The Periodontal–Cardiovascular Disease Association: Molecular Mechanisms and Clinical Implications
Abstract
1. Introduction
2. Oral Microbiome Dysbiosis and Cardiovascular Pathogenesis
- Selective inhibition of gingipain activity through structure-based protease inhibitor design.
- Targeted modulation of inflammatory cascades, with particular emphasis on NLRP3 inflammasome assembly regulation.
- Specific antioxidant interventions directed at ROS-generating enzymatic systems.
- Precise regulation of vesicle-mediated bacterial communication networks [47].
3. Molecular Mechanisms of Porphyromonas gingivalis-Induced Endothelial Dysfunction
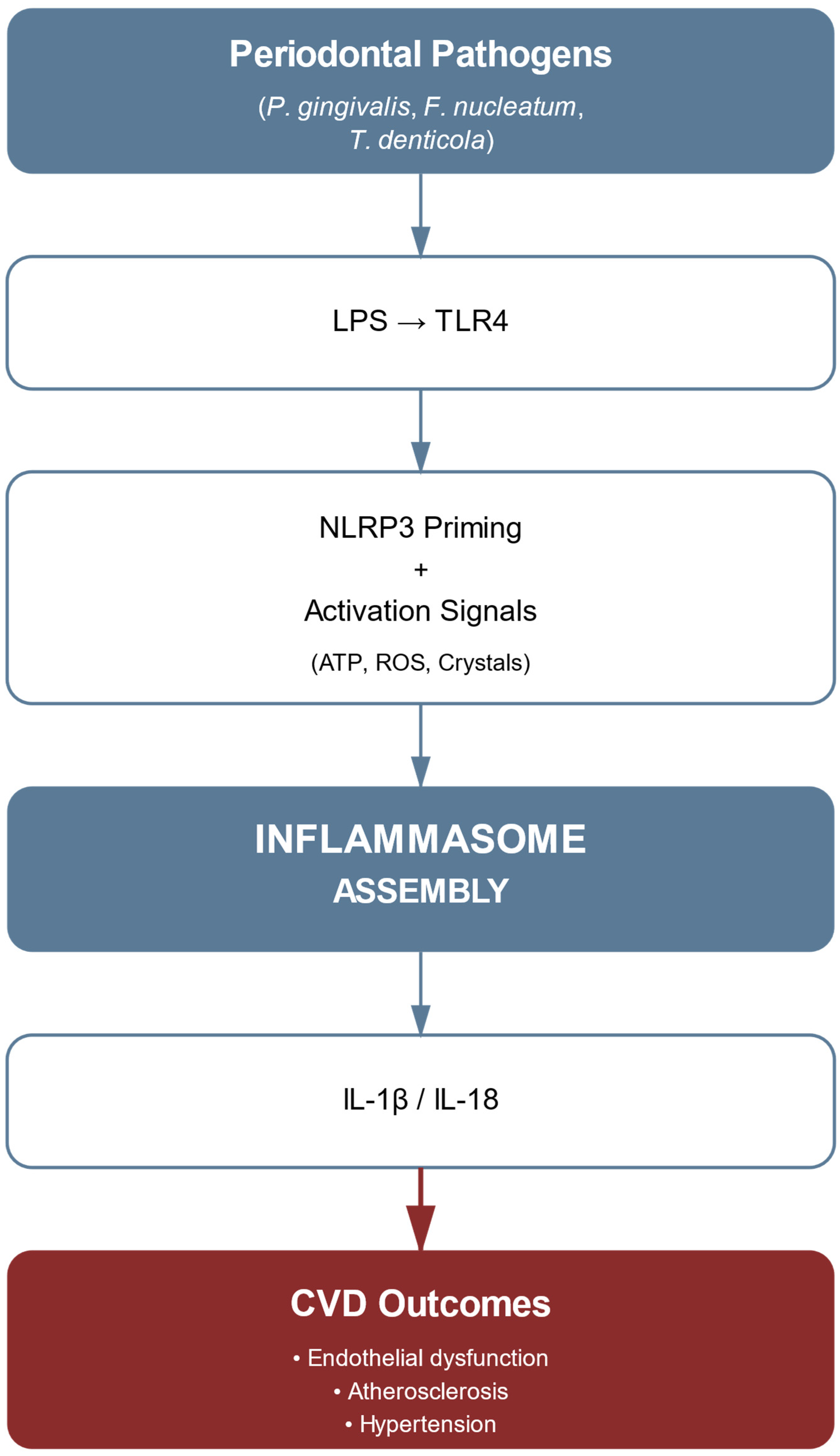
4. NLRP3 Inflammasome Activation in the Periodontitis–CVDs Link
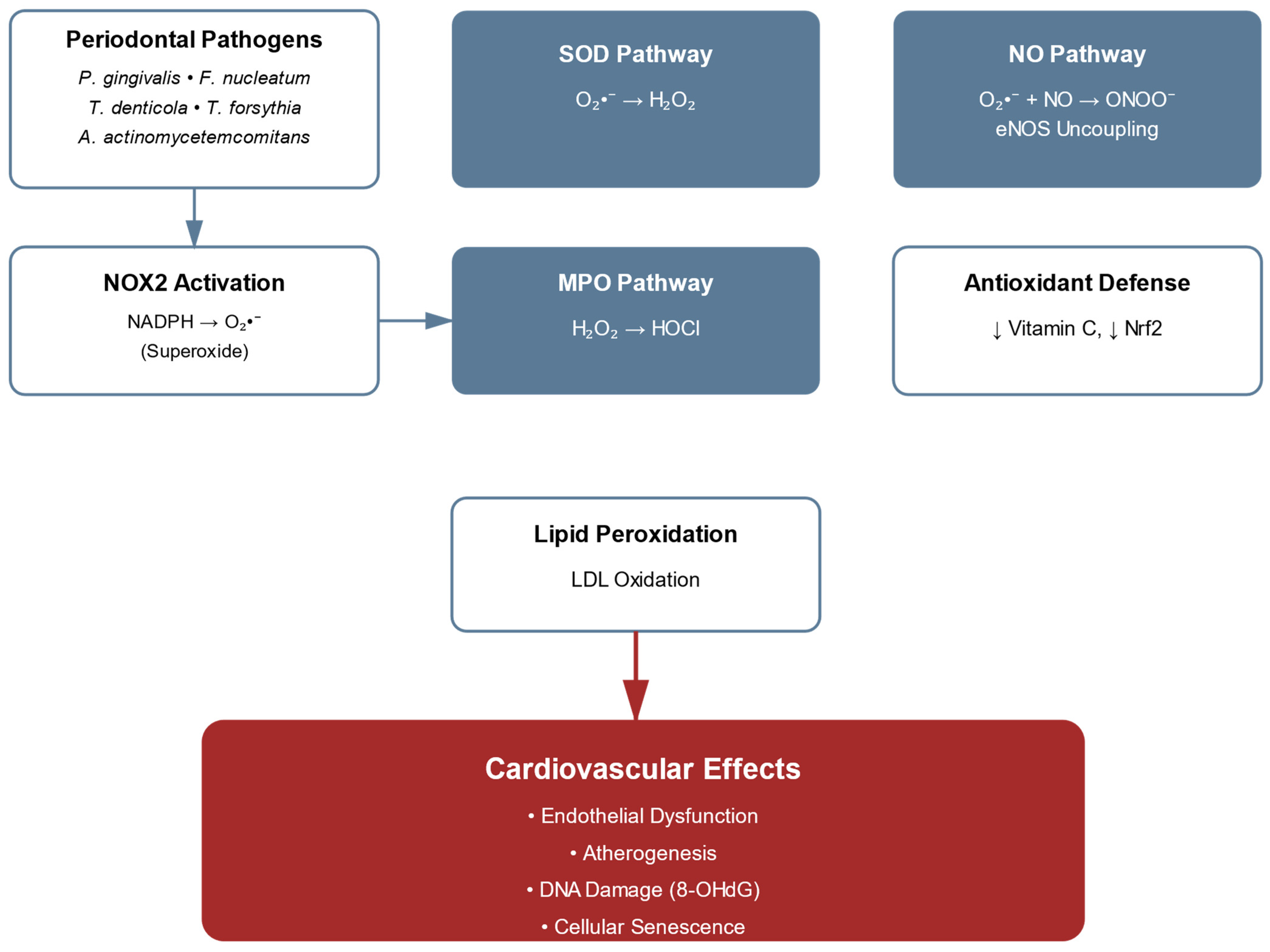
5. Oxidative Stress Mechanisms in Periodontitis-Associated Cardiovascular Disease: From ROS Generation to Vascular Dysfunction
6. Systemic Inflammatory Response to Periodontal Pathogens
7. The Role of ADMA in Periodontal–Cardiovascular Pathology
8. Epigenetic Modifications in Periodontal–Cardiovascular Pathology
9. Molecular Mechanisms of Periodontal Pathogen-Induced Vascular Smooth Muscle Cell Dysfunction: From Phenotypic Switching to Therapeutic Targets
- Antagonism of microRNA pathways (e.g., miR-21 inhibition);
- Restoration of autophagic activity;
- Modulation of EV-mediated signaling;
- Epigenetic reprogramming to reverse phenotypic plasticity [38].
10. Therapeutic Implications and Clinical Translation
11. Research Gaps and Future Directions
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Bouchard, P.; Caton, J.; Armitage, G.; Lamster, I.B.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S74–S84. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Czesnikiewicz-Guzik, M.; Osmenda, G.; Siedlinski, M.; Nosalski, R.; Pelka, P.; Nowakowski, D.; Wilk, G.; Mikolajczyk, T.P.; Schramm-Luc, A.; Furtak, A.; et al. Causal association between periodontitis and hypertension: Evidence from Mendelian randomization and a randomized controlled trial. Eur. Heart J. 2019, 40, 3459–3470. [Google Scholar] [CrossRef] [PubMed]
- Winning, L.; Patterson, C.C.; Neville, C.E.; Kee, F.; Linden, G.J. Periodontitis and risk of prevalent and incident coronary heart disease events. J. Clin. Periodontol. 2020, 47, 1446–1456. [Google Scholar] [CrossRef]
- Benahmed, A.G.; Gasmi, A.; Doşa, M.D.; Chirumbolo, S.; Mujawdiya, P.K.; Aaseth, J.; Dadar, M.; Bjørklund, G. Periodontitis continuum: Antecedents, triggers, mediators, and treatment strategies. Curr. Med. Chem. 2024, 31, 6775–6800. [Google Scholar] [CrossRef]
- Matsuda, S.; Shintani, T.; Miyagawa, T.; Yumoto, H.; Komatsu, Y.; Dewake, N.; Ohyama, T.; Naruishi, K.; Nagata, T.; Kawaguchi, H. Effect of Periodontal Treatment on Reducing Chronic Inflammation in Systemically Healthy Patients with Periodontal Disease. Am. J. Med. 2024, 137, 273–279. [Google Scholar] [CrossRef]
- Lyu, J.; Zhang, Y.; Zhou, R.; Ding, C.; Ye, H.; Fang, Q.; Jiang, C.; Chen, X.; Zhong, L. The effect of periodontal treatments on endothelial function in degrees of periodontitis patients: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0308793. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an 896 inflammatory disease of oxidative stress: We should treat it that way. Periodontol. 2000 2020, 84, 45–68. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Currò, M.; Isola, G.; Maggio, S.; Bertuccio, M.P.; Trovato-Salinaro, A.; Matarese, G.; Alibrandi, A.; Caccamo, D.; Ientile, R. Changes in the biomarkers of oxidative/nitrosative stress and endothelial dysfunction in periodontitis patients. Curr. Issues Mol. Biol. 2021, 43, 704–715. [Google Scholar] [CrossRef]
- Angjelova, A.; Jovanova, E.; Polizzi, A.; Laganà, L.; Santonocito, S.; Ragusa, R.; Isola, G. Impact of periodontitis on endothelial risk dysfunction and oxidative stress improvement in patients with cardiovascular disease. J. Clin. Med. 2024, 13, 3781. [Google Scholar] [CrossRef]
- Chen, W.A.; Monteiro, C.; Deng, Y.; van der Velden, U.; Loos, B.G. Local and systemic effects of Porphyromonas gingivalis infection. Microorganisms 2023, 11, 470. [Google Scholar] [CrossRef]
- Joshi, C.; Bapat, R.; Anderson, W.; Dawson, D.; Hijazi, K.; Cherukara, G. Detection of periodontal microorganisms in coronary atheromatous plaque specimens of myocardial infarction patients: A systematic review and meta-analysis. Trends Cardiovasc. Med. 2021, 31, 69–82. [Google Scholar] [CrossRef]
- Maeda, H.; Miyamoto, M.; Hongyo, H.; Nagai, A.; Kurihara, H.; Murayama, Y. Heat shock protein 60 (GroEL) from Porphyromonas gingivalis: Molecular cloning and sequence analysis of its gene and purification of the recombinant protein. FEMS Microbiol. Lett. 1994, 119, 129–135, Erratum in FEMS Microbiol. Lett. 1994, 124, 121–122. https://doi.org/10.1111/j.1574-6968.1994.tb07271.x. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease. Circulation 2011, 123, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.M.; Ramirez-Yanez, G.; Cornelius Timm, H.; Martínez-Lara, I. MicroRNAs: The missing link between hypertension and periodontitis? Int. J. Mol. Sci. 2024, 25, 1992. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, C.; Stafford, G.P.; Murdoch, C. Porphyromonas gingivalis outer membrane vesicles increase vascular permeability. J. Dent. Res. 2020, 99, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vargas, P.; Barloy-Hubler, F.; Acuña-Amador, L. Unveiling oral anaerobic bacteria outer membrane vesicles: A comprehensive systematic review. Odovtos. Int. J. Dent. Sci. 2024, 26, 41–61. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Kopra, E.; Pietiäinen, M.; Lehto, M.; Zaric, S.; Paju, S.; Salminen, A. Periodontitis and cardiometabolic disorders: The role of lipopolysaccharide and endotoxemia. Periodontol. 2000 2022, 89, 19–40. [Google Scholar] [CrossRef]
- Huang, X.; Xie, M.; Lu, X.; Mei, F.; Song, W.; Liu, Y.; Chen, L. The roles of periodontal bacteria in atherosclerosis. Int. J. Mol. Sci. 2023, 16, 12861. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tong, X.; Zhu, J.; Tian, C.; Jie, Z.; Zhang, D.; Lin, X.; Liang, H.; Li, W.; Ju, Y.; et al. Metagenome-genome-wide association studies reveal human genetic impact on the oral microbiome. Cell Discov. 2021, 7, 117. [Google Scholar] [CrossRef]
- Wade, W.G.; Prosdocimi, E.M. Profiling of oral bacterial communities. J. Dent. Res. 2020, 99, 621–629. [Google Scholar] [CrossRef]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontol. 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef]
- Śmiga, M.; Ślęzak, P.; Wagner, M.; Olczak, T. Interplay between Porphyromonas gingivalis Hemophore-Like Protein HmuY and Kgp/RgpA Gingipains Plays a Superior Role in Heme Supply. Microbiol. Spectr. 2023, 11, e0459322. [Google Scholar] [CrossRef]
- Sheets, S.M.; Potempa, J.; Travis, J.; Casiano, C.A.; Fletcher, H.M. Gingipains from Porphyromonas gingivalis W83 induce cell adhesion molecule cleavage and apoptosis in endothelial cells. Infect Immun. 2005, 73, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Davey, M.; Yumoto, H.; Gibson, F.C., 3rd; Genco, C.A. Fimbria-dependent activation of pro-inflammatory molecules in Porphyromonas gingivalis infected human aortic endothelial cells. Cell Microbiol. 2006, 8, 738–757. [Google Scholar] [CrossRef] [PubMed]
- Kocgozlu, L.; Elkaim, R.; Tenenbaum, H.; Werner, S. Variable cell responses to P. gingivalis lipopolysaccharide. J. Dent. Res. 2009, 88, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Tang, Q.; Yu, S.; Sun, J.; Mei, F.; Zhao, J.; Chen, L. Porphyromonas gingivalis disrupts vascular endothelial homeostasis in a TLR-NF-κB axis dependent manner. Int. J. Oral Sci. 2020, 12, 28. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Meštrović, T.; Matijević, T.; Mesarić, D.; Katalinić, D.; Erić, S.; Milostić-Srb, A.; Flam, J.; Škrlec, I. Aggregatibacter actinomycetemcomitans: From the oral cavity to the heart valves. Microorganisms 2024, 12, 1451. [Google Scholar] [CrossRef]
- Chukkapalli, S.S.; Velsko, I.M.; Rivera-Kweh, M.F.; Zheng, D.; Lucas, A.R.; Kesavalu, L. Polymicrobial Oral Infection with Four Periodontal Bacteria Orchestrates a Distinct Inflammatory Response and Atherosclerosis in ApoE null Mice. PLoS ONE 2015, 10, e0143291. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Tuomisto, K.; Jousilahti, P.; Havulinna, A.S.; Sundvall, J.; Salomaa, V. Endotoxemia, immune response to periodontal pathogens, and systemic inflammation associate with incident cardiovascular disease events. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1433–1439. [Google Scholar] [CrossRef]
- Hokamura, K.; Inaba, H.; Nakano, K.; Nomura, R.; Yoshioka, H.; Taniguchi, K.; Ooshima, T.; Wada, K.; Amano, A.; Umemura, K. Molecular analysis of aortic intimal hyperplasia caused by Porphyromonas gingivalis infection in mice with endothelial damage. J. Periodontal Res. 2010, 45, 337–344. [Google Scholar] [CrossRef]
- Yamada, M.; Ikegami, A.; Kuramitsu, H.K. Synergistic biofilm formation by Treponema denticola and Porphyromonas gingivalis. FEMS Microbiol. Lett. 2005, 250, 271–277. [Google Scholar] [CrossRef]
- Ng, H.M.; Slakeski, N.; Butler, C.A.; Veith, P.D.; Chen, Y.Y.; Liu, S.W.; Hoffmann, B.; Dashper, S.G.; Reynolds, E.C. The Role of Treponema denticola Motility in Synergistic Biofilm Formation with Porphyromonas gingivalis. Front. Cell. Infect. Microbiol. 2019, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Dashper, S.G.; Chen, Y.Y.; Crawford, S.; Slakeski, N.; Reynolds, E.C. Porphyromonas gingivalis and Treponema denticola synergistic polymicrobial biofilm development. PLoS ONE 2013, 8, e71727. [Google Scholar] [CrossRef]
- Aral, K.; Milward, M.R.; Gupta, D.; Cooper, P.R. Effects of Porphyromonas gingivalis and Fusobacterium nucleatum on inflammasomes and their regulators in H400 cells. Mol. Oral Microbiol. 2020, 35, 158–167. [Google Scholar] [CrossRef]
- Marotz, C.; Molinsky, R.; Martino, C.; Bohn, B.; Roy, S.; Rosenbaum, M.; Desvarieux, M.; Yuzefpolskaya, M.; Paster, B.J.; Jacobs, D.R.; et al. Early microbial markers of periodontal and cardiometabolic diseases in ORIGINS. npj Biofilms Microbiomes 2022, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrowicz, P.; Brzezińska-Błaszczyk, E.; Kozłowska, E.; Żelechowska, P.; Borgonovo, A.E.; Agier, J. Analysis of IL-1β, CXCL8, and TNF-α levels in the crevicular fluid of patients with periodontitis or healthy implants. BMC Oral Health 2021, 21, 120. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Filho, I.S.; Freitas Coelho, J.M.; da Cruz, S.S.; Passos, J.S.; Teixeira de Freitas, C.O.; Aragão Farias, N.S.; Amorim da Silva, R.; Silva Pereira, M.N.; Lima, T.L.; Barreto, M.L. Chronic periodontitis and C-reactive protein levels. J. Periodontol. 2011, 82, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, J.; Mahendra, L.; Felix, J.; Romanos, G. Prevelance of periodontopathogenic bacteria in subgingival biofilm and atherosclerotic plaques of patients undergoing coronary revascularization surgery. J. Indian Soc. Periodontol. 2013, 17, 719–724. [Google Scholar] [CrossRef]
- Armingohar, Z.; Jørgensen, J.J.; Kristoffersen, A.K.; Abesha-Belay, E.; Olsen, I. Bacteria and bacterial DNA in atherosclerotic plaque and aneurysmal wall biopsies from patients with and without periodontitis. J. Oral Microbiol. 2014, 6, 23408. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.F.; Lee, J.Y.; Aneja, M.; Goswami, V.; Liu, L.; Velsko, I.M.; Chukkapalli, S.S.; Bhattacharyya, I.; Chen, H.; Lucas, A.R.; et al. Polymicrobial infection with major periodontal pathogens induced periodontal disease and aortic atherosclerosis in hyperlipidemic ApoE(null) mice. PLoS ONE 2013, 8, e57178. [Google Scholar] [CrossRef]
- Ferrara, E.; Converti, I.; Scarola, R.; Tartaglia, F.C.; Gnoni, A.; Isola, G.; Rapone, B. Mechanism behind the Upregulation of Proteins Associated with the NLRP3 Inflammasome in Periodontitis and Their Role in the Immune Response in Diabetes—A Systematic Review. Appl. Sci. 2023, 13, 8278. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Nagano, K. Porphyromonas gingivalis FimA and Mfa1 fimbriae: Current insights on localization, function, biogenesis, and genotype. Jpn. Dent. Sci. Rev. 2021, 57, 190–200. [Google Scholar] [CrossRef]
- Hyink, O.; Wescombe, P.A.; Upton, M.; Ragland, N.; Burton, J.P.; Tagg, J.R. Salivaricin A2 and the novel lantibiotic salivaricin B are encoded at adjacent loci on a 190-kilobase transmissible megaplasmid in the oral probiotic strain Streptococcus salivarius K12. Appl. Environ. Microbiol. 2007, 73, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Furuta, N.; Takeuchi, H.; Amano, A. Entry of Porphyromonas gingivalis outer membrane vesicles into epithelial cells causes cellular functional impairment. Infect. Immun. 2009, 77, 4761–4770. [Google Scholar] [CrossRef] [PubMed]
- Van Damme-Ostapowicz, K.; Cybulski, M.; Kozakiewicz, M.; Krajewska-Kułak, E.; Siermontowski, P.; Sobolewski, M.; Kaczerska, D. Analysis of the Increase of Vascular Cell Adhesion Molecule-1 (VCAM-1) Expression and the Effect of Exposure in a Hyperbaric Chamber on VCAM-1 in Human Blood Serum: A Cross-Sectional Study. Medicina 2022, 58, 95. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kim, Y.G.; Park, J.W.; Suh, J.Y.; Lee, J.M. The expression of a nitric oxide derivative, tissue inhibitors of metalloproteinase-3, and tissue inhibitors of metalloproteinase-4 in chronic periodontitis with type 2 diabetes mellitus. J. Periodontal Implant Sci. 2013, 43, 87–95. [Google Scholar] [CrossRef]
- Maekawa, T.; Krauss, J.L.; Abe, T.; Jotwani, R.; Triantafilou, M.; Triantafilou, K.; Hashim, A.; Hoch, S.; Curtis, M.A.; Nussbaum, G.; et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe 2014, 15, 768–778. [Google Scholar] [CrossRef]
- Topcu Ali, O.; Akalin, F.A.; Sahbazoglu, K.B.; Yamalik, N.; Kilinc, K.; Karabulut, E.; Tözüm, T.F. Nitrite and nitrate levels of gingival crevicular fluid and saliva in subjects with gingivitis and chronic periodontitis. J. Oral Maxillofac. Res. 2014, 5, e5. [Google Scholar] [CrossRef] [PubMed]
- Rosier, B.T.; Palazón, C.; García-Esteban, S.; Artacho, A.; Galiana, A.; Mira, A. Nitrate reduction capacity of the oral microbiota is impaired in periodontitis: Potential implications for systemic nitric oxide availability. Int. J. Oral Sci. 2024, 16, 1. [Google Scholar] [CrossRef]
- Velsko, I.M.; Chukkapalli, S.S.; Rivera, M.F.; Lee, J.Y.; Chen, H.; Zheng, D.; Bhattacharyya, I.; Gangula, P.R.; Lucas, A.R.; Kesavalu, L. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS ONE 2014, 9, e97811. [Google Scholar] [CrossRef] [PubMed]
- Pietiäinen, M.; Liljestrand, J.M.; Kopra, E.; Pussinen, P.J. Mediators between oral dysbiosis and cardiovascular diseases. Eur. J. Oral Sci. 2018, 126 (Suppl. S1), 26–36. [Google Scholar] [CrossRef] [PubMed]
- Rapone, B.; Ferrara, E.; Qorri, E.; Dipalma, G.; Mancini, A.; Corsalini, M.; Fabbro, M.D.; Scarano, A.; Tartaglia, G.M.; Inchingolo, F. The Impact of Periodontal Inflammation on Endothelial Function Assessed by Circulating Levels of Asymmetric Dimethylarginine: A Single-Blinded Randomized Clinical Trial. J. Clin. Med. 2022, 11, 4173. [Google Scholar] [CrossRef]
- Palm, F.; Cederberg, J.; Hansell, P.; Liss, P.; Carlsson, P.O. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia 2003, 46, 1153–1160. [Google Scholar] [CrossRef]
- Patil, R.T.; Dhadse, P.V.; Salian, S.S.; Punse, S.D. Role of Oxidative Stress in Periodontal Diseases. Cureus 2024, 16, e60779. [Google Scholar] [CrossRef]
- Asa’ad, F.; Bollati, V.; Pagni, G.; Castilho, R.M.; Rossi, E.; Pomingi, F.; Tarantini, L.; Consonni, D.; Giannobile, W.V.; Rasperini, G. Evaluation of DNA methylation of inflammatory genes following treatment of chronic periodontitis: A pilot case-control study. J. Clin. Periodontol. 2017, 44, 905–914. [Google Scholar] [CrossRef]
- Kong, D.H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in immunological disorders and cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef]
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010, 207, 1853–1862. [Google Scholar] [CrossRef]
- Olejniczak-Kęder, A.; Szymańska, M.; Wrońska, N.; Bielecki, M.; Niedźwiedzka-Rystwej, P.; Rękawiecki, B. Extracellular DNA and markers of neutrophil extracellular traps in saliva from patients with periodontitis—A case-control study. J. Clin. Med. 2024, 13, 468. [Google Scholar]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Norhammar, A.; Näsman, P.; Buhlin, K.; de Faire, U.; Ferrannini, G.; Gustafsson, A.; Kjellström, B.; Kvist, T.; Jäghagen, E.L.; Lindahl, B.; et al. PAROKRANK Study Group. Does Periodontitis Increase the Risk for Future Cardiovascular Events? Long-Term Follow-Up of the PAROKRANK Study. J. Clin. Periodontol. 2025, 52, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lewis, H.D.; Liddle, J.; Coote, J.E.; Atkinson, S.J.; Barker, M.D.; Bax, B.D.; Bicker, K.L.; Bingham, R.P.; Campbell, M.; Chen, Y.H.; et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat. Chem. Biol. 2015, 11, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Denning, N.L.; Aziz, M.; Gurien, S.D.; Wang, P. DAMPs and NETs in Sepsis. Front. Immunol. 2019, 10, 2536. [Google Scholar] [CrossRef]
- Pai, J.K. Asymmetric dimethylarginine as a marker of metabolic dysfunction and cardiovascular disease. Curr. Cardiovasc. Risk Rep. 2008, 2, 156–160. [Google Scholar] [CrossRef]
- Vallance, P.; Leiper, J. Cardiovascular biology of the asymmetric dimethylarginine: Dimethylarginine dimethylaminohydrolase pathway. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1023–1030. [Google Scholar] [CrossRef]
- Tsikas, D. Does the Inhibitory Action of Asymmetric Dimethylarginine (ADMA) on the Endothelial Nitric Oxide Synthase Activity Explain Its Importance in the Cardiovascular System? The ADMA Paradox. J. Control. Biomed. Res. 2017, 3, 16–22. [Google Scholar] [CrossRef]
- Dowsett, L.; Higgins, E.; Alanazi, S.; Alshuwayer, N.A.; Leiper, F.C.; Leiper, J. ADMA: A Key Player in the Relationship between Vascular Dysfunction and Inflammation in Atherosclerosis. J. Clin. Med. 2020, 9, 3026. [Google Scholar] [CrossRef]
- Cibulka, R.; Široká, R.; Rajdl, D.; Racek, J.; Trefil, L.; Eiselt, J. Asymmetric dimethylarginine (ADMA) as a novel independent risk factor for cardiovascular disease in haemodialysis patients. Klin. Biochem. Metab. 2007, 15, 39–42. [Google Scholar][Green Version]
- Güney, Z.; Kurgan, S.; Önder, C.; Mammadov, C.; Serdar, M.A.; Günhan, M. Asymmetric and symmetric dimethylarginine gingival crevicular fluid levels in periodontitis. J. Periodontal Res. 2023, 58, 256–261. [Google Scholar] [CrossRef]
- Messina, B.M.; Grippaudo, C.; Polizzi, A.; Blasi, A.; Isola, G. The Key Role of Porphyromonas gingivalis in the Pathogenesis of Periodontitis Linked with Systemic Diseases. Appl. Sci. 2025, 15, 6847. [Google Scholar] [CrossRef]
- Jarzebska, N.; Mangoni, A.A.; Martens-Lobenhoffer, J.; Bode-Böger, S.M.; Rodionov, R.N. The Second Life of Methylarginines as Cardiovascular Targets. Int. J. Mol. Sci. 2019, 20, 4592. [Google Scholar] [CrossRef]
- Böger, R.H. Asymmetric dimethylarginine (ADMA) and cardiovascular disease: Insights from prospective clinical trials. Vasc. Med. 2005, 10 (Suppl. S1), S19–S25. [Google Scholar] [CrossRef]
- Jiang, Y.; Fu, J.; Du, J.; Luo, Z.; Guo, L.; Xu, J.; Liu, Y. DNA methylation alterations and their potential influence on macrophage in periodontitis. Oral Dis. 2022, 28, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Thomopoulos, C.; Tsioufis, C.; Soldatos, N.; Giamarelos, G.; Dendrinos, C.; Selima, M.; Kasiakogias, A.; Stefanadi, E.; Tousoulis, D.; Stefanadis, C. Chronic periodontal disease and systemic inflammation on endothelial dysfunction in untreated hypertensive patients: PP.2.53. J. Hypertens. 2010, 28, e63. [Google Scholar] [CrossRef]
- Zhang, K.; Li, C.; Sun, J.; Tian, X. PRMT5 inhibition ameliorates inflammation and promotes the osteogenic differentiation of LPS-induced periodontal stem cells via STAT3/NF-κB signaling. Exp. Ther. Med. 2023, 25, 264. [Google Scholar] [CrossRef]
- Hamza, S.A.; Asif, S.; Khurshid, Z.; Zafar, M.S.; Bokhari, S.A.H. Emerging Role of Epigenetics in Explaining Relationship of Periodontitis and Cardiovascular Diseases. Diseases 2021, 9, 48. [Google Scholar] [CrossRef]
- Diomede, F.; Pizzicannella, J.; Merciaro, I.; Guarnieri, S.; Trubiani, O. An intriguing relation between periodontal and cardiovascular diseases. Ital. J. Anat. Embryol. 2017, 121, 160. [Google Scholar]
- Omar, M.; Alexiou, M.; Rekhi, U.R.; Lehmann, K.; Bhardwaj, A.; Delyea, C.; Elahi, S.; Febbraio, M. DNA methylation changes underlie the long-term association between periodontitis and atherosclerotic cardiovascular disease. Front. Cardiovasc. Med. 2023, 10, 1164499. [Google Scholar] [CrossRef]
- Shi, W.; Song, J.; Weiner JM3rd Chopra, A.; Dommisch, H.; Beule, D.; Schaefer, A.S. lncRNA CDKN2B-AS1 regulates collagen expression. Hum. Genet. 2024, 143, 907–919. [Google Scholar] [CrossRef]
- Schaefer, A.S.; Richter, G.M.; Groessner-Schreiber, B.; Noack, B.; Nothnagel, M.; El Mokhtari, N.E.; Loos, B.G.; Jepsen, S.; Schreiber, S. Identification of a shared genetic susceptibility locus for coronary heart disease and periodontitis. PLoS Genet. 2009, 5, e1000378. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, H.; Park, H.J.; Kim, M.K.; Kim, Y.I.; Kim, H.J.; Bae, S.K.; Kim, Y.J.; Bae, M.K. Hispidulin Inhibits the Vascular Inflammation Triggered by Porphyromonas gingivalis Lipopolysaccharide. Molecules 2023, 28, 6717. [Google Scholar] [CrossRef] [PubMed]
- Dhungana, G.; Srisai, D.; Sampath, C.; Soliman, J.; Kelly, R.M.; Saleh, H.Y.; Sedik, A.; Raynes, E.; Ferguson, A.; Alluri, L.S.C.; et al. Unveiling the Molecular Crosstalk Between Periodontal and Cardiovascular Diseases: A Systematic Review. Dent. J. 2025, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Tero-Vescan, A.; Slevin, M.; Pușcaș, A.; Sita, D.; Ștefănescu, R. Targeting Epigenetic Plasticity to Reduce Periodontitis-Related Inflammation in Diabetes: CBD, Metformin, and Other Natural Products as Potential Synergistic Candidates for Regulation? A Narrative Review. Int. J. Mol. Sci. 2025, 26, 2853. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.W.; Huang, Y.F.; Chan, Y.; Chou, M.Y. Relationship of Porphyromonas gingivalis-derived outer membrane vesicles to calcification of vascular smooth muscle cells through ERK1/2-RUNX2. FEBS Lett. 2017, 591, 728–737. [Google Scholar]
- Su, W.; Shi, J.; Zhao, Y.; Yan, F.; Lei, L.; Li, H. Porphyromonas gingivalis triggers inflammatory responses in periodontal ligament cells by succinate-succinate dehydrogenase-HIF-1α axis. Biochem. Biophys. Res. Commun. 2020, 522, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.; Gopinathan, G.; Salapatas, A.; Nares, S.; Gonzalez, M.; Diekwisch, T.G.H.; Luan, X. SETD1 and NF-κB Regulate Periodontal Inflammation through H3K4 Trimethylation. J. Dent. Res. 2020, 99, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Shenker, B.J.; Ojcius, D.M.; Walker, L.P.; Zekavat, A.; Scuron, M.D.; Boesze-Battaglia, K. Aggregatibacter actinomycetemcomitans cytolethal distending toxin activates the NLRP3 inflammasome in human macrophages, leading to the release of proinflammatory cytokines. Infect. Immun. 2015, 83, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Kuboniwa, M.; Hendrickson, E.L.; Xia, Q.; Wang, T.; Xie, H.; Hackett, M.; Lamont, R.J. Proteomics of Porphyromonas gingivalis within a model oral microbial community. BMC Microbiol. 2009, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Liu, W.; Xue, P.; Zhang, Y.; Wang, Q.; Jin, Y. Autophagy promotes MSC-mediated vascularization in cutaneous wound healing via regulation of VEGF secretion. Cell Death Dis. 2018, 9, 58. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, S.C.; Hong, S.H.; Lee, H.J. Secretable Small RNAs via Outer Membrane Vesicles in Periodontal Pathogens. J. Dent. Res. 2017, 96, 458–466. [Google Scholar] [CrossRef]


| Biomarker Category | Marker | Normal Range | Pathological Level | Clinical Significance |
|---|---|---|---|---|
| Oxidative stress | Vitamin C | 45–80 μM | ↓ | Antioxidant depletion |
| SOD activity | 785–1570 U/g Hb | ↓ | Impaired ROS neutralization | |
| Peroxynitrite | <0.1 μM | 0.5–1.0 μM | eNOS uncoupling | |
| Inflammation | ADMA | 0.4–0.6 μM | >0.8 μM (elevated) | Endothelial dysfunction |
| VCAM-1 | 400–800 ng/mL | ↑ | Vascular inflammation | |
| CRP | <3 mg/L | >5 mg/L | Systemic inflammation | |
| Vascular function | NO bioavailability | >85% | ↓ | Endothelial dysfunction |
| BH4 levels | 5–15 nM | ↓ | eNOS cofactor depletion | |
| Flow-mediated dilation | >10% | <7% | Impaired vascular reactivity |
| Clinical Reference Values | Major Cardiovascular Effects | Changes in Periodontitis | Molecule | Mediator Category |
|---|---|---|---|---|
| Inflammatory cytokines | ||||
| Normal: <3.5 pg/mL | Endothelial dysfunction, VCAM-1 upregulation | ↑ in serum | IL-1β | |
| Normal: <10 pg/mL | Atherosclerotic plaque formation, VSMC proliferation | ↑ in serum | TNF-α | |
| Normal: <5 pg/mL | Plaque instability, CRP production | ↑ in serum | IL-6 | |
| Oxidative stress markers | ||||
| Reference: Basal activity | Sustained ROS generation, endothelial damage | ↑ activity | NOX2 | |
| Normal: <2.0 ng/mL | DNA oxidation marker, cellular damage | ↑ in serum | 8-OHdG | |
| Normal: <50 U/L | Foam cell formation, plaque progression | ↑ in plasma | MDA-LDL | |
| Epigenetic regulators | ||||
| Baseline expression | Inflammatory pathway regulation, NF-κB signaling | ↑ expression | miR-146a | |
| ChIP enrichment ratio | Pro-inflammatory gene activation | ↑ at promoters | H3K4me3 | |
| Enzymatic activity units | Altered DNA methylation patterns | ↑ activity | DNMT1 |
| Treatment Category | Specific Intervention | Mechanism of Action | Development Stage | Clinical Evidence |
|---|---|---|---|---|
| Conventional periodontal | Scaling and root planing | Mechanical disruption of biofilm, reduction of bacterial load | Standard of care | 35% reduction in CRP, improved FMD |
| Antimicrobial therapy | Direct bacterial elimination | Clinical use | Variable efficacy, resistance concerns | |
| Anti-inflammatory | MCC950 (NLRP3 inhibitor) | Selective NLRP3 inflammasome blockade | Phase II trials | Reduced IL-1β, IL-18 in pilot studies |
| Canakinumab (IL-1β antibody) | IL-1β neutralization | FDA approved (other indications) | CANTOS trial: 15% CVDs risk reduction | |
| Antioxidant | NOX2 inhibitors (GSK2795039) | Targeted ROS reduction | Preclinical | 70% reduction in vascular superoxide |
| Mitochondria-targeted antioxidants | Cellular oxidative stress reduction | Phase I | MitoQ shows promise in animal models | |
| Epigenetic modulators | HDAC inhibitors | Reversal of pathogenic gene expression | Phase I/II | Restoration of eNOS expression |
| DNMT inhibitors | DNA methylation modification | Preclinical | KLF4 promoter demethylation achieved | |
| Microbiome-based | S. salivarius M18 probiotic | Competitive exclusion, bacteriocin production | Commercial availability | 40% reduction in pathogen load |
| Gingipain inhibitors | Specific virulence factor targeting | Preclinical | COR388 in development | |
| ADMA-targeted | L-citrulline supplementation | Enhanced ADMA clearance | Clinical use | 30% ADMA reduction, improved FMD |
| DDAH enhancers | Increased ADMA metabolism | Preclinical | Proof-of-concept established | |
| Combination approaches | Periodontal therapy + statins | Synergistic anti-inflammatory effects | Observational studies | Enhanced CVD risk reduction |
| Omega-3 + periodontal treatment | Resolution of inflammation | Small RCTs | Improved clinical outcomes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, E.; D’Albenzio, A.; Bassignani, J.; Di Tanna, I.; Murmura, G.; Balice, G. The Periodontal–Cardiovascular Disease Association: Molecular Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2025, 26, 7710. https://doi.org/10.3390/ijms26167710
Ferrara E, D’Albenzio A, Bassignani J, Di Tanna I, Murmura G, Balice G. The Periodontal–Cardiovascular Disease Association: Molecular Mechanisms and Clinical Implications. International Journal of Molecular Sciences. 2025; 26(16):7710. https://doi.org/10.3390/ijms26167710
Chicago/Turabian StyleFerrara, Elisabetta, Alessandro D’Albenzio, Jessica Bassignani, Isabella Di Tanna, Giovanna Murmura, and Giuseppe Balice. 2025. "The Periodontal–Cardiovascular Disease Association: Molecular Mechanisms and Clinical Implications" International Journal of Molecular Sciences 26, no. 16: 7710. https://doi.org/10.3390/ijms26167710
APA StyleFerrara, E., D’Albenzio, A., Bassignani, J., Di Tanna, I., Murmura, G., & Balice, G. (2025). The Periodontal–Cardiovascular Disease Association: Molecular Mechanisms and Clinical Implications. International Journal of Molecular Sciences, 26(16), 7710. https://doi.org/10.3390/ijms26167710







