M.W. Nucleolar expansion and elevated protein translation in premature aging. Nat. Commun. 2017, 8, 328. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez-Gutierrez, G.E.; Zavaleta-Vásquez, T.I.; Lizcano-Meneses, J.A.; Garcia-Aguirre, I.A.; Laredo-Cisneros, M.S.; Magaña, J.J.; Winder, S.J.; Cordero-Martínez, J.; Cisneros, B. Analysis of Beta-Dystroglycan in Different Cell Models of Senescence. Int. J. Mol. Sci. 2025, 26, 7726. https://doi.org/10.3390/ijms26167726
Jimenez-Gutierrez GE, Zavaleta-Vásquez TI, Lizcano-Meneses JA, Garcia-Aguirre IA, Laredo-Cisneros MS, Magaña JJ, Winder SJ, Cordero-Martínez J, Cisneros B. Analysis of Beta-Dystroglycan in Different Cell Models of Senescence. International Journal of Molecular Sciences. 2025; 26(16):7726. https://doi.org/10.3390/ijms26167726
Chicago/Turabian StyleJimenez-Gutierrez, Guadalupe Elizabeth, Tania Ivette Zavaleta-Vásquez, Jessica Alexandra Lizcano-Meneses, Ian Alain Garcia-Aguirre, Marco Samuel Laredo-Cisneros, Jonathan J. Magaña, Steve J Winder, Joaquín Cordero-Martínez, and Bulmaro Cisneros. 2025. "Analysis of Beta-Dystroglycan in Different Cell Models of Senescence" International Journal of Molecular Sciences 26, no. 16: 7726. https://doi.org/10.3390/ijms26167726
APA StyleJimenez-Gutierrez, G. E., Zavaleta-Vásquez, T. I., Lizcano-Meneses, J. A., Garcia-Aguirre, I. A., Laredo-Cisneros, M. S., Magaña, J. J., Winder, S. J., Cordero-Martínez, J., & Cisneros, B. (2025). Analysis of Beta-Dystroglycan in Different Cell Models of Senescence. International Journal of Molecular Sciences, 26(16), 7726. https://doi.org/10.3390/ijms26167726




