ATR-CHK1 Axis Inhibitors in Gastric Cancer Treatment
Abstract
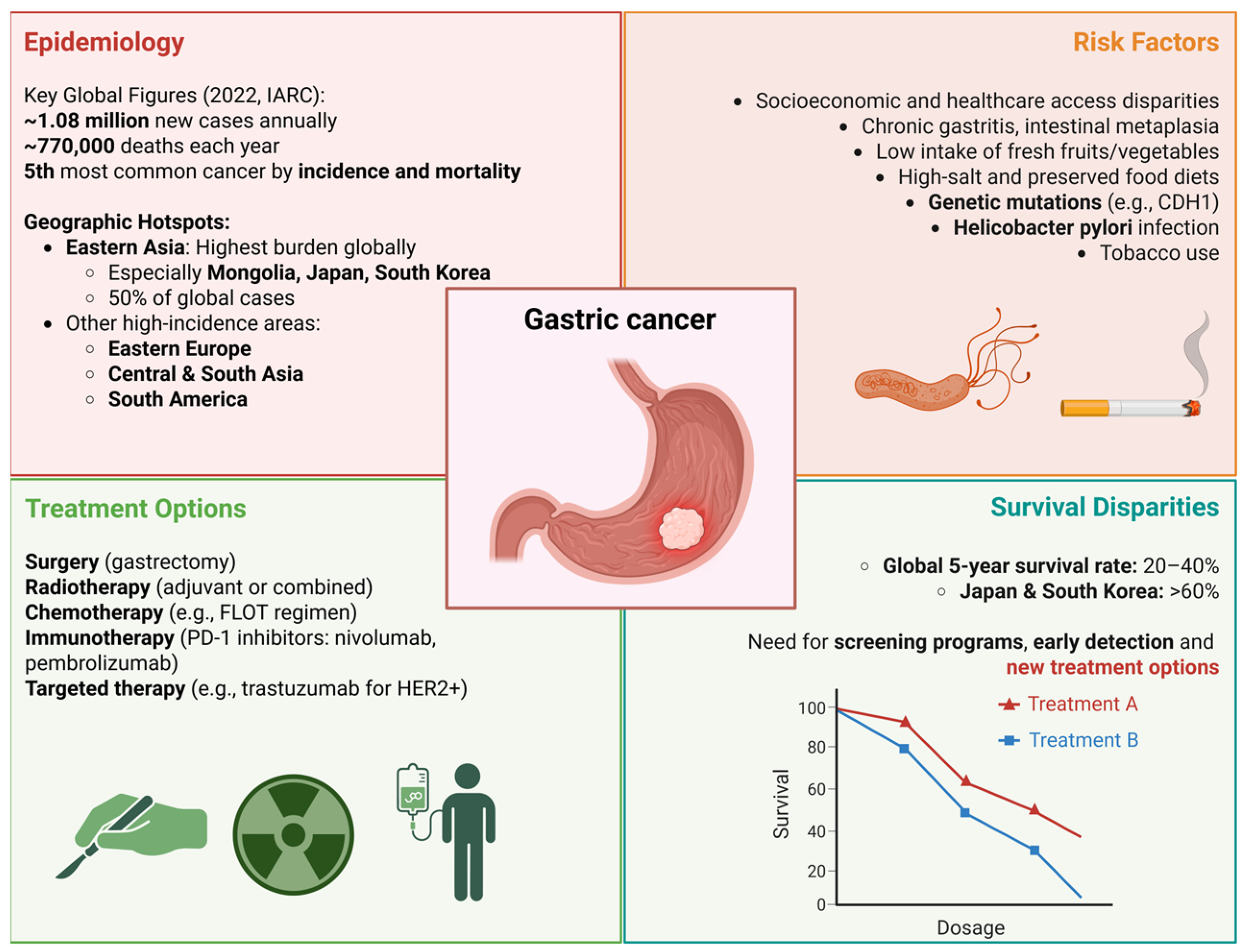
1. Introduction
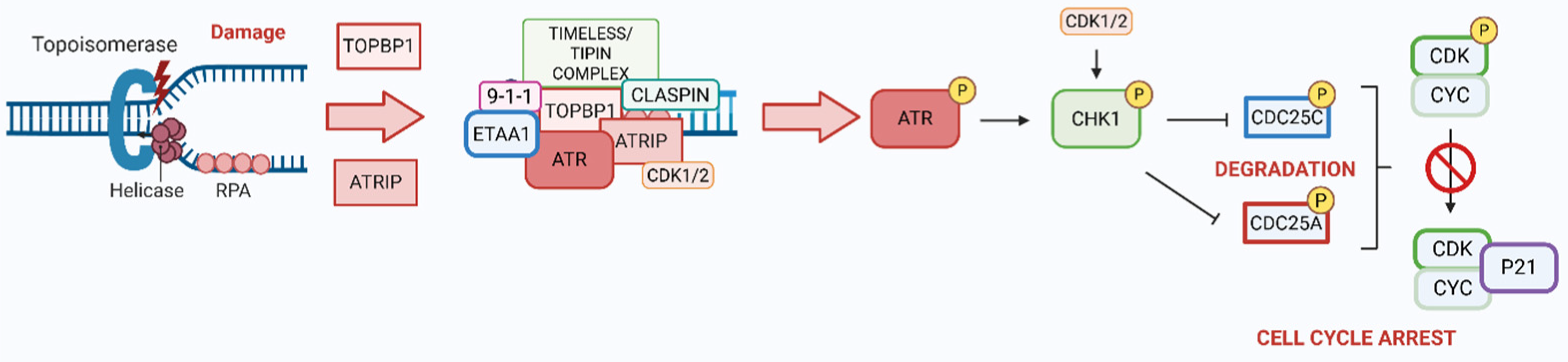
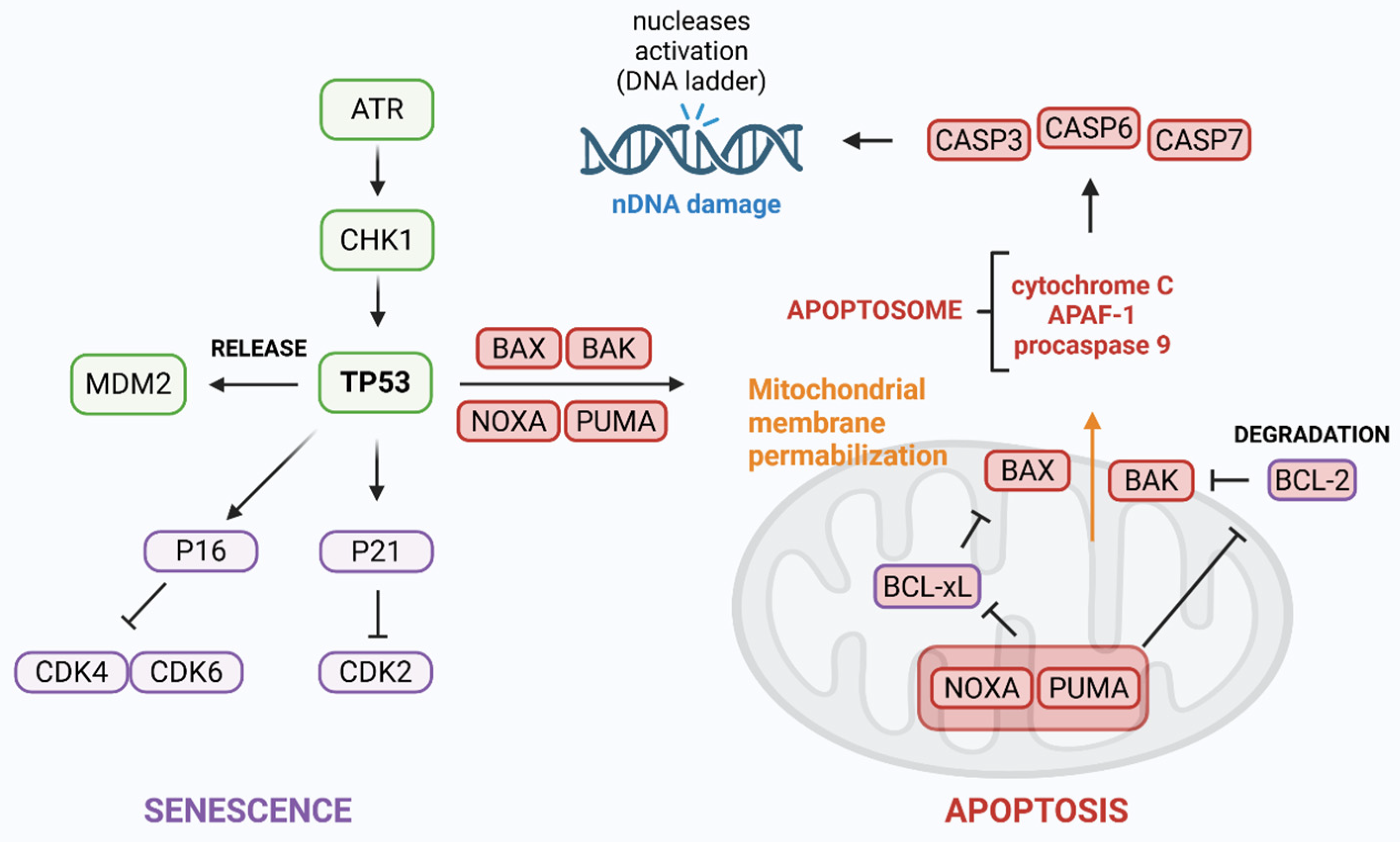
2. ATR/CHK1 Axis in DDR
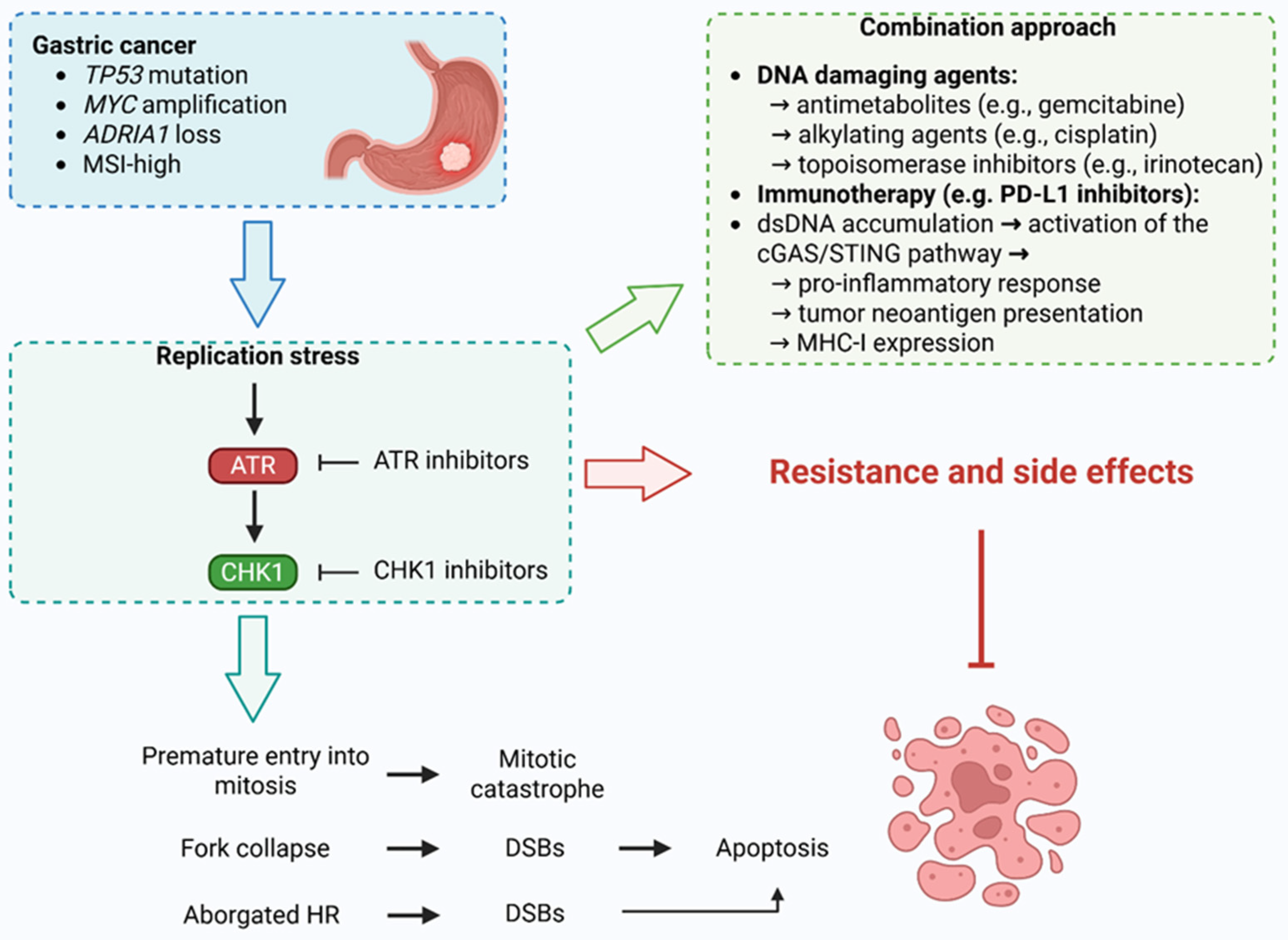
3. ATR and CHK1 Kinases in Gastric Cancer
4. ATR Kinase Inhibitors in Gastric Cancer
4.1. Pre-Clinical Studies
Completed Clinical Trials
4.2. Ongoing and Future Clinical Studies
5. CHK1 Inhibitors in Gastric Cancer
6. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| ARID1A | AT-rich interaction domain 1A |
| ATM | ataxia-telangiectasia mutated |
| ATR | ataxia telangiectasia and Rad3-related protein kinase |
| ATRIP | ATR-interacting protein |
| ATRis | ATR inhibitors |
| BAK | BCL2 antagonist/killer 1 |
| BAX | BCL2 associated X |
| BRCA1/2 | breast cancer type 1 susceptibility protein 1/2 |
| BRIP1 | BRCA1 interacting protein C-terminal helicase 1 |
| CAD | caspase-activated Dnase |
| CASPASE5 | cysteine-aspartic acid protease 5 |
| CCNC | cyclin C |
| CCNE1 | cyclin E |
| CDC25A/25C/45 | cell division cycle 25A/25C/45 |
| CDH1 | cadherin 1 |
| CDK1/2 | cyclin-dependent kinases 1/2 |
| CDR | coding region |
| cGAS-STING | cyclic GMP-AMP synthase-stimulator of interferon genes |
| CHK1/2 | checkpoint kinases 1/2 |
| ctDNA | circulating tumor DNA |
| DCR | disease control rate |
| DDK | DBF4-dependent kinase |
| DDR | DNA damage response |
| DNA-PKcs | DNA-dependent protein kinase catalytic subunit |
| DOR | duration of response |
| DSB | DNA double-strand break |
| DSBR | DNA double-strand break repair |
| EBV | Epstein–Barr virus |
| ecDNA | extrachromosomal DNA |
| ECT2 | epithelial cell transforming 2 oncogene |
| EGFR | epidermal growth factor receptor |
| ERBB2 | erb-b2 receptor tyrosine kinase 2 |
| ETAA1 | Ewing’s tumor-associated antigen 1 |
| FANCC | fanconi anemia complementation group C |
| FAS | fas cell surface death receptor |
| FGFR2 | fibroblast growth factor receptor 2 |
| FLT-PET | 3′-deoxy-3′-[18F]fluorothymidine positron emission tomo |
| FOLFIRI | leucovorin calcium, fluorouracil, and irinotecan hydrochloride |
| FOXM1 | forkhead box M1 |
| GEJ | gastric and gastroesophageal junction |
| HDAC1 | histone deacetylase 1 |
| HDGC | hereditary diffuse gastric cancer |
| HER2 | human epidermal growth factor receptor 2 |
| hMLH1 | mutL homolog 1 |
| hMSH3 | mutS homolog 3 |
| hMSH6 | mutS homolog 6 |
| HR | homologous recombination |
| HRD | homologous recombination deficiency |
| IARC | International Agency for Research on Cancer |
| IGFIIR | insulin-like growth factor II receptor |
| ILF2 | interleukin enhancer binding factor 2 |
| KU70 | X-ray repair cross-complementing 6 |
| MDC1 | mediator of DNA damage checkpoint 1 |
| MDM2 | E3 ubiquitin-protein ligase |
| MED1 | mediator complex subunit 1 |
| MHC | major histocompatibility complex |
| MLL | mixed-lineage leukemia protein |
| MMR | mismatch repair |
| MRE11 | meiotic recombination 11 homolog A |
| MSH2 | mutS homolog 2 |
| MSI | microsatellite instability |
| MTD | maximum tolerated dose |
| MYC | myelocytomatosis oncogene |
| NBS1 | Nijmegen breakage syndrome protein 1 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NGS | next-generation sequencing |
| NMD | nonsense-mediated mRNA decay |
| NOXA | phorbol-12-myristate-13-acetate-induced protein 1 |
| ORC | origin recognition complex |
| ORR | overall response rate |
| P38MAPK | p38 mitogen-activated protein kinase |
| PAK6 | p21 (RAC1) activated kinase 6 |
| PALB2 | partner and localizer of BRCA2 |
| PARP | poly (ADP-ribose) polymerase |
| PBMCs | peripheral blood mononuclear cells |
| PD-L1 | programmed death-ligand 1 |
| PDX | patient-derived xenograft |
| PFS | progression-free survival |
| PI3K | phosphoinositide 3-kinase |
| PIK3CA | phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha |
| PKB | protein kinase B |
| pRB | retinoblastoma protein |
| PUMA | BCL2 binding component 3 |
| RAC | Ras-related C3 botulinum toxin substrate |
| RAD50 | RADiation sensitive 50 |
| RAD51 | RADiation sensitive 51 |
| RAS | rat sarcoma viral oncogene homolog |
| RECQL5 | RecQ-like helicase 5 |
| RHO | ras homologous |
| ROS | reactive oxygen species |
| RPA | replication protein A |
| RS | replication stress |
| SCLC | small cell lung cancer |
| shRNA | short hairpin RNA |
| SLFN11 | schlafen family member 11 |
| ssDNA | single-stranded DNA |
| STAT3 | signal transducer and activator of transcription 3 |
| T14 | threonine 14 |
| TCF4 | T-cell factor 4 |
| TCGA | The Cancer Genome Atlas |
| TEAE | treatment-emergent adverse event |
| TGFβRII | transforming growth factor beta receptor type II |
| TOPBP1 | topoisomerase 2-binding protein 1 |
| TP53 | tumor protein p53 |
| TTP | time to progression |
| UC | urothelial carcinoma |
| UGT1A1 | uridine 5′-diphospho-glucuronosyltransferase family 1 member A1 |
| UPF2 | UP-frameshift protein 2 |
| USP1 | ubiquitin carboxyl-terminal hydrolase 1 |
| WEE1 | G2 checkpoint kinase |
| Y15/17/24 | tyrosine 15/17/24 |
| YTHDF2 | YTH N6-methyladenosine RNA binding protein 2 |
| γH2AX | phosphorylated histone H2AX on serine 139 |
References
- Inoue, M. Epidemiology of Gastric Cancer—Changing Trends and Global Disparities. Cancers 2024, 16, 2948. [Google Scholar] [CrossRef]
- Burz, C.; Pop, V.; Silaghi, C.; Lupan, I.; Samasca, G. Prognosis and Treatment of Gastric Cancer: A 2024 Update. Cancers 2024, 16, 1708. [Google Scholar] [CrossRef]
- Lin, J.-L.; Lin, J.-X.; Lin, G.-T.; Huang, C.-M.; Zheng, C.-H.; Xie, J.-W.; Wang, J.; Lu, J.; Chen, Q.-Y.; Li, P. Global Incidence and Mortality Trends of Gastric Cancer and Predicted Mortality of Gastric Cancer by 2035. BMC Public Health 2024, 24, 1763. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; He, F.; Clifford, G.M.; Li, M.; Fan, Z.; Li, X.; Wang, S.; Wei, W. A Systematic Review and Meta-Analysis on the Relative and Attributable Risk of Helicobacter pylori Infection and Cardia and Non-Cardia Gastric Cancer. Expert Rev. Mol. Diagn. 2023, 23, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Wang, T.C. Helicobacter pylori and Gastric Cancer. Gastrointest. Endosc. Clin. N. Am. 2021, 31, 451–465. [Google Scholar] [CrossRef]
- Duan, Y.; Xu, Y.; Dou, Y.; Xu, D. Helicobacter pylori and Gastric Cancer: Mechanisms and New Perspectives. J. Hematol. Oncol. 2025, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Reyes, V.E. Helicobacter pylori and Its Role in Gastric Cancer. Microorganisms 2023, 11, 1312. [Google Scholar] [CrossRef]
- Bouras, E.; Tsilidis, K.K.; Triggi, M.; Siargkas, A.; Chourdakis, M.; Haidich, A.-B. Diet and Risk of Gastric Cancer: An Umbrella Review. Nutrients 2022, 14, 1764. [Google Scholar] [CrossRef]
- Maddineni, G.; Xie, J.J.; Brahmbhatt, B.; Mutha, P. Diet and Carcinogenesis of Gastric Cancer. Curr. Opin. Gastroenterol. 2022, 38, 588–591. [Google Scholar] [CrossRef]
- Pu, K.; Feng, Y.; Tang, Q.; Yang, G.; Xu, C. Review of Dietary Patterns and Gastric Cancer Risk: Epidemiology and Biological Evidence. Front. Oncol. 2024, 14, 1333623. [Google Scholar] [CrossRef]
- Takasu, A.; Gotoda, T.; Suzuki, S.; Kusano, C.; Goto, C.; Ishikawa, H.; Kogure, H. Daily Diet and Nutrition Risk Factors for Gastric Cancer Incidence in a Japanese Population. Gut Liver 2024, 18, 602–610. [Google Scholar] [CrossRef]
- Faria, L.; Silva, J.C.; Rodríguez-Carrasco, M.; Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Libânio, D. Gastric Cancer Screening: A Systematic Review and Meta-Analysis. Scand. J. Gastroenterol. 2022, 57, 1178–1188. [Google Scholar] [CrossRef]
- Januszewicz, W.; Turkot, M.H.; Malfertheiner, P.; Regula, J. A Global Perspective on Gastric Cancer Screening: Which Concepts Are Feasible, and When? Cancers 2023, 15, 664. [Google Scholar] [CrossRef]
- Farinati, F.; Pelizzaro, F. Gastric Cancer Screening in Western Countries: A Call to Action. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2024, 56, 1653–1662. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global Surveillance of Trends in Cancer Survival 2000-14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed with One of 18 Cancers from 322 Population-Based Registries in 71 Countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Nagini, S. Carcinoma of the Stomach: A Review of Epidemiology, Pathogenesis, Molecular Genetics and Chemoprevention. World J. Gastrointest. Oncol. 2012, 4, 156–169. [Google Scholar] [CrossRef]
- Eichelberger, L.; Murphy, G.; Etemadi, A.; Abnet, C.C.; Islami, F.; Shakeri, R.; Malekzadeh, R.; Dawsey, S.M. Risk of Gastric Cancer by Water Source: Evidence from the Golestan Case-Control Study. PLoS ONE 2015, 10, e0128491. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, F.; Ding, Z.; Lian, Y.; Yang, X.; Hu, P.; Liu, Y.; Xu, L.; Li, Z.; Qiu, H. Prophylactic Antibiotic Use Is Associated with Better Clinical Outcomes in Gastric Cancer Patients Receiving Immunotherapy. Oncologist 2025, 30, oyae362. [Google Scholar] [CrossRef] [PubMed]
- Fall, K.; Ye, W.; Nyrén, O. Antibiotic Treatment and Risk of Gastric Cancer. Gut 2006, 55, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Saijo, K.; Komine, K.; Ueta, R.; Numakura, R.; Wakayama, S.; Umegaki, S.; Hiraide, S.; Kawamura, Y.; Kasahara, Y.; et al. Antibiotic Treatment Improves the Efficacy of Oxaliplatin-Based Therapy as First-Line Chemotherapy for Patients with Advanced Gastric Cancer: A Retrospective Study. Cancer Manag. Res. 2022, 14, 1259–1266. [Google Scholar] [CrossRef]
- Arai, J.; Niikura, R.; Hayakawa, Y.; Kawahara, T.; Honda, T.; Hasatani, K.; Yoshida, N.; Nishida, T.; Sumiyoshi, T.; Kiyotoki, S.; et al. Use of Antibiotics and Probiotics Reduces the Risk of Metachronous Gastric Cancer after Endoscopic Resection. Biology 2021, 10, 455. [Google Scholar] [CrossRef]
- Wang, Q.; He, X.-C.; Geng, L.-X.; Jiang, S.-L.; Yang, C.-J.; Xu, K.-Y.; Shen, S.-F.; Cao, W.-W.; Qi, W.; Zhao, S.-P. Public Awareness of Gastric Cancer Risk Factors and Screening Behaviours in Shijiazhuang, China: A Community-Based Survey. PLoS ONE 2024, 19, e0311491. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.G.; Alshareef, A.M.; Alzahrani, A.T.; Alharthi, Z.S.; Alghamdi, S.S.; Alghamdi, A.M.; Alzahrani, F.A.; Alzahrani, R.A. Knowledge and Awareness About Gastric Cancer Among the General Population in Al-Baha City, Saudi Arabia. Cureus 2023, 15, e39589. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; Choi, K.S.; Shin, H.-R.; Bang, Y.-J. Public Awareness of Gastric Cancer Risk Factors and Disease Screening in a High Risk Region: A Population-Based Study. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2009, 41, 59–66. [Google Scholar] [CrossRef][Green Version]
- Inoue, M. Public Health Interventions for Gastric Cancer Control. Gastrointest. Endosc. Clin. N. Am. 2021, 31, 441–449. [Google Scholar] [CrossRef]
- Praud, D.; Rota, M.; Pelucchi, C.; Bertuccio, P.; Rosso, T.; Galeone, C.; Zhang, Z.-F.; Matsuo, K.; Ito, H.; Hu, J.; et al. Cigarette Smoking and Gastric Cancer in the Stomach Cancer Pooling (StoP) Project. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 2018, 27, 124–133. [Google Scholar] [CrossRef]
- Jung, K.H.; Kim, S.M.; Choi, M.G.; Lee, J.H.; Noh, J.H.; Sohn, T.S.; Bae, J.M.; Kim, S. Preoperative Smoking Cessation Can Reduce Postoperative Complications in Gastric Cancer Surgery. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2015, 18, 683–690. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, M.-H.; Oh, C.-M.; Ha, E.; Yang, E.H.; Hwang, W.Y.; You, A.H.; Ryoo, J.-H. The Risk of Gastric Cancer According to Changes in Smoking Status among Korean Men. Epidemiol. Health 2022, 44, e2022086. [Google Scholar] [CrossRef] [PubMed]
- Rota, M.; Possenti, I.; Valsassina, V.; Santucci, C.; Bagnardi, V.; Corrao, G.; Bosetti, C.; Specchia, C.; Gallus, S.; Lugo, A. Dose-Response Association between Cigarette Smoking and Gastric Cancer Risk: A Systematic Review and Meta-Analysis. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2024, 27, 197–209. [Google Scholar] [CrossRef]
- Yao, K. The Endoscopic Diagnosis of Early Gastric Cancer. Ann. Gastroenterol. 2013, 26, 11–22. [Google Scholar]
- Yao, K.; Uedo, N.; Kamada, T.; Hirasawa, T.; Nagahama, T.; Yoshinaga, S.; Oka, M.; Inoue, K.; Mabe, K.; Yao, T.; et al. Guidelines for Endoscopic Diagnosis of Early Gastric Cancer. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2020, 32, 663–698. [Google Scholar] [CrossRef]
- Dohi, O.; Seya, M.; Iwai, N.; Ochiai, T.; Yumoto, J.; Mukai, H.; Yamauchi, K.; Kobayashi, R.; Hirose, R.; Inoue, K.; et al. Endoscopic Detection and Diagnosis of Gastric Cancer Using Image-Enhanced Endoscopy: A Systematic Review and Meta-Analysis. DEN Open 2025, 5, e418. [Google Scholar] [CrossRef]
- Fujishiro, M. Advanced Diagnostic and Therapeutic Endoscopy for Early Gastric Cancer. Cancers 2024, 16, 1039. [Google Scholar] [CrossRef]
- Conti, C.B.; Agnesi, S.; Scaravaglio, M.; Masseria, P.; Dinelli, M.E.; Oldani, M.; Uggeri, F. Early Gastric Cancer: Update on Prevention, Diagnosis and Treatment. Int. J. Environ. Res. Public Health 2023, 20, 2149. [Google Scholar] [CrossRef] [PubMed]
- Tastekin, D.; Paksoy, N.; Dogan, I.; Ferhatoglu, F.; Khanmammadov, N.; Bozbey, H.U.; Karabulut, S. Fluorouracil, Leucovorin, Oxaliplatin, and Docetaxel (FLOT) Regimen in the First-Line Treatment of Metastatic Gastric Cancer: A Single-Center Experience. J. Cancer Res. Ther. 2023, 19, 253–258. [Google Scholar] [CrossRef]
- Möhring, C.; Timotheou, A.; Mańczak, A.; Sadeghlar, F.; Zhou, T.; Mahn, R.; Bartels, A.; Monin, M.; Toma, M.; Feldmann, G.; et al. Efficacy and Tolerability of Fluorouracil, Leucovorin, Oxaliplatin and Docetaxel (FLOT) in Unselected Patients with Advanced Gastric and Gastroesophageal Cancer: Does Age Really Matter? J. Cancer Res. Clin. Oncol. 2023, 149, 1849–1862. [Google Scholar] [CrossRef]
- Geerts, J.F.M.; Pape, M.; Vissers, P.A.J.; Verhoeven, R.H.A.; Mostert, B.; Wijnhoven, B.P.L.; Rosman, C.; van Hellemond, I.E.G.; Nieuwenhuijzen, G.A.P.; van Laarhoven, H.W.M. Patient Access to Perioperative Chemotherapy with Fluorouracil, Leucovorin, Oxaliplatin and Docetaxel in Patients with Resectable Gastric Cancer in the Netherlands. Eur. J. Cancer 2025, 214, 115137. [Google Scholar] [CrossRef]
- Sato, Y.; Okamoto, K.; Kida, Y.; Mitsui, Y.; Kawano, Y.; Sogabe, M.; Miyamoto, H.; Takayama, T. Overview of Chemotherapy for Gastric Cancer. J. Clin. Med. 2023, 12, 1336. [Google Scholar] [CrossRef]
- Guan, W.-L.; He, Y.; Xu, R.-H. Gastric Cancer Treatment: Recent Progress and Future Perspectives. J. Hematol. Oncol. 2023, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Takei, S.; Kawazoe, A.; Shitara, K. The New Era of Immunotherapy in Gastric Cancer. Cancers 2022, 14, 1054. [Google Scholar] [CrossRef] [PubMed]
- Högner, A.; Moehler, M. Immunotherapy in Gastric Cancer. Curr. Oncol. 2022, 29, 1559–1574. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Muro, K. Updated Immunotherapy for Gastric Cancer. J. Clin. Med. 2023, 12, 2636. [Google Scholar] [CrossRef]
- Luo, D.; Liu, Y.; Lu, Z.; Huang, L. Targeted Therapy and Immunotherapy for Gastric Cancer: Rational Strategies, Novel Advancements, Challenges, and Future Perspectives. Mol. Med. Camb. Mass 2025, 31, 52. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-X.; Zhou, P.-K. DNA Damage Response Signaling Pathways and Targets for Radiotherapy Sensitization in Cancer. Signal Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef]
- Groelly, F.J.; Fawkes, M.; Dagg, R.A.; Blackford, A.N.; Tarsounas, M. Targeting DNA Damage Response Pathways in Cancer. Nat. Rev. Cancer 2023, 23, 78–94. [Google Scholar] [CrossRef]
- Huen, M.S.Y.; Chen, J. The DNA Damage Response Pathways: At the Crossroad of Protein Modifications. Cell Res. 2008, 18, 8–16. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA Damage, Repair, and Mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Chen, J.; Potlapalli, R.; Quan, H.; Chen, L.; Xie, Y.; Pouriyeh, S.; Sakib, N.; Liu, L.; Xie, Y. Exploring DNA Damage and Repair Mechanisms: A Review with Computational Insights. Bio Tech 2024, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Altieri, F.; Grillo, C.; Maceroni, M.; Chichiarelli, S. DNA Damage and Repair: From Molecular Mechanisms to Health Implications. Antioxid. Redox Signal. 2008, 10, 891–937. [Google Scholar] [CrossRef]
- Bielski, C.M.; Taylor, B.S. Homing in on Genomic Instability as a Therapeutic Target in Cancer. Nat. Commun. 2021, 12, 3663. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Chen, H.; Collins, A.R.; Connell, M.; Damia, G.; Dasgupta, S.; Malhotra, M.; Meeker, A.K.; Amedei, A.; Amin, A.; et al. Genomic Instability in Human Cancer: Molecular Insights and Opportunities for Therapeutic Attack and Prevention through Diet and Nutrition. Semin. Cancer Biol. 2015, 35, S5–S24. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, N.J.; Bailey, M.L.; Hieter, P. Synthetic Lethality and Cancer. Nat. Rev. Genet. 2017, 18, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Previtali, V.; Bagnolini, G.; Ciamarone, A.; Ferrandi, G.; Rinaldi, F.; Myers, S.H.; Roberti, M.; Cavalli, A. New Horizons of Synthetic Lethality in Cancer: Current Development and Future Perspectives. J. Med. Chem. 2024, 67, 11488–11521. [Google Scholar] [CrossRef]
- Ngoi, N.Y.L.; Gallo, D.; Torrado, C.; Nardo, M.; Durocher, D.; Yap, T.A. Synthetic Lethal Strategies for the Development of Cancer Therapeutics. Nat. Rev. Clin. Oncol. 2025, 22, 46–64. [Google Scholar] [CrossRef]
- Ryan, C.J.; Devakumar, L.P.S.; Pettitt, S.J.; Lord, C.J. Complex Synthetic Lethality in Cancer. Nat. Genet. 2023, 55, 2039–2048. [Google Scholar] [CrossRef]
- Qian, J.; Liao, G.; Chen, M.; Peng, R.-W.; Yan, X.; Du, J.; Huang, R.; Pan, M.; Lin, Y.; Gong, X.; et al. Advancing Cancer Therapy: New Frontiers in Targeting DNA Damage Response. Front. Pharmacol. 2024, 15, 1474337. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, P.-K. DNA Damage Repair: Historical Perspectives, Mechanistic Pathways and Clinical Translation for Targeted Cancer Therapy. Signal Transduct. Target. Ther. 2021, 6, 254. [Google Scholar] [CrossRef]
- Smith, G.; Alholm, Z.; Coleman, R.L.; Monk, B.J. DNA Damage Repair Inhibitors-Combination Therapies. Cancer J. Sudbury Mass 2021, 27, 501–505. [Google Scholar] [CrossRef]
- Chabanon, R.M.; Rouanne, M.; Lord, C.J.; Soria, J.-C.; Pasero, P.; Postel-Vinay, S. Targeting the DNA Damage Response in Immuno-Oncology: Developments and Opportunities. Nat. Rev. Cancer 2021, 21, 701–717. [Google Scholar] [CrossRef]
- Wang, M.; Xie, C. DNA Damage Repair and Current Therapeutic Approaches in Gastric Cancer: A Comprehensive Review. Front. Genet. 2022, 13, 931866. [Google Scholar] [CrossRef] [PubMed]
- Macheret, M.; Halazonetis, T.D. DNA Replication Stress as a Hallmark of Cancer. Annu. Rev. Pathol. 2015, 10, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Shen, R.; Mahasin, H.; Guo, Y.; Wang, D. DNA Replication: Mechanisms and Therapeutic Interventions for Diseases. MedComm 2023, 4, e210. [Google Scholar] [CrossRef]
- Friedberg, E.C.; McDaniel, L.D.; Schultz, R.A. The Role of Endogenous and Exogenous DNA Damage and Mutagenesis. Curr. Opin. Genet. Dev. 2004, 14, 5–10. [Google Scholar] [CrossRef]
- Liu, N.; Du, J.; Ge, J.; Liu, S.-B. DNA Damage-Inducing Endogenous and Exogenous Factors and Research Progress. Nucleosides Nucleotides Nucleic Acids 2024, 14, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Shiotani, B. Emerging Strategies for Cancer Therapy by ATR Inhibitors. Cancer Sci. 2023, 114, 2709–2721. [Google Scholar] [CrossRef] [PubMed]
- Anantha, R.W.; Vassin, V.M.; Borowiec, J.A. Sequential and Synergistic Modification of Human RPA Stimulates Chromosomal DNA Repair. J. Biol. Chem. 2007, 282, 35910–35923. [Google Scholar] [CrossRef]
- Myers, J.S.; Zhao, R.; Xu, X.; Ham, A.-J.L.; Cortez, D. Cyclin-Dependent Kinase 2 Dependent Phosphorylation of ATRIP Regulates the G2-M Checkpoint Response to DNA Damage. Cancer Res. 2007, 67, 6685–6690. [Google Scholar] [CrossRef] [PubMed]
- Mordes, D.A.; Glick, G.G.; Zhao, R.; Cortez, D. TopBP1 Activates ATR through ATRIP and a PIKK Regulatory Domain. Genes Dev. 2008, 22, 1478–1489. [Google Scholar] [CrossRef]
- Ohashi, E.; Takeishi, Y.; Ueda, S.; Tsurimoto, T. Interaction between Rad9-Hus1-Rad1 and TopBP1 Activates ATR-ATRIP and Promotes TopBP1 Recruitment to Sites of UV-Damage. DNA Repair 2014, 21, 1–11. [Google Scholar] [CrossRef]
- Kumagai, A.; Lee, J.; Yoo, H.Y.; Dunphy, W.G. TopBP1 Activates the ATR-ATRIP Complex. Cell 2006, 124, 943–955. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Zhou, Q.; Chen, J.; Yuan, J. RPA-Binding Protein ETAA1 Is an ATR Activator Involved in DNA Replication Stress Response. Curr. Biol. CB 2016, 26, 3257–3268. [Google Scholar] [CrossRef] [PubMed]
- Thada, V.; Cortez, D. Common Motifs in ETAA1 and TOPBP1 Required for ATR Kinase Activation. J. Biol. Chem. 2019, 294, 8395–8402. [Google Scholar] [CrossRef]
- Kumagai, A.; Kim, S.-M.; Dunphy, W.G. Claspin and the Activated Form of ATR-ATRIP Collaborate in the Activation of Chk1. J. Biol. Chem. 2004, 279, 49599–49608. [Google Scholar] [CrossRef]
- Smith-Roe, S.L.; Patel, S.S.; Simpson, D.A.; Zhou, Y.C.; Rao, S.; Ibrahim, J.G.; Kaiser-Rogers, K.A.; Cordeiro-Stone, M.; Kaufmann, W.K. Timeless Functions Independently of the Tim-Tipin Complex to Promote Sister Chromatid Cohesion in Normal Human Fibroblasts. Cell Cycle 2011, 10, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.G.; Akan, Z.; Yilmaz, S.; Grillo, M.; Smith-Roe, S.L.; Kang, T.-H.; Cordeiro-Stone, M.; Kaufmann, W.K.; Abraham, R.T.; Sancar, A.; et al. Tipin-Replication Protein A Interaction Mediates Chk1 Phosphorylation by ATR in Response to Genotoxic Stress. J. Biol. Chem. 2010, 285, 16562–16571. [Google Scholar] [CrossRef]
- Zhu, X.; Zheng, X.-Y.; Gong, P.; Xu, X. Regulation of ATR-CHK1 Signaling by Ubiquitination of CLASPIN. Biochem. Soc. Trans. 2022, 50, 1471–1480. [Google Scholar] [CrossRef]
- Yang, C.-C.; Suzuki, M.; Yamakawa, S.; Uno, S.; Ishii, A.; Yamazaki, S.; Fukatsu, R.; Fujisawa, R.; Sakimura, K.; Tsurimoto, T.; et al. Claspin Recruits Cdc7 Kinase for Initiation of DNA Replication in Human Cells. Nat. Commun. 2016, 7, 12135. [Google Scholar] [CrossRef] [PubMed]
- Ward, I.M.; Chen, J. Histone H2AX Is Phosphorylated in an ATR-Dependent Manner in Response to Replicational Stress. J. Biol. Chem. 2001, 276, 47759–47762. [Google Scholar] [CrossRef]
- Leung-Pineda, V.; Ryan, C.E.; Piwnica-Worms, H. Phosphorylation of Chk1 by ATR Is Antagonized by a Chk1-Regulated Protein Phosphatase 2A Circuit. Mol. Cell. Biol. 2006, 26, 7529–7538. [Google Scholar] [CrossRef]
- Moiseeva, T.N.; Yin, Y.; Calderon, M.J.; Qian, C.; Schamus-Haynes, S.; Sugitani, N.; Osmanbeyoglu, H.U.; Rothenberg, E.; Watkins, S.C.; Bakkenist, C.J. An ATR and CHK1 Kinase Signaling Mechanism That Limits Origin Firing during Unperturbed DNA Replication. Proc. Natl. Acad. Sci. USA 2019, 116, 13374–13383. [Google Scholar] [CrossRef]
- Li, G.; Elder, R.T.; Qin, K.; Park, H.U.; Liang, D.; Zhao, R.Y. Phosphatase Type 2A-Dependent and -Independent Pathways for ATR Phosphorylation of Chk1*. J. Biol. Chem. 2007, 282, 7287–7298. [Google Scholar] [CrossRef]
- Xu, N.; Libertini, S.; Zhang, Y.; Gillespie, D.A. Cdk Phosphorylation of Chk1 Regulates Efficient Chk1 Activation and Multiple Checkpoint Proficiency. Biochem. Biophys. Res. Commun. 2011, 413, 465–470. [Google Scholar] [CrossRef]
- Shen, T.; Huang, S. The Role of Cdc25A in the Regulation of Cell Proliferation and Apoptosis. Anticancer Agents Med. Chem. 2012, 12, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.; Pabla, N.; Dong, Z. Checkpoint Kinase 1 in DNA Damage Response and Cell Cycle Regulation. Cell. Mol. Life Sci. 2013, 70, 4009–4021. [Google Scholar] [CrossRef]
- Kotsantis, P.; Petermann, E.; Boulton, S.J. Mechanisms of Oncogene-Induced Replication Stress: Jigsaw Falling into Place. Cancer Discov. 2018, 8, 537–555. [Google Scholar] [CrossRef]
- Buisson, R.; Boisvert, J.L.; Benes, C.H.; Zou, L. Distinct but Concerted Roles of ATR, DNA-PK, and Chk1 in Countering Replication Stress during S Phase. Mol. Cell 2015, 59, 1011–1024. [Google Scholar] [CrossRef]
- Tibbetts, R.S.; Brumbaugh, K.M.; Williams, J.M.; Sarkaria, J.N.; Cliby, W.A.; Shieh, S.-Y.; Taya, Y.; Prives, C.; Abraham, R.T. A Role for ATR in the DNA Damage-Induced Phosphorylation of P53. Genes Dev. 1999, 13, 152–157. [Google Scholar] [CrossRef]
- Shieh, S.Y.; Ikeda, M.; Taya, Y.; Prives, C. DNA Damage-Induced Phosphorylation of P53 Alleviates Inhibition by MDM2. Cell 1997, 91, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Lavin, M.F.; Gueven, N. The Complexity of P53 Stabilization and Activation. Cell Death Differ. 2006, 13, 941–950. [Google Scholar] [CrossRef]
- Pabla, N.; Huang, S.; Mi, Q.-S.; Daniel, R.; Dong, Z. ATR-Chk2 Signaling in P53 Activation and DNA Damage Response during Cisplatin-Induced Apoptosis. J. Biol. Chem. 2008, 283, 6572–6583. [Google Scholar] [CrossRef] [PubMed]
- Lakin, N.D.; Hann, B.C.; Jackson, S.P. The Ataxia-Telangiectasia Related Protein ATR Mediates DNA-Dependent Phosphorylation of P53. Oncogene 1999, 18, 3989–3995. [Google Scholar] [CrossRef]
- Kciuk, M.; Marciniak, B.; Mojzych, M.; Kontek, R. Focus on UV-Induced DNA Damage and Repair—Disease Relevance and Protective Strategies. Int. J. Mol. Sci. 2020, 21, 7264. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecińska, A.; Kołat, D.; Kałuzińska, Ż.; Kontek, R. Cancer-Associated Transcription Factors in DNA Damage Response. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188757. [Google Scholar] [CrossRef] [PubMed]
- Lakin, N.D.; Jackson, S.P. Regulation of P53 in Response to DNA Damage. Oncogene 1999, 18, 7644–7655. [Google Scholar] [CrossRef]
- Pellegata, N.S.; Antoniono, R.J.; Redpath, J.L.; Stanbridge, E.J. DNA Damage and P53-Mediated Cell Cycle Arrest: A Reevaluation. Proc. Natl. Acad. Sci. USA 1996, 93, 15209–15214. [Google Scholar] [CrossRef]
- Ribezzo, F.; Shiloh, Y.; Schumacher, B. Systemic DNA Damage Responses in Aging and Diseases. Semin. Cancer Biol. 2016, 37–38, 26–35. [Google Scholar] [CrossRef]
- Williams, R.M.; Zhang, X. Roles of ATM and ATR in DNA Double Strand Breaks and Replication Stress. Prog. Biophys. Mol. Biol. 2021, 161, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Simoneau, A.; Zou, L. An Extending ATR-CHK1 Circuitry: The Replication Stress Response and Beyond. Curr. Opin. Genet. Dev. 2021, 71, 92–98. [Google Scholar] [CrossRef]
- Pećina-Šlaus, N.; Kafka, A.; Salamon, I.; Bukovac, A. Mismatch Repair Pathway, Genome Stability and Cancer. Front. Mol. Biosci. 2020, 7, 122. [Google Scholar] [CrossRef]
- Menoyo, A.; Alazzouzi, H.; Espín, E.; Armengol, M.; Yamamoto, H.; Schwartz, S. Somatic Mutations in the DNA Damage-Response Genes ATR and CHK1 in Sporadic Stomach Tumors with Microsatellite Instability. Cancer Res. 2001, 61, 7727–7730. [Google Scholar]
- Falchetti, M.; Saieva, C.; Lupi, R.; Masala, G.; Rizzolo, P.; Zanna, I.; Ceccarelli, K.; Sera, F.; Mariani-Costantini, R.; Nesi, G.; et al. Gastric Cancer with High-Level Microsatellite Instability: Target Gene Mutations, Clinicopathologic Features, and Long-Term Survival. Hum. Pathol. 2008, 39, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Z.; Liu, Y.J.C.; Yin, X.-L.; Zhan, P.; Gu, Y.; Ni, X.-Z. Loss of BRCA1 Expression Leads to Worse Survival in Patients with Gastric Carcinoma. World J. Gastroenterol. 2013, 19, 1968–1974. [Google Scholar] [CrossRef]
- Slavin, T.; Neuhausen, S.L.; Rybak, C.; Solomon, I.; Nehoray, B.; Blazer, K.; Niell-Swiller, M.; Adamson, A.W.; Yuan, Y.-C.; Yang, K.; et al. Genetic Gastric Cancer Susceptibility in the International Clinical Cancer Genomics Community Research Network. Cancer Genet. 2017, 216–217, 111–119. [Google Scholar] [CrossRef]
- Fewings, E.; Larionov, A.; Redman, J.; Goldgraben, M.A.; Scarth, J.; Richardson, S.; Brewer, C.; Davidson, R.; Ellis, I.; Evans, D.G.; et al. Germline Pathogenic Variants in PALB2 and Other Cancer-Predisposing Genes in Families with Hereditary Diffuse Gastric Cancer without CDH1 Mutation: A Whole-Exome Sequencing Study. Lancet Gastroenterol. Hepatol. 2018, 3, 489–498. [Google Scholar] [CrossRef]
- Pádua, J.D.B.; Mariano, C.F.A.; Fabro, A.T.; Tirapelli, D.P.d.C.; Sankarankutty, A.K.; Dos Santos, J.S.; Brunaldi, M.O. Prognostic Value of the Immunohistochemical Expression of RAD51 and BRCA2 in Gastric Adenocarcinoma. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2022, 70, 199–210. [Google Scholar] [CrossRef]
- Zeng, H.-H.; Yang, Z.; Qiu, Y.-B.; Bashir, S.; Li, Y.; Xu, M. Detection of a Novel Panel of 24 Genes with High Frequencies of Mutation in Gastric Cancer Based on Next-Generation Sequencing. World J. Clin. Cases 2022, 10, 4761–4775. [Google Scholar] [CrossRef]
- Pádua, J.D.B.; Mariano, C.F.A.; Fabro, A.T.; Lizarte Neto, F.S.; Zuliani, R.L.; Sares, C.T.G.; Dos Santos, J.S.; Sankarankutty, A.K.; Tirapelli, D.P.d.C.; Silveira, V.d.S.; et al. mRNA Expression and Methylation of the RAD51, ATM, ATR, BRCA1, and BRCA2 Genes in Gastric Adenocarcinoma. Biomark. Insights 2024, 19, 11772719231225206. [Google Scholar] [CrossRef]
- Su, H.; Yuan, Y.; Tang, J.; Zhang, Y.; Wu, H.; Zhang, Y.; Liang, J.; Wang, L.; Zou, X.; Huang, S.; et al. The ATR Inhibitor VE-821 Increases the Sensitivity of Gastric Cancer Cells to Cisplatin. Transl. Oncol. 2023, 36, 101743. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Han, Z.; Sun, Z.; Feng, H.; Zhao, L.; Yuan, Q.; Chen, C.; Yu, S.; Hu, Y.; Yu, J.; et al. PAK6 Promotes Homologous-Recombination to Enhance Chemoresistance to Oxaliplatin through ATR/CHK1 Signaling in Gastric Cancer. Cell Death Dis. 2022, 13, 658. [Google Scholar] [CrossRef] [PubMed]
- Min, A.; Im, S.-A.; Jang, H.; Kim, S.; Lee, M.; Kim, D.K.; Yang, Y.; Kim, H.-J.; Lee, K.-H.; Kim, J.W.; et al. AZD6738, A Novel Oral Inhibitor of ATR, Induces Synthetic Lethality with ATM Deficiency in Gastric Cancer Cells. Mol. Cancer Ther. 2017, 16, 566–577. [Google Scholar] [CrossRef]
- Kwon, M.; Kim, G.; Kim, R.; Kim, K.-T.; Kim, S.T.; Smith, S.; Mortimer, P.G.S.; Hong, J.Y.; Loembé, A.-B.; Irurzun-Arana, I.; et al. Phase II Study of Ceralasertib (AZD6738) in Combination with Durvalumab in Patients with Advanced Gastric Cancer. J. Immunother. Cancer 2022, 10, e005041. [Google Scholar] [CrossRef] [PubMed]
- Shigeishi, H.; Yokozaki, H.; Oue, N.; Kuniyasu, H.; Kondo, T.; Ishikawa, T.; Yasui, W. Increased Expression of CHK2 in Human Gastric Carcinomas Harboring P53 Mutations. Int. J. Cancer 2002, 99, 58–62. [Google Scholar] [CrossRef]
- Furlan, D.; Casati, B.; Cerutti, R.; Facco, C.; Terracciano, L.; Capella, C.; Chiaravalli, A.M. Genetic Progression in Sporadic Endometrial and Gastrointestinal Cancers with High Microsatellite Instability. J. Pathol. 2002, 197, 603–609. [Google Scholar] [CrossRef]
- Arai, H.; Wada, R.; Ishino, K.; Kudo, M.; Uchida, E.; Naito, Z. Expression of DNA Damage Response Proteins in Gastric Cancer: Comprehensive Protein Profiling and Histological Analysis. Int. J. Oncol. 2018, 52, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Bargiela-Iparraguirre, J.; Prado-Marchal, L.; Fernandez-Fuente, M.; Gutierrez-González, A.; Moreno-Rubio, J.; Muñoz-Fernandez, M.; Sereno, M.; Sanchez-Prieto, R.; Perona, R.; Sanchez-Perez, I. CHK1 Expression in Gastric Cancer Is Modulated by P53 and RB1/E2F1: Implications in Chemo/Radiotherapy Response. Sci. Rep. 2016, 6, 21519. [Google Scholar] [CrossRef]
- Kim, D.-S.; Camacho, C.V.; Kraus, W.L. Alternate Therapeutic Pathways for PARP Inhibitors and Potential Mechanisms of Resistance. Exp. Mol. Med. 2021, 53, 42–51. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, Q.; Zhang, P.; Tao, R.; Chang, W.; Li, R.; Xie, G.; Liu, W.; Zhang, L.; Kapoor, P.; et al. Chk1 Inhibition Potentiates the Therapeutic Efficacy of PARP Inhibitor BMN673 in Gastric Cancer. Am. J. Cancer Res. 2017, 7, 473–483. [Google Scholar] [PubMed]
- Zhao, Y.; Zhou, K.; Xia, X.; Guo, Y.; Tao, L. Chk1 Inhibition-Induced BRCAness Synergizes with Olaparib in P53-Deficient Cancer Cells. Cell Cycle 2023, 22, 200–212. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, J.; He, K.; Zhang, J. Ataxia Telangiectasia and Rad3-Related Inhibitors and Cancer Therapy: Where We Stand. J. Hematol. Oncol. 2019, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Khaleghian, M.; Shakoori, A.; Razavi, A.E.; Azimi, C. Relationship of Amplification and Expression of the C-MYC Gene with Survival among Gastric Cancer Patients. Asian Pac. J. Cancer Prev. 2015, 16, 7061–7069. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, J.-S.; Seo, J.; Jung, E.J.; Kim, E.J.; Lee, G.K.; Kim, W.H. C-MYC Amplification in Mucinous Gastric Carcinoma: A Possible Genetic Alteration Leading to Deeply Invasive Tumors. Anticancer Res. 2012, 32, 5031–5037. [Google Scholar]
- Graziano, F.; Fischer, N.W.; Bagaloni, I.; Di Bartolomeo, M.; Lonardi, S.; Vincenzi, B.; Perrone, G.; Fornaro, L.; Ongaro, E.; Aprile, G.; et al. TP53 Mutation Analysis in Gastric Cancer and Clinical Outcomes of Patients with Metastatic Disease Treated with Ramucirumab/Paclitaxel or Standard Chemotherapy. Cancers 2020, 12, 2049. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, J.; Kim, Y.H.; Park, J.; Shin, J.-W.; Nam, S. Clinical Relevance and Molecular Phenotypes in Gastric Cancer, of TP53 Mutations and Gene Expressions, in Combination with Other Gene Mutations. Sci. Rep. 2016, 6, 34822. [Google Scholar] [CrossRef] [PubMed]
- Mavroeidi, D.; Georganta, A.; Panagiotou, E.; Syrigos, K.; Souliotis, V.L. Targeting ATR Pathway in Solid Tumors: Evidence of Improving Therapeutic Outcomes. Int. J. Mol. Sci. 2024, 25, 2767. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Kołat, D.; Kałuzińska-Kołat, Ż.; Gawrysiak, M.; Drozda, R.; Celik, I.; Kontek, R. PD-1/PD-L1 and DNA Damage Response in Cancer. Cells 2023, 12, 530. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Zimmermann, M.; Bernier, C.; Kaiser, B.; Fournier, S.; Li, L.; Desjardins, J.; Skeldon, A.; Rimkunas, V.; Veloso, A.; Young, J.T.F.; et al. Guiding ATR and PARP Inhibitor Combinationswith Chemogenomic Screens. Cell Rep. 2022, 40, 111081. [Google Scholar] [CrossRef]
- Bradbury, A.; Hall, S.; Curtin, N.; Drew, Y. Targeting ATR as Cancer Therapy: A New Era for Synthetic Lethality and Synergistic Combinations? Pharmacol. Ther. 2020, 207, 107450. [Google Scholar] [CrossRef]
- Ngoi, N.Y.L.; Pilié, P.G.; McGrail, D.J.; Zimmermann, M.; Schlacher, K.; Yap, T.A. Targeting ATR in Patients with Cancer. Nat. Rev. Clin. Oncol. 2024, 21, 278–293. [Google Scholar] [CrossRef]
- Lu, S.; Duan, R.; Cong, L.; Song, Y. The Effects of ARID1A Mutation in Gastric Cancer and Its Significance for Treatment. Cancer Cell Int. 2023, 23, 296. [Google Scholar] [CrossRef]
- Yamada, H.; Takeshima, H.; Fujiki, R.; Yamashita, S.; Sekine, S.; Ando, T.; Hattori, N.; Okabe, A.; Yoshikawa, T.; Obama, K.; et al. ARID1A Loss-of-Function Induces CpG Island Methylator Phenotype. Cancer Lett. 2022, 532, 215587. [Google Scholar] [CrossRef]
- Angelico, G.; Attanasio, G.; Colarossi, L.; Colarossi, C.; Montalbano, M.; Aiello, E.; Di Vendra, F.; Mare, M.; Orsi, N.; Memeo, L. ARID1A Mutations in Gastric Cancer: A Review with Focus on Clinicopathological Features, Molecular Background and Diagnostic Interpretation. Cancers 2024, 16, 2062. [Google Scholar] [CrossRef]
- Baxter, J.S.; Zatreanu, D.; Pettitt, S.J.; Lord, C.J. Resistance to DNA Repair Inhibitors in Cancer. Mol. Oncol. 2022, 16, 3811–3827. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.L.; Urban, V.; Muñoz-Martínez, F.; Ayestaran, I.; Thomas, J.C.; de Renty, C.; O’Connor, M.J.; Forment, J.V.; Galanty, Y.; Jackson, S.P. Loss of Cyclin C or CDK8 Provides ATR Inhibitor Resistance by Suppressing Transcription-Associated Replication Stress. Nucleic Acids Res. 2021, 49, 8665–8683. [Google Scholar] [CrossRef]
- O’Leary, P.C.; Chen, H.; Doruk, Y.U.; Williamson, T.; Polacco, B.; McNeal, A.S.; Shenoy, T.; Kale, N.; Carnevale, J.; Stevenson, E.; et al. Resistance to ATR Inhibitors Is Mediated by Loss of the Nonsense-Mediated Decay Factor UPF2. Cancer Res. 2022, 82, 3950–3961. [Google Scholar] [CrossRef]
- Black, E.M.; Joo, Y.K.; Kabeche, L. Keeping RelApse in Chk: Molecular Mechanisms of Chk1 Inhibitor Resistance in Lymphoma. Biochem. J. 2022, 479, 2345–2349. [Google Scholar] [CrossRef]
- Zhao, X.; Kim, I.-K.; Kallakury, B.; Chahine, J.J.; Iwama, E.; Pierobon, M.; Petricoin, E.; McCutcheon, J.N.; Zhang, Y.-W.; Umemura, S.; et al. Acquired Small Cell Lung Cancer Resistance to Chk1 Inhibitors Involves Wee1 Up-Regulation. Mol. Oncol. 2021, 15, 1130–1145. [Google Scholar] [CrossRef]
- Hinds, J.W.; Ditano, J.P.; Eastman, A. Inhibition of Protein Synthesis Induced by CHK1 Inhibitors Discriminates Sensitive from Resistant Cancer Cells. ACS Pharmacol. Transl. Sci. 2021, 4, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Tolcher, A.W.; Plummer, R.; Mukker, J.K.; Enderlin, M.; Hicking, C.; Grombacher, T.; Locatelli, G.; Szucs, Z.; Gounaris, I.; et al. First-in-Human Study of the Ataxia Telangiectasia and Rad3-Related (ATR) Inhibitor Tuvusertib (M1774) as Monotherapy in Patients with Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2024, 30, 2057–2067. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.; Yap, T.A.; Lee, E.K.; Højgaard, M.; Mettu, N.B.; Lheureux, S.; Carneiro, B.A.; Plummer, R.; Fretland, A.J.; Ulanet, D.; et al. Development of a Practical Nomogram for Personalized Anemia Management in Patients Treated with Ataxia Telangiectasia and Rad3-Related Inhibitor Camonsertib. Clin. Cancer Res. 2024, 30, 687–694. [Google Scholar] [CrossRef]
- Yap, T.A.; Krebs, M.G.; Postel-Vinay, S.; El-Khouiery, A.; Soria, J.-C.; Lopez, J.; Berges, A.; Cheung, S.Y.A.; Irurzun-Arana, I.; Goldwin, A.; et al. Ceralasertib (AZD6738), an Oral ATR Kinase Inhibitor, in Combination with Carboplatin in Patients with Advanced Solid Tumors: A Phase I Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 5213–5224. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Ferraro, G.; Li, L.; Han, Y.; Varricchio, L.; Fournier, S.; An, X.; Hoffman, R.; Fretland, A.; Roulston, A.; et al. ATR Inhibitor Camonsertib (RP-3500) Suppresses Early Stage Erythroblasts By Mediating Ferroptosis. Blood 2022, 140, 8194–8195. [Google Scholar] [CrossRef]
- The Development of ATR Inhibitors in Cancer Therapy. Available online: https://www.hematologyandoncology.net/archives/may-2025/the-development-of-atr-inhibitors/ (accessed on 5 July 2025).
- Ma, C.X.; Janetka, J.W.; Piwnica-Worms, H. Death by Releasing the Breaks: CHK1 Inhibitors as Cancer Therapeutics. Trends Mol. Med. 2011, 17, 88–96. [Google Scholar] [CrossRef] [PubMed]
| Study/Trial | Focus | Model/System | Key Findings | Mechanism/Pathway | Clinical Relevance | PMID |
|---|---|---|---|---|---|---|
| VE-821 and Cisplatin in Gastric Cancer | Effect of ATR inhibitor VE-821 on cisplatin sensitivity | Gastric cancer cell lines (MKN-45, AGS), cell organoids, in vivo models | VE-821 inhibits proliferation, induces apoptosis, blocks ATR/CHK1 phosphorylation, reverses STAT3 activation, enhances DNA damage, synergizes with cisplatin | ATR-mediated DDR pathway inhibition; STAT3 signaling reversal | Higher ATR expression correlates with advanced gastric cancer stages; VE-821 enhances cisplatin sensitivity | 37517142 |
| PAK6 and Oxaliplatin Resistance | Role of PAK6 in DDR and chemoresistance to oxaliplatin | Gastric cancer clinical samples and cells | PAK6 promotes chemoresistance by activating ATR → CHK1 → RAD51 pathway; blocking ATR with AZD6738 reverses resistance | PAK6 → ATR → CHK1 → RAD51 HR repair pathway | PAK6 expression correlates with aggressive disease and oxaliplatin resistance; ATR inhibition overcomes resistance | 35902562 |
| AZD6738 in ATM-deficient Gastric Cancer | ATR inhibition efficacy in ATM-deficient models | Gastric cancer cell lines (SNU-601 ATM-deficient, SNU-484 ATM-intact), xenografts | AZD6738 causes DNA damage accumulation, cell cycle arrest, apoptosis in ATM-deficient cells; ATM inhibition sensitizes ATM-intact cells | Synthetic lethality: ATR inhibition + ATM deficiency; possible HDAC1 involvement in sensitivity | Supports biomarker-driven trials targeting ATR in ATM-deficient gastric cancers | 28138034 |
| Phase II Clinical Trial (NCT03780608) | AZD6738 + durvalumab in advanced gastric cancer (AGC) | Patients with previously treated AGC | Combination showed ORR 22.6%, disease control rate 58.1%, manageable toxicity; ATM loss or HR deficiency biomarkers predict better PFS; immune activation seen in responders | ATR inhibition → cGAS-STING pathway activation → enhanced anti-tumor immunity; checkpoint blockade synergy | DDR alterations are predictive biomarkers; supports DDR-immune checkpoint inhibitor combos in gastric cancer | 35790315 |
| Trial ID | Phase | Drug(s) Tested | Combination(s) | Cancer Type(s) | Objectives/Endpoints | Design Features | Key Biomarkers/Scientific Rationale |
|---|---|---|---|---|---|---|---|
| NCT02264678 | Phase I/1b | AZD6738 | Carboplatin (Module 1), Olaparib (Module 2), Durvalumab (Module 3), AZD5305 (Module 5) | Advanced/metastatic solid tumors, including gastric cancer | Safety, tolerability, pharmacokinetics, preliminary efficacy | Modular design, dose-escalation (Part A), cohort expansion (Part B), food effect and QT interval module | ATR inhibition disrupts DNA repair; synthetic lethality with platinum-based and PARP inhibitors; immunomodulatory effect with durvalumab; selective DDR targeting with AZD5305 |
| NCT04704661 | Phase I/Ib | AZD6738 + trastuzumab deruxtecan (DS-8201a) | Combination vs. monotherapy in dose-expansion phase | Advanced solid tumors with HER2 alterations | Safety, pharmacodynamics, preliminary efficacy; recommended Phase II dose; tolerability | Dose-escalation and dose-expansion; run-in monotherapy cycle; pharmacodynamic biomarker analysis; biorepository | HER2-targeting ADC causing DSBs + ATR inhibition blocking DDR; biomarkers include phosphorylated RAD50, SLFN11, HER2 heterogeneity, TP53, ATM, RAS mutations |
| NCT03641313 | Phase II | Berzosertib (M6620) + irinotecan | Combination | Metastatic/unresectable gastric/GEJ cancer, TP53-mutant focus | Improvement in ORR vs. historical control; secondary: PFS, OS, DOR, TTP | Biomarker-driven trial focusing on TP53 mutation; correlative biomarker analyses | TP53 mutations increase reliance on ATR G2/M checkpoint; synthetic lethality exploited by ATR inhibitor berzosertib |
| NCT04535401 | Phase I | Elimusertib (BAY 1895344) + FOLFIRI | Combination | Gastrointestinal malignancies | Determine MTD; secondary: ORR, PFS, OS, clinical benefit rate; pharmacokinetics; biomarker analyses | Dose-escalation + cohort expansion; paired biopsies and serial imaging | ATM pathway inhibition combined with chemotherapy; exploratory: UGT1A1 genotype, tumor mutation profiles, ATM expression |
| NCT04491942 | Phase I | Elimusertib + cisplatin +/− gemcitabine | Doublet: cisplatin + elimusertib; Triplet: cisplatin + gemcitabine + elimusertib | Advanced solid tumors, incl. metastatic urothelial carcinoma (UC) | Establish MTD, recommended Phase II dose; safety; pharmacokinetics; preliminary anti-tumor activity | Dose-escalation; two treatment arms | Biomarkers of DDR deficiency; somatic gene alterations; circulating tumor DNA (ctDNA); comprehensive genomic/transcriptomic profiling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kciuk, M.; Gruszka, R.; Aleksandrowicz, M.; Śliwińska, A.; Kontek, R. ATR-CHK1 Axis Inhibitors in Gastric Cancer Treatment. Int. J. Mol. Sci. 2025, 26, 7709. https://doi.org/10.3390/ijms26167709
Kciuk M, Gruszka R, Aleksandrowicz M, Śliwińska A, Kontek R. ATR-CHK1 Axis Inhibitors in Gastric Cancer Treatment. International Journal of Molecular Sciences. 2025; 26(16):7709. https://doi.org/10.3390/ijms26167709
Chicago/Turabian StyleKciuk, Mateusz, Renata Gruszka, Marta Aleksandrowicz, Agnieszka Śliwińska, and Renata Kontek. 2025. "ATR-CHK1 Axis Inhibitors in Gastric Cancer Treatment" International Journal of Molecular Sciences 26, no. 16: 7709. https://doi.org/10.3390/ijms26167709
APA StyleKciuk, M., Gruszka, R., Aleksandrowicz, M., Śliwińska, A., & Kontek, R. (2025). ATR-CHK1 Axis Inhibitors in Gastric Cancer Treatment. International Journal of Molecular Sciences, 26(16), 7709. https://doi.org/10.3390/ijms26167709






